Abstract
Background
Stereotactic brain biopsy (SBB) is a technique that allows for definitive diagnosis of brain lesions. Little information is available regarding the diagnostic utility of SBB in dogs with intracranial diseases.
Objective
To investigate the diagnostic accuracy (DA) of SBB in dogs with brain tumors.
Animals
Thirty‐one client‐owned dogs that underwent SBB followed by surgical resection or necropsy examinations.
Methods
Retrospective observational study. Two pathologists blinded to SBB and reference standard diagnoses reviewed histologic specimens and typed and graded tumors according to World Health Organization and revised canine glioma classification criteria. Agreement between tumor type and grade from SBB were compared to reference standards and assessed using kappa statistics. Patient and technical factors associated with agreement also were examined.
Results
Stereotactic brain biopsy specimens were obtained from 24 dogs with gliomas and 7 with meningiomas. Tumor type agreement between SBB and the reference standard was observed in 30/31 cases (κ = 0.95). Diagnostic concordance was perfect for meningiomas. Grade agreement among gliomas was observed in 18/23 cases (κ = 0.47). Stereotactic brain biopsy underrepresented the reference standard glioma grade in cases with disagreement. The DA of SBB was 81%, with agreement noted in 56/69 biopsy samples. Smaller tumors and fewer SBB specimens obtained were significantly associated with diagnostic discordance.
Conclusions and Clinical Importance
The DA of SBB readily allows for the diagnosis of common brain tumors in dogs. Although glioma grade discordance was frequent, diagnoses obtained from SBB are sufficient to currently inform therapeutic decisions. Multiple SBB specimens should be collected to maximize DA.
Keywords: biopsy, brain tumor, diagnosis, dog, glioma, meningioma
Abbreviations
- DA
diagnostic accuracy
- DY
diagnostic yield
- MRI
magnetic resonance imaging
- NE
necropsy examination
- ROIs
regions of interest
- SBB
stereotactic brain biopsy
- SR
surgical resection
- WHO
World Health Organization
1. INTRODUCTION
Advances in diagnostic imaging, particularly magnetic resonance imaging (MRI), have revolutionized the management of many intracranial disorders in veterinary medicine predominantly by providing information that complements historical and clinical findings and allows for the generation of a refined list of differential diagnoses. However, all of the currently available imaging modalities are sufficiently unreliable to allow for the definitive diagnosis of most intracranial disorders that are commonly encountered in veterinary neurology practice.1, 2, 3, 4 Because potential treatments and prognoses can differ substantially among the differential diagnoses generated in an individual patient based on clinical and imaging data; this diagnostic uncertainty can have profound implications on patient management. For example, in a study of dogs with focal intra‐axial brain lesions on MRI, as many as 47% of cerebrovascular accidents were misdiagnosed as gliomas, and up to 12% of gliomas were misclassified as strokes.2
Given the current limitations of diagnostic imaging, acquisition of representative samples of brain tissue that allow a neuropathologist to provide a definitive diagnosis remains integral to informing best clinical practices regarding the selection of treatment for patients with brain disease. To meet this diagnostic need, minimally invasive stereotactic methods were introduced into neurosurgical practice in human medicine over a century ago and have evolved continuously since that time. To date, stereotactic brain biopsy (SBB) remains 1 of the most commonly performed stereotactic procedures.3, 4 Diagnoses sufficient to guide therapeutic decision making are obtained in 95% of humans in which SBB is performed, with a low risk of associated adverse events.4
Few reports describe the clinical utility of SBB procedures performed in dogs with intracranial diseases.5, 6 Specific measures of the diagnostic utility of histopathological samples obtained using biopsy techniques include diagnostic yield (DY), which is the proportion of biopsy specimens producing a specific pathological diagnosis. The DY is calculated by dividing the number of biopsy specimens producing a specific pathological diagnosis by the total number of biopsies performed. In dogs with intracranial lesions, the DY of SBB has been reported to be 95%.5 Diagnostic accuracy (DA) examines the rate of concordance between samples obtained by biopsy and the final pathological diagnosis obtained by a reference standard method, such as surgical resection (SR) or necropsy examination (NE). The DA is calculated by dividing the number of biopsy specimens giving the same diagnosis as surgical or necropsy specimens by total biopsies performed. To date, very little data are available regarding the DA of SBB in dogs with naturally occurring brain diseases.6
Our objectives were to determine if the neuropathologic diagnosis obtained by SBB, using tumor type and grade as diagnostic endpoints, was an accurate representation of the entire lesion when compared to reference standard SR or necropsy specimens, and to identify any risk factors associated with diagnostic discordance. We hypothesized that the DA of SBB would be lower in dogs with gliomas than in those with meningiomas, and that DA would be positively correlated with the number of SBB specimens obtained in each patient.
2. MATERIALS AND METHODS
2.1. Study design
Retrospective observational study.
2.2. Dog and tumor characteristics
The medical records of 31 dogs that underwent SBB followed by open craniotomy and SR or NE between June 2008 and June 2018 were identified by searching a veterinary clinical trial database. Patient data were entered into the database prospectively during clinical trial participation. For the purposes of the study, information extracted from the database included the signalment, lesion side and location in the brain, T2‐weighted (T2W) volume of the target lesion at the time of SBB, number of SBB attempted, number of SBB specimens obtained, number of biopsy trajectories used during the procedure, and number of days elapsed between the performance of the SBB and the subsequent reference standard method (SR or NE).5 Volumetric tumor quantification was determined in each case using image analysis software (OsiriX MD v.6.5.2, Pixmeo SARL, Geneva, Switzerland). Volumes were defined from T2W MRI, because not all tumors demonstrated contrast enhancement. Manually defined regions of interest (ROIs) containing tumor were generated for individual contiguous T2W MRI slices, and volumes were calculated using ROI volume software.5 All dogs in the study underwent SBB to further evaluate newly MRI‐diagnosed brain mass lesions associated with clinical signs of intracranial disease.
A single clinician performed all SBB procedures in anesthetized dogs using a previously described frame‐based technique.5 Two different headframe systems were used for SBB, and all dogs in the study had SBB performed with computed tomographic (CT) guidance (Figure 1).5 In an attempt to decrease the risk of obtaining a nondiagnostic biopsy specimen, the neurosurgeon performing SBB intentionally avoided sampling necrotic or hemorrhagic areas. For intra‐axial lesions with heterogeneous or ring‐like patterns of contrast enhancement, biopsy specimens were obtained that included enhancing regions in an attempt to avoid sampling necrotic tumor areas (Figure 1).5 Hemorrhagic foci were defined by hypointensity on T2* gradient echo sequences, or hyperattenuating regions on intraoperative pre‐contrast computed tomography scans (Figure 1).
Figure 1.
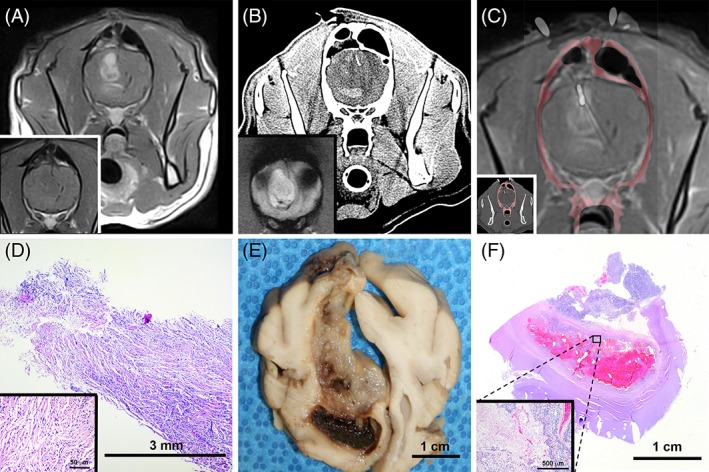
Computed tomography (CT)‐guided stereotactic brain biopsy (SBB) of canine astrocytoma illustrating underestimation of tumor grade by SBB. A, Pre‐ (inset) and post‐contrast T1‐weighted (T1W) magnetic resonance image (MRI) of heterogeneously ring‐enhancing intra‐axial mass in right frontal lobe. B, Preoperative CT with hyperattenuating region of intralesional hemorrhage corresponding with hypointensity on the T2* gradient image (inset) that is avoided during biopsy. C, Coregistered post‐contrast T1W MRI and intraoperative CT scan with biopsy needle in situ (inset) within dorsal contrast‐enhancing portion of the mass. D, Core specimen from SBB depicted in (C), demonstrating features of a low‐grade fibrillary astrocytoma. The neoplasm is composed of spindloid cells with indistinct cell borders, fibrillar eosinophilic cytoplasm, and cytoplasmic processes arranged in streams and whorls (inset) around small caliber blood vessels, hematoxylin and eosin (H&E) stain. Phenotypic heterogeneity of the tumor is evident in gross (E) and subgross (F) necropsy specimens. In the ventral hemorrhagic region, features of high‐grade astrocytoma such as geographic necrosis, cellular palisading, and microvascular proliferation are apparent (F, inset; H&E stain)
2.3. Neuropathological review and tumor classification
All archived slides representing samples obtained from the brain from each dog were collected and anonymized. A random number generator (SAS v. 9.4, SAS Institute, Cary, North Carolina) was used to assign a unique numeric identifier to all of the slides belonging to an individual dog. Anonymized slide labels contained the unique numeric identifier assigned, as well as the type of staining procedure used on the slide. Anonymized slides then were assembled into 2 sets: a set containing SBB slides from all dogs and another set containing all SR or NE or both slides from all dogs. Hematoxylin and eosin stained slides were available for all cases in both sets. When available, slides stained immunohistochemically with glial fibrillary acidic protein (GFAP; Dakoa polyclonal, 1:150), vimentin (Dako clone V9, 1:300), or oligodendrocyte transcription factor 2 (Olig‐2; Millipore polyclonal, 1:100) were included for each case in each set.7
Each slide set then was distributed to 2 veterinary pathologists who were not involved in generating the original pathology reports contained in the medical records. From the slide material provided for each case in each set, pathologists were asked to provide a neuropathologic diagnosis, and if the assigned diagnosis was neoplastic, that tumors initially be classified and graded according to World Health Organization (WHO) criteria.8 Two pathologists later were tasked with reclassifying glial tumors after the recent release of the revised classification criteria for dogs, in which gliomas are graded in a binary fashion (high‐ versus low‐grade).9 During the review process, pathologists were asked to provide specific reasons for any samples that were determined to be nondiagnostic, and any comments regarding the samples that influenced their diagnostic conclusions. At least 6 weeks lapsed between a pathologist's review of each of the slide sets. In the event of diagnostic discordance between the 2 examining pathologists, the histopathologic diagnosis coded in the original pathology report for the specimen in question was used to arrive at a majority opinion.
2.4. Statistical analyses
Means, SDs, medians, and ranges were calculated for continuous variables. Counts and proportions were calculated for discrete characteristics. The Kruskal‐Wallis test was used to compare the median tumor volume or the median number of biopsy trajectories between dogs with meningiomas and gliomas. The diagnostic concordance of tumor type or tumor grade between SBB and the reference standard was calculated using Cohen's kappa statistic and tested using the McNemar test. The 2‐sample t tests were used for the comparisons of normally distributed patient characteristics between the 2 concordance groups (agreement versus no agreement) and Kruskal‐Wallis tests were used for comparisons of non‐normally distributed patient characteristics. Fisher's exact tests were used for discrete patient characteristics. Statistical analyses were completed using SAS software version 9.4 (SAS Institute). P values <.05 were considered significant.
3. RESULTS
3.1. Dog and tumor characteristics
Thirty‐one dogs were included in the study. The median age at diagnosis by SBB was 9 years (range, 6‐12 years). There were 14 spayed females, 16 neutered males, and 1 intact male. The median body weight was 20 kg (range, 7‐43 kg). Fifteen breeds of dogs were represented including mixed breeds (n = 8), Boston Terrier (n = 6), Boxer (n = 4), American Staffordshire Terrier (n = 2), Labrador Retriever (n = 2), and 1 each of the following: Beagle, Bull Terrier, French Bulldog, German Shepherd, Golden Retriever, Miniature Schnauzer, Rat Terrier, Rottweiler, and Siberian Husky.
All tumors were located in the prosencephalon with 29 occurring on or in the cerebral hemispheres and 2 being located in the thalamus. Fourteen tumors were located on the left side of the brain, 15 on the right side, and 2 on the midline. Median tumor volume was 2.9 cm3 (range, 0.55‐12.3 cm3). The median volume of meningiomas was significantly higher than the median volume of gliomas (P = .03; Table 1).
Table 1.
Tumor and biopsy technical factor comparisons between tumor types
| Tumor type | P value | ||
|---|---|---|---|
| Glioma | Meningioma | ||
| N | 24 | 7 | |
| Median tumor volume (cm3) | 2.8 (0.55‐12.3) | 5.3 (2.9‐10.3) | .03* |
| Number of biopsy trajectories | .003* | ||
| 1 | 20 (83.3%) | 2 (28.6%) | |
| 2 | 4 (16.7%) | 2 (28.6%) | |
| 3 | 0 | 3 (42.9%) | |
| Number of biopsy specimens obtained | .26 | ||
| 1 | 6 (25.0%) | 0 | |
| 2 | 11 (45.8%) | 3 (42.9%) | |
| 3 | 6 (25.0%) | 3 (42.9%) | |
| 4 | 1 (4.2%) | 1 (14.3%) | |
P values are exact tests for categorical measures, t tests when means are presented, and Kruskal‐Wallis test when medians are presented.
Statistically significant (P < .05).
3.2. Neuropathological results and diagnostic agreement
Reference standard (SR, n = 14 or NE, n = 17) WHO neuropathological diagnoses included 10 astrocytomas (n = 1, Grade II; n = 1, Grade III; and n = 8, Grade IV), 13 oligodendrogliomas (n = 4, Grade II and n = 9, Grade III), 1 Grade III oligoastrocytoma (undefined) glioma, and 7 meningiomas (n = 4, Grade I and n = 3, Grade II). Using the revised glioma classification criteria for dogs, all tissue specimens initially graded as WHO Grade II gliomas were reported as low‐grade tumors, and all WHO Grade III and IV tumors as high‐grade gliomas by pathologists.
A Dynatech headframe (Dynatech Machining, Union City, California) was used to perform SBB in 16 dogs, and the Virginia Tech Custom headframe (Virginia Tech, Blacksburg, Virginia) was used in 15 dogs.5 Sixty‐nine individual biopsy samples were obtained from the 31 dogs. The median number of SBB obtained was 2 (range, 1‐4), and was not different between meningiomas and gliomas (P = .26; Table 1). A significantly larger (P = .003; Table 1) number of biopsy trajectories was used in dogs with meningiomas than in those with gliomas. Four of 69 SBB samples were nondiagnostic, resulting in a DY of 94.2% for the SBB procedure. Nondiagnostic biopsy specimens were obtained only from patients with glial tumors. Nondiagnostic biopsy diagnoses included normal brain tissue, hemorrhage, astrogliosis, necrosis, and amorphous eosinophilic material. The median number of days between SBB and performance of the reference standard diagnostic method was 75 days (range, 1‐372 days).
The overall DA, using the WHO grading classification scheme, was 81%, with diagnostic agreement noted between SBB and the reference standard method in 56/69 biopsy samples. Diagnostic concordance of tumor type between SBB and the reference standard was observed in 30/31 cases (κ = 0.95). None of the technical factors (headframe type or use of immunohistochemical stains), patient characteristics examined (breed, sex, age, and body weight), reference standard methods (SR or NE), SBB variables (number of biopsy trajectories, biopsies attempted, biopsy specimens obtained, and nondiagnostic biopsy specimens), or tumor features (lesion side, location, volume, and tumor type), significantly influenced tumor type concordance between SBB and the reference standard (P > .05).
Tumor grade agreements between SBB and reference standard methods were assessed for 30 cases, because a tumor grade could not be determined for 1 SBB glioma case (Table 2). Universal tumor type and grade agreement was found for all 7 meningiomas. The overall diagnostic grade concordance for all evaluable tumors using WHO classification was 80% (24/30 cases; κ = 0.73). Diagnostic grade concordance using the revised canine glioma classification was observed in 78% of cases (18/23 cases; κ = 0.47), and glioma grading agreement between WHO and revised canine glioma schema was not significantly different (McNemar P = .56). In 6/6 cases of WHO and 4/5 cases of revised canine glioma grading discordance, SBB underestimated tumor grade when compared to the reference standard (Figure 1). Smaller lesion volume was significantly associated with diagnostic discordance (Table 2) in both WHO and revised canine glioma classification systems. In addition, fewer number of biopsies attempted (P = .006) and fewer number of biopsy specimens obtained (P = .004) were factors that were significantly associated with tumor grade discordance using the WHO system (Table 2).
Table 2.
Factors associated with tumor grade agreement between World Health Organization (WHO) and revised canine glioma classification criteria
| WHO grades agree, n = 30 cases | Canine grades agree, n = 23 cases | |||||
|---|---|---|---|---|---|---|
| No | Yes | P value | No | Yes | P value | |
| N | 6 (20%) | 24 (80%) | 5 (21.7%) | 18 (78.3%) | ||
| Age (years), mean (SD) | 8.8 (2.1) | 9.1 (1.7) | .72 | 9.2 (2.2) | 8.7 (1.7) | .61 |
| Body weight (kg), mean (SD) | 20.5 (7.6) | 22.0 (11.7) | .77 | 18.0 (7.5) | 21.2 (10.9) | .55 |
| Lesion volume (cm3), mean (SD) | 1.2 (0.7) | 4.2 (3.0) | .0001* | 1.4 (0.9) | 3.4 (3.0) | .02* |
| Headframe type | 1.00 | 1.00 | ||||
| Dynatech | 3 (20%) | 12 (80%) | 3 (25%) | 9 (75%) | ||
| Virginia Tech | 3 (20%) | 12 (80%) | 2 (18.2%) | 9 (81.8%) | ||
| Sex | .49 | .31 | ||||
| Female spayed | 4 (30.8%) | 9 (69.2%) | 2 (22.2%) | 7 (77.8%) | ||
| Male neutered | 2 (12.5%) | 14 (87.5%) | 2 (15.4%) | 11 (84.6%) | ||
| Male | 0 | 1 (100.0%) | 1 (100.0%) | 0 | ||
| Reference standard (RS) method | .18 | .27 | ||||
| Surgery | 1 (7.1%) | 13 (92.9%) | 0 | 7 (100.0%) | ||
| Necropsy | 5 (31.3%) | 11 (68.8%) | 5 (31.3%) | 11 (68.8%) | ||
| Number biopsy trajectories | .26 | .54 | ||||
| 1 | 6 (28.6%) | 15 (71.4%) | 5 (26.3%) | 14 (73.7%) | ||
| 2 | 0 | 6 (100.0%) | 0 | 4 (100.0%) | ||
| 3 | 0 | 3 (100.0%) | 0 | 0 | ||
| Number biopsies attempted | .006* | .15 | ||||
| 1 | 4 (80.0%) | 1 (20.0%) | 3 (60%) | 2 (40%) | ||
| 2 | 2 (15.4%) | 11 (84.6%) | 1 (10%) | 9 (90%) | ||
| 3 | 0 | 9 (100.0%) | 1 (16.7%) | 5(83.3%) | ||
| 4 | 0 | 3 (100.0%) | 0 | 2 (100.0%) | ||
| Number biopsy specimens obtained | .004* | .07 | ||||
| 1 | 4 (80.0%) | 1 (20.0%) | 3 (60.0%) | 2 (40.0%) | ||
| 2 | 2 (14.3%) | 12 (85.7%) | 1 (9.1%) | 10(90.9%) | ||
| 3 | 0 | 9 (100.0%) | 1 (16.7%) | 5 (83.3%) | ||
| 4 | 0 | 2 (100.0%) | 0 | 1 (100.0%) | ||
| Number nondiagnostic biopsies | .56 | 1.00 | ||||
| 1 | 0 | 4 (100.0%) | 0 | 3 (100.0%) | ||
| 0 | 6 (23.1%) | 20 (76.9%) | 5 (25%) | 25 (80%) | ||
| Lesion side | .25 | .64 | ||||
| Left | 5 (35.7%) | 9 (64.3%) | 3 (27.3%) | 8 (72.7%) | ||
| Right | 1 (7.1%) | 13 (92.9%) | 2 (16.7%) | 10 (83.3%) | ||
| Midline | 0 | 2 (100.0%) | 0 | 0 | ||
| Tumor type (RS) | .41 | .052 | ||||
| Astrocytoma | 2 (20.0%) | 8 (80.0%) | 0 | 10 (100.0%) | ||
| Oligodendroglioma | 4 (33.3%) | 8 (66.7%) | 5 (41.7%) | 7 (58.3%) | ||
| Glioma undefined/oligoastrocytoma | 0 | 1 (100.0%) | 0 | 1 (100.0%) | ||
| Meningioma | 0 | 7 (100.0%) | NA | NA | ||
| GFAP/Olig‐2 immunohistochemistry (excludes meningiomas) | .56 | .07 | ||||
| SBB‐both; RS‐both | 3 (50%) | 3 (50%) | 4 (66.7%) | 2 (33.3%) | ||
| SBB‐both; RS‐GFAP | 1 (20%) | 4 (80%) | 0 | 5 (100%) | ||
| SBB‐GFAP; RS‐both | 2 (40%) | 3 (60%) | 1 (20%) | 4 (80%) | ||
| SBB‐GFAP; RS‐GFAP | 0 | 5 (100%) | 0 | 5 (100%) | ||
| SBB‐Olig‐2; RS‐GFAP | 0 | 1 (100%) | 0 | 1 (100%) | ||
| WHO tumor grade (RS) | .16 | .14 | ||||
| 1 | 0 | 4 (100.0%) | 0 | 0 | ||
| 2 | 0 | 8 (100.0%) | 1 (20%) | 4 (80%) | ||
| 3 | 4 (40.0%) | 6 (60.0%) | 4 (40.0%) | 6 (60.0%) | ||
| 4 | 2 (25.0%) | 6 (75.0%) | 0 | 8 (100.0%) | ||
| Canine glioma grade (RS) | NA | 1.00 | ||||
| Low | NA | NA | 1 (20%) | 4 (80%) | ||
| High | NA | NA | 4 (22.2%) | 14 (77.8%) | ||
| Time (days) from SBB to RS, median (IQR) | 110.5 (45.0) | 25.0 (157.0) | .25 | 126.0 (61.0) | 68.5 (158.0) | .26 |
Abbreviations: GFAP, glial fibrillary acidic protein; IQR, interquartile range; NA, not applicable; Olig‐2, oligodendrocyte transcription factor 2; SBB, stereotactic brain biopsy.
P values are exact tests for categorical measures, t tests when means are presented, and Kruskal‐Wallis test when medians are presented.
Statistically significant (P < .05).
4. DISCUSSION
In our study, the DY of SBB was 94% and overall DA was 81%. These findings are similar to those of previously published studies of dogs and humans undergoing frame‐based SBB, which report DY for neoplastic lesions ranging from 89 to 99% and DA of 63%‐90%.5, 6, 10, 11, 12, 13, 14, 15, 16 In both human and veterinary medicine, improvements in the DY and DA of brain biopsy have evolved in parallel with clinician experience, as well as with diagnostic imaging and surgical technologies.5, 6, 10, 11, 12, 13, 14, 15, 16 To date, only 2 studies have reported the DA of needle biopsy in dogs with naturally occurring brain tumors.6, 16 One investigation using a free‐hand biopsy technique resulted in a DA of 13%, with diagnostic tumor type agreement noted between biopsy and necropsy in 1/8 cases.16 Another study using a SBB procedure reported a DA of 91% (20/22 cases) in dogs with brain tumors.6 Neither of these previous studies in dogs included analyses of tumor grade as a component of diagnostic concordance. Here, we report an accuracy of 97% (30/31 cases) for determination of tumor type. The excellent agreement between SBB and the reference standard tumor type diagnoses in this, and other studies, provides additional evidence that SBB is a sufficiently accurate technique to provide definitive histologic diagnoses of brain tumors and subsequently guide patient care.6 Because meningiomas and gliomas account for 75%‐90% of all primary brain tumors in dogs, the cohort of dogs reported here is an appropriate representation of potential candidates for SBB encountered in clinical practice.6, 17
Diagnostic agreement for tumor type and grade was perfect for all meningiomas in our study, but no attempt was made to examine concordance with respect to meningioma histologic subtypes. As hypothesized, the DA of gliomas was lower than that of meningiomas. This finding is not unexpected given that meningiomas were significantly larger than gliomas, and more biopsy trajectories were used in meningioma cases. Although the number of biopsy specimens obtained was not statistically different between meningiomas and gliomas, usage of a higher number of biopsy trajectories may improve diagnostic agreement by providing a superior topographic representation of tumor histology.
All of the diagnostic discordance observed in our study occurred during evaluation of glial tumors, and the majority of disagreements were attributable to tumor grading. Glioma grade agreement was moderate and not significantly different between both grading systems (17/23 WHO graded cases and 18/23 revised canine cases). Notably, SBB frequently underestimated glioma grade compared to SR or NE. The difference in grade concordance between systems likely is explained because the WHO glioma grading system has 4 categories, whereas the revised canine classification system grades in a binary fashion (high or low). Thus, it is not unexpected that more disagreements were noted using the WHO grading system. Pathologists in our study commented that the absence of the hallmark morphologic feature of necrosis in SBB specimens often precluded assignment of a Grade IV astrocytoma or Grade III oligodendroglioma (high‐grade glioma) diagnosis.9 This may represent a form of sampling bias, because the neurosurgeon performing SBB intentionally avoided necrotic tumor regions, given that obtaining biopsy specimens from necrotic areas accounts for a substantial proportion of nondiagnostic samples in studies of SBB in dogs and humans.5, 11, 13, 15 Phenotypic heterogeneity within gliomas is well recognized to present diagnostic challenges to neuropathologists in human and veterinary medicine, especially when considering the sample size limitations inherent to SBB.9, 15 Our findings further support usage of the revised canine glioma system to streamline and standardize glioma diagnosis in dogs.
Factors that were identified to significantly influence glioma grade discordance in our study were lesion volume, number of biopsies attempted, and the number of biopsy specimens obtained. Gliomas in which grade discordance was noted were significantly smaller than those in which there was grade agreement. Previous investigations of SBB in humans and dogs have indicated that SBB of smaller lesions may result in lower diagnostic agreement for numerous reasons such as targeting inaccuracies, mechanical deformation of the target at the time of the biopsy, limited quantity or quality of biopsy specimens obtained, and higher probability of obtaining biopsy specimens outside the tumor or at its boundaries.5, 10, 13, 15 One study of humans also demonstrated that SBB of very large gliomas (>50 cm3) resulted in biopsy samples that were less representative of the entire lesion because of the extensive areas of necrosis and extreme intratumoral heterogeneity.13
The number of biopsies attempted and specimens obtained also were significant variables that affected DA of SBB in this and other studies, and are often inherently related to lesion volume.5 The median number of biopsy specimens obtained in cases of diagnostic discordance in this cohort was 1, which highlights the importance of obtaining multiple biopsy samples to improve the DA of SBB. Performance of a geographic core biopsy technique represents a possible solution for optimizing the DA of SBB by obtaining multiple samples along a single biopsy trajectory.
Several limitations of our study also may have impacted agreement. First, methods to minimize interobserver variability between pathologists classifying tumors were not included in the study design. Although pathologists participating in the study all have experience with brain tumors in dogs and SBB, similar to the results reported here, agreement among experts reviewing glioma histomorphology and grade in dogs and humans has been reported previously as moderate.5, 9, 18 Incorporation of joint training and multiple specimen review sessions for pathologists has been shown to improve DA and should be considered in future studies.19 All of the dogs in our study received interventions including stereotactic radiotherapeutic, molecularly targeted biological, and other ablative therapies between the time SBB and reference standard tissue samples were collected.20, 21, 22 Tumor phenotypes may change over time as a result of natural tumor progression or as a result of treatments administered.11, 13, 15, 21 Although time elapsed between SBB and the reference standard was not shown to significantly influence diagnostic concordance in our study, the median time elapsed between SBB and reference standard diagnosis in cases with glioma grade discordance (126 days) was nearly twice as long as in cases demonstrating agreement (68 days). In addition, the only disagreement in tumor type diagnosis observed in our study also had the longest delay (372 days) between SBB and necropsy.
5. CONCLUSIONS
The DA of SBB readily allows for the definitive diagnosis of different types of common brain tumors in dogs. Agreement between SBB and reference standard methods for glioma grading was moderate, with SBB frequently underestimating glioma grade. However, given the currently unknown influences of tumor grade on the prognosis of dogs with gliomas, SBB provides sufficient information to rationally guide therapeutic decisions in dogs with brain tumors. Multiple biopsy specimens should be obtained to maximize the DA of SBB.23
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was conducted in accordance with the guidelines of the Virginia Tech Institutional Animal Care and Use Committee (protocols 17‐011, 17‐203, 16‐017, 15‐221, 14‐235, 12‐014, and 08‐218).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health/National Cancer Institute (P01CA207206 and R01CA213423). The sponsor had no role in the design, preparation, or writing of the manuscript, or in the decision to submit the article for publication.
Kani Y, Cecere TE, Lahmers K, et al. Diagnostic accuracy of stereotactic brain biopsy for intracranial neoplasia in dogs: Comparison of biopsy, surgical resection, and necropsy specimens. J Vet Intern Med. 2019;33:1384–1391. 10.1111/jvim.15500
[Correction added on 30‐April‐2019, after first online publication: Funding Information and Acknowledgements have been added]
Funding information National Institutes of Health/National Cancer Institute, Grant/Award Number: P01CA207206 and R01CA213423
REFERENCES
- 1. Wolff CA, Holmes SP, Young BD, et al. Magnetic resonance imaging for the differentiation of neoplastic, inflammatory, and cerebrovascular brain disease in dogs. J Vet Int Med. 2012;26:589‐597. [DOI] [PubMed] [Google Scholar]
- 2. Cervera V, Mai W, Vite CH, Johnson V, Dayrell‐Hart B, Seiler GS. Comparative magnetic resonance imaging findings between gliomas and presumed cerebrovascular accidents in dogs. Vet Radiol Ultrasound. 2011;52:33‐40. [PubMed] [Google Scholar]
- 3. Friedman WA, Sceats DJ, Nestok BR, Ballinger WE. The incidence of unexpected pathological findings in an image‐guided biopsy series: a review of 100 consecutive cases. Neurosurgery. 1989;25:180‐184. [DOI] [PubMed] [Google Scholar]
- 4. Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930‐937. [DOI] [PubMed] [Google Scholar]
- 5. Rossmeisl JH, Andriani RT, Cecere TE, et al. Frame‐based stereotactic biopsy of canine brain masses: technique and clinical results in 26 cases. Front Vet Sci. 2015;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koblik PD, LeCouteur RJ, Higgins RJ, et al. CT‐guided brain biopsy using a modified Pelorus Mark III stereotactic system: experience with 50 dogs. Vet Radiol Ultrasound. 1999;40:434‐440. [DOI] [PubMed] [Google Scholar]
- 7. Rossmeisl JH Jr, Pineyro P, Sponenberg DP, et al. Clinicopathologic features of intracranial central neurocytomas in 2 dogs. J Vet Int Med. 2012;26:186‐191. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koehler JW, Miller AD, Miller CR, et al. A revised diagnostic classification of canine glioma: towards validation of the canine glioma patient as a naturally occurring preclinical model for human glioma. J Neuropathol Exp Neurol. 2018;77:1039‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moissonnier P, Blot S, Devauchelle P, et al. Stereotactic CT‐guided brain biopsy in the dog. J Small Anim Pract. 2002;43:115‐123. [DOI] [PubMed] [Google Scholar]
- 11. Aker FV, Hakan T, Karadereler S, Erkan M. Accuracy and diagnostic yield of stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimens. Neuropathology. 2005;25:207‐213. [DOI] [PubMed] [Google Scholar]
- 12. Owen CM, Linskey ME. Frame‐based stereotaxy in a frameless era: current capabilities, relative role, and the positive‐ and negative predictive values of blood through the needle. J Neurooncol. 2009;93:139‐149. [DOI] [PubMed] [Google Scholar]
- 13. Woodworth G, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Accuracy of frameless and frame‐based image‐guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res. 2005;27:358‐362. [DOI] [PubMed] [Google Scholar]
- 14. Callovini GM, Telera S, Sherkat S, Sperduti I, Callovini T, Carapella CM. How is stereotactic brain biopsy evolving? A multicentric analysis of a series of 421 cases treated in Rome over the last sixteen years. Clin Neurol Neurosurg. 2018;174:101‐107. [DOI] [PubMed] [Google Scholar]
- 15. Chandrasoma PT, Smith MM, Apuzzo ML. Stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimen. Neurosurgery. 1989;24:160‐165. [DOI] [PubMed] [Google Scholar]
- 16. Harari J, Moore MM, Leathers CW, et al. Computed tomographic‐guided, free‐hand needle biopsy of brain tumors in dogs. Prog Vet Neurol. 1994;4:41‐44. [Google Scholar]
- 17. Song RB, Vite CH, Bradley CW, Cross JR. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Int Med. 2013;27:1143‐1152. [DOI] [PubMed] [Google Scholar]
- 18. van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010;120:297‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381‐1393. [DOI] [PubMed] [Google Scholar]
- 20. Debinski W, Dickinson P, Rossmeisl JH, Robertson J, Gibo DM. New agents for targeting of IL‐13RA2 expressed in primary human and canine brain tumors. PLoS One. 2013;8:e77719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossmeisl JH, Garcia PA, Pancotto TE, et al. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative treatment of canine spontaneous intracranial gliomas. J Neurosurg. 2015;123:1008‐1025. [DOI] [PubMed] [Google Scholar]
- 22. Rossmeisl JH, Hall‐Manning K, Robertson JL, et al. Expression and activity of the urokinase plasminogen activator system in canine primary brain tumors. Onco Targets Ther. 2017;10:2077‐2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain D, Sharma M, Sarkar C, et al. Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006;23:71‐75. [DOI] [PubMed] [Google Scholar]


