Abstract
Lateral hypothalamic area (LHA) orexin neurons modulate reward-based feeding by activating ventral tegmental area (VTA) dopamine (DA) neurons. We hypothesize that signals of peripheral energy status influence rewardbased feeding by modulating the glucose sensitivity of LHA orexin glucose-inhibited (GI) neurons. This hypothesis was tested using electrophysiological recordings of LHA orexin-GI neurons in brain slices from 4 to 6 week old male mice whose orexin neurons express green fluorescent protein (GFP) or putative VTA-DA neurons from C57Bl/6 mice. Low glucose directly activated ~60% of LHA orexin-GFP neurons in both whole cell and cell attached recordings. Leptin indirectly reduced and ghrelin directly enhanced the activation of LHA orexin-GI neurons by glucose decreases from 2.5 to 0.1 mM by 53 ± 12% (n = 16, P < 0.001) and 41 ± 24% (n = 8, P <0.05), respectively. GABA or neurotensin receptor blockade prevented leptin’s effect on glucose sensitivity. Fasting increased activation of LHA orexin-GI neurons by decreased glucose, as would be predicted by these hormonal effects. We also evaluated putative VTA-DA neurons in a novel horizontal slice preparation containing the LHA and VTA. Decreased glucose increased the frequency of spontaneous excitatory post-synaptic currents (sEPSCs; 125 ± 40%, n = 9, P < 0.05) and action potentials (n = 9; P < 0.05) in 45% (9/20) of VTA DA neurons. sEPSCs were completely blocked by AMPA and NMDA glutamate receptor antagonists (CNQX 20 μM, n = 4; APV 20 μM, n = 4; respectively), demonstrating that these sEPSCs were mediated by glutamatergic transmission onto VTA DA neurons. Orexin-1 but not 2 receptor antagonism with SB334867 (10 μM; n = 9) and TCS-OX2–29 (2 μM; n = 5), respectively, blocks the effects of decreased glucose on VTA DA neurons. Thus, decreased glucose increases orexin-dependent excitatory glutamate neurotransmission onto VTA DA neurons. These data suggest that the glucose sensitivity of LHA orexin-GI neurons links metabolic state and reward-based feeding.
Keywords: Leptin, Ghrelin, Energy balance, Electrophysiology, Dopamine, Ventral tegmental area
Introduction
Most individuals in developed countries are exposed to an almost unlimited food supply largely consisting of highly palatable macronutrients such as sugar and fat. Intake of these macronutrients can trigger ingestion beyond homeostatic needs (reward-based feeding) and is one factor in the modern obesity and Type 2 Diabetes Mellitus epidemic (Fulton, 2010). The DA neurons of the ventral tegmental area (VTA) are an important part of the classical reward neurocircuitry for appetitive behavior and drug addiction (Fields et al., 2007). VTA DA neurons project to brain regions involved in motivation and positive reinforcement including the nucleus accumbens and the prefrontal cortex, which are assumed to be critical in reward-based feeding (Vittoz et al., 2008). The VTA also receives input from many brain regions involved in reward behavior including the prefrontal cortex, amygdala and lateral hypothalamic area (LHA) (Fields et al., 2007).
LHA orexin neurons activate VTA DA neurons through co-release of glutamate as well as by strengthening glutamatergic synaptic transmission (Borgland et al., 2006, 2009; Rosin et al., 2003). Thus, the LHA orexin neurons may modulate reward behavior, in part, via effects on VTA DA neurons. LHA orexin neurons are regulated by signals of metabolic status including circulating nutrients and nutrient-related hormones. The majority of orexin neurons are inhibited by glucose (i.e., glucose-inhibited or GI neurons) (Gonzalez et al., 2008). Hormonal signals of energy balance (e.g., leptin, ghrelin) also regulate the activity of LHA orexin neurons. The hormone leptin is secreted from adipose tissue in proportion to adipose tissue mass. Leptin levels increase after a meal or with weight gain (Ahima et al., 2000). In contrast, stomach secretion of the hormone ghrelin increases prior to a meal or after diet-induced weight loss (Cummings et al., 2002). Calcium imaging studies in isolated orexin neurons suggest that leptin inhibits while ghrelin activates these neurons (Yamanaka et al., 2003). Fasting (low leptin and high ghrelin) increases orexin mRNA; an effect blocked by leptin (Cai et al., 1999; López et al., 2000). Mice lacking orexin do not increase vigilance or exploration in response to a fast (Yamanaka et al., 2003). Thus, energy deficit activates LHA orexin neurons. On the other hand, hormonal (e.g., leptin) or nutrient (e.g., glucose) signals of energy sufficiency inhibit LHA orexin neurons. These data are consistent with a role for orexin in the enhanced reward-based feeding observed after food deprivation and weight loss.
We hypothesize that hormonal signals of energy status affect reward-based feeding, in part, by altering the glucose sensitivity of VTA projecting orexin neurons. Thus, LHA orexin GI neurons may link reward-based feeding with peripheral energy status. In order to test this hypothesis we first used electrophysiological techniques to determine whether ghrelin exacerbated while leptin attenuated activation of LHA orexin GI neurons by decreases in extracellular glucose concentration. We then determined whether glucose modulated putative VTA DA neurons in an orexin dependent manner using a novel horizontal brain slice containing the LHA and VTA.
Results
Low glucose directly excites LHA orexin neurons
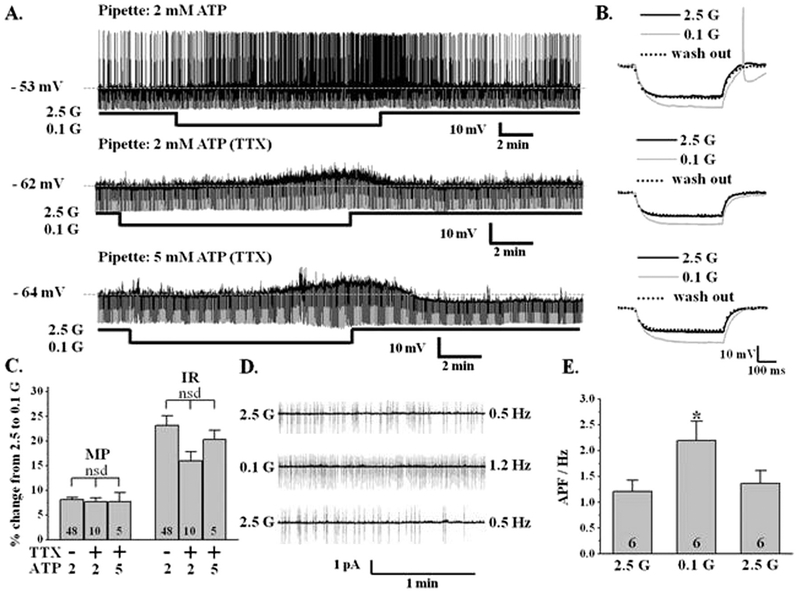
LHA orexin-GFP neurons were defined by their location lateral to the fornix in coronal brain slices. Approximately 60% (48/82) of LHA orexinGFP neurons were glucose inhibited (GI) neurons. In the whole cell current clamp recording mode decreasing glucose from 2.5 to 0.1 mM reversibly depolarized the orexin-GI neurons by 8.1 ± 0.5% (2.5 mM glucose: −56.9 ± 0.8 mV; 0.1 mM: −52.3 ± 0.8 mV; n = 48; P < 0.0001) and increased input resistance by 23 ± 2% (2.5 mM glucose: 744 ± 36 MΩ; 0.1 mM: 921 ± 47 MΩ; n = 48; P b 0.005); the latter indicating ion channel closure (Fig. 1A, B, C). Decreased glucose from 2.5 to 0.7 mM also significantly and reversibly depolarized orexin-GI neurons (3.6 ± 0.3%; P < 0.0001) and increased input resistance by 10.12 ± 0.8% (P < 0.0001; n = 6). The percent change in membrane potential and input resistance in response to decreased glucose from 2.5 to 0.7 mM was significantly smaller than that observed in response to a glucose decrease to 0.1 mM (P < 0.0001 for both variables) indicating that the effect of glucose is concentration-dependent. The reversal potential for the inhibitory effect of glucose on orexin GI neurons was −103 ± 7 mV (n = 4), which is close to the potassium equilibrium potential in our solutions (−99 mV). This suggests that low glucose excites these neurons by closing a potassium channel. The effect of glucose persisted in the presence of the sodium channel blocker, tetrodotoxin (TTX, 400 nM; n = 10; Fig. 1A, B, C), which blocks presynaptic action potentials. Thus, glucose directly inhibited these neurons.
Fig. 1. Verification of glucose sensing by orexin neurons.
A) Whole cell current-clamp recordings from representative LHA orexin-GI neurons in coronal brain slices with either 2 or 5 mM ATP in the recording pipette. For this and all subsequent current clamp traces the baseline membrane potential (MP) is indicated by a dotted line and given at the left of the trace. The upward deflections are action potentials and the downward deflections are the membrane voltage response to a constant hyperpolarizing current pulse. Input resistance (IR) is calculated according to Ohm’s Law where voltage is equal to current times resistance. A change in IR is directly proportional to a change in the voltage response. Approximately 60% (48/82) of LHA orexin- GFP neurons were inhibited by glucose indicating that they were GI neurons. Decreasing glucose (G) from 2.5 to 0.1 mM reversibly depolarized this neuron and increased action potential frequency and IR (top trace). The effects of glucose persisted in the presence of tetrodotoxin (TTX; blocks presynaptic action potentials) in the extracellular recording medium (middle trace). The effects of glucose also persisted in the presence of TTX when 5 mM ATP was included in the recording pipette (bottom trace). B) Representative traces of the voltage response to a constant hyperpolarizing pulse from each of the traces in (A) in an expanded time scale. Decreasing glucose from 2.5 to 0.1 mM reversibly increased IR as indicated by an increase in the voltage response independent of the pipette ATP concentration or the presence of TTX. C) Data bars represent the % change in MP and IR in 0.1 mM glucose relative to 2.5 mM in the presence and absence of TTX. Decreased glucose depolarized LHA orexin-GI neurons by 8.1 ± 0.5% and increased their input resistance by 23 ± 2% (n = 48). There were no significant differences in the response to decreased glucose regardless of whether TTX was present in the recording media (n = 10) or whether 2 (n = 10) or 5 (n = 5) mM ATP was included in the pipette solution (Student’s t-test, P > 0.05). Thus, decreased glucose directly excited LHA orexin neurons. D) Cell attached voltage-clamp recording of action potentials from an LHA orexin-GI neuron in a coronal brain slice. Decreasing glucose from 2.5 to 0.1 mM significantly increased action potential frequency. E) Data bars represent the action potential frequency in Hz. Decreased glucose reversibly increased action potential frequency by 82 ± 25% (Student’s t-test, *P b 0.05, n = 6). nsd: no significant difference. N values are indicated within the bar graphs.
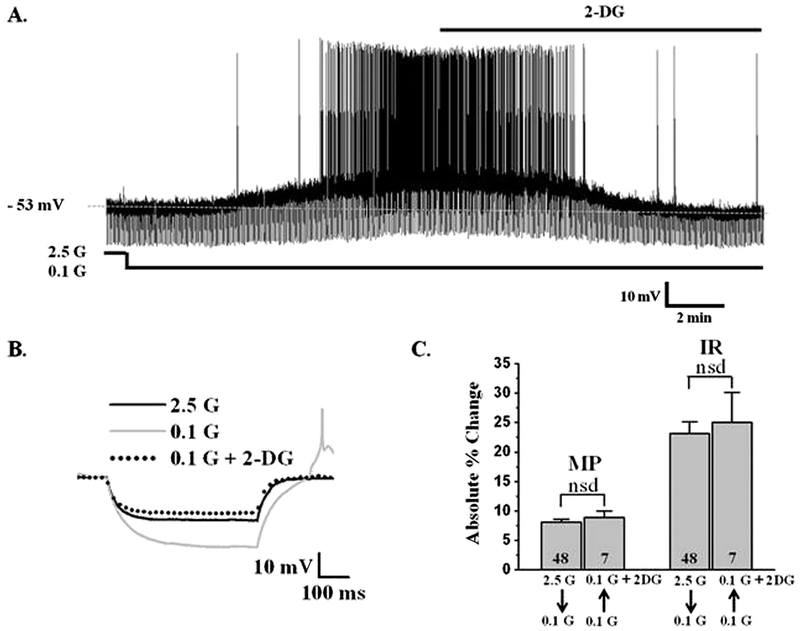
Whole cell recordings are commonly performed using 2 mM ATP in the recording pipette (Song et al., 2001; Spanswick et al., 1997; van den Pol et al., 1998). However, Liu et al. suggested that orexin neurons are more metabolically active and require higher intracellular ATP levels for normal function (Liu et al., 2011). In their hands, orexin neurons were not sensitive to decreased glucose when the pipette solution contained 5 mM ATP. In contrast, we found that glucose directly (e.g., in the presence of TTX) inhibits orexin neurons regardless of whether the recording pipette contained 2 or 5 mM ATP (Fig. 1A, B, C). Moreover, there were no significant differences between the percent change in membrane potential or input resistance in response to decreased glucose with either 2 (n = 10) or 5 (n = 5) mM ATP in the pipette with and without TTX (P > 0.05; Fig. 1C). We also evaluated orexin neurons using the cell attached patch clamp configuration in which the intracellular milieu is completely preserved. As shown in Fig. 1D and E, decreasing glucose from 2.5 mM to 0.1 mM significantly and reversibly increased the firing rate of orexin neurons by 82 ± 25% (P < 0.05, n = 6). Together, these data indicate that glucose sensing is not an artifact of intracellular dialysis during standard whole cell recording. Finally, the non-metabolizable glucose analog, 2-deoxyglucose (2DG; 2.5 mM), completely reversed the effects of decreased glucose on orexin GI neurons (n = 7; Fig. 2). This suggests that LHA orexin-GI neurons respond to the glucose molecule per se as opposed to a downstream product of glucose metabolism (e.g., ATP).
Fig. 2. 2-Deoxyglucose (2-DG) mimics the effect of glucose in LHA orexin-GI neurons.
A) Whole cell current clamp recording of an LHA orexin-GI neuron in a brain slice. Decreasing glucose (G) from 2.5 to 0.1 mM depolarized this neuron and increased input resistance (IR) and action potential frequency. The addition of 2.5 mM 2-DG to recording medium containing 0.1 mM glucose reversed the effect of low glucose. B) Representative traces of the voltage response to a constant hyperpolarizing pulse under the conditions shown in (A). Decreased glucose from 2.5 to 0.1 mM reversibly increased IR as indicated by an increase in the voltage response. The addition of 2-DG to 0.1 mM glucose completely reversed the effect of decreased glucose on IR. C) Data bars represent the absolute value of the % change in membrane potential (MP) and IR. For each variable the left bar represents the % change when glucose was lowered from 2.5 to 0.1 mM (in this case the absolute value of the % change was positive). The right bar represents the % change when 2.5 mM 2-DG was added to 0.1 mM glucose (in this case the absolute value of the % change was negative). The magnitude of the change in MP and IR when glucose was lowered from 2.5 to 0.1 mM or when 2.5 mM 2-DG was added to 0.1 mM glucose was identical (Student’s t-test, P > 0.05; n = 7). Thus, 2-DG completely reproduces the effect of glucose on orexin-GI neurons. nsd: no significant difference. N values are indicated within the bar graphs.
Metabolic hormones regulate the glucose sensitivity of LHA orexin-GI neurons
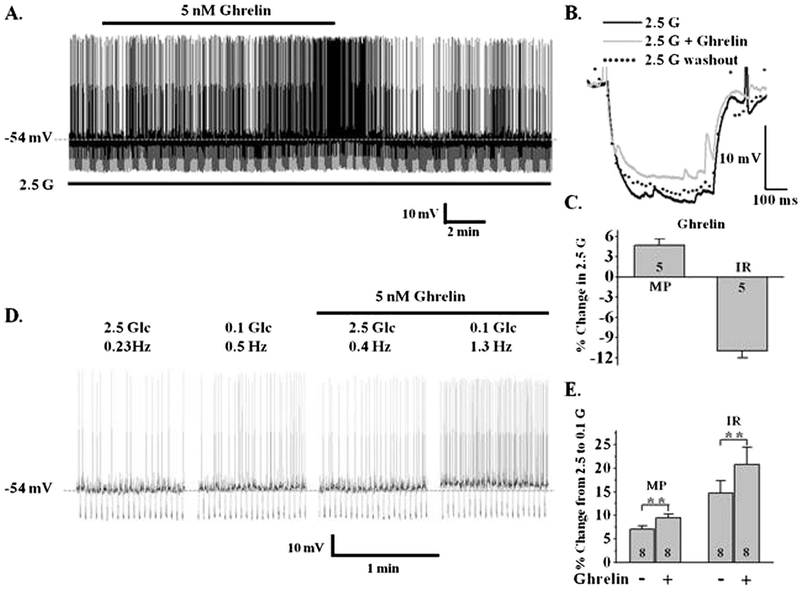
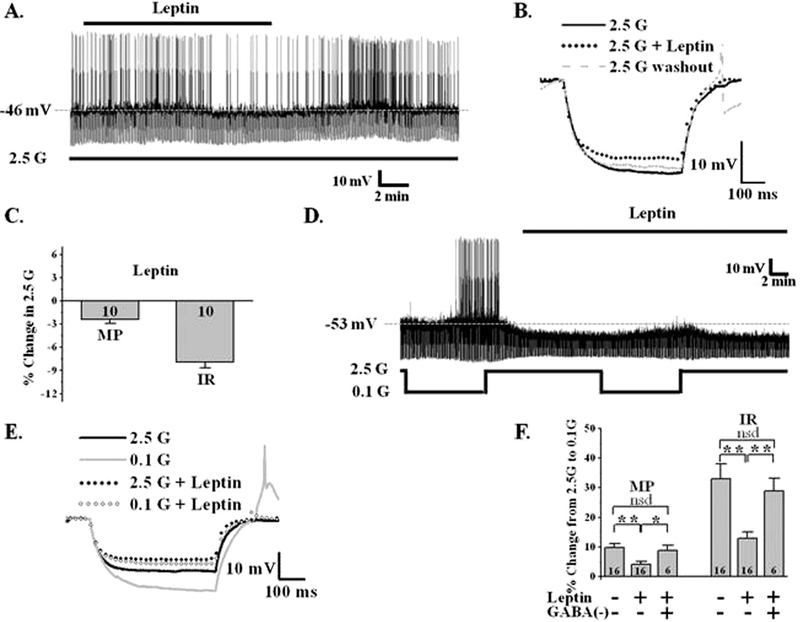
Studies using calcium imaging as a surrogate for neuronal activity suggest that the peripheral hormones of energy status, ghrelin and leptin, regulate the activity of LHA orexin neurons (Yamanaka et al., 2003). We first confirmed the effects of these hormones on neuronal activity in 2.5 mM glucose. As expected, 5 nM ghrelin depolarized orexin-GI neurons by 4.7 ± 0.9% (control: −55.4 ± 2.5 mV; ghrelin: −52.5 ± 2.7 mV, n = 6, P < 0.001, paired t-test) and increased their action potential frequency (n = 5; Fig. 3A, C). This was associated with an 11 ± 1.0% decrease in input resistance (control: 819.7 ± 97.4 MΩ; ghrelin: 743 ± 91.8 MΩ, n = 6, P < 0.001, paired t-test) indicating ion channel activation (Fig. 3B, C). The effect of ghrelin reversed at −35 ± 7 mV (n = 5), suggesting activation of a non-selective cation channel. Ghrelin’s effect persisted in TTX (n = 3) indicating that ghrelin directly activates orexin GI neurons. We next evaluated the effects of ghrelin on glucose sensitivity of orexin GI neurons. A change in glucose sensitivity was defined as a change in the magnitude of the response to a given change in extracellular glucose. As our hypothesis predicted, ghrelin enhanced the depolarization and increase in input resistance which occurs in orexin-GI neurons when glucose decreases from 2.5 to 0.1 mM by 31 ± 13% and 41 ± 24% respectively (P < 0.05, paired t-test; n = 8; Fig. 3D, E). In contrast, leptin (10 nM) hyperpolarized LHA orexin-GI neurons by 2.4 ± 0.5% (control: −58.9 ± 1.8 mV; leptin: −60.1 ± 2.0 mV, n = 10; P < 0.001, paired t-test), reduced their action potential frequency and decreased input resistance by 7.9 ± 0.7% (control: 743.6 ± 75.4 MΩ; leptin: 685.3 ± 68.2 MΩ, n = 10; P < 0.001, paired t-test; Fig. 4A, B, C). Thus, leptin inhibits LHA orexin-GI neurons by opening an ion channel. As we predicted, 10 nM leptin blunted the increased input resistance of LHA orexin-GI neurons associated with lowering glucose from 2.5 to 0.1 mM by 53 ± 12% (n = 16; P < 0.0001; paired t-test; Fig. 4D, E). The effects of both hormones on the activity and glucose sensitivity of orexin-GI neurons were reversible (Figs. 3, 4).
Fig. 3. Ghrelin enhances the activation of LHA orexin-GI neurons by decreased glucose.
A) Whole cell current-clamp recording from an LHA orexin-GI neuron in a brain slice. 5 nM ghrelin depolarized and increased the action potential frequency while decreasing input resistance (IR) of this orexin-GI neuron in 2.5 mM glucose (G). B) Representative trace of the voltage response to a constant hyperpolarizing current pulse under conditions in (A). Ghrelin reversibly decreased IR in 2.5 mM glucose as indicated with a decreased voltage response. C) Data bars represent % change in membrane potential (MP) and IR when ghrelin is added to 2.5 mM glucose. Ghrelin significantly depolarized orexin neurons by 4.7 ± 0.9% (MP) and reduced their IR by 11 ± 1.0% in 2.5 mM glucose (n = 5). D) Ghrelin enhanced the increase in action potential frequency of this LHA orexin-GI neuron occurring in decreased glucose. E) Data bars represent the % change in MP and IR as glucose was lowered from 2.5 to 0.1 mM in the presence and absence of ghrelin. Ghrelin enhanced the depolarization and increase in IR observed with decreased glucose by 31 ± 13 and 41 ± 24%, respectively (Student’s paired t-test; **P < 0.01; n = 8). N values are indicated within bars.
Fig. 4. Leptin blunts the response of LHA orexin-GI neurons to decreased glucose.
A) Whole cell current-clamp recording from an LHA orexin-GI neuron in a brain slice. Leptin hyperpolarizes this orexin-GI neuron in 2.5 mM glucose (G) and reduces the action potential frequency and input resistance (IR). B) Representative traces of the voltage response to a constant hyper- polarizing current pulse under the conditions in (A). Leptin reversibly decreased IR in 2.5 mM glucose as indicated by a decrease in the voltage response. C) Data bars represent % change in membrane potential (MP) and IR of LHA orexin-GI neurons when leptin is added to 2.5 mM glucose. Leptin significantly hyperpolarized the MP of orexin-GI neurons by 2.4 ± 0.5% and decreased their IR by 7.9 ± 0.7% (n = 10). D) Whole cell current-clamp recording from an LHA orexin-GI neuron in a brain slice. Leptin attenuates the response of this orexin-GI neuron to 0.1 mM glucose. E) Representative traces of the voltage response to a constant hyperpolarizing current pulse under the conditions in (D). Leptin blunted the increase in IR as glucose decreased from 2.5 to 0.1 mM. F) Data bars represent the % change in MP or IR as glucose was lowered from 2.5 to 0.1 mM. Leptin significantly reduced the change in MP and IR normally associated with decreased glucose (n = 16). The effect of leptin on glucose sensitivity was blocked by the GABA A and B receptor antagonists, bicucculline (20 μM) and saclofen (100 μM) (n = 6). Data were analyzed by t-test (*P < 0.05; **P < 0.01). N values are indicated within bars.
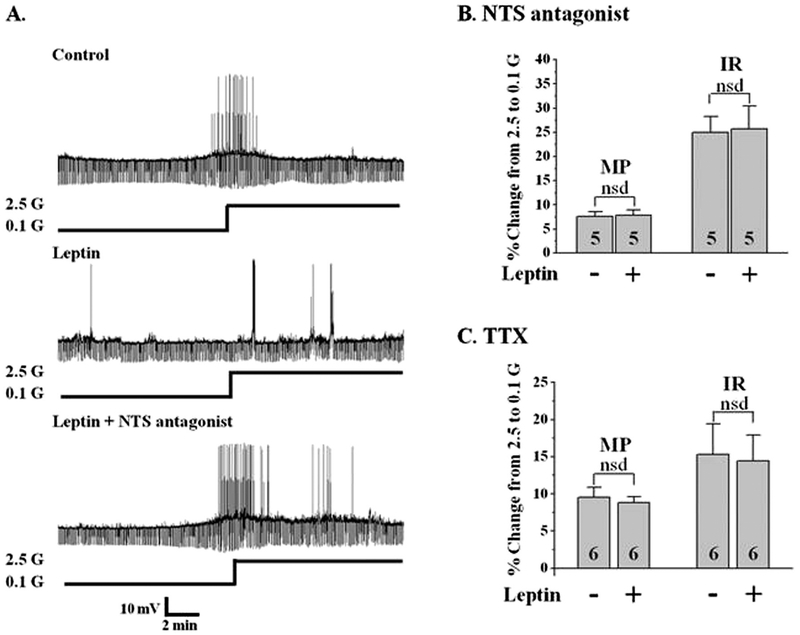
While our data and that of others support a direct effect of ghrelin on LHA orexin-GI neurons, a direct effect for leptin is controversial (Diano et al., 2003; Leinninger et al., 2011; Yamanaka et al., 2003). Diano et al. showed that orexin neurons are leptin receptor immunopositive and Yamanaka et al. showed that isolated orexin neurons are leptin sensitive (Diano et al., 2003; Yamanaka et al., 2003). However, Leinninger et al. found that orexin was not colocalized with GFP in mice with GFP labeling on leptin-receptor expressing neurons (Leinninger et al., 2011). Instead, these investigators found that the majority of LHA leptin receptor positive neurons projecting to LHA orexin neurons are GABAergic. These GABA interneurons would be present in our coronal sections of the LHA. In support of this latter concept, we found that leptin failed to alter glucose sensitivity of orexin neurons when GABA A and B receptors were blocked by bicuculline (20 μM) and saclofen (100 μM) respectively (n = 6, P > 0.05; Fig. 4F). Leptin receptors are also expressed on neurotensin neurons in the LHA (Leinninger et al., 2011), thus these neurons are also a potential site for the presynaptic effects of leptin. To test this hypothesis we evaluated the effects of leptin in the presence of the neurotensin receptor antagonist SR142948 (1 μM), which blocks both neurotensin 1 and 2 receptors. As shown in Fig. 5A, B, leptin did not blunt the effect of decreased glucose on orexin-GI neurons when neurotensin receptors were blocked (n = 5, P > 0.5). As expected from our results with GABA and neurotensin receptor blockers, leptin was also unable to blunt the effect of decreased glucose in the presence of TTX (Fig. 5C, n = 6). Finally post-recording single cell (sc) RT-PCR indicated orexin but not leptin receptor expression in LHA orexin-GI neurons (n = 4). The above data are consistent with those from the Myers laboratory and suggest that leptin modulates the glucose sensitivity of orexin-GI neurons via actions on presynaptic GABA and/or neurotensin neurons.
Fig. 5. The effect of leptin is presynaptic and dependent on neurotensin (NTS).
A) Whole cell current-clamp recordings from an LHA orexin-GI neuron in a brain slice. Leptin significantly reduced the change in membrane potential (MP) and input resistance (IR) normally associated with decreased glucose (G; top 2 traces; n = 16). The neurotensin receptor antagonist (SR142948, 1 μM) blocked the effect of leptin on the glucose sensitivity of this orexin-GI neuron (bottom trace; N = 5). B and C) Bars represent the % change in MP and IR when glucose was lowered from 2.5 to 0.1 mM in the presence or absence of leptin when either the neurotensin receptor antagonist (B) or TTX (C) was present. There was no significant effect of leptin on the response to decreased glucose in the presence of the neurotensin antagonist (Student’s t-test, P > 0.05; n = 5). Similarly, leptin had no effect on glucose sensitivity in the presence of TTX (n = 6). Post-recording single cell (sc) RT-PCR indicated orexin but not leptin receptor expression in LHA orexin-GI neurons (n = 4). Data were analyzed by paired t-test. nsd: no significant difference. N values are indicated within bars.
Fasting increases activation of LHA orexin-GI neurons by decreased glucose
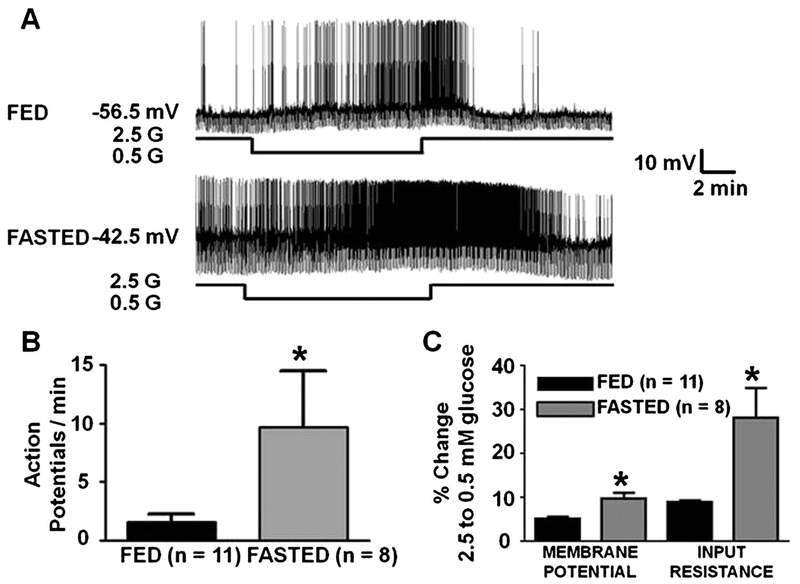
Fasting increased the overall activity of LHA orexin-GI neurons in 2.5 mM glucose as indicated by an increase in action potential frequency from 1.45 ± 0.6 (fed mice; n = 11) to 9.57 ± 5.2 (fasted mice; n = 12; P = 0.05; Fig. 6A, B) per minute. In 2.5 mM glucose, the membrane potential from orexin-GI neurons was slightly but not significantly more depolarized in the fasted (−51.8 ± 2.2 mV) vs fed (−55.7 ± 1.8 mV; P = 0.2) mice. Input resistance in 2.5 mM glucose did not differ between the groups. There was also no difference in the response of orexin-GI neurons from fed and fasted mice to a maximal glucose decrease from 2.5 to 0.1 mM. However, LHA orexin-GI neurons from fasted mice were significantly more depolarized in 0.5 mM glucose than those from fed mice (membrane potential in 0.5 mM glucose; fed: −52.6 mV, n = 11; fasted: −46.6 ± 1.8 mV, n = 8; P < 0.05). Moreover, the percent change in membrane potential and input resistance in response to decreased glucose from 2.5 to 0.5 mM was significantly greater in orexin-GI neurons from fasted vs fed mice (Fig. 6A, C). There was no significant difference in the response of orexin-GI neurons to glucose decreases from 2.5 to 0.5 mM and 2.5 to 0.7 mM (P > 0.05; see text in Low glucose directly excites LHA orexin neurons section and Fig. 6C).
Fig. 6. Fasting enhances the response of LHA orexin-GI neurons to decreased glucose.
A) Whole cell current-clamp recordings from an LHA orexin-GI neuron in brain slices from fed and fasted orexin-GFP mice. Fasting increased overall neuronal activity and enhanced the response to decreased glucose from 2.5 to 0.5 mM in these neurons. B) Action potential frequency of orexin- GI neurons from fasted mice was significantly greater in 2.5 mM glucose than in orexin-GI neurons from fed mice. C) Bars represent the % change in membrane potential and input resistance when glucose was lowered from 2.5 to 0.5 mM in orexin-GI neurons from fed and fasted mice. Fasting significantly enhanced depolarization and increased input resistance associated with decreased glucose. *Student’s t-test, P < 0.05.
Glucose-modulated orexin effects on putative VTA DA neurons
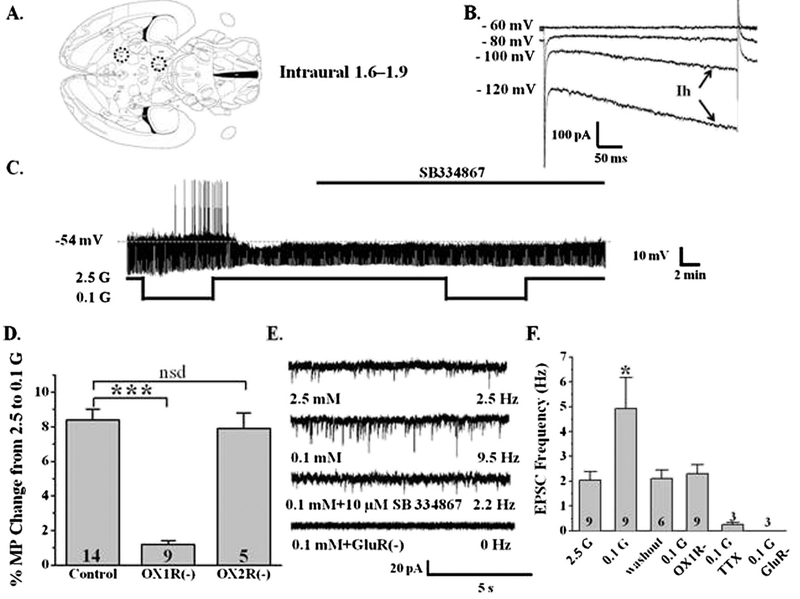
A horizontal brain slice preparation was used to preserve the neurocircuitry between the LHA and VTA (Fig. 7A). Using the whole cell voltage clamp recording mode, we identified VTA neurons from wild-type mice as putatively dopaminergic by the presence of an “H current” (Ih; Fig. 7B). Over 90% of VTA neurons exhibiting a strong Ih are DA neurons (Korotkova et al., 2003). A strong Ih was defined as a current sag greater than 150 pA at the end of a 500 ms voltage command to −120 mV as shown in Fig. 7B (Ford et al., 2006). We then used the current clamp recording mode to determine whether decreased glucose increased action potential frequency of VTA DA neurons in an orexin dependent manner. Fig. 7C shows a representative current clamp recording of a VTA DA neuron in a horizontal slice identified as above. This VTA DA neuron depolarized by 4.3 mV and increased its action potential frequency as glucose was decreased from 2.5 to 0.1 mM. Decreased glucose significantly depolarized 14 of 31 VTA DA neurons (8.3 ± 0.7%, P b 0.05) leading to increased action potential frequency (Fig. 7D). The excitatory effect of decreased glucose was blocked by the orexin 1 receptor antagonist SB334867 (10 μM) but not by the orexin 2 receptor antagonist TCS-OX2–29 (2 μM; Fig. 7D). Since orexin is only expressed in the hypothalamus, these data strongly support the presence of orexin projections from the hypothalamus to the VTA in our slice preparation. Connectivity between the LHA and VTA was also verified using whole cell current clamp recording in VTA DA neurons during electrical stimulation of the LHA. LHA electrical stimulation (5 V, 2 Hz, 5 ms duration) significantly and reversibly increased the action potential frequency of 4 out of 13 VTA DA neurons (before stimulation: 1.7 ± 0.2 Hz; during stimulation: 5.8 ± 1.1 Hz; after stimulation: 1.9 ± 0.3 Hz; N = 4, P < 0.01 during vs before stimulation).
Fig. 7. Glucose modulation of the activity of VTA DA neurons is mediated by orexin and glutamate.
A) Horizontal brain slice preparation containing the LHA and VTA as indicated by the dotted circles (modified from The Rat Brain Atlas, Page 96, George Paxinos and Charles Watson (Paxinos and Watson, 1998)). B) Whole cell voltage clamp recording illustrating a hyperpolarization-activated inwardly rectifying non-specific cation (Ih or HCN) current in a VTA neuron. Ih is visualized as the inward (downward) current in response to hyperpolarizing voltages below −100 mV. C) Whole cell current clamp recording from a VTA DA neuron. Decreasing glucose (G) increased action potential frequency. This effect was blocked by the orexin A receptor antagonist SB334867 (SB). D) Bars represent the % change in membrane potential (MP) when glucose was lowered from 2.5 to 0.1 mM. The orexin A (***P < 0.001; N = 9) but not B (TCS-OX2–29; P > 0.05; N = 5) receptor antagonist blocked the effect of decreased glucose on VTA DA neurons. E) Voltage clamp recording of spontaneous excitatory postsynaptic currents (sEPSCs) in a VTA DA neuron. sEPSCs were measured in the presence of the GABA-A receptor blocker, bicuculline. sEPSC frequency was increased when glucose was lowered from 2.5 to 0.1 mM. The effect of decreased glucose was blocked by the orexin A receptor antagonist (SB). sEPSCs were completely blocked by antagonists for the glutamate NMDA (APV) and AMPA (CNQX) receptors. F) Frequency (Hz) of sEPSCs recorded from VTA DA neurons in brain slices. sEPSC frequency was significantly enhanced (125 ± 40%; Student’s t-test, *P < 0.05; n = 9) by decreasing glucose from 2.5 to 0.1 mM. The effect of 0.1 mM G was blocked by the orexin A receptor antagonist, while sEPSCs were completely blocked by TTX and NMDA and AMPA glutamate receptor blockers (GluR-). nsd: no significant difference. N values are indicated within or above bars.
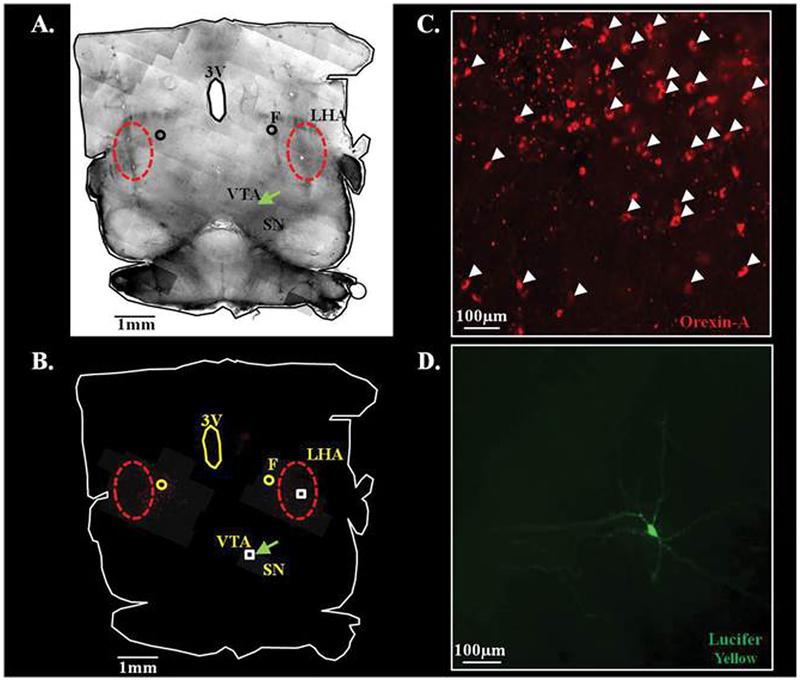
Enhanced glutamate signaling underlies orexin excitation of VTA DA neurons (Borgland et al., 2006, 2009). In order to determine whether this was also the case for glucose dependent orexin effects, voltage clamp recordings in VTA DA neurons were performed at a holding potential of −60 mV (Fig. 7E, F). Spontaneous excitatory post-synaptic currents (sEPSCs) were recorded as inward currents in the presence of the GABA A and B receptor inhibitors (20 μM bicuculline, 100 μM saclofen, respectively) which block inhibitory PSCs. Decreased glucose reversibly increased sEPSC frequency of VTA DA neurons by 125 ± 40% (Fig. 7E, F; n = 9; P b 0.05). The effect of decreased glucose on sEPSC frequency was blocked by orexin 1 receptor antagonist (n = 6; P < 0.05; Fig. 7E, F), indicating that the effects of low glucose on VTA DA neuron glutamate transmission are mediated by LHA orexin neurons. sEPSCs were completely blocked by application of glutamate receptor (AMPA: 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione [CNQX]; NMDA: 20 μM (2R)-amino-5-phosphonovaleric acid [APV]) antagonists indicating that the sEPSCs were mediated by glutamatergic synapses (n = 3; P < 0.05; Fig. 7E, F). TTX also blocked sEPSCs indicating that they were dependent on presynaptic action potentials (n = 3; P < 0.05; Fig. 7F). Finally, Fig. 8 illustrates that a horizontal slice section in which we recorded from a VTA DA neuron (labeled with Lucifer Yellow) possesses LHA orexin-immunopositive neurons. These data suggest that changes in glucose concentrations modulate the activity of VTA DA neurons by altering orexin dependent regulation of glutamatergic input onto these neurons.
Fig. 8. Immunohistochemical verification of the presence of LHA orexin neurons in a horizontal brain slice in which a VTA DA neuron was evaluated.
Composite 10× brightfield (A) and merged red- and green-filter (B) images of a horizontal brain section following electrophysiological recording of a Lucifer Yellow dialyzed VTA DA neuron (green arrow). Cortical areas have been removed and red circles define the borders of the LHA. C and D) 40× magnification of the white boxed areas indicated in B. White arrow heads indicate orexin-A immunolabeled cell bodies in C. The Lucifer Yellow containing VTA DA neuron is shown in D. 3V: 3rd ventricle, F: fornix, LH: lateral hypothalamus, SN: substantia nigra, VTA: ventral tegmental area.
Discussion
Our data support the hypothesis that peripheral signals of energy status change the magnitude of the response of LHA orexin-GI neurons to a given glucose decrease. In this way, metabolic status regulates the glucose sensitivity of LHA orexin-GI neurons such that glucose deficit reinforces activation of putative VTA DA neurons under conditions of fasting or weight loss. The cartoon in Fig. 9 summarizes our results. The satiety hormone, leptin, reduces activation of LHA orexin-GI neurons by decreased glucose. In contrast ghrelin, which is released during times of hunger, enhances the activity of LHA orexin-GI neurons in decreased glucose. Our data showing that an overnight fast increases the action potential frequency of LHA orexin-GI neurons in 2.5 mM glucose as well as enhancing their activation by decreased glucose is consistent with these hormonal effects. Thus during fasting or weight loss, decreased leptin levels and increased ghrelin levels would potentiate the response of LHA orexin-GI neurons to decreased glucose. Our data are also the first to show that activation of orexin neurons by low glucose increases glutamate signaling onto VTA DA neurons. Taken together these data suggest that increased activation of LHA orexin-GI neurons by decreased glucose could enhance reward-based feeding and contribute to the difficulty in maintaining weight loss, especially after dieting.
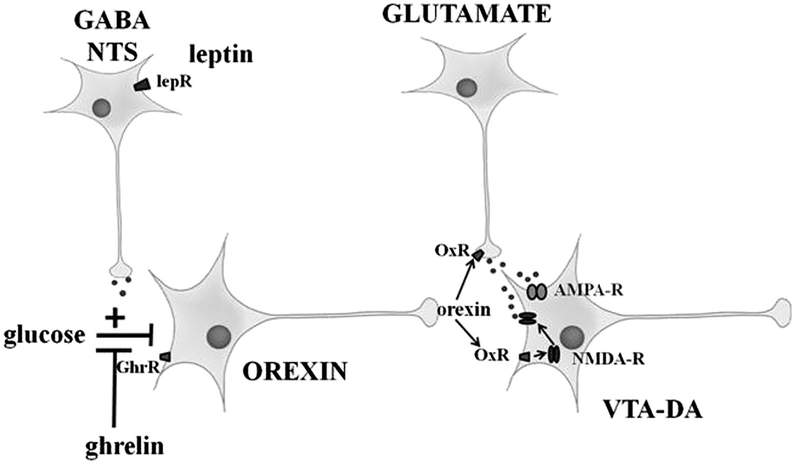
Fig. 9. Summary diagram illustrating the neurocircuitry evaluated in this study.
The ventral tegmental area (VTA) dopamine (DA) neurons project to the nucleus accumbens (Vittoz et al., 2008). Lateral hypothalamic area (LHA) orexin neurons activate VTA DA neurons by enhancing excitatory glutamate signaling (Borgland et al., 2006, 2009). Glucose directly inhibits LHA orexin neurons (as shown herein and by Burdakov et al., 2005). We hypothesize that one way that hormonal signals of energy balance influence reward pathways is by modulating the response of LHA orexin neurons to changes in glucose. Leptin indirectly potentiates (via upstream GABA and neurotensin neurons) while ghrelin directly blunts glucose inhibition of orexin neurons. OxR: orexin receptor; lepR: leptin receptor; GhrR: ghrelin receptor; NTS: neurotensin; GABA: γ-aminobutyric acid; AMPA-R: α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid receptor; NMDA-R: N-methyl-D-aspartate receptor.
There are several controversies in the literature regarding orexin-GI neurons. The first relates to whether or not they are truly glucose sensing. Over the past decade, Denis Burdakov’s laboratory has consistently shown that glucose directly inhibits LHA orexin neurons by opening a K+ channel (Burdakov et al., 2005, 2006; González et al., 2009). This inhibitory effect of glucose is not dependent on intracellular metabolism since orexin neurons were also inhibited by the non-metabolizable glucose analog, 2-DG (Gonzalez et al., 2008). However, 2 recent studies contradict these findings. Lui et al. reported that LHA orexin neurons were sensitive to intracellular ATP levels. Moreover, they showed that raising the ATP level in the whole cell recording pipette above 2 mM prevented orexin neurons from being activated by low glucose (Liu et al., 2011). In contrast, Parsons and Hirasawa showed that complete glucose deprivation (0 mM) inhibited LHA orexin neurons (Parsons and Hirasawa, 2010). Thus, we wished to reconcile these findings prior to evaluating the metabolic regulation of glucose sensing in these neurons.
We found that orexin-GI neurons make up ~60% of the LHA orexin population while the remaining 40% are glucose insensitive. Our results regarding the orexin-GI population are in mechanistic agreement with those of Burdakov’s laboratory. That is, the effects of glucose on LHA orexin-GI neurons persisted in the presence of TTX which blocks presynaptic action potentials, indicating a direct effect. LHA orexin-GI neurons responded similarly to glucose and 2-DG consistent with a metabolism independent effect. The effect of glucose reversed at the K+ equilibrium potential consistent with opening of a K+ channel. Importantly, raising the pipette ATP concentration to 5 mM did not block the response of LHA orexin-GI neurons to decreased glucose. Furthermore, the response to decreased glucose was observed using the cell attached recording configuration which preserves the integrity of the intracellular milieu and thus would not alter intracellular ATP levels. These data suggest that discrepancies in the literature may be explained by different subpopulations of orexin neurons. There is significant heterogeneity in orexin neurons in terms of projection and function (Antunes et al., 2001; Cason et al., 2010; Cheng et al., 2003; Chou et al., 2003; González et al., 2012; Horvath and Gao, 2005; Horvath et al., 1999; Jiménez et al., 2013; Nollet et al., 2011; Peyron et al., 1998; Sakurai, 2002; Tupone et al., 2011). One limitation of the orexin-GFP mouse is that our study was restricted only to the subpopulation of orexin neurons which actually express GFP (Li et al., 2002). Moreover heterogeneity with respect to glucose sensitivity has also been described for Neuropeptide Y neurons in the arcuate nucleus (Fioramonti et al., 2007). Thus, the LHA orexin neurons which we sampled may have overlapped both the glucose sensitive (GI) and insensitive populations tested by Burdakov and Lui et al., respectively. Finally, Parsons and Hirasawa (2010) show that orexin neurons are inhibited by complete glucose deprivation and activated by astrocytic lactate. Our data do not contradict these findings. First, complete glucose deprivation (0 mM) is not physiological. In our study we evaluated glucose decreases which would be relevant to a profound fast or hypoglycemia (e.g., 0.5–0.7 or 0.1 mM) (De Vries et al., 2003; Dunn-Meynell et al., 2009; Silver and Erecinska, 1994). Furthermore, activation by lactate does not preclude glucose inhibition since we find similar activation of ventromedial hypothalamic GI neurons by lactate (Song and Routh, 2005). It is also possible that the lactate sensitive orexin neurons belong to the glucose insensitive population of orexin neurons.
A second controversy in the literature relates to whether orexin neurons possess leptin receptors and thus respond directly to leptin. Using calcium imaging as an index of neuronal activity, Yamanaka et al. showed that leptin inhibits isolated orexin neurons suggesting a direct action for leptin (Yamanaka et al., 2003). In support of this Diano et al. showed leptin receptor immunoreactivity on orexin neurons (Diano et al., 2003). In contrast, the Myers laboratory did not observe an overlap between orexin immunopositive neurons and neurons in which GFP labeling indicated leptin receptor expression (Leinninger et al., 2011). Our data are consistent with those of Myers and colleagues. Like Yamanaka et al. (2003), we found that leptin inhibited LHA orexin-GI neurons. However, leptin’s effects were blocked by TTX indicating a presynaptic effect. In addition, LepR mRNA was not detected by post-recording sc-PCR analysis. Myers and colleagues have shown that LHA GABA and neurotensin co-expressing neurons possess leptin receptors and that leptin directly stimulates these neurons (Leinninger et al., 2009, 2011). Consistent with their findings the effects of leptin on LHA orexin-GI neurons were blocked by either GABA or neurotensin receptor antagonists. Our data also show that leptin opens an ion channel on orexin-GI neurons as indicated by decreased input resistance. This is consistent with leptin receptor activation of neurotensin/GABA neurons which synapse onto orexin neurons. Increased transmitter release would then activate ion channels located on orexin neurons. Unfortunately, this precludes determination of the leptin sensitive conductance/channel. In contrast to leptin, ghrelin acted directly on LHA orexin-GI neurons as shown previously (Yamanaka et al., 2003). The effect of ghrelin reversed at −35 mV suggesting activation of a non-selective cation channel. This is consistent with previous reports showing ghrelin activation of a calcium channel on growth hormone releasing hormone neurons (Osterstock et al., 2010).
Our findings show that leptin and ghrelin modulate not only the activity of orexin neurons but also their glucose sensitivity. Leptin blunts while ghrelin enhances the response of LHA orexin GI neurons to decreased glucose. These data are consistent with the effects of leptin on ventromedial hypothalamic GI neurons which we have previously reported (Murphy et al., 2009). Reciprocal modulation of the glucose sensitivity of LHA orexin-GI neurons by leptin and ghrelin is also consistent with our overall hypothesis that energy deprivation attenuates the inhibitory effect of glucose on these neurons. This metabolic regulation of glucose sensitivity could influence orexin output in several ways. First, it is possible that during energy deprivation, orexin-GI neurons may be activated by small preprandial glucose decreases. On the other hand, orexin-GI neurons may simply be more active in normal brain glucose concentrations. In both cases, increased orexin output under conditions of energy deficit could reinforce reward-based signaling in the VTA. The observation that fasting increased the action potential frequency of LHA orexin-GI neurons in 2.5 mM glucose as well as enhancing activation in low glucose is consistent with both of these scenarios. Importantly, the effect of fasting was observed in response to a decrease in glucose which is relevant to that seen in the brain of fasted animals (De Vries et al., 2003). These data also suggest that metabolic regulation of the glucose sensitivity of LHA orexin-GI neurons is physiologically relevant.
Further support for a role of LHA orexin-GI neurons in reinforcing VTA DA signaling is illustrated by our findings using a novel horizontal brain slice which contains the orexin projections from the LHA to the VTA. Using this preparation, we found that lowering glucose within the physiological range is sufficient to increase the activity of putative VTA DA neurons. The effect of glucose did not persist in the presence of the orexin 1 receptor antagonist. These data indicate that decreased glucose activates VTA DA neurons by increasing orexin release. One possibility is that the terminals of orexin neurons within the VTA are glucose sensing. However blocking action potential mediated synaptic transmission with TTX prevented the effect of glucose on sEPSCs in VTA DA neurons. This suggests that the site of glucose regulation of orexin signaling was mediated by an effect on the soma (or dendrites) of an upstream orexin neuron. Since orexin is only made in the hypothalamus these data strongly support intact hypothalamic-VTA projections in our slice preparation (Sakurai et al., 1998). Activation of VTA DA neurons by LHA electrical stimulation further confirms the presence of LHA-VTA projections in our preparation. Interestingly, the orexin 1 but not 2 receptor antagonist was effective in blocking the effects of decreased glucose on VTA neuron activity. This is consistent with reports that orexin A vs B plays a greater role in food reward (Teske et al., 2006). Our data are also consistent with literature showing that orexin enhances glutamate signaling onto VTA DA neurons since decreased glucose increased the frequency of glutamate-mediated sEPSCs recorded in VTA DA neurons (Borgland et al., 2006, 2009). In the future it will be interesting to determine whether changes in glucose influence synaptic plasticity at glutamatergic synapses in the VTA.
One caveat is that we are not able to discriminate between the LHA and the other hypothalamic orexin containing nuclei (e.g., perifornical, potentially the dorsomedial areas) in this horizontal brain slice preparation. Differentiating the glucose modulation of LHA from perifornical orexin neurons requires further investigation. Additionally dialysis of glucose into the midbrain increases dopamine release thus it is possible that some VTA neurons may be glucose-excited (GE) neurons (Levin, 2000). However, we are specifically studying excitatory responses resulting from decreased glucose. Thus it is unlikely that this will lead to artifacts in this study. Moreover, since we are evaluating presynaptic glucose effects, we are minimizing any potential postsynaptic effects of increased glucose on VTA neurons. Finally, our current clamp studies reveal minimal (<8%) occurrence of “GE”-like responses at glucose sensitive orexin-VTA synapses under our experimental conditions.
In summary, these studies are the first to show that changes in the activity of glucose sensing neurons are sufficient to influence the activity of downstream neurocircuitry. Thus, these data provide a significant advancement in understanding the relevance of neuronal glucose sensing. Our observations that leptin increases while ghrelin and fasting decrease the inhibitory effect of glucose on LHA orexin-GI neurons suggest that orexin release in the VTA would be increased during fasting or following weight loss. It is also possible that small preprandial glucose decreases might also increase orexin release to a greater extent during energy deprivation. This change in glucose sensitivity may in part explain why it is so difficult to achieve and maintain weight loss resulting from dietary restriction.
Experimental methods
Animals and tissue preparation
Male 4–6 week old C57BL6 or orexin-GFP mice were housed on a 12:12 light:dark cycle with water provided ad libitum in the Research Animal Facility at Rutgers, The State University of New Jersey, RBHSNew Jersey Medical School. All procedures were in accordance with the Institutional Animal Care and Use Committee. Most of the mice received standard rat chow ad libitum; however some mice were fasted for ~24 h (food removed at 12 noon the day prior to sacrifice). On the day of experiment mice were anesthetized and transcardially perfused with ice-cold oxygenated (95%O2/5%CO2) perfusion solution in which NaCl was replaced with sucrose; composition (in mM): 2.5 KCl, 7 MgCl2, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 glucose, 1 ascorbate, and 3 pyruvate; and osmolarity adjusted to ~300 mOsm with sucrose; pH 7.4. Brains were rapidly removed and placed in ice-cold (slushy) oxygenated perfusion solution as previously described (Song et al., 2001; Wang et al., 2004). 320 μm coronal (containing LHA orexin neurons) or horizontal sections (containing LHA orexin and VTA DA neurons) were made on a vibratome (Vibroslice, Camden Instruments, Camden, UK). The brain slices were maintained in oxygenated artificial cerebrospinal fluid (aCSF; in mM: 126 NaCl, 1.9 KCl, 1.2 KH2PO4, 26 NaHCO3, 2.5 glucose, 1.3 MgCl2, and 2.4 CaCl2; osmolarity was adjusted to ~300 mOsm with sucrose; pH 7.4) for at least 1 h at room temperature. They were then transferred to the recording chamber for remainder of the day.
Electrophysiology
Whole cell voltage and current clamp recordings were made as previously described (Song et al., 2001; Wang et al., 2004). Borosilicate pipettes (4–6 MΩ; Sutter Instruments, Novato, CA) were filled with an intracellular solution containing (in mM): 128 K-gluconate, 10 KCl, 4 KOH, 10 HEPES, 4 MgCl2, 0.5 CaCl2, 5 EGTA, 2 Na2ATP, and 0.4 Na2GTP; pH 7.2. Osmolarity was adjusted to 300 mOsm with sucrose. Cells with membrane potentials more negative than −45 mV in 2.5 mM glucose and action potentials which overshoot 0 mV were considered viable for recording. Pipette access resistance under 30 MΩ with less than a 20% change during the time course of the experiment was considered acceptable. For current clamp recordings, treatment effects were quantified using input resistance (IR) and membrane potential (MP) (Song and Routh, 2005). IR was calculated from the membrane voltage responses to a −10 or −20 pA hyperpolarizing pulse (500 ms duration). The recording pipette contained 2 mM ATP unless otherwise noted in the figures (e.g., Fig. 1B). In some experiments Lucifer Yellow (250 μg/ml) was included in pipette solution for post-recording visualization of the neuron. Voltage clamp recordings were low pass-filtered at 1 kHz and data were simultaneously digitized at 5 kHz. Neurons were clamped at −60 mV to record spontaneous excitatory postsynaptic currents (sEPSCs) in the presence of the GABA-A receptor antagonist bicuculline (20 μM). Putative VTA DA neurons from wild-type mice were identified by the presence of a hyperpolarization-activated inwardly rectifying non-specific cation current (HCN; Ih) (Korotkova et al., 2006). Cell attached voltage clamp recording was performed under the same conditions as whole cell voltage clamp except that the membrane was not ruptured. In order to verify connections between the LHA and VTA in our horizontal brain slice a platinum electrode (0.2 mm) was placed in the ipsilateral LHA (just lateral to the fornix) and pulsed electrical stimulation was performed (5 V, 2 Hz, 5 ms duration) using a Grass SD9 stimulator.
Single cell PCR (sc-PCR)
After electrophysiological recording, single cells were extracted for analysis via RT-PCR. Identified neurons were snap frozen on dry ice in an RNAseOUT™ RNase inhibitor (Invitrogen), and stored at −20 °C. cDNA synthesis was performed within three days of collection using a Superscript III first strand synthesis kit (Invitrogen) as per manufacturer’s instructions. RT reactions were performed at 45 °C for 2 h and terminated via heat inactivation at 70 °C for 15 min. PCR was performed using Taqman® primer/probes for glyceraldehyde 3- phosphate dehydrogenase (GAPDH), glial fibrillary acidic protein (GFAP), leptin receptor, and tyrosine hydroxylase (Applied Biosystems). All primers used expanded across two exons to exclude genomic DNA from amplification. PCR reactions were performed for 55 cycles on a LightCycler® (Roche) using the following protocol: denature at 95 °C for 5 min at 20 °C/s; amplify at 60 °C for 1 min at 2 °C/s; cool at 40 °C for 30 min at 20 °C/s. All PCR runs included whole hypothalamic cDNA and autoclaved nanopure water samples as positive and negative control samples, respectively. Whole hypothalamic cDNA was generated from total RNA extracted from mouse hypothalamic tissue. Tissue was collected following transcardial perfusion with 0.9% NaCl 25 U/ml Heparin and homogenized in TRIzol® using homogenization beads. Following phase separation, total RNA was precipitated, washed and re-dissolved in autoclaved nanopure water. The total amount of RNA isolated was determined on a NanoDrop (Thermo). Whole hypothalamic RNA (1–5 μg) was converted to cDNA as described above. The presence of single cell RNA was designated as positive if CT values were above the absolute lower limit determined via cDNA dilution curves for each specific primer. Any cells designated GFAP positive were not further analyzed.
Post-recording immunohistochemistry (IHC)
Following electrophysiological analysis, brain sections were fixed overnight in 4% paraformaldehyde, cyroprotected in 30% sucrose for up to three days, frozen on microscope slides in Tissue Tek and stored at −80 °C until IHC could be performed. Aldehyde conversion (15 min) was performed with fresh 1% sodium borohydride to minimize autofluorescence. Slices were blocked with 10% bovine serum albumin (BSA), 0.3% Triton-X 100 for one hour. All antibodies were freshly diluted in 10% BSA, 0.3% Triton X-100 and 0.1% sodium azide. Primary antibodies (mouse anti-orexin-A, 1:250, Santa Cruz) were applied overnight at 4 °C and, after reblocking for 1 h, secondary antibodies (anti-mouse Alexa 594, 1:300, Invitrogen) were applied for 1 h at room temperature. Slices were mounted on glass slides using an antifade mounting media (Dako). No antibodies were required for visualization of Lucifer Yellow. Images were captured with an Axiovert 200M (Zeiss) fluorescent microscope and optimized with Axiovision 4.5v software. Negative control samples lacking primary antibodies dictated the image exposure time. Composite images were compiled manually using visual landmarks within the bright field filter for each subsequent image as a point of reference.
Chemicals
Ghrelin was obtained from Phoenix Pharmaceuticals Inc. (Burlingame, CA) and leptin from ProSpec-Tany TechnoGene Ltd. (East Brunswick, NJ). Tetrodotoxin, saclofen, bicuculline and the antagonists for the orexin 1 receptor (SB334867), orexin 2 receptor (TCS-OX2–29), neurotensin receptor (SR142948), AMPA receptor (20 μM 6-cyano-7-nitroquinoxaline-2,3-dione [CNQX]) and NMDA receptor ((2R)-amino-5-phosphonovaleric acid [APV]) were obtained from Tocris Bioscience (Minneapolis, MN).
Statistical analysis
All data were expressed as means ± SE. Statistical analysis was performed using Student’s t-test (unpaired or paired). P b 0.05 was considered statistically significant.
Acknowledgments
Breeding pairs of Orexin GFP mice were generously provided by Martin Myers. Funding in part was supported by a grant from New Jersey Health Foundation (VHR) (PC 102–14).
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Ahima RS,Saper CB,Flier JS,Elmquist JK, 2000. Leptin regulation of neuroendocrine systems. Front. Neuroendocrinol 21, 263–307. [DOI] [PubMed] [Google Scholar]
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ, 2001. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol 281, R1801–R1807. [DOI] [PubMed] [Google Scholar]
- Borgland SL,Taha SA,Sarti F,Fields HL,Bonci A, 2006. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang S-J, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci 29, 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A, 2005. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J. Neurosci 25, 2429–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O,Fugger L,Verkhratsky A, 2006. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 50, 711–722. [DOI] [PubMed] [Google Scholar]
- Cai XJPSW,Harrold J,Wilson S,Buckingham RE,Arch JR,Tadayyon M,Clapham JC, Wilding J,Williams G, 1999. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes 48, 2132–2137. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G, 2010. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol. Behav 100, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SB,Kuchiiwa S,Gao HZ,Kuchiiwa T,Nakagawa S, 2003. Morphological study of orexin neurons in the hypothalamus of the Long–Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci. Res 46, 53–62. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J, 2003. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms.J. Neurosci 23, 10691–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS,Breen PA,Ma MK, Dellinger EP, Purnell JQ, 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery.N. Engl. J. Med 346, 1623–1630. [DOI] [PubMed] [Google Scholar]
- De Vries MG,Arseneau LM,Lawson ME,Beverly JL, 2003. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52, 2767–2773. [DOI] [PubMed] [Google Scholar]
- Diano S, Horvath B,Urbanski HF,Sotonyi P, Horvath TL, 2003. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology 144, 3774–3778. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA,Sanders NM,Compton D,Becker TC,Ji Eiki,Zhang BB,Levin BE, 2009. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J. Neurosci 29, 7015–7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL,Hjelmstad GO,Margolis EB,Nicola SM, 2007. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci 30, 289–316. [DOI] [PubMed] [Google Scholar]
- Fioramonti X,Contie S,Song Z,Routh VH,Lorsignol A,Penicaud L, 2007. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in NPY and POMC networks? Diabetes 56, 1219–1227. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT, 2006. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci 26, 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, 2010. Appetite and reward. Front. Neuroendocrinol 31, 85–103. [DOI] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT,Fugger L, Burdakov D, 2008. Metabolism-independent sugar sensing in central orexin neurons. Diabetes 57, 2569–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA,Burdakov D, 2009. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur. J. Neurosci 30, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA,Jensen LT,Fugger L,Burdakov D, 2012. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur. J. Neurosci 35, 1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Gao X-B, 2005. Input organization and plasticity of hypocretin neurons: possible clues to obesity’s association with insomnia. Cell Metab. 1, 279–286. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S,van den Pol AN, 1999. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J. Neurosci 19, 1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A,Caba M,Escobar C, 2013. Food-entrained patterns in orexin cells reveal subregion differential activation. Brain Res. 1513, 41–50. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE, 2003. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J. Neurosci 23, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL, 2006. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci 23, 2677–2685. [DOI] [PubMed] [Google Scholar]
- Leinninger GM,Jo Y-H,Leshan RL,Louis GW,Yang H,Barrera JG,Wilson H,Opland DM, Faouzi MA,Gong Y,Jones JC,Rhodes CJ,Chua S Jr., Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG Jr., 2009. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo Y-H, Faouzi M, Christensen L,Cappellucci Laura A., Rhodes Christopher J., Gnegy Margaret E., Becker Jill B., Pothos Emmanuel N., Seasholtz Audrey F., Thompson Robert C., Myers Jr Martin G., 2011. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, 2000. Glucose-regulated dopamine release from substantia nigra neurons. Brain Res. 874, 158–164. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao XB,Sakurai T,van den Pol AN, 2002. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron—a potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36 (6), 1169–1181. [DOI] [PubMed] [Google Scholar]
- Liu Z-W,Gan G,Suyama S,Gao X-B, 2011. Intracellular energy status regulates activity in hypocretin/orexin neurones: a link between energy and behavioural states. J. Physiol 589, 4157–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M,Seoane L,del Carmen, García M,Lago F,Casanueva FF,Señarís R,Diéguez C, 2000. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem. Biophys. Res. Commun 269, 41–45. [DOI] [PubMed] [Google Scholar]
- Murphy BA, Fioramonti X, Jochnowitz N, Fakira K, Gagen K, Contie S, Lorsignol A, Penicaud L,Martin WJ,Routh VH, 2009. Fasting enhances the response of arcuate neuropeptide Y (NPY)-glucose-inhibited (GI) neurons to decreased extracellular glucose. AJ. Cell Physiol. 296, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M,Gaillard P,Minier F,Tanti A,Belzung C,Leman S, 2011. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 61, 336–346. [DOI] [PubMed] [Google Scholar]
- Osterstock G, Escobar P, Mitutsova V, Gouty-Colomer LA, Fontanaud P, Molino F, Fehrentz JA, Carmignac D, Martinez J, Guerineau NC, Robinson IC, Mollard P, Méry PF, 2010. Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus. PLoS One 5 (2), 5 10.1371/journal.pone.0009159(e9159). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Hirasawa M, 2010. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J. Neurosci 30, 8061–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G,Watson C, 1998. The Rat Brain in Stereotaxic Coordinates, 4th ed. Academic Press. [DOI] [PubMed] [Google Scholar]
- Peyron C,Tighe DK,van den Pol AN,de Lecea L,Heller HC,Sutcliffe JG,Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL,Weston MC, Sevigny CP, Stornetta RL,Guyenet PG, 2003. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol 465, 593–603. [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2002. Roles of orexins in regulation of feeding and wakefulness. NeuroReport 13, 987–995. [DOI] [PubMed] [Google Scholar]
- Sakurai T, et al. , 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M, 1994. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci 14, 5068–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z,Routh VH, 2005. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 54, 15–22. [DOI] [PubMed] [Google Scholar]
- Song Z, Levin BE,McArdle JJ, Bakhos N, Routh VH, 2001. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50, 2673–2681. [DOI] [PubMed] [Google Scholar]
- Spanswick D,Smith MA, Groppi VE, Logan SD,Ashford MLJ, 1997. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390, 521–525. [DOI] [PubMed] [Google Scholar]
- Teske JA,Levine AS,Kuskowski M,Levine JA,Kotz CM, 2006. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesityresistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, R889–R899. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF, 2011. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci 31, 15944–15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN,Gao XB,Obrietan K,Kilduff TS,Belousov AB, 1998. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci 18, 7962–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW, 2008. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur. J. Neurosci 28, 1629–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R,Liu X,Hentges ST,Dunn-Meynell AA,Levin BE,Wang W,Routh VH, 2004. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53, 1959–1965. [DOI] [PubMed] [Google Scholar]
- Yamanaka A,Beuckmann CT,Willie JT,Hara J,Tsujino N,Mieda M,Tominaga M,K-i Yagami,Sugiyama F,Goto K,Yanagisawa M,Sakurai T, 2003. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713. [DOI] [PubMed] [Google Scholar]