Summary
Background
About 13–26% of all acute ischaemic strokes are related to non-valvular atrial fibrillation, the most common cardiac arrhythmia globally. Deciding when to initiate oral anticoagulation in patients with non-valvular atrial fibrillation is a longstanding, common, and unresolved clinical challenge. Although the risk of early recurrent ischaemic stroke is high in this population, early oral anticoagulation is suspected to increase the risk of potentially harmful intracranial haemorrhage, including haemorrhagic transformation of the infarct. This assumption, and current treatment guidelines, are based on historical, mostly observational data from patients with ischaemic stroke and atrial fibrillation treated with heparins, heparinoids, or vitamin K antagonists (VKAs) to prevent recurrent ischaemic stroke. Randomised controlled trials have subsequently shown that direct oral anticoagulants (DOACs; ie, apixaban, dabigatran, edoxaban, and rivaroxaban) are at least as effective as VKAs in primary and secondary prevention of atrial fibrillation-related ischaemic stroke, with around half the risk of intracranial haemorrhage. However, none of these DOAC trials included patients who had experienced ischaemic stroke recently (within the first few weeks). Clinicians therefore remain uncertain regarding when to commence DOAC administration after acute ischaemic stroke in patients with atrial fibrillation.
Recent developments
Prospective observational studies and two small randomised trials have investigated the risks and benefits of early DOAC-administration initiation (most with a median delay of 3–5 days) in mild-to-moderate atrial fibrillation-associated ischaemic stroke. These studies reported that early DOAC treatment was associated with a low frequency of clinically symptomatic intracranial haemorrhage or surrogate haemorrhagic lesions on MRI scans, whereas later DOAC-administration initiation (ie, >7 days or >14 days after index stroke) was associated with an increased frequency of recurrent ischaemic stroke.
Where next?
Adequately powered randomised controlled trials comparing early to later oral anticoagulation with DOACs in ischaemic stroke associated with atrial fibrillation are justified to confirm the acceptable safety and efficacy of this strategy. Four such randomised controlled trials (collectively planned to include around 9000 participants) are underway, either using single cutoff timepoints for early versus late DOAC-administration initiation, or selecting DOAC-administration timing according to the severity and imaging features of the ischaemic stroke. The results of these trials should help to establish the optimal timing to initiate DOAC administration after recent ischaemic stroke and whether the timing should differ according to stroke severity. Results of these trials are expected from 2021.
Introduction
Cerebral embolism attributable to non-valvular atrial fibrillation accounts for 13–26% of ischaemic strokes;1–3 this proportion increases with age.4 Historical observational data (including data from the control groups of randomised control trials) show that, without anti-coagulation, the risk of early recurrence in the first 14 days after atrial fibrillation-related ischaemic stroke is between about 0·5% and 1·3% per day.5 A meta-analysis of subgroups of patients with atrial fibrillation from randomised controlled trials using heparins within 48 h of ischaemic stroke did not find a significant reduction in the risk of recurrent ischaemic stroke but did find a significantly increased risk of intracranial haemorrhage.6 Vitamin K antagonists (VKAs) are still recommended as first-line therapy in patients with atrial fibrillation in many countries, although there is scarce evidence from randomised controlled trials of VKA efficacy in acute ischaemic stroke. Since 2010, four direct oral anti-coagulants (DOACs; ie, apixaban,7 dabigatran,8 edoxaban,9 and rivaroxaban10) have been approved for use in patients with non-valvular atrial fibrillation. Cochrane reviews and meta-analyses11,12 found that these DOACs have similar efficacy to VKAs in primary and secondary prevention of stroke but are associated with about half the frequency of intracranial haemorrhage. However, none of the randomised controlled trials comparing DOACs and VKAs included patients with recent ischaemic stroke associated with atrial fibrillation (within the first few weeks)—presumably because of concerns about the risk of haemorrhagic trans formation of the ischaemic brain tissue or of other intracranial haemorrhage.
In acute atrial fibrillation-related ischaemic stroke, the risk of both early recurrent ischaemic stroke5 and haemorrhagic transformation13 is highest in the days immediately after the index stroke. Integrity of the microvasculature is lost, partly because of the degradation of the basal lamina and extracellular matrix,14,15 which leads to the disruption of the blood–brain barrier16 and the haemorrhagic transformation of ischaemic brain tissue, which ranges from petechial haemorrhage to more severe parenchymal haematoma.17 Haemorrhagic transformation (termed petechial haemorrhage or parenchymal haematoma) is reported in about 9% of patients with acute ischaemic stroke and, like ischaemic stroke recurrence, is associated with large ischaemic lesions; parenchymal haematoma is associated with large cardioembolic lesions and acute recanalisation therapies given for the index stroke.18 It is suspected (though not supported by evidence) that early initiation of anticoagulation might exacerbate or cause parenchymal haemorrhage, with potentially serious clinical consequences.18 This concern has led clinicians to delay anticoagulation, although the independent contribution of haemorrhagic transformation of the infarct to clinical worsening remains uncertain19 and evidence from randomised controlled trials is not available. A lack of consensus for when to start oral anticoagulation (particularly with DOACs, the current most common standard of care) was shown in an online survey20 among UK stroke physicians, 95% of whom were uncertain about the optimal timing. In this Rapid Review, we summarise and critically review current guidelines and new published data from observational and small randomised studies, and give an overview of ongoing investigator-initiated randomised controlled trials of oral anticoagulation timing after ischaemic stroke associated with atrial fibrillation.
Guidelines
Current guidelines are imprecise and inconsistent with regards to when and how to start oral anticoagulation after the onset of atrial fibrillation-related ischaemic stroke. The “1–3–6–12 days rule” was introduced in 2013 by the European Heart Rhythm Association of the European Society of Cardiology (EHRA-ESC)21 because of evidence that large infarcts (causing severe stroke syndromes) are more likely to undergo haemorrhagic transformation than small infarcts.18 Although the timepoints and definitions of stroke severity are based only on expert consensus, this guideline has been adopted, with some variations, by various associations (including EHRA-ESC and the European Stroke Organisation) and countries (including Canada, Australia, Middle Eastern countries, and north African countries; panel). The 2018 guidelines of the American Heart Association/American Stroke Association (AHA/ASA) on early management of patients with ischaemic stroke recommend starting oral anti coagulation 4–14 days after onset of neurological symptoms.22 This recommendation was based on findings from the Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation (RAF) prospective observational study29 of 1029 consecutive patients with acute ischaemic stroke and known or newly diagnosed atrial fibrillation, only 93 (12%) of whom were treated with DOACs. UK guidelines30 recommend that oral anticoagulant administration should be deferred until at least 14 days from onset in patients with disabling ischaemic stroke, but could start earlier for non-disabling stroke, at the discretion of the clinician. A German guideline31 states that the efficacy of DOACs has not been proven within less than 14 days after a stroke, but does not make any recommendations on the basis of this statement. We found only guidelines that predated the use of DOACs for Japan (2011),32 whereas in India33 and Latin America,34 ESC, EHRA, and AHA/ASA guidelines are widely used. Repeated brain imaging before starting anticoagulation in patients with moderate and severe stroke to evaluate haemorrhagic transformation is recommended only by the ESC guidelines,23 without supporting evidence. Bridging treatment (ie, treatment after stroke onset until start of oral anticoagulation) with low molecular-weight heparins is not recommended by most guidelines, whereas the UK guidelines recommend the use of aspirin (300 mg/day) before starting oral anticoagulant treatment. All guidelines state that the level of evidence is low (mainly grade C—ie, expert opinion) and that additional studies are needed. Only the European Stroke Organisation-Karolinski Stroke Update (ESO-KSU)27 and the ASA/AHA22 guidelines cite observational data (from the RAF study)29 to support their recommendations. Importantly, none of the guidelines distinguish between use of VKAs and DOACs, despite the substantial differences in the pharmacodynamics of these compounds. For example, it could take 2–4 days after the first intake of a VKA to achieve a therapeutic international normalised ratio greater than 2·0, whereas therapeutic anticoagulation is achieved on the first day after initiating DOAC treatment.
Early anticoagulation in atrial fibrillation-related ischaemic stroke
Data from observational studies and randomised-trial control groups suggest that early recurrence after atrial fibrillation-related ischaemic stroke is between about 0.5% and 1.3% per day in the first two weeks.5,6 In observational studies, age, large ischaemic-lesion size, and atrial enlargement are risk factors for recurrent atrial fibrillation-related ischaemic stroke.29,35,36 The presence of an atrial thrombus is rare35,37 but, if detected, is associated with a high frequency of recurrence.35 Large infarction is also a risk factor for haemorrhagic transformation.18 Clinical scores, such as CHA2DS2VASc38 (which predicts the risk of ischaemic-event occurrence) and HAS-BLED39 (which predicts the risk of haemorrhagic-event occurrence), are commonly used to determine the risk of occurrence of ischaemic or haemorrhagic events in patients with atrial fibrillation who are receiving oral anticoagulants. However, these scores are not designed for use in the acute-stroke setting, have modest predictive value, and share many component risk factors (eg, hypertension, age, previous ischaemic stroke) that are highly prevalent in patients with ischaemic stroke. Indeed, a central clinical challenge in stroke medicine is the assessment, differentiation, and balancing of the risks of occurrence of ischaemic and haemorrhagic brain injury.
In patients with atrial fibrillation and a recent ischaemic stroke (who are at high risk for both recurrent ischaemia and haemorrhagic transformation), DOAC treatment—which should reduce ischaemia and has a lower bleeding risk than VKAs—is a promising strategy. However, individual net clinical benefit will vary according to the absolute risk of these events occurring in such patients, since the risk might differ depending on the timing of treatment (with early treatment being likely to reduce the risk of ischaemic stroke but potentially increasing the risk of intracranial haemorrhage). The generally higher mortality and morbidity associated with intracranial haemorrhage than with recurrent ischaemic stroke is also an important consideration.
Previous studies focused on heparins for early anti-coagulation because of the rapid onset of anti-coagulant activity with these drugs compared with VKAs (which take several days to achieve full therapeutic anti-coagulation), but did not find a significant reduction in early recurrence and found, instead, a significant in crease in haemorrhagic transformation.6 On the basis of data from studies done soon after use of DOACs entered clinical practice (RAF study,29 12% of patients on DOACs) or studies that predated DOAC use (VISTA collaboration,40 no patients treated with DOACs), the optimal timepoint to start anticoagulant treatment might be between 4–14 days after stroke onset. However, this conclusion must be interpreted cautiously since the RAF study included mixed treatment protocols with low molecular-weight heparin and warfarin as well as non-vitamin-K oral anticoagulants (NOACs), and had insufficient statistical power to evaluate the benefit of early anti-coagulation with NOACs.29 Moreover, early (2–3 days after stroke onset) initiation of VKA administration was associated with fewer recurrent ischaemic strokes than late (>3 days after stroke onset) initiation of VKA treatment in the VISTA prospective cohort study of 1644 patients with ischaemic stroke and atrial fibrillation.40
Randomised controlled trials of early DOAC treatmentin ischaemic stroke or transient ischaemic attack
Two small randomised controlled trials have focused on the early use of DOACs. In a trial41 of 195 patients with mild stroke (median National Institutes of Health Stroke Scale [NIHSS] score of 2 [IQR 0–4]), rivaroxaban had similar efficacy and safety to warfarin, when the treatment was initiated within 5 days after a mild atrial fibrillation-related ischaemic stroke (defined as infarct size on diffusion weighted imaging [DWI] of less than a third of middle cerebral artery territory, half of anterior cerebral artery territory, half of posterior cerebral artery territory, or half of one cerebellar hemisphere). The primary outcome of new ischaemic or haemorrhagic lesions on follow-up MRI scans did not differ between groups (occurrence frequency was 49·5% in the rivaroxaban group vs 54·5% in the warfarin group; p=0·49). There was no difference in clinical outcomes (each group had one clinical ischaemic stroke, whereas there were no symptomatic haemorrhages), but this study had insufficient statistical power because of the small sample size, so the results should be considered hypothesis-generating. The Dabigatran Following Acute Transient Ischemic Attack and Minor Stroke II trial (DATAS II, NCT02295826)42 randomly assigned 301 patients with transient ischaemic attack or minor stroke (NIHSS score <9, DWI lesions <25 mL) but without diagnosed atrial fibrillation to receive either aspirin or dabigatran within 72 h of stroke onset for 30 days. The primary outcome was symptomatic parenchymal haemorrhage on MRI scan at 5 weeks follow-up. There were no primary-outcome events in either group (asymptomatic haemorrhage occurred in 7·8% of the dabigatran group vs 3·5% of the aspirin group).43 However, since patients in DATAS II did not have diagnosed atrial fibrillation, these data do not provide direct evidence for risk of recurrent ischaemic stroke and haemorrhagic transformation with use of dabigatran in patients with recent ischaemic stroke and atrial fibrillation. Nevertheless, these small trials41–43 provide some reassurance about the safety of early initiation of administration of rivaroxaban or dabigatran in patients with mild-to-moderate ischaemic stroke (NIHSS score <9).
Prospective observational studies
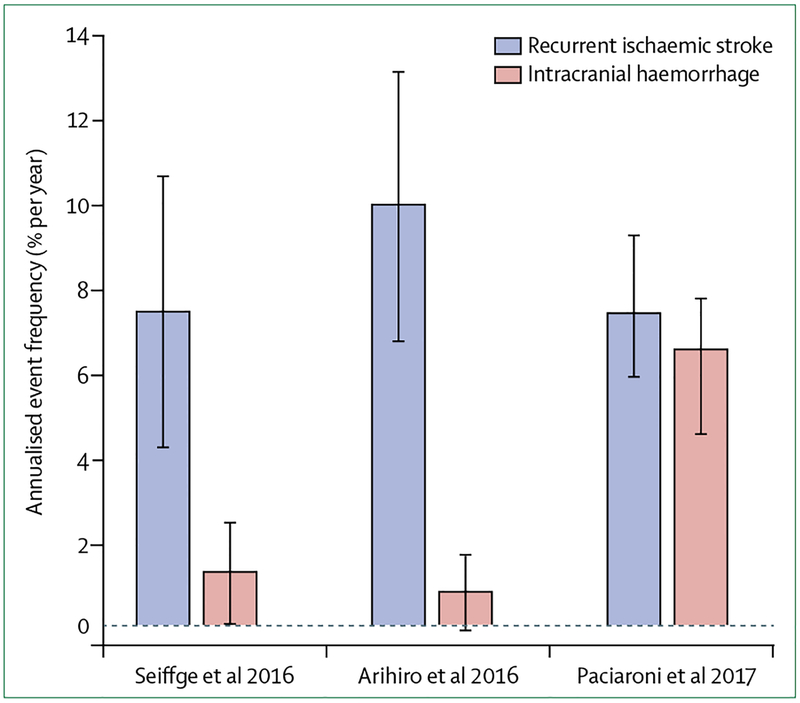
Several non-randomised, prospective observational studies44–51 have explored the potential risks and benefits of early DOAC anticoagulation in patients with atrial fibrillation-related ischaemic stroke. Three studies44–46 included patients with a recent ischaemic stroke and atrial fibrillation who were followed up for at least 3 months for clinical-outcome events (ie, recurrent ischaemic stroke and intracranial bleeding). The Swiss NOACISP44 study included 204 participants. The Japanese SAMRUAI-NVAF study45 included 1192 Japanese patients. The international (Europe and Asia) RAF-NOAC study46 included 1127 patients. All of these studies enrolled a considerable proportion of patients receiving DOACs (155 [75%] in the NOACISP study, 475 [41%] in the SAMRUAI-NVAF study, and 1127 [100%] in the RAF-NOAC study). Key study characteristics are summarised in table 1. All studies included older patients (median age 76–79 years) mainly with minor-severity to medium-severity strokes (median NIHSS score 3–8) and had a median delay between ischaemic stroke and start of DOAC administration of 5 days. The annualised risk of recurrent ischaemic stroke was consistently between 7·7% per year to 8·5% per year (figure 1). The annualised proportion of symptomatic intracranial haemorrhage was low in the NOACISP (1·3% per year) and SAMRUAI-NVAF (0·9% per year) studies (figure 1), whereas it was considerably higher in the RAF-NOAC study (6·4% per year). However, although the majority of intracranial haemorrhages in the RAF-NOAC study occurred in patients that started receiving the treatment less than 3 days after ischaemic stroke, the association of these haemorrhages with early treatment is uncertain because the haemorrhages occurred after 30 days. Two of the studies reported increased frequencies of recurrent ischaemic stroke when DOAC administration was started late: 5·1% per year, if started before 7 days versus 9·3% per year if started after 7 days (p=0·53);44 2·1% per year (within the first 3 months) if started between 3–14 days versus 9·1% per year (within the first 3 months) if started after 14 days (p<0·001).46
Table 1:
Summary of studies on early initiation of anticoagulant treatment in patients with recent atrial fibrillation-related ischaemic stroke
| Study population | Patients’ median age, median stroke severity, and infarctsize* | Median timing of anticoagulation administration initiation | Follow-up period | Recurrent ischaemic stroke† | Intracranial haemorrhage† | |
|---|---|---|---|---|---|---|
| Observational studies with clinical follow-up of ≥3 months | ||||||
| Seiffge et al44 (NOACISP) |
204 (155 DOAC treated) | 79 years; NIHSS score 4; no information on infarct size | 5 days (≤7 days for 65% [n=100] of DOAC-treated patients) | At least 3 months | 7·7% per year (5·1% per year for DOAC administration ≤7days vs 9·3% per year for DOAC administration >7days, p=0.53) | 1·3% per year |
| Arihiro et al45 (SAMURAI-NVAF) |
1192 (466 DOAC treated) | 78 years; NIHSS score 3; 24% small, 48% medium, and 28% large infarcts | 5 days for DOAC | 5 days for DOAC | 8·5% per year (VKA) and 10·1% per year (DOAC, p>0.05) | 1·2% per year (VKA) vs 0·8% per year (DOAC) |
| Paciaroni et al46 (RAF-NOAC) |
1127 (all DOAC treated) | 76 years;NIHSS score 8; 41% small, 33% medium, and 22% large infarcts | No overall median reported (8 days for dabigatran and rivaroxaban, 7 days for apixaban) | 3 months | 7·8% per year | 6·4% per year |
| Wilson et al51 | 1355 (475 DOAC) | 76 years; NIHSS score 4; 18% large infarcts | 11 days (≤4 days for 26% [n=358] of patients) | 90 days | 5·7% per year (combined DOAC and VKA) | 0.6% per year (combined DOAC and VKA) |
| Observational studies with clinical follow-up within 3 months or with surrogate outcome imaging markers | ||||||
| Macha et al47 | 243 (all DOAC treated) | 78 years; NIHSS score 5; 17% small infarct or TIA, 70% medium, and 13% large infarcts | From 1·7 days for small infarct or TlA to 6·7 days for large infarcts (≤7 days for 89·7% [n=218] of DOAC-treated patients) | In hospital | Not reported | 1 case of symptomatic and 2 cases of asymptomatic intracranial haemorrhage |
| Cappellari et al48 | 147 (all DOAC treated) | 79 years; NIHSS score 8; 54% small, 22% medium, and 24% large infarcts | 3·3 days (≤3 days for 66% [n=97] of patients; ≤7 days for all patients) | CT scan at 7 days | No case observed | 8 cases of asymptomatic intracranial haemorrhage (7 new, 1 before DOAC treatment) |
| Gioia et al49 | 60 (all rivaroxaban treated) | 74 years; NIHSS score 2; median DWI lesion volume 7·9 mL | 3 days | MRI scan at 7 days | 1 case | No cases of symptomatic intracranial haemorrhage, 8 cases of asymptomatic petechial haemorrhage |
| Deguchi et al50 | 300 (186 DOAC treated) | 77 years; NIHSS score 7; no information on infarct size | 3 days for DOAC, 7days for VKA | In hospital | No case observed | 2 cases of intracranial haemorrhage, 1 case of extracranial haemorrhage |
| Observational studies with a majority of patients receiving VKAs or heparins | ||||||
| Abdul-Rahim et al40 | 1300 (no DOAC-treated patients) | 73 years; NIHSS score 14; no information on infarct size | 2 days | 90 days | 8·2% of patients (107 events in 1300 patients) | 2·3% of patients had symptomatic intracranial haemorrhage (30 events in 1300 patients) |
| Paciaroni et al29 | 1029 (93 DOAC treated) | 77 years; NIHSS score 9; 37% small, 36% medium, and 27% large infarct | 8·5 days for DOAC, 12·1 days for VKA | 3 months | 77 events (including TIA and systemic embolism) | 37 events (including major extracranial haemorrhages) |
| Randomised controlled trials with patients receiving DOACs | ||||||
| Hong et al41 | 195 (95 rivaroxaban treated) | 70 years; NIHSS score 2; median DWI lesion volume 2·6 mL | 2 days | MRI scan at 4 weeks | 1 case | 30 new haemorrhagic lesions (all asymptomatic) |
DOAC=direct oral anticoagulant. NIHSS=National Institutes of Health Stroke Scale. VKA=vitamin K antagonist. TIA=transient ischaemic attack. DWI=diffusion weighted imaging.
Although definitions of infarct size might differ slightly between studies, small infarct size was commonly defined by lesions smaller than 1·5 cm in the anterior or posterior circulation; medium infarct size by lesions in a cortical superficial branch of middle cerebral artery (MCA), in the MCA deep branch, in the internal border zone territories, in a cortical superficial branch of posterior cerebral artery, or in a cortical superficial branch of the anterior cerebral artery; large infarct size by lesions that involve the complete territory of MCA, posterior cerebral artery, or anterior cerebral artery, in two cortical superficial branches of MCA, in a cortical superficial branch of MCA associated to the MCA deep branch, or in more than one artery territory.
Annualised event frequencies (% per year) were calculated whenever possible using published data (number of observed events divided by follow-up period).
Figure 1: Risk of recurrent ischaemic stroke and intracranial haemorrhage in patients with atrial fibrillation and a recent ischaemic stroke.
Annualised event frequencies (percentage per year with 95% CIs) of recurrent ischaemic stroke and intracranial haemorrhage in prospective observational studies44–46 of patients treated with direct oral anticoagulants with at least3 months of clinical follow-up.
Four single-centre observational studies47–50 reported on the timing of starting DOAC administration and short-term (ie, before discharge from the acute-care hospital) clinical and radiological outcomes. All studies reported early initiation (1–3 days) of DOAC treatment in patients with mild-to-moderate stroke (NIHSS score <944 or NIHSS score <8)41,42,44 or small-to-medium sized infarcts (less than a third of the affected arterial territory)45 with low frequency of symptomatic and asymptomatic intracranial haemorrhage or recurrent ischaemic stroke (table 1). Two additional studies48,49 of early DOAC-treatment initiation found that haemorrhagic transformation of the infarct (present before the start of DOAC administration) worsened in only a few patients with no new symptomatic intracranial bleeding (1 of 15 patients48 and 5 of 25 patients49 with haemorrhagic transformation before initation).
All of the reviewed observational studies have limitations: they were prone to selection bias (ie, patients judged to be at low risk of haemorrhage are more likely to start receiving treatment early, whereas patients considered to be at higher risk start receiving treatment later); initiation of DOAC administration was not standardised (although timing in most studies was guided by infarct size and stroke severity); and they were biased towards mild stroke (median NIHSS scores 3–8) and small infarcts (generally smaller than one third of the affected arterial territory). Nevertheless, these studies all suggest that early DOAC administration in patients with mild ischaemic stroke across different countries and ethnicities has a low frequency of intracranial haemorrhage (including haemorrhagic transformation).
Future directions
The persisting clinical uncertainty and the promising results of observational studies of DOAC treatment early after onset of stroke have prompted several randomised, controlled clinical trials investigating early versus late initiation of DOAC administration in patients with atrial fibrillation-related ischaemic stroke (table 2): ELAN (NCT03148457; Switzerland), OPTIMAS (EudraCT, 2018-003859-38; UK), TIMING (NCT02961348; Sweden), and START (NCT03021928; USA). A key challenge for these trials is to include a sufficiently large sample size to assess differences in the risk of occurrence of adverse-outcome events related to the timing of oral anti coagulation. All four trials have composite primary outcomes that include combinations of cerebral ischaemic or haemorrhagic events, or both, and three of them include either vascular or all-cause mortality. While using a composite endpoint that includes ischaemic stroke and intracranial haemorrhage as an outcome should increase the number of outcome events (thereby potentially increasing the statistical power), early DOAC administration might have opposite effects on these components (ie, it might reduce recurrent ischaemia, but possibly increase intracranial haemorrhage). These contrasting effects could reduce any net treatment effect, potentially increasing the probability of a neutral primary-outcome result. Nevertheless, if early DOAC treatment were shown to have the same degree of safety and efficacy as late treatment, clinical practice would probably change in favour of early treatment, given the greater convenience for patients (ie, earlier hospital discharge) and physicians (ie, improved compliance and continuation of anti-coagulant medication started in the acute-care hospital), and the economic advantage for health-care systems.
Table 2:
Summary of ongoing randomised controlled trials investigating early versus late initiation of direct oral anticoagulant treatment in patients with recent atrial fibrillation-related ischaemic stroke.
| Planned sample size | Intervention (early initiation of anticoagulant treatment) | Control (late initiation of anticoagulant treatment) | Follow-up period | Primary outcome | Patients with haemorrhagic transformation included | NIHSS exclusion criteria | Estimated end of study | |
|---|---|---|---|---|---|---|---|---|
| ELAN (NCT03148457) |
2000 | <48 h after symptom onset (minor and moderate stroke) or at day 6 (±1 day) after symptom onset (major stroke)* | Current recommendations (ie, minor stroke after day 3 [±1 day], moderate stroke after day 6 [±1 day] and major stroke after day 12 [±2 days])* | 30 days (secondary outcomes after 90 days) | Composite outcome (major bleeding, recurrent ischaemic stroke, systemic embolism, or vascular death, or a combination of these outcomes) | Yes | No exclusion criteria | October 2021 |
| OPTIMAS (EudraCT, 2018-003859-38) |
3474 | ≤4 days after acute ischaemic stroke | 7–14 days after acute ischaemic stroke | 90 days | Composite outcome at 90 days (combined incidence of recurrent symptomatic ischaemic stroke, symptomatic intracranial haemorrhage [including extradural, subdural, subarachnoid and intracerebral haemorrhage, and haemorrhagic transformation of the qualifying infarct], and systemic embolism) | Yes | No exclusion criteria | 2021–22 |
| TIMING (NCT02961348) |
3000 | ≤4 days after acute ischaemic stroke | 5–10 days after acute ischaemic stroke | 90 days | Composite outcome (recurrent ischaemic stroke, symptomatic intracerebral haemorrhage, or all-cause mortality, or a combination of these outcomes) | Yes | No exclusion criteria | December 2020 |
| START (NCT03021928) |
1500 (1000 patients with mild or moderate stroke, 500 with severe stroke)† | Time-to-treatment delay of 3, 6,10, or 14 days for mild or moderate stroke; 6,10,14, or 21 days for severe stroke† | Time-to-treatment delay of 3, 6,10, or 14 days for mild or moderate stroke; 6,10,14, or 21 days for severe stroke† | 30 days (secondary outcomes after 90 days) | Composite of any CNS haemorrhagic or other major haemorrhagic events and the ischaemic events of stroke or systemic embolism within 30 days of the index stroke | Yes | Score >3 and score <23 | August 2021 |
NIHSS= National Institutes of Health Stroke Scale. ELAN=Early Versus Late Initiation of Direct Oral Anticoagulants in Post-ischaemic Stroke Patients with Atrial fibrillation. OPTIMAS=OPtimal TIMing of Anticoagulation after AF-associated acute cardioembolic ischaemic Stroke. TIMING=Timing of oral anticoagulant therapy in acute ischemic stroke with atrial fibrillation. START=Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation.
Small infarct size is defined by lesions smaller than 1·5 cm in the anterior or posterior circulation; medium infarct size by lesions in a cortical superficial branch of middle cerebral artery (MCA), in the MCA deep branch, in the internal border zone territories, in a cortical superficial branch of posterior cerebral artery, or in a cortical superficial branch of the anterior cerebral artery; large infarct size by lesions that involve the complete territory of MCA, posterior cerebral artery, or anterior cerebral artery, in two cortical superficial branches of MCA, in a cortical superficial branch of MCA associated to the MCA deep branch, or in more than one artery territory.
Minor stroke is defined by a NIHSS score <8, mild stroke by a NIHSS score of 8–15, and severe stroke by a NIHSS score >15, as per the European Society of Cardiology and European Heart Rhythm Association definitions (panel 1).
There are some important differences between these four trials. While the OPTIMAS and TIMING trials use a binary definition of early treatment (≤4 days) in all participants across the range of stroke severity, timing of DOAC administration in the ELAN trial differs depending on the severity of the index stroke, according to the EHRA-ESC and ESO-KSU guidelines (panel), which inform practice in Switzerland and other participating countries. The START trial applies a response-adaptive randomisation design with four pre-defined time-to-treatment intervals from 2–14 days; ischaemic-outcome and haemorrhagic-outcome events will be modelled with an interim analysis after 100 patients have been assigned to different groups, to calculate new randomisation probabilities which favour the groups with the lowest-risk profiles. Together, these trials aim to recruit up to 9000 patients; all trials are ongoing and results are expected from 2021. Differences in the trial designs will need to be considered when it comes to the interpretation and comparison of the results (eg, variation in how early DOAC-administration initiation is defined). A pooled individual patient data analysis including data from all of these randomised controlled trials is planned, which should provide an ideal opportunity to assess the safety and efficacy of early DOAC treatment.
Important yet unresolved questions concern starting anticoagulation in patients for whom DOACs are considered unsuitable (eg, those with severely impaired renal function; with contraindications to oral anti-coagulants; or with severe, symptomatic haemorrhagic transformation, such as parenchymal haematoma Type 2; figure 1). As DOACs are eliminated by the kidneys (renal excretion ranges from 80% for dabigatran to 27% for apixaban), caution is warranted in patients with renal impairment. The reduced bleeding risk associated with DOAC treatment as compared with warfarin treatment can be maintained with mild-to-moderate renal impairment (with appropriate dose reduction). However, patients with creatinine clearance less than 30 mL/min were excluded from the RE-LY trial8 (which used dabigatran), the ROCKET AF trial10 (which used rivaroxaban), and the ENGAGE AF-TIMI trial9 (which used edoxaban); those with creatinine clearance less than 25 mL/min were excluded from the ARISTOTLE trial7 (apixaban). Thus, without adequate understanding of the risks associated with DOAC treatment in patients with severe renal impairment or undergoing dialysis, DOACs should be avoided and VKAs should be used instead, until future trials (to our knowledge, NCT02942407 is the only ongoing trial) can provide further insights. However, in patients with end-stage renal disease or who are undergoing dialysis, the indication and the benefit-to-risk ratio of therapy with warfarin is also unclear, as randomised controlled trials have typically excluded such patients.
The potential roles of low-dose DOACs, VKAs, combined antiplatelet therapy, or other antithrombotic strategies in patients with large infarcts also remain uncertain. Left atrial-appendage occlusion might be a promising option for patients with ischaemic stroke considered to have a contraindication to any form of oral anticoagulant.52 Planned randomised controlled trials are unlikely to answer these questions in the near future, and there is no expert consensus on how these patients should be managed. Further evidence might emerge from observational studies but, in the meantime, decisions for individual patients must be made on the basis of careful consideration of the risks of ischaemic stroke and intracranial haemorrhage.
Another topic for investigation is the use of neuroimaging biomarkers to stratify and differentiate the risks of future ischaemia and haemorrhage. In 1490 patients who received anticoagulation treatment soon after ischaemic stroke or transient ischaemic attack associated with atrial fibrillation, the baseline presence of cerebral microbleeds (seen on gradient-recalled echo T2*-weighted or magnetic resonance images), which were found in 311 (21%) participants, was independently associated with an increased risk of symptomatic intracranial haemorrhage (adjusted hazard ratio 3·67, 95% CI 1·27–10·60; p=0·016).53 These findings support the hypothesis that cerebral microbleeds are a neuroimaging biomarker of a bleeding-prone arteriopathy specifically relevant for intracranial haemorrhage associated with anticoagulation. Cerebral microbleeds might also have relevance for risk of haemorrhagic-transformation occurrence, and for the optimal timing of oral anticoagulants soon after ischaemic stroke, but there are no studies investigating this hypothesis. The relevance of cortical superficial siderosis, a much less common putative marker of cerebral amyloid angiopathy and associated risk of intracranial haemorrhage,54 has not yet been investigated. Markers of cardiac anatomy and function might have relevance for the risk of recurrent cerebral embolism (eg, transthoracic or transoesopagheal echocardiography) and deserve investigation to help select patients for early DOAC treatment. For example, left-atrial enlargement is an independent predictor of recurrent stroke of embolic subtype in patients with ischaemic stroke (even in patients without evidence of atrial fibrillation).54,55 Subgroup analysis of completed randomised controlled trials could help further understand the role of brain and cardiac biomarkers to guide oral anticoagulant treatment.
Conclusions
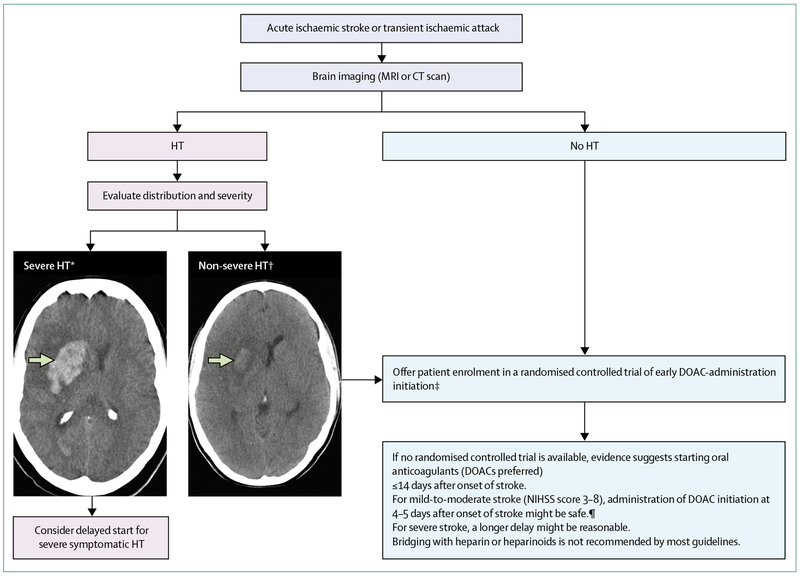
Anticoagulation using DOACs effectively prevents recurrent ischaemic stroke in patients with atrial fibrillation. However, how soon DOACs should be administered after ischaemic stroke in these patients is unclear; current guidelines vary between different organisations and countries and have been created on the basis of expert consensus rather than on empirical evidence (panel). Observational data show that many stroke physicians start DOAC treatment earlier than the timeframe tested in large randomised controlled trials and that although early treatment might be effective and acceptably safe, its safety and efficacy need to be confirmed in randomised trials. Where possible, people who had atrial fibrillation-related ischaemic stroke within the previous 2–4 days and who have no contraindications to anticoagulation should be recruited to participate in a suitable randomised controlled trial (figure 2). Where enrolment in a randomised controlled trial is not possible, clinicians will need to weigh potential risks and benefits of early DOAC administration and work within the imprecise and inconsistent advice offered by the guidelines. The currently accepted practice, based on consensus rather than empirical evidence, suggests delaying treatment with oral anticoagulants in people with severe stroke and large areas of infarction. A brain CT scan before commencing administration of oral anticoagulants can also clarify the severity and pattern of haemorrhagic transformation, so is often used in clinical practice, but whether haemorrhagic transformation should guide treatment with oral anticoagulants, and how, remains unknown.
Figure 2: Timing for initiation of direct oral anticoagulant administration.
Enrolment of eligible patients with ischaemic stroke and atrial fibrillation in ongoing randomised controlled trials is suggested on the basis of current guidelines and evidence from observational studies and clinical trials. If enrolment in a trial is not possible, clinicians will need to use the data to weigh potential risks and benefits of early DOAC administration although evidence is currently scarce to make strong recommendations. DOAC=direct oral anticoagulant. HT=haemorrhagic transformation. NIHSS= National Institutes of Health Stroke Scale. *Parenchymal haematoma Type 2 (homogeneous hyperdensity with mass effect >30% of infarct volume or extending beyond the infarct). †Petechial haemorrhage Type 1 (petechial heterogeneous hyperdensity), petechial haemorrhage Type 2 (diffuse heterogeneous hyperdensity), or parenchymal haemorrhage Type 1 (homogeneous hyperdensity <30% of infarct).56 ‡If oral anticoagulants are not contraindicated. ¶Prospective observational data and one small randomised controlled trial; grade of recommendation B, level of evidence 2a.
The ongoing clinical trials should help to establish whether early initiation of DOAC treatment in patients with atrial fibrillation is safe, prevents recurrent ischaemic stroke, shortens hospital stay, and improves the continuity of anticoagulant treatment. These trials will also highlight whether adjusting the timing of anticoagulation according to infarct size or clinical severity of the index stroke is necessary to reduce the risk of clinically harmful haemorrhagic transformation, the occurrence of which might be lower than previously estimated since use of DOACs entered clinical practice. We suggest that every effort should be made to include patients with recent ischaemic stroke and atrial fibrillation into these trials to answer the important open clinical questions.
Panel: National and international guideline recommendations on timing of anticoagulation administration after ischaemic stroke associated with atrial fibrillation.
American Heart Association/American Stroke Association (AHA/ASA)
The AHA/ASA guidelines12322 recommend that starting oral anticoagulation within4–14 days after ischaemic stroke onset is reasonable for most patients. However, a later treatment start might be considered for patients with haemorrhagic transformation.
European Society of Cardiology (ESC)23 and European Heart Rhythm Association (EHRA)24
The ESC 201623 and EHRA 201824 recommendations are endorsed by the European Stroke Organisation. The EHRA-ESC recommend giving anticoagulants 1 day after onset of transient ischaemic attack, after 3 days in patients with minor stroke (defined in these guidelines as National Institutes of Health Stroke Scale [NIHSS] score <8), after 6 days in those with mild stroke (NIHSS score 8–15), and after 12 days in those with severe stroke (NIHSS score >15).
Canadian stroke best practice recommendations 201725
Follow EHRA-ESC recommendations.
Australian guidelines for stroke management 2017
The Australian guidelines recommend giving anticoagulants 1 day after onset of transient ischaemic attack, after 5–7 days in patients with moderate stroke (not defined), and after 10–14 days in those with severe stroke (not defined).
Middle Eastern and north African consensus statement 201726
This consensus statement recommends giving anticoagulants 12 days after stroke onset in patients with moderate-to-severe ischaemic stroke (not defined), and after 2–3 weeks in patients with a large infarct (not defined).
European Stroke Organisation and Karolinksa Stroke Update 201627
These guidelines recommend giving anticoagulants 4 days after stroke onset in patients with mild stroke (not defined) and small infarct size (lesion ≤1·5 cm in the anterior or posterior circulation), after 7 days in those with moderate stroke (not defined) and medium infarct size (lesion in a cortical superficial branch of middle cerebral artery [MCA], in the MCA deep branch, in the internal border zone territories, in a cortical superficial branch of posterior cerebral artery, or in a cortical superficial branch of the anterior cerebral artery), and after 14 days in those with severe stroke (not defined) and large infarct size (lesion involves the complete territory of MCA, the posterior cerebral artery, or the anterior cerebral artery, or is in two cortical superficial branches of MCA, in a cortical superficial branch of MCA associated to the MCA deep branch, or in more than one arterial territory).
Royal College of Physicians (UK) national clinical guideline for stroke28
This guideline recommends giving anticoagulants 2 weeks after onset of disabling stroke (not defined). Earlier treatment can be considered in patients with minor, non-disabling stroke (not defined), and a low risk of haemorrhagic transformation (not defined).
Search strategy and selection criteria.
We identified references for this Rapid Review by searches of PubMed between Jan 1, 2016, and July 20, 2018, using the search terms “ischaemic stroke” OR “ischemic stroke” AND “atrial fibrillation” OR “non-valvular atrial fibrillation” OR “AF” AND “oral anticoagulation” OR “direct oral anticoagulants” OR “new oral anticoagulants” OR “novel oral anticoagulants” OR “DOAC” OR “NOAC” OR “dabigatran” OR “rivaroxaban” OR “apixaban” OR “edoxaban”. Only papers published in English were considered. Additionally, we searched our personal records and abstracts from international conferences in the past five years (2013—18; eg, the European Stroke Organisation Conference 2018 and others) for relevant publications or data. For the section on early anticoagulation, we selected publications presenting original data and published in English. In addition, we searched PubMed, personal records, and an internet search engine (ie, Google) using the search terms “guidelines”, “secondary prevention”, “stroke”, “atrial fibrillation”, and “oral anticoagulation” OR “DOAC” OR “direct oral anticoagulants” to identify guidelines from relevant organisations (national or international expert committees).
Acknowledgments
DJS was supported by the Swiss National Science Foundation (SNF), the Bangerter-Rhyner Stiftung, and the Swiss Society of Neurology. UF was supported by the SNF (33IC30_166876). DJW receives research support from the Stroke Association, the British Heart Foundation, and the Rosetrees Trust, and some of his work is undertaken in the National Institute of Health Research’s Biomedical Research Centre at UCL Hospitals NHS Foundation Trust. We wish to acknowledge all members of the trial steering committees of ELAN, OPTIMAS, TIMING and START (appendix).
Declaration of interests
DJS reports speaker honoraria from Bayer AG and Pfizer and compensation for educational efforts from STAGO. DJW reports speaker honoraria from Bayer AG. MP reports honoraria as a member of the speaker bureau of Aspen, Sanofi-Aventis, Boehringer Ingelheim, Bayer AG, Bristol-Myers Squibb, Medtronic, and Pfizer. STE reports funding for travel or speaker honoraria from Bayer AG and Boehringer Ingelheim, an educational grant from Pfizer, and compensation for educational efforts from Stago, and is on the scientific advisory boards of BayerAG, Boehringer Ingelheim, and BMS/Pfizer. UF is a consultant for Medtronic and Stryker, a Co-PI of the SWITCH trial and the ELAN trial (both supported by the Swiss National Science Foundation and the Swiss Heart Foundation) and of the SWIFT DIRECT trial (supported by Medtronic). BN received honoraria for serving on data monitoring committees from Astra Zeneca (SOCRATES and THALES trials) and Bayer AG (NAVIGATE-ESUS trial). JD reports speaker honoraria from Boehringer Ingelheim, Bayer AG, Bristol-Myer Squibb, Daiichi Sankyo, and Pfizer, and a research grant for the ELAN trial from the UK Stroke Association. TJM reports speaker honoraria from Janssen; a K23 Career Development Grant from the National Heart, Lung and Blood Institute;a research grant from the Texas Department of Health and Human Services; and an honorarium for serving on the executive committee of the ANNEXA-4 trial from McMaster’s Public Health Research Institute. TM is a member of the steering committee of the Lex-209 trial (supported by Octapharma). SW has received a research grant from Texas Department of Health and Human Resources.
References
- 1.Bejot Y, Ben Salem D, Osseby GV, et al. Epidemiology of ischemic stroke from atrial fibrillation in Dijon, France, from 1985 to 2006. Neurology 2009; 72: 346–53. [DOI] [PubMed] [Google Scholar]
- 2.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32: 2559–66. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–322. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–8. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke 1983; 14: 688–93. [DOI] [PubMed] [Google Scholar]
- 6.Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke:a meta-analysis of randomized controlled trials. Stroke 2007;38: 423–30. [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–92. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–51. [DOI] [PubMed] [Google Scholar]
- 9.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–104. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–91. [DOI] [PubMed] [Google Scholar]
- 11.Salazar CA, del Aguila D, Cordova EG. Direct thrombin inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in people with non-valvular atrial fibrillation. Cochrane Database Syst Rev 2014; 3: CD009893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2018;3: CD008980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Amelio M, Terruso V, Famoso G, et al. Early and late mortality of spontaneous hemorrhagic transformation of ischemic stroke. J Stroke Cerebrovasc Dis 2014; 23: 649–54. [DOI] [PubMed] [Google Scholar]
- 14.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 624–33. [DOI] [PubMed] [Google Scholar]
- 15.Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003; 107: 598–603. [DOI] [PubMed] [Google Scholar]
- 16.Kassner A, Merali Z. Assessment of blood–brain barrier disruption in stroke. Stroke 2015; 46: 3310–5. [DOI] [PubMed] [Google Scholar]
- 17.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–86. [DOI] [PubMed] [Google Scholar]
- 18.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008; 39: 2249–56. [DOI] [PubMed] [Google Scholar]
- 19.Kablau M, Kreisel SH, Sauer T, et al. Predictors and early outcome of hemorrhagic transformation after acute ischemic stroke. Cerebrovasc Dis 2011; 32: 334–41. [DOI] [PubMed] [Google Scholar]
- 20.Munn D, Abdul-Rahim AH, Fischer U, Werring D, Robinson TG, Dawson J. A survey of opinion: when to start oral anticoagulants in patients with acute ischaemic stroke and atrial fibrillation? Eur Stroke J 2018; published online July 10.DOI: 10.1177/2396987318787124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidbuchel H, Verhamme P, Alings M, et al. EHRA Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34: 2094–106. [DOI] [PubMed] [Google Scholar]
- 22.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines forthe early management of patients with acute ischemic stroke:a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49: e46–110. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–962. [DOI] [PubMed] [Google Scholar]
- 24.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace 2018; published online March 19. DOI: 10.1093/europace/euy054 (preprint). [DOI] [PubMed] [Google Scholar]
- 25.Wein T, Lindsay MP, Cote R, et al. Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke 2018; 13: 420–43. [DOI] [PubMed] [Google Scholar]
- 26.Hersi AS, Alhebaishi YS, Hamoui O, et al. Practical perspectives on the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation: a view from the Middle East and North Africa. J Saudi Heart Assoc 2018; 30: 122–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed N, Steiner T, Caso V, Wahlgren N. Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm13–15 November 2016. Eur Stroke J 2017; 2: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Intercollegiate Stroke Working Party. National clinical guideline for stroke. 2016. https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines (accessed on May 31, 2018).
- 29.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke 2015; 46: 2175–82. [DOI] [PubMed] [Google Scholar]
- 30.Intercollegiate Stroke Working Party. National clinical guideline for stroke. 2016. https://www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-(1).aspx (accessed May 31, 2018)
- 31.Endres M, Diener HC, Roether J, Benkhe M. Sekundärprophylaxe ischämischer Schlaganfall und transitorische ischämische Attacke (Teil 1). 2015. https://www.awmf.org/uploads/tx_szleitlinien/030-133k_S3_Sekun%C3%A4rprophylaxe_isch%C3%A4mischer_Schlaganfall_2015-02.pdf (accessed May 31, 2018)
- 32.Shinohara Y, Yanagihara T, Abe K, et al. II. Cerebral infarction/transient ischemic attack (TIA). J Stroke Cerebrovasc Dis 2011; 20: S31–73. [DOI] [PubMed] [Google Scholar]
- 33.Prasad K, Kaul S, Padma MV, Gorthi SP, Khurana D, Bakshi A. Stroke management. Ann Indian Acad Neurol 2011; 14: S82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantu-Brito C, Silva GS, Ameriso SF. Use of guidelines for reducing stroke risk in patients with nonvalvular atrial fibrillation: a review from a Latin American perspective. Clin Appl Thromb Hemost 2018; 24: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paciaroni M, Agnelli G, Falocci N, et al. Prognostic value of trans-thoracic echocardiography in patients with acute stroke and atrial fibrillation: findings from the RAF study. J Neurol 2016; 263: 231–37. [DOI] [PubMed] [Google Scholar]
- 36.Paciaroni M, Agnelli G, Caso V, et al. Prediction of early recurrent thromboembolic event and major bleeding in patients with acute stroke and atrial fibrillation by a risk stratification schema: the ALESSA score study. Stroke 2017; 48: 726–32. [DOI] [PubMed] [Google Scholar]
- 37.de Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006; 37: 2531–4. [DOI] [PubMed] [Google Scholar]
- 38.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137: 263–72. [DOI] [PubMed] [Google Scholar]
- 39.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–100. [DOI] [PubMed] [Google Scholar]
- 40.Abdul-Rahim AH, Fulton RL, Frank B, et al. Association of improved outcome in acute ischaemic stroke patients with atrial fibrillation who receive early antithrombotic therapy: analysis from VISTA. Eur J Neurol 2015; 22: 1048–55. [DOI] [PubMed] [Google Scholar]
- 41.Hong KS, Kwon SU, Lee SH, et al. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol 2017; 74: 1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng KH, Sharma M, Benavente O, et al. Dabigatran following acute transient ischemic attack and minor stroke II (DATAS II). Int J Stroke 2017; 12: 910–14. [DOI] [PubMed] [Google Scholar]
- 43.Butcher K, Ng K, Field T. The dabigatran following acute transient ischemic attack and minor stroke trial: final results. Eur Stroke J 2018; 3 (suppl 1): 3 (abstr). [Google Scholar]
- 44.Seiffge DJ, Traenka C, Polymeris A, et al. Early start of DOAC after ischemic stroke: risk of intracranial hemorrhage and recurrent events. Neurology 2016. [DOI] [PubMed] [Google Scholar]
- 45.Arihiro S, Todo K, Koga M, et al. Three-month risk-benefit profile of anticoagulation after stroke with atrial fibrillation:the SAMURAI-Nonvalvular Atrial Fibrillation (NVAF) study. Int J Stroke 2016; 11: 565–74. [DOI] [PubMed] [Google Scholar]
- 46.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) study. J Am Heart Assoc 2017; 6: e007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macha K, Volbers B, Bobinger T, et al. Early initiation of anticoagulation with direct oral anticoagulants in patients after transient ischemic attack or ischemic stroke. J Stroke Cerebrovasc Dis 2016; 25: 2317–21. [DOI] [PubMed] [Google Scholar]
- 48.Cappellari M, Carletti M, Danese A, Bovi P. Early introduction of direct oral anticoagulants in cardioembolic stroke patients with non-valvular atrial fibrillation. J Thromb Thrombolysis 2016;42: 393–98. [DOI] [PubMed] [Google Scholar]
- 49.Gioia LC, Kate M, Sivakumar L, et al. Early rivaroxaban use after cardioembolic stroke may not result in hemorrhagic transformation: a prospective magnetic resonance imaging study. Stroke 2016;47: 1917–19. [DOI] [PubMed] [Google Scholar]
- 50.Deguchi I, Tanahashi N, Takao M. Timing of treatment initiation with oral anticoagulants for acute ischemic stroke in patients with nonvalvular atrial fibrillation. Circ J 2017; 81: 180–84. [DOI] [PubMed] [Google Scholar]
- 51.Wilson D, Ambler G, Banerjee G, et al. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: multicenter cohort study. J Neurol Neurosurg Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes DR Jr., Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol 2015; 65: 2614–23. [DOI] [PubMed] [Google Scholar]
- 53.Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018; 17: 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med 1998; 128: 639–47. [DOI] [PubMed] [Google Scholar]
- 55.Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke 2015; 46: 1488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4·5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–29 [DOI] [PubMed] [Google Scholar]