Summary
Background:
We evaluated the effectiveness of integrated care centers (ICCs), which provided single-venue HIV testing, prevention, and treatment services for people who inject drugs (PWID) and men who have sex with men (MSM), in India.
Methods:
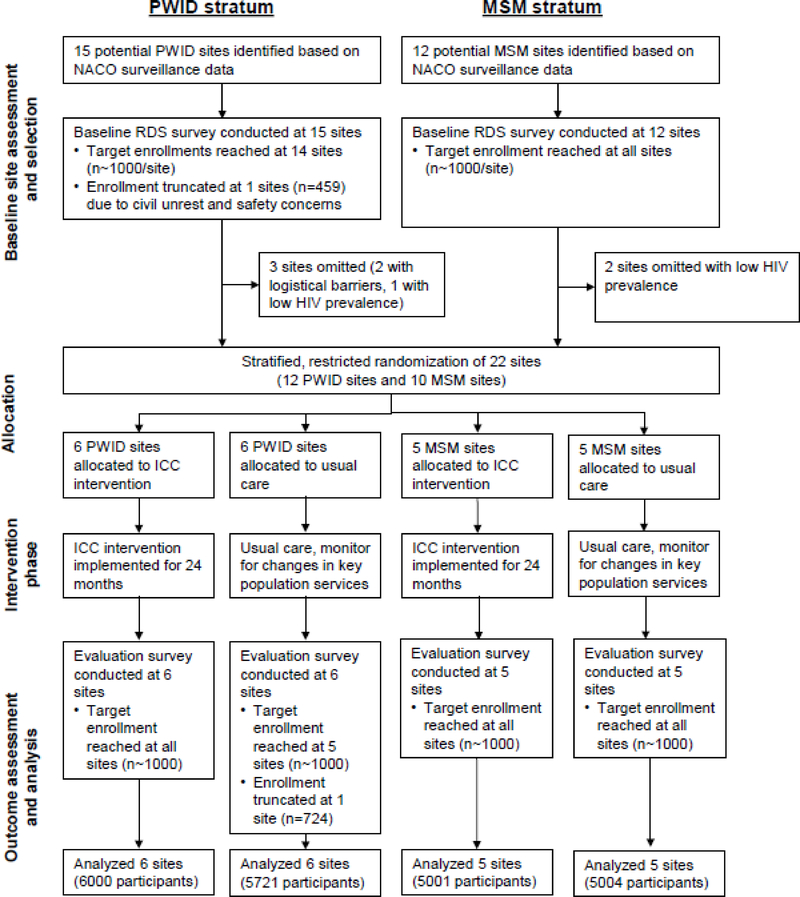
We conducted baseline respondent-driven sampling (RDS) surveys in 27 sites across India, and selected 22 of these (12 PWID and 10 MSM) for a cluster randomised trial on the basis of high HIV prevalence and logistical considerations. We used stratified (PWID and MSM), restricted randomisation to allocate sites to either the ICC intervention or usual care (11 sites per arm). We implemented ICCs in 11 cities (6 PWID ICCs embedded within opioid agonist treatment centers and 5 MSM PWIDs embedded within locations of government-sponsored health services), with a single ICC per city in all but 1 city. After a 2-year intervention phase, we conducted evaluation RDS surveys of target population members 18 years or older at all sites. The primary outcome was self-reported HIV testing in the prior 12 months (recent testing) in the evaluation survey. We used a biometric identification system to estimate ICC exposure (visited an ICC at least once) among evaluation survey participants at intervention sites. This trial is registered with ClinicalTrials.gov (NCT01686750).
Findings:
ICCs provided HIV testing for 14,689 unique clients during the intervention phase. In the evaluation phase (August 2016 to May 2017) we surveyed 11,721 PWID and 10,005 MSM participants using RDS. In the primary population-level analysis, recent HIV testing was 31% higher in ICC than usual care sites (adjusted prevalence ratio [aPR] 1·31, 95% confidence interval [CI] 0·95, 1·81, p=0·09). Among survey participants at intervention sites, ICC exposure was lower than expected (median exposure 40% at PWID sites and 24% at MSM sites). In intervention sites, survey participants who visited an ICC were 3·5-fold (95% CI 2·9, 4·1) more likely to report recent HIV testing than participants who had not. Post-hoc analyses suggested that greater ICC exposure was associated with higher recent testing (aPR for sites with >35% exposure vs. usual care 1·77, 95% CI 1·30, 2·41).
Interpretation:
Although ICCs provided HIV testing for large numbers of PWID and MSM, they were not associated with a statistically significant population-level effect on recent HIV testing at the α=0.05 level. Our finding that biometrically-tracked ICC exposure rates were low at intervention sites suggests that the scale-up of a single ICC in most cities was insufficient to serve large underlying PWID and MSM populations. Future work should address the use of population size estimates to guide the “dose” of combination HIV interventions targeting key populations.
Introduction
Outside of sub-Saharan Africa, key populations account for the majority of HIV infections worldwide. Key populations (groups at high risk for HIV due to risk behaviors) are commonly subject to stigma and criminalization of behaviors that adversely affect access to HIV services.1,2 Achieving Joint United Nations Programme on HIV/AIDS (UNAIDS) targets for HIV diagnosis and treatment3 requires new strategies for engaging key populations, particularly in low- and middle-income countries. India has an estimated 2·1 million people living with HIV.4 While overall HIV prevalence and incidence have declined in India due to successful efforts targeting heterosexual transmission routes,5 people who inject drugs (PWID) and men who have sex with men (MSM) have among the highest HIV prevalence in India and low utilization of evidence-based services. In a 2013 survey, the HIV prevalence among MSM and PWID across 27 Indian cities was 7.0% and 21.1%, respectively. Moreover, only 35.3% of MSM had 44.6% of PWID had ever been tested for HIV.6–8
In India, like many settings, HIV testing, prevention, and treatment services are supported by the government, but services are typically non-integrated and provided in separate venues.9,10 For example, although government-supported HIV testing venues are widely available in India, these venues do not provide wrap-around key population-focused services. Integrated service models that target key populations have been advocated,11 but empirical support for this strategy is limited. Non-randomized studies conducted in Ukraine11 and Greece12 found evidence that integrated interventions for PWID improved service utilization and HIV outcomes. A cluster-randomized trial targeting female sex workers in Zimbabwe found that an integrated service intervention increased HIV diagnosis rates and treatment access, but did not affect community viral load.13 We evaluated the population-level effectiveness of PWID- and MSM-focused integrated care centers (ICCs), which provided integrated HIV testing, prevention, and treatment services in a single venue.
Methods
Study Design and participants
Due to the structural nature of the intervention (designed to change physical, social, or organizational structures), we compared the ICC strategy with usual care in a stratified (PWID and MSM) cluster-randomized trial in 22 sites across India.14 We defined clusters as key population members (either PWID or MSM) that were 18 years or older residing in an Indian city. We initially identified 27 candidate sites where key populations were believed to be large or underserved (Figure 1).4 We conducted baseline respondent-driven sampling (RDS) surveys in each site (15 PWID and 12 MSM) to characterize risk behaviors, HIV testing experience, and the HIV care continuum.6–8 We selected 22 sites (12 PWID and 10 MSM) for the cluster-randomized trial based on high HIV prevalence, low access to services, and logistical considerations. Specifically, we excluded 3 sites with the lowest estimated HIV prevalence (Lucknow and Mangalore in the MSM stratum, and Bhubaneshwar in the PWID stratum), 1 PWID site with a low HIV prevalence and travel challenges (Gangtok), and 1 PWID site, in which ongoing civil unrest and militarization threatened its viability as a study site (Moreh). Following randomisation, we implemented ICCs at intervention sites for approximately 2 years, after which we conducted evaluation RDS surveys in all 22 sites for outcome assessments.
Figure 1. Cluster-randomised trial flow diagram.
PWID, people who inject drugs; MSM, men who have sex with men; NACO, National AIDS Control Organization, India; RDS, respondent-driven sampling; ICC, integrated care center
We conducted baseline RDS survey prior to the intervention and evaluation RDS survey after the intervention using identical methods. We initiated each RDS survey from a single field site in each city with 2 or 3 “seed” participants (influential and connected members of the key population, identified by research staff during focus groups and ethnography in each city prior to beginning the survey), who were given two coupons to recruit network members.15 Recruits returned to the field site with a coupon and, if eligible, were enrolled and given 2 new coupons to recruit others. We continued recruitment through successive RDS waves until a target of ~1,000 participants was enrolled at each site. “Seed” participants were excluded from analyses. We reimbursed participants for completing the study visit (INR 250 [USD 3·60]) and for each eligible participant that they recruited (INR 50 [USD 0·70], maximum 2). We used bar-coded coupons and RDS Coupon Manager software to track recruitment chains. We used a fingerprint-based biometric tracking system in the RDS surveys (Hamster Plus, SecuGen Corp., Santa Clara, CA) to prevent individuals from participating in the same survey more than once, to identify participants who sought services in an ICC, and to identify participants who participated in both the baseline and evaluation surveys.
Participants were eligible to participate in the surveys if they 1) were 18 years or older, 2) presented a valid recruitment coupon (except “seeds”), 3) spoke Hindi, English, or the local language, and 4) were competent to provide consent. Additionally, in PWID sites, participants needed to report injection drug use in the prior 2 years and in MSM sites, participants had to self-identify as male and report oral or anal intercourse with a man in the prior year. Participants provided a blood sample and completed an interviewer-administered electronic survey, which queried demographics, risk behaviors, and access to HIV testing and services. To minimize reporting bias due to social desirability, survey interviewers were not affiliated with the ICCs (in intervention sites) or with local organizations serving the key population in question.
In both the baseline and evaluation surveys, we provided pre-test counseling and rapid HIV testing using 3 rapid test kits, with Western Blot confirmation if needed (appendix p 2). Participants received their HIV test results and post-test counseling after completing the survey. HIV-positive participants were provided with referrals to government ART centers. Samples were shipped to the central laboratory in Chennai, India, where additional testing was done and aliquots stored. We measured CD4 cell counts and HIV RNA in HIV-positive participants by flow cytometry and RealTime HIV-1 (Abbott Laboratories, Abbott Park, IL, USA), respectively. Additionally, all samples from HIV-positive participants were tested with the Limited Antigen (LAg) Avidity EIA (Maxim Biomedical Inc, Rockville, MD, USA) and the JHU-modified Bio-Rad Avidity assay (Bio Rad Laboratories, Hercules, CA, USA) to identify those with recent HIV infection (appendix p 2).16 Participants provided oral consent and ethical oversight was provided by the Johns Hopkins Medicine, Johns Hopkins Bloomberg School of Public Health, and the YR Gaitonde Centre for AIDS Research and Education institutional review boards. Additionally, trial activities were reviewed by a data and safety monitoring board and an advisory board for the PWID and MSM strata, respectively.
Randomisation and masking
To minimize the likelihood of large baseline imbalances between study arms, which can occur when the number of clusters is relatively small, we used a stratified, restricted randomisation protocol.14 Briefly, after stratifying by key population (PWID and MSM), we applied restrictions to ensure both stratum-specific and overall balance across arms in geographical factors (for political reasons) and HIV related factors including recent HIV testing and HIV prevalence. Of 232,848 possible cluster allocation sequences, 596 met the restriction criteria. Of these, one allocation was selected in a number drawing ceremony on December 15, 2013 that was certified by 3 impartial observers (video available at https://www.youtube.com/watch?v=vmHYHgv_uS0). Because of the nature of the intervention, assignments were not masked.
Procedures
We designed ICCs as PWID- or MSM-focused centers that provided vertically-integrated HIV testing, prevention, and treatment assistance in a supportive setting.14 We established one ICC in each intervention site, with the exception of Imphal where we established three ICCs to accommodate a large PWID population and limited transportation in that city. PWID ICCs were established within existing opioid agonist treatment (OAT) programs. MSM ICCs were generally established in office buildings where government-sponsored health services were available. Each ICC was staffed with a supervisor, 1–2 nurses, a counselor, a phlebotomist, 1–3 outreach workers, and a part-time physician. ICCs provided services to any client seeking them (including families of PWID and MSM), without regard to risk behaviors or participation in research surveys. Clients visiting the ICCs were not compensated.
Rapid HIV counseling and testing was the cornerstone service at ICCs. The ICCs used clients’ social networks and outreach workers to identify new clients and link them to the ICCs. Outreach workers and other ICC staff accompanied HIV-positive clients on initial linkage visits to government HIV clinics, where free ART was available. Subsequently, ICC staff assisted with ART refills and tracked retention to HIV care. ICCs provided additional wrap-around services including condoms, sexually transmitted infection (STI) screening and treatment, tuberculosis screening and referral, and counseling (risk reduction, substance use, depression/anxiety). In addition, PWID ICCs provided OAT on-site and needle and syringe exchange through field-based outreach workers. The National AIDS Control Organisation (NACO) and state AIDS control societies provided ICCs with HIV test kits, condoms, STI treatment kits, OAT supplies, and needles/syringes. ICCs provided services in accordance with NACO guidelines. ICCs did not provide pre-risk exposure prophylaxis (PrEP) as this was not a component of the government HIV program and was not available free-of-charge at any site. As in the RDS surveys, we used a biometric system at ICCs to track client visits and service utilization. In the first quarter of 2016, research staff not involved in the intervention conducted brief, non-reimbursed, client satisfaction surveys in a convenience sample of ICC clients. Sites assigned to usual care received no specific intervention following the baseline RDS survey. HIV counseling and testing, ART, condoms, STI services, tuberculosis diagnosis and treatment, OAT, and needle and syringe exchange were available free of charge in usual care sites, but were not integrated.
Outcomes
We assessed study outcomes in the evaluation RDS survey, which was conducted at all sites after approximately 2 years of ICC service delivery in the intervention sites. The primary outcome was recent HIV testing at the population-level, defined as self-reported HIV testing in the prior 12 months, which was assessed in all participants, except those that reported HIV-positive status with a diagnosis more than 12 months previously. Secondary, HIV-related outcomes were assessed in participants testing positive for HIV in the study, and included awareness of status, receipt of HIV medical care in prior 6 months, CD4 cell count, use of trimethoprim-sulfamethoxazole, and current use of ART and HIV RNA <150 copies per mL among treatment-eligible participants. Consistent with Indian treatment guidelines that were in effect throughout the trial period,17 we considered HIV-positive persons to be treatment eligible if they had a current CD4 count <350 cells per μLor had ever been prescribed ART.
Additional secondary HIV-related outcomes included prevalence of viremic individuals (HIV RNA ≥150 copies per mL) in the population, a measure of community viral load,18 and HIV incidence estimated by a validated multi-assay algorithm (appendix p 2).16 Other secondary outcomes included measures of stigma,19 alcohol use,20 depression,21 self-reported spousal testing (among married participants) and transmission risk behaviors and service utilization that were population specific. For PWID this included injection abstinence, needle/syringe sharing, use of needle and syringe exchange services, and use of OAT. For MSM this included unprotected anal intercourse with a non-main partner, number of non-main male partners, STI symptoms, positive syphilis serology, and use of STI services.
Statistical analysis
With 22 sites, 1000 participants recruited per site, an RDS design effect of 2, a 2-sided α of 0·05, a within-stratum coefficient of variation of 0·25, and an outcome prevalence of 30% in the usual care arm, we had 80% power to detect a relative difference (prevalence ratio [PR]) of 1·40 or higher for recent HIV testing, which corresponds to an absolute difference of 12 percentage points or higher.14,22
In the primary analysis, we compared the RDS II-weighted prevalence23 of outcomes in ICC and usual care sites from the evaluation RDS, adjusted for baseline cluster-level prevalence. We used linear regression models that had terms for intervention status (ICC vs. usual care), stratum (PWID vs. MSM) and the baseline proportion of the outcome being assessed. Site-level proportions from both the evaluation and baseline RDS were log transformed before being entered into the regression model.22 The exponentiated coefficients for intervention status, therefore, represent the PR with 95% confidence intervals (CI) and are interpreted as the relative percentage difference in the outcome associated with the intervention.
We conducted several pre-specified sensitivity analyses14 including 1) using unweighted cluster-level estimates, 2) consideration of adjustment for demographic factors (age, sex, marital status, educational attainment) in regression models if the factor had large (odds ratio >2 or <0·5) and statistically significant (p<0·05) associations with the intervention and the outcome, 3) modeling the difference in outcome proportions between the evaluation and baseline RDS surveys, 4) using a participant-level approach with multi-level random effects regression models with a random intercept for each cluster to account for the dependence of individual-level responses within clusters24 and 5) in analyses restricted to intervention sites, we compared outcomes among evaluation participants who had and had not visited the ICC, according to biometric match. Finally, we conducted pre-specified population-stratified (PWID and MSM) analyses for all outcomes.
We defined population ICC exposure as the weighted proportion in the evaluation survey that had visited an ICC at least once. To assess for dose-response relationship at the intervention sites, we conducted a post-hoc analysis comparing site-level change in recent HIV testing (between the baseline and evaluation surveys) against population ICC exposure, using linear regression. Further, we reevaluated the primary analysis comparing the RDS II-weighted prevalence of HIV testing from the evaluation RDS, considering different levels of population ICC exposure (<20%, 20–35%, >35%). To assess if HIV testing and treatment referrals done in the baseline survey may have diluted the effect of the intervention, we conducted a post-hoc analysis in which we excluded evaluation survey participants that had also participated in the baseline survey. We used Stata version 15 software (StataCorp, College Station, TX) for statistical analyses. This trial is registered at ClinicalTrials.gov (NCT01686750).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Across the 22 trial sites, we recruited 11,993 PWID and 9,997 MSM in the baseline RDS survey (September 2012 to December 2013) and 11,721 PWID and 10,005 MSM in the evaluation survey (August 2016 to May 2017) (Table 1). A total of 1,631 PWID (14%) and 944 MSM (9%) participated in both the baseline and the evaluation surveys. Twenty-one of 22 evaluation surveys met their target enrollment (1,000 participants). We truncated the evaluation survey at the Mumbai site (PWID) at 724 participants due to slower than expected enrollment and inability to keep the survey site operating longer. In the evaluation surveys, the median number (site range) of RDS recruitment waves was 14 (9, 27) and the median time (site range) for survey enrollment was 154 days (95, 269). At the evaluation survey, the median age ranged from 26 to 36 years at PWID sites and from 23 to 32 years at MSM sites. In the PWID sites, 493 (5.8%) women were recruited. The weighted HIV prevalence ranged from 5·6% to 38·0% at PWID sites and from 3·3% to 32·8% at MSM sites (see appendix pp 3–4 for unweighted percentages).
Table 1.
Characteristics of people who inject drugs and men who have sex with men recruited in baseline and evaluation respondent-driven sampling surveys at 22 Indian sites in a cluster-randomized trial, 2012 to 2017
| PWID stratum | MSM stratum | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | ICC intervention | Usual care | ICC intervention | |||||
| Baseline | Evaluation | Baseline | Evaluation | Baseline | Evaluation | Baseline | Evaluation | |
| Participant-level Characteristics* | ||||||||
| Participants, n | 5997 | 5721 | 5996 | 6000 | 4996 | 5004 | 5001 | 5001 |
| Women, n (site % median, % range) | 341 (2·4, 0–22·7) | 272 (1·9, 0·5–22·0) | 346 (6·4, 0·2–18·7) | 221 (2·2, 0·6–15·4) | - | - | - | - |
| Age (years), site median (range) | 30 (24–34) | 29 (26–35) | 28 (26–34) | 27 (26–35) | 25 (21–30) | 27 (25–30) | 25 (23–29) | 31 (22–32) |
| At least secondary school education, n (site % median, % range) | 3331 (48·3, 30·7–95·1) | 3451 (51·1, 35·6–97·6) | 4341 (68·1, 66·1–93·8) | 4652 (73·7, 60·7–94·3) | 3868 (84·1, 65·0–85·4) | 4004 (84·1, 68·2–88·9) | 4068 (80·7, 61·7–93·2) | 4077 (83·7, 76·7–86·8) |
| Married or living with partner, n (site % median, % range) | 2331 (38·2, 26·3–48·1) | 1993 (34·9, 26·5–48·9) | 2999 (54·3, 33·4–63·9) | 2674 (47·3, 27·7–62·5) | 1997 (32, 25·7–63·0) | 2446 (43, 32·9–66·6) | 1954 (36·9, 22·4–57·8) | 2876 (46·1, 35·9–87·0) |
| Monthly household income (USD), site median (range) | 130 (92–306) | 191 (138–459) | 230 (107–389) | 275 (153–459) | 153 (122–245) | 230 (153–306) | 153 (77–184) | 230, 168–306 |
| Recent incarceration, n (site % median, % range) | 648 (6·6, 4·2–27·7) | 512 (5·9, 4·4–24·3) | 827 (8·7, 0·5–30·1) | 572 (6·2, 2·5–12·3) | 196 (3, 1·4–8·0) | 262 (5, 0·4–7·9) | 191 (2·3, 0·3–6·5) | 84 (0·7, 0·01–2·9) |
| Hazardous alcohol use1, n (site % median, % range) | 2127 (36·9, 11·5–49·3) | 2253 (38.1, 29·5–51·5) | 2721 (50·3, 22·2–66·8) | 1926 (32·2, 8·3–49·2) | 2190 (46, 26·9–57·6) | 1784 (32·1, 26·1–36·4) | 1935 (29·4, 20·4–46·9) | 1316 (18·3, 9·6–43·2) |
| Any recent substance use2, n (site % median, % range) | 5900 (99·2, 95·8–100) | 5565 (99·8, 85·8–100) | 5752 (95·7, 88·7–99·9) | 5432 (89·5, 81·8–98·1) | 3401 (71·8, 55·8–74·0) | 3506 (69·8, 56·0–83·6) | 3291 (57·1, 50·7–83·4) | 3057 (64·7, 48·5–70·1) |
| Ever tested for HIV, n (site % median, % range) | 2728 (48, 7·9–61·5) | 2940 (52·7, 16·6–68·0) | 3055 (46·6, 24·3–71·4) | 3702 (59·2, 44·3–70·2) | 2186 (40.4, 12·1–49·0) | 2722 (53·8, 31·5–61·2) | 2259 (37, 33·7–45·8) | 2751 (50·6, 45·2–61·4) |
| HIV-positive3, n (site % median, % range) | 1220 (17·5, 8·6–30·8) | 1301 (19·9, 8·9–38·0) | 1324 (20, 8·9–31·1) | 1216 (20·4, 5·6–32·2) | 565 (10·6, 4·1–13·1) | 1005 (14·9, 11·7–32·8) | 521 (7·1, 3·8–12·7) | 758 (13·6, 3·3–21·8) |
| Viral suppression among ART-eligible4, n (site % median, % range) | 185 (19·2, 1·5–60·8) | 263 (30·9, 7·4–82·9) | 290 (28·4, 1·2–52·3) | 414 (29·4, 6·7–78·6) | 154 (26·6, 18·7–71·1) | 496 (69·1, 35·2–76·1) | 164 (33·8, 22·4–74·1) | 388 (62·8, 54·7–64·0) |
| Viremic HIV-positive persons5, n (site % median, % range) | 958 (12·4, 5·2–29·7) | 985 (13·6, 1·7–35·8) | 951 (13·3, 8·0–18·8) | 763 (10·2, 4·7–19·6) | 352 (4·5, 3·2–8·5) | 467 (5·4, 4·4–15·4) | 313 (2·9, 2·3–10·1) | 329 (7·3, 1·6–8·7) |
| PWID characteristics | ||||||||
| Injection in past 6 mo., n (site % median, % range) | 5611 (94·5, 87·3–99·1) | 4975 (98·8, 57·7–98·3) | 5211 (87·5, 68·6–98·2) | 4373 (78·5, 27·1–89·6) | - | - | - | - |
| Age at first injection (years), site median (range) | 23 (18–26) | 22 (18–25) | 21 (18–21) | 20 (20–22) | - | - | - | - |
| Ever shared needle/syringe, n (site % median, % range) | 3327 (51, 37·0–72·1) | 3113 (48·4, 24·8–66·7) | 2944 (37·4, 19·9–80·4) | 3221 (42·1, 25·7–82·4) | - | - | - | - |
| Drugs injected in prior 6 mo., n (site % median, % range) | ||||||||
| Heroin | 2875 (40·1, 2·5–97·0) | 2967 (42·9, 7·4–96·0) | 1958 (14·1, 0·6–98·2) | 2699 (43·5, 0·04–89·5) | - | - | - | - |
| Buprenorphine | 2134 (32·9, 0·3–75·7) | 2066 (18·8, 0–90·9) | 2289 (31·2, 0·1–89·9) | 1640 (5·8, 0–77·2) | - | - | - | - |
| Other pharmaceutical opioids | 1634 (7·3, 0·7–88·7) | 844 (4·2, 1·2–26·2) | 1396 (6·9, 0·9–62·9) | 368 (2·1, 0–23·6) | - | - | - | - |
| Cocaine or other stimulant | 54 (0·3, 0–2·6) | 79 (1·3, 0·1–3·8) | 50 (0·5, 0·2–1·5) | 44 (0·2, 0–1·3) | - | - | - | - |
| Sedative/antianxiety | 283 (1·1, 0·1–20·3) | 311 (3·9, 0·1–27·1) | 627 (5·8, 0·4–23·6) | 66 (0·3, 0–2·3) | - | - | - | - |
| Antihistamines | 2125 (25·7, 0·01–90·1) | 2117 (13·5, 0–96·2) | 1992 (24·9, 0·04–73·0) | 1418 (3.1, 0–70·4) | - | - | - | - |
| Ever use needle exchange program, n (site % median, % range) | 2703 (40·5, 6·8–73·8) | 2520 (37·7, 8·3–63·2) | 2279 (25·8, 7·7–59·2) | 2083 (30·4, 6·1–61·1) | - | - | - | - |
| Ever use opioid agonist treatment, n (site % median, % range) | 1627 (18·9, 6·4–48·7) | 1975 (39·3, 4·7–56·4) | 1296 (21·2, 0–33·0) | 2486 (42·1, 10·7–54·2) | - | - | - | - |
| MSM characteristics | ||||||||
| Age at first sexual act with man, site median (range) | - | - | - | - | 18 (17–19) | 18 (17–18) | 18 (17–20) | 19 (18–20) |
| Number of male partners in lifetime, site median (range) | - | - | - | - | 5 (5–20) | 7 (5–40) | 12 (3–20) | 11 (1–20) |
| Unprotected anal sex with man in prior 6 mo., n (site % median, % range) | - | - | - | - | 2900 (52·2, 38·6–73·7) | 2703 (57·5, 38·4–69·4) | 2388 (38·2, 35·8–50·5) | 2209 (32·1, 17·1–61·1) |
| Ever had sex with woman, n (site % median, % range) | - | - | - | - | 3662 (83·5, 66·4–85·7) | 3381 (63·2, 56·5–87·9) | 3221 (68, 62·6–77·5) | 3448 (74·4, 71·3–77·2) |
| Ever history of sex work, n (site % median, % range) | - | - | - | - | 1640 (19·9, 10·4–33·1) | 1896 (32·8, 15·7–38·6) | 1484 (14·7, 8·4–46·8) | 1294 (14·3, 8·4–21·1) |
| Ever diagnosed with STI, n (site % median, % range) | - | - | - | - | 606 (6·7, 2·7–18·7) | 386 (4·2, 0·7–11·7) | 674 (7·2, 2·6–13·9) | 466 (5·7, 2·1–17·8) |
| Ever injected drugs, n (site % median, % range) | - | - | - | - | 90 (1·2, 0·4–3·1) | 119 (0·4, 0·3–8·6) | 48 (0·3, 0·1–3·4) | 24 (0·5, 0·1–1·7) |
| Site Recruitment Characteristics | ||||||||
| Sites, n | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 |
| Sample size per site, median (range) | 1000 (999–1000) | 1000 (722–1000) | 1000 (996–1000) | 1000 (1000–1000) | 1000 (997–1001) | 1000 (1000–1003) | 1000 (998–1003) | 1000 (1000–1001) |
| Number of seeds used per site, median (range) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (2–2) | 2 (2–2) | 2 (2–2) |
| Time to recruit sample (days), median (range) | 139 (52–190) | 171 (101–269) | 136 (89–200) | 139 (96–203) | 97 (70–157) | 175 (114–190) | 104 (87–121) | 172 (128–177) |
| Median network size, site median6 range | 16 (10–20) | 12 (7–30) | 13 (7–20) | 11 (4–20) | 10 (5–20) | 10 (5–20) | 8 (4–60) | 7 (5–21) |
| Number of recruitment waves reached, median (range) | 23 (14–31) | 18 (11–27) | 32 (14–50) | 16 (9–24) | 20 (11–23) | 11 (9–26) | 16 (13–28) | 14 (10–22) |
| Biometric overlap between evaluation and baseline RDS surveys (%), n (site %6 range) | - | 848 (15·5, 7·5–23·5) | - | 765 (13·7 5·8–18·8) | - | 480 (8·2 4·1–15·1) | - | 464 (8·9 5·7–14·6) |
| Biometric overlap between evaluation RDS and ICC registration (%), n (site %6 range) | - | - | - | 2375 (40·1, 16·7–55·5) | - | - | - | 1313 (24·1, 9·8–41·0) |
Unless noted, site-level percentages are weighted using RDS-II weights.
Measured using AUDIT, score ≥8;
Any alcohol use or illicit drug use (either injection or non-injection);
By rapid HIV testing;
CD4≤350 or prior history of ART use;
HIV-seropositive and HIV RNA >150 copies/mL
Unweighted
ICCs were operational in the 11 intervention sites for a median (site range) of 26 months (22, 29) prior to the evaluation survey. PWID ICCs registered 10,757 unduplicated clients across 6 sites (site median, 1,581, site range, 1091, 3305) and MSM ICCs registered 8,489 unduplicated clients across 5 sites (site median 1,627, site range, 1180, 2444) (Table 2). Of ICC clients that were not known to be HIV-positive at registration, 7,630 (87%) of PWID and 7,068 (92%) of MSM were tested for HIV at least once in the ICCs; 420 (5.5%) PWID and 350 (5.0%) MSM were diagnosed HIV-positive. A total of 2,636 (site median, 426, site range, 302, 684) and 1,691 (site median, 308, site range 105, 656) clients at PWID and MSM ICCs, respectively, completed anonymous client satisfaction surveys (appendix pp 5–13). On a 5-point Likert scale, 2,490 (94%) PWID clients surveyed and 1,680 (99%) MSM clients surveyed responded “strongly agree” or “agree” with the statement, “I am satisfied with the services I received today.”
Table 2.
Services provided by integrated care centers to people who inject drugs and men who have sex with men in 11 Indian sites assigned to the intervention arm of a cluster-randomized trial, 2014 to 2017
| PWID integrated care centers | MSM integrated care centers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AZ | DM | IM | BI | CD | LD | BL | BG | CH | HY | VZ | |
| ICC factors | |||||||||||
| Number of ICC sites in city | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ICC initiation date | May 1, 2014 | Jun 26, 2014 | April 1, 2014 | July 1, 2014 | Jun 7, 2014 | Jun 6, 2014 | Nov 24, 2014 | Nov 13, 2014 | Oct 14, 2014 | Feb 14, 2015 | Nov 29, 2014 |
| ICC data censor date | Nov 14, 2016 | Dec 15, 2016 | Dec 13, 2016 | Jan 14, 2017 | Jan 1, 2017 | Feb 24, 2017 | Apr 18, 2017 | May 17, 2017 | Apr 22, 2017 | May 18, 2017 | Mar 29, 2017 |
| Time provided services, months | 31 | 30 | 33 | 31 | 31 | 33 | 29 | 31 | 31 | 27 | 28 |
| Total visits, n | 42,285 | 26,214 | 210,972 | 52,196 | 71,702 | 303,501 | 6,235 | 3,878 | 4,745 | 2,227 | 4864 |
| Client characteristics | |||||||||||
| Clientsa with ≥1 visit, n | 1520 | 1641 | 3305 | 1091 | 1487 | 1713 | 1950 | 1627 | 2444 | 1180 | 1288 |
| Clientsa with ≥2 visits, n | 904 | 1168 | 2666 | 811 | 1089 | 1616 | 828 | 761 | 832 | 411 | 601 |
| Age, years, median (P25, P75) | 28 (23–32) | 30 (26–35) | 35 (28–41) | 27 (22–32) | 28 (23–35) | 26 (23–30) | 30 (25–37) | 26 (22–36) | 27 (23–34) | 26 (23–32) | 25 (21–32) |
| Women, n (%) | 234 (15·4) | 572 (34·) | 221 (6·7) | 60 (5·5) | 82 (5·5) | 207 (12·1) | 80 (4·1) | 8 (0·5) | 7 (0·3) | 58 (4·9) | 58 (4·5) |
| HIV counseling and testing | |||||||||||
| Clientsa tested for HIV at least once, n (% of clients with unknown HIV status that had an HIV test) | 929 (75·3) | 1216 (96·5) | 1677 (64·8) | 893 (94·7) | 1309 (90·2) | 1606 (99·0) | 1667 (98·8) | 1463 (97·6) | 2191 (92·2) | 918 (90·3) | 829 (82·7) |
| Clientsa tested for HIV twice or more, n | 216 | 827 | 720 | 356 | 679 | 1104 | 365 | 592 | 671 | 262 | 253 |
| Total HIV tests performed, n | 1235 | 2408 | 2880 | 1463 | 2729 | 3948 | 2216 | 2284 | 3490 | 1318 | 1242 |
| Clientsa with a positive HIV test, n | 135 | 24 | 46 | 109 | 24 | 82 | 154 | 2 | 85 | 35 | 74 |
| Clientsa with observed HIV seroconversion, n | 18 | 6 | 1 | 13 | 8 | 14 | 4 | 0 | 8 | 1 | 6 |
| Counseling servicesb | |||||||||||
| Any counseling, n | 1359 | 1603 | 2975 | 1062 | 1473 | 1693 | 1948 | 1125 | 2258 | 630 | 1125 |
| Safe sex/safe injecting, n | 257 | 1332 | 2155 | 1032 | 1460 | 1690 | 1873 | 905 | 2256 | 572 | 1125 |
| Substance use, n | 582 | 1101 | 1556 | 923 | 1069 | 1597 | 1 | 3 | 3 | 0 | 0 |
| Depression, n | 61 | 436 | 335 | 412 | 48 | 1663 | 28 | 13 | 697 | 93 | 1073 |
| Family/couple, n | 178 | 272 | 470 | 196 | 86 | 773 | 7 | 6 | 24 | 38 | 51 |
| ART adherence, n | 160 | 391 | 710 | 196 | 54 | 256 | 296 | 127 | 96 | 58 | 320 |
| Risk reduction services | |||||||||||
| Clientsa receiving condoms, n | 336 | 1261 | 1470 | 679 | 927 | 1276 | 429 | 1069 | 527 | 205 | 1004 |
| Clientsa receiving OAT, n | 456 | 188 | 1194 | 724 | 610 | 1336 | 0 | 0 | 0 | 0 | 0 |
| OST visits among those with ≥1 OAT visit, median (P25, P75) | 42 (7–158) | 15 (1–82) | 30 (8–88) | 36 (12–212) | 76 (17–269) | 134 (19–393) | -- | -- | -- | -- | -- |
| Clientsa receiving NSE services, n | 574 | 646 | 1420 | 938 | 1216 | 921 | 0 | 0 | 0 | 0 | 0 |
| HIV-positive client services | |||||||||||
| Total HIV-positive clientsc, n | 433 | 416 | 764 | 311 | 66 | 382 | 424 | 137 | 155 | 201 | 366 |
| Clientsa linked to CD4 count testing, n (% of HIV-positive) | 63 (14·6) | 90 (21·6) | 434 (56·8) | 195 (62·7) | 28 (42·4) | 162 (42·4) | 260 (61·3) | 114 (83·2) | 76 (49·0) | 34 (16·9) | 231 (63·1) |
| Clientsa linked to ART, n (% of HIV-positive) | 53 (12·2) | 48 (11·5) | 508 (66·5) | 40 (12·9) | 2 (3·0) | 259 (67·8) | 205 (48·4) | 100 (73·0) | 67 (43·2) | 38 (18·9) | 286 (78·1) |
| Other wrap-around services | |||||||||||
| Clientsa screened for tuberculosis symptoms, n | 77 | 223 | 3036 | 813 | 1459 | 1450 | 1907 | 7 | 2153 | 625 | 123 |
| Clientsa with positive tuberculosis sputum smear, n | 3 | 3 | 2 | 5 | 1 | 13 | 1 | 0 | 0 | 0 | 3 |
| Clientsa screened for an STI, n | 929 | 244 | 2971 | 965 | 1315 | 86 | 1922 | 29 | 2155 | 372 | 306 |
| Clientsa treated for an STI, n | 8 | 13 | 18 | 6 | 8 | 23 | 32 | 26 | 24 | 214 | 59 |
PWID, people who inject drugs; MSM, men who have sex with men; ICC, integrated care center; AZ, Aizawl; DM, Dimapur; IM, Imphal; BI, Bilaspur; CD, Chandigarh; LD, Ludhiana; BL, Bangalore; BG, Belgaum; CH, Chennai; HY, Hyderabad; VZ, Visakhapatnam; ICC, integrated care center; P25 and P75, 25th and 75th percentiles; ART, antiretroviral therapy; OAT, opioid agonist treatment; NSE, needle and syringe exchange; STI, sexually transmitted infection.
Unduplicated clients, biometrically defined;
Excluding counseling for HIV testing, unduplicated clients that received specific type of counseling;
Includes clients (unduplicated) with known HIV-positive status at first ICC visit and those that first tested positive for HIV in the ICC
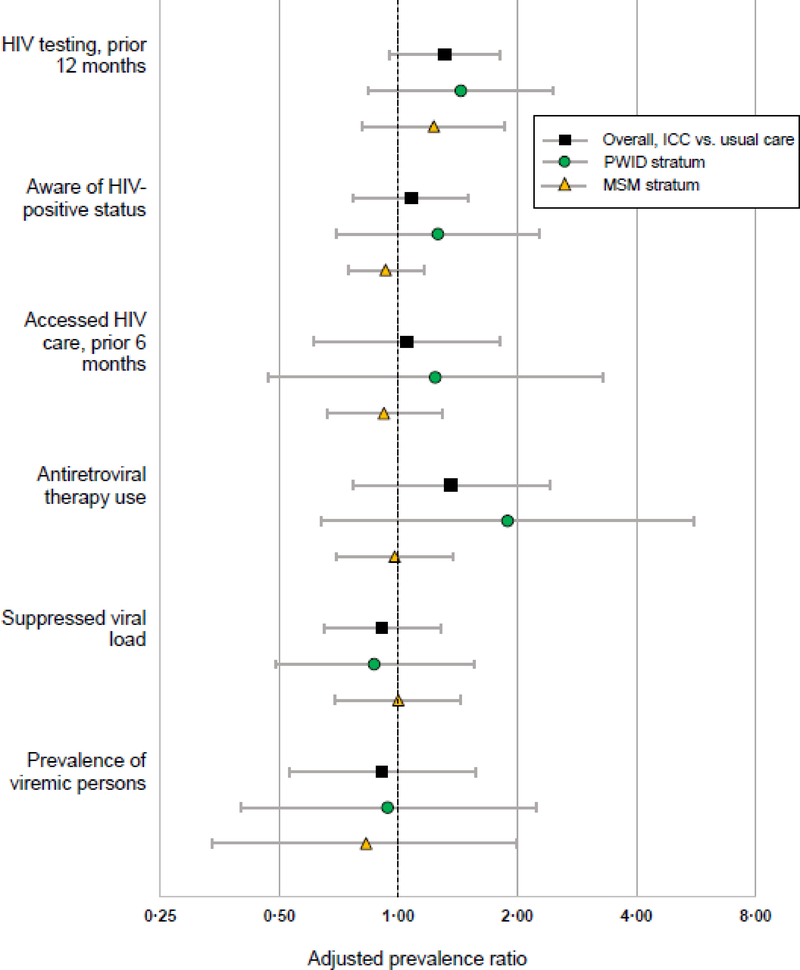
In the PWID stratum at evaluation, the average weighted percentages reporting recent HIV testing were 26·8% (95% CI 25·9, 27·8) and 39·6% (95% CI 38·5, 40·7) at usual care and ICC sites, respectively; while the corresponding values at MSM sites were 25·2% (95% CI 23·3, 27·1) and 33·9% (95% CI 32·8, 35·2) (Figure 2; appendix p 14). The within-strata coefficients of variation at the evaluation survey were 0·40 and 0·31 for PWID and MSM sites, respectively. In the primary cluster-level analysis, recent HIV testing was 31% higher in ICC compared with usual care sites (adjusted PR 1·31, 95% CI 0·95, 1·81, p=0·09, Figure 3), which corresponded to a difference of 7·7 percentage points (95% CI −1·3, 16·6). In pre-specified sensitivity analysis using a multi-level random effects model, the ICC effect on recent HIV testing was statistically significant (adjusted PR 1·31, 95% CI 1·03, 1·67, p=0·029). There was no statistical evidence of effect modification by key population stratum in either analysis (p >0·1 for interaction terms). Demographic factors did not meet pre-specified criteria for inclusion in the model. Inferences were unchanged in sensitivity analyses using unweighted sample estimates (appendix p 14). Finally, in a sensitivity analysis in which we excluded 2,557 participants (12%) from the evaluation survey who participated in the baseline survey, point estimates for ICC effect on recent HIV testing and HIV-specific outcomes were minimally changed (appendix p 15).
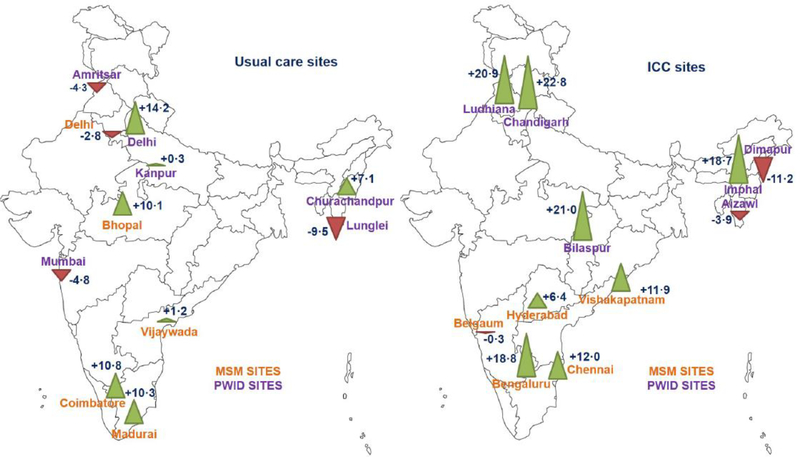
Figure 2. Indian map of study sites in cluster-randomised trial.
The left and right maps show sites assigned to usual care and ICC intervention, respectively. PWID sites are shown in purple font and MSM sites in orange font. The numbers next to each site represent the crude change in the weighted percentage of participants reporting recent HIV testing (primary outcome) between respondent-driven sampling surveys (evaluation minus baseline). The triangles also represent change in recent HIV testing (green upward pointing triangles indicate an increase in recent testing and red downward pointing triangles indicate a decreases in recent testing), with triangle height proportional to the size of the change.
Figure 3. Effect of ICC versus usual care on HIV-related outcomes.
HIV testing in the prior 12 months (primary outcome) was assessed in all participants except HIV-positive persons that reported HIV diagnosis more than 12 months prior. Awareness of status and access to HIV care were assessed in all HIV-positive participants. Antiretroviral therapy use and suppressed viral load (HIV RNA <150 copies per mL) were assessed in treatment-eligible (see text) HIV-positive participants. Prevalence of viremic persons was assessed in the complete participant sample. ICC, integrated care center; PWID, people who inject drugs; MSM, men who have sex with men.
There were no statistically significant intervention effects, overall or in key population strata, for HIV-specific outcomes, including awareness of status, accessing HIV care in the prior 6 months, use of ART among eligible persons, viral load suppression among ART-eligible persons, and prevalence of viremic individuals (Figure 3; appendix pp 16–20). Average viral suppression among those ART-eligible at baseline was 27·4% (95% CI 25·5%, 29·3%) and 40·6% 95% CI 32·8%, 48·4%) for PWID and MSM sites, respectively; at evaluation, average suppression was 34·5% (95% CI 32·6%, 36·4%) and 64·1% (95% CI 56·4, 71·9%), respectively, with no ICC effect compared with usual care (adjusted PR: 0.91, 95% CI: 0.65, 1.28). Data suggested that MSM made larger improvements in HIV care continuum outcomes than PWID (appendix pp 16–19). There were no statistically significant differences between study arms in use of trimethoprim-sulfamethoxazole or CD4 cell counts (data not shown). At the evaluation survey, the average estimated HIV incidence was 5·16 (95% CI 4·33, 5·98) and 1·44 per 100 PY (95% CI 1·05, 1·82) at PWID and MSM sites, respectively, with no evidence of an ICC compared with usual care (adjusted incidence rate ratio 0·69; 95% CI: 0·11, 4·36; appendix p 21).
There were no statistically significant intervention effects on PWID-specific risk behaviors or service use in the prior 6 months, including recent injecting, needle or syringe sharing, accessing needle and syringe exchange, or accessing OAT (appendix p 22). Similarly, there were no statistically significant intervention effects on MSM-specific risk behaviors or service use in the prior 6 months, including number of non-main sex partners, unprotected anal intercourse with a non-main partner, sexually transmitted infection symptoms or testing, or syphilis seropositivity. Among the 4 stigma indices,19 where higher scores indicate more severe stigma, vicarious stigma was significantly lower at ICC sites compared with usual care sites (score difference −1·8, 95% CI −3·0, −0·6), although this difference was driven by the PWID stratum (score difference −3·1, 95% CI −4·5, −1·7) versus the MSM stratum (score difference −0·1, 95% CI −2·0, 1·7).
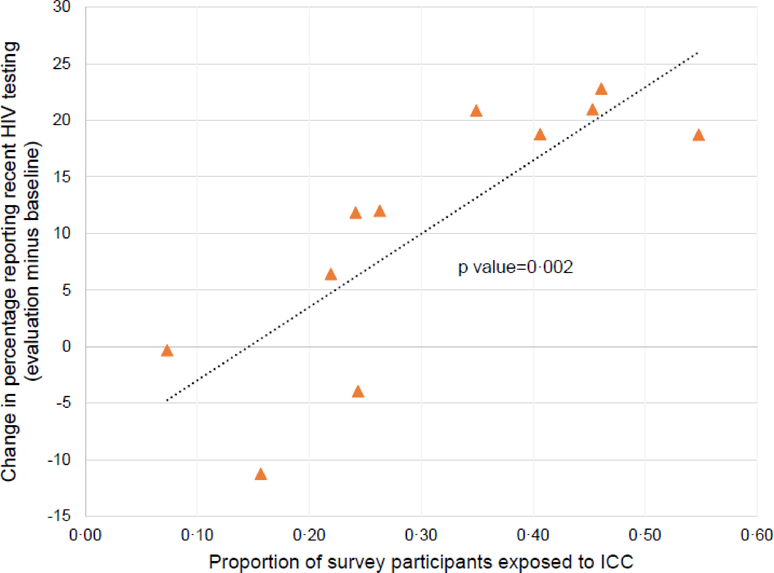
At the ICC sites, 2375 PWID and 1313 MSM who participated in the evaluation survey attended an ICC at least once, corresponding to a median (range) population ICC exposure of 40% (17, 56) at PWID sites and 24% (10, 41) at MSM sites (Table 1). Higher site-level ICC exposure at the evaluation survey was significantly associated with larger increases in recent HIV testing between the baseline and the evaluation survey (p=0·002, R-squared=0.68) (Figure 4). Moreover, in cluster-level analyses, adjusting for baseline prevalence of recent HIV testing, we observed a dose-response relationship between population ICC exposure and recent HIV testing. Compared with usual care sites, there was no statistically significant effect of intervention sites that had <20% exposure (adjusted PR 0·75, 95% CI 0·48, 1·18) or 20–35% exposure (adjusted PR 1·12, 95% CI 0·80, 1·59), but there was a statistically significant effect for sites with >35% exposure (adjusted PR 1·77, 95% CI 1·30, 2·41).
Figure 4. Association between ICC exposure among evaluation survey participants and change in recent HIV testing from baseline to evaluation.
Each triangle indicates 1 of the 11 sites assigned to the intervention. The dotted line indicates the least squares regression line, with p value from linear regression. ICC, integrated care center.
At ICC sites, evaluation survey participants that had visited an ICC at least once were over 3-fold more likely to report recent HIV testing than participants that had not visited an ICC (adjusted PR 3·46, 95% CI 2·94, 4·06). Similarly, ICC-exposed HIV-positive participants were more likely to be aware of their HIV status (adjusted PR 1·43, 95% CI 1·25, 1·64) and to be using ART if eligible (adjusted PR 1·25, 95% CI 1·02, 1·53) than unexposed participants (appendix pp 14–19). However, ART-eligible ICC-exposed participants were not more likely to have a suppressed viral load compared with unexposed persons (adjusted PR 1·07, 95% CI 0·89, 1·29). At PWID intervention sites, ICC-exposed persons were more likely than non-exposed persons to report injecting abstinence for 6 months or longer (adjusted PR 1·62, 95% CI 1·10, 2·41). Compared with non-exposed participants, ICC-exposed individuals were significantly more likely to report population-specific service use in the prior 6 months (appendix p 22), including use of needle and syringe exchange among active injectors at PWID sites (adjusted PR 2·27, 95% CI 1·76, 2·94), use of OAT at PWID sites (adjusted PR 4·25, 95% CI 3·17, 5·69), and evaluation and treatment for STI symptoms at MSM sites (adjusted PR 3·71, 95% CI 2·05, 6·73). Finally, in combined PWID and MSM strata, ICC-exposed participants were more likely than unexposed participants to report that their spouse or opposite-sex partner had been tested for HIV (adjusted PR 1·61, 95% CI 1·36, 1·91).
Discussion
In this cluster-randomised trial, we evaluated the effectiveness of PWID- and MSM-focused ICCs in India that aimed to provide vertically integrated HIV testing, risk-reduction services, and treatment linkage in non-discriminatory settings. Compared with usual care, ICCs were associated with a 31% relative population-level increase in recent HIV testing in the evaluation survey, although this difference did not achieve statistical significance at the α=0·05 level. Despite providing HIV testing to almost 15,000 unduplicated clients in a two-year period, biometric data suggested that ICC reach was suboptimal within the target populations - the median ICC exposure among evaluation survey participants was only 40% and 24% at PWID and MSM sites, respectively. However, in pre-specified individual-level analyses, we found that evaluation survey participants that had ever visited an ICC had significantly higher rates of recent HIV testing, were more likely to be aware of their status and to be taking ART (among HIV-positive persons) and had lower rates of injection-related and sexual risk behavior, compared with participants that had not visited an ICC. We also found that higher ICC exposure at intervention sites was associated with larger increases in recent HIV testing between the baseline and evaluation survey. These sensitivity analyses should be interpreted with caution given the potential for selection bias in the first analysis and the ecologic nature of the second comparison. However, the findings suggest that, relative to larger than anticipated key population sizes, a marginal ICC “dose” (all cities, except one, had only a single ICC) constrained the population-level impact of the intervention. We hypothesize that scaling up more ICCs in the intervention sites or allowing the ICCs to operate longer would have yielded larger population-level improvements. Alternatively, it may be that there are subsets within key populations that are not effectively reached by the ICC model.25
It is important to consider alternate explanations for why we did not observe a more substantial effect of the ICC intervention at the population-level. First, it is possible the ICCs were ineffective at engaging the target populations. However, this appears unlikely as large numbers of clients visited ICCs for services, the vast majority of clients that were not known to be HIV-positive at intake completed HIV testing, and clients favorably reviewed ICCs in anonymous satisfaction surveys. Second, in the baseline survey, we provided HIV counseling and testing at all sites. These baseline activities may have diluted the observed benefit of the intervention, particularly for the primary outcome, as has been observed in other combination prevention trials.26
Beyond HIV testing, the ICC intervention was not associated with improvements in HIV-specific outcomes, including awareness of status, access to care, use of ART, viral suppression, prevalence of viremic individuals, or estimated HIV incidence. We observed no significant differences between ICCs and usual care in population-specific risk behaviors or access to services. It is important to note that ICCs had only indirect ability to promote ART use among HIV-positive clients. Outreach workers linked out-of-care HIV-positive clients to government treatment clinics, but ICCs did not have their own supplies of antiretroviral drugs and could not independently initiate treatment. Work in Ukraine suggests that co-location of PWID services and ART can be successful,11 and India is increasingly open to decentralized models of ART distribution.27
Our results should be considered in the context of other trials of combination intervention strategies. A cluster-randomised trial in Zimbabwe compared the effectiveness of enhanced services (increased HIV testing, on-site ART initiation, and pre-exposure prophylaxis) with regular services in female sex worker clinics to reduce the prevalence of viremic women.13 Similar to our study, this group found that enhanced services for female sex workers were not associated with a statistically significant reduction in the prevalence of viremic women compared with regular services, despite more HIV testing and diagnoses, ART initiation, and use of pre-exposure prophylaxis at intervention sites compared with regular service sites.
HIV Prevention Trials Network (HPTN) 043 (Project Accept)28 evaluated an intervention that aimed to increase testing accessibility among young people in a cluster-randomised trial at 48 African and Thai sites. The intervention was associated with a statistically significant 25% higher HIV testing rate compared with the control condition, although the difference in estimated HIV incidence (primary outcome) was not statistically significant. The magnitude of increased HIV testing in Project Accept was similar to our finding that ICCs increased recent HIV testing by 31%. HPTN 071 (PopART) is an ongoing trial29 evaluating two interventions against standard care in 21 African sites with generalized HIV epidemics. The more intensive intervention includes health promotion efforts, annual population HIV testing, and immediate ART initiation in HIV-positive individuals.
Our study has several strengths. First, this is among the first cluster-randomised trials to evaluate the population-level effectiveness of a combination intervention strategy in key populations. Key populations account for the majority of HIV infections outside of sub-Saharan Africa, but are often stigmatized and disenfranchised, particularly in low- and middle-income countries. Second, we implemented an innovative design and sampled large numbers of PWID and MSM using RDS before and after the intervention period. Sites were separated by large distances or mountainous geography that minimized the risk of contamination. Finally, we used state-of-the-art methods to estimate changes in community viral load and HIV incidence, and we used biometric identification to allow accurate assessment of ICC exposure among survey participants.
Our trial also has limitations. First, several outcomes, including the primary outcome, were based on self-report. Misclassification of HIV testing status by self-report may have biased observed outcome association toward the null. To minimize self-report misclassification, we used identical question sequences at baseline and evaluation surveys and provided extensive interviewer training on strategies to help participants establish a timeline for past HIV testing. Second, the trial included two populations, PWID and MSM, which may have introduced heterogeneity. To mitigate this, we used identical survey questionnaires and laboratory methods in the two strata and population-stratified analyses were pre-specified.14 Point estimates for most outcomes were similar in PWID and MSM strata and there was no statistically significant effect modification for key outcomes. Third, substantial imbalances between study arms may occur in cluster randomised trials that have a relatively small number of clusters, which may reduce power or introduce bias. To mitigate this risk we stratified randomization by key population (PWID or MSM) and restricted randomization by key characteristics measured in the baseline surveys (prevalence of recent HIV testing and HIV prevalence), by establishing acceptable thresholds for imbalances between study arms and removing allocations that resulted in imbalances above the thresholds.14 Fourth, the coefficient of variation across sites was larger than projected, which reduced study power to detect a given effect size. Fifth, we surveyed PWID and MSM using RDS, a method developed for ‘hidden’ populations. Weighting procedures for RDS are proposed to produce unbiased estimates of the target population.23 However, some authors have raised questions about RDS assumptions and identified limitations.30 In our trial we sampled approximately 1,000 participants at each site and exceeded 20 recruitment waves at all sites, reducing the likelihood that samples were non-representative. Sensitivity analyses using unweighted data did not alter inferences. Sixth, our sequential cross-sectional assessments could not evaluate the effects of mortality, migration, or cessation of behaviors that could have deemed participants ineligible to participate in the evaluation assessment (e.g., discontinuation of drug injection).
In summary, we conducted a cluster-randomised trial to evaluate the population effectiveness of ICCs compared with usual care among key populations in India. We observed an overall 31% increase in HIV testing among the ICC communities compared with usual care communities, although this difference failed to achieve statistical significance at the α=0.05 level. ICCs were not associated with improved outcomes along the HIV care continuum, reductions in risk behaviors, or access to services at the population-level. However, at the individual-level, participants who visited the ICCs fared significantly better for several outcomes than those that did not visit the ICC. Although ICCs were well-accepted, and provided HIV testing and other services to large numbers of key population members, ICC exposure among evaluation survey participants at intervention sites was lower than expected. We observed a dose-response relationship between ICC exposure at the evaluation survey and increase in population-level HIV testing from baseline. These data support continued efforts to develop integrated and co-located service models for key populations and underscore the role of rigorous evaluation. Our experience also highlights the need to use key population size to guide the “dose” of a structural intervention (e.g., more centers or longer time exposure to centers) in order to effect improvements at the population-level.
Supplementary Material
Evidence before this study
We conducted a search on PubMed for articles published in English from inception through July 27, 2018, combining terms for “people who inject drugs” (PWID) or “men who have sex with men” (MSM) plus “HIV” plus “combination intervention” or “combination prevention”, which returned 291 articles. We also searched for large cluster-randomized trials of combination HIV interventions that did not specifically target PWID or MSM. Systematic reviews of HIV and risk-reduction services have identified barriers to optimal HIV prevention and treatment of PWID and MSM, particularly in low-and middle income countries.
Non-randomized studies conducted in Ukraine and Greece found evidence that integrated interventions for PWID improved service utilization and HIV outcomes. An observational study that used respondent-driven sampling to survey MSM in several Central American countries found that overall exposure to a multinational MSM-focused combination service provider was low, but that exposure was associated with higher likelihoods of condom use and recent HIV testing.
Two trials that focused on different populations than our trial are also notable. First, a cluster-randomized trial targeting female sex workers in Zimbabwe, found that a combination intervention was not significantly associated with a community-level change in the prevalence of viremic women compared with regular services, despite robust delivery of services in the intervention sites. Second, a cluster-randomized trial targeting young adults in African and Thai sites with generalized HIV epidemics found that a community-based intervention was associated with significantly increased HIV testing rates at the population level, but was not significantly associated with HIV incidence.
An individual-level randomized trial (conducted at sites in Ukraine, Vietnam, and Indonesia) compared standard care with a 2-component intervention for HIV-positive PWID (systems navigation and psychosocial counseling) that was designed to increase use of available evidence-based services. Use of antiretroviral therapy, viral suppression rates, and use of medication-assisted treatment were significantly higher and mortality was significantly lower in the intervention arm compared with standard care.
Added value of this study
In India, like many settings, HIV testing, prevention, and treatment services are supported by the government, but services are typically non-integrated, provided in separate venues, and do not explicitly target the needs of key populations. To our knowledge, ours is the first cluster-randomized trial to assess effectiveness of an integrated service model to improve HIV testing, prevention, and treatment outcomes in PWID and MSM in a low- to middle-income country. We used a novel design in which population-level outcomes were measured at the 22 trial sites using respondent-driven sampling before and after implementation of the intervention. We found evidence that integrated care centers (ICCs) were associated with population-level increases in recent HIV testing in these risk groups. However, the intervention was not associated with population-level changes in risk behaviors, HIV care continuum outcomes, or HIV incidence.
A valuable component of our study was systematic measurement of intervention exposure (using biometric matching) among key population members that participated in evaluation surveys at study conclusion. At PWID and MSM intervention sites, the median population exposure to ICCs were only 40% and 24%, respectively. In pre-specified analyses, we found that evaluation survey participants who had ever visited an ICC had significantly higher rates of recent HIV testing, were more likely to be aware of their status and to be taking ART (among HIV-positive persons) and had lower rates of injection-related and sexual risk behavior, compared with participants that had not visited an ICC. We also observed a dose-response relationship across intervention sites between higher ICC exposure at the evaluation survey and larger increases in population-level HIV testing from baseline.
Implications of all available evidence
The available evidence suggests that integrated, co-located models of care that target key populations are well-accepted and can provide services to large numbers of individuals. However, studies, such as ours, in which key outcomes were measured at the population level (i.e., included participants who were and were not exposed to the intervention), highlight the difficulty of effecting change in population-level outcomes. Our study clarifies the need to match intervention “dose” to the size and needs of the target population.
Acknowledgements
This research study was supported by the National Institutes of Health (NIH) (R01DA032059, R01MH089266, K24DA035684, T32AI102623, F31DA044046) and by the Johns Hopkins University Center for AIDS Research (P30AI094189). Additional support was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH, and by the Elton John AIDS Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Funding: National Institutes of Health and the Elton John AIDS Foundation.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing statement
Data from this study, including deidentified participant data, data dictionary, study protocol, statistical analysis plan, and informed consent documents will be made available to researchers. To access data, researchers should contact the corresponding author, Dr. Lucas (glucas@jhmi.edu). We require that researchers complete a concept sheet for their proposed analyses, to be reviewed and approved by the study investigators. The study investigators will consider overlap of the proposed project with active or planned analyses and the appropriateness of study data for the proposed analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sunil S. Solomon, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA; Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Suniti Solomon, Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Allison M. McFall, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Aylur K. Srikrishnan, Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Santhanam Anand, Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Vinita Verma, National AIDS Control Organisation, Ministry of Health and Family Welfare, New Delhi, India.
Canjeevaram K. Vasudevan, Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Pachamuthu Balakrishnan, Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Elizabeth L. Ogburn, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Lawrence H. Moulton, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Muniratnam S. Kumar, Centre for AIDS Research and Education (YRGCARE), Chennai, India.
Kuldeep Singh Sachdeva, Revised National Tuberculosis Control Programme, Ministry of Health and Family Welfare, New Delhi, India; National AIDS Control Organisation, Ministry of Health and Family Welfare, New Delhi, India.
Oliver Laeyendecker, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA; National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
David D Celentano, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Gregory M. Lucas, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Shruti H. Mehta, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
REFERENCES
- 1.Beyrer C, Sullivan PS, Sanchez J, et al. A call to action for comprehensive HIV services for men who have sex with men. Lancet 2012; 380(9839): 424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health 2017; 5(12): e1208–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. 90–90-90 An ambitious treatment target to help end the AIDS epidemic. 2014. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf (accessed 12/31 2014).
- 4.National AIDS Control Organisation, Department of AIDS Control, Ministry of Health & Family Welfare, Government of India. Annual Report 2016–17. Available at http://www.naco.gov.in/documents/annual-reports [last accessed June 2018]. 2017.
- 5.Arora P, Kumar R, Bhattacharya M, Nagelkerke NJ, Jha P. Trends in HIV incidence in India from 2000 to 2007. Lancet 2008; 372(9635): 289–90. [DOI] [PubMed] [Google Scholar]
- 6.Lucas GM, Solomon SS, Srikrishnan AK, et al. High HIV burden among people who inject drugs in 15 Indian cities. AIDS 2015; 29(5): 619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SS, Mehta SH, Srikrishnan AK, et al. High HIV prevalence and incidence among MSM across 12 cities in India. AIDS 2015; 29(6): 723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SH, Lucas GM, Solomon S, et al. HIV Care Continuum Among Men Who Have Sex With Men and Persons Who Inject Drugs in India: Barriers to Successful Engagement. Clin Infect Dis 2015; 61(11): 1732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer KH, Bekker LG, Stall R, Grulich AE, Colfax G, Lama JR. Comprehensive clinical care for men who have sex with men: an integrated approach. Lancet 2012; 380(9839): 378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latkin C, Srikrishnan AK, Yang C, et al. The relationship between drug use stigma and HIV injection risk behaviors among injection drug users in Chennai, India. Drug Alcohol Depend 2010; 110(3): 221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend 2014; 134: 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sypsa V, Psichogiou M, Paraskevis D, et al. Rapid Decline in HIV Incidence Among Persons Who Inject Drugs During a Fast-Track Combination Prevention Program After an HIV Outbreak in Athens. J Infect Dis 2017; 215(10): 1496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowan FM, Davey C, Fearon E, et al. Targeted combination prevention to support female sex workers in Zimbabwe accessing and adhering to antiretrovirals for treatment and prevention of HIV (SAPPH-IRe): a cluster-randomised trial. Lancet HIV 2018; 5(8): e417–e26. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SS, Lucas GM, Celentano DD, et al. Design of the Indian NCA study (Indian national collaboration on AIDS): a cluster randomized trial to evaluate the effectiveness of integrated care centers to improve HIV outcomes among men who have sex with men and persons who inject drugs in India. BMC Health Serv Res 2016; 16(1): 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckathorn DD. Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl 2002; 49(1): 11–34. [Google Scholar]
- 16.Laeyendecker O, Konikoff J, Morrison DE, et al. Identification and validation of a multi-assay algorithm for cross-sectional HIV incidence estimation in populations with subtype C infection. J Int AIDS Soc 2018; 21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of AIDS Control, National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India. Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents: May 2013. Available at naco.gov.in/documents/policy-guidelines [last accessed May 2018]. 2013.
- 18.Solomon SS, Mehta SH, McFall AM, et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. Lancet HIV 2016; 3(4): e183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steward WT, Herek GM, Ramakrishna J, et al. HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med 2008; 67(8): 1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders JB, Aasland OG, Babor TF, de lF Jr., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 1993; 88(6): 791–804. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes RJ, Moulton LH. Cluster Randomised Trials. Baco Raton, FL: Chapman & Hall / CRC; 2009. [Google Scholar]
- 23.Volz E, Heckathorn DD. Probability Based Estimation Theory for Respondent Driven Sampling. Journal of Official Statistics 2008; 24(1): 79–97. [Google Scholar]
- 24.Rabe-Hesketh S, Skrondal A, Pickles A. Maximum likelihood estimation of limited and discrete dependent variable models with nested random effects. Journal of Econometrics 2005; 128(2): 301–23. [Google Scholar]
- 25.Solomon SS, McFall AM, Lucas GM, et al. Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis. PLoS Med 2017; 14(11): e1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havlir D, Charlebois E, Balzer L, et al. SEARCH community cluster randomized study of HIV “test and treat” using multi-disease approach and streamlined care in rural Uganda and Kenya [Abstract WEAX0106LB]. 22nd International AIDS Conference, July 23–27, 2018, Amsterdam, Netherlands. [Google Scholar]
- 27.National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India. National Strategic Plan for HIV/AIDS and STI: 2017–2024. Available at http://naco.gov.in/national-strategic-plan-hivaids-and-sti-2017-24 [last accessed October 2018]. 2017.
- 28.Coates TJ, Kulich M, Celentano DD, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health 2014; 2(5): e267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes R, Floyd S, Schaap A, et al. A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med 2017; 14(5): e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gile KJ, Handcock MS. Respondent-Driven Sampling: An Assessment of Current Methodology. Sociol Methodol 2010; 40(1): 285–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.