Abstract
Aim:
The aim of this cross-sectional study is to explore if periodontitis is associated with alterations of the retinal microcirculation, a predictive marker of cardiovascular events.
Material and Methods:
Of 457 subjects aged 52 years and more from the ARIC cohort were included. Retinal vascular diameters were measured and summarized as central retinal arteriolar/venular equivalents (CRAE/CRVE). Periodontitis was determined by using the CDC/AAP definition. Multivariable linear regression models were used to estimate the relationships between CRAE, CRVE and periodontitis.
Results:
No association was found between CRAE and periodontal status. However, CRVE and severe periodontitis were positively and significantly associated. Mean CRVE (±SD) was 187.0 ± 17.2 μm in the health-gingivitis group, and, respectively, 188.5 ± 16.3 μm (p = 0.39) and 191.6 ± 16.8 μm (p = 0.04) in moderate and severe periodontitis groups, after adjustment for a propensity score based on confounders. Results were consistent when analyses were restricted to participants with diabetes mellitus (n = 66), but not diabetes-free subjects.
Conclusions:
Severe periodontitis is associated with larger retinal venular diameter in patients with type 2 diabetes. Further studies are needed to explore the impact of diabetes mellitus on the association between periodontitis and retinal microcirculation.
Keywords: microcirculation, periodontitis, retina
Periodontal diseases (PDs) affect almost 50% of the adult population aged 30+ in industrialized countries (Pihlstrom et al. 2005). PDs are bacterially induced inflammatory diseases of the soft and hard tissues which support the tooth (Darveau 2010). They are caused by dental plaque organized as a biofilm onto the root surface (Kolenbrander et al. 2010), which leads to the destruction of periodontal connective tissue and alveolar bone. Without treatment, PDs result in tooth loss.
Chronic periodontitis, one of the most common types of PDs, has been associated with numerous conditions, including vascular dysfunction. An association between increased carotid IMT and PDs was observed (Beck et al. 2001). NHANES data also indicate an association between hemodynamic alterations in brachial arteries and periodontal attachment loss, the landmark clinical feature of periodontitis (Lu et al. 2008).
Direct and indirect biological mechanisms are involved in the pathogenesis process linking periodontal and cardiovascular diseases. The direct mechanism is based on elevated concentrations of inflammatory biomarkers in periodontal pockets, which are responsible of an inflammatory infiltrate in the blood stream, leading to alterations of distant tissues or organs. Indirect mechanisms involve increased systemic levels of bacterial lipopolysaccharides at sites distant from the oral cavity, which may in turn lead to systemic inflammatory reactions (Schenkein & Loos 2013). Whatever the process, the up-regulation of the inflammatory cascade leads to morphological modification and permeability of the vascular endothelium. It is therefore of interest to evaluate, the association of periodontitis, not only on large vessels, but also on the microvasculature. To date, no study exists on the relationship between periodontal diseases and extra-oral microvasculature.
Retinal microcirculation appears to be a good model to explore the extra-oral microvasculature. In addition, retinal microvasculature assessment can be performed using retinal photography, which is a non-invasive and reproducible method (Sun et al. 2009). Alterations of the retinal microcirculation share common risk factors with periodontitis such as obesity (Boillot et al. 2013) or smoking (Sun et al. 2009). Changes in the retinal microcirculation are associated with an increased risk for stroke (McGeechan et al. 2009a), coronary heart disease (CHD) (McGeechan et al. 2009b) and cardiovascular mortality (Wang et al. 2007).
The aim of this analysis was to investigate the relationship between periodontal status and the retinal microcirculation.
Materials and Methods
Subjects
The design and objectives of the Atherosclerosis Risk in Communities (ARIC) Study have been reported in detail elsewhere (ARIC investigators 1989). ARIC is a longitudinal cohort of cardiovascular and other major diseases among a probability sample of 15792 men and women aged 45–64 years old at inclusion and (Visit 1, 1987–1989) from four US communities: Forsyth County, NC; Jackson, MS; the northwestern suburbs of Minneapolis, MN; and Washington County, MD. After being interviewed at home, all eligible participants were invited to a baseline clinical examination in 1987–1989. Three further 3-years intervals examinations were conducted. 6793 participants returned for the fourth examination (1996–1998) for periodontal examination and 1098 participants for retinal examination. We excluded from analyses participants who were not black or white, and the few black people in the Maryland and Minneapolis centres (n = 38). Five hundred and forty nine participants had both periodontal and retinal examinations at visit 4. Participants with less than four remaining teeth were excluded (n = 8). Eighty-four additional participants were excluded because of missing data on covariates. A final sample of 457 participants formed the analytical sample (Figure 1). The Institutional Boards of all participating centres approved the procedures. All participants gave informed consent.
Fig. 1.
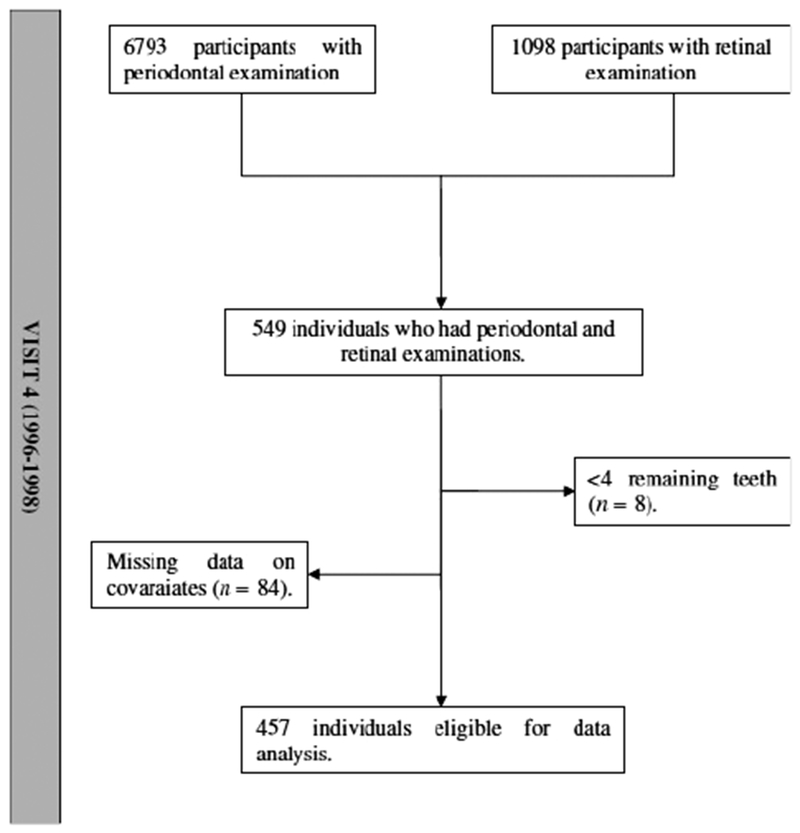
Flowchart of the study sample selection.
Measurement of retinal vascular diameters
These measurements have been described previously (Hubbard et al. 1999). Briefly, following 5 min. of dark adaptation, a 45-degree retinal film photograph was taken of one randomly selected eye, centred on the optic disc and macula. These photographs were digitized, and the calibre of individual arterioles and venules coursing through a region one half to 1 disc diameter from the optic disc margin were measured using a computer assisted method. These measurements were summarized as the central retinal arteriolar/venular equivalents (CRAE/CRVE), using the formula developed by Parr and Spears, which represented the average of estimated calibres for the central retinal vessels (Parr & Spears 1974a,b).
Measurement of periodontal conditions
The Dental ARIC, an ancillary study funded by the National Institute of Dental and Craniofacial Research, was conducted at visit 4 (1996–1998). Dental ARIC consisted of an oral examination; collection of gingival crevicular fluid, oral plaque and serum; and interviews. Persons requiring antibiotic prophylaxis for periodontal probing were excluded. Periodontal measurements (clinical attachment loss, probing depth and gingival recession) were performed on six sites for all teeth. Periodontal examiners were calibrated to a standard examiner and the percentage of agreement for attachment loss within 1 mm ranged from 83.2% to 90.2%. Weighted κ-statistics ranged from 0.76 to 0.86, and intra-examiner correlation coefficients ranged from 0.76 to 0.90 (Beck et al. 2001). We used the Centers for Disease Control and Prevention and American Academy of Periodontology (CDC/AAP) definition of periodontitis to classify participants in healthy/gingivitis, moderate and severe periodontitis. Moderate periodontitis is defined as having at least two teeth with inter-proximal attachment loss of 4 mm or more OR at least two teeth with 5 mm or more of pocket depth at inter-proximal sites. Severe periodontitis is defined as having at least two teeth with inter-proximal attachment loss of 6 mm or more AND at least one tooth with 5 mm or more of pocket depth at inter-proximal sites (Page & Eke 2007).
Definition of other covariates
Age (in years) at visit 4, centre (Forsyth County, NC; Jackson, MS; the northwestern suburbs of Minneapolis, MN; and Washington County, MD) and race (Caucasians, African Americans) were collected. Education was divided into basic (<12 years), intermediate (12–16 years) or advanced (17–21 years) levels and income into low (< $12,000), intermediate ($12,000–$50,000) and high (>$50,000) to adjust for socioeconomic status. Diabetes mellitus was defined as a fasting glucose concentration of >126.0 mg/dl (7.0 mmol/l), a non-fasting glucose concentration of >200.0 mg/dl (11.1 mmol/l), or a self-reported history of or treatment for diabetes. The question “Has your doctor ever told you that you have eye problems as a result of diabetes” was used to identify cases of diabetic retinopathy. Hypertension was defined as a systolic blood pressure >140 mm of mercury [Hg] or diastolic blood pressure >90 mm/Hg or self-reported use of antihypertensive medications in the preceding 2 weeks. Anthropometric measures were determined by trained and certified technicians according to a detailed and standardized protocol. BMI was calculated as weight(kg)/[height squared (m2)]. Participants were dichotomized as never/ever (former and current) smokers. Alcohol consumption was measured in grams per week.
Statistical analysis
Frequency distributions, means and standard deviations across periodontal status groups were determined to describe the data.
We used a propensity score because of the large number of confounders regarding the sample size, and the inherent risk of overadjustment. To generate the propensity scores, multivariate logistic models were first run in which periodontal status (CDC/AAP definition) was the dependent variable and all others confounders (age, gender, centre/race, diabetes mellitus, diabetic retinopathy, hypertension, BMI, triglycerides, HDL and LDL cholesterol, smoking, alcohol consumption, education, income and number of remaining teeth) the independent variables. Scores were then calculated for each participant. Propensity scores correspond to the probability of being exposed to periodontal diseases, taken into account the status relative to confounding variables. The c-statistics for the propensity models were 0.72 when comparing moderate periodontitis and healthy/gingivitis categories, and 0.81 when comparing severe periodontitis to healthy/gingivitis. Table S1 gives additional explanations about the propensity score. We used linear models to assess the relation between retinal arteriolar and venular diameters (dependent variables) and periodontal status, adjusting for the propensity scores as a single continuous variable of adjustment. Adjusted means across periodontal status groups were extracted from models. p values less than 0.05 were considered statistically significant. Subgroup analyses by diabetic status were conducted. To explore if a graduated association existed between retinal vascular diameters and severity of periodontal breakdown, periodontal status was coded as a continuous variable to obtain p for trend values.
In addition, because numerous studies observed narrower arteriolar and wider venular diameters with increased inflammation, CRAE and CRVE were dichotomized, with retinal arteriolar diameters classified as being in the lowest quartile of CRAE versus the other three quartiles, and CRVE being in the higher quartile of CRVE versus the other three quartiles. Retinal arteriolar and venular diameters were then regressed across periodontal status groups and other covariates using multivariate logistic models. Odds-ratios with 95% confidence intervals are presented. All statistical analyses were performed with R software (version 2.14.0, the R Development Core Team. 2013).
Results
General characteristics
Among the 457 participants included in the study, 194 subjects had moderate and 92 severe periodontitis. Mean age (±SD) was 62.5 ± 5.6 years and 211 subjects (46.2%) were male. Almost 70% were Caucasians. Half of the participants were current or former smokers. 14.4% had diabetes mellitus, 1.5% had diabetic retinopathy, 35.7% had hypertension and the mean BMI (±SD) was 28.8 ± 5.2 kg m2. Significant associations were found between periodontitis and age, gender, smoking, alcohol consumption, education, income and HDL cholesterol. Severe periodontitis was associated with retinal venular diameter (191.8 ± 18.2 μm for severe periodontitis versus 188.6 ± 16.4 μm for moderate forms and 168.8 ± 14.4 μm for the healthy/gingivitis group, p = 0.02). No statistical difference was observed for retinal arteriolar diameter across periodontal status groups. Mean probing depth, mean attachment loss and percentage of sites with bleeding on probing were significantly and positively associated with the severity of periodontal diseases according to the CDC/AAP classification, which justify the use of this specific classification (Table 1).
Table 1.
Description of sample characteristics by periodontal status
| All subjects (n = 457) |
Healthy/Gingivitis (Ref) (n = 171) |
Moderate PD (n = 194) |
Severe PD (n = 92) |
|
|---|---|---|---|---|
| Age | 62.5 ± 5.6 | 61.4 ± 5.7 | 63.1 ± 5.3† | 63.1 ± 5.9* |
| Gender (Ref: Female) | ||||
| Female | 53.8 | 69.6 | 53.1 | 26.1 |
| Male | 46.2 | 30.4 | 46.9† | 73.9† |
| Race (Ref: Jackson African Americans) | ||||
| Jackson African Americans | 28.7 | 29.2 | 25.8 | 33.7 |
| North Carolina Caucasians | 21.4 | 30.4 | 19.1 | 9.8† |
| North Carolina African Americans | 1.5 | 1.2 | 1.0 | 3.3 |
| Minnesota Caucasians | 22.1 | 17.0 | 26.3 | 22.8 |
| Washington County, MD, Caucasians | 26.3 | 22.2 | 27.8 | 30.4 |
| Diabetes mellitus | 14.4 | 11.7 | 16.0 | 16.3 |
| Diabetic retinopathy | 1.5 | 0.6 | 2.6 | 1.1 |
| Hypertension | 35.7 | 40.9 | 29.9* | 38.0 |
| BMI (kg/m2) | 28.8 ± 5.2 | 29.3 ± 6.1 | 28.4 ± 4.9 | 28.4 ± 4.2 |
| HDL Cholesterol | 49.9 ± 16.3 | 51.8 ± 16.4 | 51.0 ± 16.9 | 43.9 ± 13.6† |
| LDL Cholesterol | 122.8 ± 34.8 | 120.4 ± 35.2 | 123.4 ± 33.5 | 126.0 ± 36.5 |
| Triglycerides | 140.0 ± 81.3 | 145.5 ± 86.7 | 133.7 ± 77.9 | 142.8 ± 77.9 |
| Alcohol consumption (g per week) | 28.5 ± 69.1* | 19.8 ± 47.1 | 30.0 ± 77.7 | 41.8 ± 81.5† |
| Smoking (Ref: never smoker) | ||||
| Never smoker | 49.9 | 63.2 | 49 | 27.2 |
| Ever Smoker | 50.1 | 36.8 | 51.0† | 72.8† |
| Education level (Ref: <12 years) | ||||
| <12 years | 16.2 | 8.2 | 21.1 | 20.6 |
| 12–16 years | 42.2 | 47.4 | 36.1† | 45.7* |
| ≥17 years | 41.6 | 44.4 | 42.8† | 33.7† |
| Income (Ref: >$50,000 per year) | ||||
| <$12,000 | 33.9 | 30.4 | 36.6 | 34.8 |
| $12,000–$50,000 | 51.9 | 52.6 | 48.4 | 57.6* |
| >$50,000 | 14.2 | 17.0 | 15.0 | 7.6 |
| CRAE (μm) | 157.1 ± 14.6 | 156.1 ± 15.4 | 156.8 ± 13.7 | 159.7 ± 14.8 |
| CRVE (μm) | 188.6 ± 16.1 | 186.8 ± 14.4 | 188.6 ± 16.4 | 191.8 ± 18.2* |
| Number of teeth | 21.5 ± 6.7 | 21.7 ± 6.9 | 21.7 ± 6.6 | 20.5 ± 6.7 |
| Percentage of sites with BOP | 26.3 ± 22.2 | 20.0 ± 18.5 | 25.4 ± 19.9† | 40.2 ± 26.9† |
| Mean CAL | 1.9 ± 1.0 | 1.3 ± 0.4 | 1.8 ± 0.6† | 3.2 ± 1.2† |
| Mean PPD | 2.7 ± 0.7 | 1.6 ± 0.2 | 1.8 ± 0.3† | 2.7 ± 0.7† |
| Propensity score | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.2† | 0.7 ± 0.2† |
PD, Periodontal Disease; CRAE/CRVE, Central Retinal Arteriolar/Venular Equivalent; BOP, Bleeding On Probing; CAL, Clinical Attachment Loss; PPD, Periodontal Probing Depth.
Chi-square test and Student’s t-test.
p for statistical difference<0.05.
p for statistical difference<0.01.
Retinal arteriolar and venular diameters were associated with age and centre/race in univariate models. Retinal arteriolar diameter was additionally associated with education and alcohol consumption, whereas retinal venular diameter was associated with diabetes mellitus, BMI, smoking, triglycerides, HDL cholesterol and periodontal status (Table 2).
Table 2.
Mean Central Retinal Arteriolar Equivalent (CRAE)/Central Retinal Venular Equivalent (CRVE) across covariates
| CRAE (mean ± SD, μm) |
CRVE (mean ± SD, μm) |
|
|---|---|---|
| Age | ||
| Below median (Ref) | 159.0 ± 14.3 | 190.7 ± 15.7 |
| Above median | 155.0 ± 14.8† | 186.2 ± 16.3† |
| Gender | ||
| Female (Ref) | 158.1 ± 15.4 | 187.6 ± 16.4 |
| Male | 155.9 ± 13.7 | 189.7 ± 15.7 |
| Centre/Race | ||
| Jackson African Americans (Ref) | 160.1 ± 14.3 | 192.3 ± 16.7 |
| North Carolina Caucasians | 154.4 ± 14.8† | 186.2 ± 16.6† |
| North Carolina African Americans | 163.5 ± 7.7 | 196.5 ± 15.2 |
| Minnesota Caucasians | 156.3 ± 14.7* | 187.9 ± 15.1* |
| Washington County, MD, Caucasians | 156.3 ± 14.7* | 186.5 ± 15.3† |
| Diabetes mellitus | 158.1 ± 14.1 | 192.8 ± 15.8* |
| Diabetic retinopathy | 153.8 ± 14.7 | 195.2 ± 16.1 |
| Hypertension | 155.8 ± 15.0 | 189.8 ± 16.9 |
| BMI (kg/m2) | ||
| BMI≤25 (Ref)‡ | 157.0 ± 13.3 | 182.4 ± 15.7 |
| BMI > 25 | 157.1 ± 15.1 | 190.6 ± 15.8† |
| HDL Cholesterol | ||
| Below median (Ref) | 157.9 ± 14.6 | 191.1 ± 15.9 |
| Above median | 156.3 ± 14.6 | 186.0 ± 15.9* |
| LDL Cholesterol | ||
| Below median (Ref) | 157.2 ± 14.7 | 187.4 ± 16.1 |
| Above median | 157.0 ± 14.7 | 189.8 ± 16.1 |
| Triglycerides | ||
| Below median (Ref) | 156.1 ± 14.6 | 187.0 ± 16.1 |
| Above median | 158.1 ± 14.6 | 190.2 ± 16.1* |
| Propensity score¶ | ||
| Below median (Ref) | 156.6 ± 14.6 | 187.7 ± 16.1 |
| Above median | 157.6 ± 14.7 | 189.4 ± 16.1 |
| Smoking | ||
| Never smoker (Ref) | 156.1 ± 14.1 | 186.6 ± 15.4 |
| Ever smoker | 158.1 ± 15.1 | 190.5 ± 16.7† |
| Alcohol consumption | ||
| 0 g per week | 158.5 ± 14.5 | 189.1 ± 16.1 |
| More than 0 g per week | 153.8 ± 14.5† | 187.4 ± 16.1 |
| Education level | ||
| <12 years (Ref) | 159.9 ± 15.3 | 190.5 ± 17.4 |
| 12–16 years | 155.9 ± 14.4* | 187.7 ± 15.1 |
| ≥17 years | 157.2 ± 14.6 | 188.7 ± 16.6 |
| Periodontal status | ||
| Healthy/Gingivitis (Ref) | 156.1 ± 14.6 | 186.8 ± 16.0 |
| Moderate PD | 156.8 ± 14.6 | 188.6 ± 16.0 |
| Severe PD | 159.7 ± 14.6 | 191.8 ± 16.0* |
CRAE/CRVE, Central Retinal Arteriolar/Venular Equivalent; SD, standard deviation. n = 457
n = 114.
Median value for propensity score = 0.66. Generalized linear models.
p for statistical difference<0.05.
p for statistical difference<0.01.
Relationship between retinal arteriolar diameter (CRAE) and periodontal status
There was no association between CRAE and periodontal status (Table 2, p = 0.06), nor when adjusting for medical, dental and biological data (Table S2). Subgroup analyses by diabetic status did not change the results (Fig. 2, Table S2). Means CRAE adjusted for propensity score (maximum level of adjustment) was 156.2 ± 15.7 μm for the healthy/gingivitis group and 156.7 ± 14.8 μm and 159.6 ± 15.3 μm for moderate and severe periodontitis respectively (all p > 0.05). In spite of an apparent increase of the retinal arteriolar diameter with the severity of the periodontal breakdown, no linear association was observed (p for trend = 0.13) (Fig. 2).
Fig. 2.
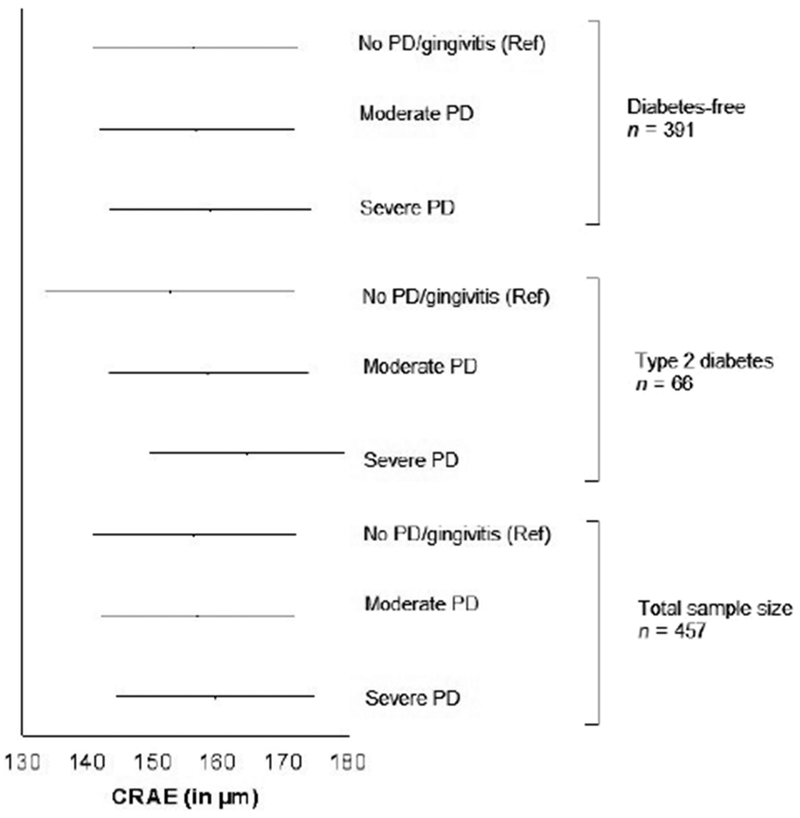
Adjusted means (with standard errors) for Central Retinal Arteriolar Equivalent (CRAE) across periodontal status categories and diabetic status. PD: Periodontal diseases. Generalized linear models. Three categories model: healthy/gingivitis (reference) versus moderate PD versus severe PD. Healthy/gingivitis. Adjustment for propensity score, maximum level of adjustment. Diabetes free, n = 391; no PD/gingivitis, n = 151; moderate PD, n = 163; severe PD, n = 77; p for linear trend = 0.32. Diabetes mellitus, n = 66; no PD/gingivitis, n = 20; moderate PD, n = 31; severe PD, n = 15; p for linear trend = 0.03. All subjects, n = 457; no PD/gingivitis, n = 171; moderate PD, n = 194; severe PD, n = 92; p for linear trend = 0.13.
Being in the lower quartile of CRAE is not significantly associated with severe periodontitis (p = 0.15), even when adjusting for the propensity score (p = 0.13).
Relationship between retinal venular diameter (CRVE) and periodontal status
A positive association was observed between CRVE and severe periodontitis (Table 2, p = 0.02). This association remained significant even when adjusting for the propensity score (maximum level of adjustment, p = 0.04) (Fig. 3). A graduated association was observed between CRVE and periodontal status, retinal venular diameters increasing across periodontal status group (adjusted m ± SD: healthy/gingivitis, 187.0 ± 17.2 μm; moderate periodontitis: 188.5 ± 16.3 μm (p = 0.39); severe periodontitis: 191.6 ± 16.8 μm (p = 0.04). p for trend<0.05) (Fig. 3). Subgroups analyses shown the association between CRVE and severe periodontitis was still significant for participants with diabetes mellitus (p = 0.01), but not for diabetes-free subjects (Fig. 3, Table S2).
Fig. 3.
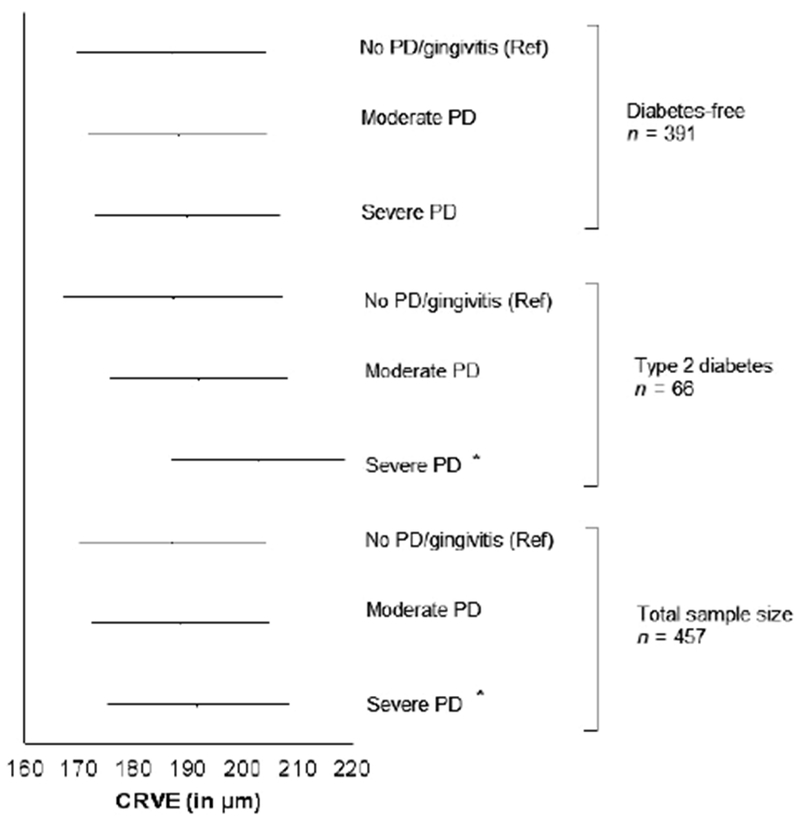
Adjusted means (with standard errors) for Central Retinal Venular Equivalent (CRVE) across periodontal status categories and diabetic status. PD: Periodontal diseases. Generalized linear models. Three categories model: healthy/gingivitis (reference) versus moderate PD versus severe PD. Healthy/gingivitis. Adjustment for propensity score, maximum level of adjustment. * p < 0.05. Diabetes free, n = 391; no PD/gingivitis, n = 151; moderate PD, n = 163; severe PD, n = 77; p for linear trend = 0.22. Diabetes mellitus, n = 66; no PD/gingivitis, n = 20; moderate PD, n = 31; severe PD, n = 15; p for linear trend< 0.01. All subjects, n = 457; no PD/gingivitis, n = 171; moderate PD, n = 194; severe PD, n = 92; p for linear trend<0.05.
Being in the higher quartile of CRVE is significantly associated with severe periodontitis (OR, 95% CI: 2.28, from 1.13 to 4.64), even when adjusting for propensity score with maximum level of adjustment (OR, 95%CI: 2.18, from 1.15 to 4.13).
Discussion
A positive and independent relationship between severe periodontitis and larger retinal venular diameters was observed in this study. No significant association was found between retinal arteriolar diameters and periodontal status.
Previous data have shown that retinal arteriolar alterations were associated with long-term high blood pressure-mediated damage, particularly in older individuals whose vessels become rigid and consequently less adapted to blood pressure changes (Leung et al. 2004, Kaushik et al. 2007). On the other hand, increased levels of serum pro-inflammatory biomarkers levels were associated with both venular diameter and periodontal attachment loss. In a large cross-sectional study based on 5279 subjects issued from the Rotterdam Study, authors observed 1-SD increments in levels of C-reactive protein in serum and plasma fibrinogen were associated with wider retinal venular diameters (de Jong et al. 2007). An increased serum level of C-reactive protein, interleukin 6, sICAM-1 and plasma fibrinogen has been also shown with an increase of the retinal venular diameter in the Multi-Ethnic Study of Atherosclerosis (MESA) (Wong et al. 2006). In a cross-sectional study conducted in 1224 French adults aged 60+, a significant and independent association was observed between larger retinal venular diameters and plasma levels of orosomucoid and C-reactive protein (Daien et al. 2013). Higher serum levels of all these inflammatory biomarkers were observed in subjects with periodontitis (Beck & Offenbacher 2002, Demmer et al. 2008, Skaleric et al. 2012, Keles et al. 2014). In a cross-sectional study conducted in ARIC and including more than 5000 participants, Beck & Offenbacher (2002) observed that extent of clinical attachment loss of 3 mm and more, extent of probing depth of 5 mm and more, and also extent of bleeding on probing, were all three associated with serum levels of C-reactive protein and sICAM-1. Finally, Range et al. (2013) observed a significant and independent association between severe periodontitis and increased levels of orosomucoid. The outcomes of the above studies suggest that systemic inflammation may be an intermediary explanatory variable in the association between the increase in the retinal venular diameters and the periodontal status, but this remains to be explored. Because only few participants had serum CRP measured with both retinal and periodontal examination, we were not able to test the hypothesis of an inflammatory pathway linking severe periodontitis to retinal microvascular alterations.
Previous studies found a positive relationship between diabetic nephropathy, glomerular filtration and periodontal diseases. In a cross-sectional analysis conducted in the ARIC study, we found a significant independent association between low glomerular filtration rate (<60 ml/min/1.73 m2) and initial (OR, 95% CI: 2.00, from 1.23 to 3.24) or severe (OR, 95%CI: 2.14, from 1.19 to 3.85) periodontitis, when compared with gingivitis or periodontally healthy participants (Kshirsagar et al. 2005). Moreover, in a longitudinal study in 529 type 2 diabetes individuals with a follow-up up of 22 years, authors found a significant and gradual association between nephropathy and periodontal diseases, assessed by the severity of alveolar bone loss. After adjustment for covariates, the hazard ratios (95% confidence intervals) for incident macroalbuminuria were 2.0 (from 1.2 to 3.5), 2.1 (from 1.2 to 3.8) and 2.6 (from 1.4 to 4.6) as high in participants with moderate, severe and edentulous, respectively, when compared with subjects with no or mild bone loss (Shultis et al. 2007). Alteration of the glomerular filtration is, with ophthalmologic disorders, one of the main complications of diabetes. Because bi-directional relationship also exists between diabetes and periodontal diseases, we decided to conduct subgroup analyses to explore the relationship between periodontal status and retinal vascular diameters across diabetic status. The non-significant association between CRAE and periodontal status was consistent across diabetic groups. On the contrary, the significant and independent association between CRVE and severe periodontitis found in the total sample size was also found in the diabetic group, but not in the diabetes-free group. The analysis conducted for all participants was adjusted for all covariates, included diabetic status and diabetic retinopathy, which means that there is an association between periodontal status and CRVE, independently of diabetes. However, because of the results from the subgroup analyses, we could hypothesize that diabetes may also mediate the relationship between CRVE and periodontal status. Future prospective studies are needed to clarify the relationship between periodontal status, diabetes and retinal microvascular alterations.
Our study has several strengths. To our knowledge, it is the first report, showing an association between periodontitis and an extraoral microvasculature. As mentioned above, numerous reports indicate an association between periodontal inflammation and cardiovascular risk but to date no study was designed to investigate the role of periodontal diseases on alterations of the microvasculature. Second, as explained above, using the propensity score, we obtained a high level of adjustment to measure the independent association between periodontal status and retinal microvasculature, and reduced the risk of overadjustment due to the number of confounding variables. This is particularly important for analyses conducted in the diabetic group in which only 66 participants were included. However, additional studies conducted in larger samples of diabetic patients are needed to confirm our results.
In our study, subjects with less than four remaining teeth and those with medical conditions prohibiting periodontal probing without prophylactic antibiotics were excluded from the analysis. These exclusions could result in a lower prevalence of diabetes, smoking and cardiovascular events which, in turns, may impact the amount of retinal microvascular alterations. Second, this study was not designed to explore the biological pathway between periodontal diseases, extra-oral microcirculation and cardiovascular events. However, it cannot be discarded that history of cardiovascular events may modify the relationship between alterations of the retinal microcirculation and severe periodontitis. Further prospective studies are needed to explore this hypothetical pathway. Finally, we had serum CRP value for only one included participants, which did not allow us to test for the inflammatory pathway hypothesis.
In conclusion, we observed severe periodontitis was associated with larger retinal venular diameter in patients with type 2 diabetes. Our data suggest that the effect of periodontal inflammation described previously on macrocirculation may be generalized to the entire circulatory system. Future studies with prospective designs are needed, to investigate the relationship between periodontal diseases, retinal micro-circulation and diabetes mellitus.
Supplementary Material
Clinical Relevance.
Scientific rationale for the study:
Periodontitis is associated with hemodynamic alterations, increased intima–media thickness in both brachial and carotid arteries and cardiovascular events. We hypothesized that periodontitis was also associated with alterations of the retinal microcirculation.
Principal findings:
We observed larger retinal venular calibres in adults with severe periodontitis. Results were consistent when limiting analyses to participants with diabetes mellitus, but not in patients without type 2 diabetes.
Practical implications:
Diabetes mellitus is associated with both periodontitis and alterations in the retinal microcirculation. Further rospective studies are needed to investigate the link between periodontal diseases, alterations of the retinal microvasculature and diabetes mellitus.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract (HHSN268200625226C). ARIC carotid MRI examination was funded by U01HL075572-01. Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917, with previous brain MRI examinations funded by R01-HL70825. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
The authors declare that they have no conflict of interests.
References
- ARIC investigators (1989) The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives.. American Journal of Epidemiology 129, 687–702. [PubMed] [Google Scholar]
- Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM & Offenbacher S (2001) Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (ARIC) study. Arteriosclerosis, Thrombosis, and Vascular Biology 21, 1816–1822. [DOI] [PubMed] [Google Scholar]
- Beck JD & Offenbacher S (2002) Relationships among clinical measures of periodontal disease and their associations with systemic markers. Annals of Periodontology/The American Academy of Periodontology 7, 79–89. [DOI] [PubMed] [Google Scholar]
- Boillot A, Zoungas S, Mitchell P, Klein R, Klein B, Ikram MK, Klaver C, Wang JJ, Gopinath B, Tai ES, Neubauer AS, Hercberg S, Brazionis L, Saw SM, Wong TY & Czernichow S (2013) Obesity and the microvasculature: a systematic review and meta-analysis. PLoS ONE 8, e52708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daien V, Carriere I, Kawasaki R, Cristol JP, Villain M, Fesler P, Ritchie K & Delcourt C (2013) Retinal vascular caliber is associated with cardiovascular biomarkers of oxidative stress and inflammation: the POLA study. PLoS ONE 8, e71089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nature Reviews. Microbiology 8, 481–490. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Kocher T, Schwahn C, Volzke H, Jacobs DR Jr & Desvarieux M, (2008) Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dentistry and Oral Epidemiology 36, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD & Cai J (1999) Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 106, 2269–2280. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, Ikram MK, Witteman JC, Hofman A, de Jong PT & Breteler MM (2007) Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Annals of Neurology 61, 491–495. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Kifley A, Mitchell P & Wang JJ (2007) Age, blood pressure, and retinal vessel diameter: separate effects and interaction of blood pressure and age. Investigative Ophthalmology & Visual Science 48, 557–561. [DOI] [PubMed] [Google Scholar]
- Keles ZP, Keles GC, Avci B, Cetinkaya BO & Emingil G (2014) Analysis of YKL-40 acute-phase protein and interleukin-6 levels in periodontal disease. Journal of Periodontology 85, 1240–1246. doi: 10.1902/jop.2014.130631. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ Jr, Periasamy S, & Jakubovics NS (2010) Oral multispecies biofilm development and the key role of cell-cell distance. Nature Reviews. Microbiology 8, 471–480. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S & Falk RJ (2005) Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 45, 650–657. [DOI] [PubMed] [Google Scholar]
- Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R & Mitchell P (2004) Impact of current and past blood pressure on retinal arteriolar diameter in an older population. Journal of Hypertension 22, 1543–1549. [DOI] [PubMed] [Google Scholar]
- Lu B, Parker D & Eaton CB (2008) Relationship of periodontal attachment loss to peripheral vascular disease: an analysis of NHANES 1999–2002 data. Atherosclerosis 200, 199–205. [DOI] [PubMed] [Google Scholar]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P & Wong TY (2009a) Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. American Journal of Epidemiology 170, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, Dejong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P & Wong TY (2009b) Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Annals of Internal Medicine 151, 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC & Eke PI (2007) Case definitions for use in population-based surveillance of periodontitis. Journal of Periodontology 78, 1387–1399. [DOI] [PubMed] [Google Scholar]
- Parr JC & Spears GF (1974a) General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. American Journal of Ophthalmology 77, 472–477. [DOI] [PubMed] [Google Scholar]
- Parr JC & Spears GF (1974b) Mathematic relationships between the width of a retinal artery and the widths of its branches. American Journal of Ophthalmology 77, 478–483. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS & Johnson NW (2005) Periodontal diseases. Lancet 366, 1809–1820. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2013) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Vienna, Austria. RC Team. http://www.R-project.org. [Google Scholar]
- Range H, Poitou C, Boillot A, Ciangura C, Katsahian S, Lacorte JM, Czernichow S, Meilhac O, Bouchard P & Chaussain C (2013) Orosomucoid, a new biomarker in the association between obesity and periodontitis. PLoS ONE 8, e57645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA & Loos BG (2013) Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. Journal of Periodontology 84, S51–S69. [DOI] [PubMed] [Google Scholar]
- Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, Knowler WC & Nelson RG (2007) Effect of periodontitis on overt nephropathy and endstage renal disease in type 2 diabetes. Diabetes Care 30, 306–311. [DOI] [PubMed] [Google Scholar]
- Skaleric E, Petelin M, Gaspirc B & Skaleric U (2012) Periodontal inflammatory burden correlates with C-reactive protein serum level. Acta Odontologica Scandinavica 70, 520–528. [DOI] [PubMed] [Google Scholar]
- Sun C, Wang JJ, Mackey DA & Wong TY (2009) Retinal vascular caliber: systemic, environmental, and genetic associations. Survey of Ophthalmology 54, 74–95. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, Klein BE, Wong TY, Burlutsky G & Mitchell P (2007) Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. European Heart Journal 28, 1984–1992. [DOI] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR & Shahar E (2006) Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Investigative Ophthalmology & Visual Science 47, 2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


