Abstract
A review on the technological development of en face optical coherence tomography (OCT) and optical coherence microscopy (OCM) is provided. The terminology originally referred to time domain OCT, where the preferential scanning was performed in the en face plane. Potentially the fastest realization of en face image recording is full-field OCT, where the full en face plane is illuminated and recorded simultaneously. The term has nowadays been adopted for high-speed Fourier domain approaches, where the en face image is reconstructed from full 3D volumes either by direct slicing or through axial projection in post processing. The success of modern en face OCT lies in its immediate and easy image interpretation, which is in particular of advantage for OCM or OCT angiography. Applications of en face OCT with a focus on ophthalmology are presented. The review concludes by outlining exciting technological prospects of en face OCT based both on time as well as on Fourier domain OCT.
1. Introduction
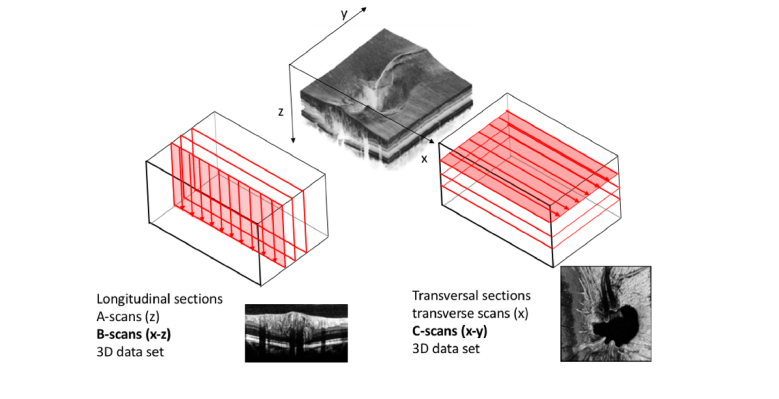
Optical coherence tomography (OCT) [1] provides image slices of tissue with high optical resolution in a non- or minimally invasive manner. There are numerous excellent reviews and book sections highlighting the many applications of OCT in medical imaging today [2], although its most successful application is still retinal imaging, in ophthalmology [3]. Conventional OCT scans an illumination spot across a tissue surface and records at each spot the depth structure. A depth scan is called amplitude scan (A-scan), analogous to ultrasound imaging. Arranging successively recorded A-scans along one scanning direction in a 2D array forms a tomogram or brightness scan (B-scan). The retina with its preferentially layered structure has been the ideal target for OCT, since this structure is well appreciated in recorded tomograms. Abnormal morphological changes can immediately be spotted as disruption of the layered structure even by non-specialists. Together with the easy operation of OCT systems this formed the basis for the huge success of this relatively young medical imaging technology. Also, the technology development coincided with perfect timing with the market release of antiVEGF drugs for treatment of wet AMD. OCT has already become the golden standard for the treatment planning and monitoring of AMD as well as of many other retinal diseases, such as diabetic retinopathy.
In the early days of OCT, the recording speed of tomograms was still slow, due to the in effect low detection efficiency of by that time established time domain OCT. Faster depth scanning critically reduced the detection sensitivity. However, for disease evaluation and treatment monitoring, it was often important to evaluate lesion extensions such as in geographic atrophy, or lesion volumes of edema or drusen. For this task it was necessary to record full tissue volumes. To keep the measurement times low, only a few tomograms were recorded for covering a volume, with increased risk of missing important pathological details. This gap was for the first time closed by en face transverse scanning (TS) time domain (TD) OCT. In en face TS OCT the preferential scanning direction is not in depth, but laterally. Equipped with resonant scanners, en face OCT was thereby capable to record already densely sampled en face images with rates of several tens of Hz. However, in this case, it is necessary to distinguish the actual coherently gated interference or OCT signal at a defined sample depth from the axially non-localized backscattered sample light intensity. This is accomplished by introducing a defined temporal modulation to the OCT signal and using a lock-in detection scheme (see section 6.2). This allows recording of a temporally gated OCT signal from a given sample depth as defined by the reference arm length. In order to produce a depth scan, the reference arm delay is changed slowly or in a stepwise manner. The resulting en face images are similar to fundus photographs, that ophthalmologists are used to, but with the additional benefit of exhibiting also information about the layered retinal structure. Figure 1 demonstrates the scanning preferences for conventional OCT to produce fast tomograms versus the TS OCT configuration for fast en face or C-scan imaging.
Fig. 1.
Scanning preference for conventional TD OCT in the axial direction (lhs). En face TDOCT uses the transversal direction for fast scanning (rhs) and is comparably slow in axial direction. This figure sets also the axis notations for the following discussions in the text.
The en face view is the standard view in most microscopy techniques. Full field (FF) OCT enhances the capabilities of standard bright field microscopy with additional coherence gating, helping to efficiently reject multiply scattered-light with gain in imaging depth and contrast [4] (see section 5). In TD FFOCT the focal plane can be kept centered with the coherence gate during reference arm scanning, thereby maintaining both gating mechanisms. In swept source (SS) FFOCT the signal is collected from all depths in parallel, causing less efficient suppression of scattering cross talk, which results in reduced image contrast as compared to TD FFOCT. The critical loss of contrast is less pronounced with line field OCT (LF OCT) that still keeps half of the confocal gate.
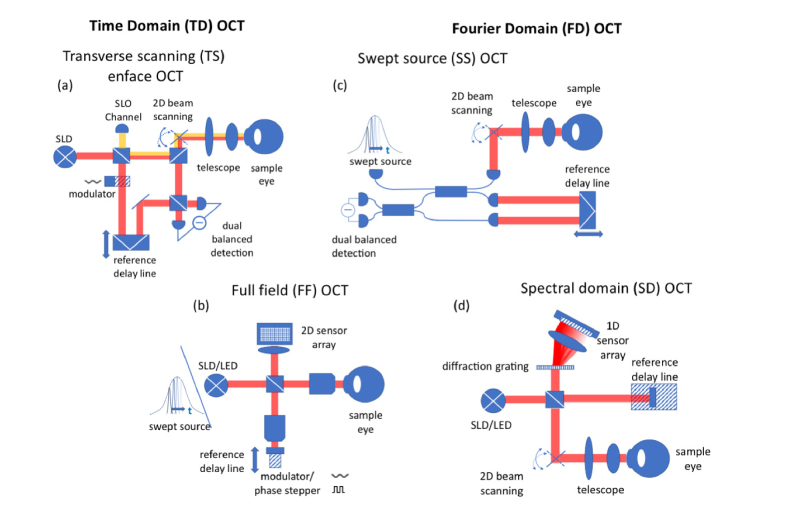
Nowadays, with ultrafast Fourier domain OCT systems, en face images slices are selected retrospectively from full recorded volumes, or are calculated by depth projection along selected depth ranges. Fourier domain system can be distinguished into the earlier spectral domain OCT and swept source OCT. In ophthalmology, en face OCT has become of particular interest for evaluating OCT angiographies or for retinal imaging with high lateral resolution. The combination of FFOCT with SS OCT is in fact the most compact OCT realization with the benefit of higher detection sensitivity. Figure 2 depicts the different OCT modalities and sets the common terms. Note that line field (LF) SS OCT, that is not shown, is similar to FF SS OCT, but with the need of a single scan axis.
Fig. 2.
Overview on OCT technology with TDOCT on the left and FDOCT on the right hand side: (a) TS en face OCT with SLO channel and balanced detection (b) FFOCT and SS FFOCT (c) SS OCT with balanced detection (d) spectral domain OCT using a spectrometer with a diffraction grating and a line array sensor.
In the following we will shortly review the historical development of en face OCT. We will further highlight some details about modulation techniques, address challenges related to axial motion artifacts, and review multimodal functional extensions of en face OCT.
2. Historical overview
As already mentioned, first en face systems were based on time-domain OCT. The advantage of en face OCT is its similarity to confocal microscopy or scanning laser ophthalmoscopy, with the additional benefit of providing coherent depth gating. The first en face OCT system was reported by Izatt el al. for optical coherence microscopy [5]. Later, Podoleanu et al. were able to image the human retina in-vivo with en face TS OCT introducing carrier modulation through lateral scanning [6,7]. The latter system was multimodal by combining OCT with scanning laser ophthalmoscopy (SLO) [8] (see section 6.1). Further improvements regarded the data visualization [1] and the extension to perform quantitative 3D tissue evaluation [2].
At the same time full field OCT emerged, exhibiting excellent resolution and image quality as first shown by Beaurepaire et al. [3] and further improved by Dubois et al. [4] and Grieve et al [5]. The highest OCT imaging speed at that time was reported by Hitzenberger et al. in 2003 [6]. They recorded a full volume in only about 1sec.
This came just before the advent of FDOCT, that had by that time already been demonstrated for high speed in-vivo retinal imaging by Wojtkowski, Leitgeb et al. in 2002 [7]. The concept of recording the interference signal as a function of optical frequency instead of time delay was introduced to biomedical imaging by Fercher et al. [8]. As already mentioned above, such FD OCT system might also employ a tunable light source such that the spectral interference signal is recorded over time. The latter modality termed swept source OCT (SSOCT) was introduced in the same year by Lexer et al. and Chinn et al. [9,10].
The intrinsic higher sensitivity of FDOCT, that was derived for the shot noise limit by Leitgeb et al [11] and was later on generalized to include other noise sources [12,13] as well as to SSOCT [14]. The recognition of the sensitivity advantage marked a paradigm change in OCT since high speed imaging came at no cost regarding sensitivity.
Volumetric imaging and the generation of arbitrary en face planes through depth projection had a strong impact on retinal diagnostics. Ophthalmologists could now use densely sampled en face maps of total retinal thickness, as well as thickness of specific layers, and could evaluate lesion size, and monitor treatment more precisely [15–17]. SS OCT pushed the speed even further into the multi MHz range through the development of novel fast light sources [18]. Especially the functional extension of OCT angiography owes much of its success to the high-speed capability and sensitivity of FDOCT. It is however difficult to name the key paper for OCTA, since the vizualisation of vascular structure and the quantification of flow had been a continuous effort from the early days of OCT on [19–21].
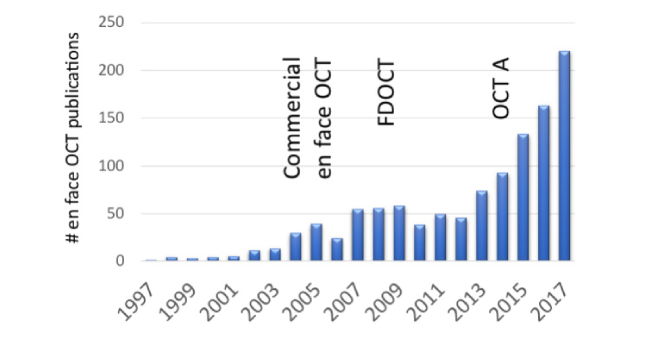
Figure 3 below plots the number of publications concerning en face OCT until 2017. There are three peaks visible that might be used to discern three eras of en face OCT. It starts with TS OCT, that was commercialized, followed by a second rise in publications after the commercialization of FDOCT, and finally the currently strongest increase to today triggered by OCT angiography.
Fig. 3.
SCOPUST search result for “en-face optical coherence tomography” OR “en face optical coherence tomography”. Three eras of en face OCT may be discerned. (OCTA – OCT angiography)
3. En face time domain OCT
3.1 Carrier frequency generation
As previously mentioned an important technical challenge of en face TDOCT is to distinguish the collected incoherent backscattered intensity from sample and reference from the actual cross correlation or interference terms, that carry the information about the axial sample structure.
Time domain FFOCT implementations use phase shifting techniques introduced by displacing the reference arm by defined fractions of the wavelength (see section 4) [3]. Those phase shifts are in general dependent on the wavelength, and are therefore intrinsically chromatic which becomes critical for large optical bandwidths. Reconstruction of the complex OCT signal would need three recordings of the interference pattern with different phase shifts. By using more than three phase shifts it is possible to reduce the chromatic error, leading however to longer measurement times [22]. The latter is critical especially for in-vivo imaging due to motion induced signal distortions.
Parallel recording of different phase shifted signal copies or employing modern sensors with fast frame rates has helped to obtain impressive results with FFOCT, even for in-vivo retinal imaging [23,24].
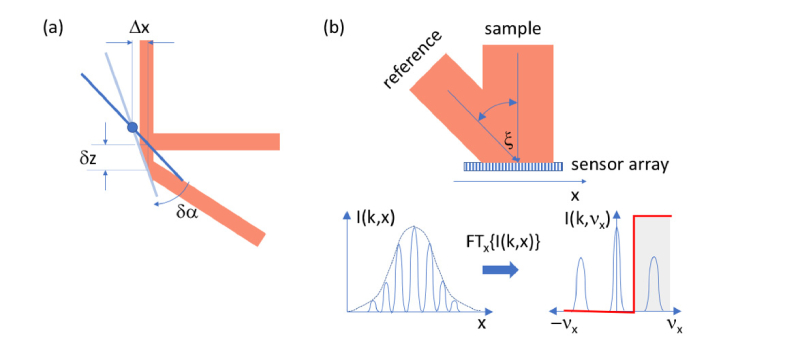
Recent FFOCT and in general parallel OCT approaches adapt techniques from holography by introducing spatial signal modulation for complex OCT signal reconstruction (Fig. 4(b)) [25–28]. Using an off-axis reference arm configuration, a spatial modulation across the parallel sensor is introduced. The spatial modulation frequency νx is proportional to the angle ξ between reference and sample beam at the sensor and inverse proportional to the center wavelength of the source. The angle is chosen such that the modulation shifts the cross-correlation signal approximately to the center of the spatial frequency axis, given that the unmodulated intensity background can be subtracted. This holographic configuration has successfully been implemented for both FFOCT and LFOCT.
Fig. 4.
Carrier frequency generation for en face TDOCT (a) setting the sample beam off the pivot position on the fast scanning galvo mirror; (b) using an off-axis reference arm configuration in parallel OCT variants for holographic signal reconstruction. The red edge shows the Fourier filter function to filter out the spatially modulated OCT signal.
For en face raster scanning OCT a faster modulation technique is needed. Resonant scanners allow already several kHz of line rates, leading to single spot transit times in the µs range. First implementations of en face TDOCT took advantage of the path length modulation during scanning, that results from off-setting the sample beam from the pivot point or rotational center of the galvo scanner mirrors. This method has been introduced by Podoleanu et al. for TS en face OCT [29–31]. The situation is plotted in Fig. 4(a). Off-setting the beam laterally by say Δx leads to an axial delay δz during scanning through an angle δα. Knowing the time δt needed for scanning through δα leads to the modulation frequency fmod = 2k δz /(2π δt) = 2 δz/(λδt), where λ is the center wavelength, and k is the center wavenumber. As a side note, a similar technique has been adapted for FD OCT to suppress complex conjugate mirror artefacts [32].
The elegance of this approach is that no additional and in general expensive fast phase shifting device such as electro-optic or acousto-optic modulators (AOM) are needed. There are however some disadvantages related to the technique. The generated carrier frequency depends on the scanning range and the acquisition or sampling rate. Changing the scanning amplitude requires a change of the sampling rate, and/or readjustment of the lock-in detection frequency band. Furthermore, the local path length shift depends on the actual position of the scanners and changes slightly across the laterally scanned range. As a result, the carrier frequency itself is not fully constant across the field of view, leading to reduced sensitivity when using a rigid lock-in technique. Another drawback is the chromaticity of the phase shift, that might become more relevant for high resolution en face OCT systems [33,34]. Despite those short-comings, such en face system was successfully commercialized (OTI OCT/SLO) and extensively used in clinical studies [35,36]. Figure 5 shows an example for results with such a clinical en face imaging system that combines SLO and TS OCT [37].
Fig. 5.
Clinical examples of combined en face TS OCT and SLO. Reproduced from [37]with permission from The Optical Society (OSA).
Despite the higher costs, acousto-optic modulation is advantageous because of the well-defined heterodyne carrier frequency. The acousto-optic effect is based on phonon-photon interaction. The phonon is produced as a travelling acoustic wave within a crystal. The travelling acoustic wave leads to periodic changes in the refractive index of the crystal medium that act as a dynamic grating structure and cause the incoming light to be diffracted and modulated. The phonon-photon interaction shifts the frequency of the diffracted light wave by the frequency of the acoustic wave as fs = fL ± fAOM = fL ± Ωt, where Ω is the modulation frequency of the AOM. The sign depends on the relative orientation between the incident light and the travelling acoustic wave. It is important to notice, that the induced modulation is independent of the wavelength avoiding therefore chromatic phase shifting errors [38]. Hitzenberger et al. reported in 2003 the fastest retinal 3D OCT system by that time using a single AOM in the reference arm [6]. The system could record a retinal volume with 256(x)x128(y)x64(z) pixels at a rate of 1.75 MVoxels/s. This speed has only been matched and exceeded by FDOCT systems. Lower modulation frequencies can be achieved using two modulators in the reference arm, and orienting them with respect to the incident light such that the first AOM induces an upshift fAOM1, whereas the second AOM induces a downshift -fAOM2. The resulting effective modulation frequency is then fAOM1-fAOM2 [39].
3.2 Focus control
As has been already outlined by Izatt in 1994 [40], OCT enhances the imaging depth range of confocal microscopy in strongly scattering media through the axial coherence gating that efficiently reduces scattered light from outside the coherence plane, in addition to the confocal effect. The optimum effect is obviously achieved when the coherence gate is axially centered with the confocal gate.
Schmitt et al. introduced the method of dynamic focusing, by observing that the confocal gate shifts axially in proportion to the square of the refractive index n, whereas the coherence gate scales axially linear with the group velocity ng. For dynamic focusing, a linear stage tunes the reference arm length by a factor of two with regard to the axial displacement of the confocal gate [41]. This approximately compensates for the different scaling factors of coherence and confocal gate over the in general small depth penetration range of OCT. Various sophisticated configurations have been developed for high speed dynamic focusing through the galvo-scanners [42] or by employing tunable lenses [43]. The possibility of dynamic focusing is in part responsible for the in general much better depth performance of time domain OCT as compared to Fourier domain OCT. Dynamic focusing has in particular been successfully applied in time domain FFOCT for high-contrast digital pathology [44,45].
In FDOCT the axial structure is recorded in parallel, with an axially fixed confocal gate. C-mode scanning enables to record several sub-volumes, where the confocal range is axially shifted between recordings [43,46]. In post-processing the in-focus sections can be extracted from each recording and synthetically reassembled using a Gabor fusion method [47] to enhance the focus depth. This comes however at the expense of time. Alternative approaches to enhance the focal depth in FDOCT used several light beams focused at different depths [48], Bessel beams [49–54] at the expense of sensitivity, or exploited the intrinsic phase stability and speed of FDOCT, and especially of LFOCT and FFOCT, to perform digital refocusing [25,55–58].
3.3 Axial tracking
Despite the high en face image rate of time domain en face OCT, the axial tuning for recording tomograms and volumes is relatively slow. The biggest challenge in time domain en face OCT is therefore the presence of axial motion artifacts. For retinal imaging axial motion speed is of the order of 1mm/s [59]. With a typical en face rate of 10Hz, and a minimum of 200 sampling points axially, the recording of a volume would take already 20 seconds. Even, when taking single tomograms at 4kHz lateral scanning, with 200 lateral sampling points, results in a tomogram rate of 20Hz with 50ms recording time per tomogram. During this time, the retina has already moved in average by an amplitude of 50µm, which is about 5 times the axial resolution of the system. Hence, for recording depth sections in-vivo it becomes evident that means for axial stabilization are necessary.
Miller et al. used axial tracking realized with an auxiliary TD interferometer to keep the coherence gate together with the confocal image plane [60]. This system was however too slow to work in-vivo. Pircher et al. demonstrated high resolution retinal en face time domain OCT in 3D using a second high-speed Fourier domain reference interferometer by axially tracking the cornea apex signal. The axial position of the reference interferometer is used for real time adjustment of the coherence gate with a rapid scanning delay line to keep it at fixed axial position in time [61] (see Fig. 6).
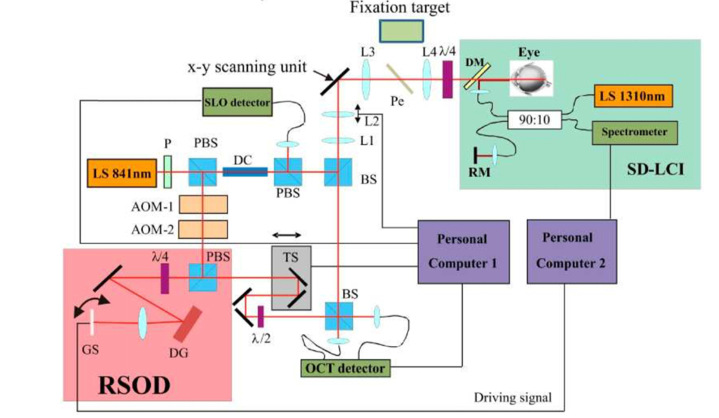
Fig. 6.
Scheme of an axially tracked SLO/TS OCT instrument. Fast axial tracking is realized via a rapid scanning optical delay line (RSOD) with the axial signal from a spectral domain low coherence interferometer (SD-LCI) operating at 1300nm. LS light source, P polarizer, DC dispersion compensation glass rods, BS beam splitter, L1-L4 lenses, AOM acousto optic modulator, TS translation stage, PBS polarizing beam splitter, DM dichroic mirror, RM reference mirror, DG diffraction grating, Pe Pellicle, GS galvo scanner including one resonant scanner (reproduced from Pircher et al. [61] with permission of The Optical Society (OSA)).
Axial tracking is less critical for SD OCT, since the axial information is recorded synchronously for all depths, avoiding axial motion distortions. The same holds in principle true for SS OCT, however motion during recording of the spectral interference pattern in time leads to a chirp, which in turn broadens the OCT signal peaks and causes loss of resolution and signal to noise ratio (SNR) [62]. Motion occurring along the slow scanning direction can be corrected by cross-registration of the tomograms in postprocessing. More sophisticated algorithms use fast reference B-scans [63] or orthogonal scanning patterns [64]. En face sections can therefore be easily extracted from 3D volumes in postprocessing and even be averaged over several registered volumes. Thereby impressive contrast has been achieved even for highly transparent neural tissue in the living human retina [65].
3.4 Phase stability
OCT is an intrinsically complex imaging technique which gives access to the full sample field information. This includes both signal amplitude as well as signal phase. Motion artifacts during in-vivo imaging in general distort the phase. Keeping the phase distortions within the unambiguous range of 2π leaves the option to correct for those motion distortions in post-processing. This has been successfully employed for Doppler OCT to correct for bulk motion phase artifacts and to distinguish them from actual blood flow induced phase changes. The only efficient way to avoid phase decorrelation due to motion is to increase the recording speed. Pircher et al. demonstrated phase contrast images of cell samples with high-speed en face OCT [66] (Fig. 7).
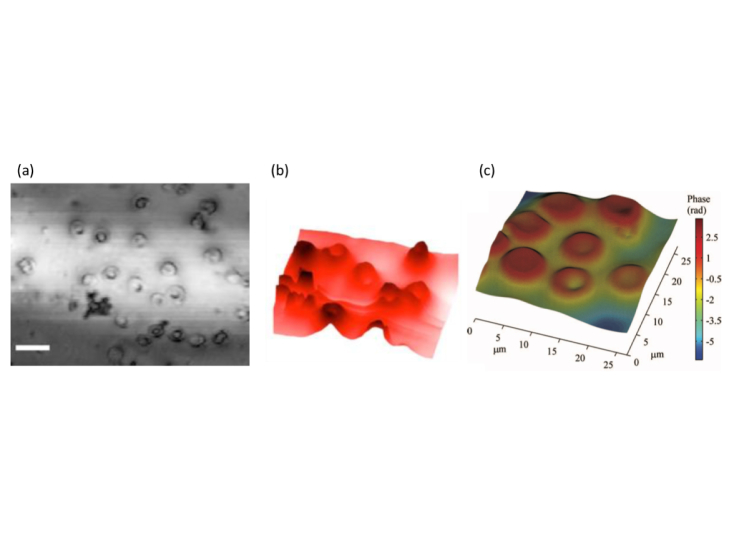
Fig. 7.
Phase contrast OCM of human erythrocytes: (a) OCT intensity image, (b) perspective quantitative phase image of the region marked with a rectangle in (a).(c) Quantitative phase image of eurythrocytes obtained with SS FF OCT(reproduced from [66] and [[67]]with permission of The Optical Society (OSA)).
As already mentioned before, en face OCT is capable to run at kHz line rate. At that speed the phase shift due to typical motion artifacts (assuming 1mm/s axial motion speed) is still within the 2π range, and thus can be corrected for in post-processing. En face phase corrected images are the basis for recently successful digital refocusing and furthermore for full aberration correction based on digital wavefront sensing (see section 6.2). Intrinsically phase stable systems in the en face plane are LFOCT and FFOCT systems. Figure 7(c) shows a quantitative phase of a blood smear obtained with FF SS OCT. The particular advantages will be reviewed in section 5.
4. En face Fourier domain OCT
The recognition of the sensitivity advantage of Fourier domain OCT as compared to time domain OCT led to a paradigm shift and set the basis for the many exciting developments in OCT [13,14,68,69]. Functional extensions such as OCT angiography or Doppler OCT, OCT elastography, but also high-speed real time volumetric OCT would have been impossible or at least impractical without the higher sensitivity and speed. Since volumetric imaging was now possible within a second and less, en face OCT became an easily accessible and versatile tool. Arbitrary en face cross sections of tissue and lesion sites could be analyzed off-line and carefully evaluated [15,16]. Lesion extension development could be followed up during treatment, and in general treatment planned more precisely. Especially the development of rapidly tuning swept sources up to the multi MHz range opens new avenues for real time volumetric imaging and thus also for en face OCT and OCT angiography [18–20,70–74] (see section 6.4). It is however outside the scope of this review article to cover all aspects of FDOCT with software extraction of en face sections, instead, the interested reader is referred to recent review papers on the development of FDOCT and its functional extensions [75,76].
Apart from software based en face FDOCT, Biedermann et al. [77] introduced a method for hardware based SSOCT signal demodulation in order to extract and visualize a single particular depth layer. In the case where the sweep is linear in wavenumber k and the interferometer dispersion balanced, a single depth layer corresponds to a narrow spectral modulation frequency band. Using lock-in detection, this frequency can be filtered out, and single en face planes be recorded. The authors state that single plane FDOCT has no sensitivity gain over en face TDOCT, however, as soon as several planes are used for averaging and speckle reduction the sensitivity will be enhanced.
The strong limitation of the method however is the importance of a constant modulation frequency across the spectrum, which might be only fulfilled by linearly sweeping swept sources without presence of strong dispersion effects [78,79]. This restriction does not apply to another hardware based FDOCT signal analysis approach coined master/slave OCT introduced by Podoleanu and Bradu [80–82]. The method employs first a training step with a mirror as a sample, that produces master copies of the spectral interference signal for all depth positions. For the actual measurement, the slave mode, the mirror is replaced by the sample and the spectral interference signal measured. However, instead of performing the mapping to k-space and taking the Fourier transform in wavenumber k, the original signal is correlated with the master signals for each depth of interest. Thereby, the method is intrinsically correcting also for system related dispersion [83]. Implementing the correlation on a CPU allows for calculating and displaying en face images in real time [84]. The system was successfully employed for various in-vivo imaging applications such as the human eye fundus, or basal cell carcinoma of the eyelid [85,86].
5. Full field OCT
Already in 1998 Beaurepaire et al. demonstrated the concept of full field time domain OCT by illuminating the full en face field of view with a low coherent light source [3]. The backscattered light is detected through a high NA microscope objective superposed with the reference light on a 2D CCD sensor. A 3D stack was recorded by moving the sample axially. The heterodyne signal in order to distinguish OCT signal from unmodulated background intensity terms is produced through photo-elastic birefringence modulator and square modulation of the linearly polarized light source [3,87]. The use of phase shifting techniques for FFOCT has been discussed in section 3.1. Images of plant cells such as onion cells were demonstrated over a field of view of 500x500µm up to a depth of 200-350µm.
Further resolution improvement was achieved by Dubois et al. using a Linnik interferometer design employing exactly matched microscope objectives in sample and reference arm [4]. Despite the by that time unrivaled lateral resolution with OCT of 0.7µm and resulting en face image quality, the low speed however limited application of the method in-vivo. Also, the depth ranging was limited to 150µm although still better than for confocal microscopy given the high numerical aperture of 0.5.
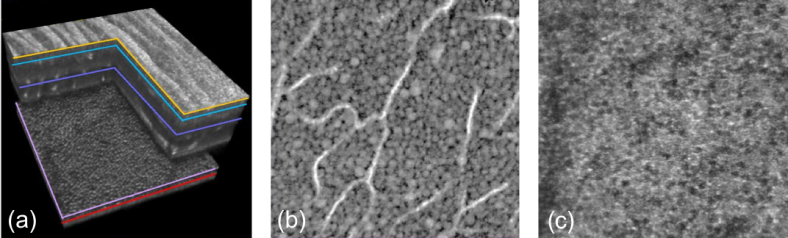
Impressive optical coherence microscopy (OCM) images of ocular tissue ex-vivo were demonstrated by Grieve et al. using a broad bandwidth tungsten halogen lamp centered at 770nm with an axial resolution of 0.7µm [5] (Fig. 8(c-f)). Better depth penetration has been shown by Oh et al. [89] by shifting to the longer wavelength range from 0.9 - 1.4 μm and employing an InGaAs array sensor. Due to reduced scattering at longer wavelengths, structures down to a depth of 800µm within human thyroid tissue ex-vivo have been successfully imaged with high isotropic resolution of about 2µm.
Fig. 8.
Imaging examples for FF OCT: SS FF OCT in-vivo results of human retina showing (a) a rendered 3D volume and (b) a corresponding tomogram (10 times averaged) exhibiting good contrast for the inner retinal layers and loss of contrast for the choroidal structures (reproduced from [88] with permission of The Optical Society); (c-f) TD FF OCT results of ex-vivo retinal tissue (Republished with permission of the Association for Research in Vision and Ophthalmology from [5]; permission conveyed through Copyright Clearance Center, Inc.)
The main drawbacks of TD FF OCT for in-vivo application are on the one hand the critical sensitivity to motion and on the other hand the low detection sensitivity. The speed limitation was relaxed by using a parallel recording of phase-shifted signal copies on two synchronized sensors as shown by Akiba et al. [23]. An interesting variant of parallel en face OCT was based on a smart pixel sensor, that allowed to perform the analog signal demodulation for each pixel on the sensor, thereby efficiently digitizing the OCT signal [90]. A sample volume of 210x210x80 μm3 (corresponding to 58x58x58 voxels) was imaged at a volume rate of 25 Hz [91]. A main challenge for TD FF OCT is the fact that incoherent background signal consumes much of the dynamic range of the CCD sensor. This results in lower detection sensitivity which challenges the application in-vivo, where speed and thus lower integration times are needed to avoid signal distortion due to involuntary patient motion.
Actual in-vivo FF TDOCT images of corneal cellular structures down to the endothelium have been demonstrated using a high-power LED together with an high speed high full-well capacity CMOS sensor running at 550Hz. The high full well capacity enabled enhanced dynamic range and therefore higher sample illuminance leading to improved sensitivity. The OCT signal was recorded through reference arm modulation with a piezo actuated mirror [92].
An attractive feature of employing a spatially incoherent source for FF OCT is the fact that the system becomes in theory insensitive to wavefront aberrations [24]. Instead of degradation of the lateral point spread function only a reduction in detected signal is observed.
Combining FFOCT with Fourier domain signal recording promised to overcome the limitation in detection sensitivity of TD OCT. However, other limitations had to be tackled before first convincing in-vivo images had been shown. Combining FF OCT with swept source OCT is probably the most compact OCT realization. It does not require any complex hardware signal modulation and demodulation, nor mechanical movement of the reference arm. A full 3D volume is recorded with a single sweep of the source as the 2D sensor records the light backscattered from the sample volume across the en face plane in parallel for each optical frequency sequentially. Hence, the spectral interference pattern is recorded in time.
The spectral interference signal can also be recorded for each wavelength in the Fresnel region or the Fourier plane instead of the image plane which is usually done in digital holography. Either in-line holography by phase stepping of the reference arm or using an off-axis configuration can be used to distinguish the cross-correlation terms from the unmodulated intensity background. Digital holographic reconstruction is then performed for each wavelength, followed by standard FDOCT signal reconstruction using the Fourier transform along the time or wavenumber coordinate.
Using such wavelength scanning wide field digital interference holography, Kim [93] demonstrated 3-D imaging of a biological sample across a millimeter with an axial resolution of only 100µm. In 2006 Povazay et al. [94] presented an improved SS FFOCT system employing a Ti:Sapphire laser and an acousto-optic tunable frequency filter. The achieved lateral and axial resolution were 3µm and 4µm, respectively. In order to reduce speckle, an acoustic mode-mixer was used with a multi-mode fiber in the illumination path. The speed, however, was too slow for in-vivo imaging, therefore only ex-vivo images of drosophila were shown.
In order to keep distortions of the spectral fringe signal during in-vivo application small, the recording time between sequential frames should ideally be below 1ms. Such high-speed sensors are existing, but expensive and complex to operate. Usually, the camera would first stream the data onto on-board memory, and then via slow connection to the computer, where the signal can be processed and displayed. Important signal preview for adjustment is therefore limited or even missing.
Employing such sensor, Bonin et al. [95] demonstrated in-vivo retinal imaging in 2010 with an equivalent A-scan rate of 1.5 MHz for a small sample volume of 640(x) x 24(y) x 512(z) pixels. The authors stated, that for keeping significant signal blurring due to involuntary motion artifacts small, a sweep rate of higher than 100Hz was needed. They developed correction algorithms in post-processing to compensate for axial motion distortions, which introduce a non-linear chirp to the spectral interference signal [96].
Hillmann et al. proposed a configuration termed [25,97] holoscopy, which combines coherent depth gating with the digital lensless holography. The lensless approach has the advantage of collecting light from the full tissue volume limited only by the apparent numerical aperture given by the size of the sensor and the distance to the sample. Post-processing applying an angular spectrum propagation on the data multiplied with the conjugate of the reference wave allows then to propagate the signal to arbitrary sample depth planes for each wavelength. For 3D imaging without defocus blur across the depth, the algorithm needs to be applied for all depth planes. The achieved axial and lateral resolution were 14.7µm and up to 7µm, respectively. With a phantom sample consisting of iron-oxide particles suspended in resin an improvement in focus depth of over 30 Rayleigh ranges was demonstrated. Later on, Hillmann et al. [97] presented an improved holoscopy reconstruction similar to the reconstruction used in inverse synthetic aperture microscopy (ISAM) as proposed for SS FFOCT [55] operating in spatial frequency space. After proper linearization of the 3D k-space following the ISAM technique, the 3D Fourier transform is taken to get an OCT volume of the sample. The proposed algorithm is different in the sense, that the recorded sample field is numerically propagated back to the sample space, instead of calculating the pseudo inverse of the forward propagating kernel. Kumar et al introduced a digital aberration correction method, which is in fact a digital scene based equivalent of a Shack-Hartmann sensor. The method senses local wavefront slopes through synthetic split of the aperture and calculating image shifts between the subapertures after 2D Fourier transform [98]. It is a non-iterative method that has equally been applied to correct also for non-isotropic aberrations across the image field [99].
Master/slave OCT was also realized in a SS FFOCT setting using a high speed 2D sensor [100]. In-vivo images at a frame rate of 10kHz of drosophila larvae and human fingertip skin have been shown.
A more severe drawback for imaging in scattering tissue is the missing confocal gating in FF OCT. It causes critical loss of contrast within tissue especially in SS FF OCT, since the individual spectral channels collect ballistic and multiply scattered light from the full depth region. This is in particular visible for imaging choroidal structures below the retinal pigment epithelium (Fig. 8(b)) or within skin tissue. The situation is slightly relaxed when scanning the axially confined coherence gate in TD FFOCT and by employing spatially incoherent illumination (Fig. 8(c-f)). LF OCT has been shown to perform better in scattering tissue since it maintains still half of the confocal gate [26,59,101–103]. Other more sophisticated solutions to overcome even this physical limitation are structured illumination as recently demonstrated experimentally for FFOCT by Grebenyuk et al. [104] or the use of the scattering matrix approach [105,106].
6. Multimodal en face OCT
Combining OCT with other modalities in general has the purpose to mitigate intrinsic shortcomings of OCT alone [35,76]. One of the limitations of OCT is the contrast mechanism that is based on backscattering differences without being highly tissue specific. Another challenge are motion distortions during recording of volumes, that could for example be corrected by correlation with a faster en face imaging modality. Confocal scanning systems also help for proper adjustment of focus positions, for example for retinal OCT. In the following a few selected examples of multimodal extensions of en face OCT are discussed.
6.1 En face OCT/scanning laser ophthalmoscopy (SLO)
Traditional en face TDOCT can be viewed as laser scanning confocal microscopy with low coherent light and a reference arm. It is of advantage to keep the confocal gate of scanning OCT axially co-centered with the coherence gate as selected by the reference arm position. Proper adjustment is particularly critical for high numerical aperture and thus high-resolution systems since slight misalignment causes strong signal loss and defocus blur.
First combined scanning laser ophthalmoscopy (SLO) [107] and OCT systems were based on TD OCT [6,108–110]. As an example from Pircher et al. [111], the setup depicted in Fig. 1 (a) accommodates both en face OCT for retinal imaging with balanced detection as well as SLO. Since SLO can potentially run faster than OCT, it may be efficiently used for correction of motion distortions of the OCT channel, when recorded synchronously. Such correction has been presented by Pircher et al. in combination with axial tracking for reconstruction of retinal volumes in 3D with negligible motion artifacts to the level of individual photoreceptors [112]. The latter included as well the correction of in-frame distortions, which is crucial for high resolution imaging [113]. Pseudo-SLO images could equally be generated in a hardware-based manner in real time from fast raster scanning OCT variants such as the system of Biedermann et al. (see chap.4), by master/slave OCT in combination with optimized GPU processing as shown by Bradu et al. [84], or by averaging over large depth ranges out of recorded volumes. Finally, SLO or line-field SLO is regularly used in commercial platforms (e.g. Spectralis OCT, Cirrus HDOCT, FLUX OPTOPOL, Canon HS100, OTI Spectral OCT/SLO) and can be used to provide the reference images for beam tracking during the OCT or OCT angiography recording.
6.2 Adaptive optics en face OCT
High resolution retinal imaging requires medium to large beam diameters at the cornea. The maximum numeric aperture that determines the lateral spot size at the retina is ultimately limited by the maximum pupil diameter. Large beam diameters however pick up critical amount of wavefront aberrations due to the cornea, lens and ocular media [114]. In healthy eyes with clear media it is possible to visualize cone photoreceptors at large eccentricities from the fovea using OCT alone [111,115]. Cone photoreceptors are responsible for photopic day and color vision, whereas rod photoreceptors enable scotopic monochrome night vision. The density of cone photoreceptors decreases with larger eccentricities and their size increases. On the other hand, the density of rod photoreceptors is lowest for the fovea and increases towards the retinal periphery.
Being able to resolve foveal cones is an indication for a diffraction limited imaging system supporting a large beam diameter. Diffraction limited imaging requires however correction of wavefront aberrations. This has been achieved by recruiting technology used in astronomy for correcting for atmospheric aberration influences. Roorda et al. demonstrated adaptive optics for scanning ophthalmoscopy on the cellular level [116]. Miller et al. and Hermann et al. were the first to demonstrate the advantage of adaptive optics correction also for OCT using a first flood illumination version of AO OCT and ultrahigh resolution TD OCT respectively [60,117].
The first of those systems included already an auxiliary axial tracking device. However, fundamental technical limits in speed precluded the clinical use. AO OCT potentially allows for a much stronger axial gating due to the short coherence of the light source than possible with SLO and allows therefore to image photoreceptors and other cellular details in 3D [118,119]. In order to appreciate the photoreceptor pattern as well as cellular details similar to microscopy fast en face recording is required keeping motion distortions small. En face OCT seems therefore to be a natural candidate for this task.
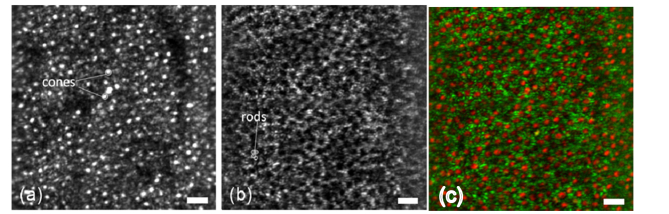
Cellular resolution imaging combining en face OCT and adaptive optics have been demonstrated by Merino et al. and Pircher et al. [120,121]. Further improvements of the latter system included fast axial tracking [61], which according to the discussion in section 3.3 is crucial for en face OCT to properly work in-vivo, as well as dynamic focusing [122] and has been realized in a compact lens-based design [123,124] (Fig. 9).
Fig. 9.
en-face SLO image (a) and averaged OCT image (b) (over 40 frames) (recorded at an imaging depth corresponding to the layer between RPE and end tips of cones at 8 degrees temporally from the fovea). (c) Composite false color image of end tips of cones (red), and layer displayed in (b) (green) (scale bars: 30μm).(reproduced from [124] with permission from The Optical Society (OSA))
Based on this system temporal photoreceptor dynamics have been studied with high resolution and image quality [125]. When combining AO correction with FDOCT the axial stabilization is no longer required, since the full depth range is recorded synchronously [126–128].
The limited tomogram rate of first AO FDOCT systems however led to strong motion distortions in the en face plane. Parallel recording using a line-field configuration helped to improve the volume rate to 6.7Hz [129]. This minimized motion artifacts and enabled en face visualization of cone photoreceptors. By interlacing four fast spectrometers [65], Kocaoglu et al. demonstrated 1MHz adaptive optics OCT [130]. The speed allowed them together with sub-pixel registration algorithms to average several volumes to enhance the sensitivity and to reduce speckle noise. With such system they were successfully visualizing mostly transparent neural cells in the human retina in-vivo [65] (Fig. 10). Apart from improving the recording speed, combining adaptive optics assisted FDOCT with simultaneous high-speed SLO allows for efficient motion-correction by image co-registration. For further details on the development of hardware-based AO OCT the reader is referred to excellent review articles [119,131,132].
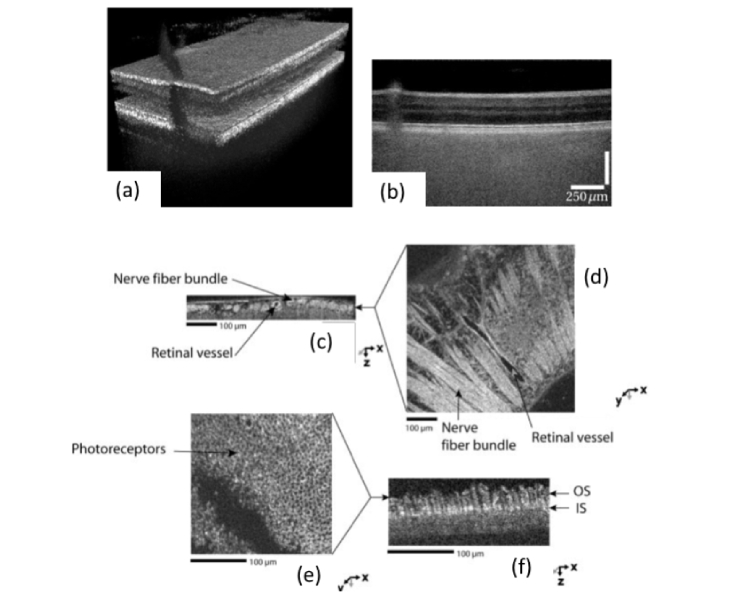
Fig. 10.
En-face images exhibiting cellular details at different depths: (a) rendered volume; (b) GCL-ganglion cell layer; (c) RPE-retinal pigment epithelium;(reproduced from [65] with permission from PNAS).
An interesting development is to perform the wavefront correction digitally by exploiting the complex valued OCT signal that gives access to the backscattered sample field. Since the reconstruction of the wavefront requires highly phase stable systems, first demonstrations of the concepts were based on high speed en face TDOCT [133], FF OCT [134] and later on LF OCT [59]. High speed OCT might however be equally apt to produce phase stable images, that can further be corrected for aberration in pure post-processing.
6.3 Polarization sensitive en face OCT
Tissue might exhibit microstructural properties that change the polarization properties of incident light. Those properties are however invisible to conventional intensity-based OCT. Fibrous tissue such as muscle or nerve fibers or collagen rich tissue exhibit form birefringence, pigmented tissue on the other hand is strongly depolarizing. Exploiting therefore also the polarization contrast potentially yields important additional information on the tissue organization and composition.
First polarization sensitive (PS) OCT devices were based on TD OCT [135] and used by de Boer et al. for skin imaging [136]. Retardation [137] and axis orientation [138] of birefringent skin and muscle were the first quantities, that were measured.
First application in the human eye was the measurement of retardation of the retinal nerve fiber layer (NFL) [139] which is the most important birefringent structure in the eye. Alteration of its thickness due to ganglion cell loss is an important indicator of neurodegenerative diseases such as glaucoma. Already with TD OCT en face maps of NFL thickness have been produced using radial scanning patterns. PSOCT added retardation contrast that correlated well with the thickness maps. Studies even indicated earlier signs of birefringence loss before NFL thinning was observed [140].
Retardation and optical axis en face maps have been produced also for the cornea [141] which can for example be used keratoconus diagnostics. PS OCT was soon adapted for a transverse scanning configuration by Pircher et al by Pircher et al. demonstrating PS OCT images of skin and the retina with high resolution [39,142–144].
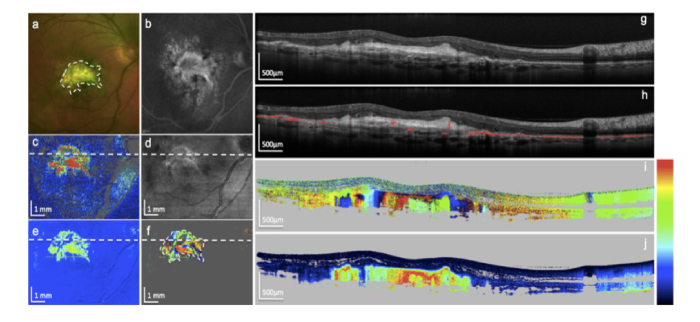
PS OCT images of skin and the retina with high resolution have been demonstrated [39,142–144]. Shifting to the faster FDOCT modality with enhanced phase stability allowed for multimodal tissue imaging including polarization contrast in 3D. It enabled much denser sampling leading to better layer segmentation and more precise en face maps of retinal thickness as well as birefringence properties [141,145–147]. An important observation was the depolarizing effect of the retinal pigment epithelium [138]. The effect of depolarization has been described by introducing the notion of degree of polarization uniformity (DOPU) that efficiently helped for detailed segmentation of the RPE and evaluation of its integrity in various retinal pathologies [148–151] (Fig. 11). High quality en face maps enabled precise tracking of nerve fiber bundles giving insight into interconnectivity of retinal areas as well as their function [153].
Fig. 11.
Images of an eye with fibrotic neovascular age-related macula degeneration. (a) Color fundus photo; (b) fluorescein angiography; (c) en face PS-OCT mean retardation map; (d) en face PS-OCT reflectivity projection map; (e) en face PS-OCT median retardation map; (f) en face PS-OCT axis orientation map; (g) PS- OCT reflectivity B-scan; (h) same B-scan with segmented retinal pigment epithelium based on its depolarization effect (red); (i) PS-OCT axis orientation B-scan, fibrotic tissue generates column-like color pattern (color bar: −90 - + 90°); (j) PS-OCT retardation B-scan, fibrotic tissue is strongly birefringent (color bar: 0 – 90°). (Republished with permission of the Association for Research in Vision and Ophthalmology from [164]; permission conveyed through Copyright Clearance Center, Inc.)
A thorough recent review on PSOCT including the various technologies as well as theory of can be found in de Boer et al. [152]. Finally, also FFOCT as an important proponent of en face OCT with its high phase stability has been enhanced to assess polarization properties of tissue [154–156]. En face images at a frame rate of 3.5 Hz of ex vivo muscle tissues have been demonstrated with an isotropic spatial resolution of ~1.0 μm.
6.4 OCT angiography
Fluorescence fundus angiography has long been the gold standard in ophthalmology for investigation of the blood circulation and perfusion dynamics in the eye fundus. For contrasting retinal vessels fluorescein is applied, whereas for the deeper choroid indocyanine green dye (ICG) is used, as it emits at longer wavelengths and suffers therefore less scattering loss. The combination of such functional imaging techniques with OCT in a co-registered manner, promised therefore enhanced diagnostic capabilities.
First combined OCT and ICG fundus imaging systems based on TS OCT were reported by Dobre et al. [157]. Later on, also commercial OCT devices included both FD OCT and fundus fluorescence imaging (SPECTRALIS HRA + OCT). The drawback of fluorescence angiography is however its invasive character, as the dyes need to be administered intra-venously. This comes with potential adverse side effects for the patients, and the method can only be applied in clinical routine in case of indications of disease.
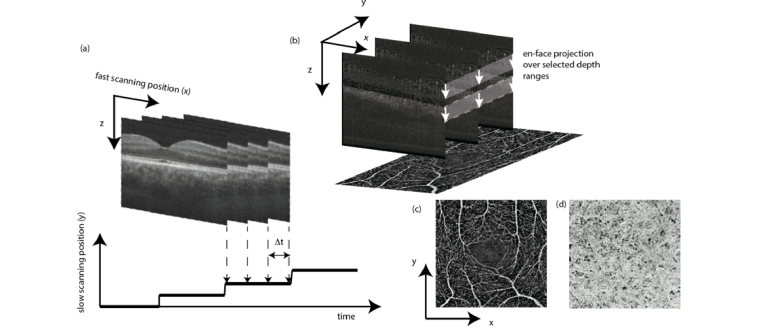
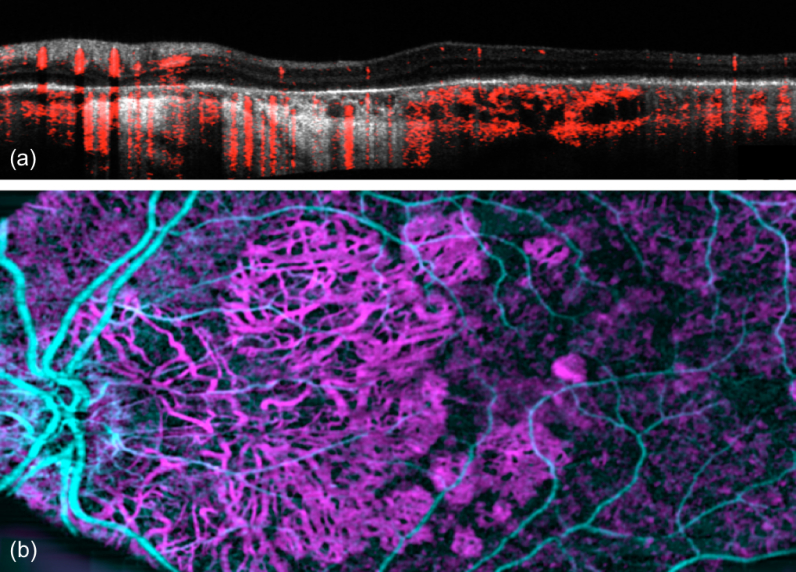
The situation changed dramatically with the introduction OCT Angiography (OCTA), which has been by far the most successful functional extension of OCT [19–21]. In ophthalmic clinical practice, the notion of en face OCT is already synonymously used for OCT angiography. OCTA provides depth resolved angiographic maps of the tissue vascular structure down to the level of smallest capillaries. The vascular contrast is fully non-invasive as it does not require any administration of dyes, with an enormous benefit for the patients by avoiding adverse side effects and the possibility of treatment monitoring and screening. The method is based on sensing OCT signal fluctuations due to moving blood constituents. The fluctuations can be equally sensed by observing intensity changes, phase decorrelation, or complex signal changes over time by taking for example the variance or simply pairwise signal differences. This suppresses static bulk tissue, but enhances structures at motion. If the difference is calculated for example between sequentially recorded tomograms at the same or close to the same position, the time period is much larger than for recorded A-scans, and thus signal decorrelation occurs already for small structural changes such as capillary flow. Angiographic en face maps are typically generated by calculating motion signal projections along a selected depth region in tissue. The en face direction is in fact the most intuitive view on the vascular structure, although the method itself would in theory allow full 3D angiographic maps (Fig. 12). However, axial OCT angiography patterns are strongly affected by shadowing artifacts, and considerable efforts are currently spent on reducing those artifacts [159].
Fig. 12.
Scheme of OCT angiography (OCTA) processing steps. (a) OCTA analysis is performed between tomograms;(b) OCTA en face maps are produced by projection over selected depth regions. (c-d) en face OCTA maps of the inner retina capillary structure and of the choroicapillary layer below the retinal pigment epithelium. (reproduced from [158] with permission from SPIE)
Current technology development aims at enhancing the field of view by employing rapidly tuning swept sources [18,20,71,74,160,161] (Fig. 13). The speed opens additional flexibility in optimizing the tomogram rate for best vascular visibility [72,73] as well as for producing quasi-quantitative en face flow maps [162].
Fig. 13.
OCTA of geographic atrophy; (a) B-scan (grey) with overlaid OCTA tomogram (red); (b) en face OCTA with depth color coding according to the color bars to the right of (a) (with permission from [160] by The Optical Society (OSA)).
Recent combination of OCT angiography with adaptive optics by Salas et al. [163,164] showed improved axial performance due to the additional strong confocal gating together with a high-resolution representation of all capillary beds within the retina. In general, the impressive vascular en face maps revived again the interest in en face OCT [165].
7. Outlook
We reviewed different technologies for structural as well as functional en face OCT. The most compact en face OCT technology is FFOCT. Advances in sensor technology and the availability of cheaper high-speed cameras will be an important driving force for translating and commercializing FF OCT for in-vivo applications. As pointed out in section 3.4 an important advantage of en face TS OCT, LFOCT and FFOCT is the intrinsically high phase correlation across the en face plane. This opens new and exciting applications for highly sensitive probing of structural and possibly even chemical changes that lead to variations in optical path length. The group of Boccara et al. used this sensitivity for label-free metabolic contrast of cells and their substructure based on their motility [166,167]. A time series of en face images was analyzed via taking the standard deviation for each pixel over time identifying various frequency ranges. Faster changes were due to moving blood cells similar to OCT angiography. At lower frequencies a different motility or correlation contrast between normal and pathological tissue has been found which could potentially help to discriminate tumor tissue during surgery without the need of exogenous contrast agents.
Recent work by Hillmann et al. demonstrated with SS FFOCT sensing of subtle changes in the retinal structure during excitation of photoreceptors with defined excitation patterns [168]. They were not only able to confirm earlier results by intrinsic signal imaging in-vivo [169,170], but could even find replicas of the illumination pattern in the response signals from the ganglion cell layer [171]. The well-defined phase across the image plane allows also for manipulation of the wavefront in the pupil plane or Fourier plane. Synthetic splitting of the pupil in postprocessing also allowed for fully directional sensing of dynamic structural changes. The ability for 3D motion vector field reconstruction in tissue during laser photocoagulation in ex-vivo porcine retina was shown recently [172].
Realizing an en face LF TDOCT with holographic reconstruction (Fig. 4(b)), Liu et al. [28] were able to record high frequency oscillations after air-pulse excitation of porcine cornea with a line rate of 200kHz. With a similar setup, Ginner et al. showed retinal imaging as well as en face Doppler OCT of retinal capillaries [27]. En face retinal images of 2048 x 1000 pixels were recorded with up to 100Hz allowing to identify flowing red blood cells.
Although the rapid development of fast light sources might allow similar en face image rates even with point scanning devices [18], parallelization of detection will play an important role. The spectrum ranges from few channels to LF or FFOCT. The latter might be problematic concerning laser safety for the anterior chamber, as the illumination field might easily be focused at strongly absorbing iris tissue. LF OCT is more relaxed in that regard, but the combination with a swept source is still limited by the availability of fast line sensors. However, such technological limitations of today might be less of an issue in the next few years.
We can certainly expect, that en face OCT will continue to play an important role in OCT. With improved performance of graphics processor units and algorithms we will however gradually see a shift from tomographic OCT and en face OCTA to full resolution volumetric real time display. The latter has the advantage to exhibit important details on axial interconnections that are less visible in en face views alone. The volumetric real time performance is certainly most critical for intra-surgical OCT [173]. Other developments aim at quantitative imaging of tissue perfusion, motility and biomechanics. Also, displaying functional en face information on top of the tissue surface in an augmented reality setting will further enhance the diagnostic capabilities in the future.
Acknowledgments
The author acknowledges funding by the Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development.
Funding
Austrian Research Foundation (FWF) (P 29093-N3); European Union’s Horizon 2020 Research and Innovation Programme (732969 MOON, 667933 MIB, 721766 FBI).
Disclosures
The author declares that there are no conflicts of interest related to this article.
References
- 1.Podoleanu A., Rogers J., Jackson D., Dunne S., “Three dimensional OCT images from retina and skin,” Opt. Express 7(9), 292–298 (2000). 10.1364/OE.7.000292 [DOI] [PubMed] [Google Scholar]
- 2.Rogers J., Podoleanu A., Dobre G., Jackson D., Fitzke F., “Topography and volume measurements of the optic nerve usingen-face optical coherence tomography,” Opt. Express 9(10), 533–545 (2001). 10.1364/OE.9.000533 [DOI] [PubMed] [Google Scholar]
- 3.Beaurepaire E., Boccara A. C., Lebec M., Blanchot L., Saint-Jalmes H., “Full-field optical coherence microscopy,” Opt. Lett. 23(4), 244–246 (1998). 10.1364/OL.23.000244 [DOI] [PubMed] [Google Scholar]
- 4.Dubois A., Vabre L., Boccara A.-C., Beaurepaire E., “High-resolution full-field optical coherence tomography with a Linnik microscope,” Appl. Opt. 41(4), 805–812 (2002). 10.1364/AO.41.000805 [DOI] [PubMed] [Google Scholar]
- 5.Grieve K., Paques M., Dubois A., Sahel J., Boccara C., Le Gargasson J.-F., “Ocular tissue imaging using ultrahigh-resolution, full-field optical coherence tomography,” Invest. Ophthalmol. Vis. Sci. 45(11), 4126–4131 (2004). 10.1167/iovs.04-0584 [DOI] [PubMed] [Google Scholar]
- 6.Hitzenberger C., Trost P., Lo P. W., Zhou Q., “Three-dimensional imaging of the human retina by high-speed optical coherence tomography,” Opt. Express 11(21), 2753–2761 (2003). 10.1364/OE.11.002753 [DOI] [PubMed] [Google Scholar]
- 7.Wojtkowski M., Leitgeb R., Kowalczyk A., Bajraszewski T., Fercher A. F., “In vivo human retinal imaging by Fourier domain optical coherence tomography,” J. Biomed. Opt. 7(3), 457–463 (2002). 10.1117/1.1482379 [DOI] [PubMed] [Google Scholar]
- 8.Fercher A. F., Hitzenberger C. K., Kamp G., Elzaiat S. Y., “Measurement of Intraocular Distances by Backscattering Spectral Interferometry,” Opt. Commun. 117(1-2), 43–48 (1995). 10.1016/0030-4018(95)00119-S [DOI] [Google Scholar]
- 9.Lexer F., Hitzenberger C. K., Fercher A. F., Kulhavy M., “Wavelength-tuning interferometry of intraocular distances,” Appl. Opt. 36(25), 6548–6553 (1997). 10.1364/AO.36.006548 [DOI] [PubMed] [Google Scholar]
- 10.Chinn S. R., Swanson E. A., Fujimoto J. G., “Optical coherence tomography using a frequency-tunable optical source,” Opt. Lett. 22(5), 340–342 (1997). 10.1364/OL.22.000340 [DOI] [PubMed] [Google Scholar]
- 11.Leitgeb R., Schmetterer L., Wojtkowski M., Hitzenberger C. K., Sticker M., Fercher A. F., “Flow velocity measurements by frequency domain short coherence interferometry,” in Proceedings of SPIE - The International Society for Optical Engineering, 2002), 16–21. 10.1117/12.470477 [DOI] [Google Scholar]
- 12.Leitgeb R., Schmetterer L., Drexler W., Fercher A., Zawadzki R., Bajraszewski T., “Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography,” Opt. Express 11(23), 3116–3121 (2003). 10.1364/OE.11.003116 [DOI] [PubMed] [Google Scholar]
- 13.de Boer J. F., Cense B., Park B. H., Pierce M. C., Tearney G. J., Bouma B. E., “Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography,” Opt. Lett. 28(21), 2067–2069 (2003). 10.1364/OL.28.002067 [DOI] [PubMed] [Google Scholar]
- 14.Choma M., Sarunic M., Yang C., Izatt J., “Sensitivity advantage of swept source and Fourier domain optical coherence tomography,” Opt. Express 11(18), 2183–2189 (2003). 10.1364/OE.11.002183 [DOI] [PubMed] [Google Scholar]
- 15.Jiao S., Knighton R., Huang X., Gregori G., Puliafito C., “Simultaneous acquisition of sectional and fundus ophthalmic images with spectral-domain optical coherence tomography,” Opt. Express 13(2), 444–452 (2005). 10.1364/OPEX.13.000444 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Erfurth U. M., Leitgeb R., Michels S., Sacu S., Povazay B., Hermann B., Ahlers C., Scholda C., Sattmann H., Drexler W., “Three-dimensional ultrahigh resolution optical coherence tomography (3D UHR OCT): A video presentation,” Invest. Ophthalmol. Vis. Sci. 46, 3393 (2005). 10.1167/iovs.05-0370 [DOI] [PubMed] [Google Scholar]
- 17.Drexler W., Fujimoto J. G., Special Section Guest Editors , “Optical coherence tomography in ophthalmology,” J. Biomed. Opt. 12(4), 041201 (2007). 10.1117/1.2773734 [DOI] [Google Scholar]
- 18.Klein T., Huber R., “High-speed OCT light sources and systems [Invited],” Biomed. Opt. Express 8(2), 828–859 (2017). 10.1364/BOE.8.000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C.-L., Wang R. K., “Optical coherence tomography based angiography [Invited],” Biomed. Opt. Express 8(2), 1056–1082 (2017). 10.1364/BOE.8.001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitgeb R. A., Werkmeister R. M., Blatter C., Schmetterer L., “Doppler optical coherence tomography,” Prog. Retin. Eye Res. 41, 26–43 (2014). 10.1016/j.preteyeres.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makita S., Hong Y., Yamanari M., Yatagai T., Yasuno Y., “Optical coherence angiography,” Opt. Express 14(17), 7821–7840 (2006). 10.1364/OE.14.007821 [DOI] [PubMed] [Google Scholar]
- 22.Schwider J., “Advanced Evaluation Techniques in Interferometry,” Prog. Opt. 28, 271–359 (1990). 10.1016/S0079-6638(08)70291-9 [DOI] [Google Scholar]
- 23.Akiba M., Chan K. P., Tanno N., “Full-field optical coherence tomography by two-dimensional heterodyne detection with a pair of CCD cameras,” Opt. Lett. 28(10), 816–818 (2003). 10.1364/OL.28.000816 [DOI] [PubMed] [Google Scholar]
- 24.Xiao P., Mazlin V., Grieve K., Sahel J.-A., Fink M., Boccara A. C., “In vivo high-resolution human retinal imaging with wavefront-correctionless full-field OCT,” Optica 5(4), 409–412 (2018). 10.1364/OPTICA.5.000409 [DOI] [Google Scholar]
- 25.Hillmann D., Lührs C., Bonin T., Koch P., Hüttmann G., “Holoscopy--holographic optical coherence tomography,” Opt. Lett. 36(13), 2390–2392 (2011). 10.1364/OL.36.002390 [DOI] [PubMed] [Google Scholar]
- 26.Fechtig D. J., Schmoll T., Grajciar B., Drexler W., Leitgeb R. A., “Line-field parallel swept source interferometric imaging at up to 1 MHz,” Opt. Lett. 39(18), 5333–5336 (2014). 10.1364/OL.39.005333 [DOI] [PubMed] [Google Scholar]
- 27.Ginner L., Schmoll T., Kumar A., Salas M., Pricoupenko N., Wurster L. M., Leitgeb R. A., “Holographic line field en-face OCT with digital adaptive optics in the retina in vivo,” Biomed. Opt. Express 9(2), 472–485 (2018). 10.1364/BOE.9.000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C.-H., Schill A., Raghunathan R., Wu C., Singh M., Han Z., Nair A., Larin K. V., “Ultra-fast line-field low coherence holographic elastography using spatial phase shifting,” Biomed. Opt. Express 8(2), 993–1004 (2017). 10.1364/BOE.8.000993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podoleanu A. G., Dobre G. M., Webb D. J., Jackson D. A., “Coherence imaging by use of a Newton rings sampling function,” Opt. Lett. 21(21), 1789–1791 (1996). 10.1364/OL.21.001789 [DOI] [PubMed] [Google Scholar]
- 30.Podoleanu A. G., Dobre G. M., Jackson D. A., “En-face coherence imaging using galvanometer scanner modulation,” Opt. Lett. 23(3), 147–149 (1998). 10.1364/OL.23.000147 [DOI] [PubMed] [Google Scholar]
- 31.Podoleanu A. G., Seeger M., Dobre G. M., Webb D. J., Jackson D. A., Fitzke F. W., “Transversal and longitudinal images from the retina of the living eye using low coherence reflectometry,” J. Biomed. Opt. 3(1), 12–20 (1998). 10.1117/1.429859 [DOI] [PubMed] [Google Scholar]
- 32.Leitgeb R. A., Michaely R., Lasser T., Sekhar S. C., “Complex ambiguity-free Fourier domain optical coherence tomography through transverse scanning,” Opt. Lett. 32(23), 3453–3455 (2007). 10.1364/OL.32.003453 [DOI] [PubMed] [Google Scholar]
- 33.Cucu R. G., Podoleanu A. G., Rogers J. A., Pedro J., Rosen R. B., “Combined confocal/en face T-scan-based ultrahigh-resolution optical coherence tomography in vivo retinal imaging,” Opt. Lett. 31(11), 1684–1686 (2006). 10.1364/OL.31.001684 [DOI] [PubMed] [Google Scholar]
- 34.Wiesauer K., Pircher M., Goetzinger E., Hitzenberger C. K., Engelke R., Ahrens G., Gruetzner G., Stifter D., “Transversal ultrahigh-resolution polarizationsensitive optical coherence tomography for strain mapping in materials,” Opt. Express 14(13), 5945–5953 (2006). 10.1364/OE.14.005945 [DOI] [PubMed] [Google Scholar]
- 35.Podoleanu A. G., Rosen R. B., “Combinations of techniques in imaging the retina with high resolution,” Prog. Retin. Eye Res. 27(4), 464–499 (2008). 10.1016/j.preteyeres.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 36.van Velthoven M. E. J., Verbraak F. D., Yannuzzi L. A., Rosen R. B., Podoleanu A. G. H., de Smet M. D., “Imaging the retina by en face optical coherence tomography,” Retina 26(2), 129–136 (2006). 10.1097/00006982-200602000-00001 [DOI] [PubMed] [Google Scholar]
- 37.Rosen R. B., Hathaway M., Rogers J., Pedro J., Garcia P., Laissue P., Dobre G. M., Podoleanu A. G., “Multidimensional en-face OCT imaging of the retina,” Opt. Express 17(5), 4112–4133 (2009). 10.1364/OE.17.004112 [DOI] [PubMed] [Google Scholar]
- 38.Bachmann A., Leitgeb R., Lasser T., “Heterodyne Fourier domain optical coherence tomography for full range probing with high axial resolution,” Opt. Express 14(4), 1487–1496 (2006). 10.1364/OE.14.001487 [DOI] [PubMed] [Google Scholar]
- 39.Pircher M., Goetzinger E., Leitgeb R., Hitzenberger C. K., “Transversal phase resolved polarization sensitive optical coherence tomography,” Phys. Med. Biol. 49(7), 1257–1263 (2004). 10.1088/0031-9155/49/7/013 [DOI] [PubMed] [Google Scholar]
- 40.Izatt J. A., Hee M. R., Owen G. M., Swanson E. A., Fujimoto J. G., “Optical coherence microscopy in scattering media,” Opt. Lett. 19(8), 590–592 (1994). 10.1364/OL.19.000590 [DOI] [PubMed] [Google Scholar]
- 41.Schmitt J. M., Lee S. L., Yung K. M., “An optical coherence microscope with enhanced resolving power in thick tissue,” Opt. Commun. 142(4-6), 203–207 (1997). 10.1016/S0030-4018(97)00280-0 [DOI] [Google Scholar]
- 42.Lexer F., Hitzenberger C. K., Drexler W., Molebny S., Sattmann H., Sticker M., Fercher A. F., “Dynamic coherent focus OCT with depth-independent transversal resolution,” J. Mod. Opt. 46(3), 541–553 (1999). 10.1080/09500349908231282 [DOI] [Google Scholar]
- 43.Murali S., Lee K. S., Rolland J. P., “Invariant resolution dynamic focus OCM based on liquid crystal lens,” Opt. Express 15(24), 15854–15862 (2007). 10.1364/OE.15.015854 [DOI] [PubMed] [Google Scholar]
- 44.Assayag O., Antoine M., Sigal-Zafrani B., Riben M., Harms F., Burcheri A., Grieve K., Dalimier E., Le Conte de Poly B., Boccara C., “Large field, high resolution full-field optical coherence tomography: a pre-clinical study of human breast tissue and cancer assessment,” Technol. Cancer Res. Treat. 13(5), 455–468 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubois A., Boccara C., “[Full-field OCT],” Med. Sci. (Paris) 22(10), 859–864 (2006). 10.1051/medsci/20062210859 [DOI] [PubMed] [Google Scholar]
- 46.Huber R., Wojtkowski M., Fujimoto J. G., Jiang J. Y., Cable A. E., “Three-dimensional and C-mode OCT imaging with a compact, frequency swept laser source at 1300 nm,” Opt. Express 13(26), 10523–10538 (2005). 10.1364/OPEX.13.010523 [DOI] [PubMed] [Google Scholar]
- 47.Rolland J. P., Meemon P., Murali S., Thompson K. P., Lee K. S., “Gabor-based fusion technique for Optical Coherence Microscopy,” Opt. Express 18(4), 3632–3642 (2010). 10.1364/OE.18.003632 [DOI] [PubMed] [Google Scholar]
- 48.Holmes J., Hattersley S., “Image blending and speckle noise reduction in multi-beam OCT,” in Progress in Biomedical Optics and Imaging - Proceedings of SPIE, 2009) [Google Scholar]
- 49.Ding Z., Ren H., Zhao Y., Nelson J. S., Chen Z., “High-resolution optical coherence tomography over a large depth range with an axicon lens,” Opt. Lett. 27(4), 243–245 (2002). 10.1364/OL.27.000243 [DOI] [PubMed] [Google Scholar]
- 50.Yi L., Sun L., Ding W., “Multifocal spectral-domain optical coherence tomography based on Bessel beam for extended imaging depth,” J. Biomed. Opt. 22(10), 1–8 (2017). 10.1117/1.JBO.22.10.106016 [DOI] [PubMed] [Google Scholar]
- 51.Leitgeb R. A., Villiger M., Bachmann A. H., Steinmann L., Lasser T., “Extended focus depth for Fourier domain optical coherence microscopy,” Opt. Lett. 31(16), 2450–2452 (2006). 10.1364/OL.31.002450 [DOI] [PubMed] [Google Scholar]
- 52.Lee K. S., Rolland J. P., “Bessel beam spectral-domain high-resolution optical coherence tomography with micro-optic axicon providing extended focusing range,” Opt. Lett. 33(15), 1696–1698 (2008). 10.1364/OL.33.001696 [DOI] [PubMed] [Google Scholar]
- 53.Curatolo A., Munro P. R. T., Lorenser D., Sreekumar P., Singe C. C., Kennedy B. F., Sampson D. D., “Quantifying the influence of Bessel beams on image quality in optical coherence tomography,” Sci. Rep. 6(1), 23483 (2016). 10.1038/srep23483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blatter C., Grajciar B., Eigenwillig C. M., Wieser W., Biedermann B. R., Huber R., Leitgeb R. A., “Extended focus high-speed swept source OCT with self-reconstructive illumination,” Opt. Express 19(13), 12141–12155 (2011). 10.1364/OE.19.012141 [DOI] [PubMed] [Google Scholar]
- 55.Marks D. L., Ralston T. S., Boppart S. A., Carney P. S., “Inverse scattering for frequency-scanned full-field optical coherence tomography,” J. Opt. Soc. Am. A 24(4), 1034–1041 (2007). 10.1364/JOSAA.24.001034 [DOI] [PubMed] [Google Scholar]
- 56.Grebenyuk A., Federici A., Ryabukho V., Dubois A., “Numerically focused full-field swept-source optical coherence microscopy with low spatial coherence illumination,” Appl. Opt. 53(8), 1697–1708 (2014). 10.1364/AO.53.001697 [DOI] [PubMed] [Google Scholar]
- 57.Fechtig D. J., Kumar A., Drexler W., Leitgeb R. A., “Full range line-field parallel swept source imaging utilizing digital refocusing,” J. Mod. Opt. 62, 1–7 (2014). [Google Scholar]
- 58.Yu L., Rao B., Zhang J., Su J., Wang Q., Guo S., Chen Z., “Improved lateral resolution in optical coherence tomography by digital focusing using two-dimensional numerical diffraction method,” Opt. Express 15(12), 7634–7641 (2007). 10.1364/OE.15.007634 [DOI] [PubMed] [Google Scholar]
- 59.Ginner L., Kumar A., Fechtig D., Wurster L. M., Salas M., Pircher M., Leitgeb R. A., “Noniterative digital aberration correction for cellular resolution retinal optical coherence tomography in vivo,” Optica 4(8), 924–931 (2017). 10.1364/OPTICA.4.000924 [DOI] [Google Scholar]
- 60.Miller D. T., Qu J., Jonnal R. S., Thorn K. E., “Coherence gating and adaptive optics in the eye,” in Coherence Domain Optical Methods and Optical Coherence Tomography in Biomedicine VII, (International Society for Optics and Photonics, 2003), 65–73. [Google Scholar]
- 61.Pircher M., Götzinger E., Sattmann H., Leitgeb R. A., Hitzenberger C. K., “In vivo investigation of human cone photoreceptors with SLO/OCT in combination with 3D motion correction on a cellular level,” Opt. Express 18(13), 13935–13944 (2010). 10.1364/OE.18.013935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun S. H., Tearney G., de Boer J., Bouma B., “Motion artifacts in optical coherence tomography with frequency-domain ranging,” Opt. Express 12(13), 2977–2998 (2004). 10.1364/OPEX.12.002977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh A. S. G., Kolbitsch C., Schmoll T., Leitgeb R. A., “Stable absolute flow estimation with Doppler OCT based on virtual circumpapillary scans,” Biomed. Opt. Express 1(4), 1047–1058 (2010). 10.1364/BOE.1.001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraus M. F., Potsaid B., Mayer M. A., Bock R., Baumann B., Liu J. J., Hornegger J., Fujimoto J. G., “Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns,” Biomed. Opt. Express 3(6), 1182–1199 (2012). 10.1364/BOE.3.001182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Z. Liu, K. Kurokawa, F. Zhang, J. J. Lee, and D. T. Miller, “Imaging and quantifying ganglion cells and other transparent neurons in the living human retina,” Proceedings of the National Academy of Sciences, 201711734 (2017). 10.1073/pnas.1711734114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pircher M., Baumann B., Götzinger E., Sattmann H., Hitzenberger C. K., “Phase contrast coherence microscopy based on transverse scanning,” Opt. Lett. 34(12), 1750–1752 (2009). 10.1364/OL.34.001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarunic M. V., Weinberg S., Izatt J. A., “Full-field swept-source phase microscopy,” Opt. Lett. 31(10), 1462–1464 (2006). 10.1364/OL.31.001462 [DOI] [PubMed] [Google Scholar]
- 68.R. Leitgeb, L. Schmetterer, M. Wojtkowski, C. Hitzenberger, M. Sticker, and A. Fercher, “Flow Velocity Measurements by Frequency Domain Short Coherence Interferometry,” SPIE Proceedings 4619, 16–21 (2002). 10.1117/12.470477 [DOI] [Google Scholar]
- 69.Leitgeb R., Hitzenberger C., Fercher A., “Performance of fourier domain vs. time domain optical coherence tomography,” Opt. Express 11(8), 889–894 (2003). 10.1364/OE.11.000889 [DOI] [PubMed] [Google Scholar]
- 70.Klein T., Wieser W., Reznicek L., Neubauer A., Kampik A., Huber R., “Multi-MHz retinal OCT,” Biomed. Opt. Express 4(10), 1890–1908 (2013). 10.1364/BOE.4.001890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srinivasan V. J., Adler D. C., Chen Y., Gorczynska I., Huber R., Duker J. S., Schuman J. S., Fujimoto J. G., “Ultrahigh-speed optical coherence tomography for three-dimensional and en face imaging of the retina and optic nerve head,” Invest. Ophthalmol. Vis. Sci. 49(11), 5103–5110 (2008). 10.1167/iovs.08-2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorczynska L., Migacz J. V., Jonnal R. S., Zawadzki R. J., Werner J. S., “Influence of speed and resolution on OCT angiography and Doppler OCT imaging in human retinal and choroidal capillary systems,” in 2016 IEEE Photonics Conference, IPC 2016, 2017), 140–141. [Google Scholar]
- 73.Poddar R., Werner J. S., “Implementations of three OCT angiography (OCTA) methods with 1.7 MHz A-scan rate OCT system on imaging of human retinal and choroidal vasculature,” Opt. Laser Technol. 102, 130–139 (2018). 10.1016/j.optlastec.2017.12.033 [DOI] [Google Scholar]
- 74.Blatter C., Klein T., Grajciar B., Schmoll T., Wieser W., Andre R., Huber R., Leitgeb R. A., “Ultrahigh-speed non-invasive widefield angiography,” J. Biomed. Opt. 17(7), 070505 (2012). 10.1117/1.JBO.17.7.070505 [DOI] [PubMed] [Google Scholar]
- 75.de Boer J. F., Leitgeb R., Wojtkowski M., “Twenty-five years of optical coherence tomography: the paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited],” Biomed. Opt. Express 8(7), 3248–3280 (2017). 10.1364/BOE.8.003248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leitgeb R. A., Baumann B., “Multimodal optical medical imaging concepts based on optical coherence tomography,” Front. Phys. 6, 114 (2018). 10.3389/fphy.2018.00114 [DOI] [Google Scholar]
- 77.Biedermann B. R., Wieser W., Eigenwillig C. M., Palte G., Adler D. C., Srinivasan V. J., Fujimoto J. G., Huber R., “Real time en face Fourier-domain optical coherence tomography with direct hardware frequency demodulation,” Opt. Lett. 33(21), 2556–2558 (2008). 10.1364/OL.33.002556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eigenwillig C. M., Biedermann B. R., Palte G., Huber R., “K-space linear Fourier domain mode locked laser and applications for optical coherence tomography,” Opt. Express 16(12), 8916–8937 (2008). 10.1364/OE.16.008916 [DOI] [PubMed] [Google Scholar]
- 79.Bonesi M., Minneman M. P., Ensher J., Zabihian B., Sattmann H., Boschert P., Hoover E., Leitgeb R. A., Crawford M., Drexler W., “Akinetic all-semiconductor programmable swept-source at 1550 nm and 1310 nm with centimeters coherence length,” Opt. Express 22(3), 2632–2655 (2014). 10.1364/OE.22.002632 [DOI] [PubMed] [Google Scholar]
- 80.Bradu A., Podoleanu A. G., “Calibration-free B-scan images produced by master/slave optical coherence tomography,” Opt. Lett. 39(3), 450–453 (2014). 10.1364/OL.39.000450 [DOI] [PubMed] [Google Scholar]
- 81.Podoleanu A. G., Bradu A., “Master-slave interferometry for parallel spectral domain interferometry sensing and versatile 3D optical coherence tomography,” Opt. Express 21(16), 19324–19338 (2013). 10.1364/OE.21.019324 [DOI] [PubMed] [Google Scholar]
- 82.Rivet S., Maria M., Bradu A., Feuchter T., Leick L., Podoleanu A., “Complex master slave interferometry,” Opt. Express 24(3), 2885–2904 (2016). 10.1364/OE.24.002885 [DOI] [PubMed] [Google Scholar]
- 83.Bradu A., Maria M., Podoleanu A. G., “Demonstration of tolerance to dispersion of master/slave interferometry,” Opt. Express 23(11), 14148–14161 (2015). 10.1364/OE.23.014148 [DOI] [PubMed] [Google Scholar]
- 84.Bradu A., Kapinchev K., Barnes F., Podoleanu A., “Master slave en-face OCT/SLO,” Biomed. Opt. Express 6(9), 3655–3669 (2015). 10.1364/BOE.6.003655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradu A., Podoleanu A. G., “Imaging the eye fundus with real-time en-face spectral domain optical coherence tomography,” Biomed. Opt. Express 5(4), 1233–1249 (2014). 10.1364/BOE.5.001233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chin C., Bradu A., Lim R., Khandwala M., Schofield J., Leick L., Podoleanu A., “Master/slave optical coherence tomography imaging of eyelid basal cell carcinoma,” Appl. Opt. 55(26), 7378–7386 (2016). 10.1364/AO.55.007378 [DOI] [PubMed] [Google Scholar]
- 87.Boccara A. C., Charbonnier F., Fournier D., Gleyze P., “Procede et dispositif de detection analogique multicanal,” (Google Patents, 1992). [Google Scholar]
- 88.Hillmann D., Spahr H., Sudkamp H., Hain C., Hinkel L., Franke G., Hüttmann G., “Off-axis reference beam for full-field swept-source OCT and holoscopy,” Opt. Express 25(22), 27770–27784 (2017). 10.1364/OE.25.027770 [DOI] [PubMed] [Google Scholar]
- 89.Oh W. Y., Bouma B. E., Iftimia N., Yelin R., Tearney G. J., “Spectrally-modulated full-field optical coherence microscopy for ultrahigh-resolution endoscopic imaging,” Opt. Express 14(19), 8675–8684 (2006). 10.1364/OE.14.008675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bourquin S., Seitz P., Salathé R. P., “Optical coherence topography based on a two-dimensional smart detector array,” Opt. Lett. 26(8), 512–514 (2001). 10.1364/OL.26.000512 [DOI] [PubMed] [Google Scholar]
- 91.Laubscher M., Ducros M., Karamata B., Lasser T., Salathé R., “Video-rate three-dimensional optical coherence tomography,” Opt. Express 10(9), 429–435 (2002). 10.1364/OE.10.000429 [DOI] [PubMed] [Google Scholar]
- 92.Mazlin V., Xiao P., Dalimier E., Grieve K., Irsch K., Sahel J.-A., Fink M., Boccara A. C., “In vivo high resolution human corneal imaging using full-field optical coherence tomography,” Biomed. Opt. Express 9(2), 557–568 (2018). 10.1364/BOE.9.000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim M.-K., “Tomographic three-dimensional imaging of a biological specimen using wavelength-scanning digital interference holography,” Opt. Express 7(9), 305–310 (2000). 10.1364/OE.7.000305 [DOI] [PubMed] [Google Scholar]
- 94.Povazay B., Unterhuber A., Hermann B., Sattmann H., Arthaber H., Drexler W., “Full-field time-encoded frequency-domain optical coherence tomography,” Opt. Express 14(17), 7661–7669 (2006). 10.1364/OE.14.007661 [DOI] [PubMed] [Google Scholar]
- 95.Bonin T., Franke G., Hagen-Eggert M., Koch P., Hüttmann G., “In vivo Fourier-domain full-field OCT of the human retina with 1.5 million A-lines/s,” Opt. Lett. 35(20), 3432–3434 (2010). 10.1364/OL.35.003432 [DOI] [PubMed] [Google Scholar]
- 96.Hillmann D., Bonin T., Lührs C., Franke G., Hagen-Eggert M., Koch P., Hüttmann G., “Common approach for compensation of axial motion artifacts in swept-source OCT and dispersion in Fourier-domain OCT,” Opt. Express 20(6), 6761–6776 (2012). 10.1364/OE.20.006761 [DOI] [PubMed] [Google Scholar]
- 97.Hillmann D., Franke G., Lührs C., Koch P., Hüttmann G., “Efficient holoscopy image reconstruction,” Opt. Express 20(19), 21247–21263 (2012). 10.1364/OE.20.021247 [DOI] [PubMed] [Google Scholar]
- 98.Kumar A., Drexler W., Leitgeb R. A., “Subaperture correlation based digital adaptive optics for full field optical coherence tomography,” Opt. Express 21(9), 10850–10866 (2013). 10.1364/OE.21.010850 [DOI] [PubMed] [Google Scholar]
- 99.Kumar A., Kamali T., Platzer R., Unterhuber A., Drexler W., Leitgeb R. A., “Anisotropic aberration correction using region of interest based digital adaptive optics in Fourier domain OCT,” Biomed. Opt. Express 6(4), 1124–1134 (2015). 10.1364/BOE.6.001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J., Bradu A., Dobre G., Podoleanu A., “Full-Field Swept Source Master-Slave Optical Coherence Tomography,” IEEE Photonics J. 7, 1–14 (2015). 10.1109/JPHOT.2015.2505145 [DOI] [Google Scholar]
- 101.Grajciar B., Pircher M., Fercher A. F., Leitgeb R. A., “Parallel Fourier domain optical coherence tomography, measurement of the human eye in vivo,” in Coherence Domain Optical Methods And Optical Coherence Tomography In Biomedicine Ix (2005), pp. 163–167. [DOI] [PubMed] [Google Scholar]
- 102.Yasuno Y., Endo T., Makita S., Aoki G., Itoh M., Yatagai T., “Three-dimensional line-field Fourier domain optical coherence tomography for in vivo dermatological investigation,” Journal of Biomedical Optics 11, 014014 (2006). 10.1117/1.2166628 [DOI] [PubMed] [Google Scholar]
- 103.Nakamura Y., Makita S., Yamanari M., Itoh M., Yatagai T., Yasuno Y., “High-speed three-dimensional human retinal imaging by line-field spectral domain optical coherence tomography,” Opt. Express 15(12), 7103–7116 (2007). 10.1364/OE.15.007103 [DOI] [PubMed] [Google Scholar]
- 104.Grebenyuk A. A., Ginner L., Leitgeb R. A., “Numerically focused full-field swept-source optical coherence microscopy with structured illumination,” Opt. Express 26(26), 33772–33782 (2018). 10.1364/OE.26.033772 [DOI] [PubMed] [Google Scholar]
- 105.Popoff S. M., Lerosey G., Carminati R., Fink M., Boccara A. C., Gigan S., “Measuring the transmission matrix in optics: an approach to the study and control of light propagation in disordered media,” Phys. Rev. Lett. 104(10), 100601 (2010). 10.1103/PhysRevLett.104.100601 [DOI] [PubMed] [Google Scholar]
- 106.Badon A., Li D., Lerosey G., Boccara A. C., Fink M., Aubry A., “Smart optical coherence tomography for ultra-deep imaging through highly scattering media,” Sci. Adv. 2(11), e1600370 (2016). 10.1126/sciadv.1600370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webb R. H., Hughes G. W., Delori F. C., “Confocal scanning laser ophthalmoscope,” Appl. Opt. 26(8), 1492–1499 (1987). 10.1364/AO.26.001492 [DOI] [PubMed] [Google Scholar]
- 108.Podoleanu A. G., Dobre G. M., Webb D. J., Jackson D. A., “Simultaneous en-face imaging of two layers in the human retina by low-coherence reflectometry,” Opt. Lett. 22(13), 1039–1041 (1997). 10.1364/OL.22.001039 [DOI] [PubMed] [Google Scholar]
- 109.Podoleanu A. G., Jackson D. A., “Combined optical coherence tomograph and scanning laser ophthalmoscope,” Electron. Lett. 34(11), 1088–1090 (1998). 10.1049/el:19980793 [DOI] [Google Scholar]
- 110.Podoleanu A. G., Dobre G. M., Cucu R. G., Rosen R., Garcia P., Nieto J., Will D., Gentile R., Muldoon T., Walsh J., Yannuzzi L. A., Fisher Y., Orlock D., Weitz R., Rogers J. A., Dunne S., Boxer A., “Combined multiplanar optical coherence tomography and confocal scanning ophthalmoscopy,” J. Biomed. Opt. 9(1), 86–93 (2004). 10.1117/1.1627778 [DOI] [PubMed] [Google Scholar]
- 111.Pircher M., Baumann B., Götzinger E., Hitzenberger C. K., “Retinal cone mosaic imaged with transverse scanning optical coherence tomography,” Opt. Lett. 31(12), 1821–1823 (2006). 10.1364/OL.31.001821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pircher M., Baumann B., Götzinger E., Sattmann H., Hitzenberger C. K., “Simultaneous SLO/OCT imaging of the human retina with axial eye motion correction,” Opt. Express 15(25), 16922–16932 (2007). 10.1364/OE.15.016922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stevenson S. B., Roorda A., “Correcting for miniature eye movements in high resolution scanning laser ophthalmoscopy,” in Progress in Biomedical Optics and Imaging - Proceedings of SPIE, 2005), 145–151. [Google Scholar]
- 114.Pallikaris A., “Adaptive optics ophthalmoscopy: results and applications,” J. Refract. Surg. 21(5), S570–S574 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Schmoll T., Leitgeb R. A., “Ocular Imaging Combining Ultrahigh Resolution and High Speed OCT,” in Handbook of Coherent-Domain Optical Methods (Springer, 2013), pp. 977–998. [Google Scholar]
- 116.Roorda A., Romero-Borja F., Donnelly Iii W., Queener H., Hebert T., Campbell M., “Adaptive optics scanning laser ophthalmoscopy,” Opt. Express 10(9), 405–412 (2002). 10.1364/OE.10.000405 [DOI] [PubMed] [Google Scholar]
- 117.Hermann B., Fernández E., Unterhuber A., Sattmann H., Fercher A., Drexler W., Prieto P., Artal P., “Adaptive-optics ultrahigh-resolution optical coherence tomography,” Opt. Lett. 29, 2142–2144 (2004). 10.1364/OL.29.002142 [DOI] [PubMed] [Google Scholar]
- 118.Pircher M., Zawadzki R. J., “Combining adaptive optics with optical coherence tomography: Unveiling the cellular structure of the human retina in vivo,” Expert Rev. Ophthalmol. 2(6), 1019–1035 (2007). 10.1586/17469899.2.6.1019 [DOI] [Google Scholar]
- 119.Pircher M., Zawadzki R. J., “Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging [Invited],” Biomed. Opt. Express 8(5), 2536–2562 (2017). 10.1364/BOE.8.002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pircher M., Zawadzki R. J., Evans J. W., Werner J. S., Hitzenberger C. K., “Simultaneous imaging of human cone mosaic with adaptive optics enhanced scanning laser ophthalmoscopy and high-speed transversal scanning optical coherence tomography,” Opt. Lett. 33(1), 22–24 (2008). 10.1364/OL.33.000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Merino D., Dainty C., Bradu A., Podoleanu A. G., “Adaptive optics enhanced simultaneous en-face optical coherence tomography and scanning laser ophthalmoscopy,” Opt. Express 14(8), 3345–3353 (2006). 10.1364/OE.14.003345 [DOI] [PubMed] [Google Scholar]
- 122.Pircher M., Götzinger E., Hitzenberger C. K., “Dynamic focus in optical coherence tomography for retinal imaging,” J. Biomed. Opt. 11(5), 054013 (2006). 10.1117/1.2358960 [DOI] [PubMed] [Google Scholar]
- 123.Felberer F., Kroisamer J. S., Hitzenberger C. K., Pircher M., “Lens based adaptive optics scanning laser ophthalmoscope,” Opt. Express 20(16), 17297–17310 (2012). 10.1364/OE.20.017297 [DOI] [PubMed] [Google Scholar]
- 124.Felberer F., Kroisamer J. S., Baumann B., Zotter S., Schmidt-Erfurth U., Hitzenberger C. K., Pircher M., “Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo,” Biomed. Opt. Express 5(2), 439–456 (2014). 10.1364/BOE.5.000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pircher M., Kroisamer J. S., Felberer F., Sattmann H., Götzinger E., Hitzenberger C. K., “Temporal changes of human cone photoreceptors observed in vivo with SLO/OCT,” Biomed. Opt. Express 2(1), 100–112 (2011). 10.1364/BOE.2.000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y., Rha J., Jonnal R., Miller D., “Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina,” Opt. Express 13(12), 4792–4811 (2005). 10.1364/OPEX.13.004792 [DOI] [PubMed] [Google Scholar]
- 127.Fernández E. J., Povazay B., Hermann B., Unterhuber A., Sattmann H., Prieto P. M., Leitgeb R., Ahnelt P., Artal P., Drexler W., “Three-dimensional adaptive optics ultrahigh-resolution optical coherence tomography using a liquid crystal spatial light modulator,” Vision Res. 45(28), 3432–3444 (2005). 10.1016/j.visres.2005.08.028 [DOI] [PubMed] [Google Scholar]
- 128.Zawadzki R. J., Jones S. M., Olivier S. S., Zhao M., Bower B. A., Izatt J. A., Choi S., Laut S., Werner J. S., “Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging,” Opt. Express 13(21), 8532–8546 (2005). 10.1364/OPEX.13.008532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y., Cense B., Rha J., Jonnal R. S., Gao W., Zawadzki R. J., Werner J. S., Jones S., Olivier S., Miller D. T., “High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography,” Opt. Express 14(10), 4380–4394 (2006). 10.1364/OE.14.004380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kocaoglu O. P., Turner T. L., Liu Z., Miller D. T., “Adaptive optics optical coherence tomography at 1 MHz,” Biomed. Opt. Express 5(12), 4186–4200 (2014). 10.1364/BOE.5.004186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zawadzki R. J., Miller D. T., “Retinal AO OCT,” in Optical Coherence Tomography: Technology and Applications, Second Edition (2015), pp. 1849–1920. [Google Scholar]
- 132.Jonnal R. S., Kocaoglu O. P., Zawadzki R. J., Liu Z., Miller D. T., Werner J. S., “A review of adaptive optics optical coherence tomography: Technical advances, scientific applications, and the future,” Invest. Ophthalmol. Vis. Sci. 57(9), OCT51–OCT68 (2016). 10.1167/iovs.16-19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shemonski N. D., South F. A., Liu Y.-Z., Adie S. G., Carney P. S., Boppart S. A., “Computational high-resolution optical imaging of the living human retina,” Nat. Photonics 9(7), 440–443 (2015). 10.1038/nphoton.2015.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hillmann D., Spahr H., Hain C., Sudkamp H., Franke G., Pfäffle C., Winter C., Hüttmann G., “Aberration-free volumetric high-speed imaging of in vivo retina,” Sci. Rep. 6(1), 35209 (2016). 10.1038/srep35209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hee M. R., Huang D., Swanson E. A., Fujimoto J. G., “Polarization-Sensitive Low-Coherence Reflectometer for Birefringence Characterization and Ranging,” J. Opt. Soc. Am. B 9(6), 903–908 (1992). 10.1364/JOSAB.9.000903 [DOI] [Google Scholar]
- 136.de Boer J. F., Milner T. E., van Gemert M. J. C., Nelson J. S., “Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography,” Opt. Lett. 22(12), 934–936 (1997). 10.1364/OL.22.000934 [DOI] [PubMed] [Google Scholar]
- 137.de Boer J. F., Milner T. E., van Gemert M. J. C., Nelson J. S., “Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography,” Opt. Lett. 22(12), 934–936 (1997). 10.1364/OL.22.000934 [DOI] [PubMed] [Google Scholar]
- 138.Hitzenberger C., Goetzinger E., Sticker M., Pircher M., Fercher A., “Measurement and imaging of birefringence and optic axis orientation by phase resolved polarization sensitive optical coherence tomography,” Opt. Express 9(13), 780–790 (2001). 10.1364/OE.9.000780 [DOI] [PubMed] [Google Scholar]
- 139.Cense B., Chen T. C., Park B. H., Pierce M. C., de Boer J. F., “Thickness and birefringence of healthy retinal nerve fiber layer tissue measured with polarization-sensitive optical coherence tomography,” Invest. Ophthalmol. Vis. Sci. 45(8), 2606–2612 (2004). 10.1167/iovs.03-1160 [DOI] [PubMed] [Google Scholar]
- 140.Fortune B., Burgoyne C. F., Cull G., Reynaud J., Wang L., “Onset and progression of peripapillary retinal nerve fiber layer (RNFL) retardance changes occur earlier than RNFL thickness changes in experimental glaucoma,” Invest. Ophthalmol. Vis. Sci. 54(8), 5653–5661 (2013). 10.1167/iovs.13-12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Götzinger E., Pircher M., Sticker M., Fercher A. F., Hitzenberger C. K., “Measurement and imaging of birefringent properties of the human cornea with phase-resolved, polarization-sensitive optical coherence tomography,” J. Biomed. Opt. 9(1), 94–102 (2004). 10.1117/1.1629308 [DOI] [PubMed] [Google Scholar]
- 142.Pircher M., Götzinger E., Leitgeb R., Sattmann H., Findl O., Hitzenberger C., “Imaging of polarization properties of human retina in vivo with phase resolved transversal PS-OCT,” Opt. Express 12(24), 5940–5951 (2004). 10.1364/OPEX.12.005940 [DOI] [PubMed] [Google Scholar]
- 143.Pircher M., Goetzinger E., Leitgeb R. A., Sattmann H., Hitzenberger C. K., “Ultrahigh resolution polarization sensitive optical coherence tomography,” in Coherence Domain Optical Methods And Optical Coherence Tomography In Biomedicine Ix (2005), pp. 257–262. [Google Scholar]
- 144.Pircher M., Goetzinger E., Leitgeb R., Hitzenberger C., “Three dimensional polarization sensitive OCT of human skin in vivo,” Opt. Express 12(14), 3236–3244 (2004). 10.1364/OPEX.12.003236 [DOI] [PubMed] [Google Scholar]
- 145.Park B., Pierce M., Cense B., de Boer J., “Real-time multi-functional optical coherence tomography,” Opt. Express 11(7), 782–793 (2003). 10.1364/OE.11.000782 [DOI] [PubMed] [Google Scholar]
- 146.Cense B., Chen T. C., Park B. H., Pierce M. C., de Boer J. F., “In vivo birefringence and thickness measurements of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography,” J. Biomed. Opt. 9(1), 121–125 (2004). 10.1117/1.1627774 [DOI] [PubMed] [Google Scholar]
- 147.Yamanari M., Makita S., Yasuno Y., “Polarization-sensitive swept-source optical coherence tomography with continuous source polarization modulation,” Opt. Express 16(8), 5892–5906 (2008). 10.1364/OE.16.005892 [DOI] [PubMed] [Google Scholar]
- 148.Michels S., Pircher M., Geitzenauer W., Simader C., Götzinger E., Findl O., Schmidt-Erfurth U., Hitzenberger C. K., “Value of polarisation-sensitive optical coherence tomography in diseases affecting the retinal pigment epithelium,” Br. J. Ophthalmol. 92(2), 204–209 (2008). 10.1136/bjo.2007.130047 [DOI] [PubMed] [Google Scholar]
- 149.Ahlers C., Götzinger E., Pircher M., Golbaz I., Prager F., Schütze C., Baumann B., Hitzenberger C. K., Schmidt-Erfurth U., “Imaging of the retinal pigment epithelium in age-related macular degeneration using polarization-sensitive optical coherence tomography,” Invest. Ophthalmol. Vis. Sci. 51(4), 2149–2157 (2010). 10.1167/iovs.09-3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Makita S., Hong Y. J., Miura M., Yasuno Y., “Degree of polarization uniformity with high noise immunity using polarization-sensitive optical coherence tomography,” Opt. Lett. 39(24), 6783–6786 (2014). 10.1364/OL.39.006783 [DOI] [PubMed] [Google Scholar]
- 151.Lippok N., Villiger M., Bouma B. E., “Degree of polarization (uniformity) and depolarization index: unambiguous depolarization contrast for optical coherence tomography,” Opt. Lett. 40(17), 3954–3957 (2015). 10.1364/OL.40.003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Boer J. F., Hitzenberger C. K., Yasuno Y., “Polarization sensitive optical coherence tomography - a review [Invited],” Biomed. Opt. Express 8(3), 1838–1873 (2017). 10.1364/BOE.8.001838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sugita M., Pircher M., Zotter S., Baumann B., Roberts P., Makihira T., Tomatsu N., Sato M., Vass C., Hitzenberger C. K., “Retinal nerve fiber bundle tracing and analysis in human eye by polarization sensitive OCT,” Biomed. Opt. Express 6(3), 1030–1054 (2015). 10.1364/BOE.6.001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moneron G., Boccara A. C., Dubois A., “Polarization-sensitive full-field optical coherence tomography,” Opt. Lett. 32(14), 2058–2060 (2007). 10.1364/OL.32.002058 [DOI] [PubMed] [Google Scholar]
- 155.Moreau J., Loriette V., Boccara A. C., “Full-field birefringence imaging by thermal-light polarization-sensitive optical coherence tomography. I. Theory,” Appl. Opt. 42(19), 3800–3810 (2003). 10.1364/AO.42.003800 [DOI] [PubMed] [Google Scholar]
- 156.Moreau J., Loriette V., Boccara A. C., “Full-field birefringence imaging by thermal-light polarization-sensitive optical coherence tomography. II. Instrument and results,” Appl. Opt. 42(19), 3811–3818 (2003). 10.1364/AO.42.003811 [DOI] [PubMed] [Google Scholar]
- 157.Dobre G. M., Podoleanu A. G., Rosen R. B., “Simultaneous optical coherence tomography--Indocyanine Green dye fluorescence imaging system for investigations of the eye’s fundus,” Opt. Lett. 30(1), 58–60 (2005). 10.1364/OL.30.000058 [DOI] [PubMed] [Google Scholar]
- 158.Drexler W., Liu M., Kumar A., Kamali T., Unterhuber A., Leitgeb R. A., “Optical coherence tomography today: speed, contrast, and multimodality,” J. Biomed. Opt. 19, 071412 (2003). [DOI] [PubMed] [Google Scholar]
- 159.Spaide R. F., Fujimoto J. G., Waheed N. K., “Image artifacts in optical coherence tomography angiography,” Retina 35(11), 2163–2180 (2015). 10.1097/IAE.0000000000000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Salas M., Augustin M., Felberer F., Wartak A., Laslandes M., Ginner L., Niederleithner M., Ensher J., Minneman M. P., Leitgeb R. A., Drexler W., Levecq X., Schmidt-Erfurth U., Pircher M., “Compact akinetic swept source optical coherence tomography angiography at 1060 nm supporting a wide field of view and adaptive optics imaging modes of the posterior eye,” Biomed. Opt. Express 9(4), 1871–1892 (2018). 10.1364/BOE.9.001871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen Z., Liu M., Minneman M., Ginner L., Hoover E., Sattmann H., Bonesi M., Drexler W., Leitgeb R. A., “Phase-stable swept source OCT angiography in human skin using an akinetic source,” Biomed. Opt. Express 7(8), 3032–3048 (2016). 10.1364/BOE.7.003032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ploner S. B., Moult E. M., Choi W., Waheed N. K., Lee B., Novais E. A., Cole E. D., Potsaid B., Husvogt L., Schottenhamml J., Maier A., Rosenfeld P. J., Duker J. S., Hornegger J., Fujimoto J. G., “Toward quantitative optical coherence tomography angiography: visualizing blood flow speeds in ocular pathology using variable interscan time analysis,” Retina 36(Suppl 1), S118–S126 (2016). 10.1097/IAE.0000000000001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salas M., Augustin M., Ginner L., Kumar A., Baumann B., Leitgeb R., Drexler W., Prager S., Hafner J., Schmidt-Erfurth U., Pircher M., “Visualization of micro-capillaries using optical coherence tomography angiography with and without adaptive optics,” Biomed. Opt. Express 8(1), 207–222 (2016). 10.1364/BOE.8.000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roberts P., Sugita M., Deák G., Baumann B., Zotter S., Pircher M., Sacu S., Hitzenberger C. K., Schmidt-Erfurth U., "Automated Identification and Quantification of Subretinal Fibrosis in Neovascular Age-Related Macular Degeneration Using Polarization-Sensitive OCTFibrosis in PS-OCT," Investigative Ophthalmology & Visual Science 57, 1699-1705 (2016). 10.1364/BOE.9.001871 [DOI] [PubMed] [Google Scholar]
- 165.Lumbroso B., Huang D., Romano A., Rispoli M., Coscas G., Clinical en Face OCT Atlas (2013).
- 166.Thouvenin O., Boccara C., Fink M., Sahel J., Pâques M., Grieve K., “Cell Motility as Contrast Agent in Retinal Explant Imaging With Full-Field Optical Coherence Tomography,” Invest. Ophthalmol. Vis. Sci. 58(11), 4605–4615 (2017). 10.1167/iovs.17-22375 [DOI] [PubMed] [Google Scholar]
- 167.Apelian C., Harms F., Thouvenin O., Boccara A. C., “Dynamic full field optical coherence tomography: subcellular metabolic contrast revealed in tissues by interferometric signals temporal analysis,” Biomed. Opt. Express 7(4), 1511–1524 (2016). 10.1364/BOE.7.001511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.D. Hillmann, H. Spahr, C. Pfäffle, H. Sudkamp, G. Franke, and G. Hüttmann, “In vivo optical imaging of physiological responses to photostimulation in human photoreceptors,” arXiv preprint arXiv:1605.02959 (2016). 10.1073/pnas.1606428113 [DOI] [PMC free article] [PubMed]
- 169.Yao X., Wang B., “Intrinsic optical signal imaging of retinal physiology: a review,” J. Biomed. Opt. 20(9), 090901 (2015). 10.1117/1.JBO.20.9.090901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schmoll T., Kolbitsch C., Leitgeb R. A., “In vivo functional retinal optical coherence tomography,” J. Biomed. Opt. 15(4), 041513 (2010). 10.1117/1.3463008 [DOI] [PubMed] [Google Scholar]
- 171.Pfäffle Clara, Hillmann Dierck, Spahr Hendrik, Kutzner Lisa, Burhan Sazan, Hilge Felix, Miura Yoko, Hüttmann G., “Functional imaging of ganglion and receptor cells in living human retina by osmotic contrast,” http://arXiv1605.02959 (2018). [DOI] [PubMed]
- 172.Spahr H., Pfäffle C., Koch P., Sudkamp H., Hüttmann G., Hillmann D., “Interferometric detection of 3D motion using computational subapertures in optical coherence tomography,” Opt. Express 26(15), 18803–18816 (2018). 10.1364/OE.26.018803 [DOI] [PubMed] [Google Scholar]
- 173.Carrasco-Zevallos O. M., Viehland C., Keller B., Draelos M., Kuo A. N., Toth C. A., Izatt J. A., “Review of intraoperative optical coherence tomography: technology and applications [Invited],” Biomed. Opt. Express 8(3), 1607–1637 (2017). 10.1364/BOE.8.001607 [DOI] [PMC free article] [PubMed] [Google Scholar]