Abstract
Background
Severity of lung lesions quantified by thoracic ultrasonography (TUS) at time of bronchopneumonia (BP) diagnosis predicted death among steers not treated for this condition. Further research is needed to confirm that lung lesions detected by TUS can be associated with negative outcomes in cattle with BP that subsequently were treated.
Objective
To quantify the effects on relapse rate and average daily gain (ADG) of lung lesions detected by TUS at first BP diagnosis in feedlot cattle.
Animals
Prospective cohort of mixed beef‐breed steers (n = 93; 243 ± 36 kg) and heifers (n = 51; 227 ± 42 kg) with BP at 4 feedlots.
Methods
Thoracic ultrasonography was performed by the same clinician and 16‐second TUS videos were evaluated offline for maximal depth and area of lung consolidation, maximum number of comet tails, and maximal depth of pleural fluid. Individual ADG was calculated between 1 and 120 days after arrival. Effects of lesions on relapse rate and ADG were investigated using mixed regression models.
Results
Maximal depth of lung consolidation was associated with a higher risk of relapse (odds ratio [OR], 1.337/cm; 95% confidence interval [CI], 1.042‐1.714) and lower ADG (− 34 g/cm; −64 to −4). Maximal area of lung consolidation also was associated with a higher relapse risk (OR, 1.052/cm2; 1.009‐1.097) but not with ADG. Comet tails and pleural fluid were not associated with risk of relapse or ADG.
Conclusions and Clinical Importance
Quantifying maximal depth and area of lung consolidation by TUS at first BP diagnosis can provide useful prognostic information in feedlot cattle.
Keywords: average daily gain, bovine respiratory disease, lung consolidation, prognosis, shipping fever
Abbreviations
- ADG
average daily gain
- BP
bronchopneumonia
- CI
confidence interval
- OR
odds ratio
- ROC curves
receiver‐operating characteristic curves
- TUS
thoracic ultrasonography
1. INTRODUCTION
Accurate diagnosis of bronchopneumonia (BP) remains a challenge in feedlot cattle.1 In a recent meta‐analysis, BP diagnosis based on clinical illness had low diagnostic sensitivity and moderate diagnostic specificity.2 Furthermore, current chute‐side confirmatory tests such as rectal temperature measurement have limited prognostic value to indicate whether calves with clinical signs of BP would recover and finish the production cycle.3
Thoracic ultrasonography (TUS) has the potential to improve diagnostic specificity of BP diagnosis4 and can be used to identify cattle at risk of unfavorable outcome.5 In a recent pilot study,5 maximal depth and area of lung consolidation in feedlot cattle with clinical BP were significantly associated with risk of dying within 15 days after diagnosis (with odds ratio [OR] estimates of negative outcome ranging from 6 to 30).5 Although very informative, this study only included 29 BP cases and enrolled cattle that did not receive antimicrobial treatment after BP diagnosis, which is not the industry standard and thus limited generalization of the findings. Therefore, the objective of our cohort study was to determine how the severity of lung lesions quantified by TUS at first BP diagnosis affected the risk of relapse and growth in feedlot cattle.
2. MATERIALS AND METHODS
2.1. Animals and study design
Mixed beef‐breed steers and heifers at high risk of developing BP (ie, recently weaned, commingled, and auction market‐derived) were studied from their arrival to 120 days after arrival at 4 commercial feedlots in Southern Alberta, Canada (1‐time capacity ranging from 5000 to 25 000 animals). At on‐arrival processing, all cattle received an SC injection of a long‐acting macrolide to control BP (tulathromycin, 2.5 mg/kg, Draxxin, Zoetis, Kirkland, QC, Canada), were weighed and vaccinated against infectious bovine herpes virus‐1, bovine viral diarrhea virus (types I and II), bovine parainfluenza‐3 virus, bovine respiratory syncytial virus, Mannheimia haemolytica, Histophilus somni, and clostridial pathogens. They also were dewormed with pour‐on ivermectin solution. In addition, heifers received a prostaglandin F2α analogue to induce abortion, as per standard feedlot procedure.
Steers and heifers were housed by sex and fed in large outdoor dirt‐floor pens with approximately 250‐300 animals per pen. Cattle twice daily were fed a concentrated barley‐based receiving and growing diet formulated to meet or exceed nutrient requirements.
Cattle were observed once or twice daily by experienced pen checkers for detection of clinical illness. Cattle with ≥1 visual signs of BP (eg, lethargy, nasal or ocular discharge, cough, increased respiratory rate, or dyspnea) were removed from their pens and clinically examined by an experienced veterinarian in a conventional hydraulic cattle squeeze chute. Cattle not previously treated for BP or another disease during the feeding period with at least 1 visual BP sign (see above) and rectal temperature ≥40°C were enrolled in the study. They were examined by TUS and subsequently treated SC in the neck area with a combination product containing an antimicrobial and a nonsteroidal anti‐inflammatory drug (40 mg/kg florfenicol and 2.2 mg/kg flunixin, 2 mL/15 kg; Resflor, Merck Animal Health). After treatment, they were walked back to their home pens.
Cattle displaying visual BP signs (see above) after a 4‐day post‐treatment interval were again pulled by experienced penriders (blinded to TUS results) and, if their rectal temperature was ≥40°C, were defined as a BP relapse and treated SC in the neck area with a second antimicrobial (enrofloxacin, 12.5 mg/kg; Baytril, Bayer Animal Health). All animals that died during the study period (0‐120 days on feed) were necropsied, and the cause of death was determined by a feedlot veterinarian based on the findings of gross postmortem examination. All cattle were individually weighed at 120 days after arrival at the feedlot.
Because of the absence of preliminary data on risk of relapse and decreased ADG associated with lung lesions detected by TUS, no sample size calculation was performed. Therefore, the number of animals enrolled in the study was decided arbitrarily based on the available time and resources (eg, number of cattle diagnosed with BP per day of visit, period of time allowed to access the squeeze chute per day of visit). The research protocol was reviewed and approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC15‐0109).
2.2. Thoracic ultrasonography
Thoracic ultrasonography was performed by the same experienced clinician using an Ibex Pro device (EI Medical Imaging, Loveland, Colorado) using a 6.2 MHz linear probe, maximal depth, 9 cm; total gain, 32 dB (far gain, 36 dB; near gain, 13 dB) as described previously.6 The thorax was neither shaved nor clipped before performing TUS, and isopropyl alcohol (70%) was used as a transducing agent, as described previously.7 Because a previous study indicated that lung lesions associated with negative outcomes in feedlot steers were located in the mid‐to‐ventral section of the thorax,5 only the mid‐to‐ventral portions of the 4th, 5th, and 6th intercostal space were examined (1st to 3rd intercostal spaces cannot be not scanned in animals of this size, particularly when they are restrained in a chute [SB, personal communication]). Both sides of the thorax were examined in 101 cattle, whereas only 1 side was examined in 43 animals (right [21] and left [22]) because of time constraints for access to the squeeze chute (see flow diagram in Supporting Information Figure S1). The clinician performing TUS stored a single 16‐second video of the most severe lung lesions per side and per animal (ie, 1 video for animals examined on only 1 side and 2 for animals examined on both sides). Videos were reviewed immediately chute‐side and videos in which ribs were observed too often because of cattle movement were deleted and recorded again to ensure proper offline evaluation.
Severity of lung lesions was evaluated offline as described7 by a clinician experienced in TUS and blinded to health and performance outcomes. Briefly, for each video, quantitative assessment of the maximal depth (in cm) and maximal area (manually counting the number of squares with consolidated lung; in cm2) of lung consolidation was performed using the squared screen (1 cm2) of the video recording as landmark. The maximum number of comet tails visible in a frozen image also was reported, as was maximal depth of pleural fluid (in cm) when pleural fluid was observed. Videos were saved in .avi format and viewed on a laptop computer without using specific software (comparable to a rapid chute‐side examination).
2.3. Statistical analyses
Data obtained from the evaluation of TUS videos were entered into a spreadsheet (Microsoft Excel, version 16.20) and assessed for normality (Shapiro‐Wilk test). Mean ± SD was used to report normally distributed data, whereas median, range, and quartiles were used for non‐normally distributed data. The maximal depth and area of lung consolidation as well as the maximal number of comet tails and depth of pleural fluid were determined for each animal.
Statistical analyses were performed using commercially available software (SAS v9.4; SAS, Cary, North Carolina). The experimental unit was the individual animal. Univariable logistic regression models were fit to assess the effects of continuous lung lesion variables (ie, depth, area, comet tails, pleural fluid) on the risk of BP relapse. To account for potential confounding by feedlot, multivariable models then were fit for each variable significantly associated with the risk of BP relapse in univariable analysis using the GLIMMIX procedure (including feedlot as a random effect). Sex was not included in the multivariable models because it was not associated with relapse (P = .28). Because of the collinearity between the maximal depth and maximal area (data not shown), these 2 variables were not combined into 1 model. To assess the diagnostic accuracy of maximal depth and area of lung consolidation to predict risk of BP relapse, receiver‐operating characteristic (ROC) curves and areas under the ROC curves (AUC) were determined for these 2 variables using MedCalc (MedCalc Statistical Software version 18.9; Ostend, Belgium; http://www.medcalc.org; 2018). Furthermore, sensitivity and specificity as well as positive and negative likelihood ratios of various thresholds of maximal depth and maximal area of lung consolidation for predicting BP relapses were determined. Likelihood ratios were based on a ratio of sensitivity and specificity and enabled clinicians to calculate posttest probability of disease based on the result of a test using Bayes' theorem (providing a pretest probability of the disease established based on a clinical expertise or a previous test).8
Average daily gain (ADG) was calculated from the individual entry body weight measured at on‐arrival processing and the individual body weight measured at the end of the 120‐day study interval. Average daily gain from animals that died during the study period (n = 6) was excluded. The effect on ADG of each lung lesion type was assessed in univariable analysis (PROC GLM). Multivariable analyses including feedlot as a random effect were conducted for each variable associated with ADG in univariable analyses (PROC MIXED Procedure). Sex was not included in final models because it was not associated with ADG (P = .29). Results were considered significant if P ≤ .05.
3. RESULTS
One hundred forty‐four mixed beef‐breed steers (n = 93; body weight [mean ± SD], 243 ± 36 kg) and heifers (n = 51; body weight, 227 ± 42 kg) were enrolled at 4 feedlots between November 2015 and January 2016. Descriptive statistics of the cattle population enrolled at each feedlot are provided (Table S1). Of these 144 cattle, 25 (17.4%) relapsed at least once after 1st BP treatment and 6 (4.2%) died of BP within 120 days after arrival at the feedlot.
Lung lesions were detected by TUS in 121 of the 144 animals. Descriptive statistics and distribution of lung lesions are provided (Table 1; Supporting Information Figures S2 and S3). In univariable analysis, both the maximal depth of lung consolidation (OR, 1.409; 95% confidence interval [CI], 1.098‐1.809; P = .007) and the area of consolidation (OR, 1.059; 95% CI, 1.018‐1.102; P = .005) were associated with the risk of relapse. Neither the maximum number of comet tails (P = .464) nor the maximal depth of pleural fluid (P = .62) were associated with the relapse rate. When the feedlot effect was included in the models as a random effect, both the maximal depth (OR, 1.337; 95% CI, 1.042‐1.714; P = .02) and the area (OR, 1.052; 95% CI, 1.009‐1.097; P = .02) remained significantly associated with the relapse risk (Table 2).
Table 1.
Descriptive statistics of lung lesions diagnosed by thoracic ultrasonography at the time of first diagnosis of bronchopneumonia in feedlot cattle (n = 144)
| Maxima | Minimum | Maximum | Median (Q1‐Q3) |
|---|---|---|---|
| Depth (cm) | 0.0 | 7.0 | 3.0 (2.0‐5.0) |
| Area (cm2) | 0.0 | 43.0 | 10.0 (3.5‐18.0) |
| No. comet tails | 0.0 | 6.0 | 0.0 (0.0‐1.0) |
| Depth of pleural effusion (cm) | 0.0 | 7.0 | 0.0 (0.0‐1.0) |
Table 2.
Effects of maximal depth and maximal area of lung consolidation at first BP diagnosis on risk of BP relapse
| Independent variable and level | Odds ratioa | 95% confidence interval | P‐value |
|---|---|---|---|
| Maximal depth (per cm) | 1.34 | 1.04‐1.71 | .02 |
| Maximal area (per cm2) | 1.05 | 1.01‐1.10 | .02 |
Results from 2 multivariable models (1 for maximal depth and 1 for maximal area) including feedlot as a random effect. All cattle were included (n = 144).
Receiver‐operating characteristic curves and AUC to predict the risk of BP relapse of the maximal depth (AUC, 0.67; 95% CI, 0.59‐0.75) and area (AUC, 0.66; 95% CI, 0.57‐0.74) of lung consolidation are presented (Figure 1). With a dichotomous approach, the optimum cutoff value of the maximal depth to predict relapse was >5 cm (sensitivity, 40.0%; 95% CI, 21.1‐61.3; specificity, 85.8%; 95% CI, 78.1‐91.5; LR+, 2.8; 95% CI, 1.5‐5.4; LR−, 0.7; 95% CI, 0.5‐1.0; Table 3). For the maximal area of lung consolidation, the optimum cutoff value was >8 cm2 (sensitivity, 80.0%; 95% CI, 59.3‐93.2; specificity, 47.9%; 95% CI, 38.7‐57.2; LR+, 1.5; 95% CI, 1.2‐2.0; LR−, 0.4; 95% CI, 0.2‐0.9; Table 4).
Figure 1.
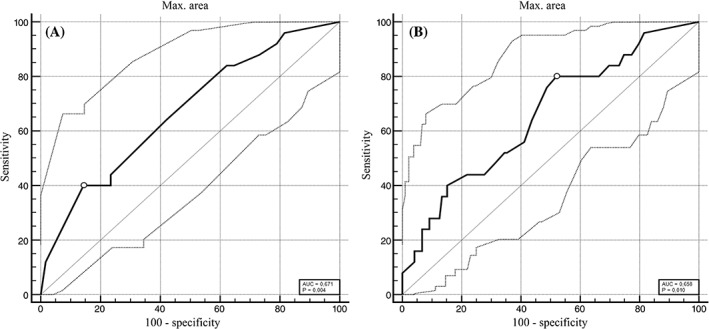
Receiver‐operating characteristic (ROC) curves (solid curves) and their 95% confidence intervals (dotted curves) to predict the relapse rate of (A) maximal depth and (B) maximal area of lung consolidation. Optimum cutoff values of maximal depth (>5 cm) and maximal area (>8 cm2) are indicated by white circles. Areas under the ROC curves (AUC), indicating how well the maximal depth and maximal area of lung consolidation separate cattle with BP that relapsed from those that did not relapse are also indicated
Table 3.
Sensitivity, specificity, and positive (LR+) and negative (LR−) likelihood ratios of different thresholds of maximal depth of lung consolidation (in cm) to predict risk of relapse in feedlot cattle with clinical BP
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | LR+ | 95% CI | LR− | 95% CI |
|---|---|---|---|---|---|---|---|---|
| ≥0 | 100.0 | 86.3‐100.0 | 0.0 | 0.0‐3.1 | 1.0 | 1.0‐1.0 | ||
| >0 | 96.0 | 79.6‐99.9 | 18.5 | 12.0‐26.6 | 1.2 | 1.0‐1.3 | 0.2 | 0.03‐1.5 |
| >0.5 | 92.0 | 74.0‐99.0 | 21.0 | 14.1‐29.4 | 1.2 | 1.0‐1.4 | 0.4 | 0.10‐1.5 |
| >1 | 88.0 | 68.8‐97.5 | 26.9 | 19.2‐35.8 | 1.2 | 1.0‐1.4 | 0.5 | 0.1‐1.3 |
| >2 | 84.0 | 63.9‐95.5 | 35.3 | 26.8‐44.6 | 1.3 | 1.0‐1.6 | 0.5 | 0.2‐1.1 |
| >2.5 | 84.0 | 63.9‐95.5 | 37.8 | 29.1‐47.2 | 1.4 | 1.1‐1.7 | 0.4 | 0.2‐1.1 |
| >3 | 64.0 | 42.5‐82.0 | 58.0 | 48.6‐67.0 | 1.5 | 1.1‐2.2 | 0.6 | 0.4‐1.1 |
| >4 | 44.0 | 24.4‐65.1 | 76.5 | 67.8‐83.8 | 1.9 | 1.1‐3.2 | 0.7 | 0.5‐1.1 |
| >4.5 | 40.0 | 21.1‐61.3 | 76.5 | 67.8‐83.8 | 1.7 | 1.0‐3.0 | 0.8 | 0.6‐1.1 |
| >5a | 40.0 | 21.1‐61.3 | 85.7 | 78.1‐91.5 | 2.8 | 1.5‐5.4 | 0.7 | 0.5‐1.0 |
| >6 | 12.0 | 2.5‐31.2 | 98.3 | 94.1‐99.8 | 7.1 | 1.3‐40.5 | 0.9 | 0.8‐1.0 |
| >7 | 0.0 | 0.0‐13.7 | 100.0 | 96.9‐100.0 | 1.0 | 1.0‐1.0 |
Optimum cutoff point based on Youden index J (ie, max sensitivity + specificity).
Table 4.
Sensitivity, specificity, and positive (LR+) and negative (LR−) likelihood ratios of different thresholds of maximal area of lung consolidation (in cm2) to predict risk of relapse in feedlot cattle with clinical BP
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | LR+ | 95% CI | LR− | 95% CI |
|---|---|---|---|---|---|---|---|---|
| ≥0 | 100.0 | 86.3‐100.0 | 0.0 | 0.0‐3.1 | 1.0 | 1.0‐1.0 | ||
| >0 | 96.0 | 79.6‐99.9 | 18.5 | 12.0‐26.6 | 1.2 | 1.0‐1.3 | 0.2 | 0.1‐1.5 |
| >0.5 | 92.0 | 74.0‐99.0 | 20.2 | 13.4‐28.5 | 1.2 | 1.0‐1.3 | 0.4 | 0.1‐1.6 |
| >1 | 88.0 | 68.8‐97.5 | 22.7 | 15.5‐31.3 | 1.1 | 1.0‐1.4 | 0.5 | 0.2‐1.6 |
| >2 | 88.0 | 68.8‐97.5 | 25.2 | 17.7‐34.0 | 1.2 | 1.0‐1.4 | 0.5 | 0.2‐1.4 |
| >3 | 84.0 | 63.9‐95.5 | 26.9 | 19.2‐35.8 | 1.2 | 0.9‐1.4 | 0.6 | 0.2‐1.5 |
| >4 | 84.0 | 63.9‐95.5 | 30.3 | 22.2‐39.3 | 1.2 | 1.0‐1.5 | 0.5 | 0.2‐1.4 |
| >5 | 80.0 | 59.3‐93.2 | 33.6 | 25.2‐42.8 | 1.2 | 1.0‐1.5 | 0.6 | 0.3‐1.4 |
| >6 | 80.0 | 59.3‐93.2 | 37.8 | 29.1‐47.2 | 1.3 | 1.0‐1.6 | 0.5 | 0.2‐1.2 |
| >8a | 80.0 | 59.3‐93.2 | 47.9 | 38.7‐57.2 | 1.5 | 1.2‐2.0 | 0.4 | 0.2‐0.9 |
| >9 | 76.0 | 54.9‐90.6 | 51.3 | 41.9‐60.5 | 1.6 | 1.2‐2.1 | 0.5 | 0.2‐1.0 |
| >10 | 64.0 | 42.5‐82.0 | 56.3 | 46.9‐65.4 | 1.5 | 1.0‐2.1 | 0.6 | 0.4‐1.1 |
| >11 | 56.0 | 34.9‐75.6 | 58.8 | 49.4‐67.8 | 1.4 | 0.9‐2.0 | 0.8 | 0.5‐1.2 |
| >12 | 52.0 | 31.3‐72.2 | 64.7 | 55.4‐73.2 | 1.5 | 0.9‐2.3 | 0.7 | 0.5‐1.1 |
| >13 | 52.0 | 31.3‐72.2 | 65.6 | 56.3‐74.0 | 1.5 | 1.0‐2.4 | 0.7 | 0.5‐1.1 |
| >14 | 48.0 | 27.8‐68.7 | 68.9 | 59.8‐77.1 | 1.5 | 0.9‐2.5 | 0.8 | 0.5‐1.1 |
| >15 | 44.0 | 24.4‐65.1 | 72.3 | 63.3‐80.1 | 1.6 | 0.9‐2.7 | 0.8 | 0.5‐1.1 |
| >16 | 44.0 | 24.4‐65.1 | 74.8 | 66.0‐82.3 | 1.8 | 1.0‐3.0 | 0.8 | 0.5‐1.1 |
| >17 | 44.0 | 24.4‐65.1 | 78.2 | 69.6‐85.2 | 2.0 | 1.2‐3.5 | 0.7 | 0.5‐1.0 |
| >18 | 40.0 | 21.1‐61.3 | 84.9 | 77.2‐90.8 | 2.6 | 1.4‐5.0 | 0.7 | 0.5‐1.0 |
| >19 | 36.0 | 18.0‐57.5 | 84.9 | 77.2‐90.8 | 2.4 | 1.2‐4.7 | 0.8 | 0.6‐1.0 |
| >20 | 36.0 | 18.0‐57.5 | 86.6 | 79.1‐92.1 | 2.7 | 1.3‐5.4 | 0.7 | 0.5‐1.0 |
| >21 | 28.0 | 12.1‐49.4 | 87.4 | 80.1‐92.8 | 2.2 | 1.0‐4.9 | 0.8 | 0.6‐1.1 |
| >22 | 28.0 | 12.1‐49.4 | 88.2 | 81.0‐93.4 | 2.4 | 1.1‐5.3 | 0.8 | 0.6‐1.1 |
| >23 | 28.0 | 12.1‐49.4 | 89.1 | 82.0‐94.1 | 2.6 | 1.1‐5.8 | 0.8 | 0.6‐1.0 |
| >24 | 28.0 | 12.1‐49.4 | 90.8 | 84.1‐95.3 | 3.0 | 1.3‐7.0 | 0.8 | 0.6‐1.0 |
| >25 | 24.0 | 9.4‐45.1 | 90.8 | 84.1‐95.3 | 2.6 | 1.1‐6.4 | 0.8 | 0.7‐1.1 |
| >27 | 24.0 | 9.4‐45.1 | 92.4 | 86.1‐96.5 | 3.2 | 1.2‐8.1 | 0.8 | 0.7‐1.0 |
| >28 | 24.0 | 9.4‐45.1 | 93.3 | 87.2‐97.1 | 3.6 | 1.4‐9.4 | 0.8 | 0.7‐1.0 |
| >30 | 16.0 | 4.5‐36.1 | 93.3 | 87.2‐97.1 | 2.4 | 0.8‐7.3 | 0.9 | 0.8‐1.1 |
| >32 | 16.0 | 4.5‐36.1 | 94.1 | 88.3‐97.6 | 2.7 | 0.9‐8.6 | 0.9 | 0.7‐1.1 |
| >33 | 16.0 | 4.5‐36.1 | 95.8 | 90.5‐98.6 | 3.8 | 1.1‐13.2 | 0.9 | 0.7‐1.0 |
| >35 | 12.0 | 2.5‐31.2 | 95.8 | 90.5‐98.6 | 2.9 | 0.7‐11.2 | 0.9 | 0.8‐1.1 |
| >36 | 8.0 | 1.0‐26.0 | 100.0 | 96.9‐100.0 | 0.9 | 0.8‐1.0 | ||
| >42 | 4.0 | 0.1‐20.4 | 100.0 | 96.9‐100.0 | 1.0 | 0.9‐1.0 | ||
| >43 | 0.0 | 0.0‐13.7 | 100.0 | 96.9‐100.0 | 1.0 | 1.0‐1.0 |
Optimum cutoff point based on Youden index J (ie, max sensitivity + specificity).
Average daily gain was calculated for 133 animals (Supporting Information Figure S1; Table S1). The maximal depth of lung consolidation was associated with ADG (P = .03), with a decrease of 34 g/cm of consolidation (95% CI, −64 to −4 g/cm; model including feedlot as a random effect). Maximal area of consolidation (P = .46), maximum number of comet tails (P = .464), and maximal depth of pleural fluid (P = .616) were not associated with ADG.
4. DISCUSSION
To the best of our knowledge, this study was the first to demonstrate that severity of lung lesions measured by TUS was associated with decreased performance and increased relapse rate in commercial feedlot cattle. Maximal depth of lung consolidation was associated with higher risk of relapse (OR, 1.337/cm) and a lower ADG (−34 g/cm). Maximal area of lung consolidation also was associated with increased relapse rate (OR, 1.052/cm2). Comet tails and pleural effusion, however, were not associated with increased risk of BP relapse or decreased ADG.
That maximal depth and area of lung consolidation were the best predictor of negative outcomes (based on P values) agrees with previous studies in beef5 and dairy9 cattle. For example, in beef cattle, a previous study5 compared the ability of various lung lesions to predict mortality in feedlot steers with BP (including the maximal depth and maximal area of consolidation per animal, number of comet tails by site, number of sites with pleural irregularity present, and maximal depth of pleural fluid accumulation per animal) and reported that the maximal depth and maximal area of consolidation were most negatively associated with clinical prognosis. They also reported that the diagnostic accuracy of maximal depth (AUC, 0.814) was slightly higher than the diagnostic accuracy of maximal area (AUC, 0.765). Interestingly, they concluded that the cutoff optimizing sensitivity and specificity of the maximal depth of consolidation was 5 cm (sensitivity, 75%; 95% CI, 43‐93; specificity, 82%; 95% CI, 57‐96), identical to the optimal cutoff obtained in our study. Because maximal depth is simple and rapid to obtain,5 future research optimizing BP management (treatment regimens, return to home pen versus hospital pen) based on the maximum depth of lung lesions at first BP diagnosis should be encouraged.
It is noteworthy that a previous study,10 which assessed the prognostic utility of TUS at the 1st BP diagnosis in 86 feedlot cattle, concluded that TUS was not particularly effective in predicting subsequent BP relapse or rate of gain. However, that study only assessed the right side of the thorax at the 3rd, 5th, and 7th intercostal spaces. Furthermore, TUS images were used for offline evaluation rather than TUS videos. Because frozen TUS image interpretation can result in a high rate of false‐positive detections,11 this approach may have affected the diagnostic accuracy of the reading. The apparent discrepancy between this previous study and our findings highlights a need for additional studies on the use of TUS for determining BP prognosis in feedlot cattle.
A potential limitation of our study was that only 1 side of the thorax was examined in 30% of the cattle. In previous studies in both beef5 and dairy12 cattle, slight to moderate agreement was found between the right and left sides of the thorax for ultrasonographic diagnosis of consolidation. Perhaps, we did not capture the maximal depth or maximal area of lung consolidation in all cattle. However, it is noteworthy that, when running the same models using only the data from cattle for which we had both lungs examined, similar negative effects of lung lesions on health and performance were found (although not significant for ADG; Supporting Information Tables S2 and S3). Another potential limitation was the relatively low number of animals enrolled in our study (and the absence of an a priori sample size calculation because of limited prior knowledge). Perhaps, the maximal area of lung consolidation would have been associated with lower ADG if a higher number of animals had been included. Indeed, to detect a difference of 130 g between cattle with a maximal area of lung consolidation ≤8 cm2 (mean ADG ± SD, 1.003 ± 0.326 kg) and cattle with a maximal area of lung consolidation >8 cm2 (mean ADG ± SD, 0.873 ± 0.374 kg), 99 animals per group would have been needed (2‐tailed test; alpha, 5%; beta, 20%; https://clincalc.com/stats/SampleSize.aspx).
Our results can help in calculating sample sizes for subsequent studies. For example, based on the relapse rate of 14% observed in cattle with lung lesions ≤5 cm deep and the relapse rate of 26% observed in cattle with lung lesions >5 cm deep, 173 animals per group would be needed to further validate the cutoff value of 5 cm for the maximal depth of lung consolidation (2‐tailed test; alpha, 5%; beta, 20%; https://clincalc.com/stats/SampleSize.aspx).
In conclusion, quantifying the maximal depth and area of lung consolidation by TUS at first BP diagnosis can provide useful prognostic information in feedlot cattle with naturally occurring BP.
CONFLICT OF INTEREST DECLARATION
Sébastien Buczinski serves as Consulting Editor for Experimental Design and Statistics for the Journal of Veterinary Internal Medicine. He was not involved in peer review of the manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The research protocol was reviewed and approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC15‐0109).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Fig S1 Flow diagram of cattle enrolled in the study.
Fig S2 Distribution of maximal depth of lung consolidation in feedlot cattle diagnosed with BP (n = 144).
Fig S3 Distribution of maximal area of lung consolidation in feedlot cattle diagnosed with BP (n = 144).
Table S1 Descriptive statistics of health and performance of the feedlot cattle (n = 144) enrolled in the study
Table S2 Effects of maximal depth and maximal area of lung consolidation at first BP diagnosis on risk of BP relapse. Only cattle for which both thoracic sides were examined by TUS were included (n = 101).
Table S3 Effects of maximal depth and maximal area of lung consolidation at first BP diagnosis on average daily gain calculated from 1 to 120 days on feed. Only cattle for which both thoracic sides were examined by TUS were included (n = 101).
Timsit E, Tison N, Booker CW, Buczinski S. Association of lung lesions measured by thoracic ultrasonography at first diagnosis of bronchopneumonia with relapse rate and growth performance in feedlot cattle. J Vet Intern Med. 2019;33:1540–1546. 10.1111/jvim.15483
Funding information University of Calgary, Faculty of Veterinary Medicine, Grant/Award Number: 10004657
Contributor Information
Edouard Timsit, Email: eftimsit@ucalgary.ca.
Sébastien Buczinski, Email: s.buczinski@umontreal.ca.
REFERENCES
- 1. Wolfger B, Timsit E, White BJ, Orsel K. A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. Vet Clin North Am Food Anim Pract. 2015;31:351‐365. [DOI] [PubMed] [Google Scholar]
- 2. Timsit E, Dendukuri N, Schiller I, Buczinski S. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: a systematic literature review and hierarchical Bayesian latent‐class meta‐analysis. Prev Vet Med. 2016;135:67‐73. [DOI] [PubMed] [Google Scholar]
- 3. Theurer ME, White BJ, Larson RL, Holstein KK, Amrine DE. Relationship between rectal temperature at first treatment for bovine respiratory disease complex in feedlot calves and the probability of not finishing the production cycle. J Am Vet Med Assoc. 2014;245:1279‐1285. [DOI] [PubMed] [Google Scholar]
- 4. Buczinski S, Menard J, Timsit E. Incremental value (bayesian framework) of thoracic ultrasonography over thoracic auscultation for diagnosis of bronchopneumonia in preweaned dairy calves. J Vet Intern Med. 2016;30:1396‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rademacher RD, Buczinski S, Tripp HM, et al. Systematic thoracic ultrasonography in acute bovine respiratory disease of feedlot steers: impact of lung consolidation on diagnosis and prognosis in a case‐control study. Bovine Pract. 2014;48:1‐10. [Google Scholar]
- 6. Buczinski S, Buathier C, Belanger AM, et al. Inter‐rater agreement and reliability of thoracic ultrasonographic findings in feedlot calves, with or without naturally occurring bronchopneumonia. J Vet Intern Med. 2018;32:1787‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ollivett TL, Caswell JL, Nydam DV, et al. Thoracic ultrasonography and bronchoalveolar lavage fluid analysis in Holstein calves with subclinical lung lesions. J Vet Intern Med. 2015;29:1728‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timsit E, Leguillette R, White BJ, et al. Likelihood ratios: an intuitive tool to incorporate diagnostic test results into decision making. J Am Vet Med Assoc. 2018;252:1362‐1366. [DOI] [PubMed] [Google Scholar]
- 9. Ollivett TL, Buczinski S. On‐farm use of ultrasonography for bovine respiratory disease. Vet Clin North Am Food Anim Pract. 2016;32:19‐35. [DOI] [PubMed] [Google Scholar]
- 10. Abutarbush SM, Pollock CM, Wildman BK, et al. Evaluation of the diagnostic and prognostic utility of ultrasonography at first diagnosis of presumptive bovine respiratory disease. Can J Vet Res. 2012;76:23‐32. [PMC free article] [PubMed] [Google Scholar]
- 11. Rosen MP, Mehta TS, Bromberg R, et al. Remote sonographic interpretation using a laser printer network: system performance and diagnostic accuracy in actual clinical practice. Am J Radiol. 2001;176:855‐860. [DOI] [PubMed] [Google Scholar]
- 12. Buczinski S, Forte G, Francoz D, et al. Comparison of thoracic auscultation, clinical score, and ultrasonography as indicators of bovine respiratory disease in preweaned dairy calves. J Vet Intern Med. 2014;28:234‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1 Flow diagram of cattle enrolled in the study.
Fig S2 Distribution of maximal depth of lung consolidation in feedlot cattle diagnosed with BP (n = 144).
Fig S3 Distribution of maximal area of lung consolidation in feedlot cattle diagnosed with BP (n = 144).
Table S1 Descriptive statistics of health and performance of the feedlot cattle (n = 144) enrolled in the study
Table S2 Effects of maximal depth and maximal area of lung consolidation at first BP diagnosis on risk of BP relapse. Only cattle for which both thoracic sides were examined by TUS were included (n = 101).
Table S3 Effects of maximal depth and maximal area of lung consolidation at first BP diagnosis on average daily gain calculated from 1 to 120 days on feed. Only cattle for which both thoracic sides were examined by TUS were included (n = 101).


