It has been many years since “the tumor necrosis factor-α-converting enzyme”, also known as ADAM17/TACE, was described as “the enzyme that does it all” because of its role in neurodegenerative diseases and in several physiological processes including proteolysis, adhesion, intracellular signaling, migration and proliferation. ADAM17/TACE is an integral membrane protein that belongs to the disintegrin and metalloprotease (ADAM) family. Several years ago, Romero-Grimaldi et al. (2011) discovered that ADAM17 was involved in the glial/neuronal fate decision of neural progenitor cells (NPCs) in vitro, and this was mediated at least in part, by its capacity to facilitate the release of the epidermal growth factor receptor (EGFR) ligand, transforming growth factor alpha (TGFα), thus regulating EGFR activation. EGFR, also referred to as ErbB1, belongs to a family of transmembrane receptors, which activate intracellular signaling cascades leading to the phosphorylation of mitogen-activated protein kinase-extracellular signal-regulated kinase or phosphoinositide 3-kinase/protein kinase B. and regulate cell cycle through cyclin expression (Rabaneda et al., 2016).
Brain injuries of different origins activate neural stem cells within neurogenic areas of the adult brain (Llorens-Bobadilla et al., 2015) in an attempt to generate new neurons. However, neurogenesis in injuries does not naturally occur because of the presence of inflammatory molecules, which generate a gliogenic environment that impairs neurogenesis. The search for new therapeutic strategies that enable neuronal preplacement by promoting neurogenesis is now the central focus within the field of neuroregeneration. These strategies include in vivo reprogramming of glial cells, exogenous neural stem cell transplants, or the promotion of endogenous neurogeneis (Torper and Gotz, 2017). In this perspective article, we discuss a recent report in which authors demonstrate that ADAM17/TACE plays a key role in impairing neurogenesis in brain injuries, thus becoming a new therapeutic target, to promote endogenous neurogenesis to regenerate brain injuries. Previous studies described that a strong expression of ADAM17 can be observed within the cerebral cortex after producing a controlled mechanical brain injury. This is accompanied by the overexpression of its substrate, the EGFR ligand TGFα (Romero-Grimaldi et al., 2011). They showed using NPC cultures that in vitro the combination of ADAM17 and TGFα activates EGFR and promotes glial cell differentiation whereas the inhibition of EGFR facilitates neuronal differentiation. The effect of the EGFR inhibitor was mimicked by ADAM17 inhibition. These suggested that ADAM17 inhibition in injuries might facilitate neurogenesis. In light of these facts, a more in-depth study was required in order to understand the effects of this metalloprotease inhibitor and to understand whether treatment of brain injuries with an ADAM17 inhibitor could facilitate neurogenesis.
The new work of Geribaldi-Doldán et al. (2018) shows that local inhibition of ADAM17 in injuries promotes the generation of neurons. In this study they also use controlled mechanical unilateral cortical injuries in which they characterize the time course of neurogenesis. They show that around the perilesional area of these cortical injuries an increase in proliferative cells can be observed from 2 to 7 days post injury (dpi), decreasing abruptly beyond 14 dpi. However, no neuroblasts are found surrounding the injured area, and most of the proliferative cells are glial fibrillary acidic portein (GFAP)+, agreeing with other models of stab wound injury in which a reactive gliosis is described in injuries (Buffo et al., 2008). In view of all evidences collected over the years they hypothesize that applying a metalloprotease inhibitor during 14 days would modify the non-neurogenic niche. Geribaldi-Doldán et al. (2018) report that the specific inhibition of ADAM17/TACE in the injured motor cortex promotes neurogenesis. Continuous infusion of a general metalloprotease inhibitor locally at the site of injury for 14 days, increases the number of proliferating cells (detected by labelling with BrdU the last day of treatment) and undifferentiated neural progenitors (nestin+ cells). Interestingly, BrdU+ cells are able to survive for another 14 days and differentiate. Considering the reactive gliosis unchained in controlled cortical impact models of injury, they study the gliogenic response and find that although metalloprotease inhibition treatment does not affect astrogliosis, it reduces the number of newly formed astrocytes. As indicated above no neuroblasts are present within the perilesional area of untreated mice. However, when lesions are treated with the metalloprotease inhibitor for 14 and 28 dpi, a great number of neuroblasts are found, which have the capacity to differentiate into mature neurons if metalloprotease activity is abolished. In agreement with other publications, authors show that this increase in proliferation and the emergence of neuroblasts is partially a consequence of cell migration from physiological neurogenic niches such as the subventricular zone (Dixon et al., 2015). Geribaldi-Doldán et al. (2018) design a refined BrdU labeling strategy to detect migrating cells. Using this approach, they do not observe any migrating cells within the injury when metalloproteases are fully active in the absence of the treatment, whereas treatment with a metalloprotease inhibitor enables migration of cells from the subventricular zone towards the perilesional area. The phenotypes of these cells included GFAP (astrocytes), nestin (neural precursors) and doublecortin (neuroblast, DCX) cells. Geribaldi-Doldán et al. (2018) finally demonstrate the specific role of ADAM17/TACE in the generation of neurons within the lesion. The results obtained in this report contrast with the report of Li et al. (2013), which shows that during development, NPCs express ADAM17 and that this enzyme participates in migration of NPCs during developmental stages. To explain these discrepancies it is reasonable to consider that Geribaldi-Doldán et al. (2018) are not blocking ADAM17 in their migration studies, they are applying a general metalloprotease inhibitor and, as they discuss in their article, it is possible that the inhibition of a matrix metalloprotease is facilitating migration. In order to specifically inhibit ADAM17, they design a lentiviral vector to overexpress the pro-domain of ADAM17 (ADAM17-Pro), a potent inhibitor of its metalloprotease activity. ADAM17-Pro transfection in vitro promotes differentiation of NPC towards a neuronal fate. Additionally, ADAM17-Pro lentiviral infection in vivo induces not only a significant increase in proliferative cells but also a large increase in DCX+ neuroblasts within the perilesional area 14 dpi. These neuroblasts exhibit a marked polarity and other features of highly differentiated neuronal progenitors. Accordingly, 28 dpi neuroblasts differentiate into mature neurons; most of these new neurons are cholinergic and a small percentage is GABAergic. These findings are in agreement with previous reports, which evidence the presence of cholinergic neurons in neurogenic niches such as the subventricular zone, where they play a significant role in the promotion of neurogenesis (Figure 1).
Figure 1.
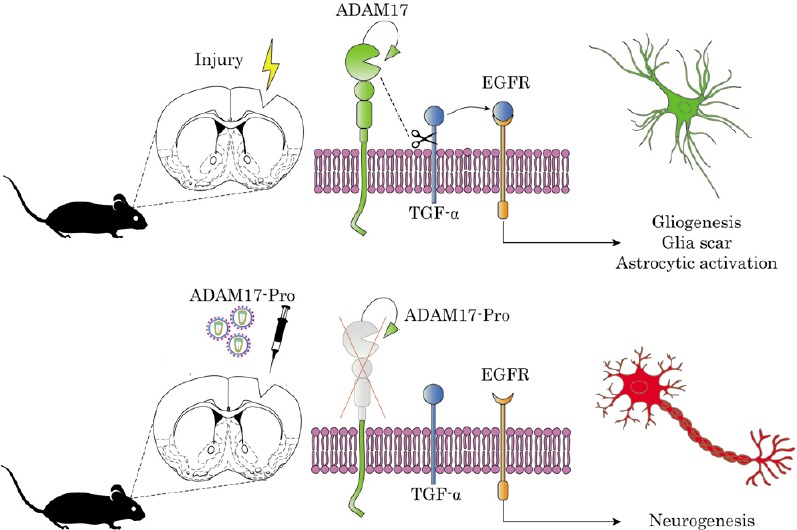
Inhibition of ADAM17 activity induces neurogenesis within the perilesional area after stab wound injury.
Injury boosts ADAM17 (tumor necrosis factor-α-converting enzyme) expression generating a gliogenic response mediated by the sheading of TGF-α ligand that activates EGFR signaling. The injection of ADAM17-Pro lentivirus in the injured cortex impairs TGFα release as well as the activation of EGFR favoring the neurogenic pathway. EGFR: Epidermal growth factor receptor; TGF-α: transforming growth factor alpha.
Collectively, these findings highlight the impact and benefits of using ADAM17 as a therapeutic target for the treatment of neuronal loss in different types of central nervous system injuries. It is now important to follow up on this study analyzing the EGFR associated signaling pathways: the mitogen-activated protein kinase-extracellular signal-regulated kinase or phosphoinositide 3-kinase/protein kinase B pathway. Both of these pathways have been described to participate in neurogenesis by determining NPC proliferation (Rabaneda et al., 2016) In addition, proteins that regulate selectivity of ADAM17 for the different EGFR ligands such as the protein kinase C (PKC) should also be considered. Activation of PKC mediated by diacilglycerol mimicking compounds promotes proliferation process in vitro in the absence of epidermal growth factor and in vivo in neurogenic niches (Geribaldi-Doldan et al., 2015; Murillo-Carretero et al., 2017). This capacity of PKC to regulate NPC proliferation might be mediated by its capacity to determine the selectivity of ADAM17 for the EGFR ligand TGFα (Dang et al., 2013), facilitating the release of this growth factor.
A role for ADAM17 in brain injuries has been reported previously. Reports show an elevated expression of ADAM17 short after producing brain injury in different models: ischemic injury, LPS induced inflammation, dentate gyrus denervation, or brain ischemic preconditioning. Elevated ADAM17 expression has been associated with the inflammatory response, the presence of reactive astrocytes, with protection against apoptosis, and in brain ischemic preconditioning models with the tolerance to subsequent injuries (Pradillo et al., 2005). Additionally, a role for ADAM17 on neurogenesis after brain injury, has only been highlighted by Romero-Grimaldi et al. (2011) and Geribaldi-Doldán et al. (2018). Results from both reports are consistent and lead to the possible use of ADAM17 as a target to regenerate brain injuries.
In relation to this, researchers worldwide are now searching for new ADAM17 inhibitors to treat a variety of disorders. Small molecule ADAM17 inhibitors have been found in the past that reached clinical trials producing some undesirable effects. For this reason more selective inhibitors are being studied recently and new clinical trials are currently being accomplished. This is the case of specific antibodies against ADAM17 or the pro-domain of ADAM17 protein used by Geribaldi et al. (2018) that are very target specific. More importantly a new small molecule ADAM17 inhibitor is currently being tested as therapeutic agent in clinical trials for cancer treatment. Notwithstanding, in order to propose ADAM17 inhibitors as therapeutic drugs to treat brain injury, it needs to be considered that although inhibition of ADAM17 may have deleterious consequences for long-term systemic administration, it may be able produce desirable effects if administered more locally (i.e. intranasal administration or local infusion to reach brain injuries). Nonetheless, targeting ADAM17 with a selective inhibitor still seems a viable and attractive approach to use in the clinic for brain injury regeneration.
This work was supported by the Spanish Consejería de Innovación, Ciencia y Empleo, Junta de Andalucía (P10CTS6639); Ministerio de Econmía y Competitividad (BFU 2015-6852-R, MINECO/FEDER) (both to CC)
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Liu XL
References
- 1.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dang M, Armbruster N, Miller MA, Cermeno E, Hartmann M, Bell GW, Root DE, Lauffenburger DA, Lodish HF, Herrlich A. Regulated ADAM17-dependent EGF family ligand release by substrate-selecting signaling pathways. Proc Natl Acad Sci U S A. 2013;110:9776–9781. doi: 10.1073/pnas.1307478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon KJ, Theus MH, Nelersa CM, Mier J, Travieso LG, Yu TS, Kernie SG, Liebl DJ. Endogenous neural stem/progenitor cells stabilize the cortical microenvironment after traumatic brain injury. J Neurotrauma. 2015;32:753–764. doi: 10.1089/neu.2014.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geribaldi-Doldan N, Carrasco M, Murillo-Carretero M, Dominguez-Garcia S, Garcia-Cozar FJ, Munoz-Miranda JP, Del Rio-Garcia V, Verastegui C, Castro C. Specific inhibition of ADAM17/TACE promotes neurogenesis in the injured motor cortex. Cell Death Dis. 2018;9:862. doi: 10.1038/s41419-018-0913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geribaldi-Doldan N, Flores-Giubi E, Murillo-Carretero M, Garcia-Bernal F, Carrasco M, Macias-Sanchez AJ, Dominguez-Riscart J, Verastegui C, Hernandez-Galan R, Castro C. 12-Deoxyphorbols promote adult neurogenesis by inducing neural progenitor cell proliferation via PKC activation. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv085. doi: 10.1093/ijnp/pyv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Erclik T, Bertet C, Chen Z, Voutev R, Venkatesh S, Morante J, Celik A, Desplan C. Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature. 2013;498:456–462. doi: 10.1038/nature12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17:329–340. doi: 10.1016/j.stem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Murillo-Carretero M, Geribaldi-Doldan N, Flores-Giubi E, Garcia-Bernal F, Navarro-Quiroz EA, Carrasco M, Macias-Sanchez AJ, Herrero-Foncubierta P, Delgado-Ariza A, Verastegui C, Dominguez-Riscart J, Daoubi M, Hernandez-Galan R, Castro C. ELAC (3, 12-di-O-acetyl-8-O-tigloilingol), a plant-derived lathyrane diterpene, induces subventricular zone neural progenitor cell proliferation through PKCbeta activation. Br J Pharmacol. 2017;174:2373–2392. doi: 10.1111/bph.13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, Davalos A, Castillo J, Lorenzo P, Lizasoain I. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- 10.Rabaneda LG, Geribaldi-Doldan N, Murillo-Carretero M, Carrasco M, Martinez-Salas JM, Verastegui C, Castro C. Altered regulation of the Spry2/Dyrk1A/PP2A triad by homocysteine impairs neural progenitor cell proliferation. Biochim Biophys Acta. 2016;1863:3015–3026. doi: 10.1016/j.bbamcr.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Grimaldi C, Murillo-Carretero M, Lopez-Toledano MA, Carrasco M, Castro C, Estrada C. ADAM-17/tumor necrosis factor-alpha-converting enzyme inhibits neurogenesis and promotes gliogenesis from neural stem cells. Stem Cells. 2011;29:1628–1639. doi: 10.1002/stem.710. [DOI] [PubMed] [Google Scholar]
- 12.Torper O, Gotz M. Brain repair from intrinsic cell sources: Turning reactive glia into neurons. Prog Brain Res. 2017;230:69–97. doi: 10.1016/bs.pbr.2016.12.010. [DOI] [PubMed] [Google Scholar]


