Abstract
Background
Reliable phenotypic antimicrobial susceptibility testing can be a challenge in clinical settings in low- and middle-income countries. WGS is a promising approach to enhance current capabilities.
Aim
To study diversity and resistance determinants and to predict and compare resistance patterns from WGS data of Acinetobacter baumannii with phenotypic results from classical microbiological testing at a tertiary care hospital in Tanzania.
Methods and results
MLST using Pasteur/Oxford schemes yielded eight different STs from each scheme. Of the eight, two STs were identified to be global clones 1 (n = 4) and 2 (n = 1) as per the Pasteur scheme. Resistance testing using classical microbiology determined between 50% and 92.9% resistance across all drugs. Percentage agreement between phenotypic and genotypic prediction of resistance ranged between 57.1% and 100%, with coefficient of agreement (κ) between 0.05 and 1. Seven isolates harboured mutations at significant loci (S81L in gyrA and S84L in parC). A number of novel plasmids were detected, including pKCRI-309C-1 (219000 bp) carrying 10 resistance genes, pKCRI-43-1 (34935 bp) carrying two resistance genes and pKCRI-49-1 (11681 bp) and pKCRI-28-1 (29606 bp), each carrying three resistance genes. New ampC alleles detected included ampC-69, ampC-70 and ampC-71. Global clone 1 and 2 isolates were found to harbour ISAba1 directly upstream of the ampC gene. Finally, SNP-based phylogenetic analysis of the A. baumannii isolates revealed closely related isolates in three clusters.
Conclusions
The validity of the use of WGS in the prediction of phenotypic resistance can be appreciated, but at this stage is not sufficient for it to replace conventional antimicrobial susceptibility testing in our setting.
Introduction
The burden of antimicrobial resistance is drastically increasing globally.1 MDR Gram-negative bacterial pathogens especially pose major health challenges worldwide.2Acinetobacter baumannii is an important nosocomial pathogen and has been reported to cause serious health problems due to its resistance to multiple antimicrobial agents.3 Its ability as a non-lactose-fermenter to grow with low nutritional requirements enables the pathogen to survive in a wide range of environments.4 Historically, A. baumannii infections have mainly been associated with severely ill patients in ICUs and surgical wards and in people exposed to medical apparatus such as ventilators and catheters.5–7 Today, A. baumannii is found across hospital wards and associated with various diseases and/or surgical sites such as respiratory tract, bloodstream, urinary tract and wound infections.8,9 It is becoming a leading cause of nosocomial infections worldwide, with high mortality and morbidity rates.3 In many cases, the infections involve MDR strains of the pathogen.10
A. baumannii possesses several mechanisms to resist a wide range of existing antibiotic classes.3,11 These include production of enzymes that can hydrolyse β-lactams, the presence of efflux pumps and modifications of the outer membrane.12 The pathogen has great capability to acquire new resistance determinants,13 which notoriously increases the threat it poses to global health.
European and other medically advanced countries started monitoring antimicrobial resistance about 20 years ago.14,15 The majority of these countries have formulated strategies to combat this life-threatening disaster.16 Regular data generation on infection patterns, aetiological agents and their susceptibility patterns over time is a core aspect of these strategies.
In developing countries, data to monitor trends and susceptibility patterns of common aetiological agents are infrequently produced,17 despite these data being crucial for formulating control strategies and treatment guidance.18 Challenges encountered in resource-limited countries include, for example, low-capacity diagnostic tools, which hamper efforts to analyse and combat antimicrobial resistance,16,19 and low availability of reliable phenotypic drug susceptibility testing resources in clinical laboratories, resulting in untraceable resistance patterns for disease-causing agents.
High-throughput diagnostic tools such as WGS hold great promise in medical diagnostics and have proven to be invaluable in the control of antimicrobial resistance.20 The use of such high-throughput technologies to study pathogens has brought a breakthrough in surveillance and monitoring in medically advanced countries. Unfortunately, such tools are rarely available in resource-limited countries.
In this study, we present what WGS can offer in tackling antimicrobial resistance by applying this technology in Tanzania where, as in the rest of the world, antimicrobial resistance is a serious and fast-growing health concern. We used WGS to identify resistance determinants and predict phenotypic resistance in A. baumannii from patients who were admitted to Kilimanjaro Christian Medical Centre (KCMC), a referral hospital in northern Tanzania. The isolates were sequenced and analysed locally at the sequencing facility in the hospital’s research centre, Kilimanjaro Clinical Research Institute (KCRI).
Materials and methods
Study design, location and sample collection
This study was part of a larger study that collected 286 wound/pus swabs from 263 inpatients admitted to the medical or surgical departments of the hospital.18 The study was a descriptive analysis to characterize isolates from clinical specimens using WGS. This study was conducted at KCMC, a tertiary healthcare facility for the northern zone of Tanzania. The clinical specimens were collected during 2013 to 2015 from patients who were admitted to the medical and surgical departments of the hospital. Informed consent was obtained for all participants. Patient hospital files were used to obtain sociodemographic and clinical characteristics of the study participants. Data were then recorded on designated case report forms.
Laboratory methods and data analysis
Clinical samples were collected during routine clinical care and transported to the Microbiology Unit of KCRI Biotechnology Laboratory for routine microbiological analysis as previously described.18 The isolates were stored at −80°C before extraction of genomic DNA using the MasterPure™ Complete DNA and RNA Purification Kit (Cat. No. MC85200; Epicentre, Illumina). The quality and quantity of genomic DNA were confirmed using a Qubit 2.0 fluorometer (Life Technologies). Library preparation (dual indexing) was done using the Nextera XT DNA Preparation Kit (Illumina Inc., San Diego, CA, USA). WGS of the library was completed on an Illumina MiSeq using the 2 × 250 bp paired-end protocol. Patient characteristics were double data-entered in OpenClinica. Excel sheets were extracted and exported to STATA 13 (StataCorp LP, TX, USA) for analyses.
MLST, antimicrobial resistance genes and SNP analysis
The raw reads were de novo assembled using SPAdes 3.6.0. The assembled sequences were analysed to identify MLST STs and antimicrobial resistance genes using offline versions of the MLST (version 1.7)21 and ResFinder (version 2.1)22 tools available online from the Centre for Genomic Epidemiology (CGE) at the Technical University of Denmark (http://cge.cbs.dtu.dk/services/). Phylogenetic distances were computed using an offline version of the CSI phylogeny pipeline23 available at CGE (https://cge.cbs.dtu.dk/services/CSIPhylogeny/). Phylogenetic trees were generated with PhyML (version 3.1, bootstrap count 100).24 The reference genome used was AYE (NC_010410.1). MLST and their allelic profiles are indicated in Table S5 (available as Supplementary data at JAC Online).
Determination of plasmids, resistance regions and ISs
A combined approach using BLAST and ISfinder25 search, contig alignment (Mauve,26 ACT27), read mapping (SRST2,28 Bowtie229), in-silico PCR (https://github.com/zwets/blast-galley), rapid annotation (Prokka)30 and manual inspection (Tablet)31 was used to analyse ISs. Unicycler32 and Bandage33 were used in the extraction of plasmid sequences.
Detection of fluoroquinolone mutations in A. baumannii
DNA gyrase gene sequences (gyrA, gyrB, parC and parE) were extracted from the A. baumannii assemblies using gene-cutter, part of blast-galley (commit 0d87ef, https://github.com/zwets/blast-galley). Reference proteins used for A. baumannii were GyrA (D0CBH9) and ParC (D0CB90). Nucleotide sequences were obtained from Ensembl Genomes (https://ensemblgenomes.org). Alignment of the protein sequences was done using MAFFT (version 7.271).34
Prediction of phenotypic drug resistance pattern using WGS
ResFinder was used to predict resistance based on phenotypes from original published studies of resistance-conferring genes.35 Prediction of fluoroquinolone resistance was done based on mutations in gyrA and parC, as there were no genes for fluoroquinolone resistance detected by ResFinder. Intrinsic genes were included if mobile genetic elements such as ISAba1 were found directly upstream of the genes. Predictions were made for antimicrobials including amoxicillin/clavulanate, ampicillin, cefazolin, ceftazidime, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid and trimethoprim/sulfamethoxazole. These antimicrobials are commonly used in our setting. Correlation between the phenotypically tested antibiotic resistance and predicted antibiotic resistance was determined using Cohen’s Kappa coefficient of agreement (κ) in eight drugs. Two drugs were dropped as they were used in combination.
Ethics
Ethics approval for the study was obtained from the National Institute for Medical Research, with certificate number NIMR/HQ/R.8a/Vol.IX/2080, and from Kilimanjaro Christian Medical University College, with certificate number 891.
Results
Characteristics of the study participants
The age range of the patients involved in this study was 11 to 92 years. Thirteen out of 14 patients were admitted due to wound infections. The causes of wounds included surgery, burns, cuts, diabetes mellitus, cancer, chronic ulcers and bedsores. The length of hospitalization, where known, was from 7 to 44 days, with most of the patients recovering from their conditions. One patient died after 20 days of hospitalization. We could not trace hospitalization length and outcome for four patients. One patient prematurely left the hospital by absconding treatment. See Table 1.
Table 1.
Sociodemographic characteristics associated with 14 A. baumannii bacterial isolates from patients who were admitted to medical and surgical departments of KCMC hospital
| ID | Collection date | Age (years) | Ward | Gender | Disease | Source of sample | Length of hospitalization (days) | Patient outcome |
|---|---|---|---|---|---|---|---|---|
| 033 | 31 July 2013 | 49 | medical 1 | male | respiratory infection | sputum | NI | NI |
| 043 | 1 August 2013 | 45 | SICU B | female | burst abdomen | wound swab | 20 | death |
| 049 | 3 August 2013 | 29 | surgical | female | burn wound | wound swab | NI | NI |
| 164C | 21 March 2014 | 92 | surgical | female | bedsores | wound swab | NI | NI |
| 518B | 30 May 2015 | 29 | surgical | female | burn wound | wound swab | 32 | absconded |
| 116 | 12 February 2014 | 20 | surgical | female | burn wound | wound swab | 9 | recovery |
| 432B | 11 March 2015 | 11 | SICU B | female | burn wound | wound swab | NI | NI |
| 186B | 4 April 2014 | 63 | surgical | male | chronic leg ulcer | wound swab | 44 | recovery |
| 123C | 18 February 2014 | 71 | surgical | female | cut wound | wound swab | 9 | recovery |
| 363B | 4 December 2014 | 58 | surgical | female | scalp tumour | wound swab | 20 | recovery |
| 309C | 16 September 2014 | 21 | surgical | male | cellulitis | wound swab | 22 | recovery |
| 028 | 30 July 2013 | 33 | surgical | male | cancerous wound | wound swab | 34 | recovery |
| 423 | 4 March 2015 | 49 | surgical | male | chronic ulcer | wound swab | 7 | recovery |
| 558 | 14 July 2015 | 76 | surgical | male | DM wound | wound swab | 34 | recovery |
DM, diabetes mellitus; NI, no information; SICU, surgical ICU.
Antimicrobial resistance pattern using classical microbiology
The isolates were tested by conventional microbiology using a panel of 10 antimicrobial agents. The overall resistance levels ranged between 50% and 92.9% across all drugs. The highest resistance level was 92.9% for cefazolin and ceftriaxone, followed by ampicillin (85.7%), ceftazidime (78.6%), chloramphenicol (71.43%) and trimethoprim/sulfamethoxazole (71.4%), gentamicin (64.3%), amoxicillin/clavulanate and nalidixic acid (57.1%) and ciprofloxacin (50%). See Table 2.
Table 2.
Phenotypic antimicrobial resistance pattern of the 14 A. baumannii isolates
| ID | AMC | AMP | CFZ | CAZ | CRO | CHL | CIP | GEN | NAL | SXT |
|---|---|---|---|---|---|---|---|---|---|---|
| 116 | I | R | R | R | R | R | R | R | I | R |
| 123C | R | R | R | R | R | R | S | S | I | R |
| 309C | R | R | R | R | R | R | S | R | S | R |
| 043 | R | R | R | R | R | R | R | R | R | R |
| 432B | R | R | R | I | R | R | R | R | R | R |
| 049 | R | R | R | R | R | S | S | S | R | S |
| 033 | I | R | R | S | R | R | S | S | S | S |
| 363 | R | R | R | R | R | R | R | R | R | R |
| 423 | I | R | R | R | R | R | R | R | R | R |
| 518B | S | S | R | R | R | I | I | R | S | R |
| 558 | R | R | R | R | R | R | R | R | R | R |
| 028 | R | R | R | R | R | R | R | R | R | R |
| 164C | I | R | R | S | R | S | S | S | S | S |
| 186B | S | S | S | R | S | S | S | S | R | S |
| R (%) | 57.1 | 85.7 | 92.9 | 78.6 | 92.9 | 71.43 | 50 | 64.3 | 57.1 | 71.4 |
| S (%) | 14.3 | 14.3 | 7.1 | 14.3 | 7.1 | 21.43 | 42.9 | 35.7 | 28.6 | 28.6 |
| I (%) | 28.6 | 0.0 | 0.0 | 7.1 | 0.0 | 7.14 | 7.1 | 0.0 | 14.3 | 0.0 |
AMC, amoxicillin/clavulanate; AMP, ampicillin; CFZ, cefazolin; CAZ, ceftazidime; CRO, ceftriaxone; CHL, chloramphenicol; CIP, ciprofloxacin; GEN, gentamicin; NAL, nalidixic acid; SXT, trimethoprim/sulfamethoxazole; R, resistant; S, susceptible; I, intermediate.
MLST STs, antimicrobial resistance genes and plasmids
MLST yielded eight different STs. The most prevalent ST (n = 4) was global clone 1 (Pasteur scheme ST1). One isolate was of global clone 2 (Pasteur scheme ST2). These global clones 1 and 2 were identified as ST405 and ST848, respectively, in the Oxford scheme. Other recurring STs as per Pasteur/Oxford schemes were: ST499/345 (n = 3), ST625/860 (n = 2), followed by single isolates for ST979/1848, ST374/1325, ST1232/1846 and ST1233/1847. The STs ST1232/1846 and ST1233/1847 were novel in both schemes, whereas ST979/1848 was novel in the Oxford scheme. More information on the allelic profiles of the isolates is in Table S4.
Several antimicrobial resistance genes representing resistance to different antimicrobial agent groups were identified, located either chromosomally or on plasmids. Chromosomally located genes included aacC1, aadA1, aphA1, catA1, sul1, tet(A), tet(B), strA and strB, while blaOXA-23, mph(E), msr(E), tet39, floR, sul2 and aadB were found on plasmids. β-Lactamase genes included class D (OXA-type) genes that are intrinsic factors in A. baumannii, blaPER-7 and the cephalosporinase-encoding gene ampC.
The three isolates of ST499/ST345 (116, 123C, 309C) were found to carry an ∼219 kb plasmid exhibiting 93% identity with the pIOMTU433 plasmid (AP014650) and 94% with the pA297-3 plasmid (KU744946). The plasmid hosted the arr-3, strB, armA, blaPER-7, cmlA1, mph(E), msr(E), strA, sul1 and dfrA26 resistance genes. See Table 3. The plasmid appeared structurally identical to the pIOMTU433 plasmid, with differences concentrated in two regions, each flanked bilaterally by ISs. The first region was adjacent to the class 1 integron-integrase gene intI1. Where pIOMTU433 contains the sul2 gene, this was replaced in pKCRI-309C-1 by a smaller segment containing the dfrA26 resistance gene. This segment is present identically on plasmid pAB04-1 (CP012007). The second difference was an ∼20 kb insert containing, among other genes, the mercury resistance operon. This transposon is present nearly identically (99.94%) on pA297-3, a plasmid that shares much of its sequence with pIOMTU433, but lacks the class 1 integron and its downstream gene cassette that pIOMTU433 and our ST499 isolates carry.
Table 3.
STs, resistance genes, mobile genetic elements and plasmids
| ID | STs (Pasteur/Oxford) | Mobile genetic elements | Intrinsic genes | Chromosomal resistance genes | Plasmids, size and associated drug resistance genes |
|---|---|---|---|---|---|
| 116 | 499/345 | CR (IS91), Intg1 | bla OXA-95, ampC-70b, ampC-181b | ND | plasmid: putative plasmid pKCRI-309C-1 |
| Size: ∼219 kb | |||||
| genes: arr-3, strB, armA, blaPER-7, cmlA1, mph(E), msr(E), strA, sul1, dfrA26 | |||||
| plasmid: pAC450 | |||||
| 123C | 499/345 | CR (IS91), Intg1 | bla OXA-95, ampC-70b, ampC-181b | ND | plasmid: putative plasmid pKCRI-309C-1 |
| Size: ∼219 kb | |||||
| genes: arr-3, strB, armA, blaPER-7, cmlA1, mph(E), msr(E), strA, sul1, dfrA26 | |||||
| plasmid: pAC450 | |||||
| 309C | 499/345 | CR (IS91), Intg1 | bla OXA-95, ampC-70b, ampC-181b | ND | plasmid: putative plasmid pKCRI-309C-1 |
| Size: ∼219 kb | |||||
| genes: arr-3, strB, armA, blaPER-7, cmlA1, mph(E), msr(E), strA, sul1 dfrA26 | |||||
| plasmid: pAC450 | |||||
| 43 | 625/860 | IS3 and IS6, ISAba1 | bla OXA-90, ampC-4 | ND | plasmid: pKCRI-43-1 |
| size: 34 935 bp | |||||
| genes: floR, sul2 | |||||
| plasmid: pRAY | |||||
| genes: aadB | |||||
| 432B | 625/860 | IS3 and IS6, ISAba1 | bla OXA-90, ampC-71b | ND | plasmid: pKCRI-432-1 |
| size: 34 935 bp | |||||
| genes: floR, sul2 | |||||
| plasmid: pRAY | |||||
| genes: aadB | |||||
| 49 | 374/1325 | ND | bla OXA-180, ampC-56 | ND | plasmid: pKCRI-49-1 |
| size: 11 681 bp | |||||
| genes: mph(E), msr(E), tet39 | |||||
| 33 | 979/1848a | ND | bla OXA-51, ampC-50 | ND | ND |
| 363 | 1/405 | ISAba1 | bla OXA-69, ampC-4 | aacC1, aadA1, aphA1, catA1, sul1, tet(A) | ND |
| 423 | 1/405 | ISAba1 | bla OXA-69, ampC-4 | aacC1, aadA1, aphA1, catA1, sul1, tet(A) | ND |
| 518B | 1/405 | ISAba1 | bla OXA-69, ampC-4 | aacC1, aadA1, aphA1, catA1, sul1, tet(A) | presence suggestive of novel plasmids, not carrying known resistance genes |
| 558 | 1/405 | ISAba1, ISAba4 | bla OXA-69, ampC-4 | aacC1, aadA1, aphA1, catA, sul1, tet(A) | plasmid: pAB14 |
| genes: blaOXA-23 | |||||
| 28 | 2/848 | ISAba1 | bla OXA-66, ampC-2 | aacC1, aadA1, strB, strA, sul1, tet(B) | plasmid: pKCRI-28-1 |
| size: 29 606 bp | |||||
| genes: mph(E), msr(E), tet39 | |||||
| 164C | 1232a/1846a | ND | bla OXA-64, ampC-68b | ND | ND |
| 186 | 1233a/1847a | ND | bla OXA-51, ampC-69b | sul2 | presence suggestive of novel plasmids, not carrying known resistance genes |
ND, not detected.
Note: pKCRI = new plasmid described in this study.
New STs identified in this study.
New ampC ST.
It was not possible to assemble the pKCRI-309C-1 plasmid into a single contig, due to the inability of short-read sequencing to ‘read through’ larger repeated sequences. We hope to perform a detailed follow-up analysis when long-read sequencing becomes available to our laboratory in the future. The plasmid has been submitted to GenBank as an assembly of six unplaced contigs (accession pending).
The mph(E), msr(E) and tet39 genes, encoding macrolide, lincosamide, streptogramin B and tetracycline resistance in isolates 28 and 49, seemed to be located on the novel plasmids pKCRI-28-1 (LR026972; 29606 bp) and pKCR-49-1 (LR026974; 11681 bp), respectively. The plasmids also harboured a higA/higB toxin–antitoxin gene pair. This gene combination has been described for other plasmids isolated from a global clone 2 strain, where the tet39 and mph(E)-msr(E) were found to be in mobile modules flanked by pdif sites.36 As in that study, tet39 was found in both plasmids to be in a 2001 bp region flanked by inverted pdif sequences. The mph(E)-msr(E) gene pair was found, together with a predicted resolvase/recombinase product (similar to WP_015060246), in 4219 and 4300 bp regions, respectively, flanked by pdif sites. The novel plasmid carried by isolate 28 additionally encoded a putative cmlA/floR chloramphenicol efflux system, located directly upstream of an ISL3-family transposase separating it from the tet39 element.
The floR and sul2 resistance genes in isolates 43 and 432B were determined to be on a novel 34 935 bp plasmid, pKCRI-43-1 (LR026973). Both genes were flanked by transposases: the floR gene by IS3 and IS6-family (IS1008) transposases; and the sul2 gene by ISAba1 upstream and by glmM followed by IS91 and IS6-family transposases downstream. The aadB gene in isolates 43 and 432B was found to be located on a pRAY plasmid present in both isolates; the plasmid and its variants are widely distributed and the most common cause of gentamicin and tobramycin resistance in Acinetobacter spp.37
The blaOXA-23 gene of isolate 558 was found to be located on plasmid pAB14, directly downstream of ISAba4, suggesting the presence of transposon Tn2007 and the possibility of elevated carbapenemase production.38
Various other plasmids were identified in the isolates; no resistance genes were detected on these plasmids. See Table 3.
AbaR resistance regions
The comM gene that is a prevalent location for insertion of AbaR resistance regions39 was found to be interrupted in the four ST1 isolates (363, 423, 518B and 558) and in the ST2 (28) isolate. The comM gene was found intact in all other isolates. In silico PCR also detected the presence of the left-hand side of Tn6019 in the ST1 and ST2 isolates and the right-hand side in the ST1 isolates.
ISs
Several isolates were found to have ISs upstream of their chromosomal β-lactamase genes. The four ST1 isolates (363, 423, 518B and 558) and isolate 28 (ST2) had ISAba1 directly upstream of the ampC gene. In the other isolates, no IS was found directly upstream of the ampC gene. In the three ST499 isolates, a common region (CR) (IS91) was found directly upstream of the blaPER-7 gene in the pKCRI-309C-1 plasmid. As pointed out above, in isolate 558 the plasmidic blaOXA-23 gene was found directly downstream of ISAba4, constituting Tn2007. Few ISs overall were found in isolates 164C, 49 and the four ST1 isolates, whereas numerous ISs were found in the three ST499 isolates (10–15 ISs) and notably isolate 186 (26 ISs).
Detection of fluoroquinolone mutations in A. baumannii
Mutations in gyrase/topoisomerase genes may confer resistance by inhibiting fluoroquinolone binding. Half (7/14) of the A. baumannii isolates were found to have mutations in gyrA (S81L) and in parC (S84L), all exhibiting resistance to ciprofloxacin and nalidixic acid. Among the other seven isolates without the double mutation, only one was found to be fluoroquinolone resistant. All other isolates had no relevant mutations. See Table 4.
Table 4.
Mutations in fluoroquinolone resistance-determining genes of A. baumannii
| ID | ST | gyrA | parC | Ciprofloxacin | Nalidixic acid |
|---|---|---|---|---|---|
| 116 | 499/345 | T82S | — | resistant | intermediate |
| 123C | 499/345 | T82S | — | susceptible | intermediate |
| 309C | 499/345 | T82S | — | susceptible | susceptible |
| 43 | 625/860 | S81L | S84L | resistant | resistant |
| 432B | 625/860 | S81L | S84L | resistant | resistant |
| 49 | 374/1325 | — | — | susceptible | susceptible |
| 33 | 979/1848a | — | — | susceptible | susceptible |
| 363 | 1/405 | S81L | S84L | resistant | resistant |
| 423 | 1/405 | S81L | S84L | resistant | resistant |
| 518 | 1/405 | S81L | S84L | resistant | resistant |
| 558 | 1/405 | S81L | S84L | resistant | resistant |
| 28 | 2/848 | S81L | S84L | resistant | resistant |
| 164C | 1232a/1846a | — | — | susceptible | susceptible |
| 186 | 1233a/1847a | — | — | susceptible | resistant |
—, no relevant substitutions.
Mutations detected in fluoroquinolone resistance-determining regions in A. baumannii. Seven out of 14 A. baumannii isolates were found to possess the mutations S81L in gyrA and S84L in parC.
New STs identified in this study.
Prediction of phenotypic drug resistance pattern using WGS
Percentage agreement between phenotypic and genotypic prediction of resistance was 57.1% for ampicillin, 64.3% for cefazolin and ceftriaxone, 71.4% for ceftazidime and nalidixic acid, 85.7% for chloramphenicol and amoxicillin/clavulanate and 92.9% for trimethoprim/sulfamethoxazole and gentamicin. Ciprofloxacin was the only drug with resistance predicted at 100%. The eight drugs for which κ was computed were cefazolin, ceftazidime, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid and ampicillin. κ ranged between 0.05 and 1, i.e. between slight and perfect agreement. Tables S1, S2, S3 and S4 summarize the correlation between phenotypic and WGS-predicted resistance as determined by Cohen’s κ.
SNP phylogenetic tree
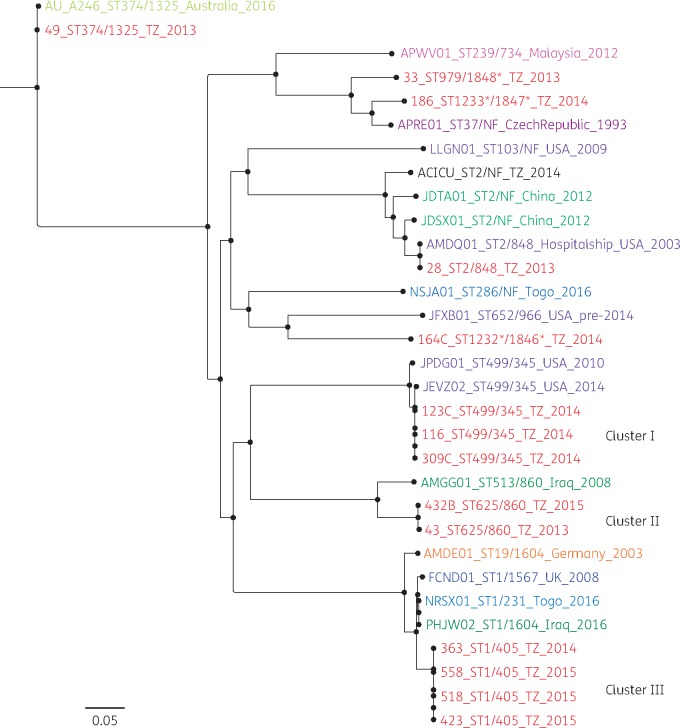
From the SNP phylogenetic tree, three clusters of Tanzanian isolates were observed. Cluster I contained the three isolates with ST499/345. All three were isolated in 2014 (14 February, 18 February and 16 September). All had the T82S mutation in gyrA and no mutations in parC. Isolates from this cluster all possessed the plasmids pKCRI-309C-1 (accession pending), containing multiple antibiotic resistance genes, and pAC450, on which no resistance genes were found. Cluster II contained the two isolates with ST625/860. The isolates were collected on the same ward (surgical ICU B), but 2 years apart, on 1 August 2013 and 11 May 2015. Both isolates had the fluoroquinolone mutations S81L in gyrA and S84L in parC and were ciprofloxacin resistant and nalidixic acid resistant. The isolates both contained the pRAY plasmid carrying aadB and pKCRI-43-1 (LR026973) containing floR and sul2. Cluster III contained the four ST1/405 isolates. See Figure 1. Isolation dates were 4 December 2014, 4 March 2015, 30 March 2015 and 14 July 2015. They all had the fluoroquinolone mutations S81L in gyrA and S84L in parC and were all found to be resistant to ciprofloxacin and nalidixic acid. One of the four isolates possessed plasmid pAB14 containing the blaOXA-23 gene. The five isolates that were not part of the above-mentioned clusters were located at independent positions in the tree. The ST374/1325 isolate clustered closely with an Australian isolate of the same ST, while the ST2/848 isolate clustered with an isolate of the same ST collected in 2003 on an American hospital ship. The remaining three isolates, which were of novel STs, were all located at phylogenetically larger distances from other isolates. Raw sequence data of the 16 A. baumannii from our study have been submitted to the European Nucleotide Archive (https://www.ebi.ac.uk/ena) under study accession number PRJEB26612. Plasmid accession numbers are: pKCRI-28-1 (LR026972); pKCRI-43-1 (LR026973); pKCR-49-1 (LR026974); and pKCRI-309C-1 (accession pending).
Figure 1.
SNP phylogeny analysis of 31 A. baumannii genomes that were isolated in 10 countries: Tanzania (TZ; current study), USA, UK, China, Malaysia, Togo, Australia, Czech Republic, Iraq and Germany. From the SNP phylogenetic tree, three clusters for Tanzanian isolates were observed. Cluster I was composed of three isolates with ST499/345 as per Pasteur/Oxford schemes. All had the T82S mutation in gyrA and had no mutations in parC. Isolates from this cluster possessed pIOMTU433-like plasmids, which contained several antibiotic resistance genes, and pAC450, which did not carry any resistance genes. Cluster II was composed of two isolates with ST625/860 as per Pasteur/Oxford schemes. Both isolates were isolated in the surgical ICU B ward. They both had fluoroquinolone mutations S81L and S84L in gyrA and parC, respectively, and were ciprofloxacin resistant and nalidixic acid resistant. The isolates contained more than one plasmid with no resistance genes on them with the exception of two: pA297-1, which carried aadB; and pKCRI-43-1, containing the genes floR and sul2. Cluster III was composed of four isolates with ST1/405 as per Pasteur/Oxford schemes. They all had fluoroquinolone mutations S81L and S84L in gyrA and parC, respectively. They were all resistant to ciprofloxacin and nalidixic acid. Three possessed a novel plasmid and one possessed plasmid pAB14 containing blaOXA-23.
Discussion
The current study aimed to investigate the diversity and resistance determinants of A. baumannii isolates collected at a tertiary hospital in Tanzania and to compare predicted resistance patterns from WGS data with phenotypic results from classical microbiological testing. The majority of patients investigated in this study were hospitalized with wound infections and had relatively long hospital stays despite the fact that many recovered. Hospitalized patients with open wounds and long hospital stays are among those who are vulnerable to A. baumannii infections. The pathogen can persist on patients’ body surfaces or in the environment for a considerable number of days, as the infections are difficult to treat.40 The pathogen has the ability to resist many antibiotics leading to fewer treatment options and sometimes death.9 It was noted from the study that some patients abscond hospitalization before recovery. In low-resource settings, patients who cannot afford hospital charges will sometimes withdraw from treatment to avoid further costs. Occasionally such patients will return to the hospital with worsened conditions. Aforesaid behaviour could cause hospital-based infections to be disseminated to the community and vice versa, with severe consequences when these patients carry drug-resistant strains, which could then easily spread to other susceptible persons.
MLST is one of the gold standards for determination of epidemiological relatedness of organisms.41 In this study MLST was performed, based on both Pasteur and Oxford schemes. Considerable genetic variation among A. baumannii strains was observed. The observed variation included STs that represent the global clones 1 and 2 of A. baumannii, as well as novel STs first identified in this study. The observed global clones are of great concern as they are disseminated worldwide and have been highly associated with MDR. They have notably been associated with carbapenem resistance and outbreaks in hospital settings.42 Among the global clones, one isolate was confirmed to possess the blaOXA-23 gene located on plasmid pAB14, directly downstream from ISAba4, suggesting the presence of transposon Tn2007 and the possibility of elevated carbapenemase production.38 Plasmid-mediated class D β-lactamase genes, particularly blaOXA-23, play a large role in mediating resistance.43 These findings are in contrast to a study conducted in Tanzania that identified predominantly IMP-type (class B) carbapenemases in A. baumannii isolates.44
SNP-based phylogenetic analysis of the A. baumannii further confirmed the diversity by revealing three major clusters containing clades of closely related isolates. This observation, particularly in the light of the time separation between isolation events, suggests existence of specific strains of A. baumannii at the KCMC hospital. The origin of these clones is not known, but it seems likely that they dwell in the hospital environment and cause infections over an extended period of time. Previous studies have reported on A. baumannii isolates from medical apparatus, water systems and handwashing sinks in the hospital environment, including ICUs and surgical wards.45,46 These isolates tend to be MDR and hence can persist for a long time if the reservoir is not destroyed, serving as a potential source for recurrent outbreaks.
Isolates in clusters I and II were found to possess plasmid-mediated resistance genes. The majority of these genes were situated in novel plasmids (pKCRI-309C-1 for cluster I and pKCRI-43-1 for cluster II). Cluster III consisted of global clone 1, for which in some isolates resistance genes were found in both plasmids and chromosomes. The coexistence of chromosomally and plasmid-encoded β-lactamases and other genes in global clone isolates has been observed in other studies.47,48 The isolate belonging to global clone 2 in this study was found to carry chromosomally mediated genes, as well as mph(E), msr(E) and tet39 genes encoding macrolide, lincosamide, streptogramin B and tetracycline resistance on a novel plasmid pKCRI-28-1.
ISs have been associated with antimicrobial resistance through multiple mechanisms: disruption of coding regions, mobilization of gene cassettes and up-regulation of gene expression by additional promoters.49 Our findings show that all global clone 1 and 2 isolates studied were resistant to ceftriaxone despite the absence of the genes responsible for resistance. However, the isolates were found to harbour ISAba1 directly upstream of the ampC gene. This configuration has been demonstrated to elevate ampC expression levels, conferring resistance to third-generation cephalosporins.50 Isolates in Cluster I were identified as belonging to ST499/345 as per Pasteur/Oxford schemes. Recent reports from other parts of the world, including Egypt in Africa,51,52 reported isolates with these STs to be associated with carbapenem resistance. In the current study, these isolates were not confirmed to have resistance to carbapenems; however, they were found to harbour the novel ampC allele ampC-70, encoding the novel amino acid sequence AmpC-181. These isolates were further confirmed to possess CR (IS91) directly upstream of the blaPER-7 gene in the pKCRI-309C-1 plasmid. These findings corroborate the observed phenotypic resistance to ceftazidime and other cephalosporins that have been reported from other studies.53
Antibiotic resistance to commonly prescribed antibiotics was found to be high, with alarming resistance rates to fluoroquinolones and third-generation cephalosporins. The high resistance rates are of great concern when it comes to A. baumannii, which is a notorious nosocomial pathogen. Overprescription of antibiotics, over-the-counter availability, self-medication, premature treatment cessation, counterfeit drugs, poor diagnostic facilities and other factors prevalent in low-income settings contribute greatly to antibiotic resistance. Empirical treatment in such settings becomes compromised and treatment options are reduced.54–56
Prediction of phenotypes from WGS data could be a powerful potential application of next-generation sequencing technology in low-resource settings, although limited studies have demonstrated this.57 Given that sequencing technology is scarcely deployed, especially in resource-limited settings, it is almost impossible to assess its power. However, the process of predicting phenotypes is complex and it is currently in limited use.35
In the current study, the percentage agreement between phenotypic and genotypic prediction of resistance ranged between 57.1% and 100%, with κ ranging between 0.05 and 1. Our prediction did not rely on the presence or absence of resistance genes alone, but considered several other aspects. In some cases, genes conferring resistance to drugs such as ciprofloxacin, nalidixic acid and cefazolin were not detected by ResFinder, yet phenotypic resistance was observed. In this case, other resistance mechanisms, such as mutations in DNA gyrase/topoisomerase IV genes, were responsible for resistance phenotypes. In the prevailing report we detected significant mutations at 81/84 in gyrA/parC in the A. baumannii isolates. Mutations at residues 81 and 84 in gyrA and parC, respectively, are most frequently observed among the strains with nalidixic acid and ciprofloxacin resistance, respectively. Other studies have reported the existence of triple mutations (gyrA, gyrB and parC) that also contribute to development of a high level of fluoroquinolone resistance in clinical isolates of A. baumannii.58 However, for convenient determination of such mutation trends, especially for the fluoroquinolones, a larger sample number is crucial in future studies.
WGS techniques have great promise in providing sufficient and reliable data for surveillance and monitoring of antimicrobial resistance. Despite the technology being robust, limited knowledge of bioinformatics data analysis is one of the major challenges. In resource-limited settings, cost challenges and a lack of sequencing expertise widen the gap to its potential utilization. These study findings support the implementation in disease-endemic regions of high-throughput techniques, to replace or add value to existing technology, and hence take a step ahead in medical diagnostics.
Conclusions
The validity of the use of WGS in prediction of phenotypic resistance can be appreciated, but at this stage is not sufficient to replace conventional antimicrobial susceptibility testing in our setting. Classical microbiology still complements the genotypically predicted resistance, as it provides better resolution of what is happening between organisms and their environment, taking into consideration that observed phenotypes are a result of interaction between genotypes and the environment.
Supplementary Material
Acknowledgements
We would like to thank all the participants who took part in this study. Thanks and appreciation to KCMC management for allowing sample collection from the hospital. Thanks to all KCRI Biotechnology Laboratory and DTU-Food staff members for their contributions.
Funding
This work was supported by the Danish International Development Agency (grant number DFC no.12–007 DTU).
Transparency declarations
None to declare.
References
- 1. Huttner A, Harbarth S, Carlet J. et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2: 31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Exner M, Bhattacharya S, Christiansen B. et al. Antibiotic resistance: what is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg Infect Control 2017; 12: Doc05.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zavascki AP, Carvalhaes CG, Picão RC. et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther 2010; 8: 71–93. [DOI] [PubMed] [Google Scholar]
- 4. Karlowsky JA, Draghi DC, Jones ME. et al. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother 2003; 47: 1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biedenbach DJ, Giao PT, Hung Van P. et al. Antimicrobial-resistant Pseudomonas aeruginosa and Acinetobacter baumannii from patients with hospital-acquired or ventilator-associated pneumonia in Vietnam. Clin Ther 2016; 38: 2098–105. [DOI] [PubMed] [Google Scholar]
- 6. Royer S, Luiza A, Faria S. et al. Spread of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clones in patients with ventilator-associated pneumonia in an adult intensive care unit at a university hospital. Brazilian J Infect Dis 2015; 19: 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vitkauskienė A, Skrodenienė E, Dambrauskienė A. et al. Characteristics of carbapenem-resistant Pseudomonas aeruginosa strains in patients with ventilator-associated pneumonia in intensive care units. Medicina (Kaunas) 2011; 47: 652–6. [PubMed] [Google Scholar]
- 8. Hussain M, Suliman M, Ahmed A. et al. Draft genome sequence of a multidrug-resistant Pseudomonas aeruginosa strain isolated from a patient with a urinary tract infection in Khartoum, Sudan. Genome Announc 2017; 5: e00203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kateete DP, Nakanjako R, Namugenyi J. et al. Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago Hospital in Kampala, Uganda (2007–2009). Springerplus 2016; 5: 1308.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andrade SS, Jones RN, Gales AC. et al. Increasing prevalence of antimicrobial resistance among Pseudomonas aeruginosa isolates in Latin American medical centres: 5 year report of the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother 2003; 52: 140–1. [DOI] [PubMed] [Google Scholar]
- 11. Milda A, Baumgart K, Molinari MA. et al. Prevalence of carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii in high complexity hospital. Braz J Infect Dis 2010; 14: 433–6. [DOI] [PubMed] [Google Scholar]
- 12. Mathlouthi N, Areig Z, Al Bayssari C. et al. Emergence of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii clinical isolates collected from some Libyan hospitals. Microb Drug Resist 2015; 21: 335–41. [DOI] [PubMed] [Google Scholar]
- 13. Fournier P, Drancourt M, Raoult D.. Bacterial genome sequencing and its use in infectious diseases. Lancet Infect Dis 2007; 7: 711–23. [DOI] [PubMed] [Google Scholar]
- 14. Bronzwaer SL, Cars O, Buchholz U. et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis 2002; 8: 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van de Sande-Bruinsma N, Grundmann H, Verloo D. et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis 2008; 14: 1722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tambo E, Ai L, Zhou X. et al. Surveillance-response systems: the key to elimination of tropical diseases. Infect Dis Poverty 2014; 3: 17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shears P. Poverty and infection in the developing world: healthcare-related infections and infection control in the tropics. J Hosp Infect 2007; 67: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumburu HH, Sonda T, Mmbaga BT. et al. Patterns of infections, aetiological agents and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop Med Int Health 2017; 22: 454–64. [DOI] [PubMed] [Google Scholar]
- 19. Okeke IN, Laxminarayan R, Bhutta ZA. et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis 2005; 5: 481–93. [DOI] [PubMed] [Google Scholar]
- 20. Köser C, Ellington M, Cartwright E.. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog 2012; 8: e1002824.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen MV, Cosentino S, Rasmussen S. et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 2012; 50: 1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaas RS, Leekitcharoenphon P, Aarestrup FM. et al. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One 2014; 9: e104984.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guindon S, Gascuel O.. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 2003; 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 25. Siguier P, Perochon J, Lestrade L. et al. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 2006; 34: D32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darling ACE, Mau B, Blattner FR. et al. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 2004; 14: 1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carver TJ, Rutherford KM, Berriman M. et al. ACT: the Artemis comparison tool. Bioinformatics 2005; 21: 3422–3. [DOI] [PubMed] [Google Scholar]
- 28. Inouye M, Dashnow H, Raven L-A. et al. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 2014; 6: 90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langmead B. (ed.). Aligning short sequencing reads with Bowtie In: Current Protocols in Bioinformatics. Hoboken, NJ, USA: John Wiley & Sons, Inc, 2010; 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30: 2068–9.24642063 [Google Scholar]
- 31. Milne I, Stephen G, Bayer M. et al. Using Tablet for visual exploration of second-generation sequencing data. Brief Bioinform 2013; 14: 193–202. [DOI] [PubMed] [Google Scholar]
- 32. Wick RR, Judd LM, Gorrie CL. et al. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 2017; 13: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wick RR, Schultz MB, Zobel J. et al. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics 2015; 31: 3350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katoh K, Standley DM.. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013; 30: 772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stoesser N, Batty EM, Eyre DW. et al. Predicting antimicrobial susceptibilities for Escherichia coli and Klebsiella pneumoniae isolates using whole genomic sequence data. J Antimicrob Chemother 2013; 68: 2234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blackwell GA, Hall RM.. The tet39 determinant and the msrE-mphE genes in Acinetobacter plasmids are each part of discrete modules flanked by inversely oriented pdif (XerC-XerD) sites. Antimicrob Agents Chemother 2017; 61: e00780–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamidian M, Nigro SJ, Hall RM.. Variants of the gentamicin and tobramycin resistance plasmid pRAY are widely distributed in Acinetobacter. J Antimicrob Chemother 2012; 67: 2833–6. [DOI] [PubMed] [Google Scholar]
- 38. Hamidian M, Kenyon JJ, Holt KE. et al. A conjugative plasmid carrying the carbapenem resistance gene blaOXA-23 in AbaR4 in an extensively resistant GC1 Acinetobacter baumannii isolate. J Antimicrob Chemother 2014; 69: 2625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Post V, Hamidian M, Hall RM.. Antibiotic-resistant Acinetobacter baumannii variants belonging to global clone 1. J Antimicrob Chemother 2012; 67: 1039–40. [DOI] [PubMed] [Google Scholar]
- 40. Sunenshine RH, Wright MO, Maragakis LL. et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007; 13: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hong JS, Yoon E-J, Lee H. et al. Clonal dissemination of Pseudomonas aeruginosa sequence type 235 isolates carrying blaIMP-6 and emergence of blaGES-24 and blaIMP-10 on novel genomic islands PAGI-15 and -16 in South Korea. Antimicrob Agents Chemother 2016; 60: 7216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zarrilli R, Pournaras S, Giannouli M. et al. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 2013; 41: 11–9. [DOI] [PubMed] [Google Scholar]
- 43. Chang Y, Luan G, Xu Y. et al. Characterization of carbapenem-resistant Acinetobacter baumannii isolates in a Chinese teaching hospital. Front Microbiol 2015; 6: 910.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mushi MF, Mshana SE, Imirzalioglu C. et al. Carbapenemase genes among multidrug resistant gram negative clinical isolates from a tertiary hospital in Mwanza, Tanzania. Biomed Res Int 2014; 2014: 303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Umezawa K, Asai S, Ohshima T. et al. Outbreak of drug-resistant Acinetobacter baumannii ST219 caused by oral care using tap water from contaminated hand hygiene sinks as a reservoir. Am J Infect Control 2015; 43: 1249–51. [DOI] [PubMed] [Google Scholar]
- 46. Wroblewska MM, Towner KJ, Marchel H. et al. Emergence and spread of carbapenem-resistant strains of Acinetobacter baumannii in a tertiary-care hospital in Poland. Clin Microbiol Infect 2007; 13: 490–6. [DOI] [PubMed] [Google Scholar]
- 47. Ramoul A, Loucif L, Bakour S. et al. Co-occurrence of blaNDM-1 with blaOXA-23 or blaOXA-58 in clinical multidrug-resistant Acinetobacter baumannii isolates in Algeria. J Glob Antimicrob Resist 2016; 6: 136–41. [DOI] [PubMed] [Google Scholar]
- 48. Atrouni A, Al Hamze M, Jisr T. et al. Wide spread of OXA-23-producing carbapenem-resistant Acinetobacter baumannii belonging to clonal complex II in different hospitals in Lebanon. Int J Infect Dis 2016; 52: 29–36. [DOI] [PubMed] [Google Scholar]
- 49. Roca I, Espinal P, Vila-Farrés X. et al. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol 2012; 3: 148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamidian M, Hall RM.. Tn6168, a transposon carrying an ISAba1-activated ampC gene and conferring cephalosporin resistance in Acinetobacter baumannii. J Antimicrob Chemother 2014; 69: 77–80. [DOI] [PubMed] [Google Scholar]
- 51. Ghaith DM, Zafer MM, Al-Agamy MH. et al. The emergence of a novel sequence type of MDR Acinetobacter baumannii from the intensive care unit of an Egyptian tertiary care hospital. Ann Clin Microbiol Antimicrob 2017; 16: 34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zowawi HM, Sartor AL, Sidjabat HE. et al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolates in the Gulf Cooperation Council States: dominance of OXA-23-type producers. J Clin Microbiol 2015; 53: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Opazo A, Sonnevend A, Lopes B. et al. Plasmid-encoded PER-7 β-lactamase responsible for ceftazidime resistance in Acinetobacter baumannii isolated in the United Arab Emirates. J Antimicrob Chemother 2012; 67: 1619–22. [DOI] [PubMed] [Google Scholar]
- 54. Ocan M, Obuku EA, Bwanga F. et al. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health 2015; 15: 742.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eticha T, Mesfin K.. Self-medication practices in Mekelle, Ethiopia. PLoS One 2014; 9: e97464.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Biswas M, Roy MN, Manik MI. et al. Self medicated antibiotics in Bangladesh: a cross-sectional health survey conducted in the Rajshahi City. BMC Public Health 2014; 14: 847.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ellington MJ, Ekelund O, Aarestrup FM. et al. The role of whole genome sequencing in antimicrobial susceptibility testing of bacteria: report from the EUCAST Subcommittee. Clin Microbiol Infect 2017; 23: 2–22. [DOI] [PubMed] [Google Scholar]
- 58. Park S, Min Lee K, Sun Yoo Y. et al. Alterations of gyrA, gyrB, and parC and activity of efflux pump in fluoroquinolone-resistant Acinetobacter baumannii. Osong Public Heal Res Perspect 2011; 2: 164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.