Abstract
Whether stress affects memory depends on which stress pathway becomes activated and which specific memory system is involved. The activation of the sympathetic nervous system (SNS), leads to a release of catecholamines. The activation of the hypothalamic-pituitary-adrenal (HPA) axis, leads to a release of glucocorticoids. In thus study, it was investigated whether SNS and/or HPA axis activation are associated with long-term memory (LTM) and/or working memory (WM) performance in humans. Thirty-three participants underwent the socially evaluated cold-pressor test. Salivary alpha-amylase (sAA) was used as a marker for the activation of the SNS and cortisol as marker for HPA axis activation. Memory was assessed by means of word lists with 15 words each. The primacy effect (i.e., the correctly recalled words from the beginning of the lists) of the serial position curve was considered as indicator for LTM. The recency effect (i.e., the correctly recalled words from the end of the lists) were used as estimator for WM performance. In sAA responders, the recency effect and, therefore, WM performance increased immediately after the stressor. This was not found in sAA non-responders. In cortisol responders, the primacy effect and, thus, LTM performance decreased 20 minutes after the stressor. No change in LTM performance was found in cortisol non-responders. Our study supports the assumptions that 1) SNS activation is associated with WM processes via stimulation of the prefrontal cortex, and 2) HPA axis activation is associated with LTM processes through interactions with the hippocampus.
Introduction
Cognitive functions–especially memory–are not entirely independent of peripheral physiological processes. Some peripherally transmitted molecules (e.g., some hormones) can pass the blood-brain barrier (BBB) and can, therefore, affect neural activity directly. Other substances indeed cannot pass the BBB but can still affect neural activity through indirect feedback loops by activating brain networks, which lead to the release of neuro modulators in brain regions involved in cognitive processing (e.g. [1–3]).
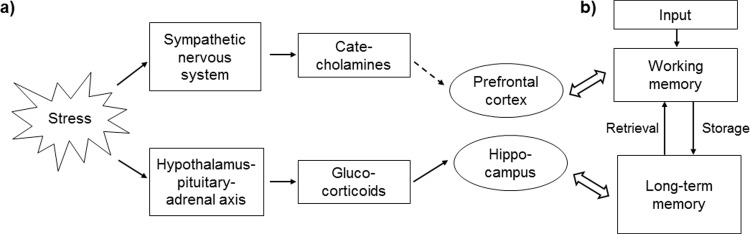
One prominent candidate which can trigger such effects is stress. Stress can be defined as “an actual or anticipated disruption of homeostasis or an anticipated threat to well-being” ([4], p. 397). The acute stress response is dominated by two pathways (e.g., [5,6]; Fig 1A). The first, which starts immediately after the onset of the stressor is the activation of the sympathetic nervous system (SNS). This leads to the release of the catecholamines adrenaline and noradrenaline which both cannot pass the BBB but can affect cognitive processing through indirect pathways [1]. The peripherally transmitted catecholamines can activate the locus coeruleus in the brainstem which stimulates the release of noradrenaline and dopamine in the prefrontal cortex (PFC) via the ventral tegmental area [3,7,8]. The PFC is involved in a variety of higher order cognitive functions, e.g., in working memory (WM) processes which are mainly controlled by noradrenaline and dopamine [9].
Fig 1.
a) Stress pathways that are activated after an acute stress situation, affected brain regions through indirect (dashed arrow) or direct pathways, and b) a simplified memory model.
The second stress response, which peaks with a short delay of a few minutes after the onset of the stressor, is the activation of the hypothalamic-pituitary adrenal (HPA) axis. This leads to the release of glucocorticoids (i.e., cortisol in humans or corticosterone in rodents) from the adrenal cortex. After threatening socially-evaluative stressors (e.g., the Trier Social Stress Test; [10]), HPA axis response peaks approximately 20 minutes after the end of the stressor (e.g., [11]). The stress hormone cortisol can pass the BBB and can, therefore, directly affect neural processing [12]. Cortisol binds to two different receptors in the brain [13,14]. The first, the mineralocorticoid receptor (MR, or type 1 receptor) can be found within the hippocampus and the prefrontal cortex [15]. The second, the glucocorticoid receptor (GR, or type 2 receptor) is widely distributed in different brain areas. Which cognitive processes (i.e., which memory functions) are affected after cortisol release depends on which receptors and, therefore, in which brain area, cortisol binds to [16]. Both receptor types have different affinity for cortisol [13]. The MRs have high affinity and are, therefore, usually occupied at basal cortisol concentrations. The GRs have a lower affinity for cortisol and are, thus, in many cases not occupied unless cortisol levels are increased. The brain structure in which both MRs and GRs are localized is the hippocampus which is also mainly involved in long-term memory (LTM) processes. Therefore, there has been a long research history in the evaluation of the effects of cortisol binding on GRs in the hippocampus and its associations with LTM processes (e.g., [17–19]).
A classical model which involves both WM and LTM, was proposed by Atkinson and Shiffrin [20]. According to this model, any new information first enters–after it has passed the so-called sensory register–WM. After this, the information is either forgotten or it is stored in LTM from where it can be retrieved at later time points (Fig 1B). One easy way to assess both WM and LTM within one experiment is to investigate the so-called serial position effects [21,22]. A typical experimental procedure is to present word lists of approximately 15 words to the participants and to let them recall as many words as they can remember immediately after the last word was presented. The classical observation is that words from the beginning as well as from the end of the word list can be better recalled than words from the middle of the list. The first effect is called the primacy effect (PE) which is associated with LTM processes. The second is called recency effect (RE) and it is associated with WM [21]. The effects of acute stress on the serial position effects have not been investigated so far.
The aims of the present study were to investigate whether SNS activation is associated with WM and whether HPA axis activation is associated with LTM performance in humans after an acute stressor. As measures of the functioning of the memory systems, the PE and the RE of the serial position curve were examined. The hypotheses were that 1) SNS activation would start immediately after the stressor and would be related with the RE and, thus, with WM and 2) that HPA axis activation would peak with a time delay of approximately 20 minutes and would be associated with the PE and, therefore, with LTM performance. It was assumed that these effects will only be found in participants who show indeed a SNS or HPA axis response, respectively (the so-called responders). Furthermore, it was hypothesized that such effects will not be found in the non-responder groups.
Materials and methods
Participants
Thirty-three healthy, German-speaking adults participated (mean age: 24.0 ± 5.7 years; eight male; BMI = 22.2 ± 2.8 kg/m2). None of them reported endocrinological, neurological, or psychological diseases. This was checked during a pre-screening. Persons with a psychiatric diagnosis (currently or in the past) were screened out. All participants gave their written and informed consent. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the local ethics committee of the Friedrich-Alexander University Erlangen-Nuremberg (protocol # 6_18 B).
Experimental procedure
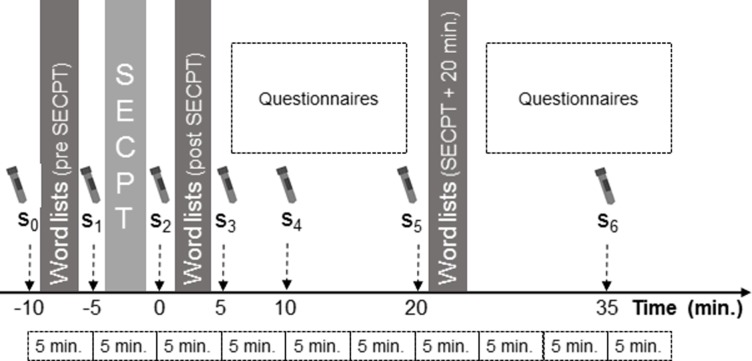
The time course of the experiment is shown in Fig 2. The whole session–including instructions–lasted 60 minutes. For memory assessment, participants were presented three word lists with 15 words each with inter-stimulus intervals of one second. The words were simple neutral words with a short pronunciation time (e.g., the German words for ‘dog’, ‘coffee’, ‘bus’, or ‘door’). After the presentation of each list, the participants were asked to immediately recall as many words as they had remembered. As measure for LTM performance, the PE was used which was defined as the sum of correctly recalled words from the first three words of the lists. Accordingly, the RE, which was used as a measure for WM performance, was defined as the sum of correctly recalled words from the last three words of the lists. Memory testing was repeated three times throughout the experimental session with three lists each time. The order of the word lists was counterbalanced between the participants and between the memory assessment time points.
Fig 2. Time course of the experimental procedure.
Stress was induced by means of the socially evaluated cold-pressor test (SECPT).
Stress was induced by means of the socially evaluated cold-pressor test (SECPT, [23]) in groups of two participants. The participants stood in front of a table on which transparent boxes filled with ice water were placed. Participants were instructed to immerse their hands in the ice water as long as possible for up to three minutes. Mean immersion time was 2:20 ± 1:06 min (min. 0:18, max: 3:00). The hand of each participant was directly opposite of the hand of the other person with the aim to introduce a competitive situation. Remaining time was displayed on a large-display digital clock that was visible for both participants. An auditory countdown announced the last five seconds. Therefore, our protocol slightly differed from that reported by Schwabe and colleagues (2008, [23]) and by Minkley and colleagues (2014) who introduced the first group version of the SECPT [24]. One experimenter who wore a medical uniform was present during the SECPT and was instructed to behave distanced and to keep a neutral mimic.
Salivary alpha-amylase (sAA) and salivary cortisol were used as measures of SNS and HPA axis activity [25–28]. Saliva was collected by means of salivettes (Sarstedt, Nümbrecht, Germany) at seven time points during the experimental session. The first saliva sample (s0) was collected immediately prior to the presentation of the first word list. The following samples were collected immediately prior (s1) and immediately after (s2) the SECPT. The following four samples were collected five (s3), ten (s4), 20 (s5), and 35 (s6) minutes after the end of the SECPT. The participants were instructed not to eat, drink (except water), smoke, or brush their teeth two hours before the start of the experimental session. Additionally, subjective stress perception was rated on a ten-point Likert-scale with the anchors “not stressed at all” and “totally stressed” during saliva collection.
Furthermore, some demographic and psychological variables were collected by means of questionnaires during waiting time between the saliva samples (when no memory tests were performed). Demographic variables that were assessed were sex, age, weight, and height. The amount of regular physical activity was measured by means of the short form of the International Physical Activity Questionnaire (IPAQ; [29,30]). Chronic stress was assessed by means of the screening scale of the Trier Inventory of Chronic Stress (TICS-SSCS; [31]) and the Perceived Stress Scale (PSS; [32]). Additionally, burnout and depression were measured by means of the Maslach Burnout Inventory [33] and the German version of the depression scale from the Center for Epidemiological Studies (CES-D, [34,35]).
Sample processing
Saliva samples were stored at -30°C after collection for later analyses. After the study was completed, samples were sent to Dresden LabService GmbH (Dresden, Germany) where they were analyzed by means of high performance liquid chromatography.
Sample analysis
For statistical analyses, IBM SPSS Statistics (version 25) was used. For evaluation of the memory test, the number of recalled words for each position (1 to 15) was summed for the three word lists at each the three measurement time points. First, only the memory tests were analyzed to ensure that primacy and recency effects were actually found.
Therefore, an analysis of variance for repeated measurement (rmANOVA) with the within-subject factors ‘position’ (‘1’, …, ‘15’) and ‘time point (‘pre SECPT’, ‘post SECPT’, and ‘SECPT + 20 min.’) was calculated. The number of correctly recalled words was averaged over the percentiles (1st to 3rd, 4th to 6th, …, 13th to 15th word position; P1, …, P5) to make the following post-hoc analysis easier to interpret. Only the percentiles were used for further statistical analysis. If necessary, sphericity violations (determined by Mauchly’s test of sphericity; [36]) were corrected by adjusting the degrees of freedom with the procedure by 36. As post-hoc tests, t-tests with adjusted alpha levels according to the Bonferroni correction were calculated. Partial eta-squares (ηp2) for ANOVAs and Cohen’s d for t-tests are reported for effect sizes. If necessary, Cohen’s d was corrected according to the method that was proposed by Morris (2008; [37]). For further analysis (after the occurrence of an PE and RE was revealed), P1 was considered as a measure of the primacy effect and, therefore, for long-term memory, and P5 was considered as a measure for the recency effect and, thus, for working memory performance. To test whether the PE and the RE differed between the three time points (‘pre SECPT’, ‘post SECPT’, and ‘SECPT + 20 min.’) and whether they were, therefore, related to the stress induction, a further rmANOVA with the factors ‘time point’ and ‘memory effect’ (‘PE’ and ‘RE’) was calculated.
Because of positive skewness, cortisol levels were transformed by means of the natural logarithm prior to further statistical analysis. Participants were classified as responders or non-responders, separately for sAA and cortisol. Participants with an increase of more than 10 percent between s1 and s2 for sAA and between s1 and s5 for cortisol were classified as responders. Further rmANOVAS with the within-subject factors ‘memory type’ and ‘time point’ and the between-subject factor ‘respondence’ were calculated. If necessary, post-hoc rmANOVAS with the factors ‘time point’ and ‘respondence’ were calculated, separately for the PE and RE.
Results
Stress effects on memory
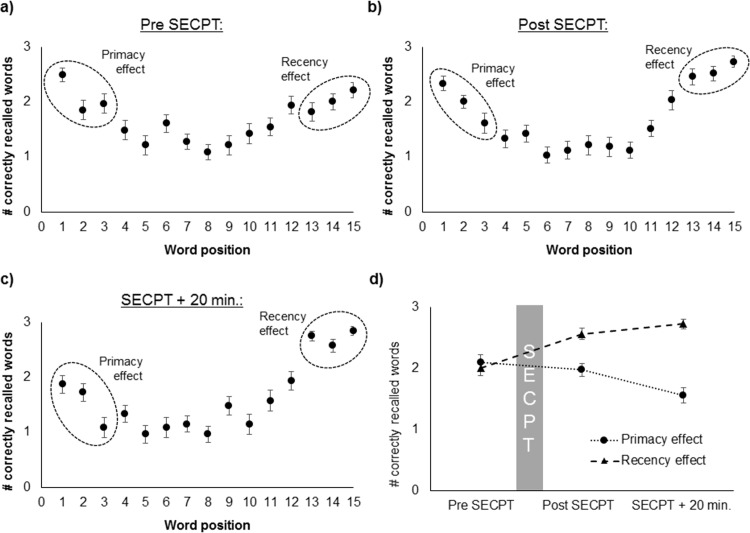
A main effect of the factor position (F(6.011, 192.35) = 24.80, p < .001, ηp2 = .44), a main effect of time point (F(2, 64) = 4.08, p = .022, ηp2 = .11), and an interaction position * time (F(14.17, 453.42) = 4.83, p < .001, ηp2 = .13) were found (Fig 3A–3C). A further rmANOVA, in which the percentiles P1, P3, and P5 were compared, revealed a significant main effect of the factor time point (F(1.61, 51.76) = 58.0, p < .001, ηp2 = .64), a marginally significant main effect of the factor percentile (F(2, 64) = 2.84, p = .066, ηp2 = .08), and a significant interaction time point * percentile (F(4, 128) = 13.19, p < .001, ηp2 = .29). Post-hoc t-tests showed that both the primacy effects (i.e., P1 > P3) and recency effects (i.e., P5 > P3) were found for all three time points (all p < .001) in this sample.
Fig 3.
Number of correctly recalled words in dependence of the word position, a) before the SECPT, b) immediately after SECPT, c) 20 minutes after the SECPT, and d) strength of the primacy and recency effect in dependence of the measurement time-point.
For further analysis, the first (P1) and the last (P5) three words were used as measures for the PE and RE. A further rmANOVA revealed main effects of the factors time point (F(2, 64) = 5.76, p = .005, ηp2 = .15) and memory effect (F(1, 32) = 17.15, p < .001, ηp2 = .35) and an interaction time point * memory effect (F(1.67, 53.4) = 20.76, p < .001, ηp2 = .39, Fig 3D). Post-hoc t-tests showed that the strength of the PE and RE was the same before the SECPT and different after the SECPT (i.e., the RE was stronger than the PE; both p < .001). Immediately after SECPT, the PE was the same as before (p = .236). The PE was significantly weaker 20 minutes after the SECPT than immediately after it (t(32) = 3.65, p = .001, d = -0.71). Therefore, LTM performance did not differ immediately after SECPT, but decreased 20 minutes after the stress induction. The RE was significantly stronger immediately after the SECPT than before (pre-post: t(32) = -5.43, p < .001, d = 0.86), but did not change further in the following 20 minutes (p = .122). Therefore, WM performance increased immediately after the SECPT, but did not change afterwards.
Subjective stress perception
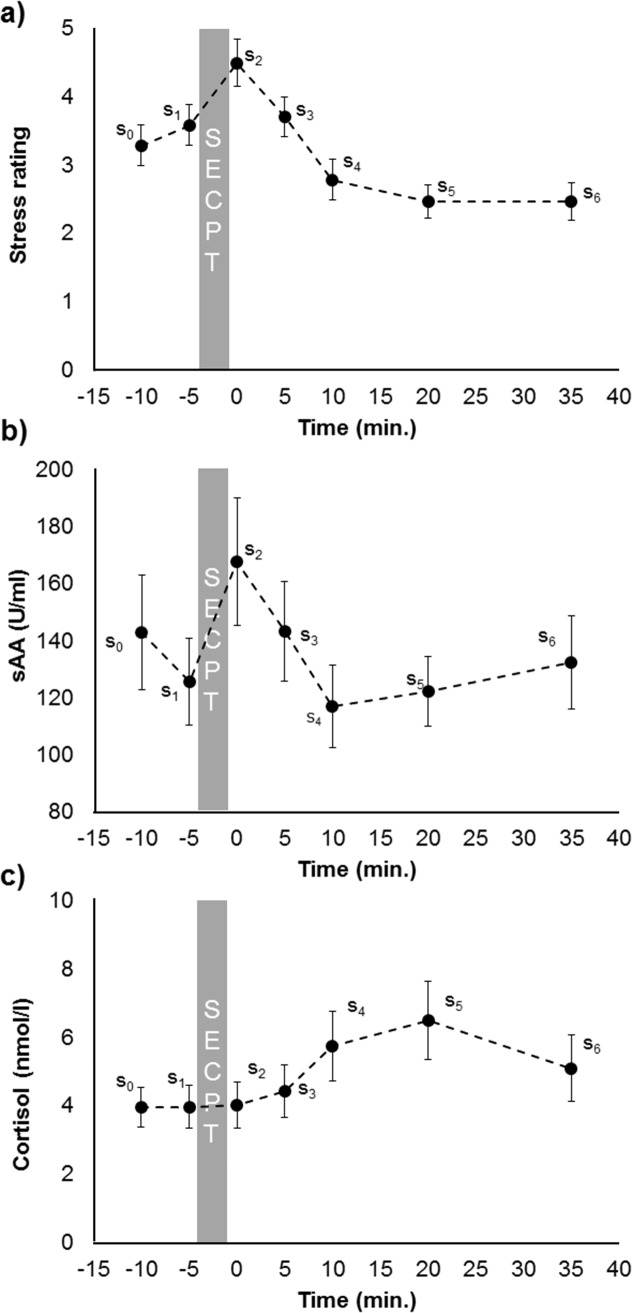
Subjective stress perception significantly differed between the seven time points (F(3.68, 117.75) = 13.56, p < .001, ηp2 = .30). Post-hoc t-tests revealed that subjective stress perception was higher immediately after the SECPT (s2) than before it (s1-s2: t(32) = -2.97, p = .006, d = 0.56) and further declined until 20 minutes after the SECPT (s5, all p < .230, Fig 4A).
Fig 4.
Time course of subjective stress ratings (a), sAA concentration (b), and cortisol concentration (c) at different time points before and after the SECPT.
Salivary alpha-amylase
Salivary alpha-amylase concentration significantly differed between the seven time points (F(6, 192) = 2.32, p = .034, ηp2 = .07, Fig 4B). Post-hoc t-tests revealed that sAA concentration increased immediately after the SECPT (s1-s2: t(32) = -2.20, p = .035, d = 0.49) and then decreased until it reached a minimum ten minutes after the SECPT (s3-s4: t(32) = 1.91, p = .033, d = -0.32). Amylase concentration did not change further after s4.
Twenty-two participants were classified as sAA-responders with an sAA-increase of more than ten percent between s1 and s2. Eleven participants were classified as sAA non-responders. A further rmANOVA with the within-subject factors memory type and time and the between-subject factor sAA respondence was calculated. This revealed a main effect of memory type (F(1, 31) = 24.25, p < .001, ηp2 = .44), a main effect of time (F(2, 62) = 4.49, p = .015, ηp2 = .13), a memory type * sAA respondence interaction (F(1, 1) = 5.29, p = .028, ηp2 = .15), and a memory type * time interaction (F(2, 62) = 15.41, p < .001, ηp2 = .33).
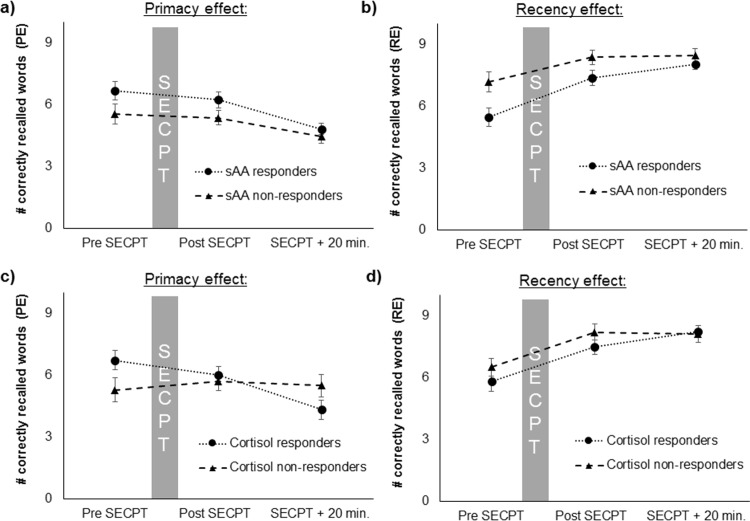
Post-hoc analysis for the PE revealed only a main effect of time (F(2, 62) = 7.55, p = .001, ηp2 = .20; Fig 5A). Thus, the PE and, therefore, long-term memory performance was not associated with the sAA response. For the RE, a main effect of time was found (F(2, 62) = 18.24, p < .001, ηp2 = .37) and a main effect of sAA respondence were found (F(1, 31) = 4.77, p = .037, ηp2 = .13; Fig 5B). Only the sAA responders showed a main effect of the factor time effect (F(2, 42) = 23.37, p < .001, ηp2 = .53), but not the sAA non-responders. Therefore, WM performance only increased in sAA responders. However, it should be noted that the sAA-non responders had higher baseline REs than the sAA-responders which might have prevented a further increase (t(31) = -2.28, p = .030, d = -0.84).
Fig 5.
Primacy and recency effects for sAA (a, b) as well as for cortisol (c, d) responders and non-responders, before, after and 20 minutes after the SECPT.
Cortisol
Cortisol concentration also differed significantly between the seven time points (F(2.02, 164.48) = 7.08, p = .001, ηp2 = .20, Fig 4C). Post-hoc t-tests revealed that cortisol concentration did not differ between before and immediately after the SECPT (p = .368). Afterwards cortisol concentration increased until it reached a maximum 20 minutes after the SECPT (s4-s5: t(32) = -3.35, p = .002, d = 0.45).
Twenty-three participants were classified as cortisol-responders with a cortisol increase of more than ten percent between s1 and s5. Ten participants were assigned to the cortisol non-responders group. A further rmANOVA with the within-subject factors memory type and time and the between-subject factor cortisol respondence was calculated. This revealed a main effect of memory type (F(1, 31) = 16.51, p < .001, ηp2 = .35), a main effect of time (F(2, 62) = 7.01, p = .002, ηp2 = .18), a memory type * time interaction (F(2, 62) = 13.12, p < .001, ηp2 = .30), and a memory type * time * cortisol respondence interaction (F(2, 1) = 4.07, p = .022, ηp2 = .12).
Post-hoc analysis for the PE revealed a main effect of time (F(2, 62) = 4.92, p = .01, ηp2 = .14) and a time * cortisol respondence interaction (F(2, 31) = 11.79, p = .005, ηp2 = .16). Post-hoc analyses showed that, for the PE, a time effect was found only for the cortisol responders (F(2, 44) = 18.28, p < .001, ηp2 = .45), but not for the cortisol non-responders (Fig 5C). Therefore, long-term memory performance only decreased in cortisol responders after the SECPT, but not in cortisol non-responders. For the RE, only a main effect of time (F(2, 62) = 15.44, p < .001, ηp2 = .51), but no interaction time * cortisol respondence or a main effect of cortisol respondence were found (Fig 5D).
Discussion
In this study, we investigated the time course of the physiological stress response and its associations with WM and LTM performance. The latter were operationalized by means of the PE and the RE of the serial position curve. Our first finding was that WM performance increased immediately after the stressor in participants who showed a sAA response. No changes in WM performance were found in sAA non-responders. This is in line with our hypothesis and with previous findings, in which improvements in WM performance after an acute stressor were found as well [38–42]. However, other studies also found the opposite, i.e. impaired WM functioning after an acute stressor (e.g., [43–47]). However, in contrast to our study, in most cases spatial WM and not verbal WM was investigated in these previous studies. One explanation that has been proposed to explain the different findings was that WM improves for simple tasks, but that it is impaired for complex tasks [2]. This explanation fits well to the results of our study because we used simple word lists with only 15 non-emotional words, and it can be assumed that this was an easy task for our participants.
Our second finding was that LTM performance did not change immediately after the stressor, but decreased 25 minutes after its onset in cortisol responders. This was also the time point of the maximal cortisol response. This decrease in LTM performance was not found in cortisol non-responders. Our finding is in line with many previous studies in which a drop in LTM performance and a relation with glucocorticoids was found (e.g., [48–52]). However, there are also a few studies which cannot support this conclusion [53,54]. It has been proposed that the timing of the glucocorticoid release (or injection for pharmacological studies), is the critical factor for the diversity of the findings [55–57]. This could also explain why we did not find an effect on LTM performance immediately after the stressor.
Our findings support the view that peripherally transmitted noradrenaline leads via indirect pathways to a release of noradrenaline and dopamine in the PFC (e.g., [2]). Furthermore, our results are in line with previous findings that have shown that peripherally released cortisol passes the BBB and binds to receptors that are located in the hippocampus (e.g., [17–19]). However, in the PFC GRs can be found as well [15] and the hippocampus also receives noradrenaline and dopamine input [58,59]. However, the association between PFC functioning and peripheral glucocorticoid release can be found for longer time delays only [60]. Furthermore, associations between hippocampal dopamine release and memory have been found for chronic stress and late long-term potentiation only [61,62].
In our study, we investigated SNS and HPA axis response to an acute stressor. However, there are further peripheral-physiological stress responses which occur with a longer time delay, but which might be related with memory processes as well (e.g., parasympathetic activation and activation of inflammatory processes; e.g., [63]). This should be investigated in future research (e.g., by means of heart-rate variability analyses and collection of blood samples).
It is important to point out that neutral words were used in our study. There is an extensive literature on the effects of (e.g., emotional) arousal or stress on emotional memory (e.g., [64,65]). It was found that emotional LTM is enhanced–and not impaired as it is for neutral stimuli [66]. For memory formation of emotional stimuli, the amygdala plays a critical role [67]. Emotional memory is indeed affected by SNS activity [68]. For example, it has been found that the enhancement in emotional LTM is eliminated through a blockade of beta-adrenergic receptors in humans [65]. Furthermore, it was shown that a noradrenaline injection after learning enhances LTM for emotional stimuli [64]. In animal studies, it has been found that noradrenaline can have long-term effects on the hippocampus [69–72]. Besides, glucocorticoids are also involved in LTM enhancement for emotional stimuli. For example, Buchanan and Lovallo [73] found that a cortisol injection during learning enhanced recall one week later. To combine these manifold findings, it has been suggested that both, the glucocorticoid and the noradrenaline pathway, interact in emotional memory formation [74,75].
Furthermore, it should be noted that, in previous studies, LTM was assessed in a different way than in our study. In most previous paradigms, participants were presented the material on one day and the recall took place on another day. Thus, the elapsed time was much longer than in our study. Our design has the advantage that learning as well as retrieval of the items could both be tested at all three time points within one person. Therefore, our design offers new insights in the effects of acute stress on memory performance.
Our study does not allow us to draw conclusions about the reasons for the sAA- or cortisol-non respondence. It has been shown previously that stress responsiveness is associated with childhood traumata and adversity (e.g., [76,77]). These might be factors underlying the non-responsiveness in our study as well. Unfortunately, we did not ask our participants for this. But, this should be investigated in future research.
Moreover, our design should be repeated with emotional stimuli in future studies. Furthermore, it should be combined with imaging techniques to get more insight into the underlying neural processes. Besides, our design should be supplemented by the collection of blood samples, because the use of sAA as marker for SNS activity is well-established [78,79], there are still some valid concerns that need to be taken into account [80]. Furthermore, our design should be supplemented by pharmacological treatments, which block either the MR or the GR, because to understand the underlying mechanisms both receptor types should be taken into account [16,81].
Conclusions
Our study supports the assumption that SNS activation after an acute stressor immediately improves WM function. This is probably related with noradrenergic and dopaminergic activation of the PFC. Furthermore, we showed that HPA axis activity is associated with LTM processes–probably through interactions with the hippocampus. Using the serial position effects to measure both WM and LTM performance within one test seems to be a very good means for further research on the effects of acute stress on memory.
Supporting information
(CSV)
(XLSX)
Acknowledgments
We thank Aylin Gögsen, Kristin von Majewski, and Yvonne Daichendt for data collection.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
LB was supported by the Bavarian Equal Opportunities Sponsorship – Förderung von Frauen in Forschung und Lehre (FFL) – Promoting Equal Opportunities for Women in Research and Teaching. The authors acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding programme Open Access Publishing.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews neuroscience. 2009;10(6):410 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience. 2009;10(6):434 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 3.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature reviews neuroscience. 2009;10(3):211 10.1038/nrn2573 [DOI] [PubMed] [Google Scholar]
- 4.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews neuroscience. 2009;10(6):397 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Jama. 1992;267(9):1244–52. [PubMed] [Google Scholar]
- 6.Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Annals of the New York Academy of Sciences. 1995;771(1):1–18. [DOI] [PubMed] [Google Scholar]
- 7.Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annual review of neuroscience. 1987;10(1):67–95. [DOI] [PubMed] [Google Scholar]
- 8.Valentino RJ, van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European journal of pharmacology. 2008;583(2–3):194–203. 10.1016/j.ejphar.2007.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Progress in neurobiology. 2007;83(2):69–91. 10.1016/j.pneurobio.2007.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- 11.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–92. 10.1016/j.psyneuen.2003.08.009 [DOI] [PubMed] [Google Scholar]
- 12.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and cognition. 2007;65(3):209–37. 10.1016/j.bandc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 13.de Kloet ER, Reul J, Sutanto W. Corticosteroids and the brain. The Journal of steroid biochemistry and molecular biology. 1990;37(3):387–94. [DOI] [PubMed] [Google Scholar]
- 14.Reul J, de Kloet E. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–11. 10.1210/endo-117-6-2505 [DOI] [PubMed] [Google Scholar]
- 15.Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, et al. Mineralocorticoid receptor stimulation improves cognitive function and decreases cortisol secretion in depressed patients and healthy individuals. Neuropsychopharmacology. 2015;40(2):386 10.1038/npp.2014.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends in neurosciences. 1999;22(10):422–6. [DOI] [PubMed] [Google Scholar]
- 17.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature neuroscience. 1998;1(1):69–73. 10.1038/271 [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS. Stress and hippocampal plasticity. Annual review of neuroscience. 1999;22(1):105–22. [DOI] [PubMed] [Google Scholar]
- 19.de Quervain J‐F de, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, et al. Glucocorticoid‐induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. European Journal of Neuroscience. 2003;17(6):1296–302. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control processes1 In: Psychology of learning and motivation: Elsevier; 1968. p. 89–195. [Google Scholar]
- 21.Atkinson RC, Shiffrin RM. The control of short-term memory. Scientific American. 1971;225(2):82–91. [DOI] [PubMed] [Google Scholar]
- 22.Murdock BB Jr. The serial position effect of free recall. Journal of experimental psychology. 1962;64(5):482. [Google Scholar]
- 23.Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33(6):890–5. 10.1016/j.psyneuen.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 24.Minkley N, Schröder TP, Wolf OT, Kirchner WH. The socially evaluated cold-pressor test (SECPT) for groups: Effects of repeated administration of a combined physiological and psychological stressor. Psychoneuroendocrinology. 2014;45:119–27. 10.1016/j.psyneuen.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 25.Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–71. 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–33. [DOI] [PubMed] [Google Scholar]
- 27.Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, et al. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol. 2005;55(3):333–42. 10.1016/j.ijpsycho.2004.09.009 . [DOI] [PubMed] [Google Scholar]
- 28.Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Annals of the New York Academy of Sciences. 2004;1032:258–63. 10.1196/annals.1314.033 . [DOI] [PubMed] [Google Scholar]
- 29.Craig CL, Marshall AL, Sjorstrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise. 2003;35(8):1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 30.Fogelholm M, Malmberg J, Suni J, Santtila M, Kyröläinen H, Mäntysaari M, et al. International physical activity questionnaire: Validity against fitness. Medicine and science in sports and exercise. 2006;38(4):753–60. 10.1249/01.mss.0000194075.16960.20 [DOI] [PubMed] [Google Scholar]
- 31.Schulz P, Schlotz W. Trierer Inventar zur Erfassung von chronischem Stress (TICS): Skalenkonstruktion und teststatistische Überprüfung; 1995.
- 32.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983:385–96. [PubMed] [Google Scholar]
- 33.Maslach C, Jackson SE. Maslach burnout inventory Consulting Psychologists Press Palo Alto MaslachMaslach Burnout Inventory: Second Edition1986. 1986. [Google Scholar]
- 34.Hautzinger M, Bailer M, Hofmeister D, Keller F. ADS: Allgemeine Depressionsskala: Testhandbuch (2te Ausgabe): Göttingen: Hogrefe; 2012. [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 36.Mauchly JW. Significance test for sphericity of a normal n-variate distribution. The Annals of Mathematical Statistics. 1940;11(2):204–9. [Google Scholar]
- 37.Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organizational research methods. 2008;11(2):364–86. [Google Scholar]
- 38.Duncko R, Johnson L, Merikangas K, Grillon C. Working memory performance after acute exposure to the cold pressor stress in healthy volunteers. Neurobiology of Learning and Memory. 2009;91(4):377–81. 10.1016/j.nlm.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henckens MJ, Hermans EJ, Pu Z, Joëls M, Fernández G. Stressed memories: How acute stress affects memory formation in humans. Journal of Neuroscience. 2009;29(32):10111–9. 10.1523/JNEUROSCI.1184-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weerda R, Muehlhan M, Wolf OT, Thiel CM. Effects of acute psychosocial stress on working memory related brain activity in men. Human brain mapping. 2010;31(9):1418–29. 10.1002/hbm.20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences. 2009:pnas-0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular psychiatry. 2011;16(2):156 10.1038/mp.2010.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–52. [DOI] [PubMed] [Google Scholar]
- 44.Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral neuroscience. 2005;119(1):98 10.1037/0735-7044.119.1.98 [DOI] [PubMed] [Google Scholar]
- 45.Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Frontiers in behavioral neuroscience. 2009;2:5 10.3389/neuro.08.005.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D-H, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. Journal of Neuroscience. 2000;20(4):1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33(5):643–53. 10.1016/j.psyneuen.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 48.Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology. 2004;29(8):1093–6. 10.1016/j.psyneuen.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 49.Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress-and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58(17):1475–83. [DOI] [PubMed] [Google Scholar]
- 50.de Quervain J-F de, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature neuroscience. 2000;3(4):313 10.1038/73873 [DOI] [PubMed] [Google Scholar]
- 51.Quesada AA, Wiemers US, Schoofs D, Wolf OT. Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology. 2012;37(1):125–36. 10.1016/j.psyneuen.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 52.Rohleder N, Wolf JM, Kirschbaum C, Wolf OT. Effects of cortisol on emotional but not on neutral memory are correlated with peripheral glucocorticoid sensitivity of inflammatory cytokine production. Int J Psychophysiol. 2009;72(1):74–80. 10.1016/j.ijpsycho.2008.03.010 . [DOI] [PubMed] [Google Scholar]
- 53.Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic–pituitary–adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 2002;27(7):843–53. [DOI] [PubMed] [Google Scholar]
- 54.Nater UM, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, et al. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 2007;32(6):758–63. 10.1016/j.psyneuen.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 55.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30(8):771–84. 10.1016/j.psyneuen.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 56.Schönfeld P, Ackermann K, Schwabe L. Remembering under stress: Different roles of autonomic arousal and glucocorticoids in memory retrieval. Psychoneuroendocrinology. 2014;39:249–56. 10.1016/j.psyneuen.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 57.Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neuroscience & Biobehavioral Reviews. 2012;36(7):1740–9. [DOI] [PubMed] [Google Scholar]
- 58.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–13. 10.1016/j.neuron.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 59.Verhage M, Ghijsen WE, Boomsma F, Lopes da Silva, Fernando H. Endogenous noradrenaline and dopamine in nerve terminals of the hippocampus: Differences in levels and release kinetics. Journal of neurochemistry. 1992;59(3):881–7. [DOI] [PubMed] [Google Scholar]
- 60.Henckens MJ, van Wingen GA, Joëls M, Fernández G. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proceedings of the National Academy of Sciences. 2011;108(14):5801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in neurosciences. 2011;34(10):536–47. 10.1016/j.tins.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. Journal of Neuroscience. 1991;11(5):1478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pellissier S, Dantzer C, Mondillon L, Trocme C, Gauchez A-S, Ducros V, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS ONE. 2014;9(9):e105328 10.1371/journal.pone.0105328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79(2):194–8. [DOI] [PubMed] [Google Scholar]
- 65.Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371(6499):702 10.1038/371702a0 [DOI] [PubMed] [Google Scholar]
- 66.Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learning & Memory. 2007;14(12):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain research reviews. 1997;24(1):1–27. [DOI] [PubMed] [Google Scholar]
- 68.Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117(1):131–43. [DOI] [PubMed] [Google Scholar]
- 69.Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain research. 1988;442(1):131–8. [DOI] [PubMed] [Google Scholar]
- 70.Neuman RS, Harley CW. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain research. 1983;273(1):162–5. [DOI] [PubMed] [Google Scholar]
- 71.Stanton PK, Sarvey JM. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. Journal of Neuroscience. 1985;5(8):2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanton PK, Sarvey JM. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain research bulletin. 1987;18(1):115–9. [DOI] [PubMed] [Google Scholar]
- 73.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26(3):307–17. [DOI] [PubMed] [Google Scholar]
- 74.Cahill L, McGaugh JL. Modulation of memory storage. Current opinion in neurobiology. 1996;6(2):237–42. [DOI] [PubMed] [Google Scholar]
- 75.Roozendaal B, Okuda S, de Quervain D-F, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138(3):901–10. 10.1016/j.neuroscience.2005.07.049 [DOI] [PubMed] [Google Scholar]
- 76.Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and anxiety. 2009;26(11):984–92. 10.1002/da.20605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological psychiatry. 2001;49(12):1023–39. [DOI] [PubMed] [Google Scholar]
- 78.Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32(4):392–401. 10.1016/j.psyneuen.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 79.Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–85. 10.1016/j.psyneuen.2008.12.004 . [DOI] [PubMed] [Google Scholar]
- 80.Bosch JA, Veerman ECI, Geus EJ de, Proctor GB. α-Amylase as a reliable and convenient measure of sympathetic activity: Don’t start salivating just yet! Psychoneuroendocrinology. 2011;36(4):449–53. 10.1016/j.psyneuen.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 81.Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: A case for the mineralocorticoid receptor. Trends in cognitive sciences. 2016;20(3):192–203. 10.1016/j.tics.2015.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.