Abstract
Objectives:
Prior authorization policies (PA) are widely used to control psychotropic medication costs by state Medicaid programs and Medicare Part D plans. The objective of this study was to examine the impact of a Maine Medicaid PA policy on initiation and switching of anticonvulsant and atypical antipsychotic treatments among patients with bipolar disorder.
Methods:
We obtained Maine and New Hampshire (comparison state) Medicaid and Medicare claims data for 2001 to 2004; the Maine PA policy was implemented in July 2003. Among continuously enrolled patients with bipolar disorder (Maine: n [H11005] 5336; New Hampshire: n [H11005] 1376), we used an interrupted times series with comparison group design to estimate changes in rates of initiating new episodes of bipolar treatment and generalized estimating equations models to examine rates of switching therapies among patients under treatment.
Results:
The Maine PA policy was associated with a marked decrease in rates of initiation of bipolar treatments; a relative reduction of 32.3% (95% CI: 24.8, 39.9) compared with expected rates at 4 months after policy implementation. This decrease was driven primarily by reductions in the initiation of nonpreferred agents. The policy had no discernable impact on rates of switching therapy among patients currently on treatment (RR: 1.03; 95% CI: 0.76, 1.39).
Conclusions:
The findings of this study provide evidence that PA implementation can be a barrier to initiation of nonpreferred agents without offsetting increases in initiation of preferred agents, which is a major concern. There is a critical need to evaluate the possible unintended effects of PA policies to achieve optimal health outcomes among low-income patients with chronic mental illness. In addition, more research is needed to understand how these barriers arise and whether specific seriously mentally ill populations or drug classes should be exempted from PA policies.
Keywords: Prior authorization, medication access, interrupted time series, bipolar disorder, Medicaid
In recent years the growth in Medicaid prescription drug expenditures has outpaced trends for other Medicaid services and has been one of the main contributing factors to overall increases in program costs.1 Medicaid spending on prescription drugs grew approximately 15.4% per year between 1994 and 2004.2 To grapple with the challenges of funding prescription medicines, state Medicaid programs commonly use prior authorization (PA) policies to manage medication use and costs.3 Many Medicare Part D plans also employ this strategy for some expensive medications, including antipsychotic agents.4 Under PA, reimbursement of a nonpreferred medication is permitted only if a prescriber requests and obtains prior approval from the Medicaid program.5 Despite the widespread use of PA policies, little is known about their effects on initiation and switching of clinically essential medications, particularly among patients with mental illness.6
Bipolar disorder is a severe and recurrent condition with manic and depressive episodes. Primary pharmacotherapies for the acute- and long-term management of this chronic illness include traditional mood stabilizers (eg, lithium), antipsychotics (eg, aripiprazole, olanzapine, risperi-done, and quetiapine) and anticonvulsant agents (eg, valproate, lamotrigine, and carbamazepine).7
Psychotropic medications account for a disproportionate share of pharmaceutical spending in Medicaid.8 In July 2003, the Maine Medicaid program implemented a PA policy affecting patients initiating treatment with a number of second-generation antipsychotic and anticonvulsant medications. For second-generation antipsychotics, a step therapy (“fail first”) was implemented which required prescribers to provide evidence that a patient had not been adequately controlled by preferred agent(s).9 Individuals already under treatment with second-generation antipsychotics or anticonvulsants (“estab lished patients”) were grandfathered under the policy and thus should not have been affected by it. The Maine Medicaid program suspended the PA requirement for second-generation antipsychotics in March 2004 and implemented a provider education program after numerous case reports of adverse effects associated with the policy.9,10 However, PA remained in effect for nonpreferred anticonvulsants.
Although the policy in Maine was intended to shift new users toward preferred agents, it may have had unintended effects. Specifically, our previous studies indicated that PA policies may inadvertently disrupt treatment among those who were the intended targets of the policy, newly treated patients with schizophrenia9 and with bipolar illness.10 The purpose of the current study was to examine potential unintended effects of the PA policy for nonpreferredsecond-generation antipsychotics and anticonvulsants in the Maine Medicaid program on medication access among patients with bipolar disorder. We hypothesized that the implementation of the PA policy may have led to a decrease in rates of treatment initiation because of administrative barriers and to an unintended increase in rates of switching of therapeutic agents among patients already established on these therapies at the time that the policy was instituted.
METHODS
Data Sources
We used data from a previous study of effects of PA on treatment discontinuation that were provided by the Centers for Medicare and Medicaid Services.10 We examined from 2001 to 2004 Medicaid claims data to identify enrollees in Maine and New Hampshire (comparison state), an adjacent state that did not have PA requirements for the medications of interest during the study period. For patients who were concurrently enrolled in Medicare, we linked Medicaid claims data with their corresponding Medicare claims using a unique patient identifier.11
Study Cohorts
We included patients (aged [H11350]18 in 2001) who were continuously enrolled in Maine or New Hampshire Medicaid for all 4 years and who had at least one inpatient or 2 outpatient diagnoses of bipolar disorder (ICD-9-CM: 296.0, 296.1, 296.4–296.8, 301.11, and 301.13).12 We excluded patients with Medicare or Medicaid managed care enrollment (Maine: n [H11005] 1 and New Hampshire: n [H11005] 17) because of the lack of claims information for patients under capitation. Within this continuously enrolled cohort, we identified analytic subgroups of potential initiators and established users of bipolar therapy (described later in the text).
Outcome Measures Treatment Initiation
We created a daily indicator to denote whether a patient was in an institution (ie, hospital, nursing home, skilled nursing facility). We spread the total days’ supply for each dispensing of a bipolar medication (ie, second-generation antipsychotic, anticonvulsant, lithium) over subsequent days in which the patient was not in an institution; generically equivalent products were treated as the same medication. This process resulted in a person-level daily indicator of medication availability for each medication dispensed.
In each state, we then identified a rolling subcohortof “potential initiators” within the continuously enrolled cohort.6 We defined potential initiators as individuals without any availability of bipolar medications and fewer than 45 institutional days during the previous 90 days at the start of a calendar month. These individuals constituted the monthly rolling denominator. Within each monthly cohort, we summed the total number of patients with an initial dispensing during the month and calculated the proportion of individuals initiating bipolar medications among potential initiators in that month. This was the primary outcome measure in a time series model estimating the changes in the level and trend in rates of initiation. We also calculated the monthly proportion of patients initiating on preferred and non-preferred therapies in each state.
Switching Among Established Users
We also identified a rolling subcohort of “established users” of any bipolar therapy within the continuously enrolled cohorts.6 For any given month, established users were defined as patients with (1) at least 2 dispensings of a single antipsychotic or anticonvulsant agent during the previous 6 months or (2) at least 2 dispensings of a combination regimen (ie, concurrent supply of an antipsychotic drug and an anticonvulsant agent). We allowed patients to be on lithium concurrently. Among these established users, we created a dichotomous indicator for whether they switched bipolar agents in each month. Switching was defined as initiation of a second medication for bipolar illness in the month in question and a maximum of one dispensing of the previous agent or regimen during the next 6 months. The total monthly number of individuals who switched medications was the numerator. The proportion of individuals who switched medications among established users was the primary outcome measure in a patient-level analysis assessing changes in the likelihood of unintended switching.
Study Design and Statistical Analysis
We used an interrupted times series with comparison group design,13 the strongest, quasi-experimental design, to estimate changes in rates of initiation of new episodes of bipolar treatment. This design can provide strong evidence of causal effects because it takes account whether a policy change causes abrupt, visible, and measurable interruptions in the pre-existing trend of the study group.13 The primary threat to the internal validity of findings is changes that occur at the same time as the policy. After interviews with Maine Medicaid officials and a thorough review of Medicaid documents, we did not identify any co-occurring policy interventions at the time of implementation of the step therapy and PA policy. We have also included a comparison group (New Hampshire) to capture regional changes in policy or practice that may confound study results. A comparison group provides information about the counterfactual inference (ie, what might have happened in the absence of policy change).13
We used segmented regression models13,14 to statistically estimate cohort-level changes in treatment initiation from the prepolicy period (July 2002 to April 2003) to the policy period (August 2003 to February 2004). To address the possibility of an anticipatory response to implementation of the policy, we considered May 2003 to July 2003 as a policy “phase-in” period and excluded these 3 data points from the time-series model examining rates of treatment initiation.
The time series models were created using the aggregated outcome measures (ie, proportion of individuals initiating bipolar medications among potential initiators) at each monthly time point; we estimated changes in level and monthly trend in treatment initiation in the months after policy implementation among the study and comparison cohorts separately. The statistical models adjusted for baseline trends with each state acting as its own control. The models also included a binary indicator denoting the months after the policy to estimate the immediate level change in the outcome measure and a term indicating the number of months after policy implementation to estimate the change in trend (slope) for the policy period. The combined change in level and trend at a given month after the policy represented the full policy effect. We controlled for all significant autocorrelation terms in the initial regression models and examined the Durbin-Watson statistic in final adjusted models to determine the nonsignificance of first-order autocorrelation of the regression residuals. For parsimony, we excluded nonsignificant (P [H11022] 0.05) time-series terms in a stepwise fashion; exclusion of the nonsignificant terms did not change the coefficients on the remaining terms.13,15
We used generalized estimating equations16 to estimate the impact of the PA policy on the likelihood of switching treatment from the current bipolar medication, overall and by preferred versus nonpreferred drug status. We created 3 time periods in the models: prepolicy(July 2002 to April 2003), phase-in (May 2003 to July 2003), and postpolicy (August 2003 to February 2004). The phase-in period was included and modeled separately to capture temporary disruptions in treatment because of confusion among providers during the early stages of policy implementation. In an earlier study,6 we observed a short-term increase in unintended switching of medications during the implementation of a similar PA policy for antidepressants in Michigan Medicaid. The models included 2 interaction terms: state (Maine [H11005] 1) with phase-in period and state with postpolicyperiod; these terms indicate short- and long-term policy effects, respectively. These models adjusted for patient-level covariates, including age, gender, Medicare/Medicaid dual enrollment status, and number of unique medications and inpatient hospitalizations during the past 6 months. We used a robust “sandwich” estimator for the covariance structure to account for correlations.17
To test the significance of differences between study cohorts, we used [H9273]2 tests for dichotomous variables and t tests for continuous variables in Table 1. We conducted all statistical analyses using SAS (version 9.1.3, SAS Institute, Cary, NC). This study was approved by the Institutional Review Board at the Harvard Pilgrim Health Care Institute.
TABLE 1.
Baseline Characteristics of the Study and Comparison Cohorts
| Study* Maine (N = 5336) |
Comparison* New Hampshire (N = 1376) |
|
|---|---|---|
| Female (%) | 67.1 | 68.6 |
| Age in January 2001 (%) | ||
| 18–34 | 33.8 | 18.8† |
| 35–54 | 49.7 | 49.4 |
| 55–64 | 8.9 | 14.5† |
| 65 + | 7.7 | 17.4† |
| White race (%) | 97.5 | 97.3 |
| Medicare/Medicaid dually enrolled (%) | 52.3 | 69.3† |
| Bipolar medications used (%) | ||
| Lithium | 17.4 | 22.6† |
| Atypical antipsychotic (AA) | 49.8 | 59.6† |
| Nonpreferred | 19.3 | 28.6† |
| Preferred | 37.6 | 38.7 |
| Anticonvulsant (AC) | 56.6 | 56.6 |
| Carbamazepine | 8.7 | 9.7 |
| Valproic acid | 23.4 | 31.5† |
| Nonpreferred | 35.9 | 27.0† |
| Lithium + AA | 10.1 | 14.9† |
| Lithium + AC | 8.4 | 10.3† |
| AA + AC | 33.9 | 36.3 |
| No. unique medications‡ | 9.2 ± 5.9 | 11.3 ± 7.0† |
| Hospital admission (%) | 31.7 | 32.7 |
Baseline period is 2002. All values are based on non-missing information.
Continuous enrollment in Medicaid from January 2001 to December 2004 and had at least one inpatient or 2 outpatient diagnoses of bipolar disorder during that period.
P [H11021] 0.05 between 2 states.
Numbers are mean and standard deviation of numbers of unique medications (distinct chemical entities) as defined by the National Drug Codes.18
RESULTS
Cohort Characteristics
We identified 6712 (Maine: n [H11005] 5336; New Hampshire: n [H11005] 1376) continuously enrolled patients with bipolar disorder (Table 1). The study and comparison cohorts were comparable overall. In both states, patients were more likely to be female ([H11022]67%); about 50% were between the ages of 35 and 54; and 32% had a hospital admission during the baseline period. In Maine, there was a slightly larger proportion of patients aged 18 to 34 years and a smaller proportion of dually enrolled patients. Combinations of drug treatments were common and medication use was similar in both states. However, baseline utilization of second-generation antipsychotics and lithium was slightly higher in New Hampshire (Table 1). Because each state acted as its own control, exact comparability in baseline characteristics between states was not required. Further, in our previous studies,13,19,20 we have found interrupted time series to be robust to differences in several characteristics of statewide study and comparison groups, especially in continuously enrolled populations.
Effects of the PA Policy on Treatment Initiation
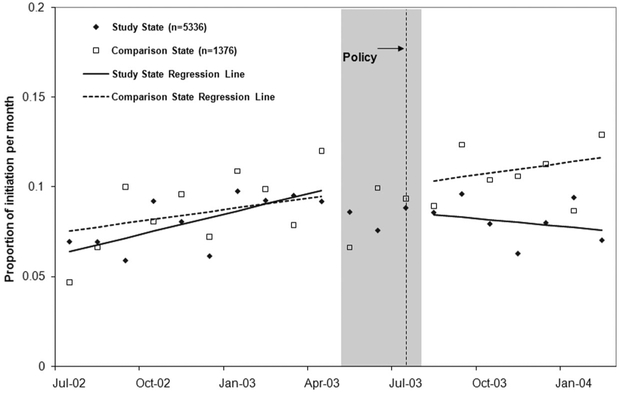
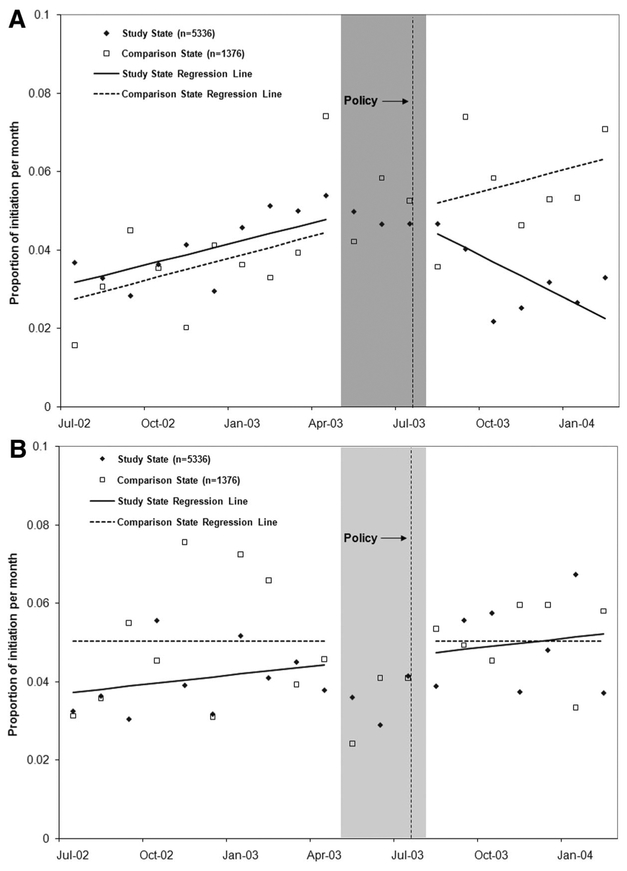
Figure 1 illustrates the impact of the PA policy on the rate of initiation of bipolar medications. After implementation of the PA in Maine, there was an immediate drop of 1.8 percentage points (95% CI: [H11002]3.20, [H11002]0.42; P [H11021] 0.05) in new starts and a decrease in slope by 0.5 percentage points (95% CI: [H11002]0.76, [H11002]0.28; P [H11021] 0.05) in treatment initiation per month, leading to an approximate relative decrease of 32.3% (95% CI: 24.8, 39.8) by 4 months after policy implementation.21 The sustained reductions in treatment initiation (about 50 fewer initiations per 10,000 patients with bipolar illness per month) were primarily driven by decreased starts on nonpreferred agents (Fig. 2A) without offsetting increases in initiation on preferred agents (Fig. 2B). The size and significance of estimated changes following the policy were nearly identical if we combined the phase-in and postpolicy periods. Rates of initiation of preferred and nonpreferred medications remained stable in New Hampshire during the study period.
FIGURE 1.
Proportion of patients initiating on bipolar medications (including antipsychotics, anticonvulsants and lithium) in the prepolicy (July 2002 to April 2003) and policy (August 2003 to February 2004) periods. Note: Interrupted time series models did not include points in the phase-in period (May 2003 to July 2003).
FIGURE 2.
Proportion of patients initiating on nonpreferred bipolar medications (A) and Proportion of patients initiating on preferred bipolar medications (B) in the prepolicy (July 2002 to April 2003) and policy (August 2003 to February 2004) periods. Note: Interrupted time series models did not include points in the phase-in period (May 2003 to July 2003).
Effects of the PA Policy on Switching Among Established Users
In Maine, the monthly average proportion of patients established on nonpreferred bipolar treatment who switched treatment was 8.6% per month prepolicy. There was no increase in the rates of switching among these patients immediately after the policy implementation. Among patients established on preferred bipolar therapy, there was also no discernable change after the policy in the rates of switching (average of 3.6% of patients switching per month prepolicy). Rates of switching among patients currently on treatment in New Hampshire were stable during the observation period (average of 4.5% of patients switching per month prepolicy and 4.9% postpolicy, respectively; data not shown).
Using generalized estimating equations models, we did not detect higher rates of switching among established users in Maine relative to New Hampshire during either the phase-in period (RR: 1.03; 95% CI: 0.76, 1.39; Table 2: column 6) or the postpolicy period (RR: 0.90; 95% CI: 0.66, 1.22 Table 2: column 7). Similarly, we also did not observe higher likelihood of switching among patients established on nonpreferred agents in Maine post versus pre policy relative to New Hampshire.
TABLE 2.
Likelihood of Switching Among Established Users of Bipolar Therapy*
| Adjusted Odds Ratios | ||||||
|---|---|---|---|---|---|---|
| Maine (n = 5336) | New Hampshire (n = 1376) | Risk Ratios (ME Versus NH) | ||||
| Phase-in Versus Prepolicy† |
Post Versus Prepolicy |
Phase-in Versus Prepolicy |
Post Versus Prepolicy |
Phase-in Versus Prepolicy |
Post Versus Prepolicy |
|
| All established users | 1.19 (1.04, 135)‡ | 1.13 (1.00, 1.29) | 1.15 (0.88, 1.51) | 1.27 (0.96, 1.67) | 1.03 (0.76, 139) | 0.90 (0.66, 1.22) |
| Using preferred agents prior to switch | 1.19 (0.90, 1.57) | 0.82 (0.63, 1.06) | 0.92 (0.56, 1.51) | 1.04 (0.64, 1.69) | 1.29 (0.73, 2.28) | 0.78 (0.45, 1.35) |
| Using nonpreferred agents prior to switch | 1.06 (0.90, 1.25) | 1.12 (0.95, 1.31) | 1.16 (0.81, 1.68) | 1.31 (0.91, 1.89) | 0.91 (0.61, 1.36) | 0.85 (0.57, 1.26) |
Generalized Estimating Equations models control for age, gender, Medicare/Medicaid dual enrollment status, number of unique medications, and inpatient hospitalizations during the past 6 months.
Prepolicy, July 2002 to April 2003; phase-in period, May 2003 to July 2003; policy, August 2003 to February 2004.
P [H11021] 0.05.
We conducted a sensitivity analysis around our definition of “established users” (ie, defined as patients with at least 3 dispensings of a bipolar drug or a regimen) and the change in definition had little effect on the results.
DISCUSSION
The Maine PA policy for new anticonvulsant and atypical antipsychotic prescriptions was associated with a sus tained reduction in treatment initiation among untreated patients with bipolar disorder. This reduction was primarily driven by a marked decrease in the rate of initiation of nonpreferred agents without an offsetting increase in initiation of preferred agents, which is a cause for concern. However, we found no evidence of unintended switching among patients already receiving bipolar therapies prior to policy implementation. This suggests that the grandfathering provisions in Maine were successful in continuing access to established medications for these patients.
PA requirements are administratively cumbersome and time-consuming.5,22 We found a sustained reduction in the overall rates of therapy initiation (about 50 fewer new starts per month per 10,000 patients with bipolar illness) after the implementation of the PA policy. Given the importance of drug therapy in this seriously mentally ill population and the fact that patients with bipolar disorder have very poor adherence with medication regimens,23 we believe that this small effect is clinically significant. Barriers to medication access may exacerbate the problem of poor adherence and may lead to declines in the health of these vulnerable patients, including higher risks of relapses, hospitalization and suicide.24 In addition, this unintended reduction in treatment initiation was greater than that observed among patients initiating antidepressant therapy in our recent study of a PA policy in Michigan for antidepressants.6 This suggests that patients with bipolar disorder may be particularly vulnerable to such policies. However, differences in policy implementation and physician response between Maine and Michigan may have also contributed to a stronger effect in Maine.
These findings indicate that PA policies may create an unintended barrier to initial treatment. One possible explanation for our finding is that physicians were confused about which medications were subject to PA and simply avoided the use of all bipolar medications. An alternative, potentially more plausible, explanation is that physicians continued to prescribe nonpreferred agents, with patients learning when they arrived at the pharmacy that their medication required prior approval. In such situations, the requirements of either completing the administrative approval process or switching medications may deter some patients from filling an initial prescription.25 Containing pharmaceutical costs while maintaining quality of care is a great challenge. Greater involvement of the medical community in the design and implementation of policies that will impact use of psychoactive medications may ensure wider policy acceptance while minimizing negative impact on patient access to essential medications.
In contrast with our previous study on the impacts of a PA policy on patients treated with antidepressants,6 we did not find any increase in rates of switching among patients previously established on therapy following implementation of the PA. It is possible that patients being treated for bipolar disorder may be monitored more aggressively than patients with depression, resulting in fewer disruptions in therapy among established users.
There are several limitations of this study that deserve discussion. First, we had no information on actual prescribing or requests for PA. As a result, we could not discern whether the observed changes in rates of initiation were due to changes in prescribing practices or patient response. Further, we could not observe medication use within inpatient facilities and thus excluded patients with long institutional stays. If our inclusion rules inadvertently selected healthier patients, our findings may not reflect the response of more severely ill bipolar patients to the PA policy. Also, medications dispensed at hospital discharge would not be captured by Medicaid data. Finally, we could not detect switching of medications that may have occurred during an institutional stay.
In conclusion, the findings of this study provide empirical evidence that PA implementation can reduce rates of initiation of nonpreferred medications without a corresponding increase in initiation of preferred agents. This presents a significant challenge to Medicaid programs and Medicare Part D plans as they strive to constrain pharmaceutical expenditure, while preserving the quality of care. This study contributes to a growing body of literature suggesting that PA policies may have unanticipated and unintended impacts on treatment when applied to antipsychotic agents,9,26 compared with other medication classes.27–30 To the extent that procedural barriers in access to essential medications contribute to adverse health events, such barriers should be avoided.5,6,9 Given the exponential growth of PA policies and recognizing their potential unintended harms, further study and evaluation of PA implementation procedures is needed to encourage optimal care of vulnerable patients with chronic mental illness.
Acknowledgments
Supported by the Robert Wood Johnson Foundation’s Changes in Health Care Financing and Organization Program, “Effects of Prior Authorization on New Medications among Medicare Beneficiaries with Bipolar Disorder” (grant No. 63213, to S.B.S.). Methods for this study were based on a previous study supported by the National Institute for Mental Health (NIMH, grant 5R01MH069776–03) conducted by the research team. Also, supported by a Fellowship in Pharmaceutical Policy at Harvard Medical School and a Sir Keith Murdoch Fellowship by the American Australian Association (to C.Y.L.) and by the Kaiser Permanente Northern California Community Benefit Program (to A.S.A.).
F.Z. and S.B.S. are investigators in the HMO Research Network Center for Education and Research in Therapeutics and are supported by the Agency for Healthcare Research and Quality.
Footnotes
This work was conducted at the Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute.
REFERENCES
- 1.Smith C, Cowan C, Heffler S, et al. National health spending in 2004: recent slowdown led by prescription drug spending. Health Aff. 2006; 25:186–196. [DOI] [PubMed] [Google Scholar]
- 2.Catlin A, Cowan C, Heffler S, et al. National Health Expenditure Accounts Team. National health spending in 2005: the slowdown continues. Health Aff. 2007;26:142–153. [DOI] [PubMed] [Google Scholar]
- 3.Crowley JS, Ashner D, Elam L. State Medicaid Outpatient Prescription Drug Policies: Findings From a National Survey, 2005 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured, Kaiser Family Foundation; 2005. [Google Scholar]
- 4.Huskamp HA, Stevenson DG, Donohue JM, et al. Coverage and prior authorization of psychotropic drugs under Medicare Part D. Psychiatr Serv. 2007;58:308–310. [DOI] [PubMed] [Google Scholar]
- 5.Soumerai SB. Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Aff (Millwood). 2004;23:135–146. [DOI] [PubMed] [Google Scholar]
- 6.Adams AS, Zhang F, LeCates R, et al. Prior authorization for antidepressants in Medicaid: effects among disabled dual enrollees. Arch Intern Med. 2009;169:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159: 1–50. [PubMed] [Google Scholar]
- 8.Koyanagi C, Forquer S, Alfano E. Medicaid policies to contain psychiatric drug costs. Health Aff. 2005;24:536. [DOI] [PubMed] [Google Scholar]
- 9.Soumerai SB, Zhang F, Ross-Degnan D, et al. Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Aff. 2008;27:w185–w195. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Adams AS, Ross-Degnan D, et al. Effects of prior authorization on medication discontinuation among Medicaid beneficiaries with bipolar disorder. Psychiatr Serv. 2009;60:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saucier P, Benzanson L, Booth M, et al. Linked data analysis of dually eligible beneficiaries in New England. Health Care Financ Rev. 1998; 20:91–108. [PMC free article] [PubMed] [Google Scholar]
- 12.Busch AB, Ling D, Frank RG, et al. Changes in the quality of care for bipolar I disorder during the 1990s. Psychiatr Serv. 2007;58:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 14.Gillings D, Makuc D, Siegel E. Analysis of interrupted time series mortality trends: an example to evaluate regionalized perinatal care. Am J Public Health. 1981;71:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinbaum DG, Kupper LL, Muller KE, et al. Applied Regression Analysis and Other Multivariable Methods. Pacific Grove, CA: Duxbury Press; 1998. [Google Scholar]
- 16.Diggle PJ, Heagerty P, Liang KY, et al. Analysis of Longitudinal Data. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 17.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 18.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–864. [DOI] [PubMed] [Google Scholar]
- 19.Soumerai SB, McLaughlin TJ, Ross-Degnan D, et al. Effects of limiting Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med. 1994;331:650–655. [DOI] [PubMed] [Google Scholar]
- 20.Wagner A, Ross-Degnan D, Gurwitz JH, et al. Effect of New York state regulatory action on benzodiazepine prescribing and hip fracture rates. Ann Intern Med. 2007;146:96–103. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Wagner A, Soumerai SB, et al. Methods for estimating confidence intervals in interrupted time series analyses of health interventions J Clin Epidemiol. 2009;62:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKinnon NJ, Kumar R. Prior authorization programs: a critical review of the literature. J Manag Care Pharm. 2001;7:297–302. [Google Scholar]
- 23.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 24.Bowden CL, Krishnan AA. Pharmacotherapy for bipolar depression: an economic assessment. Expert Opin Pharmacother. 2004;5:1101–1107. [DOI] [PubMed] [Google Scholar]
- 25.MaineCare Advisory Committee’s Prior Authorization Subcommittee Report and Recommendations on Prior Authorization for Prescription Drugs in the MaineCare and Drugs for the Elderly Programs, submitted to the Maine Department of Health and Human Services, Augusta, Maine, 19 January 2005. [Google Scholar]
- 26.Law MR, Ross-Degnan D, Soumerai SB. Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv. 2008;59:540–546. [DOI] [PubMed] [Google Scholar]
- 27.Fischer MA, Schneeweiss S, Avorn J, et al. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Eng J Med. 2004;351:2187–2194. [DOI] [PubMed] [Google Scholar]
- 28.Roughead EE, Zhang F, Ross-Degnan D, et al. Differential effect of early or late implementation of prior authorization policies on the use of Cox II inhibitors. Med Care. 2006;44:378–382. [DOI] [PubMed] [Google Scholar]
- 29.Smalley WE, Griffin MR, Fought RL, et al. Effect of a prior-authorization requirement on the use of nonsteroidal anti-inflammatory drugs by Medicaid patients. N Engl J Med. 1995;332:1612–1617. [DOI] [PubMed] [Google Scholar]
- 30.Delate T, Mager DE, Sheth J, et al. Clinical and financial outcomes associated with a proton pump inhibitor prior-authorization program in a Medicaid population. Am J Manag Care. 2005;11:29–36. [PubMed] [Google Scholar]