Identification of native Amazonian tree species with potential for phytoextraction and tolerance to cadmium (Cd) can be used to direct studies and phytoremediation programs for preservation of natural areas and restoration of environments contaminated by heavy metals. The study evaluated the physiological responses and the phytoextraction and tolerance capacity of young plants of Virola surinamensis to Cd. We founded that Virola surinamensis showed medium and high tolerance to Cd and low efficacy in Cd phytoextraction and suggest that Virola surinamensis may be promising for the phytostabilization of Cd.
Keywords: Bioconcentration factor, photosystem II, phytostabilization
Abstract
The steady increase in cadmium (Cd) levels in the environment from anthropogenic actions has contributed to environmental degradation. Virola surinamensis is a forest species that has desirable characteristics such as deep and dense roots, relatively rapid growth and high biomass production to remedy contaminated environments by Cd. The aim of this study was to assess the physiological responses and the phytoextraction and tolerance capacity of young plants of V. surinamensis submitted to Cd concentrations. The experimental design was a completely randomized design with five Cd concentrations (0, 15, 30, 45 and 60 mg L−1) for 60 days. Leaf water potential (Ψpd), stomatal conductance (gs) and transpiration (E) reduced in plants exposed to Cd. Lower values of maximum photochemical efficiency of photosystem II (Fv/Fm), electron transport rate (ETR) and photochemical quenching coefficient (qP) were accompanied by reduction of photosynthesis (A) with increasing concentrations of Cd, although the non-photochemical quenching coefficient (NPQ), and intercellular CO2 concentration (Ci) showed increase. Instantaneous water-use efficiency (A/E), net photosynthesis to intercellular CO2 concentration ratio (A/Ci) and total chlorophyll (Chl) reduced with increasing levels of Cd. Cadmium concentrations increased in different plant tissues (root > stem > leaf). The tolerance index (TI) indicated that V. surinamensis presented medium and high tolerance to Cd. The results of bioconcentration factor (BCF) and translocation factor (TF) showed low plant efficacy in Cd phytoextraction and suggest that V. surinamensis may be promising for phytostabilization of Cd.
Introduction
The constant increase in cadmium (Cd) levels in the environment from agricultural and industrial activities has contributed to the degradation and contamination of soils, surface water and groundwater (Ahmad et al. 2014). This has created a major worldwide concern, especially as it is a non-biodegradable and easily absorbed, translocated and accumulated element in plant tissues (Ali et al. 2013), making it highly bioavailable and therefore toxic even at relatively low concentrations (Bashir et al. 2015).
The symptoms of phytotoxicity by Cd in plants include a modification in the indices of chlorophyll a, b and total, resulting in significant reductions in photosynthetic activity (Elloumi et al. 2014; Hernández et al. 2015; Yang et al. 2015; Michel-López et al. 2016; Zouari et al. 2016; Nikolić et al. 2017; Silva et al. 2017), especially due to the inhibition of photosystem II (PSII) (Di Baccio et al. 2014) by changing the potential yield of the photochemical reaction (Fv/Fm) (Fernández et al. 2013; Yang et al. 2015; Solti et al. 2016) and CO2-fixing key enzymes, such as ribulose-1,5-bisphosphate carboxylase (RuBisCO) (Tran and Popova 2013). In addition, Cd in plants affects the water relations, respiration (Oláh et al. 2015), transpiration, stomatal conductance and intercellular CO2 concentration (Elloumi et al. 2014; Song et al. 2016; Zouari et al. 2016; Nikolić et al. 2017).
In the Amazon, flooded ecosystems are constantly susceptible to contamination, as they are receptors for nutrients and organic and inorganic contaminants, including heavy metals (Khan et al. 2017). High concentrations of Cd in water and sediments of these areas were demonstrated in studies by Seyler and Boaventura (2003) and Oliveira et al. (2017). Among heavy metals, Cd is considered one of the most toxic. Thus, the demand for solutions to recover soils and aquifers contaminated by metals, among them the Cd (Zhao et al. 2015).
Phytoextraction is a promising phytoremediation technique and consists of the absorption of soil or water contaminants by the plant root and its translocation to shoot (Sharma et al. 2016). The success of this technique involving forest species on Cd removal depends on the higher accumulation capacity of the metal, high biomass production and plant tolerance (Nikolić et al. 2017). However, only some plants suitable for phytoextraction of Cd are hyperaccumulating, that is, they have superior capacity to extract, accumulate and tolerate high levels of the metal (Fan et al. 2011). These plants can accumulate >100 mg Cd kg−1 (dry weight) in the aerial part (Van der Ent et al. 2013).
Studies involving woody species native to the Amazon for phytoremediation of Cd are scarce. In the present study, Swietenia macrophylla (Fan et al. 2011) and Cassia alata (Silva et al. 2017) demonstrated a capacity to accumulate and tolerate Cd, while Calophyllum brasiliense (Pereira et al. 2017) presented compromised growth, with low accumulation and greater sensitivity to Cd. To identify tree species with a capacity for phytoextraction of Cd, they can serve to direct studies and programmes on phytoremediation for the preservation of natural areas and the recomposition of environments contaminated by these metals.
In this work, we consider Virola surinamensis (Ucuúba) as a forest species with a deep and dense root system, relatively rapid growth and high biomass production. These characteristics are considered desirable and effective for woody plants to remediate metal contaminated soils, such as Cd (Abdul Qados 2015). In addition, Ucuúba is widely distributed in Amazonian floodplain and igapó ecosystems, which are potentially subject to the presence of Cd. In addition, this species has been successfully used in reclamation programmes for degraded areas, including high concentrations of copper (Cu) and zinc (Zn) in the litter (Costa et al. 2017). Suggesting that V. surinamensis develops mechanism of tolerance to environments contaminated by heavy metals.
Considering that Cd tolerance is modulated by defence mechanisms and that no studies on the behaviour of V. surinamensis exposed to Cd have been found, we tested the hypothesis that young plants of V. surinamensis trigger different physiological strategies to tolerate environments contaminated by Cd. Thus, this study aimed to assess (i) the water potential, gas exchange and the fluorescence of chlorophyll a and (ii) Cd concentration in different plant organs, bioaccumulation, translocation, and the phytoextraction and tolerance capacity of young plants of V. surinamensis submitted to Cd concentrations.
Methods
Experimental site
The experiment was conducted in a greenhouse at the Federal Rural University of Amazonia (UFRA) in Belém, State of Pará, Brazil (01°27′21″S, 48°30′16″W), from 15 September 2017 to 14 November 2017. According to the climatic classification of Köppen, the climate is type Af (Tropical rainforest), with an annual average precipitation of 2921.7 mm, average temperature of 25.9 °C, average relative humidity of 86.8 % and wind speed of 1.35 m s−1 (Ramos et al. 2009).
Plant material and growth condition
Seeds of V. surinamensis were collected in the area of the Brazilian Agricultural Research Corporation (Embrapa Eastern Amazon), located in Belém, State of Pará, Brazil (01°26′44.2″S, 48°25′03.8″W). These seeds were sown in 5-L polyethylene trays containing sand and sterilized sawdust (1:1, v/v), and maintained under mean air temperature (Tair) and relative air humidity (RH) of 28 °C and 90 %. After emergence, the seedlings containing the first pair of eophylls were transplanted to 10-L polyethylene pots containing yellow latosol and poultry litter (3:1, v/v). The seedlings grown were in a greenhouse for 180 days, being irrigated daily to replace the water lost by evapotranspiration.
Subsequently, the young plants were removed and their roots washed with deionized water and transferred to 5-L Leonard pots containing sterilized and washed sand and 800 mL of nutrient solution of Sarruge (1975), replaced weekly and constituted of (µM): KH2PO4, 400; KNO3, 2000; Ca(NO3)2·4H2O, 2000; MgSO4·7H2O, 800; FeEDTA, 400; H3BO3, 400; MnCl2·4H2O, 400; ZnCl2, 400; CuCl2·2H2O, 400; and H2MoO4·H2O, 400. The pH was maintained at 5.9 ± 0.2 using HCl and NaOH. The ionic strength was initiated in 25 % (10 days) and then increased to 50 % (35 days), remaining for a period of acclimatization of 45 days.
Experimental design and treatment evaluation
After 45 days of cultivation, we selected the most uniform seedling considering height, stem diameter, number of leaves and submitted to five Cd concentrations (treatments) as following: 0 mg L−1 of CdCl2 (control), 15, 30, 45 and 60 mg L−1 of CdCl2. The doses of Cd were determined based on the Resolution 420 of the National Council of the Environment, CONAMA (Brasil 2009), which establishes criteria and guiding values of soil quality regarding the presence of chemical substances. The experimental design was a completely randomized design with seven replications, per each treatment, totalling 35 experimental units. A single plant per pot was considered a replicate. All variables for treatment comparisons were assessed 60 days after Cd treatment differentiation.
Leaf water potential, leaf gas exchange and total chlorophyll
Leaf water potential (Ψpd) was determined in the morning between 0430 and 0530 h, using the Scholander’s pressure bomb (m 670, PMS Instrument Co., Albany, OR, USA), as described by Pinheiro et al. (2008). The third leaf from apices was used as sample.
The variables net CO2 assimilation rate (A), stomatal conductance to water vapour (gs), transpiration (E), intercellular CO2 concentration (Ci), ratio of the net photosynthesis and intercellular CO2 concentration (A/Ci) and instantaneous water-use efficiency (WUE, calculated as the ratio between A and E) were assessed using a portable infrared gas analyzer (LI-6400XT, LI-COR Biosciences Inc., Lincon, NE, USA) equipped with a blue/red light source (LI-6400-02B, LI-COR) under a photosynthetically active radiation (PAR) flux of 1000 µmol m−2 s−1 and CO2 flux of 400 ppm (Silvestre et al. 2017). The assessments of gas exchanges were carried out between 0900 and 1100 h, representing the daytime period in which photosynthesis reaches the maximum values, as determined from the diurnal curves of leaf gas exchanges. The measurements were always performed in completely expanded single sheets, located in the third node counted from the apex.
The total chlorophyll content (Chl) was determined using a portable chlorophyll meter (SPAD 502-plus, Konica Minolta, Osaka, Japan), with readings taken on the third adult leaf counted from the apex at three points on each side of the midrib of the adaxial leaf face (Jesus and Marrenco 2008). The results were expressed in SPAD (Soil Plant Analysis Development) index.
Fluorescence of chlorophyll a
The fluorescence of chlorophyll a was determined on the third adult leaf, counted from the apex, using the LI-6400XT (LI-COR Biosciences Inc., Lincon, NE, USA). Leaves adapted to the dark for 30 min were illuminated with a weak pulse of modulated radiation to obtain the initial fluorescence (F0). A saturating white light pulse of 6.000 µmol m−2 s−1 was applied for 0.8 s to ensure maximum fluorescence emission (Fm). In the dark-adapted samples, the maximum photochemical efficiency of PSII was estimated by the ratio between variable and maximum fluorescence [Fv/Fm = (Fm − F0)/Fm]. Saturating white light pulses were applied to achieve the maximum fluorescence (F′m). The actinic light was then switched off and the far-red radiation switched on to measure F0 adapted to the light (F′0). The capture efficiency of excitation energy by open PSII reaction centres (F′v/F′m) was estimated as the ratio (F′m − F′0)/F′m. The photochemical quenching coefficient (qP) was calculated as qP = (F′m − Fs)/(F′m − F′0) and the non-photochemical quenching coefficient (NPQ) was determined from the equation of Stern–Volmer [NPQ = (Fm/F′m) − 1] (Krause and Weis 1991). The actual quantum yield of PSII electron transport (ΦFSII) was calculated as (Fm′ − Fs)/Fm′ (Genty et al. 1989), where Fs is the steady state fluorescence. Electron transport rate (ETR) was calculated as ETR = ΦPSII × PPFD × f × α, where PPFD is the photosynthetic photon flux density, f is a factor that contributes to energy partitioning between PSII and PSI and is assumed to be 0.5, indicating that the excitation energy is equally distributed between the two photosystems, and α is the leaf absorbency by the photosynthetic tissues and is assumed to be 0.84 (Maxwell and Johnson 2000).
Cadmium analysis
Cadmium analysis was processed in triplicate according to the methodology described by Miyazawa et al. (2009), with adaptations. The dry matter (0.5 g) of each sample was digested in a digester tube with 8 mL of nitric acid solution (HNO3) + perchloric acid (HClO4) (3:1). After cooling, the solution in the tube was filtered and diluted with deionized water to a final volume of 50 mL. Cadmium contents were determined in this solution by atomic absorption spectrometry (Thermo Scientific ICE 3000).
Tolerance index
The tolerance index (TI) was determined to assess the plant ability to develop in the presence of Cd. The TI for Cd concentrations and for each plant organ was calculated according to Wilkins (1957), in which TI values can range from 0 (maximum sensitivity) to 1 (maximum tolerance).
| (1) |
where DMP solution with Cd is the dry mass of the plant in the solution with Cd and DMP control solution is the dry mass of the plant in the control solution.
Bioconcentration and translocation factor
To assess Cd phytoextraction capacity in V. surinamensis, the bioconcentration (BCF) and translocation factor (TF) were calculated at the end of the experiment, as in Fan et al. (2011).
| (2) |
where Cplant is the sum of the concentration of Cd (mg kg−1) in the plant organs (root, stem and leaves) and Csolution is the metal concentration of the nutrient solution (mg L−1).
| (3) |
where Caerial part is the sum of the concentration of Cd (mg kg−1) in plant organs (stem and leaves) and Croot is the concentration of the metal in the root of the plant (mg kg−1).
Data analysis
The experimental data were assessed for the normality and homogeneity of variances by the Shapiro–Wilk and Bartlett tests, respectively. For parametric variables, the means of treatments were submitted to PROC GLM, post hoc Tukey’s HSD test and correlation between variables by the PROC CORR linear of Pearson using the software SAS 9.1.3 (SAS 2007). For non-parametric variables, the data were assessed by the Kruskal–Wallis test with Bonferroni correction by the software RStudio version 1.1.383. The experimental data of all analyses were assessed at 5 % significance.
Results
Effect of Cd on water potential, gas exchange and total chlorophyll
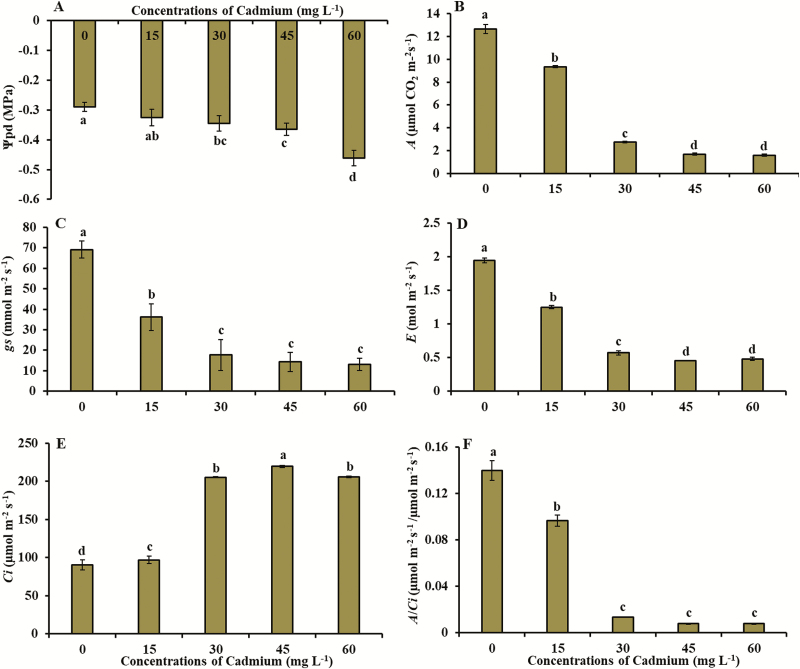
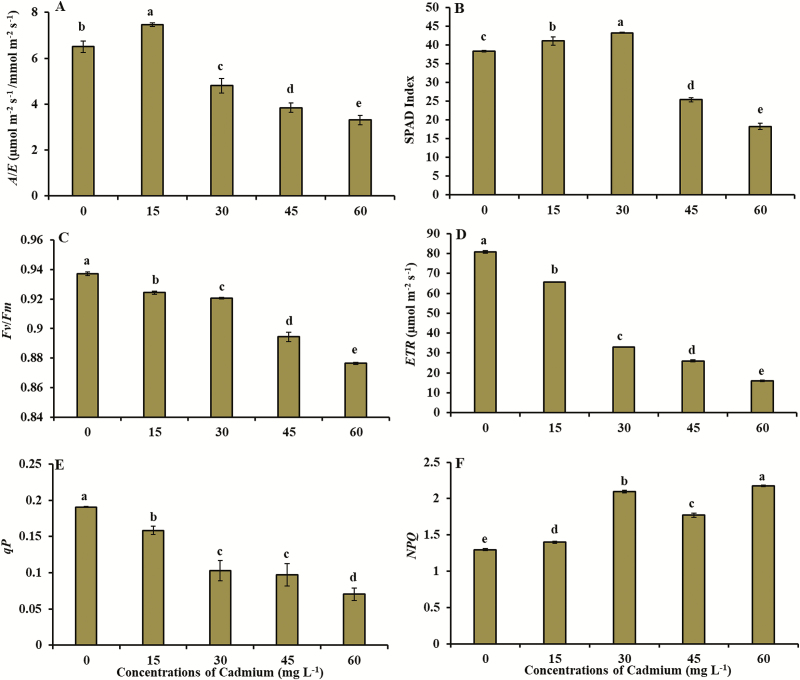
The Ψpd, gas exchange variables (A, gs, E, Ci, A/Ci and A/E) and total chlorophyll content (SPAD index) were significantly affected by exposure to Cd (Figs 1 and 2; see Supporting Information—Appendix S1). The Ψpd reduced from −0.29 MPa (control) to −0.46 MPa (concentration of 60 mg L−1 of Cd) (Fig. 1A). Lowest values of net CO2 assimilation (1.6 μmol m−2 s−1), stomatal conductance to water vapour (13.0 mmol m−2 s−1) and transpiration (0.48 mol m−2 s−1) were obtained in concentration of 60 mg L−1 of Cd (Fig. 1B–D). Intercellular CO2 concentration increased from 90.6 μmol m−2 s−1 (control) to 206.0 μmol m−2 s−1 (concentration of 45 mg L−1 of Cd) (Fig. 1E). The A/Ci ratio decreased from 0.13 µmol m−2 s−1/µmol m−2 s−1 (control) to 0.007 µmol m−2 s−1/µmol m−2 s−1 (concentration of 60 mg L−1 of Cd) (Fig. 1F). Water-use efficiency (A/E) reached the lowest value (3.3 µmol m−2 s−1/mol m−2 s−1 in concentration of 60 mg L−1 of Cd) (Fig. 2A). Total chlorophyll content (SPAD index) ranged from 38.3 (control) to 18.2 (concentration of 60 mg L−1 of Cd) (Fig. 2B).
Figure 1.
(A) Predawn water potential (Ψpd), (B) net photosynthetic rate (A), (C) stomatal conductance (gs), (D) transpiration (E), (E) internal CO2 concentration (Ci) and (F) net photosynthesis to intercellular CO2 concentration ratio (A/Ci) in young plants of V. surinamensis exposed to five concentrations of cadmium (0, 15, 30, 45 and 60 mg). Different letters for concentrations of cadmium in solution indicate significant differences in the Tukey’s test (P < 0.05). Mean ± SD, n = 7.
Figure 2.
(A) Instantaneous water-use efficiency (A/E), (B) total chlorophyll (SPAD index), (C) maximum photochemical efficiency of PSII (Fv/Fm), (D) electron transport rate (ETR), (E) photochemical quenching coefficient (qP) and (F) non-photochemical quenching coefficient (NPQ) in plants young of V. surinamensis exposed to five concentrations of cadmium (0, 15, 30, 45 and 60 mg). Different letters for concentrations of cadmium in solution indicate significant differences in the Tukey’s test (P < 0.05). Mean ± SD, n = 7.
Effect of Cd on the fluorescence of chlorophyll a
The fluorescence of chlorophyll a parameters were significantly affected by exposure to Cd (Fig. 2). Fv/Fm index decreased from 0.93 (control) to 0.87 (concentration of 60 mg L−1 of Cd) (Fig. 2C). ETR and qP reduced from 80.8 and 0.19 (control) to 15.9 and 0.07 (concentration of 60 mg L−1 of Cd), respectively (Fig. 2D and E). NPQ increased from 1.3 (control) to 2.17 (concentration of 60 mg L−1 of Cd) (Fig. 2F).
Concentration of Cd in different tissues
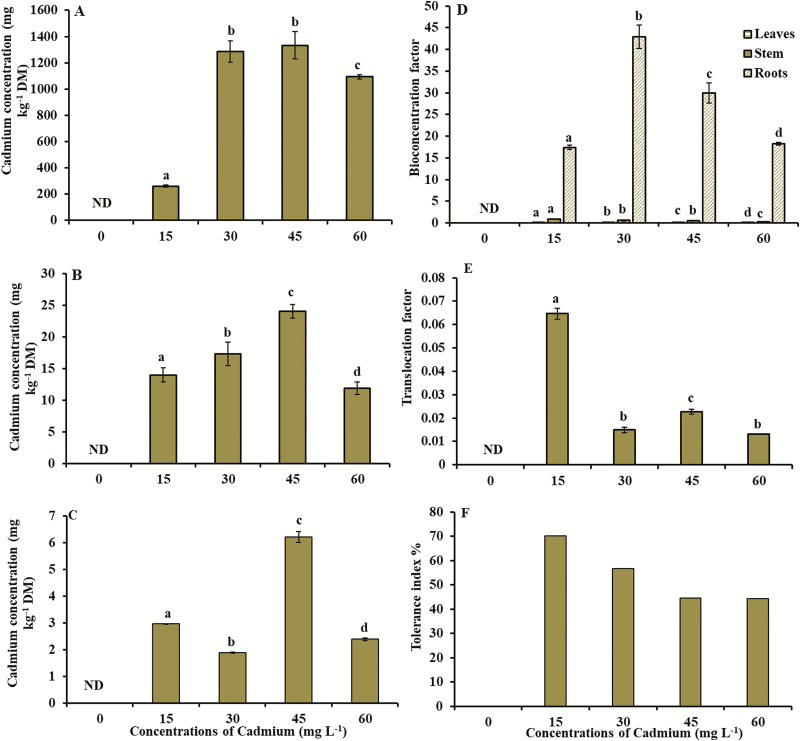
The amount of Cd in the roots and shoot of V. surinamensis increased as Cd concentrations increased in the nutrient solution (Fig. 3), being the root system the plant tissue that promoted a higher Cd accumulation, with the highest value of 1333.5 mg kg−1 DM at the concentration of 45 mg L−1 of Cd (Fig. 3A). In the stem and leaves, the highest values of Cd (23.9 and 6.2 mg kg−1 DM, respectively) were obtained at the concentration of 45 mg L−1 of Cd (Fig. 3B and C). According to Fig. 3, V. surinamensis presented Cd contents in the different plant tissues, as the order root > stem > leaf.
Figure 3.
(A) Cadmium concentration in the roots, (B) cadmium concentration in the stem, (C) cadmium concentration in the leaves, (D) bioconcentration factor, (E) translocation factor and (F) tolerance index in young plants of V. surinamensis exposed to five concentrations of cadmium (0, 15, 30, 45 and 60 mg). ND = not detected; DM = dry mass. Different letters for concentrations of cadmium in solution indicate significant differences in the Kruskal–Wallis test (P < 0.05). Mean ± SD, n = 7.
BCF, TF and TI
In the plants of V. surinamensis, BCF was higher at the concentrations of 30 mg L−1 of Cd (42.93) and 45 mg L−1 of Cd (29.95) (Fig. 3D). The maximum value of TF (0.065) and TI (70.1 %) occurred at the concentration of 15 mg L−1 of Cd (Fig. 3E and F; see Supporting Information—Appendix S2).
Discussion
The data of Ψpd (Fig. 1A) in young plants of V. surinamensis submitted to Cd concentrations indicated that, at a low variation in Ψpd value, the symptoms of water deficit began, such as the reduction of gs (Fig. 1C). The decrease of gs (Fig. 1C) in V. surinamensis submitted to Cd exposure is probably due to stomatal closure, reduction of stomata density, decrease in pore size (Elloumi et al. 2014) and decrease in stomatal size (Di Baccio et al. 2014; Nikolić et al. 2017). Reduction of stomatal conductance related to water potential was observed in Eucalyptus camaldulensis exposed to Cd (Marques et al. 2011).
According to Nikolić et al. (2017), changes in the stomata promote concomitant limitations in the diffusion of water vapour and CO2 into the cells and influence carbon assimilation and loss of the photosynthetic activity of the plant submitted to the presence of Cd. On the other hand, the reduction of gs in young plants of V. surinamensis may have been a strategy of tolerance to Cd to reduce its absorption and maintain the amount of water in the tissues for plant survival. Reduction of gs, E and A were also observed in other tree species exposed to Cd (Nikolić et al. 2017; Pereira et al. 2017).
Lowest levels of total chlorophyll in V. surinamensis exposed to Cd (Fig. 2B) suggest alteration in chlorophyll biosynthesis or degradation. Cadmium influences chlorophyll biosynthesis because the metal affects water relations (Oláh et al. 2015), limits the absorption and transport processes and utilization of Mg2+ and Fe2+ (He et al. 2013; Di Baccio et al. 2014; Huang et al. 2015) and/or replaces Mg2+ in chlorophyll molecules, causing a disturbance of glutathione availability and inhibiting in activity of δ-aminolevulinic dehydratase enzyme (ALA-D) and function of proto-chlorophyll reductase (Parmar et al. 2013). Decrease in chlorophyll levels was also observed in other studies with arboreal species submitted to Cd (Yang et al. 2015; Nikolić et al. 2017).
The reduction in chlorophyll content by increased degradation or decreased biosynthesis may have been reflected in significant reductions in photosynthetic activity of plants under stress by Cd (Elloumi et al. 2014; Hernández et al. 2015; Yang et al. 2015; Michel-López et al. 2016; Zouari et al. 2016; Nikolić et al. 2017; Silva et al. 2017).
The reduction of gs by limiting the CO2 influx in leaves may influence the reduction of photosynthetic rate (Di Bacio et al. 2014). Thus, the reduction of gs would result in low mesophilic conductance to CO2 and consequently in lower chloroplastidic CO2, justifying the decrease of photosynthesis in V. surinamensis (Fig. 1B). The influence of gs on decrease of photosynthesis in plants exposed to Cd was also observed in C. brasiliense (Pereira et al. 2017). However, the increase in intercellular CO2 concentration (Ci) (Fig. 1E) with concomitant reductions of gs and A (Fig. 1C and B) in plants exposed to Cd suggests that decline of photosynthetic activity in V. surinamensis also occurs by non-stomatal limitation. The same behaviour was observed in other tree species (Nikolić et al. 2017). It has been reported that CO2 fixation in chloroplast stroma may be affected by inhibition of enzymes of Calvin cycle (Li et al. 2015), such as RuBisCO (Parmar et al. 2013; Tran and Popova 2013; Yang et al. 2015), contributing to lower A values of plant. The inhibition of enzymes related to biochemical stage of photosynthesis, caused by exposure of plants to Cd, may have impaired the fixation and assimilation of photosynthetic CO2 and result in increase of Ci. In addition, the reduction of instantaneous efficiency of carboxylation (A/Ci) (Fig. 1F) in plants exposed to Cd reinforces the indication that high concentrations of metal may result in damage to biochemical aspects of photosynthesis causing losses in CO2 assimilation rate.
Changes in stomatal opening, water balance and photosynthetic activity are known damages in plants exposed to Cd (Di Bacio et al. 2014). In this study, the combined effect of Ψpd and gs reduction on plants exposed to Cd may have been the cause on reduction of E (Fig. 1D). The influence of stomatal conductance on transpiration of plants submitted to Cd concentrations was observed in other tree species (Nikolić et al. 2017). The reduction of transpiration in plants exposed to Cd can limit the transport of metal from roots to leaves and reduce the damage caused by photosynthetic apparatus (Gratão et al. 2015). Thus, the decrease of E in V. surinamensis in presence of Cd may have been a strategy of tolerance to metal for protection, maintenance or reduction of damages in photosystem components, in an attempt to plant survive.
The reduced values of WUE (A/E) (Fig. 2A) in plants submitted to Cd are due to the low photosynthetic rate per unit of water loss in the plants, in which V. surinamensis showed a higher sensitivity to higher Cd concentrations. According to Pajević et al. (2009), the reduction of WUE in plants exposed to Cd occurs due to the inhibition of the absorption and transport of water, which causes changes in water balance and hence a low production of photoassimilates. Different behaviour was observed in C. brasiliense exposed to Cd (Pereira et al. 2017).
The effects of Cd stress on chlorophyll fluorescence parameters (Fv/Fm, qP, ETR and NPQ) (Fig. 2) may indicate an inhibition of the activity of PSII, resulting in changes in the photosynthetic rate of plants (Pajević et al. 2009; Tang et al. 2015). The reduction of Fv/Fm values (Fig. 2C) in plants exposed to Cd were followed by a reduction of photosynthesis at a carboxylation level, evidenced by an increase in Ci (Fig. 1E). The results obtained in present study in relation to chlorophyll fluorescence were evidenced in other tree species (Pietrini et al. 2009; Di Baccio et al. 2014; Ge et al. 2015).
Although the reduction of A was followed by a significant decrease in Fv/Fm and ETR (Fig. 2C and D) in plants submitted to Cd, the effect of the metal may not have been sufficient to cause damage to Φ. This occurs because plants that present Fv/Fm values close to 0.85 are considered healthy (Kalaji and Guo 2008; Nikolić et al. 2015), i.e. the maximum photochemical quantum efficiency of PSII was not affected by Cd, indicating the stability of thylakoid structure and the efficient flux of electrons through PSII, but with disturbances at a carboxylation level (Pajević et al. 2009). According to Nikolić et al. (2015), other disturbances, in addition to those in thylakoid and chloroplast membranes, may be involved in the reduction of photosynthesis in plants exposed to heavy metals. On the other hand, significant reductions in Fv/Fm, associated with low qP values (Fig. 2E), may reduce the photosynthetic efficiency of plants, as suggested by Pietrini et al. (2009) for poplar clones and Huang et al. (2015) for Cornus controversa treated with Cd.
The reduction of qP (Fig. 2E) and increase of NPQ (Fig. 2F) showed that the damage to PSII induced by a high Cd dose was not enough to cause the photoinactivation, with PSII being protected by an effective dissipation mechanism of heat to avoid the photoinhibition at the reaction centres (Li et al. 2015; Tang et al. 2015), these findings are corroborated by Ge et al. (2015).
Concentrations of 5–10 µg Cd g−1 of DM in leaf tissue have been reported to be toxic to most plants (White and Brown 2010). Thus, tolerant plants are often exclusionary, limiting the entry and translocation of heavy metals from the root to shoot (Gallego et al. 2012). The high amount of Cd accumulated in the root of V. surinamensis (Fig. 3A) indicates the ability to absorb the Cd of the solution and retain the metal especially in the roots, which suggests exclusion and chelation of the metal in the cellular and subcellular compartments of the root system. This may have contributed to a restricted Cd transport from root to the shoot of plants, being a strategy to protect the photosynthetic apparatus, as well as the higher capacity of tolerance of the plant to Cd (Dai et al. 2013). The highest concentration of Cd in root was observed in other tree species (Nikolić et al. 2017; Pereira et al. 2017). Cadmium retention in roots occurs because the metal binds to functional groups, such as thiol, present in the cell wall components of plants (Mehes-Smith et al. 2013) and in other compounds such as glutathione (Hasanuzzaman et al. 2017), metallothioneins and phytochelatins (Hernandez et al. 2015). Some of these compounds were observed in cell wall of root system of plants exposed to Cd (Fernández et al. 2014), suggesting that cell wall of V. surinamensis root system may have functioned as a barrier to Cd translocation, justifying the higher concentration of metal in root. This is because, at least in part, the lignification can make the cell wall less penetrable, forming a barrier against the Cd influx or even bonding with the metal (Parrota et al. 2015).
The phytoextraction capacity can be evaluated by BCF and TF. The BCF evaluates the efficiency of the plant in accumulating metal in relation to the soil solution, while the TF demonstrates the ability of the plant to transport metal from root to shoot (Fan et al. 2011). Bioconcentration factor and TF, in aerial tissues of the plant, >1.0 are good indicators of the phytoextraction capacity of Cd (Dai et al. 2011). With the exception of Cd hyperaccumulating plants that have BCF and TF > 1 and accumulate >100 mg kg−1 DM of Cd in shoot, most plants have BCF < 1.0 (Van der Ent 2013). In this study, the BCF of the aerial part and the TF < 1.0 (Fig. 3D and E) indicate that V. surinamensis has low capacity of phytoextraction of Cd and do not belong to the group of hyperaccumulators of this metal. On the other hand, values of BCF and TF < 1 characterize species of metal phytostabilizing plants (Masarovičová et al. 2010). These plants accumulate more heavy metals from the substrate in their roots, but restrict their transport and entry into the aerial parts (Malik and Biswas 2012; Hosman et al. 2017). In this study, the results of BCF and TF (Fig. 3D and E) indicate the ability of the plant to bioconcentrate the Cd in the root, suggesting that V. surinamensis develop mechanisms to accumulate the metal in the root, being able to be effective for phytostabilization Cd. The values of BCF and TF in V. surinamensis are in agreement with those obtained in other studies (Michel-López et al. 2016; Nikolić et al. 2017).
The tolerance of V. surinamensis to Cd, estimated by TI, based on the total dry mass of the plants, was similar to the other tree species (Wang et al. 2016; Nikolić et al. 2017). According to the scheme proposed by Lux et al. (2004), in relation to the tolerance index, plants may have high tolerance (TI > 60), medium tolerance (TI between 0.35 and 60) and low tolerance (TI < 0.35). The results obtained in this work in relation to TI (Fig. 3F) indicate that V. surinamensis present medium and high tolerance to Cd.
Conclusion
In this study, we demonstrated that changes in Ψpd, gs and E in V. surinamenses exposed to Cd may have limited the transport of metal from roots to leaves.
The results of chlorophyll and gas exchange fluorescence parameters suggest that decrease of net CO2 assimilation in V. surinamensis is caused by stomatal limitations and changes in PSII with increasing Cd concentration.
The results of BCF and FT demonstrate low plant efficacy in Cd phytoextraction and suggest that V. surinamensis may be promising for Cd phytostabilization purposes.
Sources of Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. This study was financed in part by the Coordination of the Improvement of Higher Education Personnel in Brazil (CAPES), Finance Code 001.
Conflict of Interest
None declared.
Contributions by the Authors
W.V.A.J., C.F.O.N., and R.S.O. wrote the manuscript; W.V.A.J., and C.F.O.N. designed the study and analysed the data; B.G.S.F. analysed the data; A.V.C.B. helped in statistical analysis; C.B.A., E.D.C., D.J.P.S., J.S.A.T., and A.S.B. helped perform the experiments.
Supporting Information
The following additional information is available in the online version of this article—
Appendix S1. Data used for leaf water potential, leaf gas exchange, total chlorophyll and fluorescence of chlorophyll a.
Appendix S2. Data used for bioconcentration factor, translocation factor and tolerance index.
Acknowledgements
The authors are grateful to the Group of Studies on Biodiversity in Upper Plants of the Federal Rural University of Amazonia for the collaborations of researchers.
Literature Cited
- Abdul Qados AMS. 2015. Phytoremediation of PB and CD by native tree species grown in the Kingdom of Saudi Arabia. India Journal Scientific Research and Technology 3:22–34. [Google Scholar]
- Ahmad I, Akhtar MJ, Zahir ZA, Naveed M, Mitter B, Sessitsch A. 2014. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environmental Science and Pollution Research International 21:11054–11065. [DOI] [PubMed] [Google Scholar]
- Ali H, Khan E, Sajad MA. 2013. Phytoremediation of heavy metals–concepts and applications. Chemosphere 91:869–881. [DOI] [PubMed] [Google Scholar]
- Bashir H, Qureshi MI, Ibrahim MM, Iqbal M. 2015. Chloroplast and photosystems: impact of cadmium and iron deficiency. Photosynthetica 53:1–15. [Google Scholar]
- Brasil 2009. Ministério do Meio Ambiente. Resolução Conama n. 420, de 28 de dezembro de 2009. Diário Oficial da República Federativa do Brasil, Brasília 249:81–84. [Google Scholar]
- Costa BC, Suzuki PM, Martins WBR, Andrade VMS, Oliveira FA. 2017. Dinâmica da massa seca e propriedades químicas da liteira em Virola surinamensis e floresta sucessional na Amazônia oriental. Revista Verde de Agroecologia e Desenvolvimento Sustentável 12:23–28. [Google Scholar]
- Dai H, Shan C, Jia G, Lu C, Yang T, Wei A. 2013. Cadmium detoxification in Populus x canescens. Turkish Journal of Botany 37:950–955. [Google Scholar]
- Dai ZY, Shu WS, Liao B, Wan CY, Li JT. 2011. Intraspecific variation in cadmium tolerance and accumulation of a high-biomass tropical tree Averrhoa carambola L.: implication for phytoextraction. Journal of Environmental Monitoring 13:1723–1729. [DOI] [PubMed] [Google Scholar]
- Di Baccio D, Castagna A, Tognetti R, Ranieri A, Sebastiani L. 2014. Early responses to cadmium of two poplar clones that differ in stress tolerance. Journal Plant Physiology 171:1693–1705. [DOI] [PubMed] [Google Scholar]
- Elloumi N, Zouari M, Chaari L, Jomni C, Rouina BB, Abdallah FB. 2014. Ecophysiological responses of almond (Prunus dulcis) seedlings to cadmium stress. Biologia 69:604–609. [Google Scholar]
- Ge W, Jiao Y, Zou J, Jiang W, Liu D. 2015. Ultrastructural and photosynthetic response of Populus 107 leaves to cadmium stress. Polish Journal of Environmental Studies 24:519–527. [Google Scholar]
- Fan KC, Hsi HC, Chen CW, Lee HL, Hseu ZY. 2011. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications. Journal of Environmental Management 92:2818–2822. [DOI] [PubMed] [Google Scholar]
- Fernández R, Bertrand A, Reis R, Mourato MP, Martins LL, González A. 2013. Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. Journal of Hazardous Materials 244:555–562. [DOI] [PubMed] [Google Scholar]
- Fernández R, Fernández-Fuego D, Bertrand A, González A. 2014. Strategies for Cd accumulation in Dittrichia viscosa (L.) Greuter: role of the cell wall, non-protein thiols and organic acids. Plant Physiology and Biochemistry 78:63–70. [DOI] [PubMed] [Google Scholar]
- Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppaa MD, Benavides MP. 2012. Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environmental and Experimental Botany 83:33–46. [Google Scholar]
- Ge W, Jiao Y, Zou J, Jiang W, Liu D. 2015 Ultrastructural and photosynthetic response of Populus 107 leaves to cadmium stress. Polish Journal of Environmental Studies 24:519–527. [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990:87–92. [Google Scholar]
- Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LP, Azevedo RA. 2015. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 28:803–816. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Fujita M. 2017. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiology Molecular Biology Plants 23:249–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Li H, Luo J, Ma C, Li S, Qu L, Gai Y, Jiang X, Janz D, Polle A, Tyree M, Luo ZB. 2013. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus x canescens. Plant Physiology 162:424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández LE, Sobrino-Plata J, Montero-Palmero MB, Carrasco-Gil S, Flores-Cáceres ML, Ortega-Villasante C, Escobar C. 2015. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. Journal of Experimental Botany 66:2901–2911. [DOI] [PubMed] [Google Scholar]
- Hosman ME, El-Feky SS, Mohamed MI, Shaker EM. 2017. Mechanism of phytoremediation potential of flax (Linum usitatissimum L.) to Pb, Cd and Zn. Asian Journal of Plant Science and Research 7:30–40. [Google Scholar]
- Huang X, Jiang Y, Cheng X, Deng L, Liu X. 2015. Photosynthetic performance and anti-oxidative response of Cornus controversa seedlings under cadmium and lead stress. Bangladesh Journal of Botany 44:215–221. [Google Scholar]
- Jesus SV, Marenco RA. 2008. O SPAD-502 como alternativa para a determinação dos teores de clorofila em espécies frutíferas. Acta Amazon 38:815–818. [Google Scholar]
- Kalaji HM, Guo P. 2008. Chlorophyll fluorescence: a useful tool in barley plant breeding programs. In: Sánchez A, Gutierrez SJ, eds. Photochemistry research progress. New York: Nova Science Publishers, 12:469–463. [Google Scholar]
- Khan MA, Khan S, Khan A, Alam M. 2017. Soil contamination with cadmium, consequences and remediation using organic amendments. The Science of the Total Environment 601–602:1591–1605. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. 1991. Chlorophyll fluorescence and photosynthesis: the basics. Annual Review of Plant Physiology and Plant Molecular Biology 42:313–349. [Google Scholar]
- Li S, Yang W, Yang T, Chen Y, Ni W. 2015. Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi–a cadmium accumulating plant. International Journal of Phytoremediation 17:85–92. [DOI] [PubMed] [Google Scholar]
- Lux A, Sottníková A, Opatrná J, Greger M. 2004. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiologia Plantarum 120:537–545. [DOI] [PubMed] [Google Scholar]
- Malik N, Biswas AK. 2012. Role of higher plants in remediation of metal contaminated sites. Scientific Reviews Chemical Communications 2:141–146. [Google Scholar]
- Marques TCLLSM, Soares AM, Gomes MP, Martins G. 2011. Respostas fisiológicas e anatômicas de plantas jovens de Eucalipto expostas ao cádmio. Revista Árvore 35:997–1006. [Google Scholar]
- Masarovičová E, KráĬova´ K, Kummerová M. 2010. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiologiae Plantarum 32:823–829. [Google Scholar]
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence–a practical guide. Journal of Experimental Botany 51:659–668. [DOI] [PubMed] [Google Scholar]
- Mehes-Smith M, Nkongolo K, Cholewa E. 2013. Coping mechanisms of plants to metal contaminated soil. In: Silvern S, ed. Environmental change and sustainability . Vol. 54. Rijeka, Croatia: In Tech, 53–90. [Google Scholar]
- Michel-López CY, Gil FE, Ortíz GF, Santamaría JM, González-Mendoza D, Ceceña-Duran C, Juarez OG. 2016. Bioaccumulation and effect of cadmium in the photosynthetic apparatus of Prosopis juliflora. Chemical Speciation & Bioavailability 28:1–4, 1–6. [Google Scholar]
- Miyazawa M, Pavan MA, Muraoka T, Carmo CAFS, Melo WJ. 2009. Chemical analysis of plant tissues. In: Silva FC, ed. Manual of chemical analysis of soils, plants and fertilizers. Brasília, Brazil: Embrapa Informação Tecnológica, 191–233. [Google Scholar]
- Nikolić NP, Borišev MK, Pajević SP, Arsenov DD, Župunski MD, Orlović SS, Pilipović AR. 2015. Photosynthetic response and tolerance of three willow species to cadmium exposure in hydroponic culture. Archives of Biological Sciences 67:1411–1420. [Google Scholar]
- Nikolić N, Zorić L, Cvetković I, Pajević S, Borišev M, Orlović S, Pilipović A. 2017. Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus x euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil. Iforest 10:635–644. [Google Scholar]
- Oláh V, Hepp A, Mészáros I. 2015. Comparative study on the sensitivity of turions and active fronds of giant duckweed (Spirodela polyrhiza (L.) Schleiden) to heavy metal treatments. Chemosphere 132:40–46. [DOI] [PubMed] [Google Scholar]
- Oliveira ES, Oliveira GMTS, Melo NFAC. 2017. Concentração de hidrocarbonetos alifáticos e metais pesados na zona portuária de Vila do Conde, Rio Pará-Brasil. Revista Espacios 38:1–12. [Google Scholar]
- Pajević S, Borisev M, Nikolic N, Krstic B, Pilipović A, Orlović S. 2009. Phytoremediation capacity of poplar (Populus spp.) and willow (Salix spp.) clones in relation to photosynthesis. Archives of Biological Sciences 61:239–247. [Google Scholar]
- Parmar P, Kumari N, Sharma V. 2013. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Botanical Studies 54:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF. 2015. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Frontiers Plant Science 2:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini F, Zacchini M, Iori V, Pietrosanti L, Ferretti M, Massacci A. 2009. Spatial distribution of cadmium in leaves and its impact on photosynthesis: examples of different strategies in willow and poplar clones. Plant Biology 12:355–363. [DOI] [PubMed] [Google Scholar]
- Pereira AS, Cortez PA, Almeida AAF, Prasad MNV, França MGC, Cunha M, Jesus RM, Mangabeira PAO. 2017. Morphology, ultrastructure, and element uptake in Calophyllum brasiliense Cambess. (Calophyllaceae J. Agardh) seedlings under cadmium exposure. Environmental Science Pollution Reseach 24:15576–15588. [DOI] [PubMed] [Google Scholar]
- Pinheiro HA, Silva JV, Endres L, Ferreira VM, Camara CA, Cabral FF, Oliveira JF, Carvalho LWT, Santos JM, Santos Filho BG. 2008. Leaf gas exchange, chloroplastic pigments and dry matter accumulation in castor bean (Ricinus communis L.) seedlings subjected to salt stress conditions. Industrial Crops and Products 27:385–392. [Google Scholar]
- Ramos AM, Santos LAR, Fortes LTG. 2009. Normais climatológicas do Brasil 1961-1990, 1a edição. Brasília: INMET. [Google Scholar]
- Sarruge JR. 1975. Soluções nutritivas. Summa Phytopathologica 1:231–233. [Google Scholar]
- Seyler PT, Boaventura GR. 2003. Distribution and partition of trace metals in the Amazon basin. Hydrological Processes 17:1345–1361. [Google Scholar]
- Sharma S, Rana S, Thakkar A, Baldi A, Murthy RSR, Sharma RK. 2016. Physical, chemical and phytoremediation technique for removal of heavy metals. Journal of Heavy Metal Toxicity and Diseases 1:1–15. [Google Scholar]
- Silva JRR, Fernandes AR, Silva Junior ML, Santos CRC, Lobato AKS. 2017. Tolerance mechanisms in Cassia alata exposed to cadmium toxicity – potential use for phytoremediation. Photosynthetica 55:1–10. [Google Scholar]
- Silvestre WVD, Silva PA, Palheta LF, Oliveira Neto CF, Souza R, Festucci-Buselli RA, Pinheiro HA. 2017. Differential tolerance to water deficit in two açaí (Euterpe oleracea Mart.) plant materials. Acta Physiologiae Plantarum 39:1–10. [Google Scholar]
- Solti Á, Sárvári É, Szöllősi E, Tóth B, Mészáros I, Fodor F, Szigeti Z. 2016. Stress hardening under long-term cadmium treatment is correlated with the activation of antioxidative defence and iron acquisition of chloroplasts in Populus. Zeitschrift Fur Naturforschung. C, Journal of Biosciences 71:323–334. [DOI] [PubMed] [Google Scholar]
- Song Y, Jin L, Wang X. 2016. Cadmium absorption and transportation pathways in plants. International Journal of Phytoremediation 19:133–141. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis System Institute – SAS 2007. SAS® 9.1.3 (TS1M3) for Windows Microsoft. Cary, NC: SAS Institute. [Google Scholar]
- Tang Y, Bao Q, Tian G, Fu K, Cheng H. 2015. Heavy metal cadmium tolerance on the growth characteristics of industrial hemp (Cannabis sativa L.) in China. In: Chen S, Zhou S, eds. International Conference on Advances in Energy, Environment and Chemical Engineering. Amsterdam: Atlantis Press, 289–295. [Google Scholar]
- Tran TA, Popova LP. 2013. Functions and toxicity of cadmium in plants: recent advances and future prospects. Turkish Journal of Botany 37:1–13. [Google Scholar]
- Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H. 2013. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334. [Google Scholar]
- Wang Y, Gu C, Bai S, Sun Z, Zhu T, Zhu X, Grit DH, Tembrock LR. 2016. Cadmium accumulation and tolerance of Lagerstroemia indica and Lagerstroemia fauriei (Lythracaeae) seedlings for phytoremediation applications. International Journal of Phytoremediation 18:1104–1112. [DOI] [PubMed] [Google Scholar]
- White PJ, Brown PH. 2010. Plant nutrition for sustainable development and global health. Annals of Botany 105:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins DA. 1957. A technique for the measurement of lead tolerance in plants. Nature 180:37–38.13451634 [Google Scholar]
- Yang Y, Li X, Yang S, Zhou Y, Dong C, Ren J, Sun X, Yang Y. 2015. Comparative physiological and proteomic analysis reveals the leaf response to cadmium-induced stress in poplar (Populus yunnanensis). PLoS One 10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Shen Z, Duo L. 2015. Heavy metal uptake and leaching from polluted soil using permeable barrier in DTPA-assisted phytoextraction. Environmental Science and Pollution Research International 22:5263–5270. [DOI] [PubMed] [Google Scholar]
- Zouari M, Ben Ahmed C, Elloumi N, Bellassoued K, Delmail D, Labrousse P, Ben Abdallah F, Ben Rouina B. 2016. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicology and Environmental Safety 128:195–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.