Identification of individuals at risk for developing Alzheimer’s disease (AD) is an important issue for its pretreatment. Perspective studies predicting the disease are based on the genetic approaches emerging from the nucleotide polymorphism analysis in different loci through the entire genome in AD patients. Considerable diversity of genes located in these regions raise the question of this diversity’s peculiar role in the disease development. The mechanisms of AD have been shown to be associated with the gene variation, increased beta-amyloid production and concomitant epileptiform activity/epilepsy in particular (Palop and Mucke, 2009). In this perspective, the roles of various genes linked to epileptogenic mechanisms and the risk of AD development are analyzed. We propose that genotyping of apolipoprotein E (APOE) alleles combined with recording of electroencephalogram (EEG) can identify the early-onset AD that will allow highlighting a cohort of individuals at the risk of AD patients.
The early onset AD occurs predominantly at the age of less than 60–65 years and characterized by familial mutations in human amyloid precursor protein (APP), presenillin 1 (PSEN1), and presenillin 2 (PSEN2), which are associated with clear missense mutations in the genes (Van Cauwenberghe et al., 2016). The late onset AD (over 65 years) is characterized by a variety of genes detected by the large-scale genome-wide association studies (GWAS) (Van Cauwenberghe et al., 2016). Furthermore, the concept of the polygenic hazard score, which aims to predict the AD appearance and life expectancy was developed with the use of certain single nucleotide polymorphism (SNPs) detection assays in AD patients (Desikan et al., 2017).
In these studies, only the APOE gene has a clear amino acid variability, associated with SNPs rs429358 (R130) and rs7412 (R176), producing the APOEε4 pathogenic isoform. SNPs in other genes are located in non-coding regions (especially in introns) that make functional significance questionable. Furthermore, the results of functional annotation studies predict that certain SNPs detected in AD GWAS can affect miRNA binding, that in turn modifies miRNA interaction with the target RNAs (Han et al., 2017) and may regulate the gene expression. However, this effect needs to be proven particularly the disappointing history of research on the “regulated” TOMM40 protein expression level in AD by an intronic polyT polymorphism in TOMM40 gene (rs10524523) (Cruchaga et al., 2011). TOMM40 is located in close vicinity to APOE gene on the chromosome 19, and the link between APOE polymorphism and many AD related TOMM40 SNPs, which appear in AD GWAS studies is well explained by the genetic linkage. Specific AD polyT length differences are associated with the APOEε4 allele, the main risk factor for the late onset AD (Van Cauwenberghe et al., 2016). Interestingly, in the MAPT gene, which encodes the tau protein involved in the formation of neurofibrillary tangle (one of the major hallmarks of AD), the AD associated SNPs are also located in the introns (Zhou and Wang, 2017). The functional mutations in MAPT gene are related to several other diseases including frontotemporal dementia, Pick’s disease, progressive supranuclear palsy 1 and Parkinson-dementia syndrome. Other AD related genes include extremely rare variants with amino acid variability in cell adhesion molecules TREM2 (microglia activation), UNC5C (axonal growth), and phospholipase D3 (Del-Aguila et al., 2015).
It has been shown that 19.1–31.2% of autosomal dominant early-onset AD patients with mutations in PSEN1, PSEN2, and APP will develop seizures (Zarea et al., 2016). The subclinical epileptiform activity is associated with the APOEε4 allele (Palop and Mucke, 2009). Moreover, initially mild cognitive impairment in AD is often accompanied by temporal lobe epilepsy (Holler and Trinka, 2014). It should be mentioned, however, that non-convulsive seizures are frequently observed without overt pathological symptoms, appear episodically and remain unidentified in clinic (Horvath et al., 2016). These events are difficult to detect during standard scalp EEG while the long-term EEG recordings and their computation with the use of specialized software might be a predictive approach in the development of effective pretreatments for AD pathology (Horvath et al., 2016).
The specific mechanisms of the epileptic activity development remain unclear, but the neurodegenerative processes in AD are accompanied by accelerated degeneration of specific inhibitory GABAergic neuronal populations. Indeed, glutamate decarboxylase-65 (GAD65) immunoreactivity has been shown to be significantly reduced in AD brains (Schwab et al., 2013), indicating the severe loss of specific GABAergic interneuronal populations (in particular oriens-lacunosum moleculare neurons in the hippocampus). This disrupts the excitatory-inhibitory input balance in the brain that in turn provokes the generation of epileptiform activity and/or seizures, particularly in the hippocampus. Furthermore, though the familial AD mutations in APP, PSEN1 and PSEN2 are rare (Van Cauwenberghe et al., 2016), most of them seem to be associated with AD carriers frequently involved in the seizure development (Palop and Mucke, 2009). One of the major genetic risk factors for AD is the APOEε4 allele; however, it is able to produce the detrimental effect with the additional support from other AD risk factors (Van Cauwenberghe et al., 2016).
The meta-analysis indicates the presence of the APOEε4 allele in 9–23% of individuals in the world, among them, 15–45% APOEε4 carriers develop the AD pathology (http://www.alzgene.org/meta.asp?geneID=83). The incidences of AD are higher in APOEε4/4 carriers (25–45%) at the age 75–80 years compared with APOEε3/4 carriers (15–25%) at the same age (https://www.ncbi.nlm.nih.gov/books/NBK1161/). These studies indicate the particular importance of the APOE allele in genotyping for the AD prediction. Thus, APOEε4 appears to be a major prognostic risk factor, nevertheless, many other factors revealed in clinics are essential for the AD development (Figure 1). However, the APOEε4 characterization in combination with the EEG recordings focused on subclinical epileptiform activity seems to be the optimal approach for specific diagnosis of early-onset AD and AD treatment.
Figure 1.
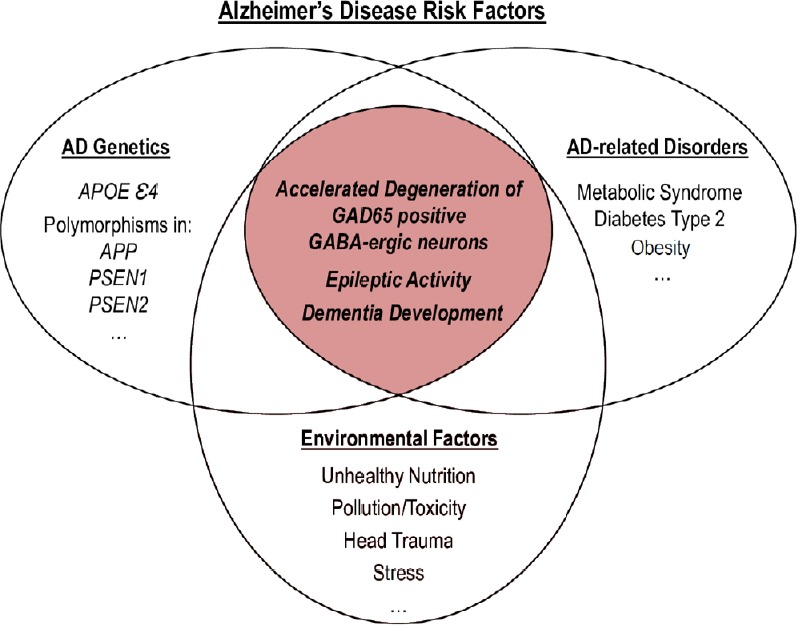
Different factors for AD onset.
One factor cannot induce AD independently and needs the support from other factors. The most pathologically mutually synergistic association occurs with APOEε4 carriers and epileptiform activity due to the accelerated degeneration of GAD65-positive GABA-ergic neurons. APOE: Apolipoprotein E; GAD65: glutamate decarboxylase-65; GABA: γ-aminobutyric acid; AD: Alzheimer’s disease.
Thus, the most effective treatment of AD is expected to be bidirectional, simultaneously affecting the intimately entwined AD and epilepsy mechanisms. However, traditional antiepileptic treatments are associated with side effects that aggravate AD-associated cognitive impairment (Horvath et al., 2016). One of the potentially effective approaches might be based on the use of nerve growth factor (NGF), which is essential for maintenance of the functioning of both cholinergic neurons in the basal nucleus of Meynert, specifically affected in AD, and the degenerated neuronal network in the neocortex and hippocampus. The most promising universal approache is the development of modern gene therapy ex vivo, for example to introduce recombinant NGF into the brain. Ex vivo cultivated fibroblasts, genetically modified for efficient NGF expression and secretion, could be delivered into the AD brain and thereby stimulates the recovery of cholinergic neurons and neuronal networks (https://clinicaltrials.gov/ct2/show/NCT00017940). This approach, aiming at a direct protection of cholinergic neurons and AD prevention, seems to be feasible for epilepsy treatment as well. Indeed, intranasal NGF administration attenuates the seizure onsets in the rat model of kindling epilepsy (Lei et al., 2017).
In conclusion, subclinical epileptiform manifestations in the EEG recordings could be a useful prognostic sign of AD development in APOEε4 carriers. Once detected, these signals might be a stimulating factor for timely prevention, or delay the development of AD.
We thank Prof. William Wisden, MA, PhD, Fellow of the Academy of Medical Sciences, from Department of Life Sciences, Imperial College of London, Faculty of Natural Sciences, for reading the manuscript and English language corrections.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Cruchaga C, Nowotny P, Kauwe JS, Ridge PG, Mayo K, Bertelsen S, Hinrichs A, Fagan AM, Holtzman DM, Morris JC, Goate AM. Association and expression analyses with single-nucleotide polymorphisms in TOMM40 in Alzheimer disease. Arch Neurol. 2011;68:1013–1019. doi: 10.1001/archneurol.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del-Aguila JL, Koboldt DC, Black K, Chasse R, Norton J, Wilson RK, Cruchaga C. Alzheimer’s disease: rare variants with large effect sizes. Curr Opin Genet Dev. 2015;33:49–55. doi: 10.1016/j.gde.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, Thompson WK, Besser L, Kukull WA, Holland D, Chen CH, Brewer JB, Karow DS, Kauppi K, Witoelar A, Karch CM, Bonham LW, Yokoyama JS, Rosen HJ, Miller BL, et al. Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score. PLoS Med. 2017;14:e1002258. doi: 10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Z, Huang H, Gao Y, Huang Q. Functional annotation of Alzheimer’s disease associated loci revealed by GWASs. PLoS One. 2017;12:e0179677. doi: 10.1371/journal.pone.0179677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holler Y, Trinka E. What do temporal lobe epilepsy and progressive mild cognitive impairment have in common? Front Syst Neurosci. 2014;8:58. doi: 10.3389/fnsys.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath A, Szucs A, Barcs G, Noebels JL, Kamondi A. Epileptic seizures in Alzheimer disease: A review. Alzheimer Dis Assoc Disord. 2016;30:186–192. doi: 10.1097/WAD.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 7.Lei J, Feng F, Duan Y, Xu F, Liu Z, Lian L, Liang Q, Zhang N, Wang F. Intranasal nerve growth factor attenuating the seizure onset via p75R/Caspase pathway in the experimental epilepsy. Brain Res Bull. 2017;134:79–84. doi: 10.1016/j.brainresbull.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab C, Yu S, Wong W, McGeer EG, McGeer PL. GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J Alzheimers Dis. 2013;33:1073–1088. doi: 10.3233/JAD-2012-121330. [DOI] [PubMed] [Google Scholar]
- 10.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarea A, Charbonnier C, Rovelet-Lecrux A, Nicolas G, Rousseau S, Borden A, Pariente J, Le Ber I, Pasquier F, Formaglio M, Martinaud O, Rollin-Sillaire A, Sarazin M, Croisile B, Boutoleau-Bretonnière C, Ceccaldi M, Gabelle A, Chamard L, Blanc F, Sellal F, et al. Seizures in dominantly inherited Alzheimer disease. Neurology. 2016;87:912–919. doi: 10.1212/WNL.0000000000003048. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F, Wang D. The associations between the MAPT polymorphisms and Alzheimer’s disease risk: a meta-analysis. Oncotarget. 2017;8:43506–43520. doi: 10.18632/oncotarget.16490. [DOI] [PMC free article] [PubMed] [Google Scholar]


