Abstract
Earlier initiation of smoking correlates with higher risk of nicotine dependence, mental health problems, and cognitive impairments. Additionally, exposure to nicotine and/or tobacco smoke during critical developmental periods is associated with lasting epigenetic modifications and altered gene expression. This study examined whether adolescent nicotine exposure alters adult hippocampus-dependent learning, involving persistent changes in hippocampal DNA methylation and if choline, a dietary methyl donor, would reverse and mitigate these alterations. Mice were chronically treated with nicotine (12.6mg/kg/day) starting at post-natal day 23 (pre-adolescent), p38 (late adolescent), or p54 (adult) for 12 days followed by a 30-day period during which they consumed either standard chow or chow supplemented with choline (9g/kg). Mice then were tested for fear-conditioning and dorsal hippocampi were dissected for whole genome methylation and selected gene expression analyses. Nicotine exposure starting at p21 or p38, but not p54, disrupted adult hippocampus-dependent fear conditioning. Choline supplementation ameliorated these deficits. 462 genes in adult dorsal hippocampus from mice exposed to nicotine as adolescents showed altered promoter methylation that was reversed by choline supplementation. Gene network analysis revealed that chromatin remodeling genes were the most enriched category whose methylation was altered by nicotine and reversed by choline dietary supplementation. Two key chromatin remodeling genes, Smarca2 and Bahccl, exhibited inversely correlated changes in methylation and expression due to nicotine exposure; this was reversed by choline. Our findings support a role for epigenetic modification of hippocampal chromatin remodeling genes in long-term learning deficits induced by adolescent nicotine and their amelioration by dietary choline supplementation.
Keywords: addiction, learning, development, epigenetics, hippocampus, adolescence
1. Introduction
Changes in cognition contributes to nicotine addiction and conversely, nicotine may disrupt cognition (Gould, 2010; Patterson et al., 2010). Both human and animal studies have shown that chronic nicotine exposure during adolescence leads to long lasting cognitive and behavioral impairments, including effects on memory and attention and reduced prefrontal cortex activation (Trauth et al., 2000; Jacobsen et al., 2005). Additionally, younger onset of nicotine use has been directly linked to severity of smoking and greater severity of nicotine dependence in adulthood (Chassin et al., 2000; Center for Disease Control, 2012).
Nicotine differentially affects the adult versus the adolescent brain and subsequently has age-dependent effects on learning. Acute nicotine exposure enhanced hippocampus-dependent learning in adult mice while withdrawal from chronic nicotine exposure produced transient impairments (Gould and Higgins, 2003; Gould and Lommock, 2003; Davis et al., 2005; Portugal and Gould, 2009). In contrast, adolescent mice exposed to chronic nicotine exhibited long-term deficits in hippocampal-dependent contextual fear manifesting in adulthood; mice exposed to nicotine in adulthood did not exhibit significant long-term contextual fear learning impairments (Portugal et al., 2012; Holliday et al., 2016; Holliday and Gould, 2017). The developmentally sensitive effects of nicotine on behavior extend to altered morphology of pyramidal cells in the hippocampus with adolescent nicotine exposure leading to atrophy of apical dendrites of pyramidal cells in the CA1 region and adult nicotine exposure leading to atrophy of basilar CA3 dendrites (Holliday et al., 2016).
The molecular mechanisms by which nicotine elicits these long-lasting changes are still poorly understood. Recently, nicotine has been shown to promote epigenetic changes in a neonatal nicotine exposure model, inducing histone methylation changes previously linked predominantly to gene activation (Jung et al., 2016). Epigenome Wide Association Studies “EWAS” comparing methylation levels of peripheral blood from adult smokers and infants exposed to tobacco smoking in-utero identified a number of genes showing developmentally specific alterations in methylation patterns, with changes associated with neonatal exposure but not adult exposure (Zeilinger et al., 2013; Pirini et al., 2015; Rzehak et al., 2016). Additionally, longitudinal analysis of methylation changes following in utero smoke exposure identified methylation changes that persisted into adolescence (Lee et al., 2015). This suggests a role for epigenetic modifications in the cognitive and behavior impairments observed after developmental chronic nicotine exposure, but the effects of adolescent exposure are unknown.
Choline, an essential nutrient, can modulate cognitive function, especially during development (Meek et al., 2008; Thomas and Tran, 2012). In animals, choline supplementation partially restored cognitive performance when administrated prenatally and even postnatally in models of prenatal alcohol exposure (Ryan et al., 2008; Schneider and Thomas, 2016). Choline may modulate neurocognitive processes via a number of different mechanisms still largely unexplored. First, choline is a precursor of acetylcholine (Murai et al., 1994) and a α7 nicotinic receptor agonist (Alkondon et al., 1997; Mike et al., 2000). Second, choline is also a precursor for phospholipids abundant in the cell membrane and myelin sheath, potentially affecting membrane potential and neuronal functions. Lastly, choline is the primary methyl donor for DNA methylation and can thereby influence gene expression across the transcriptome (Niculescu et al., 2006). In support of choline mediating epigenetic processes, changes in global DNA 5mC levels in both hippocampus and prefrontal cortex were observed in rats prenatally exposed to ethanol and then postnatally treated with a choline-supplemented diet compared with littermates with no choline supplementation (Otero et al., 2012). Given that choline has positive restorative effects on cognition in prenatal and early postnatal alcohol exposed rats (Wong-Goodrich et al., 2008; Corriveau and Glenn, 2012; Velazquez et al., 2013) and that nicotine exposure during adolescence impairs adult cognition (Portugal et al., 2012; Holliday and Gould, 2017, 2017), we investigated the long-term effects of adolescent nicotine exposure on learning and changes in hippocampal epigenetic regulation of gene expression and whether choline could reverse epigenetic changes and restore hippocampus-dependent learning in adult mice exposed to nicotine during adolescence.
2. Material and methods
2.1. Subjects and experimental conditions
Male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) at either post-natal day (p) p16, p31, or p47. P16 mice were shipped with dams and weaned at p21 into groups of four mice with a maximum of two littermates per group. Each age had four experimental groups consisting of two drug conditions (nicotine or saline) and two diet conditions (standard and choline supplemented). Thus, each age group had the following conditions: 1) Saline-Standard (SAL-CHOW; n=12–16), 2) Saline-Choline (SAL-CHOL; n = 12–16), 3) Nicotine-Standard (NIC-CHOW; n=12–16), and 4) Nicotine-Choline (NIC-CHOL; n = 12–16). Whole genome methylation and subsequent analyses were based on a subset of mice (n=4/condition) from the late adolescent cohort.
2.2. Drug and Diet Methods
One week following arrival at the Temple University animal facility, mice were subcutaneously implanted with a mini osmotic pump to deliver saline or nicotine (Sigma, St. Louis, MO; freebase, 12.6mg/kg/day) at p23 (pre-adolescent), p38 (late adolescent), and p54 (adulthood); 12.6mg/kg/day produces plasma nicotine and cotinine levels in the range seen in human smokers (Davis et al., 2005; Benowitz et al., 2009; Cole et al., 2015). Nicotine or saline was delivered continuously for 12 days at which time the mini pump was removed via a second incision. All mice underwent a 30-day prolonged abstinence period during which they were given ad libitum access to either standard mouse chow (LabDiet Mouse Chow 5015) or a choline-supplemented diet (TestDiet; Richmond, IN). Standard mouse chow had 2000ppm (2g/kg) of choline and the TestDiet choline-supplemented diet had 9000ppm (9g/kg), which was 4.5x greater than the standard diet. This concentration of choline was chosen based on previously published observations indicating reversal of cognitive deficits associated with rat models of fetal alcohol syndrome and schizophrenia (Wong-Goodrich et al., 2008; Corriveau and Glenn, 2012; Velazquez et al., 2013). Following 30 days nicotine-free with continuous access to either standard diet or choline-supplemented diet, all mice were fear conditioned with access to respective diets in between sessions.
2.3. Fear Conditioning
Training and testing procedures for fear conditioning have been described in detail previously (Gould and Higgins, 2003; Gould and Lommock, 2003; Gould et al., 2004). Briefly, mice were trained and tested in four identical chambers (17.78 cm X 19.05 cm X 38.10 cm) housed in sound attenuating boxes (Med-Associates, St. Albans, VT) for contextual fear conditioning. Mice were exposed to an auditory conditioned stimulus (CS, 85db white noise) lasting for 30 sec that co-terminated with a 2 sec shock, serving as the unconditioned stimulus (US, 0.57mA, minimum level necessary for learning) for a total of two CS-US presentations. On the second day, mice were placed in the same chamber and freezing behavior was scored for the next 5 min to measure contextual conditioning. One hour later, mice were placed in different chambers with an additional vanilla extract olfactory cue and allowed to explore for 180s with freezing behavior assessed (preCS) before presented with the auditory stimulus (CS) for another 180s. Experimenters were blind to treatment conditions. Both contextual and cued fear conditioning were assessed to examine hippocampus-dependent (contextual fear conditioning) and hippocampus-independent (cued fear conditioning) learning (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Logue et al., 1997; Fanselow, 2000)
2.4. DNA/RNA isolation and sample preparation
Immediately following cued fear conditioning, mice were euthanized via cervical dislocation. Hippocampi were extracted and rapidly dissected into dorsal and ventral portions (in a 1:1 ratio) on ice, and 20mg tissue was taken for further nucleic acid isolation. DNA and RNA were co-isolated from each sample using the ZR-Duet™ DNA/RNA kit (Zymo Reaserch) per manufacturer’s protocol. Nucleic acid concentration and purity (260/230 and 260/280 ratios) were determined using NanoDrop™ 1000 (Thermo Scientific). Purified RNA samples were flash frozen in N2 and kept frozen at −80C until use. DNA samples were further cleaned and concentrated using Genomic DNA Clean & Concentrator™−25 kit (Zymo Reaserch, USA) per manufacturer’s protocol and frozen at −20C.
2.5. Methylated DNA immunoprecipitation sequencing (MeDIP-seq)
500ng of gDNA were randomly sheared to a median fragment size of 200 bp using a Covaris™ S220 (Thermo Scientific). Quality and concentration of the sheared DNA sample were further evaluated by running on the High Sensitivity DNA 2100 Bioanalyzer chip (Agilent). Library construction was performed as described in the Fragment Library Preparation 5500 Series SOLiD™ Systems protocol (Invitrogen) with one adapted modification (Taiwo et al., 2012). To insure maximum 5mC DNA concentration and to minimize loss of DNA during the library construction process, samples were subjected to MeDIP (methylated DNA Immunoprecipitation) following the adaptor ligation step, using the Low salt binding protocol of MethylCollector™ Ultra kit (Active Motif). Samples were further purified using MiniElute PCR purification kit (Qiagen) and library construction was resumed according to the Fragment Library Preparation 5500 Series SOLiD™ Systems protocol. Following 5500 solid DNA fragment library construction libraries were converted to 5500W libraries using 5500 W Conversion kit (Thermo Fisher Scientific), loaded on 5500 W v2 FlowChips and sequenced on the 5500 Wildfire according to manufacturer’s protocol. Fragment libraries were further sequenced with SOliD 5500×1 Wildfire (Thermo Fisher Scientific) for 50 bases. The color space sequence reads were mapped to mouse genome reference UCSC’s mm10 (http://genome.ucsc.edu/) using LifeScope v2.5.1 (Thermo Fisher) with the default setting. The mapping results were stored in bam files, which were used for subsequent analyses.
2.6. Identification of Deferentially Methylated Regions (DMRs)
The R package MEDIPS, standard protocol, was used for quality control, genomic coverage estimation and differential methylation analysis using a 300bp sliding window (Lienhard et al., 2014). The Differentially Methylated Regions (DMRs) identified were filtered based on genomic location (±5kb of TSS), brain expression based on the Barres lab data base (Zhang et al., 2014) and a nominal p<0.05. Further gene specific methylation changes were evaluated based on the mean normalized methylation read values (RMS) of the most statistically significant filtered DMR compared across the different experimental groups.
2.7. Pathway enrichment analysis
GO-Ontology Panther statistical overrepresentation, database release 2017–08-14, was used to identify enrichment of biological functional pathways in the differentially methylated genes identified. Results were further Bonferroni post-hoc corrected (Thomas et al., 2003; Mi et al., 2017).
2.8. Quantitative RT-PCR
RNA was primed with 150 ng of random hexamer (Invitrogen) and reverse transcribed into first strand cDNA with Superscript RT II (Thermo Fisher scientific) in the presence of the First Strand Buffer, 10 mM DTT, and 0.5 mM dNTPs at 45 C for 1 hr in a 20 μl reaction volume. For quantitative PCR, 2 μl of cDNA was mixed with gene-specific FAM tagged primers (0.25 μΜ final concentration), TaqMan Universal PCR Mastermix Mix (Thermo Fisher Scientific) in a 10 μl volume. Reactions were carried out on the ABI 7900 real-time PCR system. Relative gene expression level for each sample was calculated using the ∆CT value. Detailed description of Taqman primers used in the study (Table 1).
Table 1: List of gene expression assays used in the study.
All Taqman primers used in the study are commercially available via the Thermo Fisher Scientific Gene expression selection web tool: https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-assays/taqman-gene-expression.html
| Gene symbol | Assay ID |
|---|---|
| Smarca2 | Mm00508992_m1 |
| Bahcc1 | Mm01253344_m1 |
| Phf8 | Mm00623340_m1 |
| Rap1gap2 | Mm01241813_m1 |
| Rnf2 | Mm00803321_m1 |
| Tnrc18 | Mm00626403_m1 |
| Trim2 | Mm00453153_m1 |
| Tshz2 | Mm03809166_m1 |
| Zcchc16 | Mm02601188_s1 |
| Zhx2 | Mm01207642_m1 |
2.9. Statistical Analysis
Separate 2×2 ANOVAs were run with two drug conditions (saline versus nicotine) and two diet conditions (standard versus choline supplemented) for contextual learning and cued learning. Main effects are presented with no additional post hocs since only two levels exist for each factor. Significant interactions were followed up with simple main effects. Data were analyzed using SPSS 18.0. Results are separated by age at which mice received nicotine treatment. Significant differences were evaluated with an alpha level set at 0.05. To determine whether the effects of nicotine and choline on specific gene methylation and expression were significant, one-way ANOVA followed by multiple comparison testing with Tukey post-hoc correction was performed on all experimental samples using GraphPad Prism 5.
3. Results
3.1. Pre-Adolescent nicotine exposure disrupts adult hippocampus-dependent learning and choline reverses the learning deficit
In the pre-adolescence cohort, mice that received nicotine and were placed on the standard diet displayed deficits in contextual fear during adulthood. Dietary choline prevented these deficits in nicotine-treated mice (Figure 1A). There was a significant interaction between diet and drug (F(1,43) = 4.74, p < 0.05). Simple main effects revealed that there was a significant drug effect in mice that received the standard diet (F(1, 43) = 7.21, p < 0.01) such that mice treated with nicotine had significantly lower contextual freezing scores in adulthood than mice treated with saline. There was no significant difference between saline-treated and nicotine-treated mice in the choline condition. This indicates that mice receiving nicotine starting at pre-adolescence and continuing into adolescence had impairments in adult hippocampus-dependent learning that were prevented with supplemental dietary choline. There was no main effect of drug and no interaction for cued fear learning but there was a significant main effect of diet (F(1,43) = 5.83, p < 0.05); mice that received the choline supplemented diet had higher freezing than mice consuming standard chow. There were no group differences in baseline freezing or freezing during the preCS (data not shown), indicating these results were not due to changes in locomotor activity. There was no significant difference in body weight between mice fed the standard chow and the choline supplemented diet.
Figure 1: Choline supplementation prevented contextual fear deficits.
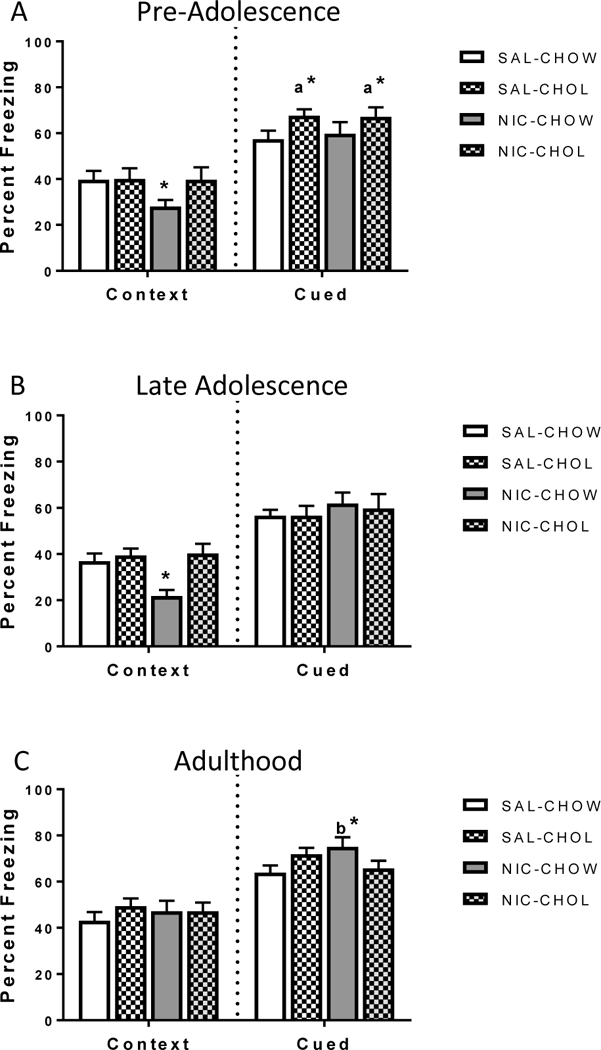
Choline (9g/kg) supplemented through the diet prevented the deficits in adult contextual fear caused by adolescent nicotine treatment. Graph titles refer to the age in which nicotine or saline treatments began. A) Choline supplementation following nicotine treatment that began in pre-adolescence prevented deficits in contextual fear learning and enhanced cued fear learning in adulthood. B) Choline supplementation following nicotine treatment in late adolescence prevented deficits in contextual fear learning in adulthood. C) There were no effects of adult nicotine exposure or choline supplementation on contextual learning later in adulthood but nicotine during adulthood increased cued fear learning. Bars represent mean percent freezing scores graphed with standard error of the mean (SEM). * p < 0.05 for simple main effects comparing diet effect within nicotine condition; a*= p < 0.05, main effect of diet condition, b* = p <0.05 for NIC-CHOW compared to SAL-CHOW conditions in adulthood. NIC = nicotine, SAL = saline, CHOW = standard diet, CHOL= choline diet
3.2. Late Adolescent nicotine exposure disrupts adult hippocampus-dependent learning and choline reverses the learning deficit
In the late adolescent cohort, adult mice that received nicotine during adolescence and were assigned to the standard diet group showed deficits in contextual fear. These deficits were prevented in adult mice that received nicotine during adolescence followed by dietary choline supplementation (Figure 1B). There was a significant diet and drug interaction, (F(1,59)= 5.89, p < 0.01). Follow-up simple main effects indicate there was no significant difference in diet condition in mice receiving saline but there was a significant difference in diet condition in mice receiving nicotine (F(1,59)=15.13, p < 0.05). Specifically, mice in the NIC-CHOW condition had significantly lower freezing scores than mice in the NIC-CHOL group. This indicates that chronic nicotine treatment during adolescence leads to deficits in contextual fear learning during adulthood, which were prevented with dietary choline. There were no differences in preCS freezing (data not shown), indicating freezing to a novel context was similar in all groups, and no differences in body weight throughout the course of diet treatment. Finally, there were no group differences in cued fear conditioning indicating hippocampus-independent learning in adulthood was not altered by late adolescent nicotine administration or choline diet supplementation.
3.3. Adult nicotine exposure does not produce long-term deficits in hippocampus-dependent learning
In mice treated with nicotine and choline during adulthood there were no significant differences between groups in contextual fear conditioning 30 days after cessation of nicotine treatment indicating hippocampal-dependent learning was not altered by either drug or diet treatments. There were no differences in baseline freezing or freezing during the preCS or in body weight (data not shown). For cued conditioning, there was a significant interaction between drug and diet condition (F(1,48) = 7.16, p < 0.01). Simple main effects indicate that within the standard chow condition, nicotine-treated mice froze more to the cue than saline-treated mice while there was no difference between drug treatment in the supplemented choline diet condition (Figure 1C).
3.4. Whole genome methylation analysis of adult dorsal hippocampus reveals 462 genes in which methylation is altered by adolescent nicotine exposure and reversed by choline
Whole genome methylation analysis was conducted on adult dorsal hippocampi from mice either exposed to nicotine or saline and choline-enriched or standard chow beginning in late adolescence. This time point was selected as it showed the most robust effects in hippocampus-dependent learning. In our methylation sequence analysis, we focused on changes in methylation that can be correlated with gene expression changes and could potentially serve as relevant targets for the restorative actions of choline in our nicotine exposure model. Three filtering criteria were used to identify relevant Differentially Methylated Regions (“DMRs”): (i) proximity to promoters. Unlike methylation changes in other genomic regions, changes in promotor associated regions have been well documented and shown to regulate proximal gene expression in a wide range of biological systems. In a majority of incidences, an inverse correlation between promotor 5mC changes and gene expression has been identified (Zemach et al., 2010; Jones, 2012). Thus, we decided to focus on methylation changes in promotor proximal regions to identify possible gene targets in which expression can be epigenetically modified, (ii) Genes that are known to be expressed in mouse brain, (iii) The significance level was set at p ≤ 0.05, due to the small sample size (n=4 per group), and statistical correction was performed on an individual gene DMR level and not on a genomic wide level. We did not use magnitude of methylation change as a selection criterion as its relationship to gene expression in a variety of biological systems is still unclear. Out of the identified genes, we further focused on possible targets that can explain the restorative action of choline in our model and selected only those gene targets whose methylation was altered by nicotine and reversed by choline. Four hundred and sixty-two genes were identified via this analytical pipeline (Figure 2 & Supplementary Figure 1).
Figure 2: Schematic representation of filtering and selection of biologically relevant Differentially Methylated Regions (DMRs).
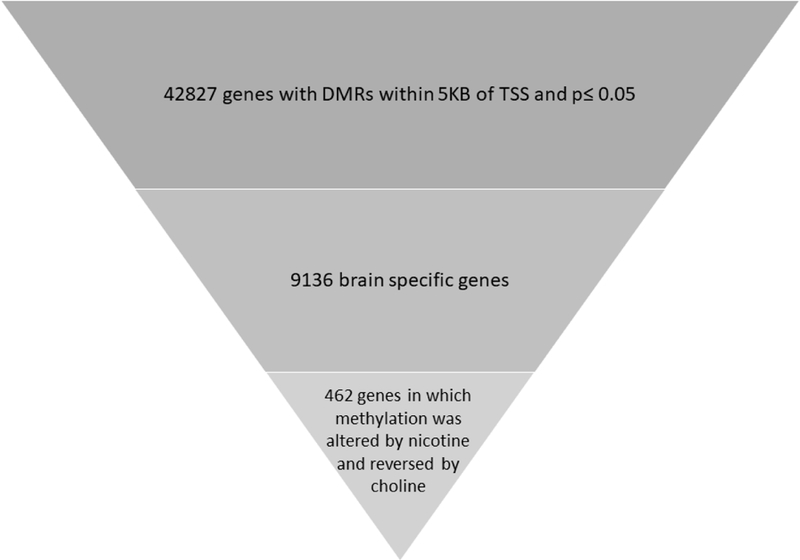
Selection of biologically relevant DMRs (Differentially Methylated Regions) from whole genome MeDIP analysis. Filtering was based on the following criteria: proximity to promotor sequences (+/− 5Kb of TSS), nominal p ≤0.05 and brain expression. Further analysis included identifying genes with methylation changes that can be potential targets of choline’s restorative action on cognition-genes in which methylation was altered by nicotine and reversed by choline.
3.5. Pathway enrichment analysis reveals chromatin remodeling genes as the most enriched category of genes whose methylation is altered by adolescent nicotine exposure and reversed by choline
To further functionally characterize the genes we identified and to gain additional insight into the molecular pathways involved in choline’s restorative action, we performed an unbiased pathway enrichment analysis using the Gene Ontology (GO) database. The most enriched categories of genes whose methylation was altered by adolescent nicotine exposure and restored by the choline-enriched diet were chromatin remodeling genes. Additionally, genes involved in nervous system development, cell migration and neuronal differentiation were also significantly enriched (Table 2 & Supplementary Table 1).
Table 2: Chromatin remodeling genes show the highest enrichment out of the differentially methylated genes identified.
PANTHER (GO-Ontology) enrichment tool was used to identify over represented functional gene categories in the 462 differentially methylated genes for which methylation was modulated by nicotine and reversed by choline. Short list of the most enriched Go Ontology gene categories, Full list can be found in Table 2–1 “Full PANTHER analysis”.
| Go term | Total genes in category | Genes in current study | Fold enrichment | P value * |
|---|---|---|---|---|
| chromatin remodeling (G0:0006338) | 122 | 14 | 5.67 | 2.70E–03 |
| positive regulation of neuron projection development (G0:0010976) | 312 | 23 | 3.64 | 1.53E–03 |
| positive regulation of nervous system development (G0:0051962) | 566 | 36 | 3.14 | 2.38E–05 |
| positive regulation of neurogenesis(G0:0050769) | 499 | 29 | 2.87 | 5.33E–03 |
| Cell migration (G0:0045666) | 744 | 43 | 2.85 | 1.12E–05 |
Bonferroni corrected
3.6. Examination of methylation and expression in a subset of chromatin remodeling genes
We next evaluated changes in expression of a subset often genes in the chromatin remodeling category, the most enriched family of genes identified in our enrichment analysis, to identify possible correlation between changes in methylation and expression that can pinpoint molecular targets involved in the restorative effects of choline observed. The subset of genes was selected to represent different chromatin remodeling factors, including canonical chromatin remodelers such as the SWI/SNF DNA helicase complex members and other factors with a broader function such as Zinc finger DNA binding proteins, (Table 3). Out of the 10 chromatin remodeling genes analyzed, 2 genes, Smarca2, a transcriptional coactivator member of the SWI/SNF complex and Bahcc1, a BAH domain containing transcriptional repressor showed significant changes in expression (Smarca2 F (3, 12) =18.8 p< 0.0001; Bahcc1 F (3,12) = 6.227 p=0.009) that were inversely correlated with methylation changes (Smarca2 F (3,12) = 15.5 p=0.0002; Bahccl F(3,12)=20.6 P<0.0001). In both genes, nicotine down-regulated expression and up-regulated methylation whereas choline up-regulated expression and down-regulated methylation in nicotine exposed mice (Figure 3).
Table 3:
Promotor methylation and expression correlation evaluation in the selected chromatin remodeling gene subset
| Gene name | Short summary of function and classification | RT PCR-methylation correlation |
|---|---|---|
| Bahcc1 | Chromatin binding protein | Expression change + positive correlative methylation change |
| Phf8 | Histone lysine demethylase | No expression change |
| Rap1gap2 | Known to facilitate recruitment of chromatin remodeling complexes e.g. NURD | No significant expression change |
| Rnf2 | Polycomb complex member Ring1 b/Rnf2, has ubiquitin E3 ligase activity towards histone H2A. | No expression change |
| Smarca2 | Part of the large ATP-dependent chromatin remodeling complex SNF/SWI, which is required for transcriptional activation of genes normally repressed by chromatin. | Expression change + positive correlative methylation change |
| Tnrc18 | Paralog of Bahcc1 | No expression change |
| Trim2 | Regulate transcription via chromatin interactions | No significant expression change |
| Tshz2 | Zinc finger DNA binding protein in mice, the known three TSHZ proteins are expressed in distinct patterns in the developing and adult brain, suggesting that they play a role in the establishment of regional identity and specification of cell types within the brain. | No expression change |
| Zcchc16 | Zinc finger DNA binding protein associated with WSTF-ISWI chromatin remodeling complex | Low expression, no detectable expression change |
| Zhx2 | zinc fingers and homeoboxes gene family nuclear homodimeric transcriptional repressors | No significant expression change |
Figure 3: Promotor methvlation inversely correlates with expression changes in the chromatin remodelers, Smarca2 and Bahcc1.
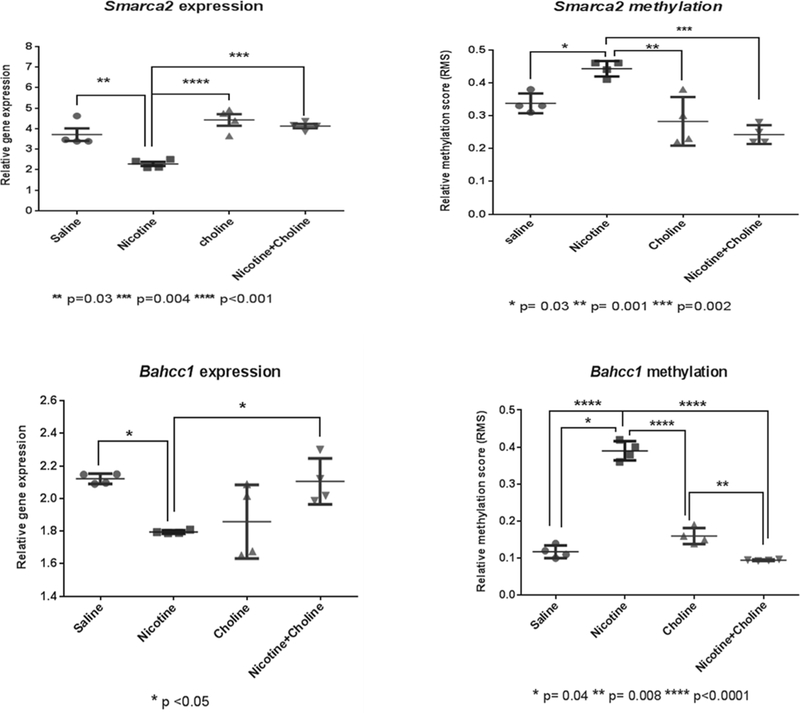
Expression and methylation changes were evaluated in a subset of chromatin remodeling genes. Two genes, Smarca2 and Bahcc1 showed significant expression changes which inversely correlated with significant methylation changes. Individual values with SEM are presented.
4. Discussion
This is the first study to investigate epigenetic changes associated with adult learning deficits induced by adolescent nicotine exposure and the effects of dietary choline supplementation on these changes. Pre-adolescent and adolescent nicotine exposure caused deficits in adult hippocampus-dependent contextual fear conditioning 30 days after cessation of nicotine treatment. This effect of nicotine was a developmental effect as adult animals that underwent the same experimental paradigm were not impaired, emphasizing the sensitivity and the importance of the maturation process of the central nervous system during adolescence and the deleterious effects that can arise from perturbation by agents such as nicotine. In addition, only contextual fear conditioning was disrupted, as cued fear conditioning was intact. The dependence of contextual fear conditioning but not cued fear conditioning upon the dorsal hippocampus (Maren, 1999; Fanselow, 2000; Lee and Kesner, 2004; Lopez-Fernandez et al., 2007) suggests the involvement of this brain region in the neurodevelopmental effects of nicotine.
Choline supplementation post nicotine exposure in the pre-adolescent and adolescent groups reversed the adult learning deficits restoring contextual fear conditioning. Interestingly, choline’s effects on contextual learning and memory were observed only in conjunction with pre-adolescent and adolescent nicotine exposure and no significant cognitive enhancement was seen with choline alone. It is unlikely this is a ceiling effect as contextual freezing was at approximately 40% and instead suggests that choline is more efficacious in a disrupted or suboptimal system. While choline alone did not alter contextual fear conditioning in controls, choline did enhance cued fear conditioning in adulthood but only for the pre-adolescent group. This suggest that choline might have developmental effects on processes specific to cued fear conditioning.
One potential mechanism of the developmental effects of nicotine exposure on contextual fear conditioning and choline reversal of these effects, explored further in this study, is epigenetic regulation of gene expression. Our whole genome methylation analysis revealed that both nicotine and choline caused changes in adult gene promoter DNA methylation, specifically known to regulate gene expression. We further focused our analyses on genes in which methylation was altered by nicotine and then reversed by choline, as these potential targets possibly explain the restorative effects of choline. The most functionally enriched group of genes identified was chromatin remodeling genes. This intriguing result reveals a possible complex multi-layer epigenetic network set into motion by adolescent nicotine exposure and reversed by choline. Changes in promoter methylation in chromatin remodeling genes can alter their expression and in turn lead to changes in chromatin genome wide (Bestor, 1998; Geiman and Robertson, 2002; Cedar and Bergman, 2009), thus impacting the expression of a multitude of genes and simultaneously altering numerous molecular networks (proposed model see Figure 4). Our gene expression analyses support this model and point to two particular genes involved in chromatin remodeling, Smarca2 and Bahccl. Both genes have recently been associated with different psychiatric disorders, as will be discussed.
Figure 4:
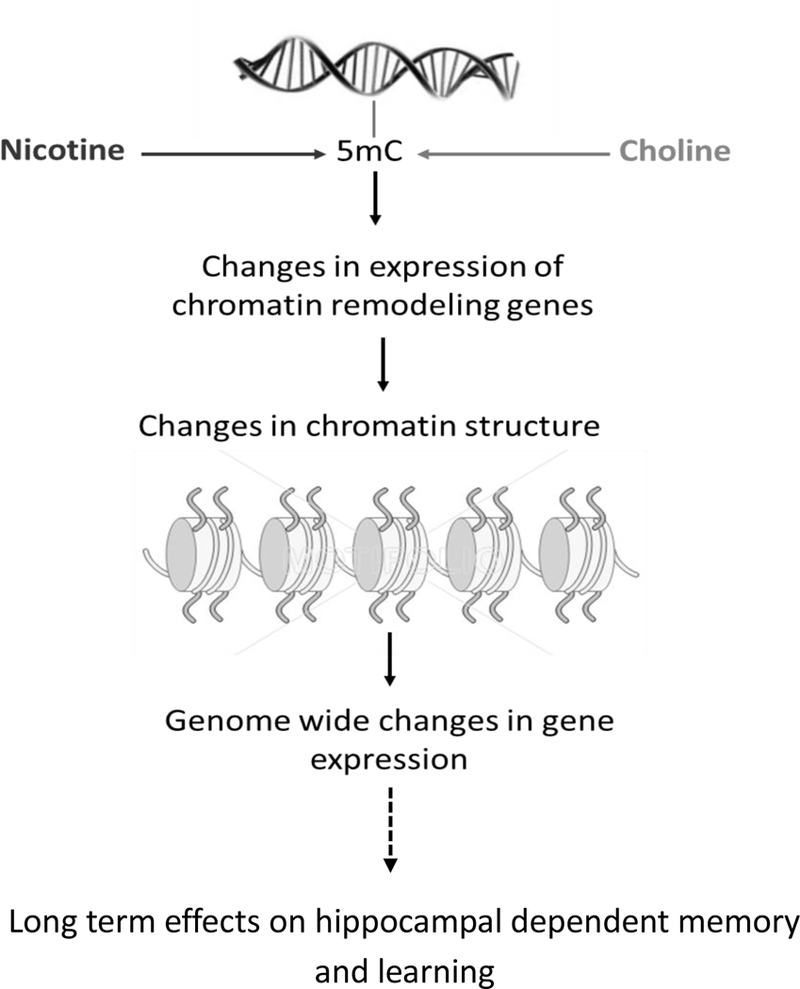
A proposed functional model for nicotine’s and choline’s Iona term stamp on hippocampal dependent memory and learning.
Smarca2/Brm is the catalytic, ATP dependent DNA Helicase, subunit of the SWI/SNF (BAF) chromatin remodeling complex. This complex can regulate neuronal transition from a stem/progenitor cell to a post-mitotic mature neuron by inducing genome wide structural chromatin changes, and has a critical role in long-term memory formation, including dendritic outgrowth (Ho et al., 2009). Genetic variation in SMARCA2 has been associated with higher risk of schizophrenia in a Japanese population and the Smarca2 knockout mouse exhibited schizophrenia-like behaviors, including impaired social interaction and prepulse inhibition (Koga et al., 2009). Furthermore, psychotogenic drugs decreased Smarca2 expression while anti-psychotic drugs increased its expression in vivo (Koga et al., 2009). Additionally, aberrant function of SMARCA2 may cause developmental disorders in humans including Nicolaides-Baraitser syndrome, Coffin-Siris syndrome, and DOORS syndrome (Kosho et al., 2014; Sousa et al., 2014).
Results from our study indicate that methylation of Smarca2 by adolescent nicotine treatment is reversed by choline supplementation concurrent with restoration of learning impairments. Recent findings from our group demonstrate that adolescent nicotine exposure results in dendritic atrophy of pyramidal cells in adulthood that correspond to hippocampus-dependent learning impairments (Holliday et al., 2016). This provides a potential link to Smarca2 as it is involved in dendritic outgrowth (Ho et al., 2009). Thus, choline supplementation may restore aberrant synaptic plasticity arising from adolescent nicotine exposure by regulating Smarca2 expression.
Bahcc1 (KIAA1447), a chromatin transcription silencer, is a member of the BAH (bromo-adjacent homology domain) family. The BAH family has been shown to be an important link between DNA methylation, cell replication and transcriptional regulation (Callebaut et al., 1999). Recently Bahcc1 downregulation in the ventral striatum was shown to be associated with altered response to dopaminergic drugs and increased anxiety in methyltransferase Kmt2alMll1 knockout mice (Shen et al., 2016).
Both Smarca2 and Bahccl showed a similar inverse correlation pattern between promoter methylation and gene expression, in which nicotine up-regulated promoter methylation and down-regulated expression and choline returned both to baseline levels by down-regulating promoter methylation and up-regulating gene expression. Although intuitively contradictory, as choline is a methyl donor and thus is assumed to increase methylation, a similar effect has been observed previously in a fetal alcohol exposure model in which global DNA methylation after choline supplementation was measured. In this model ethanol exposure induced hypermethylation and choline supplementation induced hypomethylation (Otero et al., 2012). Furthermore, prenatal choline deficiency has been found to have mixed effects on global DNA methylation, showing hypomethylation (Niculescu et al., 2006) in one study and hypermethylation in another (Kovacheva et al., 2007). Thus, these finding along with the current finding suggest that choline’s action is not as simple as previously hypothesized and may not be unidirectional, but instead may depend on the initial state of the specific genetic target.
Although our pathway enrichment analysis showed that chromatin remodeling genes were the most enriched pathway in which nicotine altered and choline reversed promoter associated methylation, additional enrichment was observed for genes involved in neurogenesis and neurodifferentiation pathways. Future prospective studies will determine the role of these and additional potentially functional pathways in the cognitive effects of both nicotine and choline and the interplay between them, and whether these are independent effects or a downstream byproduct induced by chromatin-mediated gene expression changes.
Furthermore, it will be important for future studies to examine the mechanism of action for choline, the impact of adolescent nicotine and choline on learning-related and synaptic plasticity-related processes, and the scope of other behaviors affected. Choline may counter the effects of adolescent nicotine exposure by direct changes in methylation but is it also possible that choline has indirect effects that lead to methylation changes. For example, as a a7 nicotinic receptor agonist (Alkondon et al., 1997; Mike et al., 2000), choline could stimulate processes mediated by a7 nicotinic receptors that lead to changes in methylation. However, as choline is a precursor of acetylcholine (Murai et al., 1994), it is also possible that changes could be mediated through increased signaling via other nicotinic receptors. For example, Maloku and colleagues (2011) found that α4β2 nicotinic receptors mediate epigenetic effects on cortical GABAergic neurons.
The present study found that adolescent nicotine produced changes in adult contextual fear conditioning and in dorsal hippocampus DNA methylation. The most functionally enriched group of genes identified was chromatin remodeling genes. These changes were reversed or prevented by dietary choline supplementation. This study did not attempt specifically to relate changes in methylation or gene expression to learning-related processes but instead focused on basal changes. Follow up studies need to examine if changes in chromatin remodeling genes lead to alternations in gene expression that regulate processes mediating different stages of learning and memory, such as acquisition, consolidation, and/or retrieval.
Finally, this study examined fear conditioning and found that adolescent nicotine exposure produces deficits in adult contextual fear conditioning that were reversed or prevented by choline treatment. Cued fear conditioning was not disrupted by adolescent nicotine exposure, suggesting that the effects might be specific for hippocampus-mediated processes; contextual fear conditioning is hippocampus-dependent while cued fear conditioning is hippocampus-independent (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Logue et al., 1997; Fanselow, 2000). Adolescent nicotine exposure does affect other processes including adult conditioned place preference for nicotine (de la Pena et al., 2015). Thus, it will be important to examine if similar processes mediate other long-term effects of adolescent nicotine exposure.
In summary, our study demonstrates that adolescence is a vulnerable period for the long-term impact of nicotine exposure on adult cognition and epigenetics, and that choline can prevent these changes. Importantly, adolescent nicotine exposure produced changes in adult dorsal hippocampus DNA methylation and chromatin remodeling-related genes appeared to be particularly vulnerable. To date, the ability of choline to ameliorate cognitive deficits has largely been analyzed in prenatal and postnatal animal models of alcohol exposure, where choline has shown large beneficial effects. This study is the first to examine an adolescent model, which is critical because of the link between adolescent substance use and addiction. Whether the beneficial effects of choline supplementation translate to humans is still largely unexplored but the current data suggest that choline could ameliorate deficits in learning and changes in DNA methylation associated with adolescent nicotine exposure. The few studies that have assessed the effects of developmental choline supplementation in humans have focused on Fetal Alcohol Syndrome, showing overall positive effects with a critical developmental interval for restorative intervention (Coles et al., 2015; Kable et al., 2015; Wozniak et al., 2015). Further work is needed to fully understand choline’s effects and help unravel molecular mechanisms, including the potential role of changes in expression of chromatin related genes.
Supplementary Material
Highlights:
Tobacco related deaths remain a global public health concern and adolescent nicotine abuse complicates continued abstinence efforts •
Adolescent nicotine exposure disrupts adult hippocampus learning and hippocampal gene methylation and expression
Choline supplementation ameliorates effects of adolescent nicotine exposure
This work is timely because of the increased adolescent use of nicotine via vaping
Acknowledgments
Funding: This work was funded with grant support from the National Institute of Drug Abuse (DA017949 TJG; 1U01DA041632 TJG), the Jean Phillips Shibley Endowment (TJG) and the Weinstein Summer Graduate Award presented by Roslyn and Stephen Weinstein of the Civic Foundation (EDH). Food and Drug Administration (FDA) and National Institute on Drug Abuse (NIDA) Identification of Biomarkers for Nicotine Addiction award (T-DA-1002 MG) and additionally supported by the Intramural Research Program of NIAAA NIH Project # AA00030.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflicts of interest.
Conflict of Interest: None
References:
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX (1997) Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9:2734–2742. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor TH (1998) Gene silencing. Methylation meets acetylation. Nature 393:311–312. [DOI] [PubMed] [Google Scholar]
- Callebaut I, Courvalin JC, Mornon JP (1999) The BAH (bromo-adjacent homology) domain: a link between DNA methylation, replication and transcriptional regulation. FEBS Lett 446:189–193. [DOI] [PubMed] [Google Scholar]
- Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control (2012) Smoking and Tobacco Use; 2012 Surgeon General’s Report. Smok Tob Use Available at: http://www.cdc.gov/tobacco/data_statistics/sgr/2012/ [February 23, 2016]. [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ (2000) The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol 19:223–231. [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V (2015) Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl) 232:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD, CIFASD (2015) Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern Child Health J 19: 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau JA, Glenn MJ (2012) Postnatal choline levels mediate cognitive deficits in a rat model of schizophrenia. Pharmacol Biochem Behav 103:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ (2005) Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci 25:8708–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña JB, Ahsan HM1, Tampus R, Botanas CJ, dela Peña IJ, Kim HJ, Sohn A, dela Peña I, Shin CY, Ryu JH, Cheong JH (2015) Cigarette smoke exposure during adolescence enhances sensitivity to the rewarding effects of nicotine in adulthood, even after a long period of abstinence. Neuropham 99:9–14. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2000) Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res 110:73–81. [DOI] [PubMed] [Google Scholar]
- Geiman TM, Robertson KD (2002) Chromatin remodeling, histone modifications, and DNA methylation-how does it all fit together? J Cell Biochem 87:117–125. [DOI] [PubMed] [Google Scholar]
- Gould TJ (2010) Addiction and cognition. Addict Sci Clin Pract 5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D (2004) Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res 155:167–173. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS (2003) Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem 80:147–157. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA (2003) Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci 117:1276–1282. [DOI] [PubMed] [Google Scholar]
- Holliday ED, Gould TJ (2017) Chronic Nicotine Treatment During Adolescence Attenuates the Effects of Acute Nicotine in Adult Contextual Fear Learning. Nicotine Tob Res 19:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday ED, Nucero P, Kutlu MG, Oliver C, Connelly KL, Gould TJ, Unterwald EM (2016) Long-term effects of chronic nicotine on emotional and cognitive behaviors and hippocampus cell morphology in mice: comparisons of adult and adolescent nicotine exposure. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, Lessard J, Nesvizhskii Al, Ranish J, Crabtree GR (2009) An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A 106:5181–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR (2005) Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry 57:56–66. [DOI] [PubMed] [Google Scholar]
- Jones PA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13:484–492. [DOI] [PubMed] [Google Scholar]
- Jung Y, Hsieh LS, Lee AM, Zhou Z, Coman D, Heath CJ, Hyder F, Mineur YS, Yuan Q, Goldman D, Bordey A, Picciotto MR (2016) An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior. Nat Neurosci 19:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, Kulikovsky Y, Wertelecki W, Pedersen TL, Chambers CD, CIFASD (2015) The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol Fayettev N 49:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256:675–677. [DOI] [PubMed] [Google Scholar]
- Koga M et al. (2009) Involvement of SMARCA2/BRM in the SWI/SNF chromatin-remodeling complex in schizophrenia. Hum Mol Genet 18:2483–2494. [DOI] [PubMed] [Google Scholar]
- Kosho T, Miyake N, Carey JC (2014) Coffin-Siris syndrome and related disorders involving components of the BAF (mSWI/SNF) complex: historical review and recent advances using next generation sequencing. Am J Med Genet C Semin Med Genet 166C:241–251. [DOI] [PubMed] [Google Scholar]
- Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK (2007) Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmtl expression. J Biol Chem 282:31777–31788. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP (2004) Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus 14:301–310. [DOI] [PubMed] [Google Scholar]
- Lee KWK, Richmond R, Hu P, French L, Shin J, Bourdon C, Reischl E, Waldenberger M, Zeilinger S, Gaunt T, McArdle W, Ring S, Woodward G, Bouchard L, Gaudet D, Smith GD, Relton C, Paus T, Pausova Z (2015) Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect 123:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhard M, Grimm C, Morkel M, Herwig R, Chavez L (2014) MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinforma Oxf Engl 30:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM (1997) Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci 111:104–113. [DOI] [PubMed] [Google Scholar]
- Lopez-Fernandez MA, Montaron M-F, Varea E, Rougon G, Venero C, Abrous DN, Sandi C (2007) Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J Neurosci 27:4552–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloku E, Kadriu B, Zhubi A, Dong E, Pibiri F, Satta R, Guidotti A (2011) Selective α4β2 nicotinic acetylcholine receptor agonists target epigenetic mechanisms in cortical GABAergic neurons. Neuropsychopharm 36:1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (1999) Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci 113:283–290. [DOI] [PubMed] [Google Scholar]
- Meek WH, Williams CL, Cermak JM, Blusztajn JK (2008) Developmental Periods of Choline Sensitivity Provide an Ontogenetic Mechanism for Regulating Memory Capacity and Age-Related Dementia. Front Integr Neurosci 1 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2526009/ [September 15, 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD (2017) PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45:D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX (2000) Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain Res 882:155–168. [DOI] [PubMed] [Google Scholar]
- Murai S, Saito H, Abe E, Masuda Y, Odashima J, Itoh T (1994) MKC-231, a choline uptake enhancer, ameliorates working memory deficits and decreased hippocampal acetylcholine induced by ethylcholine aziridinium ion in mice. J Neural Transm Gen Sect 98:1–13. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH (2006) Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB 20:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero NKH, Thomas JD, Saski CA, Xia X, Kelly SJ (2012) Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res 36:1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C (2010) Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend 106:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285. [DOI] [PubMed] [Google Scholar]
- Pirini F, Guida E, Lawson F, Mancinelli A, Guerrero-Preston R (2015) Nuclear and Mitochondrial DNA Alterations in Newborns with Prenatal Exposure to Cigarette Smoke. Int J Environ Res Public Health 12:1135–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ (2009) Nicotine withdrawal disrupts new contextual learning. Pharmacol Biochem Behav 92:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ (2012) Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem 97:482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD (2008) Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res 1237:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzehak P, Saffery R, Reischl E, Covic M, Wahl S, Grote V, Xhonneux A, Langhendries J-P, Ferre N, Closa-Monasterolo R, Verduci E, Riva E, Socha P, Gruszfeld D, Koletzko B, European Childhood Obesity Trial Study group (2016) Maternal Smoking during Pregnancy and DNA-Methylation in Children at Age 5.5 Years: Epigenome-Wide-Analysis in the European Childhood Obesity Project (CHOP)-Study. PloS One 11 :e0155554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD (2016) Adolescent Choline Supplementation Attenuates Working Memory Deficits in Rats Exposed to Alcohol During the Third Trimester Equivalent. Alcohol Clin Exp Res 40:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EY, Jiang Y, Javidfar B, Kassim B, Loh Y-HE, Ma Q, Mitchell AC, Pothula V, Stewart AF, Ernst P, Yao W-D, Martin G, Shen L, Jakovcevski M, Akbarian S (2016) Neuronal Deletion of Kmt2a/MII1 Histone Methyltransferase in Ventral Striatum is Associated with Defective Spike-Timing-Dependent Striatal Synaptic Plasticity, Altered Response to Dopaminergic Drugs, and Increased Anxiety. Neuropsychopharmacol 41:3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa SB, Hennekam RC, Nicolaides-Baraitser Syndrome International Consortium (2014) Phenotype and genotype in Nicolaides-Baraitser syndrome. Am J Med Genet C Semin Med Genet 166C:302–314. [DOI] [PubMed] [Google Scholar]
- Taiwo O, Wilson GA, Morris T, Seisenberger S, Reik W, Pearce D, Beck S, Butcher LM (2012) Methylome analysis using MeDIP-seq with low DNA concentrations. Nat Protoc 7:617–636. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD (2012) Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus 22:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A (2003) PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA (2000) An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res 867:29–39. [DOI] [PubMed] [Google Scholar]
- Velazquez R, Ash JA, Powers BE, Kelley CM, Strawderman M, Luscher ZI, Ginsberg SD, Mufson EJ, Strupp BJ (2013) Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis 58:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJE, Glenn MJ, Mellott TJ, Blusztajn JK, Meek WH, Williams CL (2008) Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res 1237:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, Zeisel SH, Georgieff MK (2015) Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 102:1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilinger S, Kiihnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A, Strauch K, Waldenberger M, lllig T (2013) Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PloS One 8:e63812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, McDaniel IE, Silva P, Zilberman D (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328:916–919. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


