Keywords: nerve regeneration; Parkinson's disease; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; fibroblast growth factor 20; A-type potassium current; long-term potentiation; Kv4.2; oxidative stress; malondialdehyde; motor performance; neural regeneration
Abstract
Genome-wide studies have reported that Parkinson’s disease is associated with abnormal expression of various growth factors. In this study, male C57BL/6 mice aged 10 weeks were used to establish Parkinson’s disease models using an intraperitoneal injection of 60 mg/kg 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. 28 days later, 10 or 100 ng fibroblast growth factor 20 was injected intracerebroventricularly. The electrophysiological changes in the mouse hippocampus were recorded using a full-cell patch clamp. Expression of Kv4.2 in the substantia nigra was analyzed using a western blot assay. Serum malondialdehyde levels were analyzed by enzyme-linked immunosorbent assay. The motor coordination of mice was evaluated using the rotarod test. The results showed that fibroblast growth factor 20 decreased A-type potassium current in neurons of the substantia nigra, increased long-term potentiation amplitude in the hippocampus, and downregulated Kv4.2 expression. A high dose of fibroblast growth factor 20 reduced serum malondialdehyde levels and enhanced the motor coordination of mice. These findings confirm that fibroblast growth factor 20 has a therapeutic effect on the toxicity induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, and its mechanism of action is associated with the inhibition of A-type K+ currents and Kv4.2 expression. All animal procedures were approved by the Animal Care and Use Committee of Qilu Hospital of Shandong University, China in 2017 (approval No. KYLL-2017-0012).
Chinese Library Classification No. R456; R363; R741
Introduction
Parkinson’s disease (PD), one of the most common central nervous system diseases, is currently the second most common major neurodegenerative disease, affecting more than 1% of the global population over 50 years old (Schapira and Jenner, 2011). The major pathogenesis of PD is a functional decrease, or death, of the dopaminergic neurons in the substantia nigra pars compacta and ventral tegmental area (Bjorklund and Dunnett, 2007; Gerfen and Surmeier, 2011; Shi and Chen, 2017). To date, L-dihydroxyphenylalanine (L-Dopa) is an optimal available treatment. However, the therapeutic effect of L-Dopa decreases after 4–5 years, and some patients also suffer from side-effects such as dyskinesia (Dauer and Przedborski, 2003). Therefore, it is critical to focus on the identification of novel methods or compounds to prevent the death of dopaminergic neurons and retard the progression of PD.
Multiple genetic and environmental factors contribute to the development of PD, including the gene encoding for cyclin G-associated kinase/diacylglycerol kinase theta region, human transmembrane protein 175, and fibroblast growth factor 20 (FGF20) (Pankratz et al., 2009; Jing et al., 2015). FGF20 is a member of the fibroblast growth factors which form a highly conserved polypeptide family (Dono, 2003). FGF20 regulates the development and function of the central nervous system and enhances the survival of dopaminergic neurons in the midbrain (Resnick et al., 1998; Ohmachi et al., 2000; Dauer, 2007). Although previous studies have revealed the possible associations between PD and the single nucleotide polymorphisms in the FGF20 gene (van der Walt et al., 2004; Sharma et al., 2012; Wang et al., 2017), further studies are required to determine FGF20 effects in animal models. Accordingly, in this study, we chose to assess the effects of FGF20 and assessed in a mouse model of PD.
Potassium (K+) channels are the major ion channels in controlling neuronal electrical activities and intracellular signal pathways. In dopaminergic neurons of the substantia nigra, voltage-gated A-type K+ current is one of the most important inhibitory signals and mediates intracellular calcium oscillation, and thus regulates the functions of dopaminergic neurons (Jerng and Pfaffinger, 2014; Zamponi et al., 2015; Duda et al., 2016). Conversely, this A-type K+ current, mostly mediated by Kv4.3 channels, also regulates neuronal survival, death, and apoptosis (Mathie et al., 1998; Wang et al., 2008b). A reduction of intracellular K+ concentration by the over-activation of A-type K+ channels may trigger the apoptosis of dopaminergic neurons (Shieh et al., 2000). In this study, we also tested and verified this hypothesis through the experiments.
The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a commonly used neurotoxin to establish animal models which mimic symptoms of PD. MPTP can impair the axons and synapses of substantia nigra dopaminergic neurons in the dorsal striatum, prefrontal cortex and ventral hippocampus (Zhu et al., 2011; Lee et al., 2013). Thus, in this MPTP-lesioned PD model, besides voluntary movement and goal-directed behavior, damage and injuries also occur to emotions, motivation, cognition, and memory (D’Ardenne et al., 2012; Pignatelli and Bonci, 2015). Besides studies on movement control and motor learning, experiments were performed to assess the effects of FGF20 on these non-motor symptoms of PD.
Materials and Methods
Experimental animals
Specific-pathogen-free male 10-week-old C57BL/6 mice (n = 50), weighing 20–25 g, were purchased from the Animal Center of Shanghai Laboratory, Chinese Academy of Sciences, China (license number: SCXK (Hu) 2017-0031). Mice were maintained in air-conditioned rooms at 20–25°C. Mice were housed in plastic cages and allowed free access to water and food. All experiments conformed to the Institutional Committee of Laboratory Animals. All animal procedures were approved by the Animal Care and Use Committee of Qilu Hospital of Shandong University, China in 2017 (approval No. KYLL-2017-0012). Efforts were made to minimize the number of animals used and their suffering, in accordance with ethical guidelines. The 50 mice were equally and randomly divided into five groups. Among them, 40 mice were used to establish PD models.
PD model establishment and intervention
To induce PD, C57BL/6 mice were administered with MPTP (Sigma, St. Louis, MO, USA) at an acute dose of 60 mg/kg by intraperitoneal injection (Castro-Hernández et al., 2017). At 28 days post-MPTP administration, FGF20 (GeneCopoeia, Rockville, MD, USA) was applied by the intraventricular injection. Mice were mounted in a stereotaxic apparatus (RWD Life Science, Shenzhen, China), with a guide cannula (22GA; Plastics One, Torrington, CT, USA) planted into the lateral cerebral ventricle (anteroposterior 0.3 mm, mediolateral 1.3 mm, dorsal posterior 4.0 mm). Intracerebroventricular injection of FGF20 was performed through a soft plastic catheter and this guide cannula. The injection volume was designed at 3 μL. The groups of FGF20 in low (10 ng) and high dose (100 ng) were all controlled in the above injection volume. L-Dopa (Sigma) was also administered by intraperitoneal injection in 4 mg/kg.
Preparation of brain slices
At 28 days after modeling, mice were sacrificed by cervical dislocation, and the brains were quickly removed into ice-cold cutting solution, containing sucrose 215 mM, KCl 2.5 mM, D-glucose 20 mM, NaHCO3 26 mM, NaH2PO4 1.6 mM, CaCl2 1 mM, MgCl2 4 mM, and MgSO4 4 mM, adjusted to pH at 7.4 and osmolarity at 340 mOsm, and oxygenated with 95% O2 and 5% CO2. Coronal 350 μm slices were obtained by a vibrating blade microtome (VT-1200S; Leica, Nussloch, Germany). Slices were then removed into artificial cerebrospinal fluid, supplemented with NaCl 119 mM, KCl 2.3 mM, NaHCO3 26.2 mM, D-glucose 11 mM, CaCl2 2.5 mM, NaHPO4 1 mM, and MgSO4 1.3 mM, adjusted to pH 7.3 and osmolarity of 320 mOsm, and oxygenated with 95% O2 and 5% CO2, and then incubated for at least 30 minutes at 33°C. When recording, the slice was transferred into a recording chamber and perfused with oxygenated recording solution at a rate of 5 mL/minute at 20–25°C for the electrophysiological recordings.
Electrophysiological recording
Whole-cell patch clamp recordings were performed in voltage clamp and held in −70 mV. Neurons in the hippocampus and substantia nigra were identified by infrared differential interference contrast video using an upright microscope (Nikon, Tokyo, Japan). Patch pipettes (2–5 MΩ) were pulled from borosilicate glass with a puller machine (P-97, Sutter Instruments, Novato, CA, USA). The pipettes were filled with the following internal solution: K-gluconate 125 mM, KCl 15 mM, EGTA 0.2 mM, HEPES 10 mM, Na2-phosphocreatine 10 mM, Mg-ATP 2 mM, Na2-GTP 0.3 mM, pH 7.2 and 290 mOsm. When recording A-type potassium currents, 1 μM tetrodotoxin was added to the perfusion solution to block the activities of sodium channels. When recording field excitatory postsynaptic potentials and long-term potentiation, a stimulation pipette was set on the area of a Schaffer collateral. Electrical signals were digitized and sampled at a frequency of 5 kHz with Digidata 1440A and a Multiclamp 700B patch-clamp amplifier (Molecular Devices, San Jose, CA, USA). Responses were recorded and analyzed with Clampfit 10.0 software (Molecular Devices).
Western blot assay
Since the Kv4.2 protein was located on the cell membrane (Wulff et al., 2009), protein samples from the substantia nigra were prepared using a membrane protein extraction kit (BioVision, Milpitas, CA, USA) and the concentration was measured using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Shanghai, China). After overnight incubation with the primary antibodies, including rabbit anti-Kv4.2 polyclonal antibody (1:2000; GeneTex, Irvine, CA, USA) and rabbit anti-β-actin polyclonal antibody (1:2000; GeneTex) overnight at 4°C, the polyvinylidene fluoride membranes were then incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:10,000; Santa Cruz, Dallas, TX, USA) for 2 hours at 20–25°C. Chemiluminescent signals were generated using a SuperSignal west femto maximum sensitivity substrate kit (Thermo Fisher Scientific) and detected using a FluorChem E system (ProteinSimple, San Jose, CA, USA).
Malondialdehyde measurement
At 28 days after modeling, blood samples were taken from the tails of mice. Serum samples were then separated from the clotted blood by centrifugation. The malondialdehyde levels were detected using the malondialdehyde enzyme-linked immunosorbent assay testing kit (Sigma) at 532 nm maximum light absorbance (Moridi et al., 2015; Haghdoost-Yazdi et al., 2017).
Rotarod test
At 28 days after modeling, the rotarod test (Haghdoost-Yazdi et al., 2017) was performed to evaluate the coordination of movement, which is an associated symptom of PD. This test was performed for 3 consecutive days and in 2 trials every day. Each trial was recorded for 2 minutes, during which the rotating rod underwent a linear acceleration from 5−40 r/min. The rotarod data were expressed as the passing area under the curve.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using one-way analysis of variance followed by a Tukey post hoc test with GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
FGF20 reduces A-type potassium currents after MPTP lesion
First, a ladder holding potential from −70 mV to 40 mV was used to induce the potassium currents, with a step of 10 mV. Dopaminergic neurons in the substantia nigra were recorded with a whole cell patch clamp. Only the transient section of potassium current, A-type current (IA), was used for the analysis (Figure 1A). The application of FGF20 was in two different doses (10 and 100 ng). Application of a high dose of FGF20 and L-Dopa showed powerful effects (Figure 1B). The maximal IA currents were 52% less after 100 ng FGF20 injection, and 61% less after L-Dopa injection (Figure 1C). However, a low dose of FGF20 showed 18% less IA current, but this decrease was not statistically significant. These results indicate that FGF20 might function to reduce the IA current, which was previously known to be increased in PD.
Figure 1.
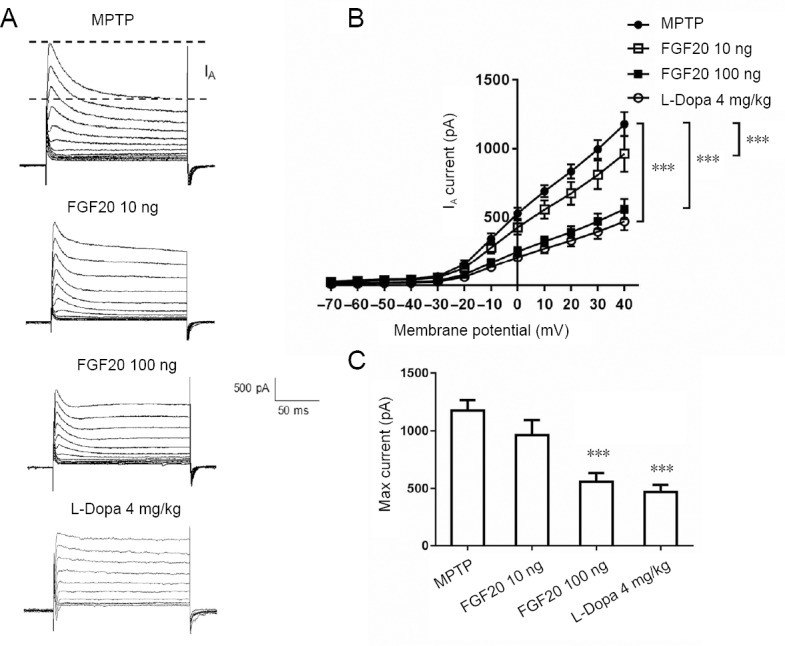
Effects of FGF20 on A-type potassium current (IA) at different doses detected by electrophysiological recordings.
(A) Typical IA traces in the MPTP model groups and three groups treated with different doses of FGF20 and L-Dopa. (B) Statistical comparison of current-voltage curves from −70 mV to 40 mV in different groups. (C) Quantitative results of the maximal IA currents, recorded at the membrane potential of 40 mV. MPTP: Intraperitoneal injection of 60 mg/kg MPTP; FGF20 10 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection 10 ng FGF20; FGF20 100 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection 100 ng FGF20; L-Dopa 4 mg/kg: intraperitoneal injection of 60 mg/kg MPTP + intraperitoneal injection of 4 mg/kg L-Dopa. Data are expressed as the mean ± SD. ***P < 0.001, vs. MPTP group (one-way analysis of variance followed by a Tukey post hoc test). MPTP: 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; FGF20: fibroblast growth factor 20; L-Dopa: L-dihydroxyphenylalanine.
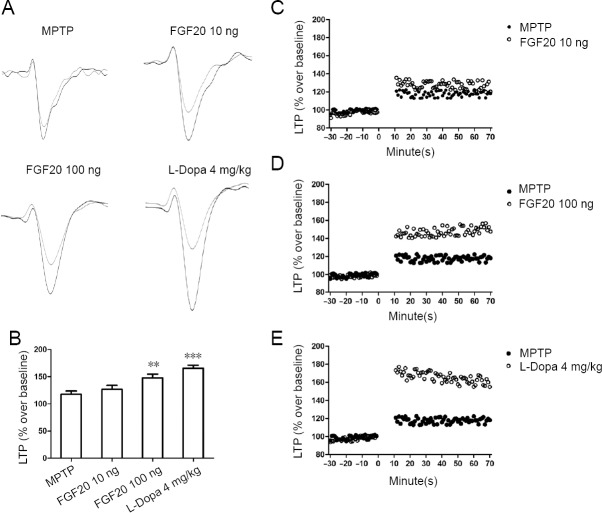
FGF20 enhances hippocampal long-term potentiation after MPTP lesion
To investigate the effect of FGF20 on synaptic plasticity and related learning skills, hippocampal long-term potentiation was selected for our study. High-frequency stimulation of the Schaffer collateral pathway was used to induce long-term potentiation, and the field excitatory postsynaptic potential was recorded in CA1 (Figure 2A). The results showed that a high dose of FGF20 enhanced the suppressed long-term potentiation after MPTP lesion, with an effect similar to L-Dopa (Figure 2B, D, and E). FGF20 at 100 ng increased long-term potentiation 63% compared to the effect of L-Dopa (long-term potentiation value, MPTP: 118%; FGF20 high: 148%; L-Dopa: 166%). However, a low dose of FGF20 showed less effect and did not alter the long-term potentiation curve when compared with the MPTP group (Figure 2B and C).
Figure 2.
Electrophysiological experiments using high-frequency stimulation-induced LTP to study the effects of FGF20 in the hippocampus detected by electrophysiological recording.
(A) Representative traces from field excitatory postsynaptic potential before (gray) and after (black) the high frequency stimulation. (B) Quantitative results of LTP. Data are expressed as the mean ± SD (one-way analysis of variance followed by a Tukey post-hoc test). **P < 0.01, ***P < 0.001, vs. MPTP group. (C–E) High-frequency stimulation-induced LTP in the MPTP group, vs. the other three treatment groups. MPTP: Intraperitoneal injection of 60 mg/kg MPTP; FGF20 10 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 10 ng FGF20; FGF20 100 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection 100 ng FGF20; L-Dopa 4 mg/kg: intraperitoneal injection of 60 mg/kg MPTP + intraperitoneal injection 4 mg/kg L-Dopa. MPTP: 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; FGF20: fibroblast growth factor 20; L-Dopa: L-dihydroxyphenylalanine; LTP: long-term potentiation.
FGF20 reduces Kv4.2 expression after MPTP lesion
The above experiments were performed on brain slices, as ex vivo research. Next, a western blot assay was performed to assess the protein expression in vivo. The brain area of the substantia nigra was divided for the assessment. Kv4.2, as the major subtype mediating the A-type current, was selected. As shown in Figure 3A, MPTP lesion increased Kv4.2 expression, whereas FGF20 (100 ng) and L-Dopa reversed this effect. A dose of FGF20 had a much greater effect (42% reduction) when compared with L-Dopa (13% reduction; Figure 3B). These results indicate that in an MPTP lesion model, up-regulation of Kv4.2 was accompanied by electrophysiological changes of IA currents, whereas high doses of FGF20 and L-Dopa reversed the above changes.
Figure 3.
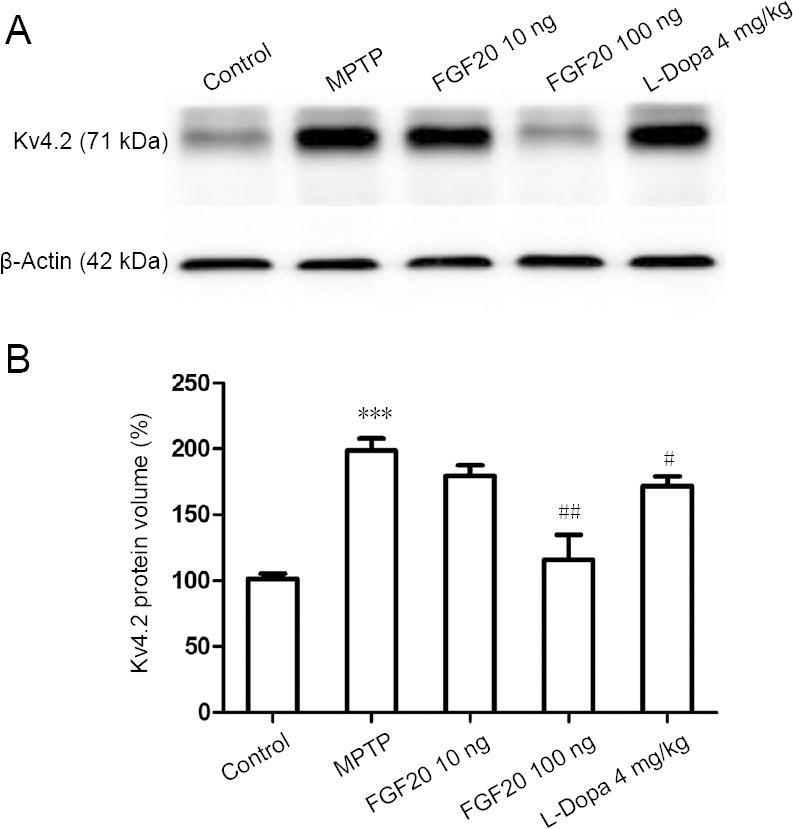
Effects of FGF20 on the expression of Kv4.2 protein at 28 days after MPTP lesion detected by western blot assay.
Both the original band (A) and statistical analysis (B) show an increased expression of Kv4.2 after MPTP lesion (***P < 0.001, vs. control group) and a reversed down-regulation of Kv4.2 after FGF20 or L-Dopa treatment (#P < 0.05, ##P < 0.01, vs. the MPTP group [one-way analysis of variance followed by a Tukey post hoc test]). Data are expressed as the mean ± SD. Control: Normal mice; MPTP: intraperitoneal injection of 60 mg/kg MPTP; FGF20 10 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 10 ng FGF20; FGF20 100 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 100 ng FGF20; L-Dopa 4 mg/kg: intraperitoneal injection of 60 mg/kg MPTP + intraperitoneal injection of 4 mg/kg L-Dopa. MPTP: 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; FGF20: fibroblast growth factor 20; L-Dopa: L-dihydroxyphenylalanine.
FGF20 reduces serum malondialdehyde concentrations after MPTP lesion
Malondialdehyde is the end product of lipoperoxidation and serves as a common biomarker of cellular oxidative stress (Gasparovic et al., 2013). The malondialdehyde levels in the serum of mice from different experimental groups were assessed. As indicated in Figure 4, after MPTP lesion, malondialdehyde concentrations were increased by 81%, but application with FGF20 and L-Dopa restored this increase.
Figure 4.
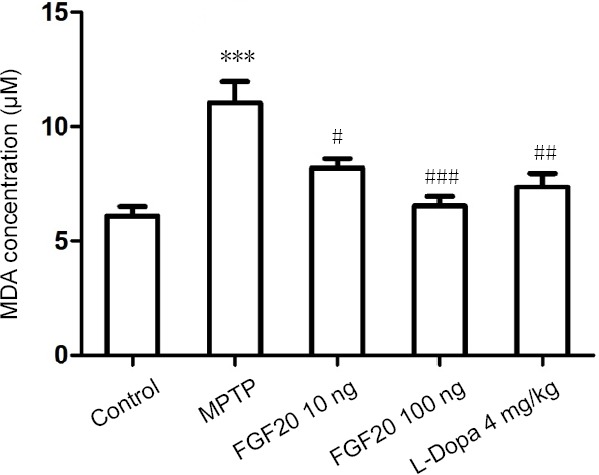
MDA levels in the serum samples of mice with MPTP lesions after FGF 20 intervention detected by enzyme-linked immunosorbent assay.
Data are expressed as the mean ± SD. ***P < 0.001, vs. the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. the MPTP group (one-way analysis of variance followed by a Tukey post hoc test). Control: Normal mice; MPTP: intraperitoneal injection of 60 mg/kg MPTP; FGF20 10 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 10 ng FGF20; FGF20 100 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 100 ng FGF20; L-Dopa 4 mg/kg: intraperitoneal injection of 60 mg/kg MPTP + intraperitoneal injection of 4 mg/kg L-Dopa. MPTP: 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; FGF20: fibroblast growth factor 20; L-Dopa: L-dihydroxyphenylalanine; MDA: malondialdehyde.
FGF20 improves motor performance after MPTP lesion
As shown in Figure 5, the motor performance of healthy mice was improved in trials 4 to 6. Additionally, both a high dose of FGF20 and L-Dopa improved the motor performance of the MPTP-treated mice, but the motor performance patterns in the low FGF20 group remained similar to those of the MPTP group.
Figure 5.
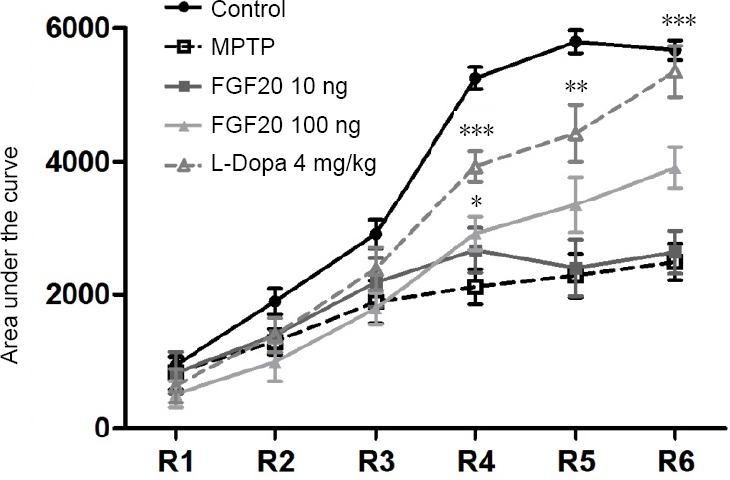
Effect of FGF20 on motor performance of mice after MPTP lesion detected by the rotarod test.
Motor performance of the mice in different groups in a rotating rod test for three consecutive days in six trials. High dose of FGF20 and L-Dopa partly recovered the learning skill of motor performance. *P < 0.05, **P < 0.01, ***P < 0.001, vs. MPTP group (one-way analysis of variance followed by a Tukey post hoc test). Data are expressed as the mean ± SD. R1−R6: Sections of the test; R1: the first section; R6: the last section. Control: Normal mice; MPTP: intraperitoneal injection of 60 mg/kg MPTP; FGF20 10 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 10 ng FGF20; FGF20 100 ng: intraperitoneal injection of 60 mg/kg MPTP + intraventricular injection of 100 ng FGF20; L-Dopa 4 mg/kg: intraperitoneal injection of 60 mg/kg MPTP + intraperitoneal injection of 4 mg/kg L-Dopa. MPTP: 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; FGF20: fibroblast growth factor 20; L-Dopa: L-dihydroxyphenylalanine.
Discussion
In this study, some evidence was provided to show the effects of FGF20 in alleviating symptoms in a mouse model of PD. FGF20 suppressed A-type K+ currents through the down-regulation of Kv4.2, enhanced long-term potentiation activities, and thus improved movement control and motor tasks. Previously, FGF20 has been reported to promote the survival of dopaminergic neurons in the substantia nigra, and therefore partly influence the occurrence and development of PD (Ohmachi et al., 2000; Murase and McKay, 2006; Sleeman et al., 2012; Duda et al., 2016). Our experimental results supported these findings and preliminarily revealed the ion channel-related mechanism underlying these effects of FGF20.
FGF20 is a member of the polypeptide fibroblast growth factor family, which extensively influences the development and function of the central nervous system (Guo et al., 2017; Sampaio et al., 2017). FGF20 is required for the efficient differentiation of dopaminergic neurons in the adult brain (Grothe et al., 2004). Generally, FGF20 is selectively expressed in calbindin-negative dopaminergic neurons, which are preferentially lost in PD. Thus, supplementation of FGF20 may help restore the homeostasis of the dopaminergic system in the early stages of PD. However, some other studies suggest that FGF20 loss is not a major risk factor for sporadic PD, and there was no association between FGF20 gene variability and PD risk (Clarimon et al., 2005; Wider et al., 2009). Therefore, the role of FGF20 in PD occurrence and aggravation remains unclear at the moment. Apart from FGF20, glial-derived neurotrophic factor, another important growth factor, can promote the maintenance and survival of substantia nigra dopaminergic neurons, through the inhibition of A-type current and the stimulation of pacemaker frequency (Yang et al., 2001; d’Anglemont de Tassigny et al., 2015; Kramer and Liss, 2015).
A-type K+ currents and delayed rectifier K+ currents are enhanced during the apoptotic process of PD in dopaminergic neurons and cortical section neurons (Wang et al., 2008a). It has been suggested that Kv4-blockers infused in vivo reduced PD symptoms in mice (Aidi-Knani et al., 2015). Moreover, tetraethylammonium and 4-aminopyridine, two potent K+ channel inhibitors, also improved the behavioral symptoms of 6-hydroxydopamine-induced Parkinsonism (Haghdoost-Yazdi et al., 2017). However, the mechanisms underlying the relationship between the inhibition of A-type currents and improvement of PD symptoms remain elusive. One possible mechanism is via the activation of voltage-gated calcium channels. The calcium influx through voltage-gated calcium channels strengthens both synaptic and neuromuscular transmissions (Hayes, 2004; Franciosi et al., 2006). Another mechanism is via the activation of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors (McBride et al., 2006). In our study, the inhibition of A-type K+ currents was associated with the downregulation of Kv4.2. However, it has been shown that Kv4.3 was the major K+ channel in the substantia nigra functionally related to PD (Liss et al., 2001). Thus, we still cannot completely confirm this result. More studies are needed to elucidate the roles of Kv4.2 and Kv4.3 in the pathogenesis of PD.
Dopaminergic synapses from the ventral tegmental area and substantia nigra to the hippocampus regulate the excitability of CA1 pyramidal neurons. Dopaminergic lesions could enhance GABA inhibition and thus suppress long-term potentiation activities (Borgkvist et al., 2015; Rosen et al., 2015; Singh et al., 2017). Moreover, hippocampal long-term potentiation can be blocked in the presence of a dopamine receptor 1 antagonist or dopamine receptor 1 knock-out mice (Muthane et al., 1994; Bach et al., 1999). In this study, FGF20 potentiated the long-term potentiation activities after MPTP lesion, indicating that FGF20 may decrease the loss of memory and learning in PD. However, it is unclear whether this effect of FGF20 is direct on the dopaminergic system or hippocampal CA1 neurons. Some studies indicated that long-term potentiation enhancement occurred after MPTP lesion, particularly in the ventral hippocampus rather than the dorsal portion (Zhu et al., 2012). Overall, although the suppression of long-term potentiation is not directly associated with PD, we posit that the modulation of long-term potentiation is still an important parameter in the assessment of FGF20. As a common biomarker of cellular oxidative stress (Haghdoost-Yazdi et al., 2017), malondialdehyde might be another indirect, but important index. We found that FGF20 reduced the serum concentration of malondialdehyde, indicating that some of the antiparkinsonian effects of FGF20 partly resulted from the inhibition of oxidative stress. Additionally, we also observed that treatment with a high dose of FGF20 remarkably improved the motor performance of the MPTP mice, further indicating the potential antiparkinsonian effects of FGF20.
However, we acknowledged some limitations remain in this study. For example, the therapeutic role of FGF20 in PD must be evaluated using in vitro experiments using human clinical samples. The progression of PD is a multifactorial process. Therefore, other molecular mechanisms underlying the anti-PD role of FGF20 should be further explored.
In summary, FGF20 suppresses A-type K+ currents, inhibits Kv4.2 expression and improves motor performance in an MPTP-induced mouse model of PD. Our findings may provide evidence of a novel cellular mechanism involved in the potential antiparkinsonian effects of FGF20.
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Institutional review board statement: All experiments conformed to the Institutional Committee of Laboratory Animals. All animal procedures were approved by the Animal Care and Use Committee of Qilu Hospital of Shandong University, China in 2017 (approval No. KYLL-2017-0012).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Rogers T, Haase R, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Aidi-Knani S, Regaya I, Amalric M, Mourre C. Kv4 channel blockade reduces motor and neuropsychiatric symptoms in rodent models of Parkinson’s disease. Behav Pharmacol. 2015;26:91–100. doi: 10.1097/FBP.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 2.Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Borgkvist A, Avegno EM, Wong MY, Kheirbek MA, Sonders MS, Hen R, Sulzer D. Loss of striatonigral GABAergic presynaptic inhibition enables motor sensitization in Parkinsonian mice. Neuron. 2015;87:976–988. doi: 10.1016/j.neuron.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro-Hernández J, Adlard PA, Finkelstein DI. Pramipexole restores depressed transmission in the ventral hippocampus following MPTP-lesion. Sci Rep. 2017;7:44426. doi: 10.1038/srep44426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarimon J, Xiromerisiou G, Eerola J, Gourbali V, Hellstrom O, Dardiotis E, Peuralinna T, Papadimitriou A, Hadjigeorgiou GM, Tienari PJ, Singleton AB. Lack of evidence for a genetic association between FGF20 and Parkinson’s disease in Finnish and Greek patients. BMC Neurol. 2005;5:11. doi: 10.1186/1471-2377-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A. 2012;109:19900–19909. doi: 10.1073/pnas.1116727109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d’Anglemont de Tassigny X, Pascual A, López-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front Neuroanat. 2015;9:10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dauer W. Neurotrophic factors and Parkinson’s disease: the emergence of a new player? Sci STKE. 2007;2007:pe60. doi: 10.1126/stke.4112007pe60. [DOI] [PubMed] [Google Scholar]
- 10.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 11.Dono R. Fibroblast growth factors as regulators of central nervous system development and function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R867–881. doi: 10.1152/ajpregu.00533.2002. [DOI] [PubMed] [Google Scholar]
- 12.Duda J, Potschke C, Liss B. Converging roles of ion channels, calcium, metabolic, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):156–178. doi: 10.1111/jnc.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franciosi S, Ryu JK, Choi HB, Radov L, Kim SU, McLarnon JG. Broad-spectrum effects of 4-aminopyridine to modulate amyloid beta1-42-induced cell signaling and functional responses in human microglia. J Neurosci. 2006;26:11652–11664. doi: 10.1523/JNEUROSCI.2490-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparovic AC, Jaganjac M, Mihaljevic B, Sunjic SB, Zarkovic N. Assays for the measurement of lipid peroxidation. Methods Mol Biol. 2013;965:283–296. doi: 10.1007/978-1-62703-239-1_19. [DOI] [PubMed] [Google Scholar]
- 15.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grothe C, Timmer M, Scholz T, Winkler C, Nikkhah G, Claus P, Itoh N, Arenas E. Fibroblast growth factor-20 promotes the differentiation of Nurr1-overexpressing neural stem cells into tyrosine hydroxylase-positive neurons. Neurobiol Dis. 2004;17:163–170. doi: 10.1016/j.nbd.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Liu T, Zhao D, Wang X, Liu D, He Y, Shan C, Kong Y, Hu W, Tao B, Sun L, Zhao H, Li S, Liu J. FGF18 protects against 6-hydroxydopamine-induced nigrostriatal damage in a rat model of Parkinson’s disease. Neuroscience. 2017;356:229–241. doi: 10.1016/j.neuroscience.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Haghdoost-Yazdi H, Piri H, Najafipour R, Faraji A, Fraidouni N, Dargahi T, Alipour Heidari M. Blockade of fast A-type and TEA-sensitive potassium channels provide an antiparkinsonian effect in a 6-OHDA animal model. Neurosciences (Riyadh) 2017;22:44–50. doi: 10.17712/nsj.2017.1.20160266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes KC. The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev. 2004;10:295–316. doi: 10.1111/j.1527-3458.2004.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández VS, Luquín S, Jáuregui-Huerta F, Corona-Morales AA, Medina MP, Ruíz-Velasco S, Zhang L. Dopamine receptor dysregulation in hippocampus of aged rats underlies chronic pulsatile l-Dopa treatment induced cognitive and emotional alterations. Neuropharmacology. 2014;82:88–100. doi: 10.1016/j.neuropharm.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Jerng HH, Pfaffinger PJ. Modulatory mechanisms and multiple functions of somatodendritic A-type K (+) channel auxiliary subunits. Front Cell Neurosci. 2014;8:82. doi: 10.3389/fncel.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing CC, Luo XG, Cui HG, Li FR, Li P, Jiang EZ, Ren Y, Pang H. Screening of polymorphisms located in the FGF20 and TMEM175 genes in North Chinese Parkinson’s disease patients. Genet Mol Res. 2015;14:13679–13687. doi: 10.4238/2015.October.28.30. [DOI] [PubMed] [Google Scholar]
- 23.Kramer ER, Liss B. GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett. 2015;589:3760–3772. doi: 10.1016/j.febslet.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee KW, Im JY, Woo JM, Grosso H, Kim YS, Cristovao AC, Sonsalla PK, Schuster DS, Jalbut MM, Fernandez JR, Voronkov M, Junn E, Braithwaite SP, Stock JB, Mouradian MM. Neuroprotective and anti-inflammatory properties of a coffee component in the MPTP model of Parkinson’s disease. Neurotherapeutics. 2013;10:143–153. doi: 10.1007/s13311-012-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4. 3L and KChip3.1 transcription. EMBO J. 2001;20:5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 28.McBride JM, Smith DT, Byrn SR, Borgens RB, Shi R. Dose responses of three 4-aminopyridine derivatives on axonal conduction in spinal cord trauma. Eur J Pharm Sci. 2006;27:237–242. doi: 10.1016/j.ejps.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Moridi H, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Yadegarazari R, Khazaei M, Khodadadi I, Tavilani H, Piri H, Asadi S, Zarei S, Rezaei A. Resveratrol-dependent down-regulation of receptor for advanced glycation end-products and oxidative stress in kidney of rats with diabetes. Int J Endocrinol Metab. 2015;13:e23542. doi: 10.5812/ijem.23542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murase S, McKay RD. A specific survival response in dopamine neurons at most risk in Parkinson’s disease. J Neurosci. 2006;26:9750–9760. doi: 10.1523/JNEUROSCI.2745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994;126:195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- 32.Ohmachi S, Watanabe Y, Mikami T, Kusu N, Ibi T, Akaike A, Itoh N. FGF-20, a novel neurotrophic factor, preferentially expressed in the substantia nigra pars compacta of rat brain. Biochem Biophys Res Commun. 2000;277:355–360. doi: 10.1006/bbrc.2000.3675. [DOI] [PubMed] [Google Scholar]
- 33.Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH PSG-PROGENI and GenePD Investigatorsm. Coordinators and Molecular Genetic Laboratories. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86:1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Resnick JL, Ortiz M, Keller JR, Donovan PJ. Role of fibroblast growth factors and their receptors in mouse primordial germ cell growth. Biol Reprod. 1998;59:1224–1229. doi: 10.1095/biolreprod59.5.1224. [DOI] [PubMed] [Google Scholar]
- 36.Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18:1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural Regen Res. 2017;12:549–557. doi: 10.4103/1673-5374.205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 39.Sharma M, Ioannidis JP, Aasly JO, Annesi G, Brice A, Van Broeckhoven C, Bertram L, Bozi M, Crosiers D, Clarke C, Facheris M, Farrer M, Garraux G, Gispert S, Auburger G, Vilarino-Guell C, Hadjigeorgiou GM, Hicks AA, Hattori N, Jeon B, et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology. 2012;79:659–667. doi: 10.1212/WNL.0b013e318264e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi CK, Chen ZQ. Effect of microglia on iron metabolismin midbrain dopaminergic neurons and theunderlying mechanism: study protocol for anin vitro cellular experiment. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:1262–1267. [Google Scholar]
- 41.Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev. 2000;52:557–594. [PubMed] [Google Scholar]
- 42.Singh S, Jamwal S, Kumar P. Neuroprotective potential of Quercetin in combination with piperine against 1-methyl-4-phenyl-1, 2, 3, 6- tetrahydropyridine-induced neurotoxicity. Neural Regen Res. 2017;12:1137–1144. doi: 10.4103/1673-5374.211194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sleeman IJ, Boshoff EL, Duty S. Fibroblast growth factor-20 protects against dopamine neuron loss in vitro and provides functional protection in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neuropharmacology. 2012;63:1268–1277. doi: 10.1016/j.neuropharm.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 44.van der Walt JM, Noureddine MA, Kittappa R, Hauser MA, Scott WK, McKay R, Zhang F, Stajich JM, Fujiwara K, Scott BL, Pericak-Vance MA, Vance JM, Martin ER. Fibroblast growth factor 20 polymorphisms and haplotypes strongly influence risk of Parkinson disease. Am J Hum Genet. 2004;74:1121–1127. doi: 10.1086/421052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G, Zeng J, Ren R, Chen S. Potassium channels in the basal ganglia: promising new targets for the treatment of Parkinson’s disease. Front Biosci. 2008a;13:3825–3838. doi: 10.2741/2971. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Sun X, Zhang X, Li H, Xie A. Quantitative assessment of the effect of FGF20 rs12720208 variant on the risk of Parkinson’s disease: a meta-analysis. Neurol Res. 2017;39:374–380. doi: 10.1080/01616412.2017.1286542. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Yang PL, Tang JF, Lin JF, Cai XH, Wang XT, Zheng GQ. Potassium channels: possible new therapeutic targets in Parkinson’s disease. Med Hypotheses. 2008b;71:546–550. doi: 10.1016/j.mehy.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Wider C, Dachsel JC, Soto AI, Heckman MG, Diehl NN, Yue M, Lincoln S, Aasly JO, Haugarvoll K, Trojanowski JQ, Papapetropoulos S, Mash D, Rajput A, Rajput AH, Gibson JM, Lynch T, Dickson DW, Uitti RJ, Wszolek ZK, Farrer MJ, et al. FGF20 and Parkinson’s disease: no evidence of association or pathogenicity via alpha-synuclein expression. Mov Disord. 2009;24:455–459. doi: 10.1002/mds.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 51.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The Physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu G, Chen Y, Huang Y, Li Q, Behnisch T. MPTP-meditated hippocampal dopamine deprivation modulates synaptic transmission and activity-dependent synaptic plasticity. Toxicol Appl Pharmacol. 2011;254:332–341. doi: 10.1016/j.taap.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhu G, Huang Y, Chen Y, Zhuang Y, Behnisch T. MPTP modulates hippocampal synaptic transmission and activity-dependent synaptic plasticity via dopamine receptors. J Neurochem. 2012;122:582–593. doi: 10.1111/j.1471-4159.2012.07815.x. [DOI] [PubMed] [Google Scholar]