Abstract
In recent years, the use of Schwann cell transplantation to repair peripheral nerve injury has attracted much attention. Animal-based studies show that the transplantation of Schwann cells in combination with nerve scaffolds promotes the repair of injured peripheral nerves. Autologous Schwann cell transplantation in humans has been reported recently. This article reviews current methods for removing the extracellular matrix and analyzes its composition and function. The development and secretory products of Schwann cells are also reviewed. The methods for the repair of peripheral nerve injuries that use myelin and Schwann cell transplantation are assessed. This survey of the literature data shows that using a decellularized nerve conduit combined with Schwann cells represents an effective strategy for the treatment of peripheral nerve injury. This analysis provides a comprehensive basis on which to make clinical decisions for the repair of peripheral nerve injury.
Keywords: nerve regeneration, peripheral nerve injury, nerve conduits, decellularization, extracellular matrix, Schwann cell, neural regeneration
Introduction
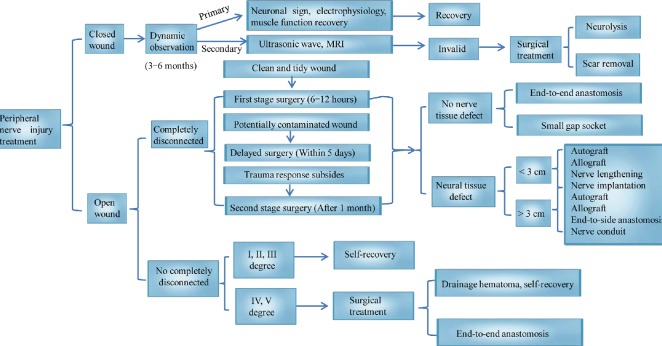
The variety of insults that cause peripheral nerve injury, include gunshot wounds, lacerations, chronic compression, tumor resection, radiotherapy, and complete transection (Secer et al., 2009; Burtt et al., 2017). Peripheral nerve injury accounts for 2.8% of all trauma cases, and severely reduces patient quality of life by damaging sensory and motor functions (Noble et al., 1998; Belkas et al., 2004; Ashley et al., 2007). Nerve injuries are classified according to the affected structures. The categories are: (I) axonal reversible conduction block, then, in terms of increasing severity, destruction of (II) axonal continuity, (III) the endoneurial tube and its contents, (IV) the funiculus and its contents, and (V) the entire nerve trunk (Sunderland, 1951; Lu et al., 2018). The main methods for the clinical treatment of a peripheral nerve injury include conservative treatment and surgical treatment. Surgical approaches rely on methods such as surgery and conduit connection, including end-to-end anastomosis, end-to-side anastomosis, autologous nerve transplantation, allogeneic nerve transplantation, nerve lengthening, and nerve transplantation (Li et al., 2014). Less severe injuries (Categories I–III) are treated using conservative methods, including physical therapy, electrical stimulation, and exercise therapy (Gordon and English, 2016), while more severe injuries (Categories IV and V) require surgical treatment (Ray and Mackinnon, 2010) (Figure 1). Among the latter, nerve transplantation is an effective strategy for the treatment of nerve defects.
Figure 1.
Main methods for the clinical treatment of different peripheral nerve injuries.
Nerve implantation (the red color) indicates that it can be used whether the nerve defect is < 3 cm or > 3 cm.
Autologous nerve transplantation is the gold standard for the treatment of peripheral nerve defects. However, it has limitations, including the needing secondary surgery to obtain the donor nerve, limited donor nerve source, donor site infection or painful neuroma formation (Sun et al., 2018). Based on the concept of tissue engineering, researchers tried various artificial nerve conduits instead of autologous nerve transplantation to repair nerve defects, including polyglycolic acid conduits (Rinker and Liau, 2011), collagen conduits (Taras et al., 2011), poly(lactic-co-glycolic acid) conduits (Bini et al., 2004), poly ε-caprolactone conduits (Reid et al., 2013), and silk conduits (Huang et al., 2012; Sarker et al., 2018). Although the above-mentioned nerve conduits repair the nerve defect to some degree, they also have attendant problems (Rebowe et al., 2018). Artificial nerve conduits can only provide mechanical support to the nerve as there is no extracellular matrix component in the lumen to promote the cavity support structure for axon growth (Jenq and Coggeshall, 1987; Chen and Shen, 2017; Liu et al., 2017).
Recently, animal-based studies (Guenard et al., 1992; Levi and Bunge, 1994; Bryan et al., 2000; Strauch et al., 2001; Mosahebi et al., 2002; Tohill et al., 2004; Hess et al., 2007; Aszmann et al., 2008; Sun et al., 2009; di Summa et al., 2011; Jesuraj et al., 2014; Hoben et al., 2015) have shown positive outcomes using decellularized nerve conduits and transplantation to repair peripheral nerve injuries. The decellularized nerve extracellular matrix has a three-dimensional network structure, which retains proteins and carbohydrates, giving structural support to the nerve. This promotes cell migration, proliferation, differentiation and regulation of intercellular communication (Gonzalez-Perez et al., 2013). Decellularized nerves, in particular, have attracted attention as a natural tubing scaffold structure. As the main glial cell and nerve regeneration coordinator of the peripheral nervous system, Schwann cells play an important role in the regeneration process of peripheral nerve injury (Fairbairn et al., 2015).
Schwann cell transplantation also offers therapeutic potential and has been employed in human clinical cases. This article reviews the methods for nerve decellularization (Table 1), the components and functions of decellularized nerves, the development of Schwann cells, and the application of Schwann cell transplantation in cases of peripheral nerve injury (Table 2).
Table 1.
Nerve decellularization methods
| Category | Mechanism of cell disruption | References | |
|---|---|---|---|
| Physical decellularization | Cold preservation | Freezing | Ide et al. (1983), Osawa et al. (1990), Evans et al. (1998), Hess et al. (2007), Jesuraj et al. (2014), Philips et al. (2018) |
| Freeze-thaw cycles | Formation of ice crystals | ||
| Non-ionic detergents | Destruction of lipid-protein interactions | Sondell et al. (1998), Haase et al. (2003), Sridharan et al. (2015), Philips et al. (2018) | |
| Chemical decellularization | Ionic detergents | Destruction of protein-protein interactions | Hudson et al. (2004), Zilic et al. (2016), Philips et al. (2018) |
| Zwitterionic detergents | Destruction of protein-protein interactions | Hudson et al. (2004) | |
| Hypertonic and hypotonic solutions | Generation of osmotic pressure | Ishida et al. (2014), Philips et al. (2018) |
Table 2.
Schwann cell transplantation in peripheral nerve injury
| Model | Nerve segment | Catheter type | Schwann cell source | Repair defect distance (mm) | References |
|---|---|---|---|---|---|
| Rat | Sciatic nerve | Acrylonitrile vinyl chloride | Allogeneic | 8 | Guenard et al. (1992) |
| Sciatic nerve | Semi-permeable guide channel | Allogeneic | 5 | Levi and Bunge (1994) | |
| Sciatic nerve | Decellularized nerves | Allogeneic | 20 | Hoben et al. (2015) | |
| Sciatic nerve | Polyhydroxybutyrate catheter | Allogeneic | 10 | Mosahebi et al. (2002), Tohill et al. (2004) | |
| Sciatic nerve | Fibrin catheter | Allogeneic | 10 | di Summa et al. (2011) | |
| Sciatic nerve | Decellularized nerves | Allogeneic | 14 | Jesuraj et al. (2014) | |
| Sciatic nerve | Decellularized nerves | Autologous | 30 | Aszmann et al. (2008) | |
| Sciatic nerve | Decellularized nerves | Allogeneic | 10 | Sun et al. (2009) | |
| Sciatic nerve | Poly(lactic-co-glycolic acid) catheter | Autologous | 10 | Bryan et al. (2000) | |
| Rabbit | Sciatic nerve | Venous blood vessels | Autologous | 60 | Strauch et al. (2001) |
| Non-human primate | Ulnar nerve | decellularized nerve | Autologous | 60 | Hess et al. (2007) |
| Human | Sciatic nerve | Autologous sural nerve | Autologous | 75 | Levi et al. (2016) |
Literature Search
The articles used an electronic search of the PubMed database for literature describing peripheral nerve injury from 1951 to 2018 were performed using search terms: “peripheral nerve disease” OR “peripheral nerve injury”, “nerve conduits”, “decellularization techniques” OR “decellularization methods”, “extracellular matrix components” OR “extracellular matrix functions”, “Schwann cell development” OR “Schwann cell myelination”, “Schwann cell transplantation”. The results were further screened by title/abstract; non-SCI experiments and review articles were excluded.
Methods for Peripheral Nerve Decellularization
Treatment with cold preservation and freeze-thaw cycles
The goal of cold preservation and freeze-thaw cycles is to induce the formation of intracellular ice crystals, which then destroy nerve cell membranes and reduce immunogenicity (Philips et al., 2018). Cold preservation and freeze-thaw cycles vary according to nerve length and diameter, as well as laboratory preferences. For example, one study described cutting a 7 mm long segment of mouse sciatic nerve and placing it on a frozen sectioning platform. This was followed by five rounds of freezing (−35°C to −40°C for 3 minutes) and thawing (2°C for 5 minutes) (Ide et al., 1983; Kaizawa et al., 2017). In a different report, cold preservation was performed by aseptically transferring the acellular nerves of Macaca fascicularis to University of Wisconsin solution supplemented with 100 U/mL of penicillin G, 40 U of normal insulin, and 16 mg/L of dexamethasone and stored at 4°C for 7 weeks (Ide et al., 1983). Another approach involved the transfer of Lewis rat sciatic nerve segments to a sterile six-well plate containing 10 mL of a solution containing University of Wisconsin solution (15 mL; NPBI International BV, Emmer Compascuum, The Netherlands), penicillin G (200,000 U/L), regular insulin (40 U/L), and dexamethasone (16 mg/L). The plate was stored at 4°C under aseptic conditions for 7 weeks (Jesuraj et al., 2014).
Cell viability post cold preservation and freeze-thaw is evaluated according to the following features: (1) morphological integrity; (2) functional integrity (e.g., Schwann cells myelinating axons); (3) replicative potential; (4) ability to synthesize DNA; (5) ability to synthesize RNA through transcription and (6) ability to transcribe constitutive or inducible proteins (e.g., trophic receptors, major histocompatibility antigens, and intercellular adhesion molecules) (Evans et al., 1998; Houschyar et al., 2016). Cryopreservation and repeated freeze-thaw cycles have less effect on the mechanical properties of the nerve, but they can damage the ultrastructure of the tissue. Thus they only destroy a portion of, for example a living Schwann cell, not the entire cell. These methods successfully reduce cell viability, but do not eliminate all cells and debris so can trigger host immune rejection (Osawa et al., 1990; Evans et al., 1998).
Chemical decellularization
Treatment with non-ionic detergents
Treatment with non-ionic detergents such as Triton X-100 disrupts lipid-lipid and lipid-protein interactions (Philips et al., 2018). Mariann Sondell first reported a method for chemical decellularization using Triton X-100 (Sondell et al., 1998). Sciatic nerves from Sprague-Dawley rats were incubated in distilled H2O, which was changed multiple times over 7 hours and immersed in 3% Triton X-100 solution overnight at room temperature. The nerves were then transferred to 4% sodium deoxycholate and washed by shaking for 24 hours. The above extraction procedure was then repeated once. After washing, the nerve was placed in phosphate-buffered saline and stored at 4°C. An in vitro evaluation of decellularized nerves (including morphology, immunohistochemistry and electrophoresis), showed a good decellularization effect while retaining the basal layer tube components. When the decellularized nerve was transplanted into the rat, the axons grew in the decellularized nerve at a growth rate of 1.2 mm/day (Sondell et al., 1998). Another published technique (Haase et al., 2003) describes the transfer of rat peroneal nerves to Dulbecco’s phosphate-buffered saline and subsequent fixation of the nerve endings to a substrate using minute dissection pins and stored in a Petri dish. The nerves were then transferred through the following solution series: (1) Solution 1 (7.3 g of ethylenediaminetetraacetic acid, 0.5 g of sodium azide, 800 mL of glycerol, and 200 mL of 0.9% NaCl) for 3 days to destroy cell membranes; (2) solution 2 (25 g of sodium deoxycholate, 0.26 g of sodium azide, and 600 mL of distilled, deionized H2O) for 3 days to dissociate intracellular proteins; (3) solution 1 for 2 days to remove lipid-soluble cell structures; (4) solution 3 (10 g of sodium dodecyl sulfate, 0.52 g of sodium azide, and 1000 mL of distilled deionized H2O) for 2 days to further denature proteins; (5) solution 5 (15 mL of Triton X-100, 0.25 g of sodium azide, and 485 mL of distilled H2O) for 2 days to preserve the decellularized nerves; (6) solution 3 for 2 days; and (7) solution 4 (0.5 g of sodium azide and 1000 mL of 0.9% saline) for 2 days followed by the removal of denatured proteins from the extracellular matrix. All operations were performed at room temperature and the entire process took 2 weeks. The report indicated that acellular nerve repair was more effective for a 2 cm nerve defect than for a 4 cm nerve defect. Although Mariann Sondell’s decellularization method has demonstrated axonal regeneration in vivo, the DNA content in the decellularized nerves is still higher than the industry standard (Crapo et al., 2011). Sridharan et al. (2015) improved Sondell’s acellular nerve technology protocols; in short, the first step is the same as Sondell’s acellular neurological regimen. The second step is a freeze-drying process of decellularized nerves perpendicular to the cooling shelf. Their method resulted in a five-fold reduction in DNA content and the complete elimination of sulfated glycosaminolycans. The effect of a decellularized nerve gap on nerve regeneration was also evaluated. However, the experiment was performed only in vitro; no in vivo experiments were performed. No In vivo experiments using Sridharan’s method have been reported to date.
Treatment with ionic detergents
Treatment with ionic detergents results in the solubilization of cell membranes and disruption of protein-protein interactions. Triton X-200, sodium deoxycholate, and sodium dodecyl sulfate are the commonly used ionic detergents for this procedure. Hudson et al. (2004) used Triton X-200 to decellularize the sciatic nerve of Sprague-Dawley rats and then performed nerve allograft transplantation. After 4 weeks, the transplanted material was examined for CD8+ cells and macrophage infiltration. The decellularized nerve grafts avoided cellular immune recognition and rejection. The sale of Triton X-200 has been discontinued, making replication of this experiment challenging (Philips et al., 2018). Another method reported by Zilic et al. (2016) incorporated a freeze-thaw approach, sodium dodecyl sulfate treatment and enzyme processing (aprotinin and Benzonase®) to decellularize relatively coarser nerves (porcine sciatic nerve branches). Decellularization was followed by a series of in vitro evaluations, including immunohistochemistry (laminin and fibronectin), biochemical analyses (collagen and sulfated sugars), and DNA quantification. The results show that this method effectively decellularizes the sciatic nerve branch but retains extracellular matrix components, including collagen, laminin and fibronectin, which may have potential clinical utility (Zilic et al., 2016).
Treatment with zwitterionic detergents
Zwitterionic detergents have dual properties of ionic and non-ionic detergents, however, their effects are relatively mild and cause less damage to the internal structure of the nerve and extracellular matrix. Zwitterionic detergents (sulfobetaine-10 and -16) in combination with Triton X-200 have been used to decellularize sciatic nerves of rats for the repair of 1 cm sciatic nerve defects. The effect was better than that observed with Sondell’s non-ionic detergent-based method or the traditional method of cold preservation and freeze-thaw cycles. This method removes the antigen that initiates cell-mediated allograft immune rejection (Hudson et al., 2004; Cai et al., 2017).
Treatment with hypertonic and hypotonic solutions
Treatment with hypertonic and hypotonic solutions generates osmotic pressure at levels lethal to cells (Philips et al., 2018). A hypertonic solution of NaCl effectively decellularized the sciatic nerve of rats and nerve allografts in rats (Ishida et al., 2014). Compared with the methods discussed above, this method was associated with less nerve destruction, increased structural maintenance and reduced immune rejection in vivo. However, the authors did not evaluate the decellularized neuronal DNA content.
Components and Functions of Decellularized Extracellular Matrix
Peripheral nerves consist of nerve fibers formed by axons and Schwann cells, connective tissue, and neuron-specific cells such as perineurial cells (Geuna et al., 2009; Assaf et al., 2017). In multicellular animals, the extracellular matrix is a three-dimensional structure found in the intercellular space that functions to support tissues and organs. Extracellular matrix is present in every tissue and organ in the body and consists of water, carbohydrates, and various proteins, including collagens, proteoglycans, non-collagenous glycoproteins, and elastins, which play an important role in cell migration, proliferation, and differentiation (Aszodi et al., 2006; Gonzalez-Perez et al., 2013; Jiang et al., 2016b). In peripheral nerves, the extracellular matrix is found in the basal lamina of Schwann cells and the endoneurium.
Collagens comprise three polypeptide chains (alpha chains) with characteristic triple helical collagenous and non-collagenous domains. In vertebrates, 27 types of collagen are classified into 9 families based on their supramolecular assembly and other features. The fibrous collagen family includes abundant type I, type II, and type III, and lower quantities of type V and type XI. Type I, type III, and type V collagens are widely distributed in the body, while types II and XI are mainly confined to cartilage, the vitreous body of the eye, and the inner ear. The basal layer produced by Schwann cells mainly consists of type IV collagen, laminin and fibronectin (Baron-Van Evercooren et al., 1986; Myllyharju and Kivirikko, 2004; Bryan et al., 2012; Gonzalez-Perez et al., 2013; Jiang et al., 2016b).
Proteoglycans consist of a core protein covalently linked to a glycosaminoglycan side chain. Glycosaminoglycans are sulfated oligosaccharides consisting of dermatan sulfate, heparan sulfate/heparin, chondroitin sulfate or keratan sulfate repeating disaccharide units. Chondroitin sulfate proteoglycans exert inhibitory effects by neutralizing factors that promote extracellular matrix production (Dingyu et al., 2016; Andries et al., 2017). In peripheral nerves, chondroitin sulfate proteoglycans may inhibit the function of laminin (Zuo et al., 1998a, b; Gordon et al., 2015; Lee et al., 2018). In addition to their known role in the hydration and permeability of the extracellular matrix, proteoglycans influence cellular activity via interactions with extracellular matrix components, growth factors, and cell surface receptors (Aszodi et al., 2006).
Laminin, a key component of the basement membrane, participates in cell migration, differentiation, and adhesion. Laminin is a heterotrimer of α, β, and γ chains, and 18 different types have been described so far (Durbeej, 2010). Fibronectin represents another key component of the extracellular matrix and forms a fibrillar matrix similar to collagen to mediate cell-cell binding. Alternative mRNA splicing results in various fibronectin isoforms. For example, 12 forms of mouse fibronectin and 20 forms of human fibronectin have been identified (Gonzalez-Perez et al., 2013). In the nervous system, Schwann cells and fibroblasts synthesize and secrete fibronectin (Baron-Van Evercooren et al., 1986; Chernousov and Carey, 2000; Sohn and Park, 2018).
Elastic fibers consist of an elastin core surrounded by a microfiber network that together contributes to tissue elasticity and resilience. To generate elasticity, soluble precursor molecules are deposited in a preformed microfibril matrix and subsequently cross-linked to form insoluble elastin polymers. Microfibrils are also present in some flexible tissues where elastin does not exist. Microfibrils are composed number of integrins including fibrillin-1 and -2, fibrin, latent transforming growth factor beta-binding proteins and EMILINs (Kielty et al., 2002).
During decellularization, whether it is physical decellularization or chemical decellularization, the purpose is to remove cellular components in the nerve but retain the extracellular matrix. The important components of the extracellular matrix, collagen, laminin, fibronectin and elastin, provide a three-dimensional scaffold structure for nerve regeneration (Werner et al., 2000; Brown and Phillips, 2007; Gardiner et al., 2007; Ingram et al., 2016).
Based on the concept of tissue engineering, decellularized nerves can be used as an effective substitute for nerve transplantation to support peripheral nerve regeneration and avoid the use of immunosuppressive agents (Pedrini et al., 2018). However, simple decellularized nerve grafts have a limited ability to promote nerve regeneration due to the lack of cellular components. Numerous studies have shown that seed cells combined with decellularized nerves have achieved good results in the repair of peripheral nerve injury (Choi et al., 2018; Huang et al., 2018). These include Schwann cells (Hess et al., 2007; Aszmann et al., 2008; Sun et al., 2009; Jesuraj et al., 2014; Hoben et al., 2015), adipose-derived stem cells (Zhang et al., 2010; Liu et al., 2011; Jiang et al., 2016a), bone marrow stromal cells (Jia et al., 2012; Zhao et al., 2014; Li et al., 2016; Wang et al., 2016), Schwann cells-like cells (Gao et al., 2014; Garcia-Perez et al., 2017) and skin derived precursor cells (Walsh et al., 2009). Of these, Schwann cell transplantation has been shown to play an important role in the repair of peripheral nerve injury. The next part of this review examines the development, secretion, myelination and transplantation of Schwann cells to promote regeneration after peripheral nerve injury.
Development, Secretion, and Myelination of Schwann Cells
During vertebrate development, neural crest cells differentiate into various cell types. In the peripheral nervous system, neural crest cells differentiate into Schwann cells and glial cells (Sommer, 2001; Le Douarin and Dupin, 2003; Suga et al., 2017). The migration of neural crest cells after delamination is regulated by the expression of specific transcription factors and tyrosine kinase receptors (Kos et al., 2001; Yu et al., 2016). The fate of neural crest cells depends on their position relative to the anterior and posterior axes of the neural tube. Schwann cells are mainly derived from neural crest-derived Schwann cell precursors; others are derived from neural crest-derived border cap cells (Monk et al., 2015). The specific mechanism of the differentiation from neural crest cells to Schwann cell precursors is unclear, but the transcription factor Sox10 plays an important role (Kuhlbrodt et al., 1998; Woodhoo and Sommer, 2008; Monk et al., 2015; Imai et al., 2017; Kim et al., 2017). During the conversion of precursor to immature Schwann cells, the Schwann cell precursors travel along the axon, under the influence of neuregulin 1, endothelins, and Notch signaling molecules (Lai, 2005; Woodhoo and Sommer, 2008; Wood and Mackinnon, 2015). Neuregulin 1 regulates Schwann cell precursor migration and proliferation through binding to ErBb2/3 on Schwann cell precursors (Newbern and Birchmeier, 2010; Raphael and Talbot, 2011; Monk et al., 2015). Notch signaling accelerates the conversion of Schwann cell precursors to immature Schwann cells (Woodhoo and Sommer, 2008; Monk et al., 2015). After Schwann cell precursors complete their migration, vascular endothelial growth factor promotes angiogenesis, and desert hedgehog promotes peripheral nerve differentiation (Mukouyama et al., 2005; Monk et al., 2015). This radial sorting process must be performed prior to myelination. Immature Schwann cells selectively encapsulate large diameter axons and eventually form a 1:1 relationship (Woodhoo and Sommer, 2008; Monk et al., 2015; Barton et al., 2017; Castelnovo et al., 2017). Only the thickest axons become myelinated as a result of radial sorting, and neuregulin 1 plays an important role in the control of this process (Perlin et al., 2011; Birchmeier and Bennett, 2016; Deng et al., 2017) (Figure 2). Studies have shown that neuregulin 1 is inhibited in uninjured nerves (Ma et al., 2018). When the nerve is injured, neuregulin 1 in Schwann cells is reactivated to promote regeneration of the myelin. Similarly after transplantation of a decellularized nerve, Schwann cells migrate into the decellularized nerve and activated neuregulin 1 promotes axonal and remyelination regeneration (Aszmann et al., 2008; Sun et al., 2009; Stassart et al., 2013; Hoben et al., 2015).
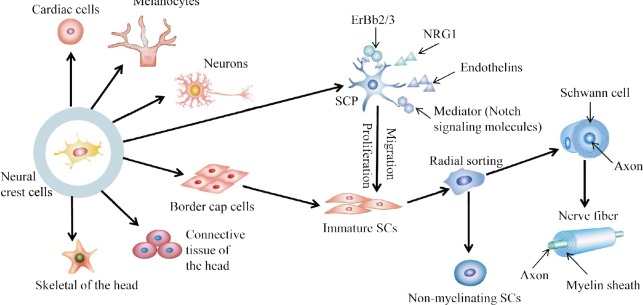
Figure 2.
Schwann cell development and myelination.
Neural crest cells can differentiate into various cell types, including cardiac cells, melanocytes, neurons, SCP, border cap cells, connective tissue of the head and skeleton tissue of the head. A small fraction of immature SCs are derived from border cap cells, but most are derived from SCP. SCP differentiates into immature SCs by proliferation and migration under the action of ErBb2/3, NRG1, endothelins and Notch signaling molecules. Immature SCs partially encapsulate the axons to form the myelin sheath through a process of radial sorting, and the other part is transformed into non-myelinating SCs. SCP: Schwann cell precursor; NRG1: neuregulin-1; SCs: Schwann cells. Adapted from Monk et al. (2015).
Application of Schwann Cell Transplantation to Nerve Regeneration
Schwann cell transplantation promotes myelination and spinal nerve regeneration in the central nervous system (Bachelin et al., 2005; Biernaskie et al., 2007; Kocsis and Waxman, 2007; Hoben et al., 2015). The transplantation of decellularized rat sciatic nerve seeded with Schwann cells repaired spinal cord injuries in rats, indicating that the decellularized extracellular matrix promotes Schwann cell survival and myelination (Cerqueira et al., 2018). Schwann cell transplantation has also shown promise as a therapy for peripheral nerve injury. Schwann cells cultured in vitro and then implanted into rat sciatic nerve formed normal myelin around the axons. Similarly, when these cultured Schwann cells were implanted into a semi-permeable guide channel a 5 mm sciatic nerve injury in rats was successfully repaired (Guenard et al., 1992). Xenografts of human peripheral nerve or guidance channel, seeded with human Schwann cells, repaired a 5-mm mouse sciatic nerve lesion. Human Schwann cells were identified immunologically in the repair 6 weeks after surgery. The Schwann cell migration, in turn, led to the formation of a myelin sheath around the regenerated mouse axons (Levi and Bunge, 1994). Experimental evidence shows that the transplantation of alginate combined with allogeneic Schwann cells in a polyhydroxybutyrate conduit repaired a 10 mm rat sciatic nerve defect, indicating its effectiveness in axonal regeneration (Mosahebi et al., 2002; Tohill et al., 2004). Strauch et al. (2001) used venous blood vessels as a conduit in combination with Schwann cells and observed the repair of a 6 cm sciatic nerve injury in rabbits. In a related study, a fibrin conduit combined with Schwann cells was used to repair 10 mm rat sciatic nerve defect (di Summa et al., 2011). Follow-up assessments at 15 weeks post-surgery showed that the Schwann cell transplantation group displayed improvements in fiber myelination, muscle atrophy, and fiber diameter relative to the conduit only group. The value of Schwann cell transplantation is further demonstrated by positive results following the removal of a 10-mm section of rat sciatic nerve for in vitro culture, followed by the inoculation of Schwann cells into a poly(lactic-co-glycolic) acid conduit during a second operation 1 week later to repair the defect. After 12 weeks, the number of myelinated nerve fibers and regenerated blood vessels increased noticeably (Bryan et al., 2000). In more recent literature (Hess et al., 2007; Aszmann et al., 2008; Sun et al., 2009; Jesuraj et al., 2014; Hoben et al., 2015), Schwann cells combined with decellularized nerve conduits to repair peripheral nerve injury have been extensively studied. Hess et al. (2007) used cryopreservation to make decellularized nerves, and when combined with autologous Schwann cells promoted remarkable regeneration of non-human primate 6 cm peripheral nerve defects. Autologous Schwann cells, obtained from a regeneration neuroma of the proximal stump, were seeded into decellularized nerves to repair the peripheral nerve injury in rats and gave good results (Aszmann et al., 2008). Schwann cells were co-cultured with allogeneic decellularized nerves in vitro to repair sciatic nerve injury in rats. The results after 12 weeks showed decellularized nerves seeded with Schwann cells could improve nerve regeneration and functional recovery after bridging the sciatic nerve gap of rats (Sun et al., 2009). Hoben et al. (2015) used the decellularized nerve complex and showed that the addition of Schwann cells was better than vascular endothelial growth factor at promoting axon regeneration. Collectively, these animal-based studies highlight the success of Schwann cell transplantation in the repair of nerve injuries. A recent report has demonstrated the therapeutic value of Schwann cell transplantation in humans (Levi et al., 2016). The sciatic nerve stump was removed from a patient’s sciatic nerve for autologous Schwann cell culture. A second operation was performed with an autologous sural nerve graft to repair the sciatic nerve injury. Autologous Schwann cells, in the presence of a collagen matrix, were injected around the graft. Follow-up evaluations at 18 months post-surgery showed recovery in sciatic nerve sensory and motor functions (Table 2).
Conclusions
Peripheral nerve injuries are common, and different treatment strategies exist for the distinctive forms of injury. In recent years, the repair of severed peripheral nerve using nerve conduits has received increasing attention. Specifically, the use of decellularized nerve conduits shows promise as a therapy for peripheral nerve damage. In addition, treatment with neural tube composite cells (e.g., Schwann cells, adipose-derived mesenchymal stem cells, and bone marrow-derived mesenchymal stem cells) represents an effective therapeutic strategy in animal models. Regardless of the repair method, there are concerns regarding biocompatibility and structural integrity. Furthermore, the long-term survival of transplanted cells is unclear. Future studies incorporating decellularized nerve conduits and neural tube composite cells should address these concerns and contribute to our knowledge regarding the therapeutic value of these techniques.
Additional file: Open peer review report 1 (102.3KB, pdf) .
Acknowledgments
The authors thank Xun Sun, MD, from Tianjin Hospital, China, for his guidance on this article.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the National Key R&D Program of China, No. 2017YFA0104701 (to YW); the National Natural Science Foundation of China, No. 31771052 (to YW); the Natural Science Foundation of Beijing of China, No. 7172202 (to YW); the PLA Youth Training Project for Medical Science of China, No. 16QNP144 (to YW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Tomohiro Torii, Baylor College of Medicine, USA.
Funding: This work was supported by the National Key R & D Program of China, No. 2017YFA0104701 (to YW); the National Natural Science Foundation of China, No. 31771052 (to YW); the Natural Science Foundation of Beijing of China, No. 7172202 (to YW); the PLA Youth Training Project for Medical Science of China, No. 16QNP144 (to YW).
P-Reviewer: Torii T; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Dawes EA, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Andries L, Van Hove I, Moons L, De Groef L. Matrix metalloproteinases during axonal regeneration, a multifactorial role from start to finish. Mol Neurobiol. 2017;54:2114–2125. doi: 10.1007/s12035-016-9801-x. [DOI] [PubMed] [Google Scholar]
- 2.Ashley WW, Jr, Baty JD, Hollander T, Noetzel MJ, Park TS. Long-term motor outcome analysis using a motor score composite following surgical brachial plexus repair. J Neurosurg. 2007;106:276–281. doi: 10.3171/ped.2007.106.4.276. [DOI] [PubMed] [Google Scholar]
- 3.Assaf K, Leal CV, Derami MS, de Rezende Duek EA, Ceragioli HJ, de Oliveira ALR. Sciatic nerve repair using poly (epsilon-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 2017;7:e00755. doi: 10.1002/brb3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aszmann OC, Korak KJ, Luegmair M, Frey M. Bridging critical nerve defects through an acellular homograft seeded with autologous schwann cells obtained from a regeneration neuroma of the proximal stump. J Reconstr Microsurg. 2008;24:151–158. doi: 10.1055/s-2008-1076091. [DOI] [PubMed] [Google Scholar]
- 5.Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 6.Bachelin C, Lachapelle F, Girard C, Moissonnier P, Serguera-Lagache C, Mallet J, Fontaine D, Chojnowski A, Le Guern E, Nait-Oumesmar B, Baron-Van Evercooren A. Efficient myelin repair in the macaque spinal cord by autologous grafts of Schwann cells. Brain. 2005;128:540–549. doi: 10.1093/brain/awh406. [DOI] [PubMed] [Google Scholar]
- 7.Baron-Van Evercooren A, Gansmuller A, Gumpel M, Baumann N, Kleinman HK. Schwann cell differentiation in vitro: extracellular matrix deposition and interaction. Dev Neurosci. 1986;8:182–196. doi: 10.1159/000112252. [DOI] [PubMed] [Google Scholar]
- 8.Barton MJ, John JS, Clarke M, Wright A, Ekberg J. The glia response after peripheral nerve injury: a comparison between schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. Int J Mol Sci. 2017;18:E287. doi: 10.3390/ijms18020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 10.Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bini TB, Gao S, Xu X, Wang S, Ramakrishna S, Leong KW. Peripheral nerve regeneration by microbraided poly(L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A. 2004;68:286–295. doi: 10.1002/jbm.a.20050. [DOI] [PubMed] [Google Scholar]
- 12.Birchmeier C, Bennett DL. Neuregulin/ErbB signaling in developmental myelin formation and nerve repair. Curr Top Dev Biol. 2016;116:45–64. doi: 10.1016/bs.ctdb.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Brown RA, Phillips JB. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol. 2007;262:75–150. doi: 10.1016/S0074-7696(07)62002-6. [DOI] [PubMed] [Google Scholar]
- 14.Bryan DJ, Holway AH, Wang KK, Silva AE, Trantolo DJ, Wise D, Summerhayes IC. Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on peripheral nerve regeneration. Tissue Eng. 2000;6:129–138. doi: 10.1089/107632700320757. [DOI] [PubMed] [Google Scholar]
- 15.Bryan DJ, Litchfield CR, Manchio JV, Logvinenko T, Holway AH, Austin J, Summerhayes IC, Rieger-Christ KM. Spatiotemporal expression profiling of proteins in rat sciatic nerve regeneration using reverse phase protein arrays. Proteome Sci. 2012;10:9. doi: 10.1186/1477-5956-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burtt KE, Badash I, Wu B. Assessing surgical methods for treatment of cubital tunnel syndrome–which is the best? Clin Trials Orthop Disord. 2017;2:123–124. [Google Scholar]
- 17.Cai M, Huang T, Hou B, Guo Y. Role of demyelination efficiency within acellular nerve scaffolds during nerve regeneration across peripheral defects. Biomed Res Int. 2017;2017:4606387. doi: 10.1155/2017/4606387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castelnovo LF, Bonalume V, Melfi S, Ballabio M, Colleoni D, Magnaghi V. Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways. Neural Regen Res. 2017;12:1013–1023. doi: 10.4103/1673-5374.211172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerqueira SR, Lee YS, Cornelison RC, Mertz MW, Wachs RA, Schmidt CE, Bunge MB. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials. 2018;177:176–185. doi: 10.1016/j.biomaterials.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Shen H. Tissue-engineered nerve conduits with internal structure in the repair of peripheral nerve defects. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:1273–1279. [Google Scholar]
- 21.Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- 22.Choi J, Kim JH, Jang JW, Kim HJ, Choi SH, Kwon SW. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural Regen Res. 2018;13:1796–1803. doi: 10.4103/1673-5374.237126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y, Wu LMN, Bai S, Zhao C, Wang H, Wang J, Xu L, Sakabe M, Zhou W, Xin M, Lu QR. A reciprocal regulatory loop between TAZ/YAP and G-protein Galphas regulates Schwann cell proliferation and myelination. Nat Commun. 2017;8:15161. doi: 10.1038/ncomms15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–291. doi: 10.1016/j.neuroscience.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 26.Dingyu W, Fanjie M, Zhengzheng D, Baosheng H, Chao Y, Yi P, Huiwen W, Jun G, Gang H. Regulation of intracellular structural tension by talin in the axon growth and regeneration. Mol Neurobiol. 2016;53:4582–4595. doi: 10.1007/s12035-015-9394-9. [DOI] [PubMed] [Google Scholar]
- 27.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 28.Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–1522. doi: 10.1002/(sici)1097-4598(199811)21:11<1507::aid-mus21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: a review of current opinion. World J Stem Cells. 2015;7:11–26. doi: 10.4252/wjsc.v7.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao S, Zheng Y, Cai Q, Deng Z, Yao W, Wang J, Wang X, Zhang P. Combination of acellular nerve graft and schwann cells-like cells for rat sciatic nerve regeneration. Neural Plast. 2014;2014:139085. doi: 10.1155/2014/139085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Perez MM, Martinez-Rodriguez HG, Lopez-Guerra GG, Soto-Dominguez A, Said-Fernandez SL, Morales-Avalos R, Elizondo-Omana RE, Montes-de-Oca-Luna R, Guzman-Lopez S, Castillo-Galvan ML, Mendoza-Lemus OF, Vilchez-Cavazos F. A modified chemical protocol of decellularization of rat sciatic nerve and its recellularization with mesenchymal differentiated schwann-like cells: morphological and functional assessments. Histol Histopathol. 2017;32:779–792. doi: 10.14670/HH-11-844. [DOI] [PubMed] [Google Scholar]
- 32.Gardiner NJ, Moffatt S, Fernyhough P, Humphries MJ, Streuli CH, Tomlinson DR. Preconditioning injury-induced neurite outgrowth of adult rat sensory neurons on fibronectin is mediated by mobilisation of axonal α5 integrin. Mol Cell Neurosci. 2007;35:249–260. doi: 10.1016/j.mcn.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M. Chapter 3: Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol. 2009;87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Perez F, Udina E, Navarro X. Extracellular matrix components in peripheral nerve regeneration. Int Rev Neurobiol. 2013;108:257–275. doi: 10.1016/B978-0-12-410499-0.00010-1. [DOI] [PubMed] [Google Scholar]
- 35.Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2016;43:336–350. doi: 10.1111/ejn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon T, Hendry M, Lafontaine CA, Cartar H, Zhang JJ, Borschel GH. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS One. 2015;10:e0127397. doi: 10.1371/journal.pone.0127397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haase SC, Rovak JM, Dennis RG, Kuzon WM, Jr, Cederna PS. Recovery of muscle contractile function following nerve gap repair with chemically acellularized peripheral nerve grafts. J Reconstr Microsurg. 2003;19:241–248. doi: 10.1055/s-2003-40580. [DOI] [PubMed] [Google Scholar]
- 39.Hess JR, Brenner MJ, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, Rickman SR, Mackinnon SE. Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plastic Reconstr Surg. 2007;119:246–259. doi: 10.1097/01.prs.0000245341.71666.97. [DOI] [PubMed] [Google Scholar]
- 40.Hoben G, Yan Y, Iyer N, Newton P, Hunter DA, Moore AM, Sakiyama-Elbert SE, Wood MD, Mackinnon SE. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand (N Y) 2015;10:396–402. doi: 10.1007/s11552-014-9720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houschyar KS, Momeni A, Pyles MN, Cha JY, Maan ZN, Duscher D, Jew OS, Siemers F, van Schoonhoven J. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int. 2016;2016:4175293. doi: 10.1155/2016/4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W, Begum R, Barber T, Ibba V, Tee NC, Hussain M, Arastoo M, Yang Q, Robson LG, Lesage S, Gheysens T, Skaer NJ, Knight DP, Priestley JV. Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials. 2012;33:59–71. doi: 10.1016/j.biomaterials.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Huang YY, Xu XL, Huang XJ, Liu JH, Qi J, Zhu S, Zhu ZW, He B, Zhu QT, Xu YB, Gu LQ, Liu XL. Various changes in cryopreserved acellular nerve allografts at -80°C. Neural Regen Res. 2018;13:1643–1649. doi: 10.4103/1673-5374.237138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 45.Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- 46.Imai S, Koyanagi M, Azimi Z, Nakazato Y, Matsumoto M, Ogihara T, Yonezawa A, Omura T, Nakagawa S, Wakatsuki S, Araki T, Kaneko S, Nakagawa T, Matsubara K. Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci Rep. 2017;7:5947. doi: 10.1038/s41598-017-05784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingram NT, Khankan RR, Phelps PE. Olfactory ensheathing cells express alpha7 integrin to mediate their migration on laminin. PLoS One. 2016;11:e0153394. doi: 10.1371/journal.pone.0153394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishida Y, Sakakibara S, Terashi H, Hashikawa K, Yamaoka T. Development of a novel method for decellularizing a nerve graft using a hypertonic sodium chloride solution. Int J Artif Organs. 2014;37:854–860. doi: 10.5301/ijao.5000365. [DOI] [PubMed] [Google Scholar]
- 49.Jenq CB, Coggeshall RE. Permeable tubes increase the length of the gap that regenerating axons can span. Brain Res. 1987;408:239–242. doi: 10.1016/0006-8993(87)90379-9. [DOI] [PubMed] [Google Scholar]
- 50.Jesuraj NJ, Santosa KB, Macewan MR, Moore AM, Kasukurthi R, Ray WZ, Flagg ER, Hunter DA, Borschel GH, Johnson PJ, Mackinnon SE, Sakiyama-Elbert SE. Schwann cells seeded in acellular nerve grafts improve functional recovery. Muscle Nerve. 2014;49:267–276. doi: 10.1002/mus.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia H, Wang Y, Tong XJ, Liu GB, Li Q, Zhang LX, Sun XH. Sciatic nerve repair by acellular nerve xenografts implanted with BMSCs in rats xenograft combined with BMSCs. Synapse. 2012;66:256–269. doi: 10.1002/syn.21508. [DOI] [PubMed] [Google Scholar]
- 52.Jiang L, Zheng Y, Chen O, Chu T, Ding J, Yu Q. Nerve defect repair by differentiated adipose-derived stem cells and chondroitinase ABC-treated acellular nerves. Int J Neurosci. 2016a;126:568–576. doi: 10.3109/00207454.2015.1048547. [DOI] [PubMed] [Google Scholar]
- 53.Jiang M, Qiu J, Zhang L, Lu D, Long M, Chen L, Luo X. Changes in tension regulates proliferation and migration of fibroblasts by remodeling expression of ECM proteins. Exp Ther Med. 2016b;12:1542–1550. doi: 10.3892/etm.2016.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaizawa Y, Kakinoki R, Ikeguchi R, Ohta S, Noguchi T, Takeuchi H, Oda H, Yurie H, Matsuda S. A Nerve conduit containing a vascular bundle and implanted with bone marrow stromal cells and decellularized allogenic nerve matrix. Cell Transplant. 2017;26:215–228. doi: 10.3727/096368916X692951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 56.Kim HS, Lee J, Lee DY, Kim YD, Kim JY, Lim HJ, Lim S, Cho YS. Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Rep. 2017;8:1714–1726. doi: 10.1016/j.stemcr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kocsis JD, Waxman SG. Schwann cells and their precursors for repair of central nervous system myelin. Brain. 2007;130:1978–1980. doi: 10.1093/brain/awm161. [DOI] [PubMed] [Google Scholar]
- 58.Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- 59.Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai C. Peripheral glia: Schwann cells in motion. Curr Biol. 2005;15:R332–R334. doi: 10.1016/j.cub.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 61.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Lee HN, Mitra M, Bosompra O, Corney DC, Johnson EL, Rashed N, Ho LD, Coller HA. RECK isoforms have opposing effects on cell migration. Mol Biol Cell. 2018;29:1825–1838. doi: 10.1091/mbc.E17-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levi AD, Bunge RP. Studies of myelin formation after transplantation of human Schwann cells into the severe combined immunodeficient mouse. Exp Neurol. 1994;130:41–52. doi: 10.1006/exnr.1994.1183. [DOI] [PubMed] [Google Scholar]
- 64.Levi AD, Burks SS, Anderson KD, Dididze M, Khan A, Dietrich WD. The use of autologous schwann cells to supplement sciatic nerve repair with a large gap: first in human experience. Cell Transplant. 2016;25:1395–1403. doi: 10.3727/096368915X690198. [DOI] [PubMed] [Google Scholar]
- 65.Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L. Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys. 2014;68:449–454. doi: 10.1007/s12013-013-9742-1. [DOI] [PubMed] [Google Scholar]
- 66.Li YJ, Zhao BL, Lv HZ, Qin ZG, Luo M. Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models. Neural Regen Res. 2016;11:1322–1326. doi: 10.4103/1673-5374.189198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011;28:565–572. doi: 10.3892/ijmm.2011.725. [DOI] [PubMed] [Google Scholar]
- 68.Liu TD, Zhang BC, Hao ML. Collagen-gelatin scaffolds for the repair of peripheral nerve defects. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:286–290. [Google Scholar]
- 69.Lu C, Sun X, Wang C, Wang Y, Peng J. Mechanisms and treatment of painful neuromas. Rev Neurosci. 2018;29:557–566. doi: 10.1515/revneuro-2017-0077. [DOI] [PubMed] [Google Scholar]
- 70.Ma KH, Duong P, Moran JJ, Junaidi N, Svaren J. Polycomb repression regulates Schwann cell proliferation and axon regeneration after nerve injury. Glia. 2018;66:2487–2502. doi: 10.1002/glia.23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376–1393. doi: 10.1002/glia.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213–223. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- 73.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 74.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21:922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Osawa T, Tohyama K, Ide C. Allogeneic nerve grafts in the rat, with special reference to the role of Schwann cell basal laminae in nerve regeneration. J Neurocytol. 1990;19:833–849. doi: 10.1007/BF01186814. [DOI] [PubMed] [Google Scholar]
- 78.Pedrini FA, Boriani F, Bolognesi F, Fazio N, Marchetti C, Baldini N. Cell-enhanced acellular nerve allografts for peripheral nerve reconstruction: a systematic review and a meta-analysis of the literature. Neurosurgery. 2018 doi: 10.1093/neuros/nyy374. doi: 10.1093/neuros/nyy374. [DOI] [PubMed] [Google Scholar]
- 79.Perlin JR, Lush ME, Stephens WZ, Piotrowski T, Talbot WS. Neuronal neuregulin 1 type III directs Schwann cell migration. Development. 2011;138:4639–4648. doi: 10.1242/dev.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Philips C, Cornelissen M, Carriel V. Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts. J Neural Eng. 2018 doi: 10.1088/1741-2552/aaa21a. doi: 10.1088/1741-2552/aaa21a. [DOI] [PubMed] [Google Scholar]
- 81.Raphael AR, Talbot WS. New insights into signaling during myelination in zebrafish. Curr Top Dev Biol. 2011;97:1–19. doi: 10.1016/B978-0-12-385975-4.00007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77–85. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rebowe R, Rogers A, Yang X, Kundu SC, Smith TL, Li Z. Nerve repair with nerve conduits: problems, solutions, and future directions. J Hand Microsurg. 2018;10:61–65. doi: 10.1055/s-0038-1626687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reid AJ, de Luca AC, Faroni A, Downes S, Sun M, Terenghi G, Kingham PJ. Long term peripheral nerve regeneration using a novel PCL nerve conduit. Neurosci Lett. 2013;544:125–130. doi: 10.1016/j.neulet.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Rinker B, Liau JY. A prospective randomized study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. J Hand Surg. 2011;36:775–781. doi: 10.1016/j.jhsa.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 86.Sarker MD, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells growth factors, and physical stimuli. Prog Neurobiol. 2018;171:125–150. doi: 10.1016/j.pneurobio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Secer HI, Solmaz I, Anik I, Izci Y, Duz B, Daneyemez MK, Gonul E. Surgical outcomes of the brachial plexus lesions caused by gunshot wounds in adults. J Brachial Plex Peripher Nerve Inj. 2009;4:11. doi: 10.1186/1749-7221-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sohn EJ, Park HT. MicroRNA mediated regulation of schwann cell migration and proliferation in peripheral nerve injury. Biomed Res Int. 2018;2018:8198365. doi: 10.1155/2018/8198365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sommer L. Context-dependent regulation of fate decisions in multipotent progenitor cells of the peripheral nervous system. Cell Tissue Res. 2001;305:211–216. doi: 10.1007/s004410000331. [DOI] [PubMed] [Google Scholar]
- 90.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 91.Sridharan R, Reilly RB, Buckley CT. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J Mech Behav Biomed Mater. 2015;41:124–135. doi: 10.1016/j.jmbbm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 93.Strauch B, Rodriguez DM, Diaz J, Yu HL, Kaplan G, Weinstein DE. Autologous Schwann cells drive regeneration through a 6-cm autogenous venous nerve conduit. J Reconstr Microsurg. 2001;17:589–595. doi: 10.1055/s-2001-18812. discussion 596-587. [DOI] [PubMed] [Google Scholar]
- 94.Suga M, Hayashi Y, Furue MK. In vitro models of cranial neural crest development toward toxicity tests: frog, mouse, and human. Oral Dis. 2017;23:559–565. doi: 10.1111/odi.12523. [DOI] [PubMed] [Google Scholar]
- 95.Sun X, Wang Y, Guo Z, Xiao B, Sun Z, Yin H, Meng H, Sui X, Zhao Q, Guo Q, Wang A, Xu W, Liu S, Li Y, Lu S, Peng J. Acellular cauda equina allograft as main material combined with biodegradable chitin conduit for regeneration of long-distance sciatic nerve defect in rats. Adv Healthc Mater. 2018;7:e1800276. doi: 10.1002/adhm.201800276. [DOI] [PubMed] [Google Scholar]
- 96.Sun XH, Che YQ, Tong XJ, Zhang LX, Feng Y, Xu AH, Tong L, Jia H, Zhang X. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol. 2009;29:347–353. doi: 10.1007/s10571-008-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 98.Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg. 2011;36:1441–1446. doi: 10.1016/j.jhsa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 99.Tohill MP, Mann DJ, Mantovani CM, Wiberg M, Terenghi G. Green fluorescent protein is a stable morphological marker for schwann cell transplants in bioengineered nerve conduits. Tissue Eng. 2004;10:1359–1367. doi: 10.1089/ten.2004.10.1359. [DOI] [PubMed] [Google Scholar]
- 100.Walsh S, Biernaskie J, Kemp SW, Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–1107. doi: 10.1016/j.neuroscience.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Jia H, Li WY, Guan LX, Deng L, Liu YC, Liu GB. Molecular examination of bone marrow stromal cells and chondroitinase ABC-assisted acellular nerve allograft for peripheral nerve regeneration. Exp Ther Med. 2016;12:1980–1992. doi: 10.3892/etm.2016.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J Neurosci. 2000;20:1822. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wood MD, Mackinnon SE. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol. 2015;265:171–175. doi: 10.1016/j.expneurol.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 105.Yu H, Pan WK, Zheng BJ, Wang HJ, Chen XL, Liu Y, Gao Y. Decreased proliferative, migrative and neuro-differentiative potential of postnatal rat enteric neural crest-derived cells during culture in vitro. Exp Cell Res. 2016;343:218–222. doi: 10.1016/j.yexcr.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B, Gao P, Hu G, Jin Y. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010;31:5312–5324. doi: 10.1016/j.biomaterials.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 107.Zhao Z, Wang Y, Peng J, Ren Z, Zhang L, Guo Q, Xu W, Lu S. Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin. Cell Transplant. 2014;23:97–110. doi: 10.3727/096368912X658845. [DOI] [PubMed] [Google Scholar]
- 108.Zilic L, Wilshaw SP, Haycock JW. Decellularisation and histological characterisation of porcine peripheral nerves. Biotechnol Bioeng. 2016;113:2041–2053. doi: 10.1002/bit.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998a;34:41–54. [PubMed] [Google Scholar]
- 110.Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998b;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.