Abstract
Background
Silent brain infarction (SBI) is defined as the presence of 1 or more brain lesions, presumed to be because of vascular occlusion, found by neuroimaging (magnetic resonance imaging or computed tomography) in patients without clinical manifestations of stroke. It is more common than stroke and can be detected in 20% of healthy elderly people. Early detection of SBI may mitigate the risk of stroke by offering preventative treatment plans. Natural language processing (NLP) techniques offer an opportunity to systematically identify SBI cases from electronic health records (EHRs) by extracting, normalizing, and classifying SBI-related incidental findings interpreted by radiologists from neuroimaging reports.
Objective
This study aimed to develop NLP systems to determine individuals with incidentally discovered SBIs from neuroimaging reports at 2 sites: Mayo Clinic and Tufts Medical Center.
Methods
Both rule-based and machine learning approaches were adopted in developing the NLP system. The rule-based system was implemented using the open source NLP pipeline MedTagger, developed by Mayo Clinic. Features for rule-based systems, including significant words and patterns related to SBI, were generated using pointwise mutual information. The machine learning models adopted convolutional neural network (CNN), random forest, support vector machine, and logistic regression. The performance of the NLP algorithm was compared with a manually created gold standard. The gold standard dataset includes 1000 radiology reports randomly retrieved from the 2 study sites (Mayo and Tufts) corresponding to patients with no prior or current diagnosis of stroke or dementia. 400 out of the 1000 reports were randomly sampled and double read to determine interannotator agreements. The gold standard dataset was equally split to 3 subsets for training, developing, and testing.
Results
Among the 400 reports selected to determine interannotator agreement, 5 reports were removed due to invalid scan types. The interannotator agreements across Mayo and Tufts neuroimaging reports were 0.87 and 0.91, respectively. The rule-based system yielded the best performance of predicting SBI with an accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 0.991, 0.925, 1.000, 1.000, and 0.990, respectively. The CNN achieved the best score on predicting white matter disease (WMD) with an accuracy, sensitivity, specificity, PPV, and NPV of 0.994, 0.994, 0.994, 0.994, and 0.994, respectively.
Conclusions
We adopted a standardized data abstraction and modeling process to developed NLP techniques (rule-based and machine learning) to detect incidental SBIs and WMDs from annotated neuroimaging reports. Validation statistics suggested a high feasibility of detecting SBIs and WMDs from EHRs using NLP.
Keywords: natural language processing, neuroimaging, electronic health records
Introduction
Background
Silent brain infarction (SBI) is defined as the presence of 1 or more brain lesions, presumed to be because of vascular occlusion, found by neuroimaging (magnetic resonance imaging, MRI or computed tomography, CT) in patients without clinical manifestations of stroke. SBIs are more common than stroke and can be detected on MRI in 20% of healthy elderly [1-3]. Studies have shown that SBIs are associated with increased risk of subsequent stroke, cognitive decline, and deficiency in physical function [1,2]. Despite the high prevalence and serious consequences, there is no consensus on the management of SBI as routinely discovering SBIs is challenged by the absence of corresponding diagnosis codes and the lack of the knowledge about the characteristics of the affected population, treatment patterns, or the effectiveness of therapy [1]. Even though there is strong evidence shows that antiplatelet and statin therapies are effective in preventing recurrent stroke in patients with prior stroke, the degree to which these results might apply to patients with SBI is unclear. Although SBI is understood by some clinicians to be pathophysiologically identical to stroke (and thus similarly treated), others view SBI as an incidental neuroimaging finding of unclear significance. The American Heart Association/American Stroke Association has identified SBI as a major priority for new studies on stroke prevention because the population affected by SBI falls between primary and secondary stroke prevention [4].
In addition to SBI, white matter disease (WMD) or leukoaraiosis is another common finding in neuroimaging of elderly. Similar to SBI, WMD is usually detected incidentally on brain scans and is commonly believed to be a form of microvascular ischemic brain damage resulting from typical cardiovascular risk factors [5]. WMD is associated with subcortical infarcts due to small vessel disease and is predictive of functional disability, recurrent stroke, and dementia [6-8]. SBI and WMD are related, but it is unclear whether they result from the same, independent, or synergistic processes [9,10]. As with SBI, there are no proven preventive treatments or guidelines regarding the initiation of risk factor–modifying therapies when WMD is discovered.
Objectives
Identifying patients with SBI is challenged by the absence of corresponding diagnosis codes. One reason is that SBI-related incidental findings are not included in a patient’s problem list or other structured fields of electronic health records (EHRs); instead, the findings are captured in neuroimaging reports. A neuroimaging report is a type of EHR data that contains the interpretation and finding from neuroimage such as CT and MRI in unstructured text. Incidental SBIs can be detected by the review of neuroradiology reports obtained in clinical practice, typically performed manually by radiologists or neurologists. However, manually extracting information from patient narratives is time-consuming, costly, and lacks robustness and standardization [11-14]. Natural language processing (NLP) has been leveraged to perform chart review for other medical conditions by automatically extracting important clinical concepts from unstructured text. Researchers have used NLP systems to identify clinical syndromes and biomedical concepts from clinical notes, radiology reports, and surgery operative notes [15]. An increasing amount of NLP-enabled clinical research has been reported, ranging from identifying patient safety occurrences [16] to facilitating pharmacogenomic studies [17]. Our study focuses on developing NLP algorithms to routinely detect incidental SBIs and WMDs.
Methods
Study Setting
This study was approved by the Mayo Clinic and Tufts Medical Center (TMC) institutional review boards. This work is part of the Effectiveness of Stroke PREvention in Silent StrOke project, which is to use NLP techniques to identify individuals with incidentally discovered SBIs from radiology reports, at 2 sites: Mayo Clinic and TMC.
Gold Standard
The detailed process of generating the gold standard is described in Multimedia Appendix 1. The gold standard annotation guideline was developed by 2 subject matter experts: a vascular neurologist (LYL) and a neuroradiologist (PHL), and the annotation task was performed by 2 third-year residents (KAK, MSC) from Mayo and 2 first-year residents (AOR, KN) from TMC. Each report was annotated with 1 of the 3 labels for SBI (positive SBI, indeterminate SBI, or negative SBI) and one of the 3 labels for WMD (positive WMD, indeterminate WMD, or negative WMD).
The gold standard dataset includes 1000 radiology reports randomly retrieved from the 2 study sites (500 from Mayo Clinic and 500 from TMC) corresponding to patients with no prior or current diagnosis of stroke or dementia. To calculate interannotator agreement (IAA), 400 out of the 1000 reports were randomly sampled and double read. The gold standard dataset was equally split to 3 subsets for training (334), developing (333), and testing (333).
Experimental Methods
We compared 2 NLP approaches. One was to define the task an information extraction (IE) task, where a rule-based IE system can be developed to extract SBI or WMD findings. The other was to define the task as a sentence classification task, where sentences can be classified to contain SBI or WMD findings.
Rule-Based Information Extraction
We adopted the open source NLP pipeline, MedTagger, as the infrastructure for the rule-based system implementation. MedTagger is a resource-driven, open source unstructured information management architecture–based IE framework [18]. The system separates task-specific NLP knowledge engineering from the generic NLP process, which enables words and phrases containing clinical information to be directly coded by subject matter experts. The tool has been utilized in the eMERGE consortium to develop NLP-based phenotyping algorithms [19]. Figure 1 shows the process workflow. The generic NLP process includes sentence tokenization, text segmentation, and context detection. The task-specific NLP process includes the detection of concept mentions in the text using regular expressions and normalized to specific concepts. The summarization component applies heuristic rules for assigning the labels to the document.
Figure 1.
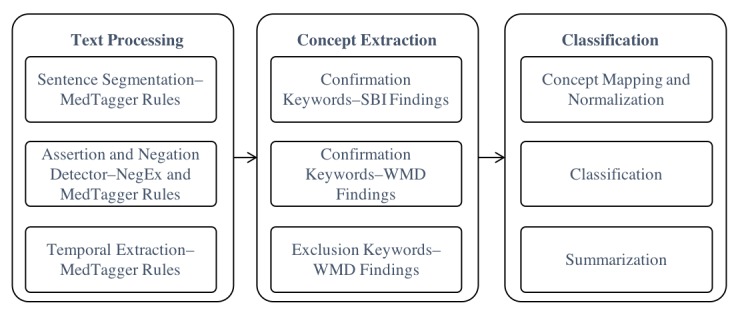
Rule system process flow. SBI: silent brain infarction; WMD: white matter disease.
For example, the sentence “probable right old frontal lobe subcortical infarct as described above,” is processed as an SBI concept with the corresponding contextual information with status as “probable,” temporality as “present,” and experiencer as “patient.”
The domain-specific NLP knowledge engineering was developed following 3 steps: (1) Prototype algorithm development, (2) Formative algorithm development using the training data, and (3) Final algorithm evaluation. We leveraged pointwise mutual information [20] to identify significant words and patterns associated with each condition for prototyping the algorithm (Multimedia Appendix 2). The algorithm was applied to the training data. False classified reports were manually reviewed by 2 domain experts (LYL, PHL). Keywords were manually curated through an iteratively refining process until all issues were resolved. The full list of concepts, keywords, modifiers, and diseases categories are listed in Textbox 1.
Silent brain infarction (SBI) and white matter disease (WMD) risk factor and indication keywords.
Confirmation keywords—disease-finding SBI: infarct, infarcts, infarctions, infarction, lacune, lacunes
Confirmation keywords—disease modifier SBI: acute, acute or subacute, recent, new, remote, old, chronic, prior, chronic foci of, benign, stable small, stable
Confirmation keywords—disease location SBI: territorial, lacunar, cerebellar, cortical, frontal, caudate, right frontoparietal lobe, right frontal cortical, right frontal lobe, embolic, left basal ganglia lacunar, basal ganglia lacunar, left caudate and left putamen lacunar
Confirmation keywords—disease-finding WMD: leukoaraiosis, white matter, microvascular ischemic, microvascular leukemic, microvascular degenerative
Exclusion WMD: degenerative changes
Machine Learning
The machine learning (ML) approach allows the system to automatically learn robust decision rules from labeled training data. The task was defined as a sequential sentence classification task. We adopted Kim’s convolutional neural network (CNN) [21] and implemented using TensorFlow 1.1.02 [22]. The model architecture, shown in Figure 2, is a variation of the CNN architecture of Collobert R [23].
Figure 2.
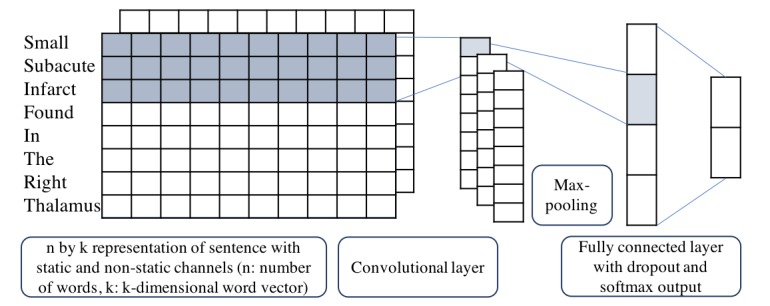
Convolutional neural network architecture with 2 channels for an example sentence.
We also adopted 3 traditional ML models—random forest [24], support vector machine [25] and logistic regression [26]—for baseline comparison. All models used word vector as input representation, where each word from the input sentence is represented as the k-dimensional word vector. The word vector is generated from word embedding, a learned representation for text where words that have the same meaning have a similar representation. Suppose x1, x2, … , xn is the sequence of word representations in a sentence where
| xi = Exi, I = 1,2, …, n. |
Here, Exi is the word embedding representation for word xi with the dimensionality d. In our ML experiment, we used Wang’s word embedding trained from Mayo Clinic clinical notes where d=100 [27]. The embedding model is the skip-gram of word2vec, an architecture proposed by Mikolov T [28]. Let xi:i+k-1 represent a window of size k in the sentence. Then the output sequence of the convolutional layer is
| coni = f(wk xi:i+k-1 + bk), |
where f is a rectify linear unit function, wk and bk are the learning parameters. Max pooling was then performed to record the largest number from each feature map. By doing so, we obtained fixed length global features for the whole sentence, that is,
| mk = max1≤i≤n-k+1(coni). |
Then the features are fit into a fully connected layer with the output being the final feature vector O=wmk + b. Finally, a softmax function is utilized to make final classification decision, that is,
| p(sbi│x,θ) = e^(Osbi)/(e^(Osbi)+e^(Oother)), |
where θ is a vector of the hyper parameters of the model, such as wk, bk, w and b.
Evaluation Metric
For evaluation of the quality of the annotated corpus, Cohen kappa was calculated to measure the IAA during all phases [29]. As the primary objective of the study is case ascertainment, we calculated the IAA at the report level.
A 2 x 2 confusion matrix was used to calculate performance score for model evaluation: positive predictive value (PPV), sensitivity, negative predictive value (NPV), specificity, and accuracy using manual annotation as the gold standard. The McNemar test was adopted to evaluate the performance difference between the rule-based and ML models [30,31]. To have a better understanding of the potential variation between neuroimaging reports and neuroimages, we compared the model with the best performance (rule-based) with neuroimaging interpretation. A total of 12 CT images and 12 MRI images were stratified—randomly sampled from the test set. A total of 2 attending neurologists read all 24 images and assigned the SBI and WMD status. The cases with discrepancies were adjudicated by the neuroradiologist (PHL) The agreement was assessed using kappa and F-measure [32].
Results
Interannotator Agreements Across Neuroimaging Reports
Among the total 400 double-read reports, 5 reports were removed because of invalid scan types. The IAAs across Mayo and Tufts neuroimaging reports were 0.87 and 0.91. Overall, there is a high agreement between readers on both reports (Tables 1 and 2). Age-specific prevalence of SBI and WMD is provided in Multimedia Appendix 2.
Table 1.
Interreader agreement across 207 Mayo neuroimaging reports.
| Interannotator agreement | Computed tomography (n=63) | Magnetic resonance imaging (n=144) | Total (n=207) | |||
|
|
% agree | kappa | % agree | kappa | % agree | kappa |
| Silent brain infarction | 98.4 | 0.92 | 97.2 | 0.83 | 97.6 | 0.87 |
| White matter disease | 100.0 | 1.00 | 98.6 | 0.97 | 99.0 | 0.98 |
Table 2.
Interreader agreement across 188 Tufts Medical Center neuroimaging reports.
| Interannotator agreement | Computed tomography (n=80) | Magnetic resonance imaging (108) | Total (n=188) | |||
|
|
% agree | kappa | % agree | kappa | % agree | kappa |
| Silent brain infarction | 98.8 | 0.79 | 99.1 | 0.94 | 99.5 | 0.91 |
| White matter disease | 100.0 | 1.00 | 99.1 | 0.98 | 99.5 | 0.99 |
Natural Language Processing System Performance
Overall, the rule-based system yielded the best performance of predicting SBI with an accuracy of 0.991. The CNN achieved the best score on predicting WMD (0.994). Full results are provided in Table 3.
Table 3.
Performance on test dataset against human annotation as gold standard.
| Evaluation of natural language processing, model name | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
| Silent brain infarction (n=333) | ||||||
|
|
Rule-based system | 0.925 | 1.000 | 1.000 | 0.990 | 0.991 |
|
|
CNNa | 0.650 | 0.993 | 0.929 | 0.954 | 0.952 |
|
|
Logistic regression | 0.775 | 0.983 | 0.861 | 0.970 | 0.958 |
|
|
SVMb | 0.825 | 1.000 | 1.000 | 0.977 | 0.979 |
|
|
Random forest | 0.875 | 1.000 | 1.000 | 0.983 | 0.986 |
| White matter disease (n=333) | ||||||
|
|
Rule-based system | 0.942 | 0.909 | 0.933 | 0.921 | 0.928 |
|
|
CNN | 0.994 | 0.994 | 0.994 | 0.994 | 0.994 |
|
|
Logistic regression | 0.906 | 0.865 | 0.896 | 0.877 | 0.888 |
|
|
SVM | 0.864 | 0.894 | 0.917 | 0.830 | 0.877 |
|
|
Random forest | 0.932 | 0.880 | 0.913 | 0.906 | 0.910 |
aCNN: convolutional neural network.
bSVM: support vector machine.
According to the McNemar test, we found the difference between rule-based system and CNN on SBI is considered to be statistically significant (P value=.03). We found no statistically significant difference between the rest of the models.
Table 4 lists the evaluation results of NLP and gold standard derived from reports against the neuroimaging interpretation for SBI and WMD. Both NLP and gold standard had moderate-high agreements with the neuroimaging interpretation, with kappa scores around .5. Our further analysis showed the practice graded findings (gold standard and NLP) achieved high precision and moderate recall scores compared with the neuroimaging interpretation. Through the confirmation with Mayo and TMC radiologists, we believed such discrepancy was because of the inconsistency in documentation standards related to clinical incidental findings, causing SBIs and WMDs underreported.
Table 4.
Comparison of the neuroimaging interpretation with gold standard and natural language processing.
| Evaluation of natural language processing against the neuroimaging interpretation | F-measure | kappa | Precision | Recall | |
| Silent brain infarction (n=24) | |||||
|
|
Gold standard | 0.74 | 0.50 | 0.92 | 0.69 |
|
|
NLPa | 0.74 | 0.50 | 0.92 | 0.69 |
| White matter disease (n=24) | |||||
|
|
Gold standard | 0.78 | 0.56 | 0.86 | 0.80 |
|
|
NLP | 0.74 | 0.49 | 0.85 | 0.73 |
aNLP: natural language processing.
Discussion
Machine Learning Versus Rule
In summary, the rule-based system achieved the best performance of predicting SBI, and the CNN model yielded the highest score of predicting WMD. When detecting SBI, the ML models were able to achieve high specificity, NPV, and PPV but moderate sensitivity because of the small number of positive cases. Oversampling is a technique to adjust the class distribution of training data to balance the ratio between positive and negative cases [33]. This technique was applied to the training data to help boost the signals of positive SBIs. The performance was slightly improved but was limited by the issue of overfitting, a situation when a model learns the training data too well. Due to that, unnecessary details and noises in the training data can create negative impact to the generalizability of the model. In our case, the Mayo reports have larger language variation (noise) because of a free style of documentation method, whereas TMC uses a template-based documentation method. According to the sublanguage analysis, Mayo had 212 unique expressions for describing no acute infarction, whereas TMC had only 12. Therefore, the model trained on oversampled data had a bias toward the expressions that only appeared in the training set. When predicting WMD, the ML model outperformed the rule-based model. The reason is because the dataset for WMD is more balanced than SBI (60% positive cases), which allows the system to equally learn from both classes (positive and negative). The overall performance on WMD is better than SBI because WMDs are often explicitly documented as important findings in the neuroimaging report.
False Prediction Analysis
Coreference resolution was the major challenge to the rule-based model for identifying SBIs. Coreference resolution is an NLP task to determine whether 2 mentioned concepts refer to the same real-world entity. For example, in Textbox 2, “The above findings” refers to “where there is an associated region of nonenhancing encephalomalacia and linear hemosiderin disposition.” To determine if a finding is SBI positive, the system needs to extract both concepts and detect their coreference relationship.
Example of coreference resolution.
“Scattered, nonspecific T2 foci, most prominently in the left parietal white matter <Concept 1>where there is an associated region of nonenhancing encephalomalacia and linear hemosiderin disposition. <Concept 1/> Linear hemosiderin deposition overlying the right temporal lobe (series 9, image 16) as well. No abnormal enhancement today. <Concept 2>The above findings are nonspecific but the evolution, hemosiderin deposition, and gliosis suggest post ischemic change. <Concept 2>”
For the ML system, the false positives from the identification of SBIs were commonly contributed by disease locations. As the keywords foci, right occipital lobe, right parietal lobe, right subinsular region, and left frontal region often coexisted with SBI expressions, the model assigned higher weights to these concepts when the model was trained. For example, the expression: “there are a bilateral intraparenchymal foci of susceptibility artifact in the right occipital lobe, right parietal lobe, right subinsular region and left frontal region” has 4 locations with no mention of “infarction” appearing in the sentence. The ML system still predicted it as SBI positive. Among all ML models, the CNN yielded the worse NPV, which suggested the CNN was more likely to receive false signals from disease locations. Our next step is to further refine the system by increasing the volume of training size through leveraging distant supervision to obtain additional SBI positive cases.
Limitations
Our study has several limitations. First, despite the high feasibility of detecting SBIs from neuroimaging reports, there is a variation between NLP-labeled neuroimaging reports and neuroimages. Second, the performances of the ML models are limited by the number of annotated datasets. Additional training data are required to have a comprehensive comparison between the rule-based and ML systems. Third, the systems were only evaluated using datasets from 2 sites; the generalizability of the systems may be limited.
Conclusions
We adopted a standardized data abstraction and modeling process to developed NLP techniques (rule-based and ML) to detect incidental SBIs and WMDs from annotated neuroimaging reports. Validation statistics suggested a high feasibility of detecting SBIs and WMDs from EHRs using NLP.
Acknowledgments
The authors would like to gratefully acknowledge National Institutes of Health grant 1R01NS102233 and Donna M Ihrke for case validation.
Abbreviations
- CNN
convolutional neural network
- CT
computed tomography
- EHR
electronic health record
- IAA
interannotator agreement
- IE
information extraction
- MRI
magnetic resonance imaging
- NLP
natural language processing
- NPV
negative predictive value
- PPV
positive predictive value
- SBI
silent brain infarction
- TMC
Tufts Medical Center
- WMD
white matter disease
Gold standard development.
Supplementary result.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke. 2014 Nov;45(11):3461–71. doi: 10.1161/STROKEAHA.114.005919.STROKEAHA.114.005919 [DOI] [PubMed] [Google Scholar]
- 2.Fanning J, Wong A, Fraser J. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014 Jul 9;12:119. doi: 10.1186/s12916-014-0119-0. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-014-0119-0 .s12916-014-0119-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer S, Longstreth JW, Koudstaal P. Silent brain infarcts: a systematic review. Lancet Neurol. 2007 Jul;6(7):611–9. doi: 10.1016/S1474-4422(07)70170-9.S1474-4422(07)70170-9 [DOI] [PubMed] [Google Scholar]
- 4.Furie K, Kasner S, Adams R, Albers G, Bush R, Fagan S, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D, American Heart Association Stroke Council‚ Council on Cardiovascular Nursing‚ Council on Clinical Cardiology‚Interdisciplinary Council on Quality of CareOutcomes Research Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011 Jan;42(1):227–76. doi: 10.1161/STR.0b013e3181f7d043.STR.0b013e3181f7d043 [DOI] [PubMed] [Google Scholar]
- 5.Gouw A, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Scheltens P, Barkhof F, LADIS Study Group Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008 May;39(5):1414–20. doi: 10.1161/STROKEAHA.107.498535.STROKEAHA.107.498535 [DOI] [PubMed] [Google Scholar]
- 6.Hijdra A, Verbeeten B, Verhulst J. Relation of leukoaraiosis to lesion type in stroke patients. Stroke. 1990 Jun;21(6):890–4. doi: 10.1161/01.STR.21.6.890. [DOI] [PubMed] [Google Scholar]
- 7.Miyao S, Takano A, Teramoto J, Takahashi A. Leukoaraiosis in relation to prognosis for patients with lacunar infarction. Stroke. 1992 Oct;23(10):1434–8. doi: 10.1161/01. [DOI] [PubMed] [Google Scholar]
- 8.Fu J, Lu C, Hong Z, Dong Q, Luo Y, Wong K. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005 Jun;76(6):793–6. doi: 10.1136/jnnp.2003.032771. http://jnnp.bmj.com/cgi/pmidlookup?view=long&pmid=15897500 .76/6/793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Wang A, Tang J, Wei D, Li P, Chen K, Wang Y, Zhang Z. Association of white matter integrity and cognitive functions in patients with subcortical silent lacunar infarcts. Stroke. 2015 Apr;46(4):1123–6. doi: 10.1161/STROKEAHA.115.008998.STROKEAHA.115.008998 [DOI] [PubMed] [Google Scholar]
- 10.Conklin J, Silver F, Mikulis D, Mandell D. Are acute infarcts the cause of leukoaraiosis? Brain mapping for 16 consecutive weeks. Ann Neurol. 2014 Dec;76(6):899–904. doi: 10.1002/ana.24285. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Jiang M, Oetjens M, Bowton E, Ramirez A, Jeff J, Basford MA, Pulley JM, Cowan JD, Wang X, Ritchie MD, Masys DR, Roden DM, Crawford DC, Denny JC. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc. 2011;18(4):387–91. doi: 10.1136/amiajnl-2011-000208. http://europepmc.org/abstract/MED/21672908 .amiajnl-2011-000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grishman R, Huttunen S, Yangarber R. Information extraction for enhanced access to disease outbreak reports. J Biomed Inform. 2002 Aug;35(4):236–46. doi: 10.1016/S1532-0464(03)00013-3. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(03)00013-3 .S1532-0464(03)00013-3 [DOI] [PubMed] [Google Scholar]
- 13.South B, Shen S, Jones M, Garvin J, Samore M, Chapman W, Gundlapalli AV. Developing a manually annotated clinical document corpus to identify phenotypic information for inflammatory bowel disease. Summit Transl Bioinform. 2009 Mar 1;2009:1–32. doi: 10.1186/1471-2105-10-S9-S12. http://europepmc.org/abstract/MED/21347157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert E, Lowenstein S, Koziol-McLain J, Barta D, Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996 Mar;27(3):305–8. doi: 10.1016/S0196-0644(96)70264-0.S0196-0644(96)70264-0 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Wang L, Rastegar-Mojarad M, Moon S, Shen F, Afzal N, Liu S, Zeng Y, Mehrabi S, Sohn S, Liu H. Clinical information extraction applications: a literature review. J Biomed Inform. 2018 Dec;77:34–49. doi: 10.1016/j.jbi.2017.11.011. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(17)30256-3 .S1532-0464(17)30256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murff H, FitzHenry F, Matheny M, Gentry N, Kotter K, Crimin K, Dittus RS, Rosen AK, Elkin PL, Brown SH, Speroff T. Automated identification of postoperative complications within an electronic medical record using natural language processing. J Am Med Assoc. 2011 Aug 24;306(8):848–55. doi: 10.1001/jama.2011.1204.306/8/848 [DOI] [PubMed] [Google Scholar]
- 17.Denny J, Ritchie M, Basford M, Pulley J, Bastarache L, Brown-Gentry K, Wang D, Masys DR, Roden DM, Crawford DC. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010 May 1;26(9):1205–10. doi: 10.1093/bioinformatics/btq126. http://europepmc.org/abstract/MED/20335276 .btq126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrucci D, Lally A. UIMA: an architectural approach to unstructured information processing in the corporate research environment. Nat Lang Eng. 1999;10(3-4):327–48. doi: 10.1017/S1351324904003523. [DOI] [Google Scholar]
- 19.McCarty C, Chisholm R, Chute C, Kullo I, Jarvik G, Larson E, Li R, Masys DR, Ritchie MD, Roden DM, Struewing JP, Wolf WA, eMERGE Team The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011 Jan 26;4:13. doi: 10.1186/1755-8794-4-13. https://bmcmedgenomics.biomedcentral.com/articles/10.1186/1755-8794-4-13 .1755-8794-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Church K, Hanks P. Word association norms, mutual information, and lexicography. Comput Linguist. 1990;16(1):22–9. https://www.aclweb.org/anthology/J90-1003 . [Google Scholar]
- 21.Kim Y. Association for Computational Linguistics. 2014. [2019-04-08]. Convolutional neural networks for sentence classification https://www.aclweb.org/anthology/D14-1181.pdf .
- 22.Abadi M, Barham P, Chen J, Chen Z, Davis A, Dean J, Devin M, Ghemawat S, Irving G, Isard M, Kudlur M, Levenberg J, Monga R, Moore S, Murray DG, Steiner B, Tucker P, Vasudevan V, Warden P, Wicke M, Yu Y, Zheng X. TensorFlow: a system for large-scale machine learning. Proceedings of the 12th USENIX conference on Operating Systems Design and Implementation; OSDI'16; November 2–4, 2016; Savannah, GA, USA. 2016. [Google Scholar]
- 23.Collobert R, Weston J. A unified architecture for natural language processing: deep neural networks with multitask learning. Proceedings of the 25th international conference on Machine learning; ICML'08; July 5-9, 2008; Helsinki, Finland. A unified architecture for natural language processing: Deep neural networks with multitask learning. Proceedings of the 25th international conference on Machine learning; 2008. [DOI] [Google Scholar]
- 24.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. https://www.stat.berkeley.edu/~breiman/randomforest2001.pdf . [DOI] [Google Scholar]
- 25.Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995;20(3):273–97. doi: 10.1023/A:1022627411411. http://image.diku.dk/imagecanon/material/cortes_vapnik95.pdf . [DOI] [Google Scholar]
- 26.Hosmer JD, Lemeshow S, Sturdivant R. Applied Logistic Regression. Hoboken, New Jersey: John Wiley & Sons; 2013. [Google Scholar]
- 27.Wang Y, Liu S, Afzal N, Rastegar-Mojarad M, Wang L, Shen F, Kingsbury P, Liu H. A comparison of word embeddings for the biomedical natural language processing. J Biomed Inform. 2018 Nov;87:12–20. doi: 10.1016/j.jbi.2018.09.008.S1532-0464(18)30182-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikolov T, Chen K, Corrado G, Dean J. arXiv. 2013. [2019-04-08]. Efficient estimation of word representations in vector space https://arxiv.org/pdf/1301.3781.pdf .
- 29.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 2016 Jul 2;20(1):37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 30.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947 Jun;12(2):153–7. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 31.Dietterich T. Approximate statistical tests for comparing supervised classification learning algorithms. Neural Comput. 1998;10(7):1895–923. doi: 10.1162/089976698300017197. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki Y. Old Dominion University. 2007. [2019-04-08]. The truth of the F-measure https://www.researchgate.net/publication/268185911_The_truth_of_the_F-measure .
- 33.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. arXiv. 2002. [2019-04-08]. SMOTE: synthetic minority over-sampling technique https://arxiv.org/pdf/1106.1813.pdf .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gold standard development.
Supplementary result.


