Abstract
Background
Hepatosplenic schistosomiasis is an important cause of variceal bleeding in low‐income countries. Randomised clinical trials have evaluated the outcomes of two categories of surgical interventions, shunts and devascularisation procedures, for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis. The comparative overall benefits and harms of these two interventions are unclear.
Objectives
To assess the benefits and harms of surgical portosystemic shunts versus oesophagogastric devascularisation procedures for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, LILACS, reference lists of articles, and proceedings of relevant associations for trials that met the inclusion criteria (date of search 11 January 2018).
Selection criteria
Randomised clinical trials comparing surgical portosystemic shunts versus oesophagogastric devascularisation procedures for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis.
Data collection and analysis
Two review authors independently assessed the trials and extracted data using methodological standards expected by Cochrane. We assessed risk of bias according to domains and risk of random errors with GRADE and Trial Sequential Analysis. We assessed the certainty of evidence using the GRADE approach.
Main results
We found two randomised clinical trials including 154 adult participants, aged between 18 years and 65 years, diagnosed with hepatosplenic schistosomiasis. One of the trials randomised participants to proximal splenorenal shunt versus distal splenorenal shunt versus oesophagogastric devascularisation with splenectomy, and the other randomised participants to distal splenorenal shunt versus oesophagogastric devascularisation with splenectomy. In both trials the diagnosis of hepatosplenic schistosomiasis was made based on clinical and biochemical assessments. The trials were conducted in Brazil and Egypt. Both trials were at high risk of bias.
We are uncertain as to whether surgical portosystemic shunts improved all‐cause mortality compared with oesophagogastric devascularisation with splenectomy due to imprecision in the trials (risk ratio (RR) 2.35, 95% confidence interval (CI) 0.55 to 9.92; participants = 154; studies = 2). We are uncertain whether serious adverse events differed between surgical portosystemic shunts and oesophagogastric devascularisation with splenectomy (RR 2.26, 95% CI 0.44 to 11.70; participants = 154; studies = 2). None of the trials reported on health‐related quality of life. We are uncertain whether variceal rebleeding differed between surgical portosystemic shunts and oesophagogastric devascularisation with splenectomy (RR 0.39, 95% CI 0.13 to 1.23; participants = 154; studies = 2). We found evidence suggesting an increase in encephalopathy in the shunts group versus the devascularisation with splenectomy group (RR 7.51, 95% CI 1.45 to 38.89; participants = 154; studies = 2). We are uncertain whether ascites and re‐interventions differed between surgical portosystemic shunts and oesophagogastric devascularisation with splenectomy. We computed Trial Sequential Analysis for all outcomes, but the trial sequential monitoring boundaries could not be drawn because of insufficient sample size and events. We downgraded the overall certainty of the body of evidence for all outcomes to very low due to risk of bias and imprecision.
Authors' conclusions
Given the very low certainty of the available body of evidence and the low number of clinical trials, we could not determine an overall benefit or harm of surgical portosystemic shunts compared with oesophagogastric devascularisation with splenectomy. Future randomised clinical trials should be designed with sufficient statistical power to assess the benefits and harms of surgical portosystemic shunts versus oesophagogastric devascularisations with or without splenectomy and with or without oesophageal transection.
Surgical treatment (shunts compared with devascularisation) for preventing variceal rebleeding due to schistosomiasis of the liver and spleen
Background
Schistosomiasis ('bilharzia' or 'snail fever') is a water‐borne disease caused by parasites known as blood flukes. Blood flukes are released by fresh water snails and penetrate the skin of humans (swimmers and others in close contact with water). Here, they migrate into the venous circulation, settling in various typical sites such as the gut, the urinary bladder, and the liver, where they cause local inflammation. In the liver, they result in Symmer's pipe‐stem periportal fibrosis, with the consequent complication of increased portal blood pressure. Infected people may develop varices (enlarged blood vessels within the wall of the oesophagus and stomach). Bleeding from these varices is not uncommon and can result in death. Although several methods exist to stop the initial bleeding, it may recur with the same risk of death as during the initial bleed without further treatment.
The first‐line treatment to prevent variceal rebleeding is with medications (non‐selective beta‐blockers to lower the portal blood pressure) combined with endoscopic method (use of a long tube fitted with a camera to locate and close the varices with elastic bands). This involves repeated treatment sessions, hence treatment success is heavily dependent on patient compliance, which in low income countries may be adversely affected by eco‐social factors such as transport costs.
Surgery is an alternative treatment option. There are two broad surgical categories to decrease the risk of repeat bleeding from varices: these are either shunts (a channel that diverts all or part of the bloodstream from the liver to the general blood circulation) or devascularisation surgery (disconnection of the enlarged blood vessels in the walls of the oesophagus and stomach). Either treatment may be performed as a once‐off procedure to prevent variceal rebleeding. However, it is not clear which of these treatments offers the best result.
We aimed to determine the benefits and harms of shunts compared with devascularisation in preventing variceal rebleeding due to schistosomiasis of the liver and spleen.
Study characteristics
We found two randomised clinical trials (types of studies in which participants are assigned to treatment group using a random method) involving a total of 154 adult participants who received either a non‐selective shunt surgery, a selective shunt surgery, or devascularisation surgery. However, the design of both trials was of insufficient quality, as the numbers of trial participants were small, and some participant information was lacking. One of the trials was funded by an institutional grant, and how funding was obtained for the other trial was not clear. We assessed both trials as at high risk of bias.
Key results
There were no significant differences in the number of participants who had repeat bleeding, adverse effects of treatment, or deaths between the shunt surgery and the devascularisation group, but participants who had devascularisation were less likely to suffer encephalopathy (disease of the brain due to damage from toxins produced by the liver). Neither of the trials addressed quality of life after treatment.
Conclusions
Given the very low certainty of the evidence due to the way the clinical trials were performed, limited trial data and trial participants, we were unable to determine whether one treatment is better than the other. We suggest that future trials include a sufficient number of randomised participants to be able to obtain meaningful results on patient‐relevant outcomes and allow objective comparison of these two surgery types.
Summary of findings
Summary of findings for the main comparison.
Surgical portosystemic shunts compared to devascularisation with splenectomy for prevention of variceal rebleeding in people with hepatosplenic schistosomiasis
| Surgical portosystemic shunts compared to devascularisation with splenectomy for prevention of variceal rebleeding in people with hepatosplenic schistosomiasis | ||||||
| Patient or population: people with hepatosplenic schistosomiasis Setting: health institutions in Brazil and Egypt Intervention: surgical portosystemic shunts Comparison: devascularisation with splenectomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with devascularisation with splenectomy | Risk with surgical portosystemic shunts | |||||
| All‐cause mortality follow‐up: range 5 years to 10 years | Study population | RR 2.35 (0.55 to 9.92) | 154 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 48 per 1000 | 114 per 1000 (27 to 480) | |||||
| Serious adverse events follow‐up: range 5 years to 10 years | Study population | RR 2.26 (0.44 to 11.70) | 154 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 16 per 1000 | 36 per 1000 (7 to 189) | |||||
| Quality of life | ‐ | ‐ | 154 (2 RCTs) | ‐ | None of the trials reported on quality of life. | |
| Variceal rebleeding rate follow‐up: range 5 years to 10 years | Study population | RR 0.39 (0.13 to 1.23) | 154 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 145 per 1000 | 57 per 1000 (19 to 179) | |||||
| Encephalopathy follow‐up: range 5 years to 10 years | Study population | RR 7.51 (1.45 to 38.89) | 154 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Due to lack of events in the devascularisation arms, we derived the risk with shunts by summing the events and sample sizes from shunt treatment arms across the studies. | |
| 1774 per 1000 | 1000 per 1000 (1000 to 1000) | |||||
| Ascites follow‐up: range 5 years to 10 years | Study population | RR 0.11 (0.01 to 1.98) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 133 per 1000 | 15 per 1000 (1 to 264) | |||||
| Any re‐intervention follow‐up: range 5 years to 10 years | Study population | RR 3.00 (0.13 to 70.83) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Due to lack of events in the devascularisation arms, we derived the risk with shunts by summing the events and sample sizes from shunt treatment arms across the studies. | |
| 1033 per 1000 | 1000 per 1000 (134 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for risk of bias due to high risk of bias in overall assessment of both trials. 2Downgraded two levels for imprecision due to small sample size and few events.
Background
Description of the condition
Schistosomiasis is a parasitic disease that is endemic in poor communities with inadequate sanitation and lack of access to potable water (Steinmann 2006). The World Health Organization (WHO) estimates that the total number of people infected worldwide is over 200 million, with more than 90% of total infected people living in Africa (WHO 2014). Despite schistosomiasis control activities, current evidence suggest high prevalence of the disease in Africa, and the burden of undiagnosed hepatosplenic form remains high (Payne 2013). The life cycle of this parasite involves two hosts, namely humans (the definitive host) and snails (the intermediate host). Human hosts become infected by contact with infested water, where cercariae released by infected snails of the genus Bomphalaria penetrate the human skin or mucosa, or both. Further maturation takes place in the lungs and liver to produce adult worms that migrate to the mesenteric veins where they mate and deposit their eggs. There are several species of the blood fluke (Schistosomatidae family), but two species, namely Schistosoma mansoni (found predominantly in Africa, Arabia, and South America) and Schistosoma japonicum (found in South‐East Asia, especially mainland China), are responsible for the hepatosplenic form of the disease (Colley 2014b). The available evidence suggests that an immune‐mediated granulomatous inflammatory reaction to the trapped eggs in portal vein radicles results in periportal fibrosis known as Symmer's pipe‐stem fibrosis (Symmers 1904; Burke 2009; Colley 2014a). This fibrosis subsequently results in the development of pre‐sinusoidal portal hypertension, which leads to variceal bleeding, ascites, and death (Ross 2002).
Portal hypertension refers to the pathological increase of the hepatic venous pressure gradient above 8 mmHg, and clinically obvious variceal bleeding occurs when the pressure gradient exceeds 12 mmHg (Sanyal 2008). This is a hallmark of liver cirrhosis. However, bleeding may also be caused by non‐cirrhotic conditions such as hepatosplenic schistosomiasis. The sequelae of portal hypertension include the development of varices, encephalopathy, hypersplenism, and ascites (Goff 1993). The prevalence of portal hypertension in schistosomiasis endemic areas approaches 18% in the absence of a schistosomiasis control programme (Mudawi 2007); of these 30% to 60% will develop varices (De Cock 1982; Saad 1991).
Oesophagogastric variceal bleeding is the most lethal complication of portal hypertension, as the mortality from the first bleeding episode is approximately 15% to 20% in cirrhotic portal hypertension (Chalasani 2003; Carbonell 2004; Villanueva 2006), and 10% in non‐cirrhotic portal hypertension (Chofle 2014). Recurrent bleeding is not uncommon in survivors of the first episode and is also associated with a similar mortality. The greatest risk of rebleeding is during the first 30 days following the initial variceal bleed (Smith 1982). Diagnosis of acute variceal bleeding is established by upper gastrointestinal endoscopy (Gonzalez 2008; de Franchis 2010). The histopathological confirmation of hepatosplenic schistosomiasis remains the gold standard for diagnosis and is based on demonstrating the typical pipe‐stem fibrosis from wedge liver biopsy (Symmers 1904; Burke 2009; Colley 2014a). However, with advancements in radiology, ultrasound is increasingly being used for diagnosis of hepatosplenic schistosomiasis by demonstrating changes in portal vein radicles (Hatz 1992; Abdel‐Wahab 1993).
Although portal hypertension is established as a risk factor for variceal bleeding (Goff 1993; Sanyal 2008), the status of varices, particularly variceal size, is another important risk factor for bleeding (Lebrec 1980; Garcia‐Tsao 2010).
Description of the intervention
The initial treatment for acute variceal bleeding is the same for both cirrhotic and non‐cirrhotic portal hypertension, consisting of a combination of pharmacological (vasoactive agent) and endoscopic therapies. Using this management, bleeding will stop in 90% of people (D'Amico 2003; Gonzalez 2008; de Franchis 2010). The remaining 10% are classified as having refractory bleeding, and further management options include radiologically placed transjugular intrahepatic portosystemic shunt (TIPS) or surgical interventions (shunts, devascularisation procedures, or liver transplantation) (de Franchis 2010). The risk of long‐term rebleeding without further intervention following the control of acute variceal bleeding is approximately 80% (Kiire 1989; D'Amico 1995; Vleggaar 1998). Hence, secondary prevention is considered to be required in any person who has suffered a variceal bleed (de Franchis 2010).
Medical treatment (combined endoscopic band ligation and non‐selective beta‐blockers) is an effective first‐line modality to prevent variceal rebleeding (de Franchis 2010; Sarin 2010). However, approximately 20% to 30% of people will still have recurrent variceal bleeding while undergoing medical therapy (Kiire 1989; Bhargava 1990; Vleggaar 1998; Sarin 2010). Patients are therefore usually offered repeated sessions of endoscopy with sclerotherapy or banding to obliterate the varices. This may have cost and travel implications for the person and the health system, specifically in resource‐poor areas where schistosomiasis is endemic.
Transjugular intrahepatic portosystemic shunt has an efficacy of more than 90% rebleed‐free rate and is recommended as rescue therapy following the failure of medical treatment (Rossle 2006; Boyer 2010; de Franchis 2010). Although it is a less invasive procedure than a surgical procedure, it may have more complications and requires more re‐interventions than surgical procedures. The occlusion and stenosis rate for TIPS is 17% compared with 9% for surgical shunts (Rosemurgy 2012); rebleeding occurs in 20% to 30% of people with TIPS compared to less than 10% for surgical shunts; there is a median survival of 26 months for TIPS compared to 52 months for surgical shunts (Rikkers 1998; Costa 2010; Rosemurgy 2012); and postshunt encephalopathy ranges between 18% and 45% (Rossle 1994; Deng 2006). In addition, TIPS requires more intensive long‐term surveillance than surgical shunts due to its higher occlusion rates and resulting more frequent need for re‐intervention, up to 21% for TIPS versus 6% for surgical shunts (Toomey 2013). To our knowledge there is no literature supporting the use of TIPS in schistosomal portal hypertension (Conn 1993; Eesa 2011).
The surgical interventions for prevention of variceal rebleeding are shunts, devascularisation procedures, or liver transplantation (de Franchis 2010). Liver transplantation is an effective treatment for the definitive control of variceal rebleeding in people with end‐stage liver disease (de Franchis 2010; Rosemurgy 2012), yet only 3% to 14% of people with cirrhosis complicated by variceal bleeding eventually receive transplantation (Stanley 1996; Tripathi 2004; Rossle 2006; Rosemurgy 2012; Toomey 2013). Moreover, people with hepatosplenic schistosomiasis do not meet liver transplantation criteria, as their liver function is usually preserved in the absence of cirrhosis (De Cock 1986; Denié 1996; Bica 2000; Rosemurgy 2012).
Several studies have compared surgical interventions (either shunt or devascularisation) with placebo or endoscopic therapy for the prevention of variceal bleeding due to cirrhotic portal hypertension, and one systematic review concluded that surgical intervention is a better strategy to prevent variceal bleeding but with a higher morbidity (D'Amico 1995; Khan 2006). These studies lack statistical power to determine true intervention effect, yet endoscopic therapy is recommended as first‐line treatment for the prevention of variceal rebleeding (Garcia‐Tsao 2017). A recent randomised clinical trial concluded that a combination of surgical intervention and endoscopic treatment provided the best initial strategy for preventing variceal rebleeding in hepatosplenic schistosomiasis (Costa 2016). Although surgical options for preventing variceal rebleeding are only considered as an alternative strategy when medical therapy fails, they may well be a one‐stop procedure which entails fewer hospital visits and less intensive follow‐up (Henderson 2005; Pal 2012).
Surgical shunts are considered for people with good performance status and Child‐Pugh class A or early B (Child 1964; Pugh 1973; Garcia‐Tsao 2010; Orloff 2012; Gur 2014). Shunts are surgically created conduits that divert some or all of portal venous blood away from the liver into the systemic circulation. These conduits may be an autogenous vein graft or polytetrafluoroethylene prosthesis. Commonly created surgical portosystemic shunts include the H‐shunt and the selective distal splenorenal shunt (Warren 1967; Sarfeh 1986; Sarfeh 1994). The H‐shunt is created between the portal vein and the inferior vena cava using an 8‐ to 16‐millimetre ringed polytetrafluoroethylene prosthesis. This prosthesis is non‐expansible, and by diminishing its diameter from 16 mm to 8 mm, a partial portal decompression is achieved (Sarfeh 1986; Sarfeh 1994). The distal splenorenal shunt is created by anastomosing the distal splenic vein to the left renal vein with or without disconnecting the splenopancreatic and gastric venous connections to the portal system while preserving portal venous blood flow and hepatic function (Warren 1967). On the basis of their haemodynamic effect on portal circulation, surgical shunts are divided into selective and non‐selective types. Non‐selective shunts totally bypass portal blood flow into the systemic circulation, while selective shunts maintain nutrient hepatic blood flow. A recent meta‐analysis concluded that rebleeding rate, encephalopathy, and late mortality are comparable for selective and non‐selective shunts (Yin 2013). However, it has been argued that total portal decompression may precipitate encephalopathy in up to 39% of people with preserved liver function, such as people with hepatosplenic schistosomiasis (Raia 1994). Selective shunts are therefore considered to be superior to non‐selective shunts in this subgroup (Henderson 1988; Da Silva 1992; Conn 1994; Raia 1994; Andersson 2007). Overall perioperative mortality following shunt procedures is 6% to 15%, with five‐year survival rates approaching 80% in cirrhotics, in whom mortality occurs as a result of progressive hepatic decompression (Rosemurgy 2012; Gur 2014). The survival for non‐cirrhotics such as hepatosplenic schistosomiasis exceeds that of cirrhotics (Raia 1994; Gawish 2000).
Oesophagogastric devascularisation procedures is another intervention that has been used in people with hepatosplenic schistosomiasis. It involves transhiatal devascularisation of the lower oesophagus and proximal half of the stomach, with ligation of branches of left gastric, short gastric, left gastroepiploic, and perforating oesophageal arteries and veins. There are several modifications of devascularisation, including one‐stage devascularisation and splenectomy without oesophageal transection (Hassab 1967), or with oesophageal transection plus splenectomy in a two‐stage operation (Sugiura 1973). The original Sugiura procedure was subsequently modified into a one‐stage abdominal procedure (Peracchia 1980; Inokuchi 1985), or without oesophageal transection (Jin 1996; Johnson 2006), or without truncal vagotomy (Ginsberg 1982), and without splenectomy (Orozco 1998). One randomised clinical trial comparing devascularisation with splenectomy versus without splenectomy showed no evidence of a difference in overall outcomes between the two methods, although participants who had splenectomy received more blood transfusion compared with no splenectomy. This demonstrates that addition of splenectomy to devascularisation procedures may not always be necessary (Orozco 1998). However, the further role of devascularisation procedures remains controversial due to their high morbidity and mortality when compared to surgical shunts in certain patient series (Selzner 2001). Overall perioperative mortality following devascularisation procedures ranges from 13% to 24% (Rikkers 1998; Qazi 2006; Voros 2012), and variceal rebleeding of up to 40% has been reported (Henderson 1988; Orozco 1992; Johnson 2006). However, the risk of encephalopathy is rare (Conn 1994; Raia 1994), and there is no need for postprocedure surveillance. Overall five‐year survival is approximately 75% and is comparable to shunt procedures (Ezzat 1990; Orozco 1992). A significant procedure‐specific morbidity is oesophageal anastomotic leak, which may occur in as many as 10% (Voros 2012).
How the intervention might work
Surgical portosystemic shunts decrease portal venous pressure, and hence decrease portal hypertension by diverting all or part of portal blood flow into systemic circulation. The consequent decrease in portal venous pressure below the threshold of 12 mmHg will prevent variceal bleeding (Warren 1967; Sarfeh 1986; Sarfeh 1994).
Oesophagogastric devascularisation with or without splenectomy reduces portal hypertension by decreasing portal blood flow, but with a compensatory increase in hepatic artery flow. This maintains effective hepatic blood flow and preserves liver function. The procedure normalises the hyperdynamic circulatory state present in hepatosplenic schistosomiasis (Brandt 1995; de Cleva 2007; Zhang 2009; Evangelista‐Neto 2012).
Why it is important to do this review
Previous meta‐analyses have compared portosystemic shunts with devascularisation procedures for the prevention of variceal rebleeding in cirrhotic portal hypertension (Yin 2013; Zong 2015). Unlike liver cirrhosis in which there is destruction of liver architecture with concomitant loss of hepatocyte function, in hepatosplenic schistosomiasis hepatic architecture and function are usually preserved in the absence of concomitant liver disease such as viral hepatitis infection (De Cock 1986; Denié 1996; Bica 2000; Ross 2002). It is therefore important to conduct a systematic review with meta‐analysis and Trial Sequential Analysis to compare surgical portosystemic shunts with devascularisation procedures for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis.
Objectives
To assess the benefits and harms of surgical portosystemic shunts versus oesophagogastric devascularisation procedures for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomised clinical trials in which surgical portosystemic shunts were compared with oesophagogastric devascularisation with or without splenectomy, and with or without oesophageal transection for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis. For assessment of harms, we intended to include quasi‐randomised studies and observational studies identified during our search for randomised clinical trials. We are aware that this approach increases the risk of overlooking harms of the intervention.
Types of participants
We included participants with hepatosplenic schistosomiasis confirmed by liver biopsy, irrespective of age and sex, who have experienced variceal rebleeding. We excluded participants with concomitant liver cirrhosis and those who received TIPS.
Types of interventions
We considered the following types of surgical shunt interventions as experimental interventions:
portacaval shunt (connecting the portal vein and the vena cava);
mesocaval shunt (connecting the mesenteric vein and the vena cava);
central (proximal) splenorenal shunt (connecting proximal splenic vein to left renal vein with or without splenopancreatic and gastric disconnection or splenectomy);
distal splenorenal shunt (connecting distal splenic vein to left renal vein with or without splenopancreatic and gastric disconnection);
large‐diameter H‐graft shunt (16 mm, externally reinforced polytetrafluorethylene either as mesocaval or portocaval shunt);
small‐diameter H‐graft shunt (8 mm, externally reinforced polytetrafluorethylene either as mesocaval or portacaval shunt).
We considered oesophagogastric devascularisation with or without splenectomy, and with or without oesophageal transection as control interventions.
Types of outcome measures
Primary outcomes
-
All‐cause mortality
immediate (30 days)
intermediate (one year)
long term (five years)
Serious adverse events (procedure‐related complications). We used the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice's definition of a serious adverse event (ICH‐GCP 1997), that is any untoward medical occurrence that resulted in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or is a congenital anomaly or birth defect. We considered all other adverse events as non‐serious.
Quality of life. We defined quality of life as the extent to which a person's usual or expected physical, emotional, and social well‐being has been affected by the intervention (Cella 1995). Since trial authors are likely to use different instruments to measure quality of life, we planned to use the recommendation for choosing a statistical method to enhance interpretability to evaluate quality of life estimates in this meta‐analysis (Thorlund 2011b).
Secondary outcomes
Variceal rebleeding rate (diagnosed clinically by haematemesis, melena, or blood in gastric aspirate, and confirmed by endoscopy)
-
Number of people who developed encephalopathy, defined by any of the following:
classical signs detected on physical examination (change in mental status examination in association with elevated ammonia, and asterixis);
signs unequivocally described by the participant's relatives;
psychometric testing; or
electroencephalogram.
Development of new or worsening of pre‐existing ascites detected clinically or radiologically
Number of people requiring any re‐intervention
Search methods for identification of studies
Electronic searches
We performed electronic searches for relevant trials in the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2018), (up until 11 January 2018); Cochrane Central Register of Controlled Trials (CENTRAL), (up until 2017, Issue 12) in the Cochrane Library; MEDLINE Ovid (1946 to 11 January 2018); Embase Ovid (1974 to 11 January 2018); LILACS (Latin American and Caribbean Health Sciences Literature) (BIREME; 1982 to 11 January 2018); Science Citation Index Expanded (Web of Science; 1900 to 11 January 2018), and Conference Proceedings Citation Index – Science (Web of Science; 1990 to 11 January 2018) (Royle 2003). The search strategies and the time spans of the searches are listed in Appendix 1.
Searching other resources
We handsearched reference lists of identified studies for further relevant trials.
We also searched conference/meeting proceedings and abstracts published by International Hepato‐Pancreato Biliary Association (IHPBA) (1994 to January 2018), the American Association for the Study of Liver Diseases (AASLD) (1994 to 11 January 2018), and other relevant organisations.
We also searched on‐line trial registries such as ClinicalTrials.gov (clinicaltrials.gov), the European Medicines Agency (EMA) (www.ema.europa.eu/ema), the World Health Organization, International Clinical Trials Registry Platform (www.who.int/ictrp), and the Food and Drug Administration (FDA) (www.fda.gov) for ongoing or unpublished trials on 11 January 2018.
Data collection and analysis
Selection of studies
Two review authors (CJE and MB) independently screened the list of titles and abstracts retrieved by the search in order to identify potentially eligible studies. We retrieved the full‐text articles of those studies deemed potentially eligible, and two review authors (CJE and MB) reviewed the full‐text articles for inclusion in the review. We resolved any areas of disagreement through discussion. We sought unpublished data by writing to the authors of included studies.
Data extraction and management
Two review authors (CJE and MB) independently extracted data from included trials using a standardised data collection form (Appendix 2), which included the following.
Name of first author
Date of trial publication
Country of trial and maximum duration of follow‐up
Inclusion and exclusion criteria
Demographic data
Biochemical data
Method of diagnosis of schistosomiasis
Number of participants randomised, number excluded with reasons, number analysed, and number of withdrawals
Assessment of risk of bias
Outcomes: number of participants with events for dichotomous outcome, mean and standard deviation for continuous outcome at maximal follow‐up
Assessment of risk of bias in included studies
Two review authors (CJE and MB) independently assessed the risk of bias of each included trial using the following domains, as recommended in the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011), the Cochrane Hepato‐Biliary Group Module (Gluud 2018), and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Hrobjartsson 2012; Savović 2012a; Savović 2012b; Hrobjartsson 2013; Hrobjartsson 2014a; Hrobjartsson 2014b; Lundh 2017). We used the following definitions to assess the risk of bias in included trials.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, coin tossing, shuffling cards or envelopes, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We only used such studies for the assessment of harms.
Allocation concealment
Low risk of bias: participant allocation could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants. We only used such studies for the assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judged that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judged that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: no missing outcome data, or missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods such as multiple imputations were employed to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trials reported all the predefined outcomes in their method. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, we would not consider those outcomes to be reliable.
Unclear risk: the study authors do not report all predefined outcomes fully, or it is unclear whether the study authors recorded data on these outcomes.
High risk: the study authors do not report one or more predefined outcomes.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conductance, or results of the trial (industry‐sponsored trials overestimate the efficacy by about 25%) (Lundh 2017).
Unclear risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received another type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other factors that could put it at risk of bias.
Unclear risk of bias: the trial may or may not be free of other factors that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall risk of bias
We judged trials to be at an overall low risk of bias if they were assessed as at low risk of bias in all 'Risk of bias' domains. We judged trials to be at an overall high risk of bias if they were judged to be at unclear risk of bias or high risk of bias in one or more 'Risk of bias' domains.
Measures of treatment effect
We measured intervention effects for dichotomous outcomes using risk ratios (RR) with 95% confidence interval (CI), and for continuous outcomes using mean difference (MD) with 95% CI across studies. We planned to report Trial Sequential Analysis‐adjusted CI.
Unit of analysis issues
The unit of analysis was trial participants as randomised to the trial groups. When a trial had more than two groups, we extracted data from the groups that corresponded to the treatment options considered as experimental, and used half of the participants in the control group for the respective comparisons.
As expected, we did not find any cross‐over or cluster‐randomised trials.
Dealing with missing data
We performed an intention‐to‐treat analysis.
Dealing with missing data using sensitivity analysis
We contacted trial authors to obtain missing data but we could not obtain all missing data. We therefore dealt with missing data by considering participants as treatment failures or treatment successes by imputing them according to the following two scenarios for our primary outcomes:
'extreme case' analysis favouring the experimental intervention ('best‐worst' case scenario): none of the participants who dropped out of the experimental group experienced the outcome, but all the participants who dropped out of the control group experienced the outcome; including all randomised participants in the denominator;
'extreme case' analysis favouring the control ('worst‐best' case scenario): all participants who dropped out of the experimental group, but none of the participants who dropped out of the control group experienced the outcome; including all randomised participants in the denominator.
Assessment of heterogeneity
We assessed heterogeneity between trials using the Chi2 test and I2 statistic. The degree of heterogeneity observed was measured using the I2 statistic (Higgins 2002; Sterne 2011). The values of the I2 statistic were as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: represent considerable heterogeneity.
An I2 statistic above 50% was considered as significant, and the possible cause of heterogeneity explored further.
Assessment of reporting biases
We planned to investigate reporting bias by visual inspection of funnel plot asymmetry if we included at least 10 trials. For dichotomous outcomes, we planned to use the Harbord test for asymmetry (Harbord 2006), and for continuous outcomes we used the regression asymmetry test, Egger 1997, and the adjusted rank correlation (Begg 1994).
Data synthesis
We performed meta‐analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Hepato‐Biliary Group Module (Higgins 2011; Gluud 2018). We meta‐analysed data according to the eight‐step procedure for validation of meta‐analytic results in systematic reviews as suggested by Jakobsen and colleagues (Jakobsen 2014). For our data analysis we used the software packages Review Manager 5 provided by Cochrane, RevMan 2014, and Trial Sequential Analysis Version 0.9.5.10 Beta provided by the Copenhagen Trial Unit (Thorlund 2011a; TSA 2011). We used both fixed‐effect and random‐effects meta‐analyses, and presented the data with the most conservative estimate of the two. The most conservative estimate of the two is the one closest to 1.0 for dichotomous or 0.0 (zero) for continuous outcomes (Jakobsen 2014). If the two point estimates were equal, we used the estimate with the widest CI as our main result of the two analyses (Jakobsen 2014). We presented heterogeneity using the I2 statistic (Higgins 2002).
Where data were available from only one trial, we used Fisher's exact test for dichotomous data (Fisher 1922), and planned to use Student's t‐test for continuous data to present the results narratively (Student 1908).
Trial Sequential Analysis
Cumulative meta‐analyses can introduce random errors because of sparse data and repetitive testing of accumulating data (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Higgins 2011; Wetterslev 2017); hence we used Trial Sequential Analysis (TSA 2011) to control for random errors (Thorlund 2011a). The diversity‐adjusted required information size (DARIS) was calculated for all outcomes in order to control random errors (Wetterslev 2008; Wetterslev 2009). The DARIS calculation took into account the following: control group event proportion observed in the meta‐analysis; a plausible relative risk reduction of 20%; a risk of type I error of 2.5% due to three primary outcomes and 2% due to four secondary outcomes; a risk of type II error of 10% (Castellini 2017); and the adjusted diversity from the meta‐analysis (Wetterslev 2008; Wetterslev 2009; Jakobsen 2014; Wetterslev 2017). We also planned to calculate and report the Trial Sequential Analysis‐adjusted CI (Thorlund 2011a). We assumed that testing for statistical significance was performed with each new trial added to the trial sequential meta‐analysis. On the basis of the calculated DARIS, we planned to construct trial sequential monitoring boundaries. If the Z‐curve crossed the trial sequential monitoring boundary for benefit or harm before the DARIS was reached, we planned to conclude evidence of benefit or harm of the intervention. In contrast, if the boundary was not surpassed, we planned to conclude that further trials needed to be conducted in order to attain true intervention effect. However, where the Z‐curve crossed the monitoring boundary for futility, we planned to conclude futility of the comparison (Wetterslev 2008; Thorlund 2011a; Wetterslev 2017).
A more detailed description of Trial Sequential Analysis can be found at www.ctu.dk/tsa/ (Thorlund 2011a).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Trials at low risk of bias compared to trials at high risk of bias.
Non‐selective surgical shunts versus devascularisation procedures compared to selective surgical shunts versus devascularisation procedures.
Surgical shunts versus devascularisation with splenectomy compared to surgical shunts versus devascularisation without splenectomy.
Age of participants: less than 65 years compared to greater than 65 years.
Length of follow‐up: less than 30 days compared to greater than 30 days.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing missing data in a best‐worst case scenario assuming participants with missing data for dichotomous outcome experienced a good outcome in the experimental group and a poor outcome in the control group. We also considered a worse‐best case scenario by assuming participants with missing data had a poor outcome in the experimental group and a good outcome in the control group (Gamble 2005). As we included only two trials, we did not perform the following sensitivity analyses: assessment of the search method for the included trials; exclusion criteria; the type of data analysed; process of data analysis; and measure of intervention outcome at 30 days.
'Summary of findings' table
We designed one 'Summary of findings' table for our review comparison, using GRADEpro GDT software (GRADEpro GDT 2015). Using GRADE (Guyatt 2011), we graded the certainty of evidence for all Primary outcomes and Secondary outcomes based on five domains: risk of bias, indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results; and a high probability of publication bias. We defined the levels of evidence as 'high', 'moderate', 'low', or 'very low'. We followed the recommendations of Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We defined the levels of evidence as follows.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
We identified 2014 references through the database searches (Figure 1). We excluded 771 duplicate records. After reading the titles and abstracts of the remaining 1243 references, we selected only 14 references for full manuscript review. We excluded 10 studies, which are presented with reasons for their exclusion in the Characteristics of excluded studies tables. We were thus left with four full‐text publications (Da Silva 1992; Raia 1994; Strauss 1999; Gawish 2000), referring to two trials that met the inclusion criteria of our review (Raia 1994; Gawish 2000). Da Silva 1992 was an interim report of Raia 1994 at two years of follow‐up, and Strauss 1999 evaluated changes in variceal size following different interventions in a subset of 73 participants of the Raia 1994 trial. Raia 1994 presented data at maximal follow‐up of 10 years.
Figure 1.
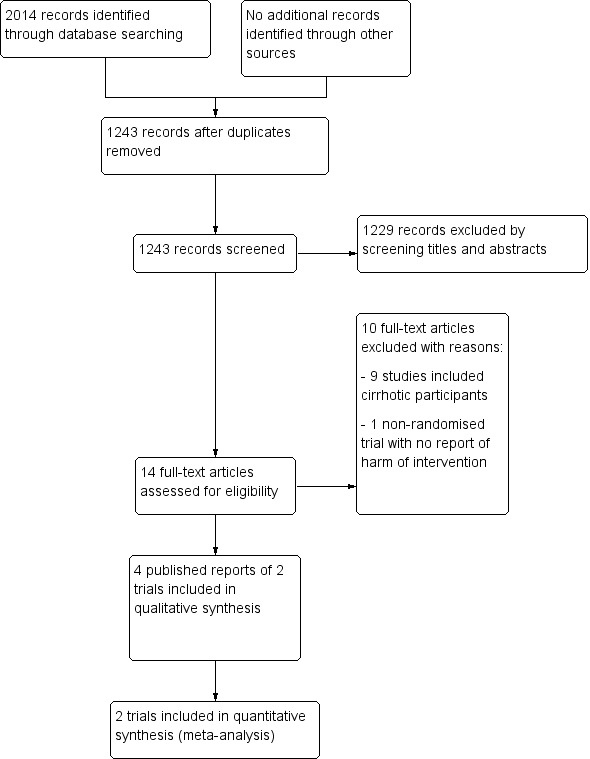
Study flow diagram.
We identified no other references of interest through other sources or through screening the reference lists of the identified randomised clinical trials.
Included studies
The two randomised clinical trials are presented in the Characteristics of included studies tables.
Raia 1994 and Gawish 2000 randomised a total of 154 participants diagnosed with hepatosplenic schistosomiasis. Raia 1994 randomised 94 participants into three intervention groups (proximal splenorenal shunt versus distal splenorenal shunt versus oesophagogastric devascularisation with splenectomy), and Gawish 2000 randomised 60 participants into two intervention groups (distal splenorenal shunt versus oesophagogastric devascularisation with splenectomy). The age range of the participants was 18 years to 55 years in the Raia 1994 trial and 23 years to 65 years in the Gawish 2000 trial. Raia 1994 was conducted in Brazil, and Gawish 2000 in Egypt. Due to increased postprocedural encephalopathy events in the proximal splenorenal shunt group, Raia 1994 was terminated at the end of the second year of recruitment, however participants were followed up for 10 years. None of the trials performed their analyses using the intention‐to‐treat principle. Raia 1994 was funded by an institutional grant; the source of funding was not mentioned in Gawish 2000. The diagnosis of hepatosplenic schistosomiasis was made based on clinical and biochemical assessments in both trials. Liver biopsy was performed during the time of surgical intervention in order to exclude cirrhosis.
Excluded studies
Following review of the full‐text articles, we excluded 10 studies with reasons (Characteristics of excluded studies). We excluded nine studies that included cirrhotic participants (Callow 1970; Jackson 1971; Galambos 1976; House 1980; Fischer 1981; Langer 1985; Nussbaum 1993; Mercado 1996; Xiong 2002). One study was a prospective and non‐randomised study that evaluated haemodynamic changes following surgical interventions in hepatosplenic schistosomiasis but failed to report harms of the interventions (de Cleva 2007).
Risk of bias in included studies
We assessed both included trials as at overall high risk of bias because we one or more 'Risk of bias' domains was at either unclear or high risk of bias (Figure 2; Figure 3).
Figure 2.
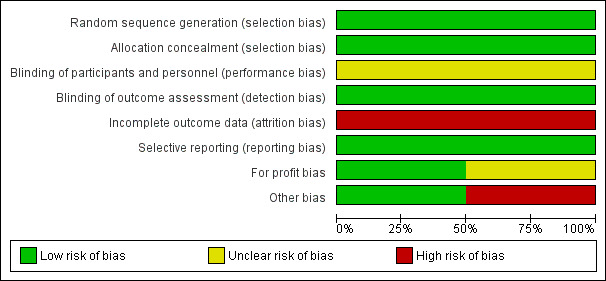
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
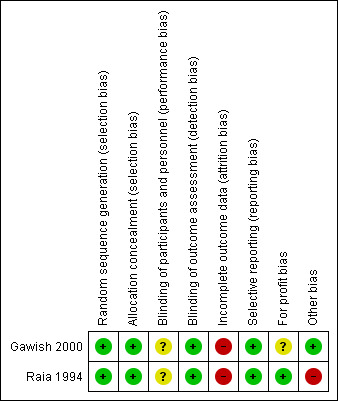
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged the methods of allocation sequence generation and allocation concealment to be adequate for Raia 1994 and Gawish 2000, so that both trials were at low risk of selection bias.
Blinding
We judged both trials to be at high risk of performance and detection bias because they did not mention if blinding was performed.
Incomplete outcome data
Raia 1994 excluded all participants with missing data in their analysis. Gawish 2000 excluded all participants for whom procedure was regarded as not properly done and replaced them with others. Neither the method of selection of these replacement participants nor the adverse events in the participants who were replaced was clearly documented in their publication. We contacted the author, and he replied that hospital records of participants could no longer be retrieved because it had been a long time since the trial was conducted. We judged both trials to be at high risk of attrition bias.
Selective reporting
We found no evidence of published protocols for the two trials, but it appears that the authors of each trial reported all outcomes in their method. Therefore, we judged both trials to be at low risk of reporting bias.
Other potential sources of bias
For‐profit bias
We assessed possible funding sources for both trials. It was clear that Raia 1994 obtained funding through an institutional grant, but it was not clear how Gawish 2000 obtained their funding.
Early‐stopping bias
Raia 1994 terminated recruitment of participants after two years because of concerns about an increased number of participants developing encephalopathy in the proximal splenorenal shunt group. We assessed this trial as at risk of early‐stopping bias. Gawish 2000 did not mention early stopping of their trial.
Effects of interventions
See: Table 1
Surgical portosystemic shunts versus devascularisation with splenectomy
All‐cause mortality
Both trials reported on mortality (Raia 1994; Gawish 2000). None of the trials reported mortality at 30 days or one year, therefore we could not perform these analyses as planned in our protocol. The maximum follow‐up was five years in Gawish 2000 and 10 years in Raia 1994. We found no evidence of a difference between the shunts (analysed together) versus devascularisation with splenectomy at 10‐year follow‐up (risk ratio (RR) 2.35, 95% confidence interval (CI) 0.55 to 9.92; participants = 154; studies = 2; I2 = 16%; Analysis 1.1). The test for subgroup differences showed no difference (P = 0.23).
Analysis 1.1.
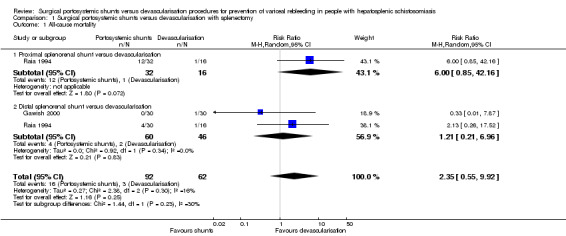
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 1 All‐cause mortality.
Serious adverse events
When reporting on adverse events, neither of the trials defined adverse events as serious or non‐serious (Raia 1994; Gawish 2000). Following the ICH‐GCP 1997 definition for serious adverse events, we determined that total shunt occlusion, total portal vein thrombosis, and death were serious adverse events (ICH‐GCP 1997). However, as death occurred after the occurrence of a serious adverse event, we did not include mortality in our analysis on serious adverse events in order to avoid double counting. We found no evidence of a difference between the shunts (analysed together) versus devascularisation with splenectomy on serious adverse events (RR 2.26, 95% CI 0.44 to 11.70; participants = 154; studies = 2; I2 = 0%; Analysis 1.2). The test for subgroup differences showed no difference (P = 0.55).
Analysis 1.2.
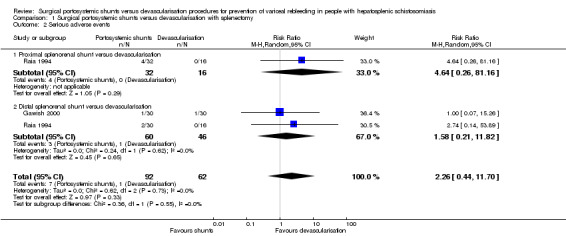
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 2 Serious adverse events.
We presented non‐serious adverse events in Table 3.
Table 1.
Non‐serious adverse events
| Non‐serious adverse events | Intervention | Study | Number of participants with an event | Total number of participants | Proportion (%) |
| Partial portal vein thrombosis | Devascularisation | Gawish 2000 | 17 | 30 | 57 |
| Haemoglobinaemia | Distal splenorenal shunt | Raia 1994 | 14 | 30 | 47 |
Quality of life
Neither of the included trials provided data on quality of life.
Variceal rebleeding
Both trials provided data on variceal rebleeding. We found no evidence of a difference between the shunts (analysed together) versus devascularisation with splenectomy in the rate of variceal rebleeding (RR 0.39, 95% CI 0.13 to 1.23; participants = 154; studies = 2; I2 = 0%; Analysis 1.3). The test for subgroup differences showed no difference (P = 0.31).
Analysis 1.3.
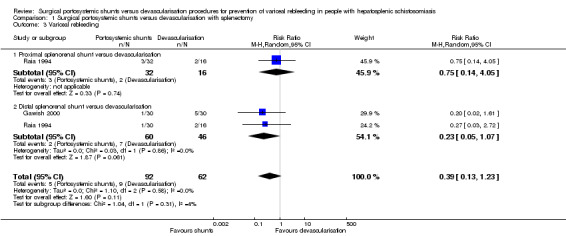
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 3 Variceal rebleeding.
Encephalopathy
Both trials reported on encephalopathy. We found evidence suggesting an increase in encephalopathy in the shunts (analysed together) group versus devascularisation with splenectomy group (RR 7.51, 95% CI 1.45 to 38.89; participants = 154; studies = 2; I2 = 0%; Analysis 1.4). The test for subgroup differences showed no difference (P = 0.69).
Analysis 1.4.
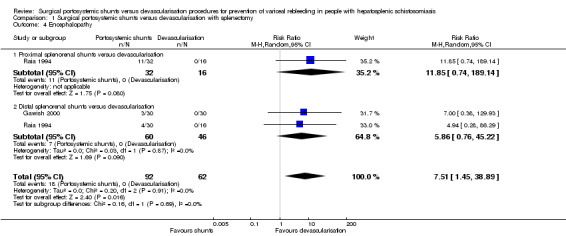
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 4 Encephalopathy.
Ascites
Only one trial reported on new onset of ascites (Gawish 2000). We found no evidence of a difference in the occurrence of ascites between the shunts (distal splenorenal) group versus devascularisation with splenectomy group using Review Manager 5 calculations (RR 0.11, 95% CI 0.01 to 1.98; participants = 60; studies = 1; I2 = 0%; Analysis 1.5), and also when we applied Fisher's exact P, two‐tailed test (P = 0.11).
Analysis 1.5.
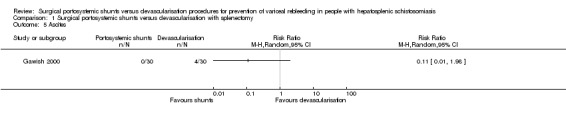
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 5 Ascites.
Any re‐intervention
Only one trial provided data on any re‐intervention (Gawish 2000). We found no evidence of a difference between the shunts (distal splenorenal) group versus devascularisation with splenectomy group on any re‐intervention using Review Manager 5 calculations ((RR 3.00, 95% CI 0.13 to 70.83; participants = 60; studies = 1; I2 = 0%; Analysis 1.6), and also when we applied Fisher's exact P, two‐tailed test (P = 0.5).
Analysis 1.6.
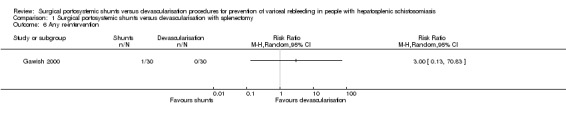
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 6 Any re‐intervention.
Subgroup analysis
We only performed a subgroup analysis of non‐selective (proximal splenorenal) shunts versus devascularisation procedures compared to selective surgical (distal splenorenal) shunts versus devascularisation procedures (see previous analyses (Analysis 1.1 through Analysis 1.4)).
We could not conduct the remaining prespecified subgroup analyses because of the paucity of data.
Sensitivity analysis
We performed a best‐worse case sensitivity analysis to evaluate the impact of missing participants on our estimates for all‐cause mortality at 10‐year follow‐up, finding no evidence of a difference between surgical portosystemic shunts versus oesophagogastric devascularisation procedures (RR 1.20, 95% CI 0.52 to 2.77; participants = 154; studies = 2; I2 = 0%; Analysis 1.7). We also found no evidence of a difference in a worst‐best case scenario analysis (RR 3.15, 95% CI 0.66 to 15.03; participants = 154; studies = 2; I2 = 30%; Analysis 1.8).
Analysis 1.7.
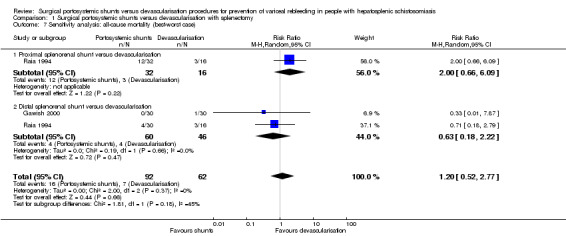
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 7 Sensitivity analysis: all‐cause mortality (best‐worst case).
Analysis 1.8.
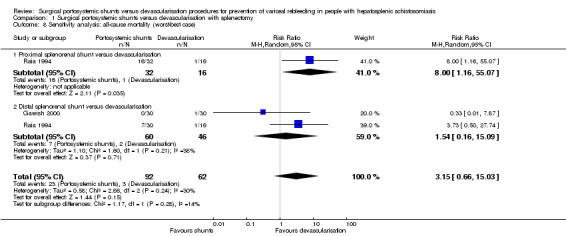
Comparison 1 Surgical portosystemic shunts versus devascularisation with splenectomy, Outcome 8 Sensitivity analysis: all‐cause mortality (worst‐best case).
Trial Sequential Analysis
We attempted to perform Trial Sequential Analysis for all of our review outcomes, but the alpha spending boundaries could not be drawn for any of them because of the small event rates and small sample size of the trials.
Certainty of the evidence
We have presented the certainty of the evidence in Table 1. We judged the overall certainty of the evidence of all review outcomes as very low because of the overall high risk of bias of the trials (downgraded two levels) and imprecision (downgraded two levels because of wide confidence intervals, small sample sizes, and few events). We did not downgrade the evidence for inconsistency, indirectness of evidence, and publication bias.
Discussion
Summary of main results
We identified only two small single‐centre randomised clinical trials at high risk of bias that compared surgical portosystemic shunts versus oesophagogastric devascularisation with splenectomy for the prevention of variceal rebleeding in 154 people with hepatosplenic schistosomiasis. The trials were conducted in Brazil and Egypt. The small event rates and small sample size of the trials prevented us from producing meaningful analyses and constructing Trial Sequential Analysis monitoring boundaries. Both trials compared non‐selective shunts (proximal splenorenal shunt) or selective shunts (distal splenorenal shunt) versus devascularisation with splenectomy. We found no evidence of a difference between shunts versus devascularisation with splenectomy on all‐cause mortality, serious adverse events (poorly reported), variceal rebleeding, ascites, and any re‐intervention. There appeared to be some evidence suggesting an increase in the development of encephalopathy in the shunts group in comparison to the devascularisation with splenectomy group. Neither of the trials provided data on health‐related quality of life. Our sensitivity analyses showed no evidence of a difference regarding all‐cause mortality when missing data were imputed in a best‐worse and worst‐best scenario, which again could be due to the insufficient sample size and few dropouts.
Due to the very low certainty of the evidence related to incomplete outcome data and small sample size, we are very uncertain of our outcome results.
Overall completeness and applicability of evidence
Given that we included only two trials at high risk of bias and random error with an insufficient number of participants, our confidence in the estimate of intervention effects is very low. One of the trials terminated recruitment prematurely because of concerns about an increased number of participants who developed encephalopathy in the proximal splenorenal shunt group, however our analysis did not show differences between proximal splenorenal shunts compared with distal splenorenal shunts for any of the review outcomes. The other trial included participants with Child‐Pugh class B that suggested coexistence of hepatic cirrhosis with hepatosplenic schistosomiasis. Although the trial authors used wedge liver biopsy and histopathological investigations to exclude liver cirrhosis, it is not uncommon to have coexistence of viral hepatitis with hepatosplenic schistosomiasis in communities where schistosomiasis is endemic (Gasim 2015). A coexistence of a disease may make a strict selection of people with isolated hepatosplenic schistosomiasis difficult to perform, thereby lowering the external validity of the trial results. The available evidence appeared to be inadequately powered to address our review questions.
Quality of the evidence
We judged both trials as at high risk of bias, mostly due to incomplete outcome data. One trial obtained an institutional grant, while the source of funding was not declared for the other trial.
We graded the overall certainty of the evidence for all outcomes as very low because of overall risk of bias and imprecision (due to small sample sizes of the individual trials and few events). Moreover, we are not able to exclude publication bias.
Potential biases in the review process
We performed an extensive search of databases according to Cochrane recommendations. We searched electronic databases for any randomised clinical trials including participants with hepatosplenic schistosomiasis who were treated with surgical portosystemic shunts or oesophagogastric devascularisation procedures to prevent variceal rebleeding. Our search strategies were very broad, including any language and publication date, and a vast number of references were retrieved (n = 1243), however only two randomised clinical trials fulfilled the inclusion criteria of our review. A reason for the paucity of trials of interest to our review could be the wide availability and popularity of non‐surgical interventions, such as endoscopy and transjugular intrahepatic portosystemic shunt (TIPS). Among the retrieved study references was one comparative observational study assessing shunts and devascularisation that included participants with hepatosplenic schistosomiasis, but the study did not report on harm (de Cleva 2007). The remaining study references and the respective references to the included trials did not provide any further references to the topic of our review. We found no relevant observational studies reporting on harm among the search results for randomised clinical trials either, and this is a known limitation for meta‐analyses with randomised clinical trials alone. The very small fraction of the required information size observed when we attempted to perform Trial Sequential Analysis also underlined the high risk of random error, again due to the paucity of trials and small number of participants. We could not construct funnel plots in order to look for publication bias. The mentioned biases thus contributed to the inconclusiveness of our review findings.
Agreements and disagreements with other studies or reviews
We found no systematic reviews comparing surgical portosystemic shunts versus oesophagogastric devascularisation procedures for prevention of variceal rebleeding in people with hepatosplenic schistosomiasis. However, we found two "systematic reviews with meta‐analyses" comparing shunts versus devascularisation procedures for the prevention of variceal rebleeding in people with hepatic cirrhosis and non‐cirrhotic conditions, including hepatosplenic schistosomiasis (Yin 2013; Zong 2015). Evaluating the studies included in these two meta‐analyses, we found out that Yin 2013 included 16 studies with 1042 participants from 1970 to 2010, but the majority of the studies were quasi‐randomised, and the study participants were not divided in terms of cirrhosis and hepatosplenic schistosomiasis. We also observed some discrepancies between the interpretation of the results of the statistical analysis and the data used for analysis in Yin 2013, which questions their reported findings that are otherwise very similar to our outcome results regarding the comparison of shunts versus devascularisation procedures. Zong 2015 included 11 quasi‐randomised studies with 1716 participants from 1980 to 2013, and found that when compared with devascularisation, shunts seemed to decrease variceal rebleeding and ascites, but increased encephalopathy. However, we observed similar statistical problems in Zong 2015 as with Yin 2013. Though the authors state that they followed Cochrane methods to produce their meta‐analyses, these reviews have nothing in common with the strict criteria and requirements of performing a Cochrane Review with meta‐analyses of randomised clinical trials, 'Risk of bias' assessment, etc.
Authors' conclusions
Given the very low certainty of the available body of evidence and the low number of clinical trials, we could not determine an overall benefit or harm of surgical portosystemic shunts compared with oesophagogastric devascularisation procedures for the prevention of variceal rebleeding in people with hepatosplenic schistosomiasis.
Future randomised clinical trials are required to assess the overall benefits and harms of surgical portosystemic shunts versus oesophagogastric devascularisations with or without splenectomy, and with or without oesophageal transection for prevention of variceal rebleeding in people with hepatosplenic schistosomiasis. Given that endoscopic therapy is accepted as first‐line therapy for the treatment and prevention of variceal rebleeding, future trials should be designed to randomise participants after initial endoscopic treatment into groups for repeat endoscopy or to surgery (either shunts or devascularisation procedures). Participants must be strictly Child‐Pugh class A and be properly screened to exclude all types of liver cirrhosis. Adequate information about the benefits and harms of each intervention should be provided to participants for informed consent. Outcomes should include health‐related quality of life and costs in order to ensure a balanced comparison. Also needed are randomised clinical trials and systematic reviews assessing these interventions individually versus sham surgery. Such trials should be multicentre located in schistosomiasis endemic areas in order to achieve sufficient statistical power to produce true intervention effects. These trials should be registered and given open access (Skoog 2015), with their protocol drafted according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement (Chan 2013), and their reporting according to the CONSORT statement (Schulz 2010).
Acknowledgements
We thank Christain Gluud and Sarah L. Klingenberg from the Cochrane Hepato‐Biliary Group for their professionalism and co‐operation.
CJE was supported by a fellowship offered by the Effective Health Care Research Consortium through the grant to Cochrane South Africa, South African Medical Research Council. This Consortium is funded by UK aid from the UK Government for the benefit low‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Peer reviewers: Delcio Matos, Brazil Contact editor: Steffani Trastulli, Italy Sign‐off editor: Christian Gluud, Denmark
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | 11 January 2018 | (((port*systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt* or anastomos*)) or ('dean warren shunt*' or H‐shunt* or PSS or devasculari*ation)) AND (varic* and (h*emorrhag* or bleed* or rebleed*)) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2017, Issue 12 | #1 MeSH descriptor: [Portasystemic Shunt, Surgical] explode all trees #2 ((port*systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt* or anastomos*)) or ('dean warren shunt*' or H‐shunt* or PSS or devasculari*ation) #3 #1 or #2 #4 MeSH descriptor: [Esophageal and Gastric Varices] explode all trees #5 MeSH descriptor: [Schistosomiasis] explode all trees #6 varic* and (h*emorrhag* or bleed* or rebleed*) #7 #4 or #5 or #6 #8 #3 and #7 |
| MEDLINE Ovid | 1946 to 11 January 2018 | 1. exp Portasystemic Shunt, Surgical/ 2. (((port*systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt* or anastomos*)) or ('dean warren shunt*' or H‐shunt* or PSS or devasculari*ation)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp "Esophageal and Gastric Varices"/ 5. exp Schistosomiasis/ 6. (varic* and (h*emorrhag* or bleed* or rebleed*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 7. 4 or 5 or 6 8. 3 and 7 9. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 10. 8 and 9 |
| Embase Ovid | 1974 to 11 January 2018 | 1. exp portosystemic anastomosis/ 2. (((port*systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt* or anastomos*)) or ('dean warren shunt*' or H‐shunt* or PSS or devasculari*ation)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp esophagus varices/ 5. exp schistosomiasis/ 6. (varic* and (h*emorrhag* or bleed* or rebleed*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 7. 4 or 5 or 6 8. 3 and 7 9. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 10. 8 and 9 |
| Science Citation Index Expanded (Web of Science) | 1900 to 11 January 2018 | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(varic* and (h*emorrhag* or bleed* or rebleed*)) #1 TS=(((port*systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt* or anastomos*)) or ('dean warren shunt*' or H‐shunt* or PSS or devasculari*ation)) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to 11 January 2018 | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(varic* and (h*emorrhag* or bleed* or rebleed*)) #1 TS=(((port*systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt* or anastomos*)) or ('dean warren shunt*' or H‐shunt* or PSS or devasculari*ation)) |
| LILACS (Bireme) | 1982 to 11 January 2018 | ((port$systemic or portacaval or mesocaval or splenorenal or surgical or selective or non‐selective or partial or total) and (shunt$ or anastomos$)) or (dean warren shunt$ or H‐shunt$ or PSS or devasculari$ation) [Words] and varic$ and (h$emorrhag$ or bleed$ or rebleed$) |
Appendix 2. Data collection form
Data Extraction Form
Review title: Surgical portosystemic shunts versus devascularisation procedures for variceal bleeding due to hepatosplenic schistosomiasis
Review authors: 1. Chikwendu J Ede; 2. Martin Brand
Date:
Study title:
| First author | Journal/Conference Proceedings | Date of Publication |
Contact address first author:
Email address first author:
Source of sponsorship:
Study eligibility
| RCT | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear | Yes / No / Unclear | Yes / No / Unclear | Yes / No* / Unclear |
(* Possible selective reporting bias. Awaiting assessment until clarified with trial authors.)
| Inclusion criteria: | |
| Exclusion criteria: |
Demographic data
| Participant characteristics | ||||||
| Whole study (N) | Shunts | Devascularisation | Combined/Others | |||
| Non‐selective | Selective | OGD | OGDS | |||
| Age (mean±SD, median, range, ) | ||||||
| Sex of participants (n) (Male/Female) | Male/Female | |||||
| Child‐Pugh Class(A,B,C) | ||||||
PSRS= proximal splenorenal shunt DSRS= distal splenorenal shunt
OGD = Oesophagogastric devascularisation alone. OGDS= oesophagogastric devascularisation with splenectomy
| Shunts | Devascularisation | Combined/Others | |||
| Parameters of Liver function before intervention. (Mean ± SD) | Non‐selective | Selective | OGD | OGDS | |
| Total bilirubin | |||||
| Conjugated bilirubin | |||||
| AST | |||||
| ALT | |||||
| Prothrombin time/ INR | |||||
| Serum Albumin | |||||
| Others | |||||
| Parameters of Liver function after intervention. (Mean±SD) | |||||
| Total bilirubin | |||||
| Conjugated bilirubin | |||||
| AST | |||||
| ALT | |||||
| Prothrombin time/INR | |||||
| Serum Albumin | |||||
| Others | |||||
Trial characteristics
Study design: Ο Parallel group Ο Cross‐over Ο Open label
Comments:
Intervention: Ο Treatment Ο Other
| Trial characteristics | |
| Further details | |
| Single centre/Multicentre | |
| Country/Countries | |
| Number of participant recruited | |
| Number excluded before randomisation | |
| Reasons for exclusion | |
| Number randomised | |
| Number of participants in each intervention group (Shunt versus devascularisation) | |
| Number of participants who received intended treatment | |
| Maximum follow‐up duration ( weeks, months or years,) | |
| Number of participants lost to follow‐up | |
| Time from bleeding to randomisation (mean ± SD; range) | |
| Method to establish diagnosis hepatosplenic schistosomiasis. | |
Risk of bias
| Random sequence generation | |
| Method: | Grade (circle) |
| Sequence generation was achieved using computer random number generation or a random number table. Drawing lots, coin tossing, shuffling cards or envelopes, and throwing dice by an independent person | Yes/Unclear/No |
| Allocation concealment | |
| Method: | Grade (circle) |
| The participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes) | Yes/Unclear/No |
| Blinding of participants and personnel | ||
| Method: | Grade (circle) | |
| Blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding | Yes/Unclear/No | |
| Blinding of outcome assessment | ||
| Method: | Grade (circle) | |
| Blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding | Yes/Unclear/No | |
| Incomplete outcome data (Yes/No) | ||
| Method: | Grade (circle) | |
| Missing data were unlikely to make treatment effects depart from plausible values. | Yes/Unclear/No* (* Trial authors to be contacted for information on missing data) |
|
| What method was used to handle missing data? | ||
| Selective outcome reporting | ||
| Method | Grade (circle) | |
| Study protocol available and all pre‐specified outcomes of interest in the review have been reported in the pre‐specified way | Yes(Low risk) / No(High risk / Unclear | |
| Study protocol is not available but is clear that published reports include all expect outcomes, including those that were pre‐specified | Yes / No / Unclear | |
| For‐profit bias | ||
| Method: | Grade | |
| The study is free of industry sponsorship or other for profit support that may manipulate design, conductance or result. | Yes/No/Unclear | |
| Other bias | ||
| Study appears to be free of other sources of bias | Yes / No / Unclear | |
| Give example. | ||
Were withdrawals described? Yes ..... No..... Not Clear......
Discuss if appropriate…………………………………………………………………………………………
…………………………………………………………………………………………………………
Data extraction
| Primary outcomes |
| Secondary outcomes |
| Other outcomes |
Results
Seious adverse events: Described Yes No
If Yes Procedure related Overall
Serious adverse events (SAE):
Number of SAE :
| Number | Shunts | Devascularization | Combined procedure | Total | ||
| Non‐selective | Selective | OGD | OGDS | |||
| Length of Hospital stay due to adverse events (Mean, Standard deviation, and median) | ||||||
| Total | ||||||
Comments:
Withdrawals due to serious adverse events:
| Shunts | Devascularisation | Combined | |
| Number of withdrawals |
Outcomes for Patient Subgroups: specify subgroups
Outcome
| For Dichotomous data | |||||
| Unit of measurement (n) |
Shunts | Devascularisation | Details if outcome only described in text | ||
| Non‐selective | Selective | OGD | OGDS | ||
n = number of participants, not number of events
| For Continuous data | |||||
| Unit of measurement (Mean ± SD) |
Shunts | Devascularisation | Details if outcome only described in text | ||
| Non‐selective | Selective | OGD | OGDS | ||
| Other information which are relevant to the results or any other comment that should be followed up |
Data and analyses
Comparison 1.
Surgical portosystemic shunts versus devascularisation with splenectomy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [0.55, 9.92] |
| 1.1 Proximal splenorenal shunt versus devascularisation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 6.0 [0.85, 42.16] |
| 1.2 Distal splenorenal shunt versus devascularisation | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.21, 6.96] |
| 2 Serious adverse events | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.44, 11.70] |
| 2.1 Proximal splenorenal shunt versus devascularisation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 4.64 [0.26, 81.16] |
| 2.2 Distal splenorenal shunt versus devascularisation | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.21, 11.82] |
| 3 Variceal rebleeding | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.13, 1.23] |
| 3.1 Proximal splenorenal shunt versus devascularisation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.14, 4.05] |
| 3.2 Distal splenorenal shunt versus devascularisation | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.05, 1.07] |
| 4 Encephalopathy | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 7.51 [1.45, 38.89] |
| 4.1 Proximal splenorenal shunts versus devascularisation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 11.85 [0.74, 189.14] |
| 4.2 Distal splenorenal shunts versus devascularisation | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 5.86 [0.76, 45.22] |
| 5 Ascites | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Any re‐intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Sensitivity analysis: all‐cause mortality (best‐worst case) | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.52, 2.77] |
| 7.1 Proximal splenorenal shunt versus devascularisation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.66, 6.09] |
| 7.2 Distal splenorenal shunt versus devascularisation | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.18, 2.22] |
| 8 Sensitivity analysis: all‐cause mortality (worst‐best case) | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.66, 15.03] |
| 8.1 Proximal splenorenal shunt versus devascularisation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 8.00 [1.16, 55.07] |
| 8.2 Distal splenorenal shunt versus devascularisation | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.16, 15.09] |
Differences between protocol and review
We changed the title of the published protocol from "Surgical portosystemic shunts versus devascularisation procedures for variceal bleeding due to hepatosplenic schistosomiasis" to "Surgical portosystemic shunts versus devascularisation procedures for prevention of variceal rebleeding in people with hepatosplenic schistosomiasis".
We improved the wording of the review objectives as follows: "To assess the benefits and harms of surgical portosystemic shunts versus oesophagogastric devascularisation procedures for prevention of variceal rebleeding in people with hepatosplenic schistosomiasis". (The protocol objectives read: "To determine if surgical portosystemic shunts have better overall outcomes compared with oesophagogastric devascularisation procedures in the prevention of variceal rebleeding due to schistosomal portal hypertension".)
We also improved the wording of the Background and Methods sections to improve readability and to follow Cochrane recommendations.
We moved the outcome "variceal rebleeding rate (diagnosed clinically by haematemesis, melena, or blood in gastric aspirate, and confirmed by endoscopy)" to the secondary outcomes to comply with Cochrane recommendations for three primary outcomes.
We added Dimitrinka Nikolova as an author because of her invaluable contributions in writing and discussing the review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial | |
| Participants |
|
|
| Interventions | Distal splenorenal shunt (30 participants) versus oesophagogastric devascularisation with splenectomy (30 participants) | |
| Outcomes | Variceal rebleeding, duplex data, and encephalopathy | |
| Notes | Duplex data include:
We contacted 1 of the publication authors (Youssri Gaweesh) on 21 October 2015 by post and received a reply via email on 25 October 2015. Hepatic schistosomiasis was confirmed by liver biopsy, and cirrhosis was excluded. It is not clear why some participants were Child B class. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The word "randomization" was used in the study. This was also confirmed through personal communication with the trial author. |
| Allocation concealment (selection bias) | Low risk | The authors used sealed envelopes to conceal participant allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not mentioned in the trial publication if any blinding was done. Given the nature of the interventions, it was unrealistic to blind participants and study personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) (Mortality, Variceal rebleeding, Encephalopathy) | Low risk | It was not mentioned in the trial if any blinding was done. We concluded that outcome assessment was unlikely to be influenced by lack of blinding since all outcomes were objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants lost to follow‐up were replaced: 7 participants in the devascularisation and 6 participants in the shunt group. However, the method of replacement or potential adverse events experienced by these participants were not mentioned. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was not available, but the outcomes reported were the same as those predefined in the methods section. |
| For profit bias | Unclear risk | The source of funding was unclear. |
| Other bias | Low risk | 'Trial stopped early for benefit or harm': the trial was run as planned. |
| Methods | Randomised clinical trial | |
| Participants |
|
|
| Interventions | Proximal splenorenal shunt (32 participants); distal splenorenal shunt (30 participants); and oesophagogastric devascularisation with splenectomy (32 participants) | |
| Outcomes | Survival, variceal rebleeding, and adverse events: encephalopathy and haemolysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This trial used a random number table to randomise participants. |
| Allocation concealment (selection bias) | Low risk | This trial used sealed envelopes to conceal participant allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not mentioned in the trial if any blinding was performed. Given the nature of the interventions, it was unrealistic to blind participants and study personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) (Mortality, Variceal rebleeding, Encephalopathy) | Low risk | It was not mentioned in the trial if any blinding was performed. We concluded that outcome assessment was unlikely to be influenced by lack of blinding since all outcomes were objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants lost to follow‐up were excluded in the computation of intervention effect. |
| Selective reporting (reporting bias) | Low risk | The protocol was not available, but all outcomes in the methods section were reported on. |
| For profit bias | Low risk | The trial obtained an institutional grant, therefore we assumed it to be free of industry sponsorship. |
| Other bias | High risk | 'Trial stopped early for benefit or harm': recruitment of trial participants was terminated after 2 years due to concerns about an increased number of participants who developed encephalopathy in the proximal splenorenal shunt group. The trial was thus stopped early for harm. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Callow 1970 | Included cirrhotic participants |
| de Cleva 2007 | Prospective observational study of haemodynamic changes associated with different interventions. No harms reported. |
| Fischer 1981 | Included cirrhotic participants |
| Galambos 1976 | Included cirrhotic participants |
| House 1980 | Included cirrhotic participants |
| Jackson 1971 | Included cirrhotic participants |
| Langer 1985 | Included cirrhotic participants |
| Mercado 1996 | Included cirrhotic participants |
| Nussbaum 1993 | Included cirrhotic participants |
| Xiong 2002 | Included cirrhotic participants |
Contributions of authors
CJE drafted the review. CJE and MB conducted the searches and data extraction. CJE entered the data into Review Manager 5 and performed data analysis and interpretation. CJE, DN, and MB discussed and wrote the review. CJE, DN, and MB approved the review.
Sources of support
Internal sources
-
None, Other.
None
External sources
-
None, Other.
None
Declarations of interest
CJE has nothing to declare. DN has nothing to declare. MB has nothing to declare.
Notes
None.
New
References
References to studies included in this review
- Gawish Y, El‐Hammadi HAB, Kotb M, Awad AT, Anwar M. Devascularization procedure and DSRS: a controlled randomized trial on selected haemodynamic portal flow pattern in schistosomal portal hypertension with variceal bleeding. International Surgery 2000;85(4):325‐30. [PubMed] [Google Scholar]
- Raia S, Silva LC, Gayotto LCC, Forster SC, Fukushima J, Strauss E. Portal hypertension in schistosomiasis: a long‐term follow‐up of a randomized trial comparing three types of surgery. Hepatology (Baltimore, Md.) 1994;20(2):398‐403. [PubMed] [Google Scholar]
References to studies excluded from this review
- Callow AD, Resnick RH, Chalmers TC, Ishihara AM, Garceau AJ, O'Hara ET. Conclusions from a controlled trial of the prophylactic portacaval shunt. Surgery 1970;67(1):97‐103. [PUBMED: 5308037] [PubMed] [Google Scholar]
- Cleva R, Herman P, Carneiro D'Albuquerque LA, Pugliese V, Santarem OL, Saad WA. Pre‐ and postoperative systemic hemodynamic evaluation in patients subjected to esophagogastric devascularization plus splenectomy and distal splenorenal shunt: a comparative study in schistomomal portal hypertension. World Journal of Gastroenterology 2007;13(41):5471‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JE, Bower RH, Atamian S, Welling R. Comparison of distal and proximal splenorenal shunts: a randomized prospective trial. Annals of Surgery 1981;194(4):531‐44. [PUBMED: 6974543] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos JT, Warren WD, Rudman D, Smith RB 3rd, Salam AA. Selective and total shunts in the treatment of bleeding varices. A randomized controlled trial. New England Journal of Medicine 1976;295(20):1089‐95. [PUBMED: 1086428] [DOI] [PubMed] [Google Scholar]
- House AK, Turner VF. Bleeding oesophageal varices treated by a selective or total protal systemic shunt. Journal of the Royal College of Surgeons of Edinburgh 1980;25(2):117‐24. [PUBMED: 6965985] [PubMed] [Google Scholar]
- Jackson FC, Perrin EB, Felix WR, Smith AG. A clinical investigation of the portacaval shunt. V. Survival analysis of the therapeutic operation. Annals of Surgery 1971;174(4):672‐701. [PUBMED: 5098227] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer B, Taylor BR, Mackenzie DR, Gilas T, Stone RM, Blendis L. Further report of a prospective randomized trial comparing distal splenorenal shunt with end‐to‐side portacaval shunt. An analysis of encephalopathy, survival, and quality of life. Gastroenterology 1985;88(2):424‐9. [PUBMED: 3880715] [DOI] [PubMed] [Google Scholar]
- Mercado MA, Morales‐Linares JC, Granados‐Garcia J, Gomez‐Mendez TJ, Chan C, Orozco H. Distal splenorenal shunt versus 10‐mm low‐diameter mesocaval shunt for variceal hemorrhage. American Journal of Surgery 1996;171(6):591‐5. [PUBMED: 8678206] [DOI] [PubMed] [Google Scholar]
- Nussbaum MS, Schoettker PJ, Fischer JE. Comparison of distal and proximal splenorenal shunts: a ten‐year experience. Surgery 1993;114(4):659‐65; discussion 665‐6. [PUBMED: 8211679] [PubMed] [Google Scholar]
- Xiong BX, Xie M, Sun JY, Li HL. Effect of combined operation and portaazygous devascularization in the treatment of esophageal variceal bleeding. Acta Academiae Medicinae Jiangxi VL 2001;41(5):63‐5. [Google Scholar]
Additional references
- Abdel‐Wahab MF, Strickland GT. Abdominal ultrasonography for assessing morbidity from schistosomiasis. 2. Hospital studies. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(2):135‐7. [PUBMED: 8337709] [DOI] [PubMed] [Google Scholar]
- Andersson KL, Chung RT. Hepatic schistosomiasis. Current Treatment Options in Gastroenterology 2007;10:504‐12. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PubMed] [Google Scholar]
- Bhargava DK, Dasarathy S, Atmakuri SP, Dwivedi M. Comparative efficacy of emergency endoscopic sclerotherapy for active variceal bleeding due to cirrhosis of the liver, non‐cirrhotic portal fibrosis and extrahepatic portal venous obstruction. Journal of Gastroenterology and Hepatology 1990;5:432‐7. [DOI] [PubMed] [Google Scholar]
- Bica I, Hamer DH, Stadecker MJ. Hepatic schistosomiasis. Infectious Disease Clinics of North America 2000;14:583‐604. [DOI] [PubMed] [Google Scholar]
- Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology (Baltimore, Md.) 2010;51:306. [DOI] [PubMed] [Google Scholar]
- Brandt CT, Caneca OA, Tavares DJ, Avila Junior L. Surgical hepatosplenic schistosomiasis mansoni in children: a Doppler duplex study of the portal vein and the hepatic artery. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(1):70‐1. [PUBMED: 7747313] [DOI] [PubMed] [Google Scholar]
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunology 2009;31(4):163‐76. [PUBMED: 19292768] [DOI] [PubMed] [Google Scholar]
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology (Baltimore, Md.) 2004;40:652‐9. [DOI] [PubMed] [Google Scholar]
- Castellini G, Nielsen EE, Gluud C. Comment on: "Cell therapy for heart disease: trial sequential analyses of two Cochrane reviews". Clinical Pharmacology and Therapeutics 2017;102(1):21‐4. [DOI] [PubMed] [Google Scholar]
- Cella DF. Measuring quality of life in palliative care. Seminars in Oncology 1995;22:73‐81. [PubMed] [Google Scholar]
- Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. American Journal of Gastroenterology 2003;98:653‐9. [DOI] [PubMed] [Google Scholar]
- Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ (Clinical Research Ed.) 2013;346:e7586. [PUBMED: 23303884] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Problems in Clinical Surgery 1964;1:1‐85. [PubMed] [Google Scholar]
- Chofle AA, Jaka H, Koy M, Smart LR, Kabangila R, Ewings FM, et al. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infectious Diseases 2014;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunology 2014;36(8):347‐57. [PUBMED: 25142505] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014;383(9936):2253‐64. [PUBMED: 24698483] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn HO. Transjugular intrahepatic portal‐systemic shunts: the state of the art. Hepatology (Baltimore, Md.) 1993;17:148‐58. [PubMed] [Google Scholar]
- Conn HO. A randomized comparison of three types of surgery in schistosomal portal hypertension: many fewer answers than questions. Hepatology (Baltimore, Md.)1994; Vol. 20, issue 2:526‐8. [PUBMED: 8045513] [PubMed]
- Costa G, Cruz RJ Jr, Abu‐Elmagd KM. Surgical shunt versus TIPS for treatment of variceal hemorrhage in the current era of liver and multivisceral transplantation. Surgical Clinics of North America 2010;90:891‐905. [DOI] [PubMed] [Google Scholar]
- Costa Lacet CM, Neto JB, Ribeiro LT, Oliveira FS, Wyszomirska RF, Strauss E. Schistosomal portal hypertension: randomized trial comparing endoscopic therapy alone or preceded by esophagogastric devascularization and splenectomy. Annals of Hepatology 2016;15(5):738‐44. [PUBMED: 27493113] [DOI] [PubMed] [Google Scholar]
- D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta‐analytic review. Hepatology (Baltimore, Md.) 1995;22:332‐54. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Franchis R. Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology (Baltimore, Md.) 2003;38:599‐612. [DOI] [PubMed] [Google Scholar]
- Silva LC, Carrilho FJ. Hepatosplenic schistosomiasis. Pathophysiology and treatment. Gastroenterology Clinics of North America 1992;21:163‐77. [PubMed] [Google Scholar]
- Cock KM, Awadh S, Raja RS, Wankya BM, Lucas SB. Esophageal varices in Nairobi, Kenya: a study of 68 cases. American Journal of Tropical Medicine and Hygiene 1982;31:579‐88. [DOI] [PubMed] [Google Scholar]
- Cock KM. Hepatosplenic schistosomiasis: a clinical review. Gut 1986;27:734‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of Hepatology 2010;53:762‐8. [DOI] [PubMed] [Google Scholar]
- Deng D, Liao MS, Qin JP, Li XA. Relationship between pre‐TIPS hepatic hemodynamics and postoperative incidence of hepatic encephalopathy. Hepatobiliary & Pancreatic Diseases International 2006;5:232‐6. [PubMed] [Google Scholar]
- Denié C, Vachiery F, Elman A, Soupison T, Gadano A, Moreau R, et al. Systemic and splanchnic hemodynamic changes in patients with hepatic schistosomiasis. Liver 1996;16:309‐12. [DOI] [PubMed] [Google Scholar]
- Eesa M, Clark T. Transjugular intrahepatic portosystemic shunt: state of the art. Seminars in Roentgenology 2011;46:125‐32. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista‐Neto J, Pereira FF, Franca ST, Amaral FJ, Brandt CT, Fonseca‐Neto OC, et al. Splenectomy and gastric vein ligature in hepatosplenic schistosomiasis: effects upon esophageal variceal pressure and endoscopic risk factors of esophageal variceal bleeding. Arquivos Brasileiros de Cirurgia Digestiva 2012;25:41‐8. [DOI] [PubMed] [Google Scholar]
- Ezzat FA, Abu‐Elmagd KM, Aly MA, Fathy OM, el‐Ghawlby NA, el‐Fiky AM, et al. Selective shunt versus nonshunt surgery for management of both schistosomal and nonschistosomal variceal bleeders. Annals of Surgery1990; Vol. 212, issue 1:97‐108. [DOI] [PMC free article] [PubMed]
- Fisher RA. On the interpretation of X^2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87‐94. [Google Scholar]
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [PUBMED: 15878471] [DOI] [PubMed] [Google Scholar]
- Garcia‐Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. New England Journal of Medicine 2010;362:823‐32. [DOI] [PubMed] [Google Scholar]
- Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md.) 2017;65(1):310‐35. [PUBMED: 27786365] [DOI] [PubMed] [Google Scholar]
- Gasim GI, Bella A, Adam I. Schistosomiasis, hepatitis B and hepatitis C co‐infection. Virology Journal 2015;12:19. [PUBMED: 25889398] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg RJ, Waters PF, Zeldin RA, Spratt EH, Shandling B, Stone RM, et al. A modified Sugiura procedure. Annals of Thoracic Surgery 1982;34:258‐64. [DOI] [PubMed] [Google Scholar]
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2018, Issue 3. Art. No.: LIVER. [PubMed]
- Goff JS. Gastroesophageal varices: pathogenesis and therapy of acute bleeding. Gastroenterology Clinics of North America 1993;22:779‐800. [PubMed] [Google Scholar]
- Gonzalez R, Zamora J, Gomez‐Camarero J, Molinero LM, Banares R, Albillos A. Meta‐analysis: combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Annals of Internal Medicine 2008;149:109‐22. [DOI] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT. Version accessed January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
- Gur I, Diggs BS, Orloff SL. Surgical portosystemic shunts in the era of TIPS and liver transplantation are still relevant. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16:481‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JAC. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
- Hassab MA. Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations. Surgery 1967;61:169‐76. [PubMed] [Google Scholar]
- Hatz C, Jenkins JM, Ali QM, Abdel‐Wahab MF, Cerri GG, Tanner M. A review of the literature on the use of ultrasonography in schistosomiasis with special reference to its use in field studies. 2. Schistosoma mansoni. Acta Tropica 1992;51(1):15‐28. [PUBMED: 1351352] [DOI] [PubMed] [Google Scholar]
- Henderson JM. Surgical methods for the prevention of first and recurrent variceal bleeding. Zeitschrift für Gastroenterologie 1988;26(Suppl 2):49‐53. [PubMed] [Google Scholar]
- Henderson JM, Yang Y. Is there still a role for surgery in bleeding portal hypertension?. Nature Clinical Practice. Gastroenterology & Hepatology 2005;2(6):246‐7. [PUBMED: 16265209] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ (Clinical Research Ed.) 2012;344:e1119. [PUBMED: 22371859] [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ: Canadian Medical Association Journal 2013;185(4):E201‐11. [PUBMED: 23359047] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrobjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub‐studies. International Journal of Epidemiology 2014;43(4):1272‐83. [PUBMED: 24881045] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Rasmussen JV, Hilden J, et al. Observer bias in randomized clinical trials with time‐to‐event outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. International Journal of Epidemiology 2014;43(3):937‐48. [PUBMED: 24448109] [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
- Inokuchi K. Present status of surgical treatment of esophageal varices in Japan: a nationwide survey of 3,588 patients. World Journal of Surgery 1985;9:171‐80. [DOI] [PubMed] [Google Scholar]
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Rikkers LF. Transabdominal esophagogastric devascularization as treatment for variceal hemorrhage. Surgery 1996;120:641‐7; discussion 647‐9. [DOI] [PubMed] [Google Scholar]
- Johnson M, Rajendran S, Balachandar TG, Kannan D, Jeswanth S, Ravichandran P, et al. Transabdominal modified devascularization procedure with or without esophageal stapler transection ‐ an operation adequate for effective control of a variceal bleed. Is esophageal stapler transection necessary?. World Journal of Surgery 2006;30:1507‐18. [DOI] [PubMed] [Google Scholar]
- Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD000553.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiire CF. Controlled trial of propranolol to prevent recurrent variceal bleeding in patients with non‐cirrhotic portal fibrosis. BMJ (Clinical Research Ed.) 1989;298:1363‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [DOI] [PubMed] [Google Scholar]
- Lebrec D, Fleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology 1980;79(6):1139‐44. [PUBMED: 6969201] [PubMed] [Google Scholar]
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
- Mudawi HM, Ibrahim KB. Endoscopic variceal sclerotherapy in patients with Symmers periportal fibroses. Tropical Doctor 2007;37:179‐81. [DOI] [PubMed] [Google Scholar]
- Orloff MJ, Isenberg JI, Wheeler HO, Daily PO, Girard B. Budd‐Chiari syndrome revisited: 38 years' experience with surgical portal decompression. Journal of Gastrointestinal Surgery 2012;16:286‐300. [DOI] [PubMed] [Google Scholar]
- Orozco H, Mercado MA, Takahashi T, Hernandez‐Ortiz J, Capellan JF, Garcia‐Tsao G. Elective treatment of bleeding varices with the Sugiura operation over 10 years. American Journal of Surgery 1992;163:585‐9. [DOI] [PubMed] [Google Scholar]
- Orozco H, Mercado MA, Martinez R, Tielve M, Chan C, Vasquez M, et al. Is splenectomy necessary in devascularization procedures for treatment of bleeding portal hypertension?. Archives of Surgery 1998;133:36‐8. [DOI] [PubMed] [Google Scholar]
- Pal S. Current role of surgery in portal hypertension. Indian Journal of Surgery 2012;74(1):55‐66. [PUBMED: 23372308] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L, Turner‐Moss E, Mutengo M, Asombang AW, Kelly P. Prevalence of schistosome antibodies with hepatosplenic signs and symptoms among patients from Kaoma, Western Province, Zambia. BMC Research Notes 2013;6:344. [PUBMED: 23987918] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia A, Ancona E, Battaglia G. A new technique for the treatment of esophageal bleeding in portal hypertension. International Surgery 1980;65:401‐4. [PubMed] [Google Scholar]
- Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60:646‐9. [DOI] [PubMed] [Google Scholar]
- Qazi SA, Khalid K, Hameed AM, Al‐Wahabi K, Galul R, Al‐Salamah SM. Transabdominal gastro‐esophageal devascularization and esophageal transection for bleeding esophageal varices after failed injection sclerotherapy: long‐term follow‐up report. World Journal of Surgery 2006;30:1329‐37. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, Cochrane. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, Cochrane, 2014.
- Rikkers LF. The changing spectrum of treatment for variceal bleeding. Annals of Surgery 1998;228:536‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemurgy AS, Frohman HA, Teta AF, Luberice K, Ross SB. Prosthetic H‐graft portacaval shunts vs transjugular intrahepatic portasystemic stent shunts: 18‐year follow‐up of a randomized trial. Journal of the American College of Surgeons 2012;214:445‐53; discussion 453‐5. [DOI] [PubMed] [Google Scholar]
- Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. New England Journal of Medicine 2002;346:1212‐20. [DOI] [PubMed] [Google Scholar]
- Rossle M, Haag K, Ochs A, Sellinger M, Noldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic stent‐shunt procedure for variceal bleeding. New England Journal of Medicine 1994;330:165‐71. [DOI] [PubMed] [Google Scholar]
- Rossle M, Siegerstetter V, Euringer W, Olschewski M, Kromeier J, Kurz K, et al. The use of a polytetrafluoroethylene‐covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): long‐term follow‐up of 100 patients. Acta Radiologica 2006;47:660‐6. [DOI] [PubMed] [Google Scholar]
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19:591‐603. [DOI] [PubMed] [Google Scholar]
- Saad AM, Homeida M, Eltom I, Nash T, Bennett JL, Hassan MA. Oesophageal varices in a region of the Sudan endemic for Schistosoma mansoni. British Journal of Surgery 1991;78:1252‐3. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology 2008;134:1715‐28. [DOI] [PubMed] [Google Scholar]
- Sarfeh IJ, Rypins EB, Mason GR. A systematic appraisal of portacaval H‐graft diameters. Clinical and hemodynamic perspectives. Annals of Surgery 1986;204(4):356‐63. [PUBMED: 3490229] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfeh IJ, Rypins EB. Partial versus total portacaval shunt in alcoholic cirrhosis. Results of a prospective, randomized clinical trial. Annals of Surgery 1994;219(4):353‐61. [PUBMED: 8161260] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin SK, Gupta N, Jha SK, Agrawal A, Mishra SR, Sharma BC, et al. Equal efficacy of endoscopic variceal ligation and propranolol in preventing variceal bleeding in patients with noncirrhotic portal hypertension. Gastroenterology 2010;139:1238‐45. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157:429‐38. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [PUBMED: 20334633] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzner M, Tuttle‐Newhall JE, Dahm F, Suhocki P, Clavien PA. Current indication of a modified Sugiura procedure in the management of variceal bleeding. Journal of the American College of Surgeons 2001;193:166‐73. [DOI] [PubMed] [Google Scholar]
- Skoog M, Saarimäki JM, Gluud C, Scheinin M, Erlendsson K, Aamdal S, et al. The Nordic Trial Alliance Working Group on Transparency and Registration. Transparency and registration in clinical research in the Nordic countries. nta.nordforsk.org/projects/nta_transparency_report.pdf 2015 (accessed 12 June 2018).
- Smith JL, Graham DY. Variceal hemorrhage: a critical evaluation of survival analysis. Gastroenterology 1982;82:968‐73. [PubMed] [Google Scholar]
- Stanley AJ, Jalan R, Forrest EH, Redhead DN, Hayes PC. Long term follow up of transjugular intrahepatic portosystemic stent shunt (TIPSS) for the treatment of portal hypertension: results in 130 patients. Gut 1996;39:479‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta‐analysis, and estimates of people at risk. Lancet Infectious Diseases 2006;6:411‐25. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sakai P, Gayotto LC, Cardoso RA, Forster S, Raia S. Size of gastroesophageal varices: its behavior after the surgical treatment of portal hypertension. Revista do Hospital das Clinicas; Faculdade de Medicina da Universidade de Sao Paulo 1999;54(6):193‐8. [DOI] [PubMed] [Google Scholar]
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25. [Google Scholar]
- Sugiura M, Futagawa S. A new technique for treating esophageal varices. Journal of Thoracic and Cardiovascular Surgery 1973;66:677‐85. [PubMed] [Google Scholar]
- Symmers WSC. Note on a New Form of Liver Cirrhosis Due to the Presence of the Ova of Bilharzia Haematobia. 9th Edition. London (UK): Young J. Pentland, 1904. [Google Scholar]
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 May 2016).
- Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health‐related quality of life outcomes in meta‐analysis ‐ a tutorial and review of methods for enhancing interpretability. Research Synthesis Methods 2011;2:188‐203. [DOI] [PubMed] [Google Scholar]
- Toomey PG, Ross SB, Golkar FC, Hernandez JM, Clark WC, Luberice K, et al. Outcomes after transjugular intrahepatic portosystemic stent shunt: a "bridge" to nowhere. American Journal of Surgery 2013;205:441‐6. [DOI] [PubMed] [Google Scholar]
- Tripathi D, Helmy A, Macbeth K, Balata S, Lui HF, Stanley AJ, et al. Ten years' follow‐up of 472 patients following transjugular intrahepatic portosystemic stent‐shunt insertion at a single centre. European Journal of Gastroenterology & Hepatology 2004;16:9‐18. [DOI] [PubMed] [Google Scholar]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
- Villanueva C, Piqueras M, Aracil C, Gómez C, López‐Balaguer JM, Gonzalez B, et al. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. Journal of Hepatology 2006;45:560‐7. [DOI] [PubMed] [Google Scholar]
- Vleggaar FP, Buuren HR, Schalm SW. Endoscopic sclerotherapy for bleeding oesophagogastric varices secondary to extrahepatic portal vein obstruction in an adult Caucasian population. European Journal of Gastroenterology & Hepatology 1998;10:81‐5. [DOI] [PubMed] [Google Scholar]
- Voros D, Polydorou A, Polymeneas G, Vassiliou I, Melemeni A, Chondrogiannis K, et al. Long‐term results with the modified Sugiura procedure for the management of variceal bleeding: standing the test of time in the treatment of bleeding esophageal varices. World Journal of Surgery 2012;36:659‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WD, Zeppa R, Fomon JJ. Selective trans‐splenic decompression of gastroesophageal varices by distal splenorenal shunt. Annals of Surgery 1967;166:437‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Schistosomiasis: number of people receiving preventive chemotherapy in 2012. Weekly Epidemiological Record 2014;89:21‐8. [PubMed] [Google Scholar]
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Liu H, Zhang Y, Rong W. The surgical treatment for portal hypertension: a systematic review and meta‐analysis. Gastroenterology 2013;2013:464053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wen T, Yan L, Chen Z, Yang H, Deng X, et al. The changes of hepatic hemodynamics and functional hepatic reserve after splenectomy with periesophagogastric devascularization. Hepato‐gastroenterology 2009;56:835‐9. [PubMed] [Google Scholar]
- Zong GQ, Fei Y, Liu RM. Comparison of effects of devascularization versus shunt on patients with portal hypertension: a meta‐analysis. Hepato‐gastroenterology 2015;62(137):144‐50. [PUBMED: 25911885] [PubMed] [Google Scholar]
References to other published versions of this review
- Ede CJ, Brand M. Surgical portosystemic shunts versus devascularisation procedures for variceal bleeding due to hepatosplenic schistosomiasis. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011717] [DOI] [PMC free article] [PubMed] [Google Scholar]


