Abstract
Background
Restrictive cardiomyopathy (RCM) is a common primary cardiomyopathy of cats. However, little information is available regarding prognostic variables in large populations of cats with RCM.
Objectives
To characterize the epidemiological, clinical, and echocardiographic features of cats with RCM and to document their survival times and risk factors for cardiac death (CD).
Animals
Ninety‐two cats with RCM.
Methods
Retrospective study. Diagnosis of RCM was based on echocardiographic and Doppler criteria. Median survival time to CD and adjusted hazard ratios (HR) were estimated by the Kaplan‐Meier method and multivariate Cox models, respectively.
Results
The feline population (median age [interquartile range], 8.6 years [4.1‐12.4]; body weight, 4.0 kg [3.3‐4.7]) included 83 cats (90%) with the myocardial RCM form and 9 (10%) with the endomyocardial fibrosis RCM form. Most RCM cats (64/92, 70%) were symptomatic at the time of diagnosis, with dyspnea related to congestive heart failure in 57 of 64 cats (89%). The median survival time of the 69 cats with the myocardial RCM form and available follow‐up was 667 days (range, 2‐3710 days) considering CD. Independent of age, biatrial enlargement, and arrhythmias, increase of the left atrium (LA)‐to‐aorta (Ao) ratio (hazard ration [HR], 2.5 per 0.5‐unit increase; 95% confidence interval [CI], 1.5‐4.2; P < .001) and presence of severe LA enlargement (end‐diastolic LA : Ao ≥2; HR, 3.4; 95% CI, 1.3‐8.7; P = .01) were significantly associated with shorter time to CD.
Conclusions and Clinical Importance
Cardiac death is common in RCM cats, and LA enlargement seems independently associated with decreased survival time in these cats.
Keywords: echocardiography, feline, heart, myocardium
Abbreviations
- 2D
2‐dimensional mode
- A
peak velocity of late diastolic transmitral flow
- Ao
aorta
- ATE
aortic thromboembolism
- CD
cardiac death
- CHF
congestive heart failure
- CI
confidence interval
- E
peak velocity of early diastolic transmitral flow
- E : A
ratio of E to A
- EMF
endomyocardial fibrosis
- HCM
hypertrophic cardiomyopathy
- HR
hazard ratio
- IQR
interquartile range
- IRIS
International Renal Interest Society
- IVSd
end‐diastolic interventricular septal thickness
- LA
left atrium
- LV
left ventricle
- LVD
left ventricular diameter at end‐diastole
- LVFWd
left ventricular free wall thickness at end‐diastole
- LVs
left ventricular diameter at end‐systole
- RA
right atrium
- RCM
restrictive cardiomyopathy
- SD
standard deviation
- SF%
shortening fraction (%)
1. INTRODUCTION
Restrictive cardiomyopathy (RCM) in humans represents a heterogeneous group of myocardial diseases characterized by impaired ventricular filling because of decreased myocardial compliance of either or both ventricles with normal or near‐normal systolic function and wall thicknesses.1, 2, 3 In humans, RCM varies considerably according to its origin (inherited or not), histopathological features, pathogenesis, clinical presentation, diagnostic criteria, and prognosis.3
Spontaneously occurring RCM also has been reported in cats.4, 5, 6, 7, 8, 9, 10 Two different histopathological forms of RCM in cats have been described (ie, the “myocardial” form characterized by patchy to diffuse interstitial myocardial fibrosis and, less commonly, the “endomyocardial fibrosis” form [EMF]).4 The hallmark feature of the EMF form is a severe endomyocardial scar that may appear as a distinct “tubular” lesion bridging the interventricular septum (IVS) and the left ventricular free wall (LVFW), or less frequently as diffuse endomyocardial scarring resulting in a decrease or obliteration of the left ventricular (LV) cavity.4
Although RCM currently is considered as a common primary cardiomyopathy of cats,6, 7 little information regarding prognostic factors predictive of survival is available.
The aim of our retrospective study was therefore to (1) assess the main epidemiological and clinical features of a large population of cats with RCM recruited over a 15‐year period (2001‐2015), (2) describe the echocardiographic and Doppler alterations associated with the disease, and (3) analyze the clinical course of the cats investigated and identify factors associated with cardiac death (CD).
2. MATERIALS AND METHODS
2.1. Animals
The case records of client‐owned cats, diagnosed with RCM based on conventional echocardiographic and Doppler examination at the Alfort Cardiology Unit (UCA, National Veterinary School of Alfort, France) between October 2001 and December 2015 were retrospectively reviewed.
The main epidemiological (age, body weight, sex, and breed) and clinical characteristics of the included animals were extracted from computerized databases. The presence of concurrent systemic disease and treatment at diagnosis were recorded. Cats with hyperthyroidism and those with systemic arterial hypertension (systolic arterial blood pressure measured in awake cats by the standard Doppler method [811‐BL; Parks Medical Electronics, Inc, Aloha, Oregon] >160 mm Hg in unstressed animals)11, 12 were not included in the study, nor were cats with systolic cranial motion of the mitral valve or focal hypertrophy of the IVS. The International Renal Interest Society (IRIS) serum creatinine cutoff concentrations were used to assess the renal status of patients (IRIS stages 1‐4), when corresponding data were available.13
2.2. Conventional echocardiography and Doppler examination
Standard transthoracic M‐mode, 2‐dimensional (2D), and Doppler examinations with concomitant ECG tracings were performed by trained observers (Dipl. ECVIM‐CA Cardiology, last year resident, clinician with at least 3 years of experience at our unit) in awake cats by the use of ultrasound units (Vingmed System 5, Vivid 5, Vivid 7, Vivid E9; General Electric Medical System, Waukesha, Wisconsin) equipped with phased‐array transducers, as previously described and validated.14 Data from the original echocardiographic reports were collected. All images additionally were reviewed by 2 board‐certified specialists (V. Chetboul and V. Gouni).
2.2.1. Two‐dimensional and M‐mode measurements
All echocardiographic measurements were performed directly on screen freeze‐frame images and then averaged (3 measurements for each value). Left ventricular end‐diastolic and end‐systolic internal diameters (LVDd and LVDs respectively), LVFW thicknesses at end‐diastole (LVFWd) and end‐systole (LVFWs), IVS thicknesses at end‐diastole (IVSd) and end‐systole (IVSs) were measured by the use of 2D‐guided M‐mode,15, 16 and the LV shortening fraction (SF%) then was calculated. The subaortic interventricular septal (SA‐IVSd) thickness also was measured at end‐diastole by 2D mode from the right parasternal 5‐chamber view at the mitral valve‐chordae tendineae interface and compared with reference ranges, to exclude subaortic focal myocardial hypertrophy.17, 18
For each cat, all M‐mode measurements (IVSd, IVSs, LVFWd, LVFWs, LVDd, LVDs, and SF%) were compared to the 95% prediction intervals assessed according to body weight from a large population of healthy cats.19 The SF% thus was considered as normal if contained within the corresponding 95% prediction interval, and as mildly decreased if below the lower bound of the interval but above the lower bound of the interval minus the interday standard deviation (SD) (5.7%) of SF% assessed by a trained observer from our group.14 The same method was used to analyze myocardial thicknesses and LV diameters and define mild myocardial hypertrophy and mild LV dilatation, while taking into account corresponding interday SD values and the upper bound of the 95% prediction interval.14, 19
The left atrial (LA) and aortic (Ao) diameters were measured at end‐diastole by a 2D method from the right parasternal short axis view, as previously described (Figure 1).14, 17 The LA : Ao ratio then was calculated, and LA enlargement was defined as an LA : Ao > 1.2 (upper cutoff obtained from a population of 100 prospectively recruited healthy cats).18 The right end‐diastolic atrial diameter also was measured at the level of the tricuspid annulus from the 2D right parasternal 4‐chamber view. The right atrium (RA) was judged as dilated if its diameter was wider than the upper reference limit (>15 mm) obtained from a population of 120 healthy adult (≥12 months) cats recruited at the UCA and determined as previously described by applying the statistical procedures recommended by the Clinical and Laboratory Standards Institute guidelines,20, 21 and by using the Tukey method for identifying outliers and the Anderson‐Darling test for evaluating normality of the distribution (Xlstat‐Biomed, Version 2016, Addinsoft, Data Analysis and Statistical Solution for Microsoft Excel, Paris, France). The 2.5th and 97.5th percentiles were used to determine the limits of the reference interval, and the 90% confidence interval (CI) was calculated by a parametric approach.22 The results were as follows: mean RA diameter ± SD = 11.9 ± 1.4 mm; (minimum ‐ maximum) = (7.0‐15.7 mm); lower limit (90% CI) = 9.1 mm (8.8‐9.5); upper limit (90% CI) = 14.7 mm (14.3‐15.1).
Figure 1.
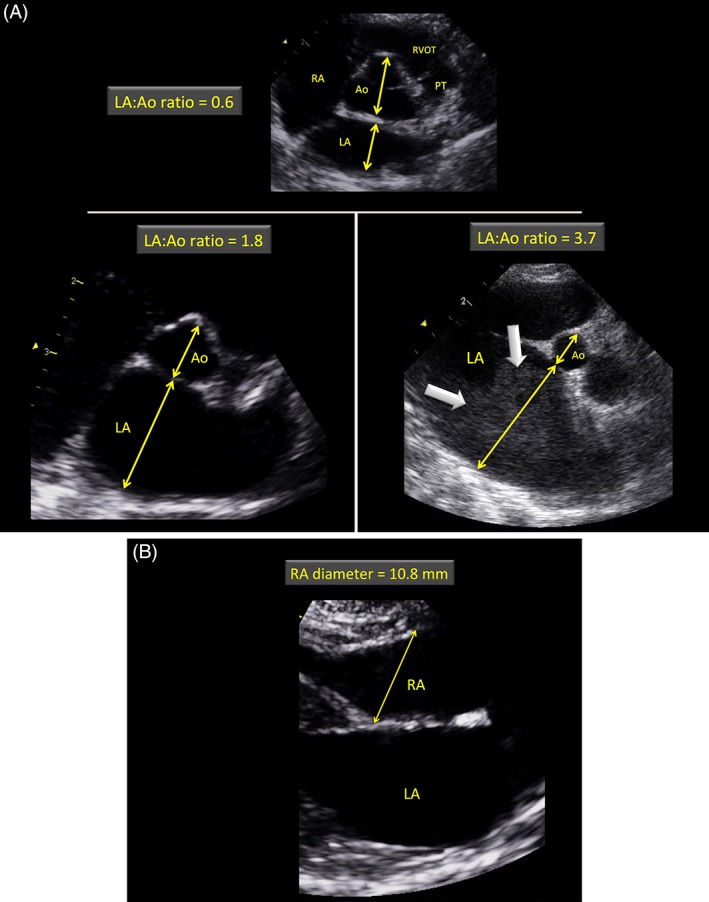
A and B, Examples of echocardiograms demonstrating the left atrial and aortic measurements (A) and the right atrial diameter measurement (B) at end‐diastole, as performed in the present study. A, Short‐axis right‐sided parasternal views obtained at the level of the aortic valve where commissures of the valve cusps are visualized in a healthy cat with a normal left atrium (LA) (top) and 2 cats affected by restrictive cardiomyopathy with moderate (bottom left) and severe (bottom right) left atrial enlargement. The internal short‐axis diameter of the aorta (Ao) was measured along the commissure between the noncoronary and left coronary aortic valve cusps at end‐diastole (ie, first frame before aortic valve opening). The internal short‐axis diameter of the LA was measured using the same frame in a line extending from and parallel to the same commissure to the distant margin of the LA. Note the presence of echo contrast for the cat with severe left atrial dilatation (thick arrows). B, Long‐axis right‐sided parasternal 4‐chamber view optimized for the left (LA) and right atrial (RA) cavities at end‐diastole in a normal cat. The right atrial diameter was measured as shown by the line crossing the tricuspid annulus at end‐diastole (ie, first frame showing tricuspid valve closure). PT, pulmonary trunk; RA, right atrium; RVOT, right ventricular outflow tract
2.2.2. Conventional Doppler examination
Pulsed‐wave and continuous‐wave Doppler modes were used to record blood flow velocities. Maximum early (E) and late (A) diastolic mitral flow velocities were determined using pulsed‐wave Doppler mode from the left apical 4‐chamber view, and the mitral E : A ratio then was calculated. Continuous‐wave Doppler recorded using the left apical 5‐chamber view was used to exclude LV outflow tract obstruction characterized by turbulent aortic flow of high velocity (>2 m/s).18 The absence of systolic cranial motion of the mitral valve also was evaluated using both 2D and M‐modes.23 Color‐flow and spectral Doppler modes also were used to identify potential mitral valve regurgitation and exclude shunting and stenotic lesions.
2.3. Inclusion criteria
Cats in which RCM was diagnosed during the study period were included in the study. The RCM diagnostic criteria were as follows (Figures 1 and 2): included cats should have a restrictive mitral inflow Doppler pattern, characterized by a mitral E : A ratio > 2,5, 24 associated with LA or biatrial enlargement, and a normal or mildly modified LV as defined above. Because tachycardia is a common occurrence in cats, leading to partial or complete fusion of early and late filling waves (thus preventing calculation of E and A waves),24 cats with EA fusion could be included in the study, but only if LA or biatrial enlargement was associated with normal 2D and M‐mode LV variables (ie, IVSd, IVSs, LVFWd, LVFWs, SA‐IVSd, LVDd, LVDs, and SF% within normal ranges),18, 19 so that misdiagnosis with other cardiomyopathies (eg, hypertrophic cardiomyopathy [HCM], dilated cardiomyopathy) would not be possible.
Figure 2.
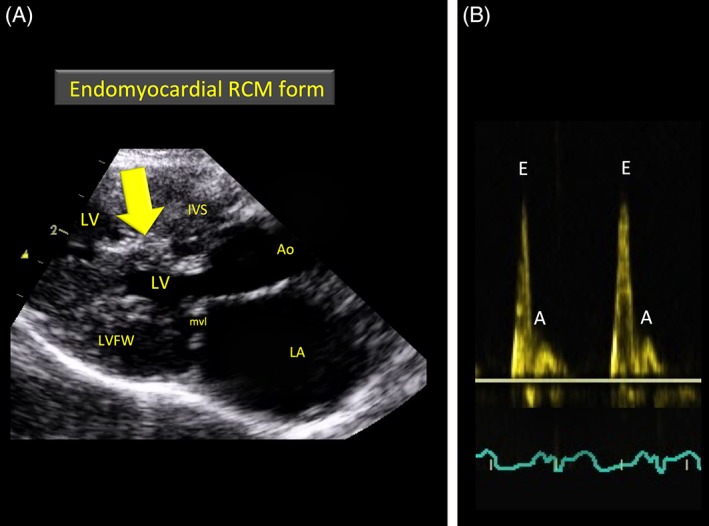
A and B, Representative echocardiograms recorded in 2 cats with restrictive cardiomyopathy. A, Long‐axis right‐sided parasternal 5‐chamber view showing a large bridging scar (arrow) between the interventricular septum (IVS) and left ventricular free wall (LVFW) in a cat with an endomyocardial form of restrictive cardiomyopathy. B, Transmitral pulsed wave Doppler examination showing a typical restrictive filling pattern characterized by an increased E : A ratio (=4.6). Ao, aorta; LA, left atrium; LV, left ventricle; mvl, mitral valve leaflets
The differential diagnosis of RCM forms was established on the 2D morphological aspect of the LV endocardium and myocardium. Unlike the myocardial RCM form, the EMF RCM form typically is characterized by a severe endomyocardial scar that diffusely involves the LV leading to obliteration of the LV cavity or that appears as a distinct lesion bridging the IVS and LVFW at the level of the mid to apical LV chamber (Figure 2).4
2.4. Radiography and ECG
In the case of respiratory signs (eg, dyspnea), thoracic radiographs (lateral and dorsoventral views) were obtained to confirm signs (eg, pulmonary edema) of congestive heart failure (CHF).
All cats with suspected arrhythmias on cardiac auscultation underwent an ECG in addition to concomitant ECG tracings performed during echocardiographic examinations.
2.5. Follow‐up
Follow‐up data were collected by reviewing the paper and electronic records of cats diagnosed with RCM as defined above. At the time of diagnosis, cats were classified as either clinically or subclinically affected, that is, with or without clinical signs attributed to RCM, respectively (eg, dyspnea, ascites, syncope, aortic thromboembolism [ATE]). Owners of cats for which the outcome could not be found in the database at the time of writing were contacted by telephone or e‐mail to determine the current status of their animals: alive (clinically or subclinically affected) or dead (date and cause of death, defined as cardiac or noncardiac). Cases of animals dying suddenly, euthanized for cardiac reasons or dying from nonresponsive CHF or ATE, were considered as CD. Sudden deaths included sudden witnessed deaths and suspected sudden deaths (cats found dead at home without an obvious cause and completely asymptomatic during the previous 24 hours). Cats for which the outcome could not be obtained at the time of writing were considered lost to follow‐up and consequently were censored at the time of their last examination. Cats that survived <24 hours or that had no available follow‐up were excluded from the survival analysis.
2.6. Statistical analysis
Commercially available software (Addinsoft (2019). XLSTAT statistical and data analysis solution. Long Island, NY, USA) was used for the statistical analyses. Data were expressed as proportions (%), medians, and interquartile ranges (IQR). Median survival times to all‐cause death and to cardiac death both were estimated using the Kaplan‐Meier method and compared by applying a log rank test. Regarding CD, adjusted hazard ratios (HR) were estimated using multivariate Cox proportional hazard models. We hypothesized that the following variables were risk factors for CD: end‐diastolic LA : Ao ratio (as quantitative variable and binary variable using LA : Ao ratio ≥ 2 corresponding to severe LA enlargement versus LA : Ao ratio < 2), biatrial enlargement (ie, presence of an RA dilatation), arrhythmias, RCM form (myocardial versus EMF), signs of CHF, increased IRIS stages (IRIS stages ≥2 versus IRIS stage = 1), and ATE. A Cox regression model was performed for each of the above exposures. Each model was adjusted for age (ie, age was a “forced” variable in each model) and for all variables considered potential confounding factors (ie, variables statistically associated with CD and associated with each above‐mentioned variable of interest at P < .20). Hazard ratios and 95% CI were calculated. The level of significance was set at P < .05.
3. RESULTS
3.1. Study population
Between October 2001 and December 2015, 131 cats were diagnosed with RCM. After a final review, 92 of them met the inclusion criteria and 39 were not included in the study either as a consequence of IVS or LVFW thicknesses being greater than the chosen cutoff values (n = 7) or because of mildly modified LV without any mitral E and A waves values (EA fusion, n = 7; data unavailable, n = 11). The remaining 14 cats were not included in the study owing to hyperthyroidism (n = 13) and the absence of LA enlargement despite the presence of a large lesion bridging the IVS and LVFW, consistent with an EMF RCM form (n = 1). After exclusion of the 39 cats initially recruited for the study, all cats finally included (n = 92) had normal 2D and M‐mode LV variables (ie, IVSd, IVSs, LVFWd, LVFWs, SA‐IVSd, LVDd, LVDs, and SF% within normal ranges)18, 19 associated with LA or biatrial enlargement (see details below).
The study sample thus consisted of 92 cats with RCM (median age [IQR], 8.6 years [4.1‐12.4]; body weight, 4.0 kg [3.3‐4.7]), with 45 males (17 intact and 28 neutered) and 47 females (12 intact and 35 neutered) from 9 different breeds with a majority of Domestic Shorthairs (64/92, 70%), followed by Birmans (9/92, 10%), Persians (7/92, 8%), Siamese cats (6/92, 7%), and other breeds (6/92, 7%).
3.2. Clinical findings at the time of diagnosis
Most recruited cats (64/92, 70%) had at least 1 clinical sign related to RCM at the time of diagnosis. The most common reported clinical sign was dyspnea related to CHF, found in 57 of 64 (89%) cats, either alone (47/57, 82%) or associated with syncope (1/57, 2%), ascites (8/57, 14%) or bilateral hind limb paralysis as a result of ATE (1/57, 2%). The remaining 7 cats with clinical signs related to RCM had ascites (n = 3) or bilateral hind limb paralysis caused by ATE (n = 4). In addition to the 60 cats with clinical signs of CHF at diagnosis (ie, dyspnea, ascites or both), 3 other cats (3%) received furosemide because of CHF before inclusion and had no clinical signs at the time of diagnosis. Therefore, 63 of 92 RCM cats (68%) included in the present study had past (3%) or current (65%) CHF signs.
Data regarding cardiac auscultation at the time of diagnosis were available for 86 of the 92 included RCM cats. Cardiac auscultation was abnormal for most cats (70/86, 81%), with 1 to 3 abnormalities for each. The most common abnormality was a left apical systolic heart murmur (54/70, 77%). A gallop sound was recorded in 22 cats (31%) and arrhythmias in 16 cats (23%), including supraventricular (n = 4) and ventricular premature beats (n = 6), atrial fibrillation (n = 1), and third‐degree atrioventricular block (n = 5).
In 54 of 92 cats (59%), RCM was associated with a concomitant systemic disease, of which 19 (35%) were infectious diseases (eg, feline immunodeficiency virus infection, pyometra, urinary infection, bronchitis, feline infectious peritonitis, pyothorax).
Treatment status at the time of RCM diagnosis was known for all cats. Of 92 cats, 48 (52%) cats were not being treated, whereas 44 (48%) cats were receiving 1 or a combination of the following drugs that had been prescribed previously by referring veterinarians: furosemide (n = 31), benazepril (n = 16), spironolactone (n = 2), diltiazem (n = 3), aspirin (n = 4), clopidogrel (n = 2), and heparin (n = 2).
3.3. Conventional echocardiographic findings
All included cats (n = 92) had myocardial thicknesses as well as LV diameters and SF% within normal ranges, including 41 cats with an available E : A ratio. Echocardiographic and Doppler variables obtained in the study population are listed in Table 1. Two‐dimensional and M‐mode echocardiography determined that the most common RCM form was myocardial RCM (83/92, 90%), followed by the EMF RCM form characterized by a tubular lesion bridging the IVS and LVFW (9/92, 10%). As an inclusion criterion, all recruited cats showed at least LA enlargement, which was considered severe in 22 of 92 cases (24%). Additionally, biatrial enlargement was detected in 31 of the 81 cats (38%) for which the RA diameter was measured.
Table 1.
Echocardiographic and Doppler variables assessed in the whole restrictive cardiomyopathy (RCM) study population (n = 92) and in each RCM form
| Whole study population (n = 92) | Myocardial form (n = 83) | EMF (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | 1st‐3rd quartile | n | Median | 1st‐3rd quartile | n | Median | 1st‐3rd quartile | |
| M‐mode echocardiographic variables | |||||||||
| IVSd (mm) | 92 | 4.3 | 3.6‐4.8 | 83 | 4.4 | 3.7‐4.9 | 9 | 3.9 | 3.6‐4.4 |
| LVd (mm) | 92 | 16.2 | 14.0‐17.8 | 83 | 16.1 | 14.0‐17.7 | 9 | 18.5 | 16.5‐19.5 |
| LVFWd (mm) | 92 | 4.4 | 3.7‐4.8 | 83 | 4.6 | 3.7‐5.0 | 9 | 4.3 | 3.9‐4.5 |
| IVSs (mm) | 92 | 7.0 | 5.8‐7.6 | 83 | 7.0 | 6.1‐7.7 | 9 | 6.1 | 5.2‐7.2 |
| LVs (mm) | 92 | 8.0 | 6.6‐9.9 | 83 | 7.8 | 6.6‐9.2 | 9 | 11.2 | 7.6‐11.9 |
| LVFWs (mm) | 92 | 7.6 | 6.4‐8.2 | 83 | 7.7 | 6.5‐8.3 | 9 | 7.5 | 6.9‐8.1 |
| SF% | 92 | 48 | 43‐53 | 83 | 49 | 44‐55 | 9 | 42 | 40‐45 |
| Two‐dimensional echocardiographic variables | |||||||||
| SA‐IVSd (mm) | 79 | 4.1 | 3.5‐4.7 | 70 | 4.1 | 3.5‐4.7 | 9 | 4.0 | 2.9‐4.8 |
| LA : Ao ratio at end‐diastole | 91 | 1.7 | 1.4‐1.9 | 82 | 1.7 | 1.4‐1.9 | 9 | 2.0 | 1.7‐2.1 |
| RA (mm) | 81 | 13.6 | 11.9‐16.1 | 73 | 13.8 | 12.0‐16.1 | 8 | 12.0 | 11.0‐14.0 |
| Conventional Doppler variable | |||||||||
| Mitral E : A ratio | 41 | 2.8 | 2.5‐3.3 | 35 | 2.8 | 2.6‐3.3 | 6 | 2.6 | 2.3‐3.5 |
Abbreviations: Ao, aorta; EMF, endomyocardial fibrosis; IVSd, end‐diastolic interventricular septal thickness; IVSs, end‐systolic interventricular septal thickness; LA, left atrium; LVd, end‐diastolic left ventricular internal diameter; LVFWd, end‐diastolic left ventricular free wall thickness; LVFWs, end‐systolic left ventricular free wall thickness; LVs, end‐systolic left ventricular internal diameter; RA, end‐diastolic right atrial diameter; SA‐IVSd, subaortic interventricular septal thickness measured at end‐diastole; SF%, shortening fraction.
A spontaneous echo contrast (smoke) was found in 7 of the 92 included cats (8%), either in the left (n = 4) or in both (n = 3) atrial cavities. One of these cats had an ATE episode at the time of diagnosis.
Finally, as assessed by color‐flow Doppler mode, mild mitral valve regurgitation, most commonly detected in early systole or seen just beyond the coaptation point of the mitral valve leaflets, associated with irregular and slightly thickened mitral valve leaflets, was detected in all cats with left apical systolic heart murmurs.
3.4. Cardiac events and survival
At the time of writing, the alive versus dead status was known for 72 of 92 cats (78%). Among these cats, 58 of 72 (81%) had died, 21 (36%) from noncardiac‐related causes (cancer [n = 6], nephrologic and urologic [n = 3], gastrointestinal [n = 3], neurological [n = 1], endocrine [n = 2], infectious diseases [n = 3], and unknown [n = 3]), and 37 (64%) from cardiac‐related causes with 16% (6/37) occurring within the first 24 hours after the initial RCM diagnosis (spontaneous death or euthanasia for acute CHF or ATE). Among the 37 cats with CD, 34 (92%) died or were euthanized because of progressive and drug refractory CHF as confirmed for all cats by thoracic radiographs (n = 33; 89%) or ATE (n = 1; 3%), and only 3 of 37 (8%) died suddenly. Among the 14 cats of the 19 RCM cats with infectious diseases and available follow‐up, 13 cats died, including 5 (36%) for cardiac reasons (progressive and drug refractory CHF).
Fourteen of the 92 cats included in the study were excluded from survival analysis, either because of early death within the first 24 hours (n = 7, including 6 cardiac deaths and 1 noncardiac death, that is, euthanasia after polytraumatic shock) or because of unavailable follow‐up (n = 7). Among the 78 remaining cats with available follow‐up (9 cats with the EMF RCM form and 69 with the myocardial RCM form), 60 had mild to moderate LA enlargement (LA : Ao < 2). For all cats with mild LA enlargement (ie, LA : Ao ratio within the first quartile; n = 17), the actual LA diameter could have been underestimated to an unknown extent because of dehydration (n = 6), ongoing treatments (n = 17), or both prescribed by referring veterinarians, including furosemide (n = 13; mean [range] dosage, 3.4 mg/kg/day [2‐6 mg/kg/day]), benazepril (n = 2), and spironolactone (n = 2). Among these 17 cats, 13 had past or current CHF related to RCM and 11 also had RA enlargement.
The median survival time of the 69 cats with the myocardial RCM form included in the survival analysis was 436 days (range, 2‐3710 days) and 667 days (range, 2‐3710 days) considering all‐cause death and CD, respectively (Figures 3 and 4). Regarding survival, no significant difference was observed among cats with available E : A ratio and those without (P = .40).
Figure 3.
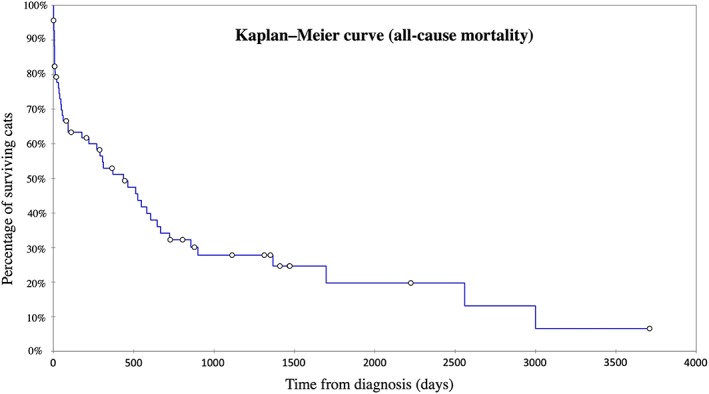
Kaplan‐Meier curve illustrating survival time from initial restrictive cardiomyopathy diagnosis to all‐cause death for 69 cats out of the 83 cats included in the study with the myocardial restrictive cardiomyopathy form (excluding the 7 cats that died <24 hours after diagnosis and the 7 cats with unavailable follow‐up). Median (min‐max) median survival time from diagnosis was 436 days (2‐3710). Circles denote censored observations
Figure 4.
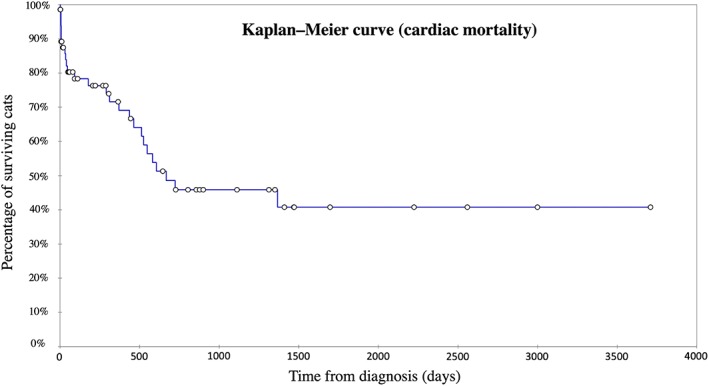
Kaplan‐Meier curve illustrating survival time from initial restrictive cardiomyopathy diagnosis to cardiac‐related death for 69 of the 83 cats included in the study with the myocardial restrictive cardiomyopathy form (excluding the 7 cats that died <24 hours after diagnosis and the 7 cats with unavailable follow‐up). Median (min‐max) median survival time from diagnosis was 667 days (2‐3710). Circles denote censored observations
Results of univariate and multivariate Cox proportional hazards analyses performed on the 69 cats with the myocardial RCM form are presented in Table 2.
Table 2.
Hazard ratios (HRs) for cardiac death associated with variables of interest (LA : Ao ratio, arrhythmia, congestive heart failure, biatrial enlargement, RCM form, aortic thromboembolism, and International Renal Interest Society [IRIS] stagea)
| Exposures | Exposed cats (n/N) | Crude associations | Adjusted associations | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| LA : Ao ratio (per 0.5‐unit increase) | ‐ | 2.7 | 1.4‐3.4 | <.001 | 2.5b | 1.5‐4.2 | <.001 |
| Left atrial enlargement (LA : Ao ≥ 2 versus LA : Ao < 2) | 15/69 | 2.5 | 1.1‐5.7 | .02 | 3.4b | 1.3‐8.7 | .01 |
| Arrhythmia (yes versus no) | 14/69 | 2.46 | 1.1‐5.9 | .02 | 2.0c | 0.8‐4.9 | .13 |
| Congestive heart failure (yes versus no) | 50/69 | 1.6 | 0.6‐4.0 | .31 | 2.3d | 0.8‐6.6 | .11 |
| Biatrial enlargement (yes versus no) | 28/69 | 1.7 | 0.8‐3.6 | .20 | 1.0e | 0.4‐2.2 | .98 |
| Aortic thromboembolism (yes versus no) | 5/69 | 0.5 | 0.1‐3.6 | .48 | 0.8c | 0.1‐5.8 | .78 |
| IRIS stage (IRIS stage ≥2 versus IRIS stage = 1) | 21/46 | 0.7 | 0.3‐1.6 | .38 | 0.6f | 0.2‐1.8 | .39 |
Abbreviations: Ao, aorta; CI, confidence interval; LA, left atrium; RCM, restrictive cardiomyopathy.
Bold values indicate statistically significant P‐value.
Univariate and multivariate Cox proportional hazards analyses were performed on the 69 cats with the myocardial RCM form and an available follow‐up.
Adjusted for age, biatrial enlargement, and arrhythmia.
Adjusted for age, LA : Ao ratio.
Adjusted for age and biatrial enlargement.
Adjusted for age, LA : Ao ratio, and arrhythmia.
Adjusted for age, LA : Ao ratio and biatrial enlargement.
The univariate analysis showed that arrhythmia and LA enlargement, as assessed by the LA : Ao ratio, were associated with shorter time to CD. After adjustment for potential confounders, only the association between LA : Ao ratio and CD remained statistically significant. Of note, body weight and sex were not identified as confounders. Thus, independently of age, biatrial enlargement, and arrhythmias, the time to CD was significantly shorter per each 0.5‐unit increase in LA : Ao ratio (HR, 2.5; 95% CI, 1.5‐4.2; P < .001). In other words, the LA : Ao ratio was identified as a risk factor for CD, with a 0.5‐unit increase in LA : Ao ratio associated with a >2‐fold increased risk of CD. Similarly, time to CD was significantly shorter for cats with severe LA enlargement (LA : Ao ratio ≥ 2) than for others (LA : Ao ratio < 2; adjusted HR, 3.4; 95% CI, 1.3‐8.7; P = .01).
No statistical association was found between CD and the other tested exposures (biatrial enlargement, arrhythmias, CHF, IRIS stage [IRIS stage ≥2 versus IRIS stage = 1], and ATE).
4. DISCUSSION
Previous reports have provided relevant information on clinical, echocardiographic, and pathological features of RCM in cats.4, 5, 6, 7, 8, 9, 10, 25, 26 Survival times also have been reported in several studies.5, 6, 7, 8, 10 Our study provides additional information, particularly regarding the effects of epidemiological, clinical, and echocardiographic variables on prognosis in a large population of cats with RCM.
Most cats with RCM in our study were middle‐aged animals (median age, 8.6 years), as reported by others (mean or median age from 6 to 10 years).5, 6, 7, 8, 10 Nevertheless, like RCM cats in previous studies,5, 6, 7, 8, 25 the age range was wide, thus highlighting the presence of RCM in very young (<1 year old) but also in very old (>15 years old) cats, similar to the most common cardiomyopathy of cats (HCM).27, 28, 29
No breed predisposition to RCM was found in our study. No sex predisposition was found either, as 49% of the included RCM cats were males. By comparison, in another report involving a similar population of cats with RCM, 59% of the RCM cats were males.10
Interestingly, in our study, RCM was associated with a concomitant systemic disease in >50% of the 92 included cats, of which 35% had infectious diseases (eg, feline immunodeficiency virus infection, pyometra, urinary infection, bronchitis, feline infectious peritonitis, pyothorax). Similarly, in another report, extracardiac inflammatory lesions (eg, interstitial pneumonia) were found in nearly 30% of cats with LV endocardial fibrosis.25 Additionally, Bartonella spp. recently has been identified as a possible cause or cofactor of the endomyocarditis–LV endocardial fibrosis complex in cats.30
Most cats (70%) included in our study had at least 1 clinical sign related to RCM at the time of diagnosis, with a clear predominance of CHF signs detected in 94% of these cats. These results are in accordance with those of other reports, showing that most cats with RCM show clinical signs at the time of diagnosis, predominantly related to CHF.5, 8, 10 This high proportion of cats with CHF may be related, at least in part, to the chosen diagnostic criteria of RCM: all RCM cats in our study had to present with LA enlargement (which was considered severe in 24% of cases and associated with RA enlargement in >33% of cases). In another report, hind limb paresis or paralysis associated with ATE was very common at presentation (41%),8 whereas only 5 cats (5%) were diagnosed with ATE in our study, and 12% to 14% were previously reported.5, 10 Nevertheless, cats from the former report8 were recruited from necropsies performed on cats with heart diseases (and not from clinical examinations as in our study and the 2 other studies5, 10).
In our study, cardiac auscultation also was abnormal in the majority of RCM cats (81%), owing to left apical systolic heart murmurs caused by mild mitral valve regurgitation, and a gallop sound and various supraventricular and ventricular arrhythmias occurred in about 33% and 25% of the cases, respectively. Such auscultation abnormalities also have been described by other authors but in variable proportions.5, 6, 7, 8, 10 The likelihood of having included cats with pure mitral regurgitation in our study, instead of cats with RCM, is not high. According to our inclusion criteria, all included cats should have had an enlarged LA, which is not possible with mild mitral valve regurgitation.
As shown by other authors,5, 6, 7, 8, 10 our data confirm that RCM in cats has a major impact on the clinical course and survival of affected animals, although overall survival time is highly variable. More than 67% of the recruited cats had past or current CHF at the time of RCM diagnosis. Therefore, as expected, CD was very common despite medical treatment, occurring in >50% of RCM cats with an available follow‐up, nearly 20% of them experiencing CD within the first 24 hours owing to acute CHF or ATE. Similarly, in another report,5 91% of RCM cats were presented with CHF at diagnosis, and their survival thereafter was limited, with 60% of CDs reported 0.1 to 52 months after diagnosis and a median survival time of only 3.4 months. Similarly, in another report, CD occurred in 83% of the 60 cats with an available follow‐up,10 and in another series most of the cats with RCM died of cardiac‐related causes (86%), compared with 44% of cats with HCM and only 20% of cats with secondary cardiomyopathy.7 Another study also reported a longer survival time for cats with unclassified cardiomyopathy (925 days) and HCM (492 days), as compared with those with RCM (132 days).6 However, the wide range of survival times reported in these retrospective studies, including our study, has originated from single‐site referral centers, leading to potential referral bias. Further multicenter studies thus are needed to assess cardiovascular morbidity and survival in RCM cats from large cohorts recruited in many different countries around the world, as was done recently for cats with HCM.31
Lastly, in our study, LA enlargement was significantly associated with decreased survival time in cats with RCM, as also reported for cats with HCM.27 Similarly, in another report, in a population including 60 RCM cats with an available follow‐up, in the univariate Cox analysis, LA enlargement (mild‐to‐moderate versus severe) as well as respiratory distress and pleural effusion showed statistically significant effects on survival (although in the multivariate Cox regression backward analysis, only respiratory distress showed a statistically significant effect on survival).10 In our study, independent of age, biatrial enlargement, and arrhythmias, the risk of CD was increased by 2.5‐fold for a 0.5‐unit LA : Ao ratio increase. In other words, from a practical point of view, a 0.5‐unit difference in LA : Ao ratio between 2 RCM cats was associated with a >2‐fold increased risk of CD (eg, when comparing cats with LA : Ao values of 2.0 and cats with LA : Ao values of 1.5). Additionally, severe LA enlargement was associated with a 3.4‐fold increase in the risk of CD.
Our study had several limitations. As mentioned above, the RCM diagnosis was based on echocardiographic and Doppler criteria and was not confirmed by pathological examination of the heart. Moreover, owing to E and A wave fusion, Doppler evaluation of mitral valve inflow (which is 1 of the main imaging diagnostic tools used for RCM diagnosis) could be obtained for only 41 of the 92 cats included in the study. However, as an inclusion criterion (in order to avoid any misdiagnosis with other cardiomyopathies), the remaining 51 cats with fused E and A waves all showed LA or biatrial enlargement associated with normal 2D and M‐mode LV variables (ie, myocardial thicknesses, LV diameters, and SF% within normal ranges). Doppler evaluation of mitral valve inflow however presents several limitations, which have been well described in human patients: only advanced RCM stages are characterized by restrictive patterns (indicative of markedly increased LV filling pressures) with, additionally, some overlap between RCM patients and healthy controls and some variations according to ongoing medical treatments.32, 33, 34, 35, 36 This explains the development of complementary diagnostic criteria such as pulmonary venous Doppler, tissue Doppler imaging, and more recently speckle tracking imaging criteria that were not used in our study, and this may represent another limitation.
Moreover, in our study, the chosen imaging criteria were very strict, thus limiting the number of RCM cats included (39 cats were not included due either to myocardial thicknesses greater than the chosen cutoff values or to the absence of mitral E and A waves values, concomitant hyperthyroidism, and absence of LA enlargement despite the presence of a large lesion bridging the IVS and LVFW consistent with the EMF RCM form). Other limitations are related to the retrospective nature of the study. Values for several clinical variables such as auscultation were unavailable for some animals. Regarding LA measurement, a recurring problem is left auricular enlargement that is commonly disproportionate as compared to atrial size. Nevertheless, in our study, all images were reviewed by 2 board‐certified specialists, who thoroughly determined that the left auricle was not taken into account in left atrial measurements (as shown in Figure 1). Additionally, such measurement errors would have been “nondifferential misclassification errors”, leading to nondifferential misclassification bias. Therefore, without such errors, the association between the LA : Ao ratio and CD would have remained significant.
Conversely, the long survival of some cats observed in our study could raise the possibility of having included normal animals as mildly affected RCM cats, leading to a significant association between LA : Ao ratio and time to CD, all the more because “supernormal” filling patterns closely resembling restrictive filling already have been described in healthy young cats.24 However, this hypothesis seems unlikely because all cats with mild LA enlargement were not healthy: >75% of them had past or current CHF related to RCM, >50% also showed RA enlargement, and for all of these cats, the actual LA diameter could have been underestimated to an unknown extent owing to ongoing medical treatments at the time of diagnosis, dehydration, or both.
Lastly, in our study, each multivariate model was based on the assumption of the absence of interaction among exposures included in the model. Therefore, such potential interactions should be further investigated.
In conclusion, despite the above‐noted limitations, some important conclusions can be drawn from our data. Although the overall survival time was highly variable, CD was common in RCM cats, representing 64% of all‐cause deaths, with LA enlargement being significantly associated with decreased survival time. Because these results were obtained from a single‐site retrospective study, further prospective multicenter studies involving large cohorts of cats from different countries are now needed to more accurately evaluate short‐ and long‐term survival associated with RCM in cats.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
The authors sincerely acknowledge Dr. Justine Bellin as well as the Regional Council of the Martinique for the research grant funded to Dr. Peggy Passavin at the Alfort Cardiology Unit (UCA). Part of this study was presented as a poster at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, MD.
Chetboul V, Passavin P, Trehiou‐Sechi E, et al. Clinical, epidemiological and echocardiographic features and prognostic factors in cats with restrictive cardiomyopathy: A retrospective study of 92 cases (2001‐2015). J Vet Intern Med. 2019;33:1222–1231. 10.1111/jvim.15464
REFERENCES
- 1. Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841‐842. [DOI] [PubMed] [Google Scholar]
- 2. Falk RH, Hershberger RE. The dilated, restrictive, and infiltrative cardiomyopathies Braunwald's Heart Disease. 10th ed. Philadelphia, PA: Elsevier Saunders; 2015:1551‐1573. [Google Scholar]
- 3. Muchtar E, Blauwet LA, Gertz MA. Restrictive cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121:819‐837. [DOI] [PubMed] [Google Scholar]
- 4. Fox PR. Endomyocardial fibrosis and restrictive cardiomyopathy: pathologic and clinical features. J Vet Cardiol. 2004;6:25‐31. [DOI] [PubMed] [Google Scholar]
- 5. Fox PR, Basso C, Thiene G, Maron BJ. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: a new animal model of human disease. Cardiovasc Pathol. 2014;23:28‐34. [DOI] [PubMed] [Google Scholar]
- 6. Ferasin L, Sturgess CP, Cannon MJ, Caney SMA, Gruffydd‐Jones TJ, Wotton PR. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg. 2003;5:151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spalla I, Locatelli C, Riscazzi G, Santagostino S, Cremaschi E, Brambilla P. Survival in cats with primary and secondary cardiomyopathies. J Feline Med Surg. 2016;18:501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura Y, Fukushima R, Hirakawa A, et al. Epidemiological and clinical features of the endomyocardial form of restrictive cardiomyopathy in cats: a review of 41 cases. J Vet Med Sci. 2016;78:781‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimura Y, Karakama S, Hirakawa A, Tsuchiaka S, Kobayashi M, Machida N. Pathological features and pathogenesis of the endomyocardial form of restrictive cardiomyopathy in cats. J Comp Pathol. 2016;155:190‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Locatelli C, Pradelli D, Campo G, et al. Survival and prognostic factors in cats with restrictive cardiomyopathy: a review of 90 cases. J Feline Med Surg. 2018;20:1138‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21:542‐558. [DOI] [PubMed] [Google Scholar]
- 12. Gouni V, Tissier R, Misbach C, et al. Influence of the observer's level of experience on systolic and diastolic arterial blood pressure measurements using Doppler ultrasonography in healthy, conscious cats. J Feline Med Surg. 2015;17:94‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polzin DJ. Chronic kidney disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. Philadelphia, PA: WB Saunders; 2010:1990‐2021. [Google Scholar]
- 14. Chetboul V, Concordet D, Pouchelon JL, et al. Effects of inter‐ and intra‐observer variability on echocardiographic measurements in awake cats. J Vet Med a Physiol Pathol Clin Med. 2003;50:326‐331. [DOI] [PubMed] [Google Scholar]
- 15. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography committee of the specialty of cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 16. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072‐1083. [DOI] [PubMed] [Google Scholar]
- 17. Chetboul V, Petit A, Gouni V, et al. Prospective echocardiographic and tissue Doppler screening of a large Sphynx cat population: reference ranges, heart disease prevalence and genetic aspects. J Vet Cardiol. 2012;14:497‐509. [DOI] [PubMed] [Google Scholar]
- 18. Chetboul V, Carlos Sampedrano C, Tissier R, et al. Quantitative assessment of velocities of the annulus of the left atrioventricular valve and left ventricular free wall in healthy cats by use of two‐dimensional color tissue Doppler imaging. Am J Vet Res. 2006;67:250‐258. [DOI] [PubMed] [Google Scholar]
- 19. Häggström J, Andersson ÅO, Falk T, et al. Effect of body weight on echocardiographic measurements in 19,866 pure‐bred cats with or without heart disease. J Vet Intern Med. 2016;30:1601‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misbach C, Lefebvre HP, Concordet D, et al. Echocardiography and conventional Doppler examination in clinically healthy adult cavalier king Charles spaniels: effect of body weight, age, and gender, and establishment of reference intervals. J Vet Cardiol. 2014;16:91‐100. [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute (CLSI) . Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guidelines, CLSI Document C28‐A3, vol. 28, no. 3. 3rd ed. Wayne, PA: Clinical and Laboratory Standard; 2008. [Google Scholar]
- 22. Geffre A, Concordet D, Braun JP, Trumel C. Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol. 2011;40:107‐112. [DOI] [PubMed] [Google Scholar]
- 23. Schober K, Todd A. Echocardiographic assessment of geometry and the mitral valve apparatus in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2010;12:1‐16. [DOI] [PubMed] [Google Scholar]
- 24. Schober KE, Chetboul V. Echocardiographic evaluation of left ventricular diastolic function in cats: hemodynamic determinants and pattern recognition. J Vet Cardiol. 2015;17(Suppl 1):S102‐S133. [DOI] [PubMed] [Google Scholar]
- 25. Stalis IH, Bossbaly MJ, Van Winkle TJ. Feline endomyocarditis and left ventricular endocardial fibrosis. Vet Pathol. 1995;32:122‐126. [DOI] [PubMed] [Google Scholar]
- 26. McEndaffer L, Molesan A, Erb H, Kelly K. Feline panleukopenia virus is not associated with myocarditis or endomyocardial restrictive cardiomyopathy in cats. Vet Pathol. 2017;54:669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2013;27:1427‐1436. [DOI] [PubMed] [Google Scholar]
- 28. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990‐1999). J Am Vet Med Assoc. 2002;220:202‐207. [DOI] [PubMed] [Google Scholar]
- 29. Trehiou‐Sechi E, Tissier R, Gouni V, et al. Comparative echocardiographic and clinical features of hypertrophic cardiomyopathy in 5 breeds of cats: a retrospective analysis of 344 cases (2001‐2011). J Vet Intern Med. 2012;26:532‐541. [DOI] [PubMed] [Google Scholar]
- 30. Donovan TA, Balakrishnan N, Carvalho Barbosa I, McCoy T, Breitschwerdt EB, Fox PR. Bartonella spp. as a possible cause or cofactor of feline endomyocarditis‐left ventricular endocardial fibrosis complex. J Comp Pathol. 2018;162:29‐42. [DOI] [PubMed] [Google Scholar]
- 31. Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the REVEAL study. J Vet Intern Med. 2018;32:930‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321‐1360. [DOI] [PubMed] [Google Scholar]
- 33. Klein AL, Hatle LK, Taliercio CP, et al. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991;83:808‐816. [DOI] [PubMed] [Google Scholar]
- 34. Sasaki N, Garcia M, Ko HH, Sharma S, Parness IA, Srivastava S. Applicability of published guidelines for assessment of left ventricular diastolic function in adults to children with restrictive cardiomyopathy: an observational study. Pediatr Cardiol. 2015;36:386‐392. [DOI] [PubMed] [Google Scholar]
- 35. Satpathy C, Mishra TK, Satpathy R, Satpathy HK, Barone E. Diagnosis and management of diastolic dysfunction and heart failure. Am Fam Physician. 2006;73:841‐846. [PubMed] [Google Scholar]
- 36. Hunt SA. American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to update the 2001 guidelines for the evaluation and management of heart failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2005;46:e1‐e82. [DOI] [PubMed] [Google Scholar]


