Abstract
Transient ischemia in the whole brain leads to neuronal loss/death in vulnerable brain regions. The striatum, neocortex and hippocampus selectively loose specific neurons after transient ischemia. Just 5 minutes of transient ischemia can cause pyramidal neuronal death in the hippocampal cornu ammonis (CA) 1 field at 4 days after transient ischemia. In this study, we investigated the effects of 5-minute (mild), 15-minute (severe), and 20-minute (lethal) transient ischemia by bilateral common carotid artery occlusion (BCCAO) on behavioral change and neuronal death and gliosis (astrocytosis and microgliosis) in gerbil hippocampal subregions (CA1–3 region and dentate gyrus). We performed spontaneous motor activity test to evaluate gerbil locomotor activity, cresyl violet staining to detect cellular distribution, neuronal nuclei immunohistochemistry to detect neuronal distribution, and Fluoro-Jade B histofluorescence to evaluate neuronal death. We also conducted immunohistochemical staining for glial fibrillary acidic protein and ionized calcium-binding adapter molecule 1 (Iba1) to evaluate astrocytosis and microgliosis, respectively. Animals subjected to 20-minute BCCAO died in at least 2 days. BCCAO for 15 minutes led to pyramidal cell death in hippocampal CA1–3 region 2 days later and granule cell death in hippocampal dentate gyrus 5 days later. Similar results were not found in animals subjected to 5-minute BCCAO. Gliosis was much more rapidly and severely progressed in animals subjected to 15-minute BCCAO than in those subjected to 5-minute BCCAO. Our results indicate that neuronal loss in the hippocampal formation following transient ischemia is significantly different according to regions and severity of transient ischemia. The experimental protocol was approved by Institutional Animal Care and Use Committee (AICUC) of Kangwon National University (approval No. KW-180124-1) on May 22, 2018.
Keywords: transient global brain ischemia, delayed neuronal death, glial activation, ischemic duration, hippocampus, spontaneous motor activity, Mongolian gerbil, histology, neural regeneration
Chinese Library Classification No. R446; R364; R741
Introduction
Transient ischemia in the brain is caused by temporary obstruction of blood flow in the cerebrovascular system. Temporary hindrance of blood supply to the brain can increase the risk of ischemia/reperfusion (IR) injury to the brain (Park et al., 2014; Zhang et al., 2017). Transient global brain ischemia occurs under particular ischemic conditions such as return of spontaneous circulation after cardiac arrest. It leads to selective neuronal death/loss in the specific vulnerable substructures (region or areas) of the brain (i.e., the neocortex, striatum and hippocampus) (Nakayama et al., 2013; Park et al., 2017b). Among these hippocampal structures, pyramidal cells or neurons in the CA1 region will die several days after transient global brain ischemia. This is called “delayed neuronal death” (Kirino, 2000).
It is well known that astrocytes perform many functions, including biochemical support of endothelial cells to constitute the blood-brain barrier (BBB), maintenance of extracellular ion balance, provision of nutrients to nervous tissues, and participation in the repair of the central nervous system (CNS) following injuries (Horner and Palmer, 2003; Daneman and Prat, 2015). In the BBB, astrocytes form neurovascular coupling by provision of cellular link between neuronal circuitry and blood vessels (Daneman and Prat, 2015). Astrocyte activity is related to blood flow in the CNS. Thus it can be measured by functional magnetic resonance imaging (fMRI) (Swaminathan, 2008; Figley and Stroman, 2011).
Microglia are immune cells in the CNS that secrete various kinds of inflammatory cytokines under some conditions such as cerebral ischemia (Yan et al., 2012b; Gulke et al., 2018). In addition, microglia communicate with neurons to maintain a healthy environment in the CNS (Suzumura, 2013). Both astrocytes and microglia are activated in the pathological status of the CNS, such as ischemic insults and neurodegenerative diseases. They release various factors to contribute to neuronal death as well as neuronal survival when nervous tissues are in the pathological status (Burda and Sofroniew, 2014).
It is well known that just 5-minute bilateral common carotid artery occlusion (BCCAO) in gerbils can cause death/loss of pyramidal neurons in the hippocampal CA1 region 4–5 days later (Kirino, 1982; Kirino and Sano, 1984; Park et al., 2015, 2017a). Furthermore, spontaneous hyperactivity following 5-minute BCCAO is generally shown in gerbils (Janac et al., 2006; Park et al., 2017b). To our best knowledge, previous studies on ischemic injuries after mild, severe, or lethal transient ischemia were performed in the septum and striatum (Ohk et al., 2012; Park et al., 2013). Chronological alterations of neuronal loss and gliosis in the hippocampal formation, which consists of hippocampal CA1–3 regions and dentate gyrus, following mild, severe, or lethal transient ischemia have not been fully investigated yet. We chose 5, 15 and 20 minutes of BCCAO to induce mild, severe and lethal ischemic injury, respectively, as previously reported (Park et al., 2013). The main objective of this study was to investigate the differences in degree of behavioral alterations, neuronal death/loss, and gliosis in the hippocampus CA1–3 region and dentate gyrus of gerbils subjected to 5, 15 and 20 minutes of BCCAO. Gerbils were chosen because they had been demonstrated as an excellent animal model of transient global cerebral ischemia (Li et al., 2011; Kim et al., 2017).
Materials and Methods
Experimental animals
One hundred and five 6-month-old male gerbils, weighing ~70 g were provided by the Experimental Animal Center of Kangwon National University located at Chuncheon, Republic of Korea. These animals were randomly divided into sham operated (n = 28), 5-minute BCCAO (n = 28), 15-minute BCCAO (n = 28), 20-minute BCCAO (n = 21) groups. They were housed with an optimum status under suitable temperature (about 23°C) and humidity (about 60%). Twelve hours of light and dark cycle was controlled. They were provided free access to water and feed. The experimental protocol was approved by Institutional Animal Care and Use Committee (AICUC) of Kangwon National University (approval No. KW-180124-1) on May 22, 2018. The study protocol adhered to guidelines from the current international laws and policies in “Guide for the Care and Use of Laboratory Animals” (The National Academies Press, 8th Ed., 2011).
BCCAO procedure
Surgical procedure of BCCAO was carried out as we previously described (Yoo et al., 2016; Park et al., 2017d). Briefly, using an inhaler, gerbils were anesthetized with a mixture of 2.5% isoflurane (Hana Pharmaceutical Co., Ltd., Seoul, Republic of Korea) in 33% oxygen and 67% nitrous oxide (Carpenter, 2013). Common carotid arteries (CCA) were exposed and ligated with aneurysm clips (0.69 N; Yasargil FE 723K; Aesculap, Tuttlingen, Germany) for 5, 15, or 20 minutes. Thereafter, these clips were removed for reperfusion. Complete occlusion and reperfusion of the CCA was confirmed by interruption of blood flow in the central retinal artery using an ophthalmoscope (HEINE K180®, Heine Optotechnik, Herrsching, Germany). Normal rectal temperature (37 ± 0.5°C) was controlled during the surgery. Temperature was monitored using a rectal temperature probe (TR-100; Fine Science Tools Inc., Foster City, CA, USA). Sham operated animals underwent the same surgical procedure without BCCAO. After the surgical procedure, to maintain body temperature, animals were kept at 23°C with 60% humidity in a thermal incubator (Mirae Medical Industry, Seoul, Republic of Korea) until they were euthanized.
Determination of spontaneous motor activity
To elucidate hyperactivity induced by BCCAO, spontaneous motor activity (SMA) was measured according to a published study (Yan et al., 2011) at 1 day after BCCAO operartion because SMA peaked at 1 day after transient ischemia/reperfusion. Briefly, each gerbil was placed in a Plexiglas cage (25 cm × 20 cm × 12 cm) located inside a soundproof chamber (Kinder Scientific, Poway, CA, USA). SMA was recorded using a Photobeam Activity System-Home Cage (San Diego Instruments, San Diego, CA, USA). Each gerbil was examined continuously via a 4 × 8 photobeam configuration. Score for each gerbil was generated under live observation. Video sequences were used for subsequent analysis.
Tissue preparation for histological examination
Tissue preparation was done at sham, 1, 2 and 5 days after ischemia/reperfusion according to our previous study (Lee et al., 2016a). Briefly, the ischemic gerbils (n = 7 at each time point after ischemia/reperfusion) were anesthetized by intraperitoneal injection of pentobarbital sodium (70 mg/kg, JW Pharm. Co., Ltd., Seoul, Republic of Korea) (Carpenter, 2013) and perfused via the ascending aorta with 0.1 M phosphate-buffered saline (PBS; pH 7.4) followed by fixation with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed after fixation and post-fixed in the same fixative for 7 hours. The fixed brains were infiltrated with 30% sucrose solution (in 0.1 M phosphate buffer, pH 7.4) for 12 hours for cryoprotection. Finally, these brains were coronally and serially sectioned (25 μm in thickness) in a cryostat (Leica, Nussloch, Germany).
Cresyl violet staining
To observe cellular distribution and change in the hippocampus following BCCAO, Cresyl violet (CV) staining was done according to a published method (Park et al., 2015). Briefly, sections were mounted onto microscopy slides coated with gelatin. These sections were stained with 1.0% (w/v) CV acetate (Sigma, St. Louis, MO, USA) and subsequently incubated in serial ethanol bath for dehydration. Finally, cover glasses were mounted onto these stained sections with Canada balsam (Kanto, Tokyo, Japan).
Fluoro Jade B histofluorescence
Fluoro Jade B (FJB), a high affinity fluorescent marker for degenerative neuron, histofluorescence staining was carried out to investigate neuronal death/degeneration after BCCAO as described previously (Ahn et al., 2016a). Briefly, brain sections were immersed in a solution of 0.06% potassium permanganate. Subsequently, these sections were incubated in a solution of 0.0004% FJB (Histochem, Jefferson, AR, USA). These stained sections were dehydrated and mounted by cover glasses using dibutylphthalate polystyrene xylene (DPX, Sigma, St. Louis, MO, USA) as a mounting medium. For observation, we used an epifluorescent microscope (Carl Zeiss, Oberkochen, Germany) equipped with 450–490 nm blue excitation light.
Immunohistochemistry
Neuronal damage/death and gliosis after BCCAO operation were examined by immunohistochemistry according to a published protocol (Park et al., 2015). Briefly, sections were exposed to 0.3% H2O2 (in 0.01 M PBS, pH 7.4) for 25 minutes and then treated with 10% normal horse or goat serum (in 0.01 M PBS, pH 7.4) for 40 minutes at room temperature. Subsequently, they were incubated for 24 hours at room temperature with primary antibodies: mouse anti-NeuN (1:1100; Chemicon, Temecula, CA, USA) for analyzing neuronal cells, mouse anti-glial fibrillary acidic protein (GFAP) (1:700; Chemicon) for measuring astrocytes, and rabbit anti-Iba1 (1:700, Wako, Osaka, Japan) for examining microglia. These incubated sections were reacted with biotinylated horse anti-mouse IgG or goat anti-rabbit IgG (1:250, Vector, Torrance, CA, USA) as a secondary antibody and exposed to avidin-biotin complex (1:300, Vector). Finally, these immunoreacted sections were visualized by reacting with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) in 0.1 M PBS (pH7.4).
Data analysis
Data were analyzed as described previously (Park et al., 2017c). For quantitative analyses of NeuN-immunoreactive (ir) neurons, FJB-positive cells, GFAP-ir astrocytes and Iba1-ir microglia, we selected seven sections/gerbil (a total of 49 sections) and took digital images of each immunostained structure in hippocampal sub-regions using an optical (BX53, Olympus, Hamburg, Germany) or epifluorescent microscope (Carl Zeiss, Göttingen, Germany) equipped with a camera (Olympus). We carried out cell counting in a 250 μm × 250 μm area of each hippocampal subregion using an Optimas 6.5 software (CyberMetrics, Scottsdale, AZ, USA) for image analysis.
To quantitatively analyze GFAP and Iba1 immunoreactivity, we captured digital images of each immunoreactivity in a tissue area of 140 μm × 140 μm and calibrated the image into an array of 512 × 512 pixels. We measured the immunoreactivity of each abovementioned index by 0 to 255 gray-scale system. The ratio of relative optical density (ROD) of each immunoreactivity was calibrated as percentage using Adobe Photoshop (version 8.0, Adobe, San Jose, CA, USA). Finally, we analyzed RODs using NIH Image software version 1.59 (National Institutes of Health, Bethesda, MD, USA). A ratio of the ROD was calibrated as percentage of the sham operated group, which was designated as 100%.
Statistical analysis
Data shown in this study represent the mean ± standard error of the mean (SEM) among experimental groups. They were statistically analyzed using SPSS 18.0 (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) with a post hoc Bonferroni’s multiple comparison test was done to determine differences among groups. Statistical significance was considered at P < 0.05.
Results
SMA
SMA was determined at 1 day after BCCAO operation (Figure 1). Seven animals were applied in each group. SMA was significantly increased in the 5-minute BCCAO group compared with the sham group (P < 0.05). SMA was significantly increased in the 15-minute BCCAO group than in the 5-minute BCCAO group (P < 0.05). In the 20-minute BCCAO group, we did not obtain any data because these animals died.
Figure 1.
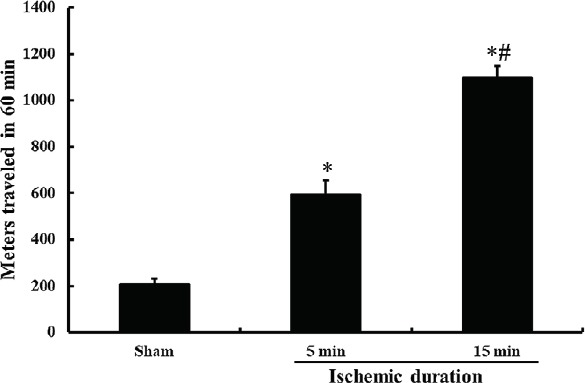
SMA of gerbils subjected to 5- and 15-min BCCAO at 1 day after BCCAO.
*P < 0.05, vs. sham operated (sham) group, #P < 0.05, vs. 5-min BCCAO group. The bars indicate the mean ± SEM (n = 7/group). SMA: Spontaneous motor activity; BCCAO: bilateral common carotid artery occlusion; min: minute.
CV-stained cells
As shown in Figure 2A, in the sham operated group, CV-stained cells were easily identified in all sub-regions of hippocampal formation (hippocampal CA1–3 region and dentate gyrus) (Figure 2Aa). CV-stained cells were typically distributed in the pyramidal layer in hippocampal CA1–3 regions (Figure 2Ab and Ac) and in the granule cell layer in the dentate gyrus (Figure 2Ad).
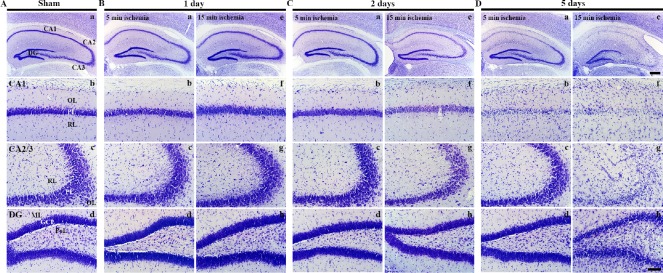
Figure 2.
CV staining in the hippocampal formation of gerbils at different time after 5- and 15-min BCCAO.
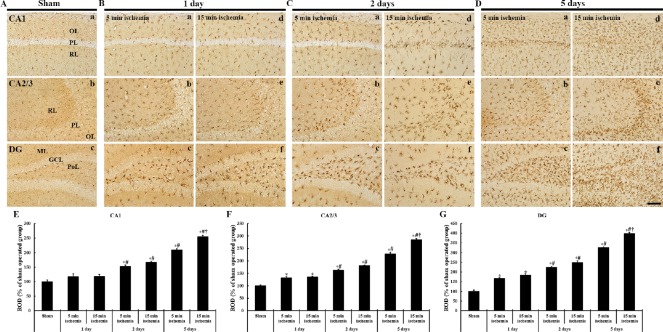
(A) Sham operation; (B) 1 day after BCCAO, (C) 2 days after BCCAO, (D) 5 days after BCCAO. CV-stained cells are not changed in all subregions in the hippocampus at 1 day after BCCAO. At 2 days after BCCAO, CV staining is markedly weakened in the PL of the CA1 region of gerbils from the 15-min BCCCAO group. Five days after BCCAO, CV-stained cells are decreased in number in the PL of the CA1 region of gerbils from the 5-min BCCAO group. The numbers of CV-stained cells in the 15-min BCCAO group were reduced in the PL of the CA1–3 regions. In the DG, changes in the number of CV-stained cells are detected only in the 15-min BCCAO group. Scale bars: 400 µm (Aa, Ba, Be, Ca, Ce, Da, De) and 100 µm (Ab–Ad, Bb–Bd, Bf–Bh, Cb–Cd, Cf–Ch, Db–Dd, Df–Dh). CV: Cresyl violet; BCCAO: bilateral common carotid artery occlusion; PL: pyramidal layer; DG: dentate gyrus; GCL: granule cell layer; ML: molecular layer; OL: oriens layer; PoL: polymorphic layer; RL: radiant layer; min: minute.
One day after BCCAO operation, the distribution of CV-stained cells in BCCAO groups was not altered compared to the sham operated group (Figure 2B).
Two days after BCCAO, the distribution of CV-stained cells in the 5-minute BCCAO group was not changed (Figure Figure 2Ca–Cd). CV staining in the 15-minute BCCAO group was weakened in hippocampal CA1–3 regions while CV staining in the dentate gyrus was not changed compared to that in the sham operated group (Figure 2Cd).
Five days after BCCAO, CV-stained cells in the 5-minute BCCAO group were rarely observed in the hippocampal CA1 pyramidal layer. However, the distribution of CV-stained cells in the hippocampal CA2–3 region and dentate gyrus was similar to that in the sham operated group (Figure 2Da–Dd). In the 15-minute BCCAO group, CV-stained cells were rarely detected in the hippocampal CA1–3 pyramidal layer. They were significantly decreased in the dentate granule cell layer (Figure 2De–Dh).
FJB-positive cells
As shown in Figure 3, FJB-positive cells in the sham operated group were not observed in any sub-regions of the gerbil hippocampal formation (Figure 3A).
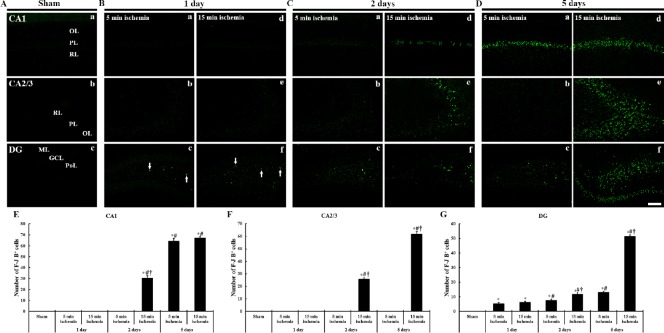
Figure 3.
FJB histofluorescence in the hippocampal formation of gerbils at different time after 5- and 15-min BCCAO.
(A) Sham operation; (B) 1 day after BCCAO, (C) 2 days after BCCAO, (D) 5 days after BCCAO. Degenerative neurons are positive to FJB and marked in green fluorescence. No FJB-positive cells are shown in the sham operated group. One day after BCCAO, FJB-positive cells (arrows) are detected in the dentate PoL. Two days after BCCAO, FJB-positive cells are additionally detected in the CA1 PL of gerbils from the 15-min BCCAO group. Five days after BCCAO, FJB-positive cells in the 5-min BCCAO group are significantly increased in the CA1 PL, and FJB-positive cells in the 15-min BCCAO group are significantly increased in the CA1 PL, dentate PoL and GCL. Scale bar: 100 µm. (E–G) The mean number of FJB- positive cells in the CA1 (E), CA2/3 (F) and DG (G) (n = 7 at each time after BCCAO; *P < 0.05, vs. the sham operated group, #P < 0.05, vs. the prior time point of corresponding BCCAO group, †P < 0.05, vs. the same time point of 5-min BCCAO group). The bars indicate the mean ± SEM. FJB: Fluoro Jade B; BCCAO: bilateral common carotid artery occlusion; OL: oriens layer; PL: pyramidal layer; PoL: polymorphic layer; RL: radiant layer; DG: dentate gyrus; GCL: granule cell layer; ML: molecular layer; min: minute.
One day after BCCAO, FJB-positive cells were not found in the hippocampal CA1–3 regions in both 5-minute and 15-minute BCCAO groups. However, a few FJB-positive cells were found in the dentate polymorphic layer (Figure 3Bc, Bf and G).
Two days after BCCAO, in the 5-minute BCCAO group, FJB-positive cells were slightly increased in the dentate polymorphic layer (Figure 4Cc and G). In the 15-minute BCCAO group, many FJB-positive cells were found in the hippocampal CA1 pyramidal layer, and the numbers of FJB-positive cells were increased in the dentate polymorphic layer compared with the 5-minute BCCAO group (Figure 3Cc, Cd–Cf and E–G).
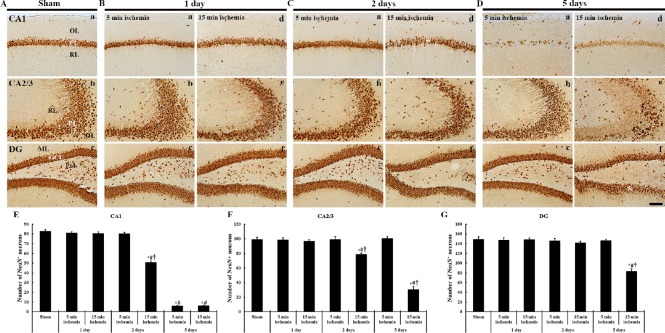
Figure 4.
Immunohistochemistry (brown color) for NeuN in the hippocampal formation of gerbils at different time after 5- and 15-min BCCAO.
(A) Sham operation; (B) 1 day after BCCAO, (C) 2 days after BCCAO, (D) 5 days after BCCAO. At 1 day after BCCAO, NeuN-ir neurons are not altered in all subregions. At 2 days after BCCAO, NeuN-ir neurons are decreased in the pyramidal layer (PL, asterisk) of the CA1 region alone. At 5 days after BCCAO, NeuN-ir neurons are rarely shown in the CA1 PL of gerbils from both 5- and 15-min BCCAO groups. In the 15-min BCCAO group, NeuN-ir are significantly decreased in number in the CA2/3 PL, dentate polymorphic layer (PoL) and lower granule cell layer (GCL, asterisk). Scale bar: 100 µm. (E–G) The mean number of NeuN-ir neurons in the CA1 (E), CA2/3 (F) and dentate gyrus (G) (n = 7 at each time after BCCAO; *P < 0.05, vs. the sham operated group, #P < 0.05, vs. the prior time point of corresponding BCCAO group, †P < 0.05, vs. the same time point of 5 min-BCCAO group). The bars indicate the mean ± SEM. BCCAO: Bilateral common carotid artery occlusion; OL: oriens layer; PL: polymorphic layer; RL: radiant layer; DG: dentate gyrus; GCL: granule cell layer; ML: molecular layer; min: minute.
Five days after BCCAO, in the 5-minute BCCAO group, FJB-positive cells were increased in the dentate polymorphic layer (Figure 3Dc and G) and many FJB-positive cells were found in the hippocampal CA1 pyramidal layer alone (Figure 3Da and E). In the 15-minute BCCAO group, the numbers of FJB positive cells were significantly increased in all hippocampal CA regions (Figures 3Dd, De and E–4G). In addition, FJB-positive cells in the dentate polymorphic layer were significantly increased in number, and many FJB-positive cells were shown in the lower blade of the granule cell layer (Figure 3Df and G).
NeuN-ir neurons
NeuN-ir neurons, in sham operated gerbils, were easily observed in all sub-regions of the hippocampal formation. Hippocampal CA1–3 pyramidal cells, dentate granule cells and polymorphic cells were all immunoreactive for NeuN (Figure 4A).
One day after BCCAO, NeuN-ir neurons in the gerbils from 5- or 15-minute BCCAO groups were not different from those identified in the sham operated group (Figure 4B).
Two days after BCCAO, in the 5-minute BCCAO group, the numbers of NeuN-ir neurons in all hippocampal sub-regions were similar to those in the sham operated group (Figure 4Ca–Cc). However, in the 15-minute BCCAO group, the numbers of NeuN-ir neurons were significantly decreased in the hippocampal CA1–3 regions compared with the 5-minute BCCAO group, although no significant difference in such number was found in the dentate gyrus (Figure 4Cd–Cf and E–G).
Five days after BCCAO, NeuN-ir neurons in the 5-minute BCCAO group were rarely detected in the hippocampal CA1 region. The number of NeuN-ir neurons was not altered in the hippocampal CA2–3 regions or dentate gyrus (Figure 4Da–Dc and E–G). In the 15-minute BCCAO group, the numbers of NeuN-ir neurons were significantly reduced in all hippocampal subregions compared to those in the sham operated group. Especially, NeuN-ir neurons in the dentate granule cells layer were significantly reduced in the lower blade of the granule cell layer (Figure 4A, D and E–G).
GFAP-ir astrocytes
In the sham operated group, GFAP-ir astrocytes were in the resting form and distributed in all layers of all subregions of the gerbil hippocampal formation (Figure 5A).
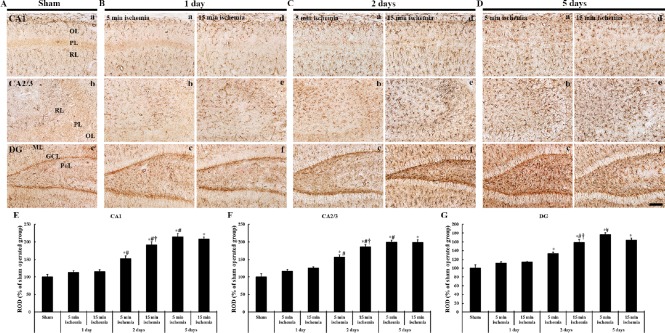
Figure 5.
Immunohistochemistry (brown color) for GFAP in the hippocampal formation of gerbils at different time after 5- and 15-min BCCAO.
(A) Sham operation; (B) 1 day after BCCAO, (C) 2 days after BCCAO, (D) 5 days after BCCAO. One day after BCCAO, the distribution of GFPA-ir astrocytes is not significantly altered in all subregions. At 2 days after BCCAO, GFAP-ir cells are significantly hypertrophied in all subregions. Five days after BCCAO, GFAP-ir cells are significantly hypertrophied in all subregions of gerbils from the 5- and 10-min BCCAO groups. Scale bar: 100 µm. (E–G) ROD of GFAP-ir cells in the CA1 (E), CA2/3 (F) and DG (G) (n = 7 at each time after BCCAO; *P < 0.05, vs. the sham operated group, #P < 0.05, vs. the prior time point of corresponding BCCAO group, †P < 0.05, vs. the same time point of 5 min-BCCAO group). The bars indicate the means ± SEM. GFAP: Glial fibrillary acidic protein; ir: immunoreactive; BCCAO: bilateral common carotid artery occlusion; OL: oriens layer; PL: polymorphic layer; RL: radiant layer; DG: dentate gyrus; GCL: granule cell layer; ML: molecular layer; PoL: polymorphic layer; min: minute.
One day after BCCAO, GFAP immunoreactivity in 5- and 15-minute BCCAO groups was slightly increased. They showed bulky cytoplasm (hypertrophic state) in all subregions (Figure 5B and E–G). In addition, the numbers of GFAP-ir astrocytes in both groups were significantly increased in all subregions (Table 1). In the hippocampal CA2–3 regions, the numbers of GFAP-ir astrocytes were significantly increased in the 15-minute group than in the 5-minute BCCAO group (Table 1).
Table 1.
The mean number of GFAP-ir astrocytes in the gerbil hippocampal formation of gerbils from the 5- and 15-minute BCCAO groups at sham, 1, 2 and 5 days after BCCAO
| Region | Sham | 1 day | 2 days | 5 days | |||
|---|---|---|---|---|---|---|---|
| 5-minute ischemia | 15-minute ischemia | 5-minute ischemia | 15-minute ischemia | 5-minute ischemia | 15-minute ischemia | ||
| CA1 | 43±2.9 | 81±3.1* | 84±3.4* | 107±2.0*# | 121±2.4*#† | 131±2.3*# | 126±1.8* |
| CA2/3 | 22±1.9 | 68±2.4* | 80±3.5*† | 95±3.1*# | 121±2.7*#† | 137±2.3*# | 134±3.5*# |
| DG | 48±2.5 | 73±3.7* | 75±3.2* | 88±3.5*# | 123± 4.0*#† | 130±3.4*# | 127±2.8* |
GFAP-ir astrocytes, expressed as the mean ± SEM, are counted in a 250 μm x 250 μm square in hippocampal subregions at sham, 1, 2 and 5 days after 5- and 15-minute BCCAO operation. n = 7 at each time point in each group. *P < 0.05, vs. sham operated group; #P < 0.05, vs. prior time point of the corresponding BCCAO group; †P < 0.05, vs. the same time point of the 5-minute BCCAO group. GFAP-ir: Glial fibrillary acidic protein-immunoreactive; BCCAO: bilateral common carotid artery occlusion; DG: dentate gyrus.
Two days after BCCAO, GFAP-ir astrocytes were significantly hypertrophied in all hippocampal subregions of the gerbils from the 15-minute BCCAO group. The ROD was 125.6% in the CA1 region, 119.6% in the CA2–3 regions, and 135.9% in the dentate gyrus of gerbils in the 15-minute BCCAO group compared to the 5-minute BVVAO group (Figure 5Ca–Cf and E–G). In addition, the numbers of GFAP-ir astrocytes in both groups were significantly increased in all hippocampal subregions compared to the pre-time point (Table 1). Furthermore, in the 15-minute BCCAO group, their numbers at each time point after BCCAO were significantly increased in all subregions compared to those in the 5-minute BCCAO group (Table 1).
In the 5-minute BCCAO group, GFAP immunoreactivity at 5 days after BCCAO was increased significantly compared to the prior time point (Figure 5C and D). However, GFAP immunoreactivity in the 15-minute BCCAO group was slightly lower than that in the 5-minute BCCAO group. The ROD of GFAP immunoreactivity in the 15-minute BCCAO group was 96.7% in the hippocampal CA1 region and 99.8% in the hippocampal CA2–3 regions of gerbils and it was 92.9% in the 5-minute BCCAO group (Figure 5Ca–Cf and E–G). In addition, the numbers of GFAP-ir astrocytes were significantly increased in the hippocampal CA1–3 regions and dentate gyrus of gerbils in the 5-minute BCCAO group compared with those at 2 days after BCCAO operation (Table 1).
Iba1-ir microglia
Iba1-ir microglia in the sham operated gerbils were in the resting form and evenly distributed throughout all layers in all hippocampal subregions (Figure 6A). One day after BCCAO, Iba1-ir microglia in both 5- and 15-minute BCCAO groups were slightly activated in all subregions compared to those in the sham operated gerbils (Figure 6B and E–G). Moreover, the numbers of Iba1-ir microglia in both 5- and 15-minute BCCAO groups were significantly increased in all hippocampal subregions (Table 2). In the 15-minute BCCAO group, the numbers of Iba1-ir microglia were more significantly increased in any subregion compared to those in the 5-minute BCCAO group (Table 2).
Figure 6.
Immunohistochemistry for Iba1 (brown color) in the hippocampal formation of gerbils at different time after 5- and 15-min BCCAO.
(A) Sham operation; (B) 1 day after BCCAO, (C) 2 days after BCCAO, (D) 5 days after BCCAO. At 1 day after BCCAO, Iba1-ir cells are hypertrophied in all subfields. At 2 and 5 days after BCCAO, Iba1-ir microglia are more hypertrophied in all subregions of both groups: in particular, they are much more hypertrophied in the 15-min BCCAO group than in the 5-min BCCAO group. (E–G) ROD of Iba1-ir cells in the CA1 (E), CA2/3 (F) and DG (G) (n = 7 at each time after BCCAO; *P < 0.05, vs. the sham operated group, #P < 0.05, vs. the prior time point of corresponding BCCAO group, †P < 0.05, vs. the same time point of 5 min BCCAO operated group). The bars indicate the mean ± SEM. BCCAO: Bilateral common carotid artery occlusion; ir: immunoreactive; OL; oriens layer; PL: polymorphic layer; RL: radiant layer; DG: dentate gyrus; GCL: granule cell layer; ML: molecular layer; PoL: polymorphic layer; ROD: relative optical density; min: minute.
Table 2.
The mean number of Iba1-ir microglia in the gerbil hippocampal formation of gerbils from the 5- and 15-minute BCCAO groups at sham, 1, 2 and 5 days after BCCAO
| Region | Sham | 1 day | 2 days | 5 days | |||
|---|---|---|---|---|---|---|---|
| 5-minute ischemia | 15-minute ischemia | 5-minute ischemia | 15-minute ischemia | 5-minute ischemia | 15-minute ischemia | ||
| CA1 | 33±3.7 | 54±2.7* | 59±3.5* | 68±3.1*# | 81±3.1*#† | 106±3.0*# | 123±3.3*#† |
| CA2/3 | 30±2.6 | 59±2.5* | 62±2.6* | 72±2.4*# | 78±2.8*# | 95±4.0*# | 103±6.1*#† |
| DG | 31±3.0 | 57±2.5* | 61±2.4* | 74±3.5*# | 74±3.1*# | 102±3.0*# | 118±1.3*#† |
Iba1-ir microglia, expressed as the mean ± SEM, are counted in a 250 μm x 250 μm square of the hippocampal subregions. n = 7 at each time point in each group. *P < 0.05, vs. the sham operated group, #P < 0.05, vs. the prior time point of corresponding BCCAO group; †P < 0.05, vs. the same time point of 5-minute BCCAO group. BCCAO: bilateral common carotid artery occlusion; ir: Immunoreactive; DG: dentate gyrus.
Two days after BCCAO, Iba1-ir microglia in the 15-minute BCCAO group were more activated compared to those in the 5-minute BCCAO group. The ROD of Iba1 immunoreactivity in the 15-minute BCCAO group was 109.0% in the hippocampal CA1 region, 111.9% in the hippocampal CA2–3 regions and 110.5% in the dentate gyrus compared to the 5-minute BCCAO group (Figure 6B, C and E–G). Furthermore, the numbers of Iba1-ir microglia in both 5- and 15-minute BCCAO groups were significantly increased in all hippocampal subregions compared to pre-time point after BCCAO (Table 2).
Iba1-ir microglia in both 5- and 15-minute BCCAO groups were more activated at 5 days after BCCAO than at 2 days after BCCAO (Figure 6D). At this time point after BCCAO, the ROD of Iba1 immunoreactivity in the 15-minute BCCAO group was 121.6% in the hippocampal CA1 region, 125.0% in the hippocampal CA2–3 regions, and 121.9% in the dentate gyrus compared to the 5-minute BCCAO group (Figure 6E–G). In addition, the numbers of Iba1-ir microglia in both 5- and 15-minute BCCAO groups were significantly increased in all hippocampal subregions compared to those at 2 days after BCCAO (Table 2).
Discussion
Mongolian gerbil has a unique hallmark in arteries of the cerebrovascular system. In the base of the brain, the Willis’ circle frequently has no posterior communicating arteries (Kuchinka et al., 2008). Therefore, only ligation of bilateral common carotid arteries in the gerbil can lead to ischemia in the forebrain which is supplied by common carotid arteries (Martinez et al., 2012). Based on this characteristic, gerbils have widely been used to study ischemic damage and related mechanisms (Candelario-Jalil et al., 2001; Yan et al., 2012a; Park et al., 2014, 2017a; Ahn et al., 2016b; Lee et al., 2016b). In particular, neuronal loss or death occurs selectively in vulnerable gerbil brain regions, such as the striatum, cerebral cortex and hippocampal formation, by transient ligation of bilateral CCA. In the case of transient ischemia for 5 minutes, hippocampal CA1 pyramidal neurons will die at 4–5 days after 5 minutes of transient ischemia (Kirino, 1982; Nakayama et al., 2013; Park et al., 2017b).
In this study, we modulated durations of transient ischemia to be 5, 15 and 20 minutes to compare the effects on locomotor activity, neuronal death, and glial activation among the three groups through SMA test, CV staining, immunohistochemistry for NeuN, FJB histofluorescence and immunohistochemistry for GFAP and Iba1. Above all, in this study, we found that animals died in at least 2 days after 20 minutes of BCCAO. This finding indicates that transient ischemia for 20 minutes in the forebrain or telencephalon is lethal in gerbils.
The SMA test has been considered as a useful behavioral estimation after transient global cerebral ischemia. Many researches have reported significant hyperactivity after BCCAO operation in gerbils (Janac et al., 2006; Park et al., 2017b). In the present study, SMA was evaluated at 1 day after BCCAO operation. It was found that SMA was significantly increased in gerbils subjected to 15-minute BCCAO.
The present study revealed that pyramidal neurons of CA1–3 regions showed NeuN immunoreactivity. In addition, NeuN immunoreactivity in the pyramidal neurons of gerbils in the 15-minute BCCAO group was significantly weaker than that in the 5-minute BCCAO group at 5 days after BCCAO. Such a phenomenon might be related to the severe neuronal damage induced by longer ischemia duration. Previous studies have reported that hyperthermia can evoke severer damage of pyramidal neurons after 5 minutes of BCCAO in gerbils (Noor et al., 2003; Kim et al., 2015). NeuN immunoreactivity reported in the previous studies was similar to our present result. In this study, 15 minutes of BCCAO operation led to loss of pyramidal neurons in the hippocampal CA1–3 regions at 2 days after BCCAO. This study is the first to use FJB histofluorescence staining. It has been reported that pyramidal cells of the CA1 will die at 5 days after 5 minutes of BCCAO. However, at this time point after BCCAO, pyramidal neurons of CA2–3 regions were not lost (Cho et al., 2013). This finding indicates that 15 minutes of BCCAO is severe enough to evoke pyramidal cell death in all hippocampal CA regions. The above-mentioned studies and our present study indicate that 15 minutes of transient ischemia in the forebrain under hyperthermia could evoke much severer damage or death compared to that under normothermic condition.
On the other hand, neuronal death or loss in the dentate gyrus occurs in the polymorphic layer at about 6 hours after transient cerebral ischemia. These cells are considered to be very vulnerable to transient ischemic insults (Moon et al., 2009). In this respect, we have found that polymorphic cells of the dentate gyrus were lost from 1 day after 5 minutes of BCCAO (Ahn et al., 2016a). This is consistent with our present finding observed by FJB histofluorescence staining. Furthermore, for the first time, we found that granule cells of the dentate gyrus died at 5 days after 15 minutes of BCCAO, rather than after 5 minutes of BCCAO in this study.
Granule cells located in the dentate gyrus give mossy fibers to the CA3 region. They are very resistant against transient cerebral ischemia (Schmidt-Kastner and Freund, 1991; McAuliffe et al., 2011). It is well known that the subgranular zone, which is a narrow zone of the granule cell layer, offers neurogenesis for granule cells in lifespan (Kempermann et al., 2015). To study neurogenesis of granule cells in the dentate gyrus, researchers have applied adrenalectomy to induce death of granule cells (Brunson et al., 2005; Spanswick et al., 2011). However, it is very hard to study neurogenesis because animals subjected to adrenalectomy cannot survive for long time. In this regard, our present findings might be a tool to study the mechanisms of neurogenesis in the dentate gyrus (Brunson et al., 2005). Based on this finding, gerbils subjected to 15-minute BCCAO might be used as a tool to study neurogenesis in the dentate gyrus, since granule cell loss is induced in the 15-minute BCCAO group.
Several studies have demonstrated that brain ischemia leads to excessive activation of glial cells (astrocytes and microglia) (Ordy et al., 1993; Ahn et al., 2016a; Park et al., 2018). This phenomenon is termed gliosis. It is accompanied by neuroinflammatory responses through secretion of various inflammatory factors induced by ischemic insults (Lambertsen et al., 2004; Yoo et al., 2011). Many studies have reported that neuroprotective materials against brain ischemic insults can attenuate gliosis as a yardstick of neuroprotection against ischemic insults (Melani et al., 2014; Park et al., 2015, 2017d).
In this study, we compared patterns of astrocyte and microglial activations among the three ischemia groups. Results from the 5-minute BCCAO group were consistent with the findings in the hippocampal CA1 region from a precedent study (Park et al., 2017d). That is to say, with time after 5 minutes of ischemia, astrocytes (GFAP-ir cells) and microglia (Iba1-ir cells) were increased in numbers. They became hypertrophied in the CA1 region, suggesting that gliosis was significantly weak in other subregions.
Results of this study revealed that in the 15-minute BCCAO group, GFAP-ir astrocytes were hypertrophied earlier, and their GFAP immunoreactivity was slightly reduced at 5 days after BCCAO, compared to the 5-minute BCCAO group. Sugawara et al. (2002) have reported that GFAP immunoreactivity in astrocytes is decreased after onset of neuronal death induced by transient ischemia. In this respect, our results suggest that earlier neuronal death in the 15-minute BCCAO group might have occurred at 2 days after BCCAO. Such death might have caused a decrease in GFAP immunoreactivity at 5 days after 15-minute BCCAO.
Microglial cells participate in the clearance of dead cells and tissue debris after ischemic insults (Schilling et al., 2005). In the present study, we found that pyramidal cells of the CA1–3 regions in the gerbils from the 15-minute BCCAO group died at 2 days after BCCAO and granule cells of the dentate gyrus died 5 days after BCCAO. Together with neuronal loss, many Iba1-ir microglia became hypertrophied significantly earlier in all hippocampal subregions of gerbils in the 15-minute BCCAO group than in the 5-minute BCCAO group. In particular, many hypertrophied microglia in the 15-minute BCCAO group accumulated in the pyramidal layer of CA1–3 regions at earlier time points compared to those in the 5-minute BCCAO group. When neuronal death occurred, many hypertrophied microglia accumulated in the pyramidal layer or granule layer to remove tissue debris. Furthermore, congregation of microglia in those layers occurred earlier in the 15-minute BCCAO group than in the 5-minute BCCAO group. This finding indicates that, to remove tissue debris, microglia in the 15-minute BCCAO group became hypertrophied and proliferated more rapidly than those in the 5-minute BCCAO group.
Our results focused on the behavioral and histological analysis and showed the differences among mild, severe or lethal transient global cerebral ischemia. However, these results are difficult to reveal molecular mechanisms underlying abovementioned differences. Therefore, in-depth molecular mechanism studies about differences among mild, severe or lethal transient cerebral ischemia are needed.
In summary, this study showed that 15 minutes of BCCAO evoked earlier neuronal death/loss in hippocampal subregions than 5 minutes of BCCAO. Together with neuronal death/loss, astrocytes and microglia were markedly proliferated and hypertrophied in hippocampal regions after 15 minutes of BCCAO compared to those after 5 minutes of BCCAO. Furthermore, gerbils died in at least 2 days after 20 minutes of BCCAO. These findings imply that we must consider the effect of different ischemic durations when we treat transient brain ischemia injury.
Additional file: Open peer review reports 1 (75.1KB, pdf) and 2 (109KB, pdf) .
Footnotes
Conflicts of interest: The authors have declared that there is no conflict of interest.
Financial support: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B01011790; to JHC); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1A2B4009079; to MHW), and by Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01329401; to MHW) Rural Development Administration, Republic of Korea. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: The experimental protocol was approved by Institutional Animal Care and Use Committee (AICUC) of Kangwon National University (approval No. KW-180124-1) on May 22, 2018. The study protocol adhered to guidelines from the current international laws and policies in “Guide for the Care and Use of Laboratory Animals” (The National Academies Press, 8th Ed., 2011).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Eduardo Puelles, Institute of Neurosciences, UMH-CSIC, Spain; Angélica Zepeda, UNAM, Mexico.
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B01011790; to JHC); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1A2B4009079; to MHW), and by Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01329401; to MHW) Rural Development Administration, Republic of Korea.
P-Reviewers: Puelles E, Zepeda A; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Liu XL
References
- 1.Ahn JH, Shin BN, Park JH, Kim IH, Cho JH, Chen B, Lee TK, Tae HJ, Lee JC, Kang IJ, Kim YM, Lee YL, Won MH, Seo JY. Long-term observation of neuronal degeneration and microgliosis in the gerbil dentate gyrus after transient cerebral ischemia. J Neurol Sci. 2016a;363:21–26. doi: 10.1016/j.jns.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Ahn JH, Chen BH, Shin BN, Cho JH, Kim IH, Park JH, Lee JC, Tae HJ, Lee YL, Lee J, Byun K, Jeong GB, Lee B, Kim SU, Kim YM, Won MH, Choi SY. Intravenously infused F3. Olig2 improves memory deficits via restoring myelination in the aged hippocampus following experimental ischemic stroke. Cell Transplant. 2016b;25:2129–2144. doi: 10.3727/096368916X692230. [DOI] [PubMed] [Google Scholar]
- 3.Brunson KL, Baram TZ, Bender RA. Hippocampal neurogenesis is not enhanced by lifelong reduction of glucocorticoid levels. Hippocampus. 2005;15:491–501. doi: 10.1002/hipo.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candelario-Jalil E, Mhadu NH, Al-Dalain SM, Martinez G, Leon OS. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res. 2001;41:233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JW. Exotic animal formulary (ED 4) J Exotic Pet Med. 2013;22:308–309. [Google Scholar]
- 7.Cho JH, Yan BC, Lee YJ, Park JH, Ahn JH, Kim IH, Lee JC, Kim YM, Lee B, Won MH. Reduced beta-catenin expression in the hippocampal CA1 region following transient cerebral ischemia in the gerbil. Neurochem Res. 2013;38:1045–1054. doi: 10.1007/s11064-013-1015-2. [DOI] [PubMed] [Google Scholar]
- 8.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figley CR, Stroman PW. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci. 2011;33:577–588. doi: 10.1111/j.1460-9568.2010.07584.x. [DOI] [PubMed] [Google Scholar]
- 10.Gulke E, Gelderblom M, Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 2018;11:1756286418774254. doi: 10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horner PJ, Palmer TD. New roles for astrocytes: the nightlife of an ‘astrocyte’. La vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Janac B, Radenovic L, Selakovic V, Prolic Z. Time course of motor behavior changes in Mongolian gerbils submitted to different durations of cerebral ischemia. Behav Brain Res. 2006;175:362–373. doi: 10.1016/j.bbr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim IH, Lee TK, Cho JH, Lee JC, Park JH, Ahn JH, Shin BN, Chen BH, Tae HJ, Kim YH, Kim JD, Kim YM, Won MH, Kang IJ. Pretreatment with Chrysanthemum indicum Linne extract protects pyramidal neurons from transient cerebral ischemia via increasing antioxidants in the gerbil hippocampal CA1 region. Mol Med Rep. 2017;16:133–142. doi: 10.3892/mmr.2017.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MJ, Cho JH, Park JH, Ahn JH, Tae HJ, Cho GS, Yan BC, Hwang IK, Lee CH, Bae EJ, Won MH, Lee JC. Impact of hyperthermia before and during ischemia-reperfusion on neuronal damage and gliosis in the gerbil hippocampus induced by transient cerebral ischemia. J Neurol Sci. 2015;348:101–110. doi: 10.1016/j.jns.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 17.Kirino T. Delayed neuronal death. Neuropathology. 2000;20 Suppl:S95–97. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- 19.Kuchinka J, Nowak E, Szczurkowski A, Kuder T. Arteries supplying the base of the brain in the Mongolian gerbil (Meriones unguiculatus) Pol J Vet Sci. 2008;11:295–299. [PubMed] [Google Scholar]
- 20.Lambertsen KL, Gregersen R, Meldgaard M, Clausen BH, Heibol EK, Ladeby R, Knudsen J, Frandsen A, Owens T, Finsen B. A role for interferon-gamma in focal cerebral ischemia in mice. J Neuropathol Exp Neurol. 2004;63:942–955. doi: 10.1093/jnen/63.9.942. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Park JH, Ahn JH, Kim IH, Cho JH, Choi JH, Yoo KY, Lee CH, Hwang IK, Kwon YG, Kim YM, Kang IJ, Won MH. New GABAergic neurogenesis in the hippocampal CA1 region of a gerbil model of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016a;26:581–592. doi: 10.1111/bpa.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TK, Park JH, Ahn JH, Shin MC, Cho JH, Bae EJ, Kim YM, Won MH, Lee CH. Pretreated duloxetine protects hippocampal CA1 pyramidal neurons from ischemia-reperfusion injury through decreases of glial activation and oxidative stress. J Neurol Sci. 2016b;370:229–236. doi: 10.1016/j.jns.2016.09.059. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Park JH, Yan B, Yoo KY, Lee CH, Choi JH, Hwang IK, Won MH. Neuroprotection of alpinia katsumadai seed extract against neuronal damage in the ischemic gerbil hippocampus is linked to altered brain-derived neurotrophic factor. Lab Anim Res. 2011;27:67–71. doi: 10.5625/lar.2011.27.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez NS, Machado JM, Perez-Saad H, Coro-Antich RM, Berlanga-Acosta JA, Salgueiro SR, Illera GG, Alba JS, del Barco DG. Global brain ischemia in Mongolian gerbils: assessing the level of anastomosis in the cerebral circle of Willis. Acta Neurobiol Exp (Wars) 2012;72:377–384. doi: 10.55782/ane-2012-1909. [DOI] [PubMed] [Google Scholar]
- 25.McAuliffe JJ, Bronson SL, Hester MS, Murphy BL, Dahlquist-Topala R, Richards DA, Danzer SC. Altered patterning of dentate granule cell mossy fiber inputs onto CA3 pyramidal cells in limbic epilepsy. Hippocampus. 2011;21:93–107. doi: 10.1002/hipo.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melani A, Corti F, Cellai L, Vannucchi MG, Pedata F. Low doses of the selective adenosine A2A receptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res. 2014;1551:59–72. doi: 10.1016/j.brainres.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Moon JB, Lee CH, Park CW, Cho JH, Hwang IK, Yoo KY, Choi JH, Shin HC, Won MH. Neuronal degeneration and microglial activation in the ischemic dentate gyrus of the gerbil. J Vet Med Sci. 2009;71:1381–1386. doi: 10.1292/jvms.001381. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama S, Vest R, Traystman RJ, Herson PS. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J Mol Neurosci. 2013;51:92–98. doi: 10.1007/s12031-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noor R, Wang CX, Shuaib A. Effects of hyperthermia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett. 2003;349:130–132. doi: 10.1016/s0304-3940(03)00802-4. [DOI] [PubMed] [Google Scholar]
- 30.Ohk TG, Yoo KY, Park SM, Shin BN, Kim IH, Park JH, Ahn HC, Lee YJ, Kim MJ, Kim TY, Won MH, Cho JH. Neuronal damage using fluoro-jade B histofluorescence and gliosis in the striatum after various durations of transient cerebral ischemia in gerbils. Neurochem Res. 2012;37:826–834. doi: 10.1007/s11064-011-0678-9. [DOI] [PubMed] [Google Scholar]
- 31.Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol. 1993;119:128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- 32.Park CW, Lee JC, Ahn JH, Lee DH, Cho GS, Yan BC, Park JH, Kim IH, Lee HY, Won MH, Cho JH. Neuronal damage using fluoro-Jade B histofluorescence and gliosis in the gerbil septum submitted to various durations of cerebral ischemia. Cell Mol Neurobiol. 2013;33:991–1001. doi: 10.1007/s10571-013-9967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CW, Lee TK, Cho JH, Kim IH, Lee JC, Shin BN, Ahn JH, Kim SK, Shin MC, Ohk TG, Won MH, Lee YJ, Seo JY, Park JH. Rufinamide pretreatment attenuates ischemia-reperfusion injury in the gerbil hippocampus. Neurol Res. 2017a;39:941–952. doi: 10.1080/01616412.2017.1362189. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Kim YH, Ahn JH, Choi SY, Hong S, Kim SK, Kang IJ, Kim YM, Lee TK, Won MH, Lee CH. Atomoxetine protects against NMDA receptor-mediated hippocampal neuronal death following transient global cerebral ischemia. Curr Neurovasc Res. 2017b;14:158–168. doi: 10.2174/1567202614666170328094042. [DOI] [PubMed] [Google Scholar]
- 35.Park JH, Park OK, Yan B, Ahn JH, Kim IH, Lee JC, Kwon SH, Yoo KY, Lee CH, Hwang IK, Choi JH, Won MH, Kim JD. Neuroprotection via maintenance or increase of antioxidants and neurotrophic factors in ischemic gerbil hippocampus treated with tanshinone I. Chin Med J (Engl) 2014;127:3396–3405. [PubMed] [Google Scholar]
- 36.Park JH, Shin BN, Chen BH, Kim IH, Ahn JH, Cho JH, Tae HJ, Lee JC, Lee CH, Kim YM, Lee YL, Kim SK, Won MH. Neuroprotection and reduced gliosis by atomoxetine pretreatment in a gerbil model of transient cerebral ischemia. J Neurol Sci. 2015;359:373–380. doi: 10.1016/j.jns.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Cho JH, Ahn JH, Choi SY, Lee TK, Lee JC, Shin BN, Hong S, Jeon YH, Kim YM, Hwang IK, Lee YJ, Won MH, Kang IJ. Neuronal loss and gliosis in the rat striatum subjected to 15 and 30 minutes of middle cerebral artery occlusion. Metab Brain Dis. 2018;33:775–784. doi: 10.1007/s11011-018-0192-8. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Lee TK, Ahn JH, Shin BN, Cho JH, Kim IH, Lee JC, Kim JD, Lee YJ, Kang IJ, Hong S, Kim YH, Jeon YH, Lee YL, Won MH. Pre-treated Populus tomentiglandulosa extract inhibits neuronal loss and alleviates gliosis in the gerbil hippocampal CA1 area induced by transient global cerebral ischemia. Anat Cell Biol. 2017c;50:284–292. doi: 10.5115/acb.2017.50.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JH, Park CW, Ahn JH, Choi SY, Shin MC, Cho JH, Lee TK, Kim IH, Lee JC, Kim YH, Kim YM, Kim JD, Tae HJ, Shin BN, Bae EJ, Chen BH, Won MH, Kang IJ. Neuroprotection and reduced gliosis by pre- and post-treatments of hydroquinone in a gerbil model of transient cerebral ischemia. Chem Biol Interact. 2017d;278:230–238. doi: 10.1016/j.cbi.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 42.Spanswick SC, Epp JR, Sutherland RJ. Time-course of hippocampal granule cell degeneration and changes in adult neurogenesis after adrenalectomy in rats. Neuroscience. 2011;190:166–176. doi: 10.1016/j.neuroscience.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr Protein Pept Sci. 2013;14:16–20. doi: 10.2174/1389203711314010004. [DOI] [PubMed] [Google Scholar]
- 44.Swaminathan N. Brain-scan mystery solved. Sci Am Mind. 2008;19:7–7. [Google Scholar]
- 45.Yan BC, Choi JH, Yoo KY, Lee CH, Hwang IK, You SG, Kang IJ, Kim JD, Kim DJ, Kim YM, Won MH. Leptin’s neuroprotective action in experimental transient ischemic damage of the gerbil hippocampus is linked to altered leptin receptor immunoreactivity. J Neurol Sci. 2011;303:100–108. doi: 10.1016/j.jns.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Yan BC, Kim SK, Park JH, Ahn JH, Lee CH, Yoo KY, Choi JH, Lee DS, Kim MJ, Kim YM, Won MH. Comparison of inflammatory cytokines changes in the hippocampal CA1 region between the young and adult gerbil after transient cerebral ischemia. Brain Res. 2012a;1461:64–75. doi: 10.1016/j.brainres.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Yan BC, Park JH, Ahn JH, Choi JH, Yoo KY, Lee CH, Cho JH, Kim SK, Lee YL, Shin HC, Won MH. Comparison of glial activation in the hippocampal CA1 region between the young and adult gerbils after transient cerebral ischemia. Cell Mol Neurobiol. 2012b;32:1127–1138. doi: 10.1007/s10571-012-9837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo DY, Lee KY, Park JH, Jung HY, Kim JW, Yoon YS, Won MH, Choi JH, Hwang IK. Glucose metabolism and neurogenesis in the gerbil hippocampus after transient forebrain ischemia. Neural Regen Res. 2016;11:1254–1259. doi: 10.4103/1673-5374.189189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo KY, Yoo DY, Hwang IK, Park JH, Lee CH, Choi JH, Kwon SH, Her S, Lee YL, Won MH. Time-course alterations of Toll-like receptor 4 and NF-kappaB p65, and their co-expression in the gerbil hippocampal CA1 region after transient cerebral ischemia. Neurochem Res. 2011;36:2417–2426. doi: 10.1007/s11064-011-0569-0. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Park JH, Maharjan S, Park JA, Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, Cho JH, Lee IK, Lee CH, Hwang IK, Kim YM, Suh YG, Won MH, Kwon YG. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J Neuroinflammation. 2017;14:122. doi: 10.1186/s12974-017-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.