Abstract
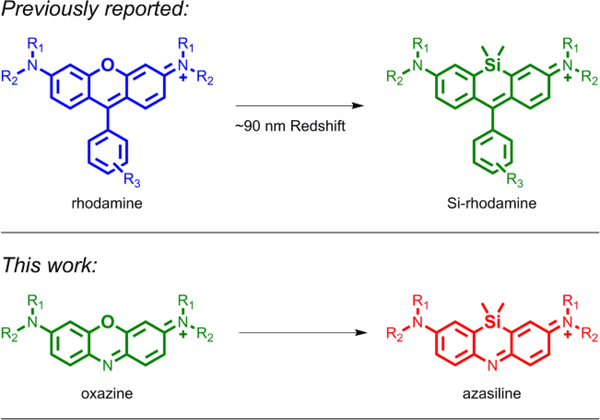
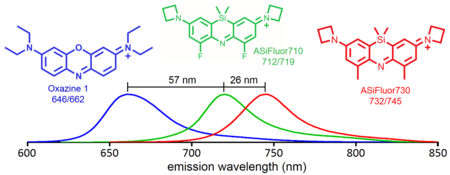
Exchanging the bridging oxygen atom in rhodamine dyes with a dimethylsilyl group red-shifts their excitation and emission spectra, transforming orange fluorescent rhodamines into far-red Si-rhodamines. To study the effect of this substitution in other dye scaffolds, synthetic approaches to incorporate silicon into the bridging position of oxazine dyes were developed. The fluorescence of the compact azasiline dyes ASiFluor710 and ASiFluor730 is red-shifted by 57–83 nm from that of Oxazine 1.
Graphical Abstract
Fluorescent probes are among the simplest and most convenient chemical tools used to investigate biological processes. In particular, near infrared (NIR) fluorophores with excitation and emission between 650 nm to 900 nm are becoming increasingly popular. Unlike visible-light fluorophores, NIR light can effectively penetrate live tissues, lessening phototoxicity and improving contrast.1 Although the benefits of employing NIR fluorophores are well recognized, NIR scaffolds have been dominated by just a few structural classes, particularly cyanines.2 Expanding the repertoire of scaffolds for constructing NIR fluorophores and chromophores can improve our ability to fine-tune spectral properties and develop novel chemical sensors.
Substitution of the bridging oxygen in xanthene fluorophores with silicon or other group 14 elements has been shown to dramatically red-shift their excitation and emission wavelengths, and thus has received much attention in the past decade (Figure 1).3,4 Among the group 14 elements, silicon produces the most red-shifted fluorophores.5 For example, the excitation and emission wavelengths of tetramethylrhodamine (548ex/572em) are red-shifted by ~90 nm in Si-tetramethylrhodamine (643ex/662em).6 Moreover, Si-rhodamines (Figure 1) retain the high photostability of rhodamines with quantum yields comparable to popular near-IR cyanine fluorophores.7,8 Hence, numerous Si-rhodamines have been reported with advantageous properties for live cell and in vivo imaging techniques.9–12
Figure 1.
Scope of this work.
Clearly, Si incorporation has expanded the applications and potential of rhodamine fluorophores, and the incorporation of other elements at this position is a burgeoning area of research.13–16 Less well studied is the effect of these modifications on other dye scaffolds. Oxazine dyes already have typical excitation and emission wavelengths around 640–660 nm.17 We hypothesized that silicon substitution in oxazines would yield analogously red-shifted “Si-oxazine” (azasiline) dyes (Figure 1).
Here, we report the synthesis and photophysical characteristics of two azasiline fluorophores with excitation and emission wavelengths over 700 nm.
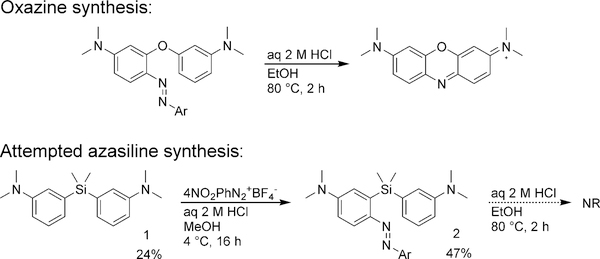
On the basis of the structural similarities between oxazines and the envisioned azasilines, we first designed a synthetic scheme modeled after known synthetic routes to oxazine dyes17,18 (Scheme 1). Silane 1 was obtained by treating 3-bromo-N,N-dimethylaniline with n-BuLi and dichlorodimethylsilane.19 Analogously to oxazine dye synthesis, a diazonitrobenzene moiety was added to yield 2. However, acid-catalyzed cyclization conditions that readily form oxazine dyes failed for the corresponding azasilines. The lack of reaction could reflect the lowered nucleophilicity of the aryl silane compared to the aryl ether and/or the longer C-Si bond length.
Scheme 1:
Modeling Azasiline Synthesis after Oxazines
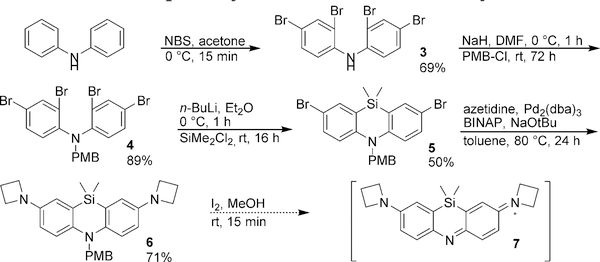
We next adopted a synthetic route predicated on the synthesis of azasilines as blue fluorescent emitters for OLEDs.20,21 In this revised scheme, diphenylamine was tetrabrominated with NBS to yield 3 (Scheme 2). The nitrogen was then protected with a PMB group using NaH to yield 4. Lithium-halogen exchange with n-BuLi followed by dichlorodimethylsilane treatment afforded the cyclized product 5.
Scheme 2:
Attempted synthesis of an azetidenyl azasiline
Azetidines, which have been shown to increase the quantum yields of rhodamines,6 were then installed using Buchwald-Hartwig coupling to yield the protected leuco-dye 6. However, attempted deprotection of 6 with TFA to yield azasiline 7 resulted in decomposition after solvent removal. In an alternative method for deprotection, iodine treatment of 6 in methanol yielded the crude putative azasiline fluorophore as a green compound, and HPLC purification encouragingly revealed a peak with an absorption maxima of 730 nm. However, upon solvent removal, decomposition again occurred to yield a possibly polymeric product that was insoluble in acetone, chloroform, methanol, water and DMSO.
Stymied by the apparent instability of 7, we next tested whether the stability of the azasiline dye could be modulated by altering the sterics and/or electronics of the azasiline with different substituents. Two different derivatives were designed: one that includes inductively-withdrawing but π-donating fluorine groups10 (13), and one that contains bulkier and electron-donating methyl groups (18).
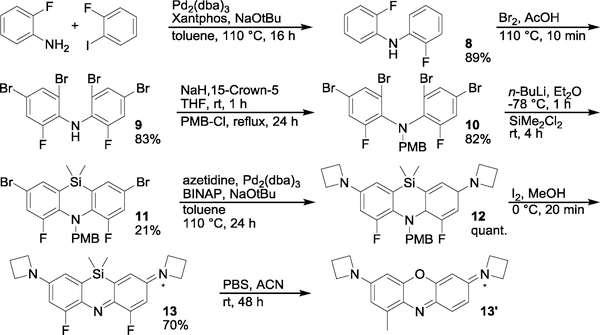
To access the difluoro azasiline 13, bis(2-fluorophenyl)amine 8 was synthesized through Buchwald-Hartwig coupling of 2-fluoroaniline with 1-bromo-2-fluorobenzene (Scheme 3). As with diphenylamine in Scheme 2, 8 was tetrabrominated, protected with PMB, and cyclized to yield the core brominated azasiline. Transformation to the azetidine leuco-dye 12 was then performed by Buchwald-Hartwig amination. Deprotection and oxidation was achieved in one step using I2 in methanol to give the final difluorinated azasiline 13 (ASiFluor710).
Scheme 3:
Synthesis of Difluoro-Azasiline (ASiFluor710)
To our delight, 13 readily oxidized and precipitated out of solution during I2 treatment. Simple filtering and washing of the precipitant with hexanes and methanol afforded pure product. In ethanol, the dye exhibited absorption and fluorescence at wavelengths > 700 nm (Figure S1), with an extinction coefficient of 60,000 M−1 cm−1 and quantum yield of 0.11 (Figure S2). Unfortunately, the dye slowly degraded in PBS (Figure S3). LC/MS analysis of ASiFluor710 incubated in PBS for 48 hours revealed a new peak with absorbance maxima of ~640 nm and a mass of 306, compared to 370 for the parent dye (Figure S3b). 1H and 19F NMR indicated the loss of both fluorines and the dimethylsilyl group. The data are consistent with the unexpected rearrangement to oxazine dye 13’, highlighting the need to further explore the unique chemistry of silicon, especially in the context of multi-functional heterocycles.22
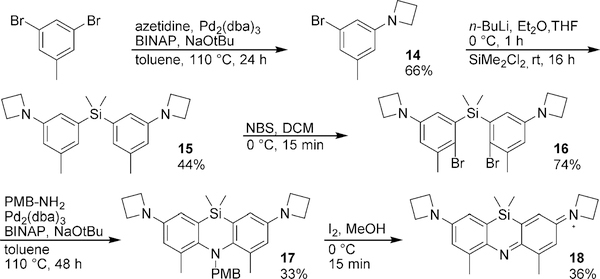
Although the incorporation of the π-donor fluorine allowed the synthesis of an azasiline dye, its reactivity in SNAr reactions likely leads to fluorine substitution and subsequent fluoride-mediated reaction with the silyl group. Methyl groups are more benign electron-donors that may help stabilize the oxidized azasiline dye without this liability. To access the dimethyl azasiline derivative, we first followed the same general synthetic route used to synthesize 13. However, that scheme failed at the PMB protection step, presumably due to the increased steric hindrance posed by the methyl groups. We therefore revisited our original synthetic scheme and postulated that cyclization could be achieved via Buchwald-Hartwig coupling (Scheme 4). First, Buchwald-Hartwig coupling of azetidine with 3,5-dibromotoluene yielded 14. The symmetric silane 15 was then formed by lithium-halogen exchange and treatment with dichlorodimethylsilane. Subsequent dibromination with NBS produced silane 16, which underwent Buchwald-Hartwig coupling with 4-methoxybenzylamine to effect ring closure, yielding the protected leuco dye 17. Iodine treatment of 17 yielded the final product 18 (ASiFluor730).
Scheme 4:
Synthesis of Dimethyl-Azasiline (ASiFluor730)
ASiFluor730 (18) fluoresces at > 730 nm in both organic and aqueous solvents (Table 1, Figure S1). Gratifyingly, HPLC and LC/MS analyses of 18 incubated in PBS for 24 hours showed no degradation (Figure S3). The quantum yield and molar extinction coefficient of 18 were calculated to be 0.11 and 121,000 M−1 cm−1 in ethanol (Table 1), which is comparable to that of Oxazine 1 (0.14 and 118,000 M−1 cm−1).23,24 In ethylene glycol and 1,4-dioxane, 18 has quantum yields of 0.13 and 0.11, respectively. However, the quantum yield of 18 dropped to approximately 0.01 in deionized water and PBS, which is 11-fold lower than in ethanol (Table 1, Figure S2). The addition of 0.05% Tween-20 increased the quantum yield to 0.06, suggesting that the reduced quantum yield in aqueous solvent is due in part to aggregation.25 (Table 1, Figure S2). Future incorporation of polar groups such as sulfonates should increase aqueous solubility and may, in turn, increase quantum yields in water.26,27
Table 1:
Photophysical Properties of Azasilines
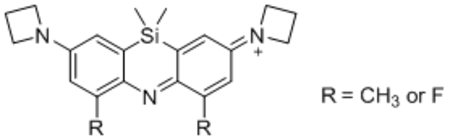 | |||||
|---|---|---|---|---|---|
| R | solvent | λ abs (nm) | λem (nm) | ε (M−1 cm−1) | Φ |
| CH3a | EtOH | 732 | 745 | 121,000 | 0.11 |
| PBS | 725 | 739 | 53,000 | 0.01 | |
| H2O | 725 | 739 | 70,000 | 0.009 | |
| 0.05% Tween-20 | 730 | 745 | 95,000 | 0.06 | |
| Ethylene Glycol | 736 | 747 | 109,000 | 0.13 | |
| 1,4-Dioxane | 701 | 734 | 46,000 | 0.11 | |
| Fb | EtOH | 709 | 719 | 60,000 | 0.11 |
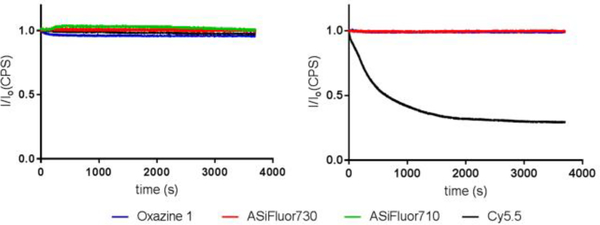
Lastly, we compared the photostability of the ASiFluors to Oxazine 1 and Cy5.5. Each fluorophore was subjected to continuous irradiation at its peak absorption wavelength for one hour. All fluorophores were photostable in ethanol. However, in PBS, Cy5.5 lost about 60% of its initial fluorescence, whereas Oxazine 1 and ASiFluor730 were unaffected (Figure 2).
Figure 2: Photostability of Azasilines.
Each fluorophore was irradiated continuously for one hour at their max absorbance wavelength (Oxazine 1 = 646 nm; Cy5.5 = 684 nm; ASiFluor710 = 710 nm; ASiFluor730 = 730 nm) in ethanol (left panel) and PBS (right panel). ASiFluor710 was not tested in PBS due to its instability in aqueous solution.
In conclusion, we have developed synthetic approaches to a new class of near IR-fluorophores based on azasilines, the Si-analogues of oxazine dyes. Although not as bright as cyanines, ASiFluor730 is one of the most compact near-IR fluorophores that absorbs beyond 700 nm (Table S1). The NIR excitation and emission wavelength, photostability, and compact size show promise for the future development of azasiline-based optical probes and suggests that silicon can be incorporated more broadly into chromophore scaffolds to further expand the diversity of NIR fluorophores, chromophores, and luminescent molecules.28–30
Supplementary Material
Acknowledgements
We thank members of the Kelch and Ryder labs at UMass Medical School for the use of equipment. This work was supported in part by a grant from the US National Institutes of Health (DA039961).
Footnotes
Supporting Information Available. Experimental procedures, supplemental tables and figures, and full spectroscopic data for all new compounds.
References
- (1).Hong G; Antaris AL; Dai H Near-Infrared Fluorophores for Biomedical Imaging. Nature Biomedical Engineering 2017, 1, 0010. [Google Scholar]
- (2).Pansare VJ; Hejazi S; Faenza WJ; Prud’homme RK Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores, and Multifunctional Nano Carriers. Chem. Mater 2012, 24, 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fu M; Xiao Y; Qian X; Zhao D; Xu Y A Design Concept of Long-Wavelength Fluorescent Analogs of Rhodamine Dyes : Replacement of Oxygen with Silicon Atom. Chemical Communications 2008, 0, 1780–1782. [DOI] [PubMed] [Google Scholar]
- (4).Umezawa K; Citterio D; Suzuki K New Trends in Near-Infrared Fluorophores for Bioimaging. Anal Sci 2014, 30, 327–349. [DOI] [PubMed] [Google Scholar]
- (5).Koide Y; Urano Y; Hanaoka K; Terai T; Nagano T Evolution of Group 14 Rhodamines as Platforms for Near-Infrared Fluorescence Probes Utilizing Photoinduced Electron Transfer. ACS Chem. Biol 2011, 6, 600–608. [DOI] [PubMed] [Google Scholar]
- (6).Grimm JB; English BP; Chen J; Slaughter JP; Zhang Z; Revyakin A; Patel R; Macklin JJ; Normanno D; Singer RH; et al. A General Method to Improve Fluorophores for Live-Cell and Single-Molecule Microscopy. Nature Methods 2015, 12, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ikeno Takayuki; Nagano Tetsuo; Hanaoka Kenjiro. Silicon‐substituted Xanthene Dyes and Their Unique Photophysical Properties for Fluorescent Probes. Chemistry – An Asian Journal 2017, 12, 1435–1446. [DOI] [PubMed] [Google Scholar]
- (8).Koide Y; Urano Y; Hanaoka K; Piao W; Kusakabe M; Saito N; Terai T; Okabe T; Nagano T Development of NIR Fluorescent Dyes Based on Si–Rhodamine for in Vivo Imaging. J. Am. Chem. Soc 2012, 134, 5029–5031. [DOI] [PubMed] [Google Scholar]
- (9).Wang T; Zhao Q-J; Hu H-G; Yu S-C; Liu X; Liu L; Wu Q-Y Spirolactonized Si-Rhodamine: A Novel NIR Fluorophore Utilized as a Platform to Construct Si-Rhodamine-Based Probes. Chem. Commun 2012, 48, 8781–8783. [DOI] [PubMed] [Google Scholar]
- (10).Grimm JB; Brown TA; Tkachuk AN; Lavis LD General Synthetic Method for Si-Fluoresceins and Si-Rhodamines. ACS Cent. Sci 2017, 3, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lukinavičius G; Umezawa K; Olivier N; Honigmann A; Yang G; Plass T; Mueller V; Reymond L; Jr IRC; Luo Z-G; et al. A Near-Infrared Fluorophore for Live-Cell Super-Resolution Microscopy of Cellular Proteins. Nature Chemistry 2013, 5, 132–139. [DOI] [PubMed] [Google Scholar]
- (12).Kolmakov K; Hebisch E; Wolfram T; Nordwig LA; Wurm CA; Ta H; Westphal V; Belov VN; Hell SW Far-Red Emitting Fluorescent Dyes for Optical Nanoscopy: Fluorinated Silicon-Rhodamines (SiRF Dyes) and Phosphorylated Oxazines. Chemistry 2015, 21, 13344–13356. [DOI] [PubMed] [Google Scholar]
- (13).Liu J; Sun Y-Q; Zhang H; Shi H; Shi Y; Guo W Sulfone-Rhodamines: A New Class of Near-Infrared Fluorescent Dyes for Bioimaging. ACS Appl Mater Interfaces 2016, 8, 22953–22962. [DOI] [PubMed] [Google Scholar]
- (14).Zhou X; Lai R; Beck JR; Li H; Stains CI Nebraska Red: A Phosphinate-Based near-Infrared Fluorophore Scaffold for Chemical Biology Applications. Chem. Commun. (Camb.) 2016, 52, 12290–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhou X; Lesiak L; Lai R; Beck JR; Zhao J; Elowsky CG; Li H; Stains CI Chemoselective Alteration of Fluorophore Scaffolds as a Strategy for the Development of Ratiometric Chemodosimeters. Angew. Chem. Int. Ed. Engl 2017, 56, 4197–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chai X; Cui X; Wang B; Yang F; Cai Y; Wu Q; Wang T Near-Infrared Phosphorus-Substituted Rhodamine with Emission Wavelength above 700 Nm for Bioimaging. Chemistry 2015, 21, 16754–16758. [DOI] [PubMed] [Google Scholar]
- (17).Pauff SM; Miller SC Synthesis of Near-IR Fluorescent Oxazine Dyes with Esterase-Labile Sulfonate Esters. Org. Lett 2011, 13, 6196–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Anzalone AV; Wang TY; Chen Z; Cornish VW A Common Diaryl Ether Intermediate for the Gram-Scale Synthesis of Oxazine and Xanthene Fluorophores. Angew. Chem. Int. Ed 2013, 52, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wang B; Chai X; Zhu W; Wang T; Wu Q A General Approach to Spirolactonized Si-Rhodamines. Chem. Commun. (Camb.) 2014, 50, 14374–14377. [DOI] [PubMed] [Google Scholar]
- (20).Suzuki R; Tada R; Hosoda T; Miura Y; Yoshioka N Synthesis of Ester-Substituted Dihydroacridine Derivatives and Their Spectroscopic Properties. New J. Chem 2016, 40, 2920–2926. [Google Scholar]
- (21).Sun JW; Baek JY; Kim K-H; Moon C-K; Lee J-H; Kwon S-K; Kim Y-H; Kim J-J Thermally Activated Delayed Fluorescence from Azasiline Based Intramolecular Charge-Transfer Emitter (DTPDDA) and a Highly Efficient Blue Light Emitting Diode. Chem. Mater 2015, 27, 6675–6681. [Google Scholar]
- (22).Barraza SJ; Denmark SE Synthesis, Reactivity, Functionalization, and ADMET Properties of Silicon-Containing Nitrogen Heterocycles. J. Am. Chem. Soc 2018, 140, 6668–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Rurack K; Spieles M Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600−1000 Nm. Anal. Chem 2011, 83, 1232–1242. [DOI] [PubMed] [Google Scholar]
- (24).Würth C; Grabolle M; Pauli J; Spieles M; Resch-Genger U Relative and Absolute Determination of Fluorescence Quantum Yields of Transparent Samples. Nature Protocols 2013, 8, 1535–1550. [DOI] [PubMed] [Google Scholar]
- (25).Buschmann V; Weston KD; Sauer M Spectroscopic Study and Evaluation of Red-Absorbing Fluorescent Dyes. Bioconjugate Chemistry 2003, 14, 195–204. [DOI] [PubMed] [Google Scholar]
- (26).Mujumdar RB; Ernst LA; Mujumdar SR; Lewis CJ; Waggoner AS Cyanine Dye Labeling Reagents - Sulfoindocyanine Succinimidyl Esters. Bioconjugate Chem. 1993, 4, 105–111. [DOI] [PubMed] [Google Scholar]
- (27).Panchuk-Voloshina N; Haugland RP; Bishop-Stewart J; Bhalgat MK; Millard PJ; Mao F; Leung W-Y; Haugland RP Alexa Dyes, a Series of New Fluorescent Dyes That Yield Exceptionally Bright, Photostable Conjugates. J Histochem Cytochem. 1999, 47, 1179–1188. [DOI] [PubMed] [Google Scholar]
- (28).Adams ST; Miller SC Beyond D-Luciferin: Expanding the Scope of Bioluminescence Imaging in Vivo. Curr Opin Chem Biol 2014, 21, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sharma DK; Adams ST; Liebmann KL; Miller SC Rapid Access to a Broad Range of 6′-Substituted Firefly Luciferin Analogues Reveals Surprising Emitters and Inhibitors. Org. Lett 2017, 19, 5836–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Miller SC; Mofford DM; Adams ST Lessons Learned from Luminous Luciferins and Latent Luciferases. ACS Chem. Biol 2018, doi: 10.1021/acschembio.7b00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.