Abstract
Resident and inflammatory macrophages are essential effectors of the innate immune system. These cells provide innate immune defenses and regulate tissue and organ homeostasis. In addition to their roles in diseases such as cancer, obesity and osteoarthritis, they play vital roles in tissue repair and disease rehabilitation. Macrophages and other inflammatory cells are recruited to tissue injury sites where they promote changes in the microenvironment. Among the inflammatory cell types, only macrophages have both pro-inflammatory (M1) and anti-inflammatory (M2) actions, and M2 macrophages have four subtypes. The co-action of M1 and M2 subtypes can create a favorable microenvironment, releasing cytokines for damaged tissue repair. In this review, we discuss the activation of macrophages and their roles in severe peripheral nerve injury. We also describe the therapeutic potential of macrophages in nerve tissue engineering treatment and highlight approaches for enhancing M2 cell-mediated nerve repair and regeneration.
Keywords: nerve regeneration, macrophage, origin, polarization, function, nerve injury, nerve repair, tissue engineering, neural regeneration
Introduction
Macrophages have the powerful ability to phagocytize foreign bodies (Wynn et al., 2013). They are found in all tissues, where they act as sentinels that protect the tissue and ensure organ homeostasis. Tissue-specific macrophage types originate during development and include osteoclasts (bone), alveolar macrophages (lung), histiocytes (interstitial connective tissue), Kupffer cells (liver and gut), splenic macrophages (spleen), microglia (brain) and differentiated Schwann cells (peripheral nervous system) (Chen et al., 2015). Macrophages function as phagocytic antigen-presenting cells and have key roles in scavenging heterologous pathogens and transmitting danger signals; they are also involved in the formation of memory cells (Iijima and Iwasaki, 2014). However, they may also contribute to secondary infections (Gaya et al., 2015) and cancer metastasis (Keklikoglou and De Palma, 2014). Because of their roles in tissue repair, macrophages represent viable targets for disease treatment (Suzuki et al., 2014; Sadtler et al., 2016). A study by Suzuki et al. (2014) has indicated the potential therapeutic use of pulmonary macrophage transplantation for the treatment of hereditary pulmonary alveolar proteinosis. The application of macrophage-based therapies against peripheral nerve injury is attractive because the proximal and distal ends of the peripheral nerve participate in completely distinct events: proximal regeneration and distal denaturation (DeFrancesco-Lisowitz et al., 2015). Macrophages involved in distal degeneration promote the switch from the pro-inflammatory (M1) to the anti-inflammatory (M2) phenotype, which enhances proximal nerve regeneration (Mokarram et al., 2012). In the M2 macrophage subtypes, in addition to their common anti-inflammatory effects, they also have their respective functions. Briefly, M2a, M2b, M2c and M2d macrophages promote cell proliferation, cell maturation, resolution of inflammation and angiogenesis, respectively (Ferrante and Leibovich, 2012; Novak and Koh, 2013a, b; Chen et al., 2015; Gensel and Zhang, 2015). The M2 macrophage subtypes have great therapeutic potential given their documented anti-inflammatory effects and their respective functions in promoting tissue regeneration in engineered nerve tissue models (Mokarram et al., 2012).
In this review, we discuss the origin, polarization, type and function of macrophages, highlight their roles in peripheral nerve injury and describe their therapeutic value in neural tissue engineering.
We performed a literature search in PubMed from 2002 to December 2018 of studies published in English. The key words were macrophages [MeSH Terms], macrophage polarization and function, and peripheral nerve injuries [MeSH Terms]. The results were further screened by title/abstract, and non-Scientific Citation Index experiments and review articles. Furthermore, studies concerning central nervous system diseases and neoplasms were excluded.
Origin, Polarization, and Function of Macrophages
Tissue-resident macrophages and circulating macrophages (Geissmann et al., 2010; Davies and Taylor, 2015) originate from bone marrow hematopoietic stem cells (Wynn et al., 2013) (Figure 1). Before circulating macrophages enter the bloodstream, they must be stimulated by cytokines such as multi-colony stimulating factor (multi-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) (Wu et al., 2017). Upon stimulation, these cells develop into monocytes, and then differentiate further into pre-macrophages. Finally, they become mature macrophages and are released into the circulation (Chen et al., 2015; Helft et al., 2015).
Figure 1.
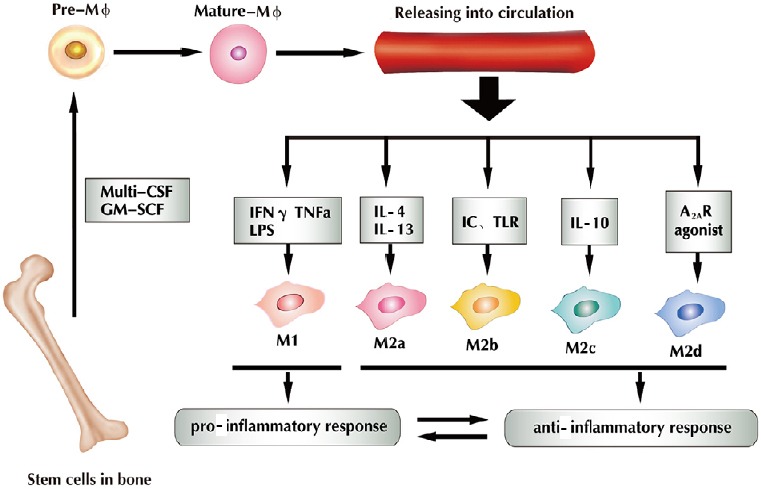
Under the regulation of cytokines (multi-CSF and GM-SCF), bone marrow-derived macrophages differentiate into mononuclear cells, and then gradually become mature macrophages that can be released into the circulation.
IFN-γ, TNFα and LPS stimulate macrophages into M1, IL-4 and IL-13 into M2a, IC and TLR into M2b, and IL-10 into M2c; A2AR agonist stimulates them into M2d. M1 macrophages induce a pro-inflammatory response, whereas M2 macrophages induce an anti-inflammatory response, and both exist in dynamic equilibrium (Chen et al., 2015). Multi-CSF: Multi-colony stimulating factor; GM-CSF: granulocyte macrophage colony stimulating factor; IFN-γ: interferon gamma; TNFα: tumor necrosis factor alpha; LPS: lipopolysaccharides; IC: immune complexes; TLR: toll-like receptor; A2AR: adenosine A2A receptor; IL: interleukin; IL-1R: IL-1 receptor. Adapted from Chen et al. (2015) and Helft et al. (2015).
The macrophage polarization process transforms the macrophages’ functions according to their current environment (Li et al., 2018). M1 macrophages are pro-inflammatory that secrete cytokines. M2 macrophages are anti-inflammatory macrophages that promote tissue repair. Because of this dual function, macrophages serve as potent immune effector cells. They play important roles in tissue homeostasis and disease rehabilitation, e.g., promoting the initiation and progression of tissue injury, as well as promoting wound healing and tissue remodeling. Macrophages show different functions at different disease stages; therefore their proper induction and elimination are necessary for efficient recovery (Duffield et al., 2005; Mantovani and Locati, 2013; Zhou et al., 2014). Heterogeneity and plasticity are hallmarks of macrophages (Gordon and Plűddemann, 2013; London et al., 2013) (Table 1). A variety of stimuli can induce different degrees of phenotypic polarization. In general, macrophage activation is classified as classic activation (M1) and selective activation (M2). M1 macrophages are induced by Toll-like receptor (TLR), ligands and interferon (IFN)-γ, and express CD32, chemokine (C-C motif) ligand 2 (CCL2), CD86, inducible nitric oxide synthase, HLA-DR, CD197, hypoxia inducible factor-1α (HIF-1α), IFN regulatory factor 1, myeloid differentiation primary response 88, TLR-2 and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (Mueller and Schultze-Mosgau, 2011; Gensel and Zhang, 2015; Bardi et al., 2018). Polarized M1 macrophages mainly secrete pro-inflammatory factors, which aggravate inflammation and promote debris removal, sterilization and elimination of apoptotic cells (Gensel and Zhang, 2015). Compared with M1 macrophages, the activation pathway of M2 macrophages is complex. MS-275, a histone deacetylase inhibitor, induces the transformation of M1 to M2 type macrophages (Zhang and Schluesener, 2012). Additional studies have indicated that M2 macrophages can be induced by interleukin (IL)-4/IL-13 (M2a) to express insulin-like growth factor-1 (IGF-1), IL1RN and CD206. M2a macrophages promote anti-inflammatory effects such as cell proliferation and migration, production of growth factors and removal of apoptotic cells. M2b macrophages are induced by an immune complex and express CD86, TNFα, CD64, vascular endothelial growth factor (VEGF) and IGF-1. M2b macrophages promote cell maturation, tissue stabilization, angiogenesis and extracellular matrix synthesis. M2c macrophages are induced by the anti-inflammatory cytokines, IL-10 and transforming growth factor-β (TGF-β), expressing SLAM, Sphk-1, THBS1, HMOX-1, TGF-β, CD206 and CD163. They accelerate the resolution of inflammation, tissue repair and extracellular matrix synthesis, and produce growth factors. M2d macrophages are induced by the A2AR agonist and express high levels of VEGF, IL-10 and inducible nitric oxide synthase, and low levels of TNFα, IL-12 and arginase-1 (Gordon, 2003; Biswas and Mantovani, 2010; Fujiu et al., 2011; Ferrante et al., 2013; Sudduth et al., 2013; Chen et al., 2015). M2d macrophages derived from M1 macrophages have roles in angiogenesis and wound healing (Ferrante and Leibovich, 2012; Chen et al., 2015; Gensel and Zhang, 2015). With changes in the environment, M1 and M2 macrophages maintain a state of dynamic equilibrium. The dynamic switching of phenotype and function in macrophages is regulated by modulating signals (Jang et al., 2013; Mercalli et al., 2013). Recent studies have reported that M2 macrophages could also differentiate into M1 macrophages upon stimulation with lactoferrin-containing immune complexes, thus emphasizing their dynamic nature (Gao et al., 2018).
Table 1.
Characteristics of macrophages
| Activation pathway | Stimulating factors | Surface markers/common features | Release factors | Functions | |
|---|---|---|---|---|---|
| M1 | Classic activation | TLR ligands, TNFα, INF-γ | CD32, CCL2, CD86, Nos2, HLA-DR, CD197, HIF-1α | TNFα, IL-1α, IL-1β, nitric oxide, metalloproteinases, reactive oxygen species | Pro-inflammatory, phagocytosis |
| M2 | |||||
| M2a Selective activation | IL-10, IL-4 | IGF-1, IL1RN, CD 206 | IL-10, TGF-β | Cell proliferation and migration, removal of apoptotic cells | |
| M2b | Immune complex | CD86, TNFα, CD64, VEGF, IGF-1 | IL-10, VEGF | Cell maturation, issue stabilization, ECM synthesis | |
| M2c | IL-10/TGF-β | SLAM, Sphk-1, THBS1, TGF-β, HMOX-1, CD206, CD163 | IL-10 | Resolution of inflammation, ECM synthesis, production of growth factors | |
| M2d | A2AR agonist | TNFα, IL-12, arginase-1 | IL-10, VEGF | Angiogenesis, wound healing | |
IL: Interleukin; IL1RN: interleukin 1 receptor antagonist; TGF-β: transforming growth factor-β; NOS: nitric oxide synthase; IGF-1: insulin-like growth factor-1; SLAM: signaling lymphocytic activation molecule; Sphk-1: sphingosine kinase 1; HMOX1: heme oxygenase 1; VEGF: vascular endothelial growth factor; IFN-γ: interferon gamma; TNFα: tumor necrosis factor-α; A2AR: adenosine A2A receptor; HLA-DR: human lymphocyte antigen class II beta chain paralogs; THBS1: thrombospondin 1.
Recruitment of Macrophages to Injury Sites
The processes of macrophage recruitment and polarization are necessary for macrophage-mediated injury repair (Kucharova and Stallcup, 2018). Recruitment requires the help of chemokines or other signaling proteins. For example, AMPKa1 serves as a recruitment signal in the regeneration of skeletal muscle. In the absence of AMPKa1, macrophages do not acquire the M2 phenotype and have impaired phagocytosis (Lawrence and Fong, 2010). The CC chemokine, CCL2, and its receptor, CCR2, are responsible for monocyte trafficking in response to bone fractures (Xing et al., 2010). Mice deficient in CCR suffer from osteosclerosis (Binder et al., 2009). As a consequence, macrophage recruitment to the site of injury is necessary. After a peripheral nerve injury, monocyte chemoattractant protein-1 (MCP-1, also called CCL2), leukemia inhibitory factor, IL-1α, IL-1β and pancreatitis-associated protein III serve as the major monocyte/macrophage recruitment signals (Shamash et al., 2002; Tofaris et al., 2002; Namikawa et al., 2006; Van Steenwinckel et al., 2015). Macrophage infiltration is first observed at the site of injury 2–3 days post-injury and peaks at 7 days post-injury (Perry et al., 1987; Taskinen and Röyttä, 1997; Bendszus and Stoll, 2003; Mueller et al., 2003; Namikawa et al., 2006). To boost recruitment, infiltrated macrophages may secrete CCL2, TNFα, IL-1α and IL-1β (Shamash et al., 2002; Kiguchi et al., 2013). In nerve injury models, macrophage recruitment occurs even in the absence of the key recruitment signals, MCP-1 and IL-1β, as injured nerves also produce recruitment signals (Shamash et al., 2002). In Schwann cells, macrophage recruitment is regulated by the miR-327/CCL2 axis. When stimulated by the outside microenvironment, the increase in miR-327 expression inhibits macrophage recruitment, while CCL2 promotes macrophage recruitment (Zhao et al., 2017). In CCR2-deficient mice, macrophage infiltration to injured nerve sites is significantly decreased (Siebert et al., 2000; DeFrancesco-Lisowitz et al., 2015), which indicates that CCL2 is a primary pro-recruitment molecule. In summary, cytokine signaling is necessary for proper macrophage recruitment and subsequent injury repair.
After recruitment, the number of macrophages in the injured site increases. The macrophage subtypes do not increase simultaneously. Previous studies have indicated that macrophages, in most tissues, transit from M1 to M2 macrophages over time (Arnold et al., 2007; Dal-Secco et al., 2015). In addition to the above-mentioned polarization stimulation factors derived from injury, the M2a subtype can secret cytokines such as IL-10 and TGF-β, polarizing macrophages toward the M2c subtype directly (David and Kroner, 2011). When recruited and polarized, macrophages secrete cytokines to prepare for tissue repair.
Roles of Macrophages in Peripheral Nerve Injury
Nerve injury includes central nerve injury and peripheral nerve injury, both of which present challenges with respect to clinical treatment and functional recovery (Burtt et al., 2017), particularly those associated with the spinal cord. By contrast, skin and muscle tissues respond well to tissue repair mechanisms and show good recovery. The mechanisms governing these tissue-specific differences are not well understood, but it is clear that macrophages are key mediators of repair in all tissue types. Spinal cord injury can induce a chronic wound state that undergoes expansion and maintains demyelination, leading to impaired recovery and progressive tissue degeneration (Chiu et al., 2018). A spinal cord injury consists of three phases: inflammation, proliferation and remodeling (Gensel and Zhang, 2015). The central nervous system is unique in that complete regeneration after injury is rare, and relative to other injury forms, the repair process is complex and poorly understood. By contrast, peripheral nerves have strong regenerative capacity and macrophages play a core role in their repair. Injuries will induce distal segment of the nerve undergoing a series of cellular and molecular events that result in breakdown of the distal nerve segment, which is called Wallerian degeneration (Rotshenker, 2011), thereby promoting the regeneration of peripheral nerve. For these reasons, we will focus primarily on macrophage-mediated repair in the peripheral nerve injury setting. Unlike the central nervous system, where damaged neurons are usually unable to regenerate, axons in the peripheral nervous system can regenerate after injury (Figure 2).
Figure 2.
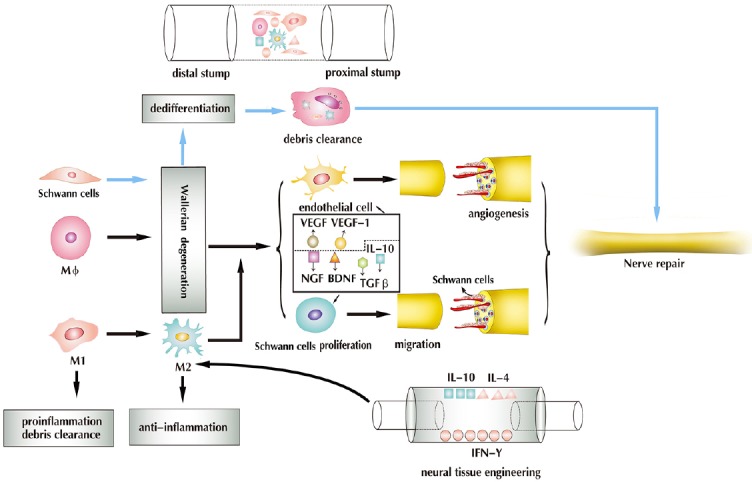
Injury alters the peripheral nerve microenvironment.
Peripheral nerve damage promotes Wallerian degeneration, axonal degeneration, BNB compromise, myelin breakdown and macrophage infiltration. In Wallerian degeneration, Schwann cells undergo dedifferentiation, and along with macrophages, participate in the clearance of debris. Macrophages promote Wallerian degeneration, detect ischemia and promote angiogenesis by releasing VEGF and VEGF-1, all of which serve to promote the migration and proliferation of Schwann cells by releasing IL-10, TGF-β, NGF and BDNF, and working as a scavenger, while maintaining an M1/M2 dynamic balance. In neural tissue engineering, the adsorption of cytokines on the nerve scaffold (e.g., IL-10, IL-4, IFN-γ) increases the ratio of M2 macrophages, thereby promoting nerve regeneration. VEGF: Vascular endothelial growth factor; TGF-β: transforming growth factor-β; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; IL: interleukin; IFN-γ: interferon-γ; BNB: blood-nerve barrier. Adapted from Cattin et al. (2015) and Chen et al. (2015).
After nerve injuries, especially severe and long-distance nerve injuries, local hypoxia and tissue necrosis secondary to inflammation are major obstacles for nerve repair and regeneration, which require a good microenvironment that is clean of necrotic tissue fragments, to promote angiogenesis, and the proliferation and migration of glial cells (Schwann cells). However, macrophages have the capacity to promote nerve repair (Cattin et al., 2015).
Macrophages respond to hypoxia by promoting angiogenesis
Damage to peripheral nerves promotes the recruitment and infiltration of many inflammatory cell types. Among the inflammatory cell types, only macrophages can sense (Cattin et al., 2015) and respond to hypoxic conditions through activation of the transcription factor, HIF-1α, which ultimately stimulates angiogenesis via VEGF activation (Pugh and Ratcliffe, 2003; Krock et al., 2011). In this case, macrophages in both the distal and proximal stumps are in hypoxic conditions (pO2 < 10 mmHg) (Young and Moller, 2010). At 2 days post-injury and before vascularization, a large number of hypoxic cells are present at the site of injury. This triggers HIF-1α synthesis in macrophages, followed by the expression of VEGF-A, which stimulates endothelial cell proliferation and migration. By day 3 post-injury, the proportion of hypoxic cells is substantially decreased, which indicates vascularization of the injured site (Cattin et al., 2015). Macrophages promote angiogenesis (Fantin et al., 2010) and maintain the health of endothelial cells by secreting VEGF-A (Lee et al., 2007). Macrophage-induced angiogenesis, which provides nutrition for the repair of the nerve tissue, is a critical step in injury repair. In addition, as macrophages are polarized into different subtypes, they can release cytokines to accelerate angiogenesis. For example, M2d macrophages secrete IL-10 and VEGF, which facilitate angiogenesis and the repair of blood vessels (Ferrante and Leibovich, 2012). Furthermore, angiogenesis provides a route for the migration of Schwann cells to the injury site.
Macrophages contribute to the migration and proliferation of Schwann cells
Macrophages promote inflammation through the production and release of pro-inflammatory cytokines. In addition, they serve as important antigen-presenting cells, stimulating an immune response to repair damage and accelerate recovery (Mueller et al., 2003). In addition to these roles, macrophages activate the proliferation and division of Schwann cells, which are vital glial cells in the peripheral nerve tissue (Armstrong et al., 2003). A previous study has indicated that after injury, Wallerian denaturation in the distal stump nerve occurs, and Schwann cells begin to dedifferentiate into Schwann cell precursors (Chen et al., 2015). After dedifferentiation, progenitor Schwann cells have a stronger phagocytosis and secretory capacity. These features aid in debris removal from the injury site and increase the release of nerve growth factor (NGF), brain-derived growth factor and ciliary nerve growth factor, which are key growth factors involved in nerve repair, or regeneration and proliferation of Schwann cells (Gong et al., 2014; Hoyng et al., 2014). Wallerian denaturation is also associated with axonal degeneration, compromise of the blood-nerve barrier, myelin breakdown and macrophage infiltration (Stoll and Muller, 1999). Therefore, it effectively promotes macrophage infiltration, which subsequently promotes migration and proliferation of Schwann cells. Additional studies have reported that M2a macrophages boost cell proliferation and cell migration by releasing IL-10 or TGF-β (Ferrante and Leibovich, 2012; Chen et al., 2015; Gensel and Zhang, 2015). In the hypoxic nerve injury microenvironment, macrophages sense low oxygen levels and promote angiogenesis, which allows endothelial cells to guide the direction of Schwann cell migration. Schwann cells use the polarized vasculature as a support to accelerate nerve repair and regeneration (Cattin et al., 2015), and synthesize the basement membrane for axon regeneration (Gong et al., 2014). Therefore, the macrophage-mediated migration and proliferation of Schwann cells has important implications in peripheral nervous system injuries.
Macrophages promote anti-inflammation and display enhanced scavenger function
In response to injury, macrophages are polarized into different subtypes to participate in damage repair. After activation, M1 macrophages show enhanced phagocytic ability along with increased scavenging of cellular debris and bacteria via upregulation of the scavenger receptor, CD36 (Sindrilaru and Scharffetter-Kochanek, 2013). M2a, M2b and M2c macrophages release anti-inflammatory cytokines, and increase cell proliferation and migration, tissue remodeling (via release of Arginase and Ym1) and secretion of growth factors (Novak and Koh, 2013a, b). Therefore, when macrophages retain the M1 state, injured nerve tissues remain in an inflammatory and chronic wound state (Wang et al., 2007; Hu et al., 2011; Sindrilaru et al., 2011; Rigamonti et al., 2014). In addition, it has been reported that macrophages modulate the expression of galectin-1 (Gaudet et al., 2015), which has been implicated in the regulation of phagocytosis, inflammation/gliosis and axon growth after spinal cord injury. Moreover, galectin-1 has been shown to promote neural stem cell proliferation (Sakaguchi et al., 2006; Yamane et al., 2010).
Macrophages Application in Neural Tissue Engineering
Self-repair is not an option when faced with severe and long-distance nerve injuries. Fortunately, significant technological progress has been made in the field of neural tissue engineering. Typical engineered nerve tissue includes a nerve scaffold, seed cells and nerve factors (Gu et al., 2014). The nerve scaffold provides support and protection, and the seed cells and related nerve factors accelerate nerve regeneration. Tissue engineering has become particularly applicable for the treatment of severe peripheral nerve damage. Although great progress has been made in nerve tissue engineering for long-distance nerve defects, improvements to optimize nerve regeneration and function restoration in target organs are still needed. Engineering involves the fabrication of a biocompatible artificial nerve scaffold that mimics the natural extracellular matrix (Gu et al., 2014), and the addition of seed cells and related factors to the nerve scaffold to promote nerve regeneration. Often, stem cells are selected as seed cells for nerve tissue engineering. Stem cells have the potential to differentiate into Schwann cells, secrete neurotrophic factors, and assist in nerve regeneration and myelin formation (Ren et al., 2012), but they cannot regulate inflammation or clean up necrotic debris (Mokarram et al., 2012). Therefore, efforts to incorporate macrophages into the engineered nerve tissue are of great importance.
Artificial nerve conduits can be used to bridge the ends of damaged peripheral nerves. Macrophages are recruited to the lesion under the action of chemokines (Shamash et al., 2002; Tofaris et al., 2002; Namikawa et al., 2006; Van Steenwinckel et al., 2015). Recruitment to the injury site leads to activation of both the M1 and M2 subtypes. The coordination of M1 and M2 regulates the initial events of local inflammation (Mokarram et al., 2012). The distal segment of the nerve undergoes a series of cellular and molecular events that result in Wallerian degeneration (Rotshenker, 2011). During this process, cytokine production contributes to the recruitment of inflammatory cells such as M1 cells (Chen et al., 2007). M1 cells release additional pro-inflammatory cytokines such as TNFα, IL-1α and IL-1β, nitric oxide, reactive oxygen species and metalloproteinases (Murray and Wynn, 2011), which promote further tissue destruction. To control this process, activated M2 macrophages release the anti-inflammatory cytokines, IL-4/IL-13 and IL-10, which mediate tissue restoration and suppression of pro-inflammatory responses (Gordon, 2003). Therefore, engineered nerve tissues would benefit from the incorporation of macrophages, given their key roles in the regulation of inflammation (Mokarram et al., 2012). The ratio of pro-healing (M2) to pro-inflammatory (M1) macrophages directly correlates with the number of regenerated axons (Mokarram et al., 2012). Thus, methods that increase the ratio of M2/M1 subtypes and promote nerve regeneration have become increasingly popular. There are two ways to increase the M2/M1 ratio in nerve injury or artificial nerve conduits. The first method incorporates the chemotaxis and polarization of macrophages by cytokines, and the second method involves direct injection of M2 into the nerve conduit. For the former approach, physical methods have been used to adsorb macrophage chemotactic factors onto nerve conduits. However, the physically adsorbed components are free to diffuse from the scaffold surface into the surrounding tissue shortly after implantation. Therefore, the chance of recruiting and polarizing macrophages to the M2 state is small (Wang et al., 2012). To address this challenge, a combination of conjugated and adsorbed IL-10 was found to be an effective approach for inducing macrophage M2 polarization in the vicinity of scaffolds (Potas et al., 2015). IL-10 desorbed from the scaffold is free to promote the polarization of macrophages beyond the scaffold surface. Macrophage stimulation by exogenous IL-10 subsequently triggers the production of endogenous IL-10 (Mantovani et al., 2002), which is significantly increased in M2 macrophages. It is commonly thought that M2 macrophages respond after M1 macrophages have fulfilled their roles. However, the presence of M2 macrophages at early time points post-injury indicates that M2 cell activation is promoted by extrinsic factors. M2 macrophages not only overcome axonal growth inhibition, but also promote regeneration (Kigerl et al., 2009). Further studies have shown that engineered scaffolds treated with IL-4 or IFN-γ directly polarized macrophages toward the M2a subtype, and indirectly toward the M2c subtype. M2a macrophages secrete cytokines such as IL-10 and TGF-β (David and Kroner, 2011), and function in cell proliferation and migration, removal of apoptotic cells, cell maturation, tissue stabilization, angiogenesis, resolution of inflammation and tissue regeneration (Ferrante and Leibovich, 2012; Chen et al., 2015; Gensel and Zhang, 2015). These results indicate that adsorption of cytokines on the scaffolds increases the ratio of M2 macrophages, leading to significantly enhanced nerve regeneration. The explanation for this observation is associated with the vital functions of macrophages in wound healing. Specifically, the optimal balance of macrophage types promotes healing by regulation of the injury microenvironment, detection of hypoxia, induction of angiogenesis (Cattin et al., 2015; Moore et al., 2018) and preparation of the surrounding parenchyma for regeneration.
The second method for enhancing the local M2 macrophage population involves direct injection of macrophages into the injured nerve or nerve conduit. This method was validated by a recent study that incorporated a model of ischemia developed by unilateral femoral artery excision. Macrophages (M0, M1, M2) and phosphate-buffered saline were injected into the ischemic muscle 24 h after injury. Four days after treatment, M1 macrophage therapy restored perfusion and the number of endothelial cells, improved muscle morphology and fibrosis, and promoted functional recovery (Lu et al., 2011). The transplantation of M1 macrophages promoted a faster transition from the M1-like to M2-like phenotype (Gensel and Zhang, 2015). Although this application cannot be used to promote nerve tissue repair or regeneration, the results indicate that during damage repair no single macrophage subtype can effectively promote the repair of damaged tissue.
Conclusion and Perspective
Macrophages are key mediators of inflammation. Macrophage recruitment to the site of injury initiates a cascade of events that contribute to proper nerve tissue repair. Macrophages not only play an important role in the repair of damaged nerves, but also represent a therapeutic target for treatment of peripheral nerve injury. Owing to their pro- and anti-inflammatory nature, macrophages are able to protect tissues and promote repair. During tissue repair, M1 macrophages secrete pro-inflammatory cytokines, thereby enhancing inflammatory reactions and tissue necrosis. By contrast, M2 macrophages induce an anti-inflammatory response, accelerate tissue repair and represent a target for therapeutic treatment. Effective tissue repair requires coordination of M1 and M2 activities. M1 macrophages have powerful phagocytic effects and promote a suitable microenvironment for M2 macrophages to repair the injured tissue. Interventions that involve injection of M1 macrophages into the sites of injury appear to have the best therapeutic effects. The injected M1 cells accelerated both the removal of necrotic tissue and transformation of the M1 to M2 phenotype. While promising, these outcomes have only been demonstrated in a model of ischemic muscle injury, and have not been observed in the context of peripheral nerve injury. In some immune system diseases, M2 polarization is suppressed and the number of M1 macrophages is increased, leading to increased inflammation and the improper repair of the injured tissues.
Because the underlying mechanisms governing this process are poorly understood, further studies in this area are required for the successful development of novel therapeutic strategies. For example, a clear understanding of the specific therapeutic targets of macrophages is critical for the development of effective treatment strategies that involve macrophage activation. There is also uncertainty regarding the best methods for ascertaining what types of stimuli effectively promote macrophage M2 polarization. To be considered valuable for clinical applications, it is important to elucidate the regulatory mechanisms that control the transformation of macrophages to M2, and promote nerve repair and regeneration.
These findings shed light on improving peripheral nerve regeneration after scaffold modification by adsorption of cytokines such as IL-4, IFN-γ or IL-10, to promote macrophage M2 polarization. However, to promote nerve regeneration in neural tissue engineering, we need a good microenvironment, as well as good seed cells and nerve regeneration-related growth factors. Adsorption of M1 and M2 macrophage chemokines on scaffolds, or addition of polarized M1, and M1 macrophages as seed cells, may provide novel therapeutic targets for severe and long-distance nerve injuries. However, the amount and proportion of the two kinds of chemokines adsorbed on the scaffolds, and of polarized M1 and M2 macrophages added to the scaffolds need further study. The macrophage subtypes that respond to hypoxia, and promote the migration and proliferation of Schwann cells, and the mechanism remain to be further studied.
Additional files: Open peer review reports 1 (75.1KB, pdf) –3 (70.9KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing interests.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 31771052 (to YW); the National Key Research & Development Program of China, No. 2017YFA0104701, 2017YFA0104702 and 2016YFC1101601; the National Basic Research Program of China (973 Program), No. 2014CB542201 (to JP); the Natural Science Foundation of Beijing, No. 7172202 (to YW); and the PLA Youth Training Project for Medical Science, No. 16QNP144 (to YW). None of the funding bodies plays any role in the study other than to provide funding.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Ozgur Boyraz, Gulhane Military Medical Academy, Turkey; Sheng Yi, Nantong University, China; Mark A. Yorek, University of Iowa, USA.
Funding: This work was supported by the National Natural Science Foundation of China, No. 31771052 (to YW); the National Key Research & Development Program of China, No. 2017YFA0104701, 2017YFA0104702 and 2016YFC1101601; the National Basic Research Program of China (973 Program), No. 2014CB542201 (to JP); the Natural Science Foundation of Beijing, No. 7172202 (to YW); and the PLA Youth Training Project for Medical Science, No. 16QNP144 (to YW).
P-Reviewers: Boyraz Q, Sheng Y, Yorek MA; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Bell M, Wang L, Song LP; T-Editor: Liu XL
References
- 1.Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 2.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendszus M, Stoll G. Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging. J Neurosci. 2003;23:10892–10896. doi: 10.1523/JNEUROSCI.23-34-10892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15:417–424. doi: 10.1038/nm.1945. [DOI] [PubMed] [Google Scholar]
- 6.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 7.Burtt KE, Badash I, Wu B. Assessing surgical methods for treatment of cubital tunnel syndrome - which is the best? Clin Trials Orthop Disord. 2017;2:123–124. [Google Scholar]
- 8.Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–453. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–618. doi: 10.1007/s00401-015-1482-4. [DOI] [PubMed] [Google Scholar]
- 11.Chiu CW, Huang WH, Kuo HS, Tsai MJ, Chen CJ, Lee MJ, Cheng H. Local inhibition of matrix metalloproteinases reduced M2 macrophage activity and impeded recovery in spinal cord transected rats after treatment with fibroblast growth factor-1 and nerve grafts. Neural Regen Res. 2018;13:1447–1454. doi: 10.4103/1673-5374.235302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–456. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 14.Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience. 2015;302:174–203. doi: 10.1016/j.neuroscience.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 2012;1:10–16. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao CH, Dong HL, Tai L, Gao XM. Lactoferrin-containing immunocomplexes drive the conversion of human macrophages from M2- into M1-like phenotype. Front Immunol. 2018;9:37. doi: 10.3389/fimmu.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudet AD, Sweet DR, Polinski NK, Guan Z, Popovich PG. Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair. Mol Cell Neurosci. 2015;64:84–94. doi: 10.1016/j.mcn.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A, Batista FD. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347:667–672. doi: 10.1126/science.aaa1300. [DOI] [PubMed] [Google Scholar]
- 24.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Gong L, Zhu Y, Xu X, Li H, Guo W, Zhao Q, Yao D. The effects of claudin 14 during early Wallerian degeneration after sciatic nerve injury. Neural Regen Res. 2014;9:2151–2158. doi: 10.4103/1673-5374.147946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 28.Gordon S, Plűddemann A. Tissue macrophage heterogeneity: issues and prospects. Semin Immunopathol. 2013;35:533–540. doi: 10.1007/s00281-013-0386-4. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Zhu J, Xue C, Li Z, Ding F, Yang Y, Gu X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35:2253–2263. doi: 10.1016/j.biomaterials.2013.11.087. [DOI] [PubMed] [Google Scholar]
- 30.Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis e Sousa C. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015;42:1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM, Tannemaat MR, Malessy MJ, Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol. 2014;261:578–593. doi: 10.1016/j.expneurol.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Zhang H, Lu Y, Bai H, Xu Y, Zhu X, Zhou R, Ben J, Xu Y, Chen Q. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res Cardiol. 2011;106:1311–1328. doi: 10.1007/s00395-011-0204-x. [DOI] [PubMed] [Google Scholar]
- 33.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang E, Lee S, Kim JH, Kim JH, Seo JW, Lee WH, Mori K, Nakao K, Suk K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013;27:1176–1190. doi: 10.1096/fj.12-222257. [DOI] [PubMed] [Google Scholar]
- 35.Keklikoglou I, De Palma M. Cancer: Metastasis risk after anti-macrophage therapy. Nature. 2014;515:46–47. doi: 10.1038/nature13931. [DOI] [PubMed] [Google Scholar]
- 36.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiguchi N, Kobayashi Y, Saika F, Kishioka S. Epigenetic upregulation of CCL2 and CCL3 via histone modifications in infiltrating macrophages after peripheral nerve injury. Cytokine. 2013;64:666–672. doi: 10.1016/j.cyto.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucharova K, Stallcup WB. Dissecting the multifactorial nature of demyelinating disease. Neural Regen Res. 2018;13:628–632. doi: 10.4103/1673-5374.230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42:519–523. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Peng Y, Bao FK, Liu AH. Correlation between gene polymorphism of macrophage migration inhibitory factor and disease susceptibility: recognition, target and significance. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:4574–4579. [Google Scholar]
- 43.London A, Benhar I, Mattapallil MJ, Mack M, Caspi RR, Schwartz M. Functional macrophage heterogeneity in a mouse model of autoimmune central nervous system pathology. J Immunol. 2013;190:3570–3578. doi: 10.4049/jimmunol.1202076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25:358–369. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478–1483. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 47.Mercalli A, Calavita I, Dugnani E, Citro A, Cantarelli E, Nano R, Melzi R, Maffi P, Secchi A, Sordi V, Piemonti L. Rapamycin unbalances the polarization of human macrophages to M1. Immunology. 2013;140:179–190. doi: 10.1111/imm.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore EM, Suresh V, Ying G, West JL. M0 and M2 macrophages enhance vascularization of tissue engineering scaffolds. Regen Eng Transl Med. 2018;4:51–61. [Google Scholar]
- 50.Mueller CK, Schultze-Mosgau S. Histomorphometric analysis of the phenotypical differentiation of recruited macrophages following subcutaneous implantation of an allogenous acellular dermal matrix. Int J Oral Maxillofac Surg. 2011;40:401–407. doi: 10.1016/j.ijom.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83:175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- 52.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Namikawa K, Okamoto T, Suzuki A, Konishi H, Kiyama H. Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J Neurosci. 2006;26:7460–7467. doi: 10.1523/JNEUROSCI.0023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013a;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013b;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potas JR, Haque F, Maclean FL, Nisbet DR. Interleukin-10 conjugated electrospun polycaprolactone (PCL) nanofibre scaffolds for promoting alternatively activated (M2) macrophages around the peripheral nerve in vivo. J Immunol Methods. 2015;420:38–49. doi: 10.1016/j.jim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 59.Ren Z, Wang Y, Peng J, Zhao Q, Lu S. Role of stem cells in the regeneration and repair of peripheral nerves. Rev Neurosci. 2012;23:135–143. doi: 10.1515/revneuro-2011-0069. [DOI] [PubMed] [Google Scholar]
- 60.Rigamonti E, Zordan P, Sciorati C, Rovere-Querini P, Brunelli S. Macrophage plasticity in skeletal muscle repair. Biomed Res Int. 2014;2014:560629. doi: 10.1155/2014/560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352:366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakaguchi M, Shingo T, Shimazaki T, Okano HJ, Shiwa M, Ishibashi S, Oguro H, Ninomiya M, Kadoya T, Horie H, Shibuya A, Mizusawa H, Poirier F, Nakauchi H, Sawamoto K, Okano H. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc Natl Acad Sci U S A. 2006;103:7112–7117. doi: 10.1073/pnas.0508793103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siebert H, Sachse A, Kuziel WA, Maeda N, Brück W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol. 2000;110:177–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- 66.Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the Culprits: Macrophages-Versatile Regulators of Wound Healing. Adv Wound Care (New Rochelle) 2013;2:357–368. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sudduth TL, Greenstein A, Wilcock DM. Intracranial injection of Gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Aβ in APP/PS1 mice along a different time course than anti-Aβ antibodies. J Neurosci. 2013;33:9684–9692. doi: 10.1523/JNEUROSCI.1220-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki T, Arumugam P, Sakagami T, Lachmann N, Chalk C, Sallese A, Abe S, Trapnell C, Carey B, Moritz T, Malik P, Lutzko C, Wood RE, Trapnell BC. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450–454. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taskinen HS, Röyttä M. The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol. 1997;93:252–259. doi: 10.1007/s004010050611. [DOI] [PubMed] [Google Scholar]
- 72.Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Steenwinckel J, Auvynet C, Sapienza A, Reaux-Le Goazigo A, Combadiere C, Melik Parsadaniantz S. Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun. 2015;45:198–210. doi: 10.1016/j.bbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 74.Wang TY, Forsythe JS, Nisbet DR, Parish CL. Promoting engraftment of transplanted neural stem cells/progenitors using biofunctionalised electrospun scaffolds. Biomaterials. 2012;33:9188–9197. doi: 10.1016/j.biomaterials.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 76.Wu B, Graff R, Badash I, Skeate JG, Lane C, Mansour I, Rao R, Yi A, Merriman J, Vangsness CT, Hatch III GR, Dorr L, Gilbert P, Schroeder ET. Effect of tourniquet use during total knee arthroplasty on global inflammatory cytokine changes associated with ischemia-reperfusion injury. Clin Trials Orthop Disord. 2017;2:1–10. [Google Scholar]
- 77.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xing Z, Lu C, Hu D, Yu YY, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, Nomura T, Okano HJ, Kanemura Y, Toyama Y, Okano H. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010;88:1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 80.Young RJ, Moller A. Immunohistochemical detection of tumour hypoxia. Methods Mol Biol. 2010;611:151–159. doi: 10.1007/978-1-60327-345-9_12. [DOI] [PubMed] [Google Scholar]
- 81.Zhang ZY, Schluesener HJ. HDAC inhibitor MS-275 attenuates the inflammatory reaction in rat experimental autoimmune prostatitis. Prostate. 2012;72:90–99. doi: 10.1002/pros.21410. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Y, Wang Y, Gong J, Yang L, Niu C, Ni X, Wang Y, Peng S, Gu X, Sun C, Yang Y. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials. 2017;134:64–77. doi: 10.1016/j.biomaterials.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 83.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


