Abstract
Anxiety and stress disorders have been linked to deficits in fear extinction. Our laboratory and others have demonstrated that acute nicotine impairs contextual fear extinction, suggesting that nicotine exposure may have negative effects on anxiety and stress disorder symptomatology. However, the neurobiological mechanisms underlying the acute nicotine-induced impairment of contextual fear extinction are unknown. Therefore, based on the previous studies showing that brain-derived neurotrophic factor is central for fear extinction learning and acute nicotine dysregulates brain-derived neurotrophic factor signaling, we hypothesized that the nicotine-induced impairment of contextual fear extinction may involve changes in tyrosine receptor kinase B signaling. To test this hypothesis, we systemically, intraperitoneally, injected C57BL/6J mice sub-threshold doses (2.5 and 4.0 mg/kg) of 7,8-dihydroxyflavone, a small-molecule tyrosine receptor kinase B agonist that fully mimics the effects of brain-derived neurotrophic factor, or vehicle an hour before each contextual fear extinction session. Mice also received injections, intraperitoneally, of acute nicotine (0.18 mg/kg) or saline 2–4 min before extinction sessions. While the animals that received only 7,8-dihydroxyflavone did not show any changes in contextual fear extinction, 4.0 mg/kg of 7,8-dihydroxyflavone ameliorated the extinction deficits in mice administered acute nicotine. Overall, these results suggest that acute nicotine-induced impairment of context extinction may be related to a disrupted brain-derived neurotrophic factor signaling.
Keywords: Nicotine, tyrosine receptor kinase B receptors, brain-derived neurotrophic factor, extinction, post-traumatic stress disorder
Previous studies from our laboratory and others have shown that acute nicotine modulates fear learning (Davis et al., 2006; Gould and Higgins, 2003; Gould and Wehner, 1999; Wehner et al., 2004), fear extinction (Kutlu and Gould, 2014; Kutlu et al., 2016a,b, 2017a), and safety learning (Connor et al., 2017; Haaker et al., 2017; Kutlu et al., 2014) in rodents and humans. Specifically, these studies have shown that acute nicotine administered before fear conditioning and testing enhanced contextual fear learning (Davis et al., 2006; Gould and Higgins, 2003; Gould and Wehner, 1999; Wehner et al., 2004). Moreover, acute nicotine injected prior to each extinction session resulted in impaired contextual fear extinction without affecting general freezing behavior (Kutlu and Gould, 2014) and augmented spontaneous recovery of contextual fear following extinction, but did not affect fear memory recall (Kutlu et al., 2016b). Although nicotine mediates a variety of neuromodulatory mechanisms such as cholinergic, dopaminergic and gama-aminobutyric acid ergic pathways as well as cell signaling cascades (see Kutlu and Gould, 2016 for a review), the underlying neurobiological mechanisms responsible for the impairing effects of nicotine on contextual fear extinction have not been elucidated. In this report, we describe findings suggesting that tyrosine receptor kinase B (trkB) receptors are involved in acute nicotine-dependent fear extinction deficits.
Given its central role in fear extinction, one of the candidate mechanisms that may explain nicotine’s impairing effects on contextual fear extinction is brain-derived neurotropic factor (BDNF) signaling. BDNF has been shown to positively modulate fear extinction (Andero and Ressler, 2012). For example, when infused into the medial prefrontal cortex (mPFC), BDNF improved fear extinction (Peters et al., 2010), whereas deletion of BDNF in the hippocampus disrupted fear extinction (Heldt et al., 2007). Moreover, not only were levels of BDNF elevated in the hippocampus during extinction; but BDNF infused into the hippocampus increased firing rates of infralimbic cortex neurons in the mPFC (Rosas-Vidal et al., 2014), suggesting that BDNF signaling between the hippocampus and mPFC is essential for fear extinction. Finally, there is also evidence showing that activation of trkB, the high affinity receptor for BDNF, also enhanced cued fear extinction (Andero et al., 2011). Importantly, acute nicotine injections have been shown to decrease BDNF mRNA levels in the hippocampus (Kenny et al., 2000), suggesting direct nicotinic control over hippocampal BDNF signaling. Overall, these results suggest that BDNF/trkB signaling is required for successful fear extinction learning, and it is possible that the impairing effects of acute nicotine on contextual fear extinction may be due to the nicotine-induced disruption of BDNF/trkB signaling. Therefore, in the present study we examined the effects of systemic injections of 7,8-dihydroxyflavone (7,8-DHF), a small molecule trkB-agonist that mimics the effects of BDNF (Jang et al., 2010; Liu et al., 2016), on the acute nicotine-induced impairment of contextual fear extinction. We hypothesized that when administered at sub-threshold doses that would not affect baseline fear extinction, 7,8-DHF would reverse acute nicotine’s impairing effects.
Methods
Subjects
Subjects were eight-week old C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine, USA) that were group-housed in a colony room maintained on a 12-hour light/dark cycle and had access to food and water ad libitum. All training and testing was done between 09:00–18:00. Behavioral procedures used in this study were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Contextual fear conditioning, training and testing, occurred in four identical conditioning chambers (18.8×20×18.3 cm) contained within sound-attenuating boxes (MED Associates, St. Albans, Vermont, USA). Background noise (65 dB) was produced by a ventilation fan located in the back of each box; while a white noise conditioned stimulus (CS; 85 dB) was produced by a speaker located on the right wall of the conditioning chambers. The front and back walls and the ceiling of the conditioning chambers were composed of Plexiglas and the floors were metal grids (0.20 cm and 1.0 cm apart) connected to a shock generator which produced a two-second long, 0.57 mA foot-shock unconditioned stimulus (US). The stimuli were controlled by an IBM-PC compatible computer running MED-PC software. All chambers were cleaned with 70% ethanol between each subject.
Drugs and administration
The mice were injected intraperitoneally (i.p.) with either 2.5 or 4.0 mg/kg 7,8-DHF (TCI America, Portland, Oregon, USA) or vehicle (17%, dimethyl sulfoxide) one hour prior to each extinction session followed by i.p. injections of 0.18 mg/kg nicotine (freebase, Sigma, St Louis, Missouri, USA) or saline 2–4 min prior to each extinction session. We chose the 0.18 mg/kg nicotine dose because nicotine at this dose delays contextual fear extinction (Kutlu and Gould, 2014). Also, because the half-life of nicotine in blood is approximately six minutes in C57BL/6J mice (Petersen et al., 1984), we expected it to be fully effective during extinction sessions. The doses for 7,8-DHF treatment were chosen based on Andero et al. (2011) showing that 5.0 mg/kg 7,8-DHF administered i.p. one hour prior to behavioral testing enhanced cued fear extinction, as well as a dose-response study we conducted where mice received the three doses of 7,8-DHF (1.25, 2.50, 5.0 mg/kg) or vehicle injections (Supplementary Material Figure 1). Therefore, we chose doses lower than 5.0 mg/kg in order to achieve a sub-threshold dose not affecting baseline fear extinction. This injection regiment resulted in a total of six experimental groups; vehicle-saline, vehicle-nicotine, 2.5 mg/kg 7,8-DHF-saline, 2.5 mg/kg 7,8-DHF-nicotine, 4.0 mg/kg 7,8-DHF-saline, and 4.0 mg/kg 7,8-DHF-nicotine. All injection volumes were 10 mL/kg.
Behavioral procedures
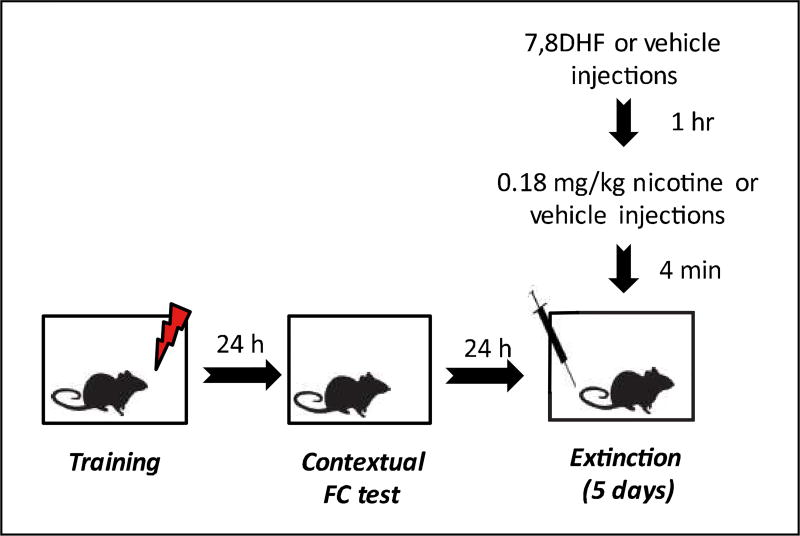
During training, mice were placed in the conditioning chambers and baseline freezing was assessed for 120 s. Following previous studies showing acute nicotine-induced impairment of contextual fear extinction (Kutlu and Gould, 2014; Kutlu et al., 2016a), subjects were trained in background fear conditioning where they received two CS-US pairings in which a 30 s CS co-terminated with a two-second 0.57 mA foot-shock. After the first CS-US pairing, freezing was assessed for 120 s as a measure of immediate freezing to the US. Animals remained in the chamber for 30 s after the second CS-US pairing and were then removed. For contextual testing, the animals were placed hack in the same context they were exposed to during training, and freezing was measured for five minutes in the absence of both the auditory CS and US. During the next five days, the animals were re-exposed to the same context as they were exposed to during training for contextual fear extinction (Figure 1). Contextual fear extinction sessions were identical to the initial testing session. Before each extinction session mice received 7,8-DHF or vehicle (one hour pre-treatment) and then nicotine or saline (four-minute pre-treatment). Each session occurred 24 h after the previous session. Freezing was used as the dependent variable, scored by using a time-sampling procedure. Each subject was observed every 10 s for a duration of one second and scored as either freezing or active. Freezing was defined as the absence of voluntary movement except respiration (Blanchard and Blanchard, 1969). These scores were then converted to percentage freezing. During scoring, experimenters were blinded to the drug conditions.
Figure 1.
The schematic of experimental designs. While each box represents a phase of the experiment, the syringe represents 7,8-dihydroxyflavone (7,8-DHF), vehicle, nicotine, or saline injections and the lightning bolt symbol indicates the presentations of the footshocks.
FC=Fear Conditioning
Statistical analysis
For statistical analysis, a three-way mixed-design analysis of variance (ANOVA) examined two levels of nicotine (0.18 mg/kg or saline), three levels of 7,8-DHF (2.5 mg/kg 7,8-DHF, 4.0 mg/kg 7,8-DHF, or vehicle), across six trials (one testing and five extinction trials) as our independent variables. In order to eliminate potential between-group baseline differences in contextual freezing, which may affect subsequent fear extinction curves, the dependent variable was percentage freezing to the context normalized to the individual freezing levels at the initial testing session (freezing×100/ initial freezing; Kutlu et al., 2016a; Tian et al., 2008). We used Bonferroni-corrected t-tests for comparisons for freezing responses between individual extinction sessions. We preferred this method over other post-hoc analyses as our hypothesis did not necessitate computing all possible comparisons. Raw percentage freezing scores were also analyzed in the same way described for normalized freezing. As mentioned above, we also ran a dose-response study to examine the effects of 7,8-DHF on baseline contextual fear extinction. We analyzed the results of this study using a two-way mixed-design ANOVA with four levels of drug (1.25, 2.50, 5.0 mg/kg 7,8-DHF, or saline) across testing and five extinction trials. Finally, we binned number of freezing scores within the test session as well as each extinction session into three data points (average of number of freezing responses during the first 100, second 100, and third 100 s periods). We analyzed this data for only the saline-vehicle, nicotine-vehicle, saline-4.0 mg/kg 7,8-DHF, and nicotine-4.0 mg/kg 7,8-DHF groups using a three-way mixed-design ANOVA with two levels of nicotine (0.18 mg/kg or saline), 2 levels of 7,8-DHF (4.0 mg/kg 7,8-DHF or vehicle), across 18 bins (three testing and 15 extinction trial bins) as our independent variables.
Results
For the dose-response study examining the effects of 7,8-DHF on baseline contextual fear extinction, our statistical analysis yielded the finding that the main effect of drug was significant (F(3,24)=3.244; p<0.05). We also ran a repeated-measures ANOVA comparing only the saline and 5 mg/kg 7,8-DHF groups. According to this analysis, the main effect of drug for 5 mg/kg 7,8-DHF-vehicle groups approached significance (F(1,12)=4.371, p=0.059) suggesting that the higher dose of 7,8-DHF may have extinction-enhancing effects whereas lower doses of 7,8-DHF did not have an effect (Supplementary Material Figure 1).
Then, in a separate cohort of mice, we examined whether subthreshold doses of 7,8-DHF (2.5 and 4.0 mg/kg) reversed the acute nicotine-induced impairment of contextual fear extinction. For this study, we first ran a repeated-measures ANOVA only in the saline controls to test baseline fear extinction, which showed that the trial main effect was significant in the control group mice suggesting robust baseline fear extinction learning (F(5,45)=8.613; p<0.01 for normalized freezing; F(5,45)=11.983; p<0.01 for raw freezing). Second, replicating our previous results, we found that the nicotine×trial interaction was significant in the vehicle-treated groups demonstrating that acute nicotine treatment impaired contextual fear extinction in the absence of 7,8-DHF (Figure 2; F(5,90)=8.450; p<0.05 for normalized freezing; F(5,90)=8.605; p<0.01 for raw freezing). In addition, we tested whether the 7,8-DHF doses that we used in our study affected baseline fear extinction in saline controls. In the saline controls, the interaction between 7,8-DHF and trial was not significant when 2.5 and 4.0 mg/kg doses were tested together (F(10,130)=l.106; p>0.05 for normalized freezing; F(10,130)=1.334; p>0.05 for raw freezing) or when 4.0 mg/kg dose was tested alone (F(5,90)=l.467; p>0.05 for normalized freezing; F(5,90)=1.619; p>0.05 for raw freezing) suggesting that 7,8-DHF did not affect baseline contextual fear extinction at these doses.
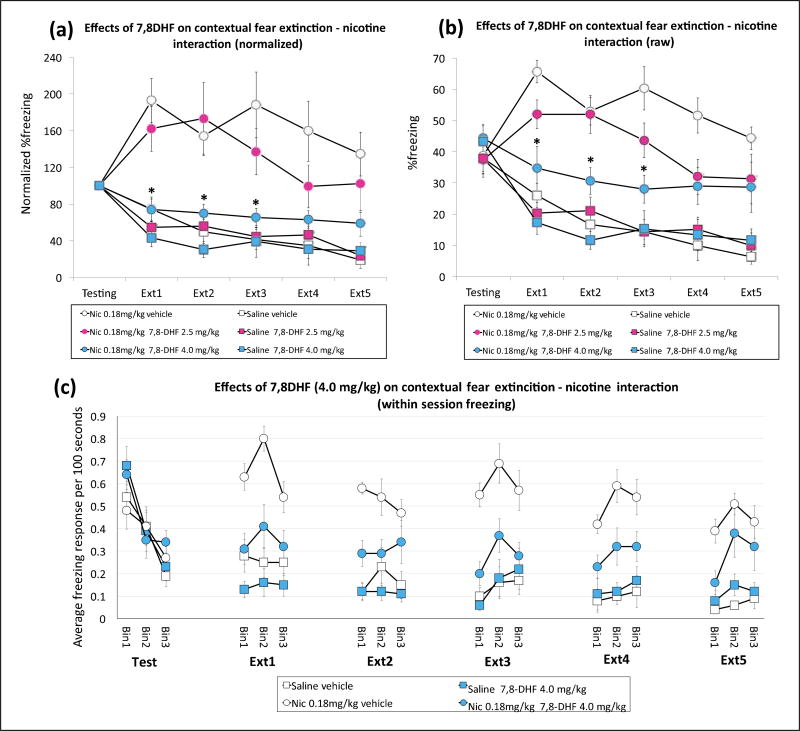
Figure 2.
7,8-Dihydroxyflavone (7,8-DHF) reverses acute nicotine-induced impairment of contextual fear extinction. Acute nicotine (0.18 mg/kg) impairs contextual fear extinction and 7,8-DHF at the 4.0 mg/kg dose reverses the impairment (n=9–10 per group), (a) Normalized percentage freezing responses during testing and extinction phases, (b) Raw percentage freezing responses during testing and extinction phases, (c) Average number of freezing responses during the first (Binl), second (Bin2), and third (Bin3) hundred of seconds of testing and five extinction sessions. *p<0.05 on Bonferroni-corrected t-tests comparing the nicotine 0.18 mg/kg (Nic 0.18 mg/kg)-7,8-DHF 4.0 mg/kg and Nic 0.18 mg/kg-vehicle groups.
Finally, we tested the effect of 7,8-DHF administration on acute nicotine-induced contextual fear extinction. A three-way mixed-design ANOVA showed that the nicotine (nicotine and saline)×7,8-DHF (2.5 mg/kg 7,8-DHF, 4.0 mg/kg 7,8-DHF, and vehicle)×trial interaction was significant for normalized freezing (F(10,265)=2.419; p<0.05) but not for raw freezing (F(10,265)=1.608; p=0.10). However, the nicotine ×7,8-DHF interaction was significant for both normalized (F(10,265)=4.255; p<0.05) and raw (F(2,53)=4.728; p<0.05) freezing scores. Bonferroni-corrected t-tests indicated that the difference between nicotine-vehicle and nicotine-4.0 mg/kg 7,8-DHF group raw and normalized freezing scores was significant for the first three extinction trials (Figure 2(a) and (b); p<0.05). The 2.5 mg/kg 7,8-DHF dose did not result in significant differences in any of the trials (p>0.05). This suggests that nicotine treatment resulted in impaired contextual fear extinction and 7,8-DHF injections at the 4.0 mg/kg dose reversed the impairment. Moreover, our binned data over testing and five extinction session also showed a significant nicotine×7,8-DHF interaction (F(l,36)=10.395; p<0.05; Figure 2(c)) indicating a within session alterations in the freezing response between groups that received nicotine alone and nicotine with 7,8-DHF. Overall, these results suggest that 7,8-DHF injections reduced the acute nicotine-induced impairment of contextual fear extinction without affecting baseline fear extinction.
Discussion
Herein we show that trkB activation via systemic 7,8-DHF administration dose-dependently ameliorates acute nicotine-induced impairment of contextual fear extinction. Most interestingly, the ameliorating effects of 7,8-DHF were not additive. That is, 7,8-DHF alone did not enhance fear extinction. Therefore, 7,8-DHF specifically reversed acute nicotine’s effects on fear extinction without altering general freezing behavior or contextual fear extinction.
There are several potential mechanisms that may explain our results. First, acute nicotine decreases BDNF mRNA levels in the hippocampus (Kenny et al., 2000). Acute nicotine may also decrease BDNF activation of trkB in the hippocampus leading to disruption of hippocampal-mPFC processes involved in extinction. Thus, 7,8-DHF-induced trkB receptor activation might reverse acute nicotine’s effects on contextual fear extinction by compensating for nicotine-associated decreases in trkB activation. However, although Kenny et al. (2000) showed that acute nicotine decreased BDNF mRNA levels in the hippocampus at two- and 24-hour timepoints, another study showed that BDNF protein levels were increased at the eight-hour time-point and no change was detected at the 24-hour timepoint (French et al., 1999). This suggests that there may be a discrepancy between the effects of acute nicotine on BDNF mRNA and protein levels. Alternatively, acute nicotine might directly alter trkB receptor expression. For example, French et al. (1999) showed that acute nicotine results in increased trkB receptor mRNA in the hippocampus, which may indicate an acute nicotine-induced dysregulation of trkB receptors. However, given that 7,8-DHF reverses nicotine’s effects on contextual fear extinction rapidly starting from the first extinction session, trkB upregulation may not be the likely mechanism underlying these effects. Nevertheless, future studies are required to better understand how nicotine alters BDNF/trkB receptor signaling.
It is important to note that we observed 7,8-DHF reversal of nicotine-induced impairment of contextual fear extinction in a relatively small range of doses where 4.0 mg/kg dose effectively reversed the extinction deficit, the 2.5 mg/kg dose was only transiently effective. Furthermore, additional statistical analysis showed that the main effect of drug for only saline-vehicle and nicotine-4.0 mg/kg 7,8-DHF group comparison was trending towards significance (F(l,18)=3.215, p=0.090). This suggests that neurobiological mechanisms outside the trkB-nicotine interaction may be involved in the acute nicotine-induced impairment of contextual fear extinction. Finally, we observed the reversal effects of 7,8-DHF starting from the first extinction session. This may suggest that within session encoding of extinction memories rather than between session consolidation of these memories were affected by 7,8-DHF administration. We recently showed that acute nicotine has impairing effects on contextual fear extinction when injected before or after fear extinction (Kutlu et al., 2017b), which shows that nicotine may modulate both encoding and consolidation of extinction memories. Therefore, one potential explanation is that 7,8-DHF reverses nicotine’s effects only on encoding but not consolidation. Finally, another possibility is that 7,8-DHF reduces acute nicotine-enhanced spontaneous recovery of contextual fear. Previously, we showed that acute nicotine enhanced spontaneous recovery of extinguished contextual fear when administered prior to re-testing (Kutlu et al., 2016b). In addition, we have shown that acute nicotine augmented within-session spontaneous recovery during its impairing effects on contextual fear extinction (Kutlu and Gould, 2014). Thus, it is possible that the impaired extinction we observed as a result of acute nicotine administration may be a product of augmented spontaneous recovery. Supporting this argument, in the present study, we replicated our results showing acute nicotine-induced enhancement of within-session spontaneous recovery. Moreover, we showed that 7,8-DHF reduces this effect (Figure 2(c)). This suggests that the 7,8-DHF-induced reversal of contextual fear extinction deficits may be due to reduced recovery of contextual fear.
Nicotine use has been associated with an increased risk of developing anxiety and stress disorders (e.g. post-traumatic stress disorder (PTSD); Breslau et al., 1991; Koenen et al., 2005), and is a strong modulator of fear and trauma-related symptoms of these disorders (Thorndike et al., 2006). Together with clinical studies showing that nicotine administration may exacerbate trauma related memories (e.g. Hawkins and Cougle, 2013), our preclinical results demonstrate that nicotine may prolong the exposure therapy process by disrupting fear extinction (Kutlu and Gould, 2014; Kutlu et al., 2016a,b, 2017a). Therefore, these results have implications for understanding the relationship between nicotine and anxiety and stress disorders. Specifically, our results suggest that 7,8-DHF may be employed as an effective pharmacological intervention for nicotine’s effects on trauma-related disorders. Importantly, a 7,8-DHF prodrug is currently under development for treatment of Alzheimer’s disease (Liu et al., 2016) and thus, in the future, this drug may also be included as a pharmacological treatment targeting exposure therapy in smokers.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded with grant support from the National Institute on Drug Abuse (T.J.G., DA017949; 1U01DA041632), Jean Phillips Shibley Endowment, and Penn State Biobehavioral Health Department.
Footnotes
Declaration of Conflicting Interests
The authors) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Andero R, Heldt SA, Keqiang Y, et al. Effect of 7,8-dihydroxy-flavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Connor DA, Kutlu MG, Gould TJ. Nicotine disrupts safety learning by enhancing fear associated with a safety cue via the dorsal hippocampus. J Psychopharmacol. 2017;31:934–944. doi: 10.1177/0269881117695861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both fore-ground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Humby T, Homer CH, et al. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Schümann D, et al. Where there is smoke there is fear—impaired contextual inhibition of conditioned fear in smokers. Neuropsychopharmacology. 2017;42:1640–1646. doi: 10.1038/npp.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KA, Cougle JR. The effects of nicotine on intrusive memories in nonsmokers. Exp Clin Psychopharmacol. 2013;21:434–442. doi: 10.1037/a0033966. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, et al. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 2000;85:234–238. doi: 10.1016/s0169-328x(00)00246-1. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Garrett B, Gadiwalla S, et al. Acute nicotine disrupts consolidation of contextual fear extinction and alters long-term memory-associated hippocampal kinase activity. Neurobiol Learn Mem. 2017b;145:143–150. doi: 10.1016/j.nlm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. An acute dose of nicotine delays extinction of contextual fear in mice. Behav Brain Res. 2014;263:133–137. doi: 10.1016/j.bbr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiol Behav. 2016;155:162–171. doi: 10.1016/j.physbeh.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Oliver C, Gould TJ. The effects of acute nicotine on contextual safety discrimination. J Psychopharm. 2014;28:1064–70. doi: 10.1177/0269881114552743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Holliday E, Gould TJ. High-affinity α4β2 nicotinic receptors mediate the impairing effects of acute nicotine on contextual fear extinction. Neurobiol Learn Mem. 2016a;128:17–22. doi: 10.1016/j.nlm.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Tumolo JM, Holliday E, et al. Acute nicotine enhances spontaneous recovery of contextual fear and changes c-Fos early gene expression in infralimbic cortex, hippocampus, and amygdala. Learn Mem. 2016b;23:405–414. doi: 10.1101/lm.042655.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Zeid D, Tumolo JM, et al. Pre-adolescent and adolescent mice are less sensitive to the effects of acute nicotine on extinction and spontaneous recovery. Brain Res Bull. 2017a doi: 10.1016/j.brainresbull.2017.06.010. Epub ahead of print 15 June 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2–9. doi: 10.1186/s40035-015-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, et al. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, et al. Hippocampal–prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike FP, Wernicke R, Pearlman MY, et al. Nicotine dependence, PTSD symptoms, and depression proneness among male and female smokers. Addict Behav. 2006;31:223–231. doi: 10.1016/j.addbeh.2005.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Gao J, Han L, et al. Prior chronic nicotine impairs cued fear extinction but enhances contextual fear conditioning in rats. Neuroscience. 2008;153:935–943. doi: 10.1016/j.neuroscience.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]