Abstract
Purpose
Given the pivotal role of epidermal growth factor receptor (EGFR) inhibitors in advanced EGFR-mutant non–small-cell lung cancer (NSCLC), we tested adjuvant erlotinib in patients with EGFR-mutant early-stage NSCLC.
Materials and Methods
In this open-label phase II trial, patients with resected stage IA to IIIA (7th edition of the American Joint Committee on Cancer staging system) EGFR-mutant NSCLC were treated with erlotinib 150 mg per day for 2 years after standard adjuvant chemotherapy with or without radiotherapy. The study was designed for 100 patients and powered to demonstrate a primary end point of 2-year disease-free survival (DFS) greater than 85%, improving on historic data of 76%.
Results
Patients (N = 100) were enrolled at seven sites from January 2008 to May 2012; 13% had stage IA disease, 32% had stage IB disease, 11% had stage IIA disease, 16% had stage IIB disease, and 28% had stage IIIA disease. Toxicities were typical of erlotinib; there were no grade 4 or 5 adverse events. Forty percent of patients required erlotinib dose reduction to 100 mg per day and 16% to 50 mg per day. The intended 2-year course was achieved in 69% of patients. The median follow-up was 5.2 years, and 2-year DFS was 88% (96% stage I, 78% stage II, 91% stage III). Median DFS and overall survival have not been reached; 5-year DFS was 56% (95% CI, 45% to 66%), 5-year overall survival was 86% (95% CI, 77% to 92%). Disease recurred in 40 patients, with only four recurrences during erlotinib treatment. The median time to recurrence was 25 months after stopping erlotinib. Of patients with recurrence who underwent rebiopsy (n = 24; 60%), only one had T790M mutation detected. The majority of patients with recurrence were retreated with erlotinib (n = 26; 65%) for a median duration of 13 months.
Conclusion
Patients with EGFR-mutant NSCLC treated with adjuvant erlotinib had an improved 2-year DFS compared with historic genotype-matched controls. Recurrences were rare for patients receiving adjuvant erlotinib, and patients rechallenged with erlotinib after recurrence experienced durable benefit.
INTRODUCTION
Of the estimated 160,000 patients with non–small-cell lung cancer (NSCLC) who will be diagnosed in the United States in 2018,1 only approximately one third will be candidates for surgical resection. Even after resection, many patients will relapse, with recurrence rates varying from 10% in stage IA to 1% to 75% in stage IIIB NSCLC.2 This high metastatic potential, even in early-stage disease, creates a clinical need for adjuvant treatment to eradicate microscopic residual disease. Multiple trials have demonstrated that adjuvant cisplatin-based chemotherapy after resection in NSCLC improves the chance of overall survival (OS) at 5 years by approximately 5% to 10%, with greater benefit in patients with more advanced-stage disease.3,4
The limited benefit of adjuvant chemotherapy is likely related to the inherent chemoresistance of NSCLC. Indeed, older platinum doublet regimens, such as those used in the adjuvant trials mentioned previously, have only 17% to 22% objective response rates when used in advanced disease,5 and even a modern chemotherapy regimen for advanced nonsquamous NSCLC, cisplatin and pemetrexed, seems comparable to older regimens in the adjuvant setting.6 The current treatment paradigm in advanced NSCLC incorporates histology, tumor programmed death-ligand 1 expression,7 and tumor genotype, none of which were recognized as important variables when the pivotal adjuvant chemotherapy trials were designed in the late 1990s. The most common actionable somatic genomic alteration in NSCLC is the epidermal growth factor receptor (EGFR) mutation, which is observed in approximately 15% of patients with lung adenocarcinoma in the United States and up to 50% of patients with lung adenocarcinoma in Asia. In patients with metastatic lung cancers harboring an EGFR mutation, the small molecule EGFR tyrosine kinase inhibitor (TKI) erlotinib has superior response rates and progression-free survival compared with platinum doublet chemotherapy.8,9
On the basis of the distinct mechanism of action and superior efficacy in advanced disease, it is possible that adjuvant EGFR TKIs would improve outcomes in early-stage EGFR-mutant lung cancer. Targeted treatments have been shown to improve survival in the adjuvant setting in other oncogene-driven malignancies, such as human epidermal growth factor receptor 2 (HER2)-positive breast cancer10,11 and cKIT-positive GI stromal tumors.12 Several clinical trials have been designed to investigate EGFR TKIs in the adjuvant setting, but the majority have not selected patients by EGFR-mutant genotype; the one exception is the ADJUVANT/CTONG1104 trial,13-17 which was a randomized phase III trial of adjuvant platinum doublet chemotherapy versus gefitinib in patients with resected stage II to IIIA EGFR-mutant lung cancer. Although this trial did show a statistically significant prolongation of disease-free survival (DFS) with gefitinib, the benefit seemed to diminish over time, and there was no clear difference in long-term outcomes. In fact, to date, none of the published adjuvant trials have been able to show convincing clinical benefit from adjuvant EGFR TKIs, leaving this question a significant unmet need. Here, we report the results of the Surgically Resected EGFR-Mutant Lung Cancer With Adjuvant Erlotinib Cancer Treatment (SELECT) study, a single-arm, open-label phase II clinical trial to assess the efficacy and tolerability of adjuvant erlotinib in patients with surgically resected EGFR-mutant stage IA to IIIA NSCLC.
MATERIALS AND METHODS
Study Design and Treatment
This study was approved by local institutional review boards, and all patients provided written informed consent before enrollment. This was a single-arm, open-label, phase II study. Patients with completely resected stage IA to IIIA NSCLC (using the American Joint Committee on Cancer 7th edition staging criteria2) with sensitizing EGFR mutations who had completed planned adjuvant chemotherapy with or without radiotherapy as appropriate for their stage received erlotinib 150 mg tablets by mouth, once per day, for up to 2 years. Dose reductions were allowed for toxicity. Treatment was continued until the completion of a 2-year course, disease recurrence, discontinuation because of toxicity, withdrawal of consent, or failure to follow up.
Follow-up visits occurred monthly for the first 3 months, then every 3 months while receiving study therapy (total duration of 2 years). After completion of study therapy, visits occurred every 6 months for 1 year and then annually. Patients were observed for toxicity related to treatment and symptoms of recurrence. Imaging for recurrence included computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast every 6 months for the first 3 years and then annually through the fifth year; other imaging was ordered as clinically indicated. Recurrence was confirmed with a biopsy when feasible.
Patients
Eligible patients were ≥ 18 years old and had completely resected NSCLC with stage IA to IIIA with a sensitizing mutation in EGFR. Local EGFR testing results were used for enrollment. EGFR exon 19 deletion and exon 21 L858R mutations were allowed; other EGFR mutations were accepted on a case-by-case basis after discussion with the study lead (L.V.S.). Patients who had previously received an EGFR TKI were excluded. All patients had to have an Eastern Cooperative Oncology Group performance status of 0 to 2 and no evidence of recurrent cancer by CT of the chest, abdomen, and pelvis before enrollment. Surgical resection had to be within 9 months of enrollment if adjuvant chemotherapy and radiotherapy were given or within 6 months if only adjuvant chemotherapy was given, and recovery from the toxicities of adjuvant therapy was required. The enrollment window for patients receiving chemotherapy and radiotherapy was extended from 6 to 9 months after the original pilot study of 36 patients because of the difficulty in completing sequential chemoradiotherapy within 6 months. There was no minimum time from surgery to enrollment. Patients with known interstitial lung were excluded. See the full eligibility criteria in the Data Supplement.
Study Assessments
Enrolled patients were treated with erlotinib 150 mg tablet by mouth once per day in continuous monthly treatment cycles for up to 2 years. Toxicities were graded by Common Terminology Criteria for Adverse Events (version 3).18 Dose reductions for adverse events were specified in the clinical protocol and were recorded. Disease reassessment by CT scan of the chest, abdomen, and pelvis was performed at routine intervals as described previously; disease recurrence was defined as the earliest radiographic evidence of disease and was confirmed by biopsy where feasible. Molecular information on recurrence specimens as well as postrecurrence treatment was collected.
Statistical Analysis
The primary objective was DFS at 2 years from enrollment, with secondary objectives of safety, tolerability, and OS. The trial was powered to demonstrate a 10% improvement in the 2-year DFS rate compared with historical controls.19 The trial was initially designed as a pilot study with a goal enrollment of 36 patients, but once it was clear that enrolling a higher number of patients was feasible, the sample size was expanded to 100. With 100 patients, the study had a 90% power to observe an improvement in DFS at 2 years from the historical control of 76% to 86% for patients treated with adjuvant erlotinib, using the one-sided binomial proportion hypothesis test with a significance level of 0.1. The historical benchmark in the original pilot study design used a 2-year DFS estimate of 70%, but this was updated to 76%, reflecting the final published Memorial Sloan Kettering Cancer Center cohort data that served as our benchmark.
RESULTS
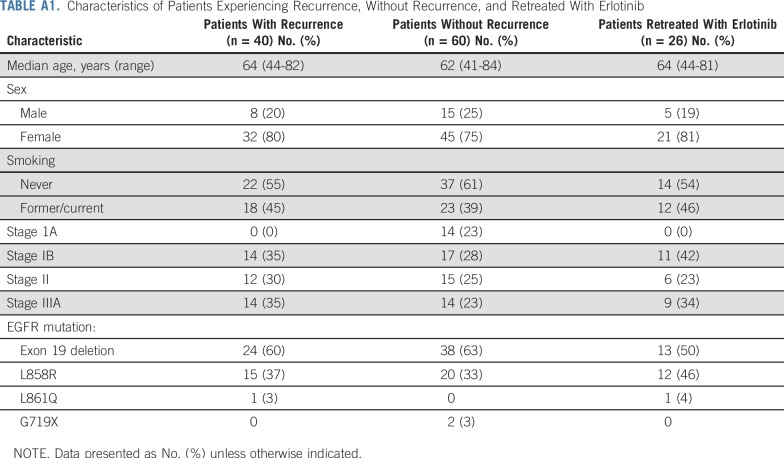
Patients (N = 100) with EGFR-mutant NSCLC were enrolled at seven sites between January 2008 and May 2012. The baseline patient characteristics are listed in Table 1. The stage distribution was similar to other NSCLC adjuvant trials, with roughly 30% stage IB, 30% stage II, and 30% stage IIIA.
TABLE 1.
Baseline Patient Characteristics
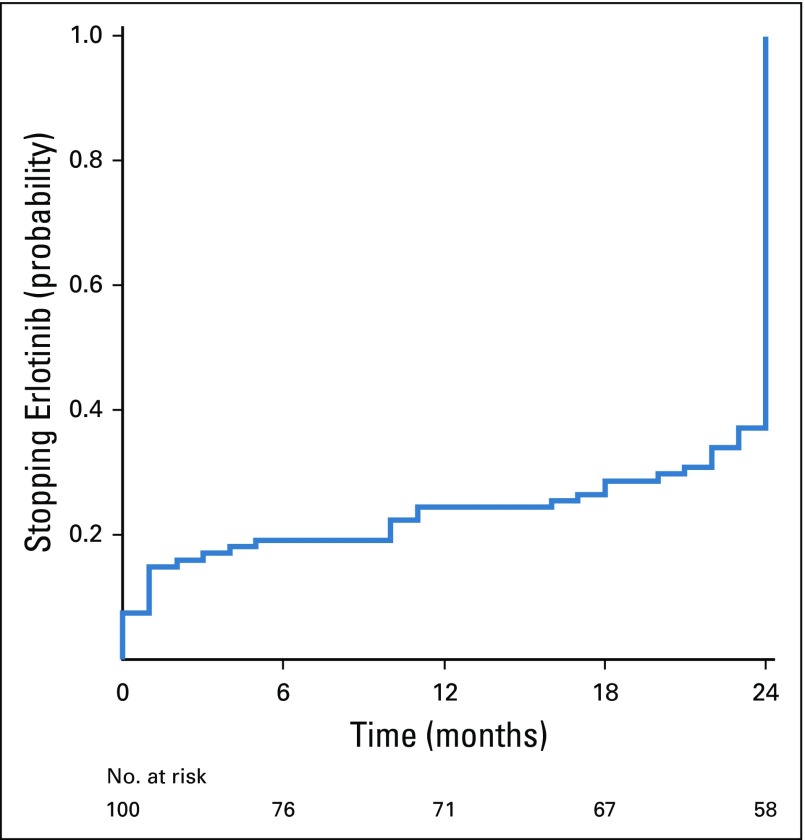
The median duration of treatment with adjuvant erlotinib was 23 months (range, 0 to 24 months), and the average duration was 19 months, with 69% of patients completing at least 22 months of treatment (Fig 1). Note that because of differences in interpretation of protocol instructions, it was possible to confuse the treatment course length of 24 months to mean 24 cycles, and because each cycle was 28 days in length, 24 cycles was equivalent to 22 calendar months. All patients who discontinued treatment between months 22 and 24 did so because they had completed 24 cycles and were considered by their study doctors as completing the treatment per protocol. Eleven patients discontinued erlotinib within the first month of treatment because of intolerance, whereas four patients continued erlotinib beyond 2 years (off study) because of patient or physician preference.
FIG 1.
Probability of discontinuing adjuvant erlotinib in the study population.
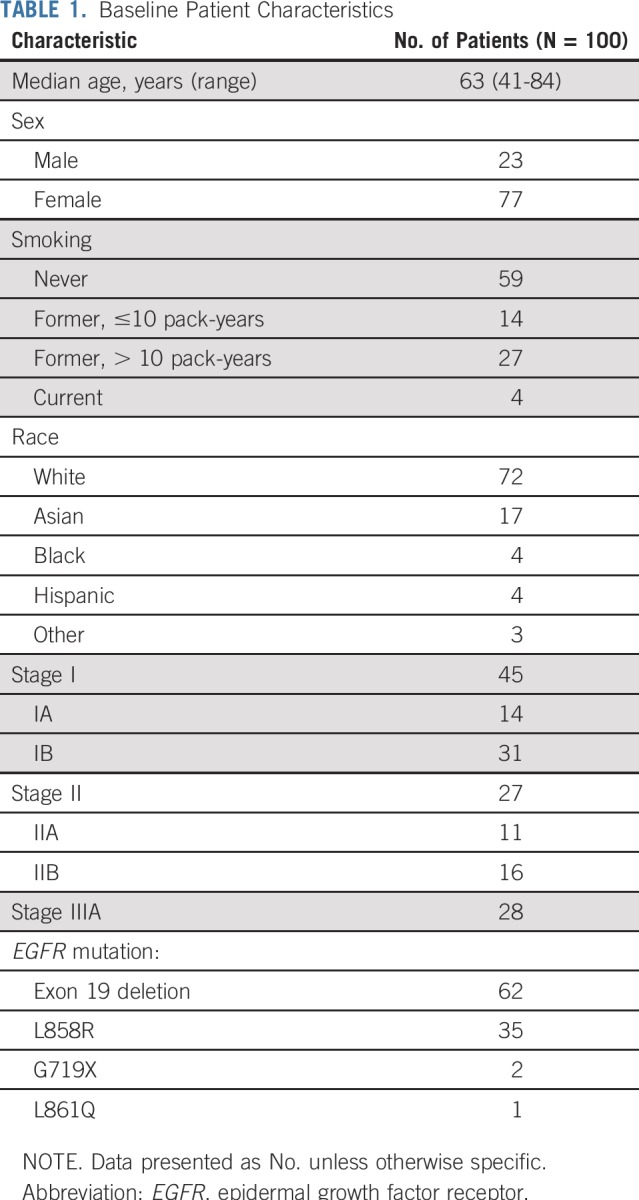
Forty percent of patients required a dose reduction to 100 mg, and 16% needed a second dose reduction to 50 mg. Toxicities were typical of erlotinib; the most common adverse events were rash, diarrhea, dry skin, fatigue, nausea/vomiting, nail changes, pruritis, stomatitis, and transaminitis (Table 2). There were no grade 4 or 5 events, and only one patient had grade 2 pulmonary fibrosis.
TABLE 2.
Adverse Events Felt to Be Related to Treatment at > 25% Frequency or Grade 3 or Higher
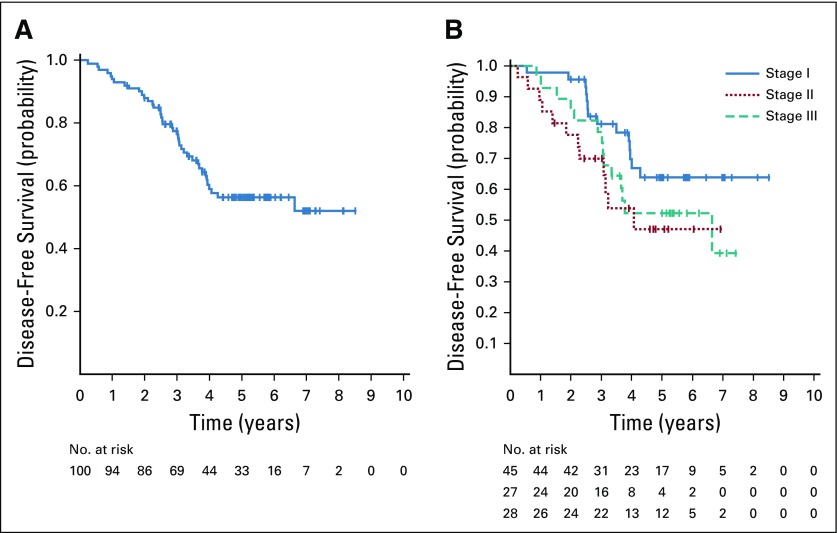
The median follow-up was 5.2 years (range, 17 to 105 months); 40 patients recurred and 17 patients with recurrence died. All patients were evaluable for the primary end point of 2-year DFS, which was 88% (95% CI, 80% to 93%). This is significantly higher than the historical control19 of 76% (P = .0047). The 2-year DFS by stage was 96% for patients with stage I, 78% for stage II, and 91% with stage IIIA. The median DFS and OS have not yet been reached (Fig 2). Five-year DFS was 56% (95% CI, 45% to 66%), and 5-year OS was 86% (95% CI, 77% to 92%).
FIG 2.
(A) Disease-free survival in the overall study population. (B) Disease-free survival by pathologic stage.
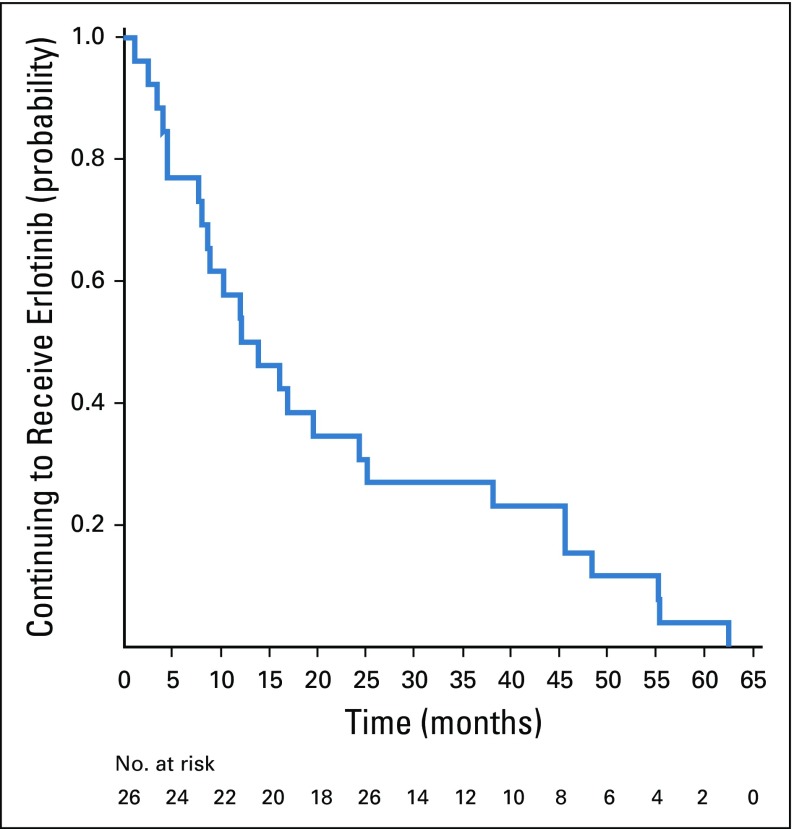
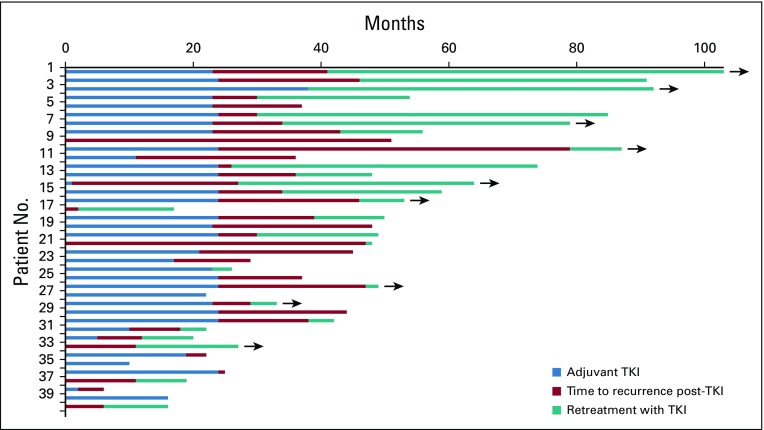
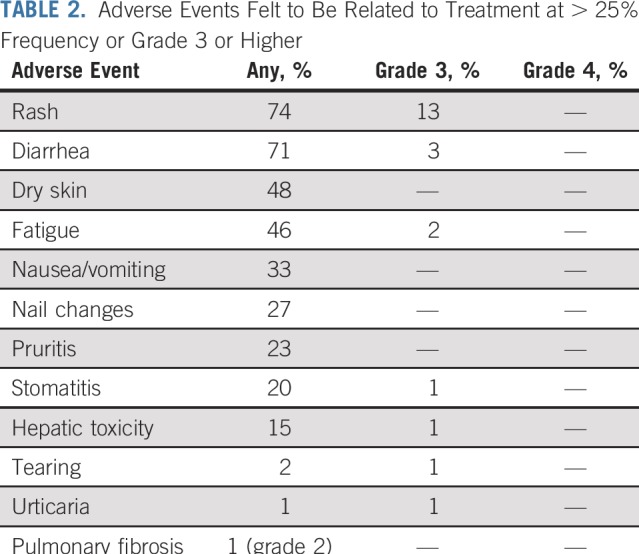
Recurrent cancer was noted in 40 patients, four while receiving erlotinib treatment and 36 after stopping erlotinib (Appendix Table A1, online only). The median time to recurrence after stopping erlotinib was 25.4 months (range, 0 to 79 months). Seventeen recurrences (42%) were at a single site, including eight (20%) that were in the CNS only. Patients who experienced disease recurrence had received a significantly shorter duration of adjuvant erlotinib treatment than those who remained disease free (Wilcoxon rank sum P = .027). Twenty-four patients (60%) with recurrent disease underwent a repeat biopsy, and 20 had successful EGFR mutation testing of the recurrence. All 20 patients maintained the original canonical EGFR mutation, and only one recurrent tumor (5%) acquired a T790M mutation. The patient with T790M recurred while still taking erlotinib. Among the 40 patients who recurred, 26 (65%) were retreated with erlotinib after recurrence, yielding a median duration of treatment of 13.1 months (range, 0 to 62 months; Figs 3 and 4); nine patients continued to take erlotinib at the data cutoff. Formal radiographic tumor measurements were not performed as part of this study after recurrence; therefore progression-free survival (PFS) could not be determined in this group. However, the majority of patients derived benefit from re-exposure to erlotinib as clinically determined by the treating physicians.
FIG 3.
Probability of continuing to take a second course of erlotinib in patients with recurrence.
FIG 4.
Swimmer plot of all patients who experienced disease recurrence (n = 40). The time receiving adjuvant erlotinib (light blue), time after adjuvant treatment before disease recurrence (red), and time receiving retreatment with erlotinib (teal) are illustrated. Arrows indicate that the patient continues to take erlotinib at data cutoff. TKI, tyrosine kinase inhibitor.
DISCUSSION
This prospective trial of adjuvant erlotinib in patients with resected, early-stage EGFR-mutant NSCLC showed that most patients were compliant and able to complete up to 2 years of therapy. The 2-year DFS of 88% in the SELECT trial is promising and exceeds that of a large retrospective cohort of patients with resected early-stage EGFR-mutant NSCLC who received no adjuvant EGFR TKI.19 Our results are also notable for a low rate of recurrence (4%), whereas actively taking adjuvant TKI and a significant inverse relationship between the length of adjuvant erlotinib and the likelihood of recurrence. All of these data suggest that adjuvant erlotinib may delay recurrence in patents with EGFR-mutant NSCLC and that length of therapy may be related to outcomes.
The use of targeted therapies as adjuvant treatment of oncogene-driven cancers is well established in other diseases. Endocrine therapy has been used in hormone receptor–positive breast cancer for decades, with more effective and longer duration of therapy leading to longer survival times.20,21 Adjuvant HER2-directed therapy with the monoclonal antibody trastuzumab or, more recently, with the HER2 TKI neratinib is the standard of care for HER2-amplified breast cancer because of improvements in both DFS and OS.10,11 In perhaps the closest analogy to EGFR-mutant NSCLC, the use of adjuvant imatinib in KIT-mutant GI stromal tumor was first shown to improve DFS with 1 year of therapy,22 and then extension of treatment to 3 years was shown to improve OS.12
These study results are consistent with retrospective data in the EGFR-mutant population, as well as EGFR-mutant subgroups, from prior trials in broader NSCLC populations. The BR.19 trial of adjuvant gefitinib identified only 15 patients with EGFR-mutant NSCLC, and the IFCT-0801 TASTE trial treated only seven patients with adjuvant erlotinib, which is too few to draw conclusions about efficacy.14,15 As the use of EGFR TKIs became widespread in advanced disease, these drugs have been offered in the off-label adjuvant setting. In a retrospective cohort study from Memorial Sloan Kettering, 167 patients with completely resected stage I to III NSCLC with EGFR mutations were identified, with 56 of them having received adjuvant erlotinib.19 The reported 2-year DFS was 89% in the patients who received perioperative EGFR TKI, compared with 72% in the group that did not, with an observed hazard ratio of 0.53 (P = .06), despite a much higher percentage of patients with stage II and III disease in the treated versus control arms (77% v 31%). Interestingly, an update in 2012 of the Memorial Sloan Kettering cohort showed the long-term DFS in the EGFR-mutant subgroups that received adjuvant TKI. The estimated 5-year DFS was approximately 55%,23 comparable to the 56% seen in SELECT.
During the conduct of our study, two phase III trials were ongoing and have since been reported. The Randomized Double-Blind Trial in Adjuvant NSCLC With Tarceva (RADIANT) trial investigated the efficacy of 2 years of adjuvant erlotinib versus placebo in patients with resected NSCLC who had overexpression of EGFR protein by immunohistochemistry or amplification of EGFR gene copy number by fluorescent in situ hybridization analysis.17 There was no difference in PFS or OS between arms in the overall intention-to-treat population. Retrospective detection of EGFR mutation status was planned within this randomized, controlled trial, but only 161 of 973 patients were found to have EGFR-mutant tumors, and the statistics were not designed to directly compare outcomes within this subgroup. Despite this, a nonsignificant but clinically meaningful difference in median DFS was observed between the EGFR-mutant group treated with erlotinib and placebo (46.4 months v 28.5; hazard ratio, 0.61; 95% CI, 0.38 to 0.98; P = .039). In RADIANT, the 2-year DFS was 89% in the erlotinib-treated arm, which is similar to our SELECT results.
In the recently published phase III ADJUVANT trial, 222 patients with resected stage II to IIIA EGFR-mutant NSCLC were randomly assigned to up to 2 years of adjuvant gefitinib versus platinum doublet chemotherapy, with a primary end point of DFS.24 This trial demonstrated an improved DFS in the adjuvant TKI arm compared with the chemotherapy arm (hazard ratio, 0.6; 95% CI, 0.42 to 0.87; P = .005); however, by 3 years, the difference in DFS proportion was small: 34% in the TKI arm compared with 27% in the control arm, suggesting that recurrences were delayed but not necessarily prevented by gefitinib. This trial enrolled a large percentage of patients with stage IIIA disease (64%) and did not require positron emission tomography scans for staging; therefore, the poor 3-year DFS in both arms raises the possibility that many patients may have had metastatic disease at the time of enrollment. Another point of contrast was that patients in SELECT could be enrolled up to 9 months after surgery, which could potentially exclude patients with early recurrence. The ADJUVANT trial design of randomly assigning patients to immediate adjuvant therapy has the advantage of capturing this higher-risk population and also contributes to the high rate of recurrence in this trial.
One major concern with prolonged treatment in an adjuvant trial is cumulative toxicity and its impact on patient compliance. In the RADIANT trial, the median duration of treatment with erlotinib was only 11.9 months compared with 22 months with placebo, presumably because of patient intolerance. In SELECT, the median duration of erlotinib was 23 months, which may be attributed to aggressive symptom management and dose reductions when needed, as well as patients’ knowledge of their EGFR-mutant status and the open-label nature of this trial. Although the majority of patients completed prescribed therapy, dose reductions were still required in 40% of patients, and an additional 11% stopped treatment shortly after starting because of intolerance, suggesting that toxicity is likely to be a significant issue in any adjuvant trial using first-generation EGFR TKIs. Ongoing adjuvant trials with third-generation EGFR TKIs, such as osimertinib (NCT02511106), may have improved compliance because of relative sparing of the wild-type EGFR receptor with this class of agents,25 resulting in lower rates of rash and diarrhea.
Although there were few recurrences while patients received active TKI treatment in SELECT, the number of recurrences after stopping erlotinib calls into question whether adjuvant EGFR TKIs are capable of altering the natural history of the disease to improve cure rates or are merely delaying recurrence. In advanced disease, EGFR TKI therapy is cytotoxic to a population of cells but cytostatic to other more inherently resistant cells, which may persist during adjuvant therapy as well. Ultimately, randomized trials will be needed to demonstrate clinically meaningful benefit of adjuvant targeted therapies. One such trial is the EGFR-mutant arm of the ongoing Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (ALCHEMIST) trial (NCT02193282), which randomly assigns patients with resected stage IB to IIIA NSCLC harboring EGFR mutations to 2 years of erlotinib or observation with a primary end point of OS. The ongoing ADAURA trial (NCT02511106) is testing 3 years of adjuvant osimertinib versus placebo. These studies will answer important questions; however, the optimal duration of adjuvant EGFR TKI therapy will remain undefined.
Another hypothetical concern with adjuvant EGFR TKI treatment is whether such treatment would lead to early emergence of resistance mechanisms, such as T790M, and thus diminished efficacy of a TKI in the recurrent setting. In this article, we report that only one patient with recurrence was found to have T790M on rebiopsy. This patient also developed progression while receiving adjuvant erlotinib, suggesting that for the remaining patients with recurrence, the underlying cause was stopping the suppressive erlotinib rather than truly acquiring resistance to erlotinib. Among patients with recurrence, 65% were retreated with erlotinib, and although formal response data are not available, the median duration of treatment of 13.1 months approximates the PFS of erlotinib in a de novo metastatic EGFR-mutant population.8 Importantly, this indicates that adjuvant TKI treatment does not lead to clinically resistant disease on recurrence, consistent with a prior retrospective report in this setting.26
In conclusion, 2 years of adjuvant erlotinib after surgery and standard adjuvant treatment of patients with early-stage EGFR-mutant NSCLC resulted in a higher 2-year DFS rate than historical controls. Recurrences generally remained sensitive to retreatment with erlotinib. Ongoing and future randomized trials will formally define the clinical benefit of adjuvant EGFR TKIs in this setting.
Appendix
TABLE A1.
Characteristics of Patients Experiencing Recurrence, Without Recurrence, and Retreated With Erlotinib
Footnotes
Presented in abstract form at the 2012 ASCO Annual Meeting, Chicago, IL, June 1 to 5, 2012, and the 2014 ASCO Annual Meeting, Chicago, IL, May 30 to June 3, 2014.
The study was an investigator-initiated trial funded by Genentech. J.E.C., V.W.R., and M.G.K. are supported in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant No. P30 CA008748. The work of D.B.C. is in part funded by National Institutes of Health/National Cancer Institute Grants No. P50 CA090578 and R01 CA218707.
AUTHOR CONTRIBUTIONS
Conception and design: Nathan A. Pennell, Joel W. Neal, Jamie E. Chaft, Christopher G. Azzoli, Valerie W. Rusch, Mark G. Kris, Lecia V. Sequist
Administrative support: Lecia V. Sequist
Provision of study materials or patients: Nathan A. Pennell, Christopher G. Azzoli, Ramaswamy Govindan, Tracey L. Evans, Daniel B. Costa, Heather A. Wakelee, Mark G. Kris, Lecia V. Sequist
Collection and assembly of data: Nathan A. Pennell, Joel W. Neal, Jamie E. Chaft, Christopher G. Azzoli, Pasi A. Jänne, Ramaswamy Govindan, Tracey L. Evans, Daniel B. Costa, Heather A. Wakelee, Rebecca S. Heist, Marc A. Shapiro, Valerie W. Rusch, Mark G. Kris, Lecia V. Sequist
Data analysis and interpretation: Nathan A. Pennell, Joel W. Neal, Jamie E. Chaft, Christopher G. Azzoli, Daniel B. Costa, Rebecca S. Heist, Alona Muzikansky, Sudish Murthy, Michael Lanuti, Valerie W. Rusch, Mark G. Kris, Lecia V. Sequist
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Nathan A. Pennell
Consulting or Advisory Role: AstraZeneca, Eli Lilly, Regeneron, Cota Healthcare
Research Funding: Genentech (Inst), Celgene (Inst), AstraZeneca (Inst), Pfizer (Inst), Merck, Loxo Oncology (Inst), Boehringer Ingelheim (Inst)
Joel W. Neal
Consulting or Advisory Role: ARIAD/Takeda, AstraZeneca, Genentech, Eli Lilly, Exelixis, Loxo Oncology, Jounce Therapeutics, Genentech (Inst), Merck (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Exelixis (Inst), ARIAD/Takeda (Inst), Nektar (Inst)
Jamie E. Chaft
Consulting or Advisory Role: Genentech, AstraZeneca/MedImmune, Merck, Bristol-Myers Squibb
Research Funding: Genentech (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst)
Christopher G. Azzoli
Consulting or Advisory Role: Merck, ARIAD/Takeda
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, ARIAD, Chugai Pharma, Genentech, Loxo Oncology, Mirati Therapeutics, Araxes Pharma, Ignyta, Eli Lilly, AstraZeneca, Astellas Pharma, Daiichi Sankyo, Eli Lilly, Boehringer Ingelheim, Puma Biotechnology
Patents, Royalties, Other Intellectual Property: I am a co-inventor on a Dana-Farber Cancer Institute–owned patent on EGFR mutations licensed to Lab Corp. I receive postmarketing royalties from this invention
Ramaswamy Govindan
Honoraria: Boehringer Ingelheim, Genentech/AbbVie
Consulting or Advisory Role: GlaxoSmithKline, Boehringer Ingelheim, Genentech, AbbVie, Celgene, Novartis, AstraZeneca/MedImmune, Inivata, Merck Serono, Pfizer, Bristol-Myers Squibb, EMD Serono
Research Funding: Bayer (Inst), GlaxoSmithKline (Inst), MethylGene (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Celgene, Merck, Amgen, Genentech, GlaxoSmithKline
Tracey L. Evans
Honoraria: Genentech, Genentech (I), Merck, AstraZeneca
Consulting or Advisory Role: Genentech, Genentech (I), AstraZeneca
Speakers' Bureau: Genentech, Genentech (I), Merck, AstraZeneca
Travel, Accommodations, Expenses: Genentech, Genentech (I)
Daniel B. Costa
Honoraria: Takeda/Millennium Pharmaceuticals, Pfizer
Consulting or Advisory Role: AstraZeneca, Takeda/Millennium Pharmaceuticals
Research Funding: Genentech (Inst), Takeda/Millennium Pharmaceuticals (Inst), AstraZeneca (Inst), Pfizer (Inst), Merck (Inst), Merrimack (Inst), Bristol-Myers Squibb (Inst), Clovis Oncology (Inst)
Heather A. Wakelee
Honoraria: Novartis, AstraZeneca
Research Funding: Genentech (Inst), Pfizer (Inst), Eli Lilly (Inst), Celgene (Inst), AstraZeneca/MedImmune (Inst), Exelixis (Inst), Novartis (Inst), Clovis Oncology (Inst), Xcovery (Inst), Bristol-Myers Squibb (Inst), Gilead Sciences (Inst), Pharmacyclics (Inst), ACEA Biosciences (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Rebecca S. Heist
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, Tarveda Therapeutics
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Incyte (Inst), Celgene (Inst), Mirati Therapeutics (Inst), Peregrine Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Debiopharm Group (Inst), Corvus Pharmaceuticals (Inst)
Marc A. Shapiro
Consulting or Advisory Role: Anthem
Alona Muzikansky
Consulting or Advisory Role: Sofregen Medical
Michael Lanuti
Honoraria: Holy Cross Hospital
Research Funding: National Institutes of Health, Stand Up to Cancer
Valerie W. Rusch
Research Funding: Genelux (Inst)
Travel, Accommodations, Expenses: da Vinci Surgery
Other Relationship: Bristol-Myers Squibb
Mark G. Kris
Consulting or Advisory Role: AstraZeneca, Regeneron, Pfizer
Lecia V. Sequist
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, Genentech, Bristol-Myers Squibb, Pfizer, Merrimack
Research Funding: Boehringer Ingelheim (Inst), Clovis Oncology (Inst), Genentech (Inst), Merrimack (Inst), Novartis (Inst), AstraZeneca (Inst), Johnson & Johnson (Inst), Merck (Inst), Pfizer (Inst), Guardant Health (Inst), Incyte (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. American Cancer Society: Cancer Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
- 2.Goldstraw P, Crowley J, Chansky K, et al. : The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2:706-714, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pignon JP, Tribodet H, Scagliotti GV, et al. : Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552-3559, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Gaspar LE, Chaft JE, et al. : Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol 35:2960-2974, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. : Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92-98, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Wakelee HA, Dahlberg SE, Keller SM, et al: E1505: Adjuvant chemotherapy +/- bevacizumab for early stage NSCLC—Outcomes based on chemotherapy subsets. J Clin Oncol 34, 2016 (suppl; abst 8507)
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, et al. : Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239-246, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, et al. : Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735-742, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Moja L, Tagliabue L, Balduzzi S, et al. : Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 4:CD006243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan A, Delaloge S, Holmes FA, et al. : Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:367-377, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Joensuu H, Eriksson M, Sundby Hall K, et al. : One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 307:1265-1272, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Huang Q, Li J, Sun Y, et al. : Efficacy of EGFR tyrosine kinase inhibitors in the adjuvant treatment for operable non-small cell lung cancer by a meta-analysis. Chest 149:1384-1392, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Goss GD, O’Callaghan C, Lorimer I, et al. : Gefitinib versus placebo in completely resected non-small-cell lung cancer: Results of the NCIC CTG BR19 study. J Clin Oncol 31:3320-3326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wislez M, Barlesi F, Besse B, et al. : Customized adjuvant phase II trial in patients with non-small-cell lung cancer: IFCT-0801 TASTE. J Clin Oncol 32:1256-1261, 2014 [DOI] [PubMed] [Google Scholar]
- 16. Wu Y ZW, Wang Q, Xu S, et al: Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): A randomized, Phase III trial (CTONG 1104). J Clin Oncol 35, 2017 (suppl; abst 8500) [Google Scholar]
- 17.Kelly K, Altorki NK, Eberhardt WEE, et al. : Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): A randomized, double-blind, phase III trial. J Clin Oncol 33:4007-4014, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Trotti A, Colevas AD, Setser A, et al. : CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176-181, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Janjigian YY, Park BJ, Zakowski MF, et al. : Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 6:569-575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies C, Godwin J, Gray R, et al. : Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 378:771-784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies C, Pan H, Godwin J, et al. : Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805-816, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dematteo RP, Ballman KV, Antonescu CR, et al. : Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet 373:1097-1104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Angelo SP, Janjigian YY, Ahye N, et al. : Distinct clinical course of EGFR-mutant resected lung cancers: Results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 7:1815-1822, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Zhong WZ, Wang Q, Mao WM, et al. : Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet Oncol 19:139-148, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Jänne PA, Yang JC, Kim DW, et al. : AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372:1689-1699, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Oxnard GR, Janjigian YY, Arcila ME, et al. : Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res 17:6322-6328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]