Abstract
Between 2006 and 2017, frailty prevalence decreased in HIV-positive individuals aged 50 years but presented a 3-fold increase among those 75 years of age. This dynamic relationship, defined as the frailty compression ratio, represents the net result of gero-inducing and gero-protective competing forces, described in the cohort.
Keywords: aging, frailty, frailty compression, gero-protector, gero-inducer, HIV
Projections estimate that the number of people aged 60 years and older is expected to reach 2 billion worldwide by 2050 [1]. Global aging is accompanied by an increased prevalence of age-related conditions, which tend to accumulate, occur concurrently, and interact between themselves [2].
Frailty may replace the metric of chronological age by providing a more accurate measure of an individual’s overall biological and functional status [3]. Frailty may also be considered as a public health outcome, promoting interventions aimed at postponing frailty, thereby reducing the number of years spent with disabilities and comorbidities.
At a clinical level, the geriatric construct of “frailty” is the net result of competing forces, some acting as gero-protectors others as gero-inducers, unbalancing the homeostatic reserves against stressors and exposing the individual to a higher risk of negative outcomes, including falls, disability, comorbidities, cognitive impairment, and death [4].
In the last decade, the management of people living with HIV (PLWH) has significantly improved. The “test and treat” paradigm has reduced the proportion of PLWH experiencing a low nadir CD4 and resultant poor immunological recovery. This has occurred alongside the availability of less toxic and more effective antiretrovirals (ARVs). This has allowed the withdrawal of toxic nucleoside reverse transcriptase inhibitors (d-drugs) and the progressive introduction of integrase inhibitors as the base of antiretroviral therapies (ARTs) [5].
The changing demographics in PLWH are evolving in a similar manner and represent a paradigm of this global scenario. The early introduction of effective and less toxic ART has progressively improved overall survival in PLWH; this may be considered a gero-protective phenomenon [6]. Contrarily, the increase in the median age (currently about 50 years in high-income countries), along with other factors, is the main driving force behind the increasing prevalence of noncommunicable diseases (NCDs), which can be considered a gero-inducing phenomenon [7].
We hypothesized that this evolving clinical scenario could be represented over the past 10 years by a change in the frailty prevalence, delaying frailty onset to an older age in this population.
We explored the dynamic relationship between chronologic age and frailty by assessing the prevalence of being frail at the ages of 50 and 75 years in PLWH over the period 2006–2017. To operationalize this relationship, we developed a novel metric, the frailty compression ratio (FCR).
METHODS
We performed a retrospective evaluation of 3321 PLWH on stable ART attending the Modena HIV Metabolic Clinic (MHMC; Modena, Italy). This is a tertiary-level referral center specifically dedicated to the prevention and treatment of NCDs, multimorbidity (defined as the simultaneous presence of more than 3 NCDs), and frailty in Italian PLWH.
Frailty was described using the Frailty Index (FI), a tool computing the accumulation of age-related health deficits (ie, clinical signs, symptoms, comorbidities, and laboratory abnormalities), captured during a standard comprehensive evaluation at the MHMC. We used a previously validated FI, based on 37 health variables routinely collected at each clinical visit [8].
The FI has been found to predict risk in increments as low as 0.01, as well as 0.10. For a clinical study, we chose increments of 0.10. Recognizing that there would be very few people with low FI counts in a clinical sample and that there is poor survival at high FI scores, we grouped participants into the following 3 categories: fit <0.3, frail 0.31–0.39, and frailest >0.40 [9].
In this study, a novel metric, termed the frailty compression ratio (FCR), was developed. The FCR was defined as the ratio between the probability of being in the frailest category at age of 75 years and the probability of being in the frailest category at the age of 50 years. The probability of being frailest as a function of age was estimated using separate fourth-order polynomial logistic regression (most frail vs fit/frail) for each year between 2006 and 2017, based on the population of the MHMC in that year. An increase in this ratio represents a shift of the frailty prevalence toward an older age. Predictors of FCR were explored with linear regression analysis.
RESULTS
Participants were predominantly men (68.1%), with a median age (interquartile range [IQR]) of 49 (45–54) years. The median time since HIV diagnosis (IQR) was 19.7 (12.8–24.4) years, and the median nadir CD4 cell count (IQR) was 200 (86–300) cells/µL. The HIV viral load was undetectable in 92% of the cohort.
In the whole cohort, 32.5% in 2006 and in 16.4% in 2017 were in the frailest category (P < .001), suggesting an overall decrease in the frailty prevalence in this cohort.
Supplementary Table 1 describes the main demographic and HIV characteristics of the cohort in 2006 and 2017 and at equal intervals during this observation period.
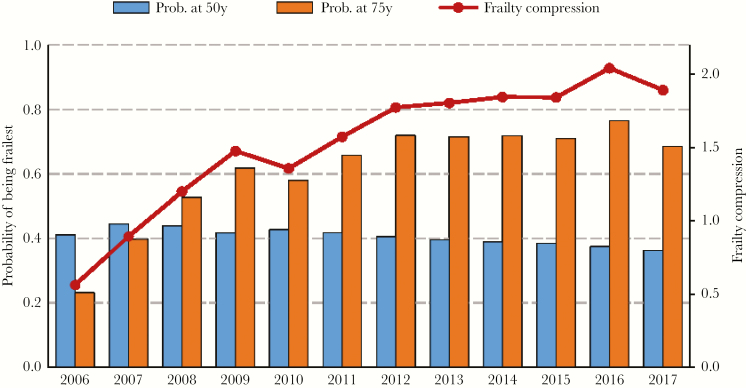
From 2006 to 2017, the probability of being frailest at the age of 50 years decreased from 41.1% to 36.2% over the period of observation, whereas the probability of being frailest at the age of 75 years increased from 23.1% to 68.5% during this time period. The FCR thus increased from 0.56 to 1.89. Figure 1 depicts the change in FCR by calendar year.
Figure 1.
Frailty prevalence at the ages of 50 and 75 years and frailty compression ratio per calendar year.
Predictors of frailty compression were explored with a linear regression model. FCR was independently associated with proportion of male individuals (beta = 0.71, P < .01), age (beta = 0.56, P = .056), nadir CD4+ T-cell count (beta = 0.86, P < .001), and proportion of PLWH with HIV duration >20 years (beta = 0.84, P < .001).
DISCUSSION
In the present analysis, we documented that the frailty prevalence over the past decade has decreased in PLWH aged 50, whereas it increased in those aged 75. We describe these trends in terms of the FCR in PLWH.
The attempt to shift frailty to advanced age through the promotion of healthy life styles, effective disease prevention strategies, and appropriate therapeutic interventions is the main goal of considering aging in the geriatric population in terms of health span, rather than life span.
This study introduced an innovative metric that describes at a population level a shift in the prevalence of frailty toward an older age. This tool has potential use in monitoring public health interventions but cannot be considered at the individual level, and therefore it cannot be used to monitor aging in association with cellular damage, organ pathology, or disease progression.
The public health attempt to describe the number of years spent with or without disability or comorbidities in advanced age was conceptualized several decades ago by the notion of the compression of morbidity, which refers to delaying the onset of NCDs [10]. In the geriatric setting, it is now assumed that age-related disease accumulation is unavoidable, but frailty compression may reduce premature disabling conditions.
To our knowledge, the significant shift in delaying frailty observed in our HIV cohort is unprecedented and has never been previously described, even for other chronic diseases (eg, Parkinson’s, rheumatological or cardio-metabolic conditions; all of these also share a notable frailty burden).
In our cohort, predictors of FCR included HIV duration and CD4 nadir. This new epidemiological scenario is consistent with contemporary HIV care, which is characterized by the early diagnosis and treatment of HIV and use of more effective and less toxic ARVs [5, 11]. We cannot prove causation between FCR and contemporary care. Nevertheless, the association between ARV use and HIV demographic changes seems quite evident. From this perspective, it might be argued that contemporary HIV care acts in a gero-protective manner, and thus ARVs can be considered gero-protective agents.
Gero-protectors are drugs capable of slowing down the underlying biologic processes of aging and extending healthy life span [1]. To date, more than 200 compounds have been implicated as potential gero-protective agents. For example, in a clinical trial, investigators reported that everolimus was able to boost immune responses when administered in combination with an influenza vaccine in people older than age 65 years [12].
With the early initiation of modern ART, the vast majority of newly diagnosed PLWH avoid the immunological burden of low nadir CD4 count and rapidly achieve an undetectable HIV viral load after starting ARVs. This immunological benefit may counteract any possible ARV-related toxicity and be the main driver of the shift in onset of frailty toward an older age.
This hypothesis should be confirmed in further studies. Given global aging and the weakness of some traditional clinical paradigms, including CD4 cell count, it is perhaps time to add frailty, rather than age or viro-immunological status, as a relevant outcome for HIV medicine. Its adoption as an additional criterion in the evaluation of new ARVs and strategies might be clinically useful. It is also important to agree on reliable metrics for capturing the gero-protection of interventions for both research and clinical settings.
Our data raise a new debate on the ambivalent nature of ARVs. A “Dr. Jekyll ARV” may act as a gero-protector, delaying frailty to older age. Meanwhile, a “Mr. Hyde ARV” is a gero-inducer, associated with age-related diseases (e.g., cardiovascular, bone, or renal disease). HIV remains a unique model of aging as a paradigm of the competing and opposite forces involved in epidemiological transitions due to aging.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This study was not sponsored.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bellantuono I. Find drugs that delay many diseases of old age. Nature 2018; 554:293–5. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. World report on ageing and health. Geneva: World Health Organization; 2015. http://www.who.int/ageing/events/world-report-2015-launch/en/. Assessed 15 August 2018. [Google Scholar]
- 3. Brothers TD, Kirkland S, Guaraldi G, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis 2014; 210:1170–9. [DOI] [PubMed] [Google Scholar]
- 4. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–7. [DOI] [PubMed] [Google Scholar]
- 5. Sereti I, Krebs SJ, Phanuphak N, et al. ; RV254/SEARCH 010, RV304/SEARCH 013 and SEARCH 011 protocol teams Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis 2017; 64:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guaraldi G, Rockwood K. Geriatric-HIV medicine is born. Clin Infect Dis 2017; 65:507–9. [DOI] [PubMed] [Google Scholar]
- 7. Althoff KN, Jacobson LP, Cranston RD, et al. ; Multicenter AIDS Cohort Study (MACS) Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guaraldi G, Brothers TD, Zona S, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015; 29:1633–41. [DOI] [PubMed] [Google Scholar]
- 9. Hubbard RE, O’Mahony MS, Woodhouse KW. Characterising frailty in the clinical setting–a comparison of different approaches. Age Ageing 2009; 38:115–9. [DOI] [PubMed] [Google Scholar]
- 10. Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med 1980; 303:130–5. [DOI] [PubMed] [Google Scholar]
- 11. Guaraldi G, Zona S, Brothers TD, et al. Aging with HIV vs. HIV seroconversion at older age: a diverse population with distinct comorbidity profiles. PLoS One 2015; 10:e0118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014; 6:268ra179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.