Abstract
The aged population is constantly growing, thus fostering an increase in age-dependent diseases. Among these, diabetic retinopathy (DR) along with age-related macular degeneration entails progressive vision loss. Since such conditions are associated with the proliferation of novel vessels, their pharmacotherapeutic management consists of the intravitreal injection of anti-vascular endothelial growth factor drugs, able to hinder the driving of vascular proliferation prompted by vascular endothelial growth factor. The humanized anti-vascular endothelial growth factor monoclonal antibody ranibizumab provided evidence for efficacy in several trials, hence earning approval by the US Food and Drug Administration for therapeutic use in all the stages of DR. Due to the lack of epidemiologic and pharmacoeconomic evaluation in the local Calabria Region context, the present retrospective observational study focused on prevalence of DR and age-related macular degeneration, treatment and cost of therapy with ranibizumab in 870 patients arriving to clinical observation at the “Mater Domini” University Hospital in Calabria, Italy from January 2014 to June 2017. Data were extracted from the database of ophthalmology ward and subjected to statistical analysis. The results suggest that the most frequent retinal diseases are age-related macular degeneration and DR and that the use of ranibizumab has been decreasing over the 4-year study period together with the associated cost per patient which was similar for both disorders. Therefore, appropriateness of treatment with drugs other than ranibizumab needs to be assessed in this setting and deep monitoring of pharmacologic treatment for retinal diseases is necessary to prevent or delay visual acuity decrease and complete vision loss. Study procedures were performed in accordance with the “Mater Domini” University Hospital ethical standards of the responsible committee on human experimentation
Keywords: age-related macular degeneration, diabetic retinopathy, pharmacovigilance, ranibizumab, anti-VEGFs, retrospective study
Chinese Library Classification No. R453; R774
Introduction
The increasing life expectancy poses a continuously growing risk to develop age-related pathological conditions. One of the most common is represented by diabetes mellitus, a systemic disease responsible for serious impairment of several physiologic functions, such as vision till complete blindness. In particular, the Centers for Disease Control and Prevention provided a worrisome estimate (Atlanta, 2017; Zhao and Singh al., 2018): 9.4% of the US population suffers from diabetes. Considering the global population suffering from diabetes amounting to some 415 million in 2015 with an expected increase to 642 by 2040 (Herat et al., 2018), more than one-third is affected by diabetic retinopathy (DR), often with vision-threatening diabetic retinopathy (severe non-proliferative or proliferative DR) or diabetic macular oedema (DME) (Yau et al., 2012). Moreover, about 11 million of US inhabitants and 170 million people all over the world suffer from age-related macular degeneration (AMD) (Pennington and DeAngelis, 2016) and these numbers are destined to increase up to 196 million in 2020 and 288 million in 2040 (Jonas et al., 2017), also in this case due to the longer life span.
DR is one of the most common microvascular complications of diabetes and the number of affected patients is predicted to amount to 191.0 million in 2030 (Zheng et al., 2012), although there is evidence that DR is also a neurodegenerative disease of the retina. Microaneurysms, intraretinal microvascular abnormalities as dilated preexisting capillaries, capillary occlusion, exudates, oedema, and intraretinal neoangiogenesis leading to the formation of tortuous blood vessels are typical of DR. This disease is characterized by an early asymptomatic stage in which patients do not realize their condition followed by progression to nonproliferative DR (mild, moderate, severe, and very severe), severe proliferative DR (when neoangiogenesis occurs in the innermost retina) and macular oedema with consequent vision loss (Herat et al., 2018). This occurs with AMD characterized by degeneration of retinal pigment epithelium cells and choroidal neovascularization as well (Jonas et al., 2017). New vessels are more fragile, thus being subjected to leakage and inducing the formation of scars that can cause tractional retinal detachment (Herat et al., 2018). Another important cause of visual morbidity is branch or central retinal vein occlusion (RVO) with abrupt onset (Petrella et al., 2012). Because of the typical alterations of these ophthalmological diseases and the capillary occlusion found in early DR, a pathogenetic mechanism has been hypothesized: capillary occlusion induces hypoxia increasing the vascular endothelial growth factor (VEGF) expression and, consequently, the retinal proliferation of new vessels (Aiello et al., 1995; Herat et al., 2018). Therefore, among others (see also steroids, though less frequently used) the pharmacological treatment mainly consists in the utilization of anti-VEGF drugs, able to inhibit neoangiogenesis and to stabilize vascular leakage, thus reducing oedema.
Indeed, panretinal photocoagulation was the gold standard, since it reduces neovascularization, until intravitreal injections of anti-VEGF agents were demonstrated to be useful even without photocoagulation (Zhao and Singh, 2018). There are several isoforms of VEGF, among which VEGF-A165 splice variant of VEGF-A is thought to be responsible for retinal pathologic vascularization (Ishida et al., 2003; Zhao et al., 2018). Ranibizumab, approved by the US Food and Drug Administration for AMD in 2006 and for all the stages of DR in 2017, is a recombinant antibody fragment of the humanized anti-VEGF monoclonal antibody, based on bevacizumab but with affinity to all isoforms of VEGF (Zhao and Singh, 2018). Anti-VEGF drugs used include ranibizumab, bevacizumab and aflibercept. A recent Cochrane network meta-analysis reported the effectiveness of anti-VEGF drugs in improving vision in people with DME, with moderate-certainty evidence of some advantage at 1 year of aflibercept over ranibizumab and bevacizumab (Virgili et al., 2017). Some studies carried out in Italy have shown that 3.5 million people suffer from DM (Vujosevic and Midena, 2016); DR prevalence has been highlighted to amount to 27.6% in the studied population of a decade long telemedicine screening (Vujosevic et al., 2017) and to 15.5% in the No Blind telemedicine study (Sasso et al., 2018); 10.4% and 4.1% of the population in the PAMDI study suffers from intermediate or serious AMD, respectively (Piermarocchi et al., 2011).
Hence, whilst a few studies have been carried out in other areas of Italy, the regional Calabrian context has never been assessed for epidemiology and pharmacotherapy of retinal diseases. Therefore, the present retrospective observational study aims at assessing the prevalence of DR and AMD as well as attempts some pharmacoeconomic analysis of pharmacotherapeutic management of these diseases using ranibizumab in the population in Calabria, Italy.
Materials and Methods
A survey has been conducted in collaboration with the “Mater Domini” University Hospital (Calabria, Italy), and the prescriptions of ranibizumab from January 2014 to June 2017 have been taken into consideration. Data were extracted from the database of ophthalmology ward. The database belongs to the Pharmacy Unit, “Mater Domini” University Hospital, Catanzaro, Italy and the relevant personell is in the authorship. The distribution for frequencies of the diseases within the cohort has been assessed as well as the frequency of use of ranibizumab per disease during the period of study. The cost of ranibizumab injection per patient in the given period was calculated through Microsoft Office Excel (2010) spreadsheet. Procedures were performed in accordance with the “Mater Domini” University Hospital ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Results
Distribution of ophthalmological diseases
The cohort of patients considered during this retrospective observational study was characterized based on the disease. In particular, the 870 patients in this study consisted of: 377 patients (184 males and 193 females) suffering from AMD; 183 patients (123 males and 60 females) affected by DR; 185 patients (136 males and 49 females) suffering from DME; 125 patients (63 males and 62 females) affected by RVO. The percentage distribution of these pathological conditions is shown in Figure 1.
Figure 1.
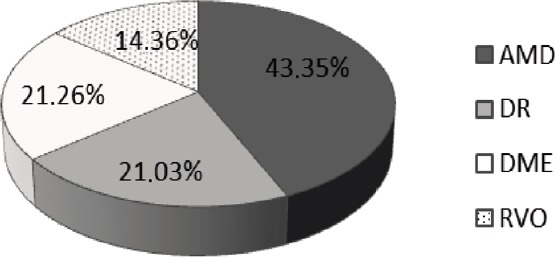
Percentage distribution of retinal diseases in the cohort of patients of “Mater Domini” University Hospital.
Data were collected during a retrospective observational survey from January 2014 to June 2017. AMD: Age-related macular degeneration; DR: diabetic retinopathy; DME: diabetic macular edema; RVO: retinal vein occlusion.
Accordingly, the most frequent retinal diseases are AMD and DR and the DME group can be considered as a progression of DR during the course of the diseases. Sex distribution of these retinal pathologies is displayed in Table 1.
Table 1.
Distribution of AMD, DR, DME, and RVO among sexes in the cohort of patients
| Year | AMD group | DR group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | TOT | F | M | TOT | ||||||||
| 2014 | 14 | 20 | 34 | 9 | 23 | 32 | |||||||
| 2015 | 47 | 37 | 84 | 28 | 48 | 76 | |||||||
| 2016 | 64 | 56 | 120 | 16 | 32 | 48 | |||||||
| 2017 | 68 | 71 | 139 | 7 | 20 | 27 | |||||||
| TOT | 193 | 184 | 377 | 60 | 123 | 183 | |||||||
| Year | DME group | RVO group | |||||||||||
| F | M | TOT | F | M | TOT | ||||||||
| 2014 | 0 | 0 | 0 | 8 | 12 | 20 | |||||||
| 2015 | 1 | 6 | 7 | 12 | 21 | 33 | |||||||
| 2016 | 17 | 42 | 59 | 21 | 19 | 40 | |||||||
| 2017 | 31 | 88 | 119 | 21 | 11 | 32 | |||||||
| TOT | 49 | 136 | 185 | 62 | 63 | 125 | |||||||
AMD: Age-related macular degeneration; DR: diabetic retinopathy; DME: diabetic macular oedema; RVO: retinal vein occlusion; F: female; M: male; TOT: total.
Pharmacotherapeutic treatment with ranibizumab
According to the prescriptions, the use of intravitreal injections of ranibizumab in the regimen of 0.5 mg administered by intravitreal injection once a month (approximately every 28 days) (Hernandez et al., 2018), decreased from 2014 to June 2017 in both DR and AMD. It remained more frequent in AMD patients compared in DR patients, as highlighted in Figure 2.
Figure 2.
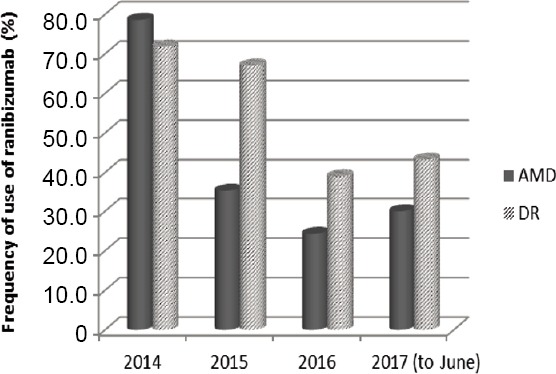
Frequency (%) of therapeutic use of ranibizumab in the most common retinal diseases.
The utilization of ranibizumab is more frequent in AMD than in DR, but it is subjected to a reduction over time. AMD: Age-related macular degeneration; DR: diabetic retinopathy.
Pharmacoeconomic analysis
The cost of the intravitreal administration of ranibizumab in AMD and DR was analyzed and the cost per patient calculated on the bases of the number of injections. The cost per person ranked almost the same value in the two retinopathies and it decreased (Figure 3), likely due to the reduced frequency of treatment with ranibizumab, previously underlined.
Figure 3.
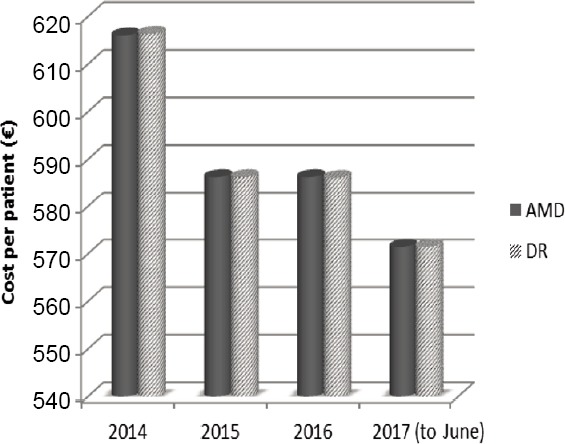
Cost per patient with AMD or DR receiving treatment with ranibizumab from January 2014 to June 2017.
The cost per patient is quite equal in AMD and DR and it is subjected to a reduction over time. AMD: Age-related macular degeneration; DR: diabetic retinopathy.
Discussion
Neovascularization, vitreous haemorrage and macular oedema are characteristic features of retinal diseases such as DR, AMD and RVO that can impair visual acuity up to complete vision loss and blindness. The burden of these diseases is even more remarkable if it is considered that one of the most relevant risk factors is represented by older age (also responsible for the rising incidence of diabetes), since life expectancy has become significantly longer over the last years. Furthermore, DR represents the leading cause of vision loss also in working middle-aged people (Cheung et al., 2010).
Another noteworthy issue is the late diagnosis of these retinal diseases and this may stem from the lack of symptoms in the early stages. Indeed, early detection and prompt settlement of appropriate treatment are fundamental to minimize vision loss worsening (Ding and Wong, 2012). Risk factors for the pathogenesis and development of DR reside in inflammation and endothelial cell dysfunction (van Hecke et al., 2005; Spijkerman et al., 2007), together with dyslipidemia for the development of DME (Ding and Wong, 2012). The clinical research effort has provided data about the prevalence of retinal diseases worldwide (Yau et al., 2012), in all the different continents (Rogers et al., 2010; Pennington and DeAngelis, 2016; Colijn et al., 2017) and ethnic groups, since AMD, in particular, results more predominant in Caucasian populations (la Cour et al., 2002). Going more in depth into the Italian situation, it is possible to find out that 3.5 million people are afflicted by DM though data concerning DR at national level are lacking (Vujosevic and Midena, 2016). However, some Italian data about DR have emerged more recently: DR prevalence has resulted the 27.6% in the studied population of a decade long telemedicine screening (Vujosevic et al., 2017) and the 15.5% in the No Blind telemedicine study (Sasso et al., 2018). However, the PAMDI Study assessed AMD prevalence in Italy on 845 patients enrolled in Northeast Italy: the 10.4% of the studied population presented intermediate AMD, while the 4.1% suffered from serious forms of AMD (Piermarocchi et al., 2011). Our retrospective observational study considered the population arriving to clinical observation at “Mater Domini” University Hospital to have an insight on the local Calabrian landscape. The results showed that the 43.35% of the 870 patients studied from January 2014 to June 2017 were affected by AMD, the 21.03% by DR, the 21.26% by DME as progression of DR, and the 14.36% by RVO, thus confirming that RVO represents the second most common cause of retinal vascular disease after DR (Keane and Sadda, 2011). The pharmacotherapy of retinal diseases consists in an anti-angiogenic therapy. Therefore, the use of ranibizumab in the two most common pathological conditions in our cohort of patients was analyzed within the considered time period. The results show that ranibizumab is more often used in AMD than in DR, though a trend towards a decreased pharmacoutilization was observed during the period 2014–2017. Pharmacoeconomic assessment shows that the cost per patient is almost equivalent for treatment of AMD and DR with a trend towards reduction within the considered period. These findings may be explained by a shift of the therapy to other medications. In particular, the therapeutic approach to AMD has remarkably changed over the years: from photodynamic therapy the therapy moved to the intravitreal administration of anti-VEGF drugs with better efficacy, from the oldest pegabptanib to the newest aflibercept (Vottonen, 2018). Endowed with the effectiveness of its predecessor ranibizumab, but needing fewer administrations, aflibercept is a fully human decoy receptor; at variance with other agents, it binds and inhibits the activity of not only all the VEGF-A isoforms but also of the VEGF-B and placental growth factor 1 and 2 (Vottonen, 2018). Under a cost perspective, according to the National Institute for Health and Care Excellence (NICE; [TA294]) the price of a vial of ranibizumab was established to amount to 742.17 £ and of a vial of aflibercept to 816.00 £ in 2013, suggesting that further studies are needed to better assess accuracy of prescription in the pharmacotherapy of retinal diseases. Indeed, pharmacoeconomic aspects of the use of anti-VEGFs in DR and AMD, i.e. incremental cost-effectiveness ratio, depending on treatment cost and quality-adjusted life-years, are also important.
Footnotes
Conflicts of interest: The authors declare no conflict of interest and no proprietary interest.
Financial support: None.
Institutional review board statement: The study followed the principles of the Declaration of Helsinki and guidelines on Good Clinical Practice. Procedures followed were in accordance with the “Mater Domini” University Hospital ethical committee standards of the human experimentation.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The biostatisticians of University of Calabria reviewed the statistical methods of this study.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, and tables), will be available upon request. Data will be available immediately following publication, no end date; for anyone who wishes to access the data. In order to gain access, data requestors will need to sign a data access agreement. Proposals should be directed to wibrahim@med.wayne.edu.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M; S-Editor: Li CH; T-Editor: Liu XL
References
- 1.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 2.Atlanta GCfDCaP US Department of Health and Human Services. Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2017 [Google Scholar]
- 3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 4.Colijn JM, Buitendijk GHS, Prokofyeva E, Alves D, Cachulo ML, Khawaja AP, Cougnard-Gregoire A, Merle BMJ, Korb C, Erke MG, Bron A, Anastasopoulos E, Meester-Smoor MA, Segato T, Piermarocchi S, de Jong PTVM, Vingerling JR, Topouzis F, Creuzot-Garcher C, Bertelsen G, et al. Prevalence of age-related macular degeneration in europe: the past and the future. Ophthalmology. 2017;124:1753–1763. doi: 10.1016/j.ophtha.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diabetes Rep. 2012;12:346–354. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 6.Herat LY, Matthews VB, Rakoczy PE, Carnagarin R, Schlaich M. Focusing on sodium glucose cotransporter-2 and the sympathetic nervous system: potential impact in diabetic retinopathy. Int J Endocrinol. 2018;2018:9254126. doi: 10.1155/2018/9254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez L, Lanitis T, Cele C, Toro-Diaz H, Gibson A, Kuznik A. Intravitreal aflibercept versus ranibizumab for wet age-related macular degeneration: a cost-effectiveness analysis. J Man Care Special Pharm. 2018;24:608–616. doi: 10.18553/jmcp.2018.24.7.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Inv Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6:493–497. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 10.Keane PA, Sadda SR. Retinal vein occlusion and macular edema - critical evaluation of the clinical value of ranibizumab. Clin Ophthalmol. 2011;5:771–781. doi: 10.2147/OPTH.S13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.la Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–133. doi: 10.2165/00002512-200219020-00003. [DOI] [PubMed] [Google Scholar]
- 12.Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 2016;3:34. doi: 10.1186/s40662-016-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrella RJ, Blouin J, Davies B, Barbeau M. Incidence and characteristics of patients with visual impairment due to macular edema secondary to retinal vein occlusion in a representative canadian cohort. J Ophthalmol. 2012;2012:723169. doi: 10.1155/2012/723169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piermarocchi S, Segato T, Scopa P, Masetto M, Ceca S, Cavarzeran F, Peto T PAMDI Study Group. The prevalence of age-related macular degeneration in Italy (PAMDI) study: report 1. Ophthal Epidemiol. 2011;18:129–136. doi: 10.3109/09286586.2011.574334. [DOI] [PubMed] [Google Scholar]
- 15.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319.e311. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, Sardu C, Rinaldi L, Galiero R, Acierno C, de Sio C, Adinolfi LE on behalf of NO BLIND Study Group. Applicability of telemedicine in the screening of diabetic retinopathy (DR): The first multicentre study in Italy. The No Blind Study. Diabetes Metab Res Rev. 2018:e3113. doi: 10.1002/dmrr.3113. [DOI] [PubMed] [Google Scholar]
- 17.Spijkerman AM, Gall MA, Tarnow L, Twisk JW, Lauritzen E, Lund-Andersen H, Emeis J, Parving HH, Stehouwer CD. Endothelial dysfunction and low-grade inflammation and the progression of retinopathy in Type 2 diabetes. Diabet Med. 2007;24:969–976. doi: 10.1111/j.1464-5491.2007.02217.x. [DOI] [PubMed] [Google Scholar]
- 18.van Hecke MV, Dekker JM, Nijpels G, Moll AC, Heine RJ, Bouter LM, Polak BC, Stehouwer CD. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia. 2005;48:1300–1306. doi: 10.1007/s00125-005-1799-y. [DOI] [PubMed] [Google Scholar]
- 19.Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2017;6:CD007419. doi: 10.1002/14651858.CD007419.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vottonen P. Anti-vascular endothelial growth factors treatment of wet age-related macular degeneration: from neurophysiology to cost-effectiveness. Acta Ophthalmologica. 2018;96(Suppl A109):1–46. doi: 10.1111/aos.13706. [DOI] [PubMed] [Google Scholar]
- 21.Vujosevic S, Midena E. Diabetic retinopathy in Italy: epidemiology data and telemedicine screening programs. J Diabetes Res. 2016;2016:3627465. doi: 10.1155/2016/3627465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vujosevic S, Pucci P, Casciano M, Daniele A, Bini S, Berton M, Cavarzeran F, Avogaro A, Lapolla A, Midena E. A decade-long telemedicine screening program for diabetic retinopathy in the north-east of Italy. J Diabetes Complications. 2017;31:1348–1353. doi: 10.1016/j.jdiacomp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Singh RP. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context. 2018;7:212532. doi: 10.7573/dic.212532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]


