Abstract
Background
During antiretroviral therapy (ART), HIV-1-infected patients may present with ultralow (UL) HIV-RNA viral loads (VLs) below quantification levels of current assays. Reasons for UL-VL detection and its relation to virological rebound (VR) are unclear.
Methods
HIV-1-infected, ART-naïve patients followed at 2 university hospitals were included. All participants had an HIV-RNA >200 copies/mL at ART initiation and achieved a VL <50 copies/mL during ART. UL-VL was determined by the presence/absence of polymerase chain reaction signal detected using a commercially available assay (COBAS, TaqMan, Roche). Random-effects Poisson regression was used for assessing determinants of UL-VL not detected overtime and conditional risk set analysis for VR (1 VL > 200 copies/mL or 2 VL > 50 copies/mL) while accounting for frequency of VL measurements.
Results
Between 2009 and 2013, 717 patients initiated ART containing 2 nucleos(-t)ide reverse transcriptase inhibitors (NRTIs) plus a non-NRTI (29.4%), a protease inhibitor (58.4%), or an integrase-strand transfer inhibitor (INSTI; 12.1%). During a median (interquartile range) 3.4 (2.3–4.6) years, 676 (94.3%) patients achieved UL-VL not detected. In multivariable analysis, UL-VL not detected overtime was associated with younger age (P < .001), female gender (P = .04), lower baseline VL (P < .001), baseline CD4+ >500 vs <350/mm3 (P < .001), and INSTI-containing ART (P = .009). One hundred thirty-one (18.3%) patients had VR during follow-up, which was independently associated with a CD4/CD8 ratio <0.8 during follow-up (P = .01) and time spent with UL-VL not detected (P < .001). When UL-VL not detected occurred for ≥50% of the follow-up duration (n = 290), faster time to reach UL-VL not detected (P < .001), faster CD4+ T-cell count increase (P = .03), and faster CD4/CD8 ratio increase (P = .001) were observed.
Conclusions
VL suppression at an ultralow level is associated with INSTI-class ART initiation. Extensive VL suppression below ultralow detection could improve immune reconstitution.
Keywords: HIV, initiation antiretroviral therapy, integrase inhibitor, residual viremia
Antiretroviral therapy (ART) effectively controls HIV infection, suppressing HIV viral loads (VL) below levels of quantification in the majority of patients. Ultrasensitive assays, with detection thresholds as low as 0.3 copies/mL, reveal the presence of residual viremia in treated patients [1]. The source of persistent ultralow (UL)-level viremia remains unclear; research suggests that it could be the result of ongoing rounds of viral replication, virus released from induced latently infected cells, or both [2].
HIV-RNA levels are associated with immunological and clinical outcomes in HIV-infected patients, and hence their quantification in plasma is an essential tool for monitoring ART efficacy. Achieving and maintaining VL “undetectability” (<20 or 50 copies/mL) is the recommended target for combined ART in all international guidelines [3, 4].
For firstline regimens, patients with lower baseline HIV-RNA VL, increased genotypic susceptibility, and ART containing an integrase-strand transfer inhibitor (INSTI) or efavirenz (EFV) are more likely to exhibit virological success and less likely to encounter virological rebound (VR), defined as thresholds above 50 copies/mL [5, 6]. No evidence has supported the role of HIV subtypes in virological control [7]. As most commercially available assays have HIV-RNA quantification thresholds at 20–50 copies/mL, it remains to be determined if these factors, and possibly others, are associated with rates of achieving HIV-RNA levels below ultrasensitive thresholds [8, 9]. Furthermore, previous epidemiological studies have suggested that HIV-RNA replication detected at lower thresholds (20–40 copies/mL) increases risk of VR [10], yet whether this pertains to detection at UL-VL levels is unclear. Finally, the time-varying nature of UL-VL detectability during effective treatment has not been appropriately assessed in previous studies.
The purpose of this study was to determine the virological, immunological, and therapeutic correlates of VL below ultrasensitive detection levels (UL-VL) in a large group of ART-naïve patients from outpatient clinics initiating more recent ART combinations. Furthermore, we aimed to use long-term repeated sampling of UL-VLs during treatment to more concretely establish the effect of persistent residual viremia on VR.
METHODS
Study Design and Population
Between 2009 and 2013, HIV-1-infected antiretroviral-naïve patients were selected from 2 French university hospitals (Pitié-Salpêtrière and Saint-Antoine Hospitals). Inclusion criteria were as follows: initiating triple ART regimen with a nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) backbone, HIV-RNA >200 copies/mL at ART initiation, achieved <50 copies/mL at least once during ART, and ≥2 available UL-VLs during follow-up. The study was carried out in accordance with the Declaration of Helsinki. As this was a retrospective, noninterventional study with no additional procedures from standard of care and use of biological samples was permitted after receiving ethical approval (La Pitié-Salpêtrière and Saint-Antoine Hospitals, Paris, France), this study was exempt from informed consent according to French Public Health Code (CSP Article L.1121–1.1).
Quantification of HIV Viral Load
Plasma HIV-1 RNA was measured using a commercially available polymerase chain reaction (PCR) assay (Ampliprep COBAS TaqMan V2.0, Roche, Meylan, France). This assay provides quantitative results for HIV-RNA values ≥20 copies/mL. Qualitative results are also given as HIV-RNA detected (but <20 copies/mL) or when the PCR target was not detected (UL-VL not detected).
Clinical and Therapeutic Characteristics
The following patient characteristics were obtained from computerized medical records: age, gender, date of first HIV-positive serology, AIDS-defining illness, HIV subtype, CD4+ and CD8+ T-cell counts, and nadir CD4+ T-cell count. CD4/CD8 ratio was dichotomized at 0.8 (median level 1 year after ART initiation). Individual antiretroviral agents were recorded, along with their dates of initiation and discontinuation, if applicable. All patients gave written informed consent that a de-identified, electronic version of their medical chart could be used for research purposes.
Statistical Analysis
Analysis intended to reflect “real-life” therapeutic options and hence follow-up incorporated patients undergoing sequential lines of combined ART. Baseline was defined as the visit before ART initiation. Patients were followed while on continuous ART until ART discontinuation, last follow-up visit, or no follow-up within 12 months after last VL measurement, whichever occurred first. Follow-up was not censored if individuals switched to another therapeutic line of ART.
Two primary outcomes (based exclusively on HIV RNA levels) were analyzed in the study: (1) rate of UL-VL not detected and (2) time until VR (defined as having 2 consecutive HIV-RNA VL measurements >50 copies/mL or 1 measurement >200 copies/mL after a period of undetectability). First, in a risk factor analysis examining rate of UL-VL not detected, univariable incidence rate ratios (IRRs) comparing levels of determinants and their 95% confidence intervals (CIs) were calculated using a random-effects Poisson regression model accounting for patient variability at baseline. Second, risk factors of VR were examined in the cohort. As VR can occur several times during the course of ART, we modeled rates of VR in a repeat-event analysis [11]. Follow-up was divided into intervals of virological suppression. To summarize, the initial follow-up period began at the first visit with HIV-RNA <50 copies/mL and ended at first VR. Additional periods of follow-up were incorporated, beginning at the visit when HIV <50 copies/mL was next achieved after VR and ending at the visit with subsequent VR, until the last VR. For individuals without VR, follow-up continued until the last visit. A conditional risk set, Cox proportional model with gap time was used to assess determinants of VR, from which univariable hazard ratios (HRs) and their 95% CIs were calculated. All parameter estimates were adjusted for number of tests per year to account for HIV-RNA testing frequency.
For both the random-effect Poisson regression and conditional risk set proportional hazards models, determinants with a P ≤.1 in univariable analysis were retained, and backwards selection was used to create a final multivariable model. Individual antiretroviral classes were compared with all classes combined as a reference group, which is denoted as the grand mean.
Several secondary outcomes were used to describe other measures of UL-VL detection over time. First, we examined the cumulative proportion achieving first UL-VL not detected. Second, we calculated the duration of follow-up with UL-VL not detected by summing, within individuals, the time intervals beginning at UL-VL not detected and ending at the next detectable UL-VL. The highest interval was taken for each individual and median (interquartile range [IQR]) time calculated. Third, the duration of follow-up with UL-VL not detected was divided by total follow-up time for each individual to estimate the overall proportion of follow-up with UL-VL not detected. We assessed the effect of changing VL levels to <20 and <50 copies/mL for all 3 outcomes.
In secondary analysis, we assessed the association between proportion of follow-up time with UL-VL not detected on virological and immunological progression during treatment. Based on the upper tertile, we constructed 2 groups of “high” or “low” UL HIV-RNA suppression (defined at ≥50% or <50% of follow-up with UL-VL not detected, respectively). We then compared UL HIV-RNA groups with respect to time to achieving first UL-VL not detected (while testing for differences in hazards using proportional hazards regression adjusted for baseline HIV-RNA VL) and changes in CD4+ cell count and CD4/CD8 ratio from baseline (modeled as a square root function of time using mixed-effects linear regression with random intercept and tested as a time–group interaction).
All analyses were performed using STATA (version 12.1; StataCorp, College Station, TX). All statistical tests were 2-sided, and a P value of <.05 was considered significant.
RESULTS
Description of the Study Population
Between 2009 and 2013, 717 patients initiating ART were included (519 from Pitié-Salpêtrière Hospital and 198 from Saint-Antoine Hospital). The characteristics of the study population are presented in Table 1. Most patients were male (73.5%) with median age (IQR) of 39 (32–46) years. Patients initiated ART shortly after HIV diagnosis (median [IQR], 0.2 [0.1–2.4] years). Before ART initiation, median CD4 T-cell counts (IQR) were not severely low, at 306/mm3 (178–441/mm3), and only 14.4% of patients had an AIDS-defining illness. The median baseline HIV-1 VL was 4.84 log10 copies/mL, and 40.7% of patients presented with a VL >5.00 log10 copies/mL. Firstline ART contained 2 NRTIs plus either a non-NRTI (NNRTI; 29.4%), a protease inhibitor (PI; 58.4%), or an INSTI (12.1%) (antiretroviral agents are detailed in Supplementary Table 1).
Table 1. .
Description of the Study Population at Antiretroviral Therapy Initiation
| N = 717 | Initial ART Backbone | P | |||
|---|---|---|---|---|---|
| NRTI + NNRTI (n = 211) | NRTI + PI (n = 419) | NRTI + INSTI (n = 87) | |||
| Male/female (% male) | 527/190 (73.5) | 171/40 (81.0) | 293/126 (69.9) | 63/24 (72.4) | .01 |
| Age (n = 716), y | 39 (32–46) | 39 (32–47) | 38 (32–45) | 42 (34–51) | .009 |
| Hospital | .005 | ||||
| La Pitié-Salpétrière | 519 (72.4) | 160 (75.8) | 286 (68.3) | 73 (83.9) | |
| Saint-Antoine | 198 (27.6) | 51 (24.2) | 133 (31.7) | 14 (16.1) | |
| Time since first positive HIV serology, y | 0.2 (0.1–2.4) | 0.5 (0.1–2.5) | 0.2 (0.1–2.3) | 0.1 (0.1–2.9) | .2 |
| AIDS-defining illness | 103 (14.4) | 16 (7.8) | 75 (17.9) | 12 (13.8) | .002 |
| CD4+ T-cell count (n = 666), /mm3 | 306 (178–441) | 366 (268–467) | 275 (137–408) | 322 (210–408) | <.001 |
| Nadir CD4+ T-cell count, mm3 | 275 (157–374) | 328 (242–410) | 238 (110–349) | 278 (168–360) | <.001 |
| CD8+ T-cell count (n = 643), /mm3 | 780 (538–1141) | 822 (586–1241) | 764 (497–1115) | 780 (564–1167) | .10 |
| CD4:CD8 ratio (n = 643) | 0.35 (0.21–0.57) | 0.41 (0.24–0.63) | 0.31 (0.17–0.51) | 0.35 (0.21–0.57) | <.001 |
| HIV-RNA viral load, log10 copies/mL | 4.84 (4.36–5.24) | 4.68 (4.17–5.08) | 4.90 (4.41–5.33) | 4.84 (4.49–5.29) | <.001 |
| HIV-RNA viral load >105 copies/mL | 292 (40.7) | 66 (31.3) | 187 (44.6) | 39 (44.8) | .004 |
| HIV subtype (n = 105) | .7 | ||||
| B | 43 (41.0) | 12 (48.0) | 25 (37.3) | 6 (46.2) | |
| CRF02_AG | 34 (32.4) | 6 (24.0) | 25 (37.3) | 3 (23.1) | |
| Other | 28 (26.7) | 7 (28.0) | 17 (25.4) | 4 (30.8) | |
| No. of antiretroviral agents | 3 (3–3) | 3 (3–3) | 3 (3–3) | 3 (3–3) | .6 |
| Positive HCV RNA (n = 291) | 18 (6.2) | 4 (5.5) | 7 (3.9) | 7 (18.9) | .002 |
| HCV RNA viral load, log10 IU/mLa | 6.20 (5.70–6.55) | 6.20 (5.52–6.56) | 6.55 (5.88–6.71) | 6.15 (5.63–6.39) | .5 |
| Positive HBsAg serology (n = 705) | 23 (3.3) | 5 (2.4) | 18 (4.3) | 0 (0) | .09 |
| Switching ART regimen during follow-up | 425 (59.3) | 92 (43.6) | 284 (67.8) | 49 (56.3) | <.001 |
| No. of switches during follow-upb | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | .8 |
All statistics are No. (%), except for continuous variables, where data are reported as median (interquartile range).
Abbreviations: ART, antiretroviral therapy; HCV, hepatitis C virus; NNRTI, non–nucleoside/nucleotide reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor.
aOnly among patients with positive HCV RNA viral loads.
bOnly among patients switching ART regimens at least once.
Study characteristics at ART initiation are also compared between initial ART regimens in Table 1. A significantly higher proportion of females commenced PI-containing ART, whereas the median age was higher in individuals commencing INSTI-containing ART. Most differences in HIV-related parameters were observed in individuals initiating PI-containing ART, with a significantly higher proportion having an AIDS-defining illness, lower median current and nadir CD4+ T-cell counts, and a higher median HIV-RNA viral load.
Antiretroviral Therapy and Virological Response During Follow-up
Patients were followed for a median (IQR) of 3.4 (2.3–4.6) years. Firstline therapy was continued until the end of follow-up in 292 (40.7%) patients. The remaining 425 (59.3%) patients had 1 (n = 287), 2 (n = 78), or ≥3 (n = 60) treatment switches during follow-up, whereas the median time until first treatment switch (IQR) was 1.6 (0.8–2.6) years. Among these, there were 670 switches to the following ART combinations: 2 NRTIs + 1 NNRTI (n = 264), 2 NRTIs + 1 PI (n = 194), 2 NRTIs + 1 INSTI (n = 122), other combination ART with an NRTI backbone (n = 41), dual therapy with 2 NRTIs (n = 16) or with INSTI + another agent (n = 11), or PI monotherapy (n = 22). Overall, the median follow-up time (IQR) under the following backbones was as follows: 2 NRTIs + 1 NNRTI, 2.0 (1.2–3.3) years; 2 NRTIs + 1 PI, 2.2 (1.3–3.6) years; 2 NRTIs + 1 INSTI, 1.6 (0.7–3.1) years; and other, 1.6 (0.6–3.0) years. Individuals initiating PI-containing ART were more likely to switch regimens; however, there was no difference in the number of switches between ART regimens (Table 1). Furthermore, individuals more likely to switch ART regimens (Supplementary Table 2) were those with an AIDS-defining illness (P < .001), lower median current and nadir CD4+ T-cell counts (P = .008 and P = .02, respectively), and higher HIV-RNA viral load (P < .001).
A median number (IQR) of 11 (8–14) HIV-RNA VL measurements per person was taken during follow-up, resulting in a median (IQR) of 2 (1–3) tests per year per person. VL <50 copies/mL was achieved in all 717 patients as part of the inclusion criteria, with 85.1%, 12.4%, and 2.5% occurring during firstline, second-line, and third-line ART, respectively. The cumulative proportion obtaining HIV-RNA VL <50 copies/mL was 78.1% at month 12, 86.1% at month 18, and 92.8% at month 24 of follow-up (with the last patient achieving <50 copies/mL at 37 months).
Suppression Below Ultrasensitive Detection Thresholds During Follow-up
The cumulative proportion obtaining UL-VL not detected was 42.3%, 57.7%, and 70.3% at months 12, 18, and 24 of follow-up, respectively. Of the 676 (94.3%) patients achieving UL-VL not detected, first UL-VL not detected occurred a median (IQR) of 1.1 (0.6–2.0) years after ART initiation. Duration of UL-VL not detected lasted at most a median (IQR) of 1.4 (0.7–2.3) years. The median proportion of follow-up with UL-VL not detected within patients (IQR) was 40.0% (20.0%–60.0%), whereas this proportion was lower when assessed at <20 copies/mL (median [IQR], 61.5% [47.1%–75.0%]) or <50 copies/mL (median [IQR], 71.4% [60.0%–80.0%]).
Clinical, immunological, and virological factors associated with having a UL-VL not detected during the course of follow-up are shown in Table 2. In multivariable analysis, UL-VL not detected over time was associated with younger age (P < .001), female gender (P = .04), lower baseline VL (P < .001), baseline CD4+ >500 vs <350/mm3 (P < .001), and ART containing an INSTI (P = .009). Analysis restricted to firstline therapy among individuals with virological response to their firstline regimen gave similar results (Supplementary Table 3).
Table 2. .
Determinants of UL-VL Not Detected During Antiretroviral Therapy
| Univariable | Multivariablea | |||
|---|---|---|---|---|
| IRR (95% CI) | P | IRR (95% CI) | P | |
| Age (n = 716), per 10 y | 0.88 (0.83–0.92) | <.001 | 0.89 (0.85–0.94) | <.001 |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.13 (1.00–1.28) | .06 | 1.14 (1.01–1.29) | .04 |
| Baseline HIV-RNA | ||||
| <105 copies/mL | 1.00 | 1.00 | ||
| ≥105 copies/mL | 0.67 (0.60–0.75) | <.001 | 0.72 (0.64–0.80) | <.001 |
| Baseline CD4+ T-cell count (n = 665) | ||||
| >500 cells/mm3 | 1.00 | 1.00 | ||
| 350–500 cells/mm3 | 0.91 (0.77–1.08) | .3 | 0.90 (0.76–1.06) | .2 |
| <350 cells/mm3 | 0.63 (0.54–0.74) | <.001 | 0.66 (0.57–0.77) | <.001 |
| Baseline CD4+ T-cell nadir | ||||
| ≥250 cells/mm3 | 1.00 | |||
| <250 cells/ mm3 | 0.68 (0.61–0.76) | <.001 | ||
| Time-varying CD4:CD8 ratio (n = 681) | ||||
| ≥0.8 | 1.00 | |||
| <0.8 | 0.89 (0.81–0.99) | .03 | ||
| Anchor drug of ART during follow-upb | ||||
| NNRTI | 0.99 (0.91–1.07) | .8 | 0.99 (0.91–1.07) | .8 |
| PI | 0.98 (0.91–1.07) | .7 | 0.96 (0.89–1.05) | .4 |
| Integrase inhibitor | 1.13 (1.01–1.25) | .03 | 1.15 (1.04–1.28) | .009 |
| Other combination | 0.91 (0.79–1.06) | .2 | 0.91 (0.78–1.06) | .2 |
| Concomitant treatment switch | ||||
| No | 1.00 | |||
| Yes | 0.97 (0.86–1.09) | .6 |
Analysis includes 717 HIV-infected patients initiating antiretroviral therapy and accounts for subsequent lines of ART after switching regimens. Incidence rate ratios and 95% confidence intervals were calculated from a random-effects Poisson regression accounting for patient variability at baseline.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HCV, hepatitis C virus; IRR, incidence rate ratio; NNRTI, non–nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; UL-VL, ultralow viral load.
aIn multivariable analysis, baseline nadir CD4+ was closely linked to baseline CD4+ T-cell count and hence excluded. Time-varying CD4:CD8 ratio (preferred over CD4:CD8 ratio at baseline) was excluded because its associated P value was below the prespecified threshold (P = .402). A total of 52 patients had missing data on baseline CD4 T-cell count (n = 51) or age (n = 1) and were not included the multivariable model.
bIndividual antiretroviral classes were compared with all classes combined as the reference group.
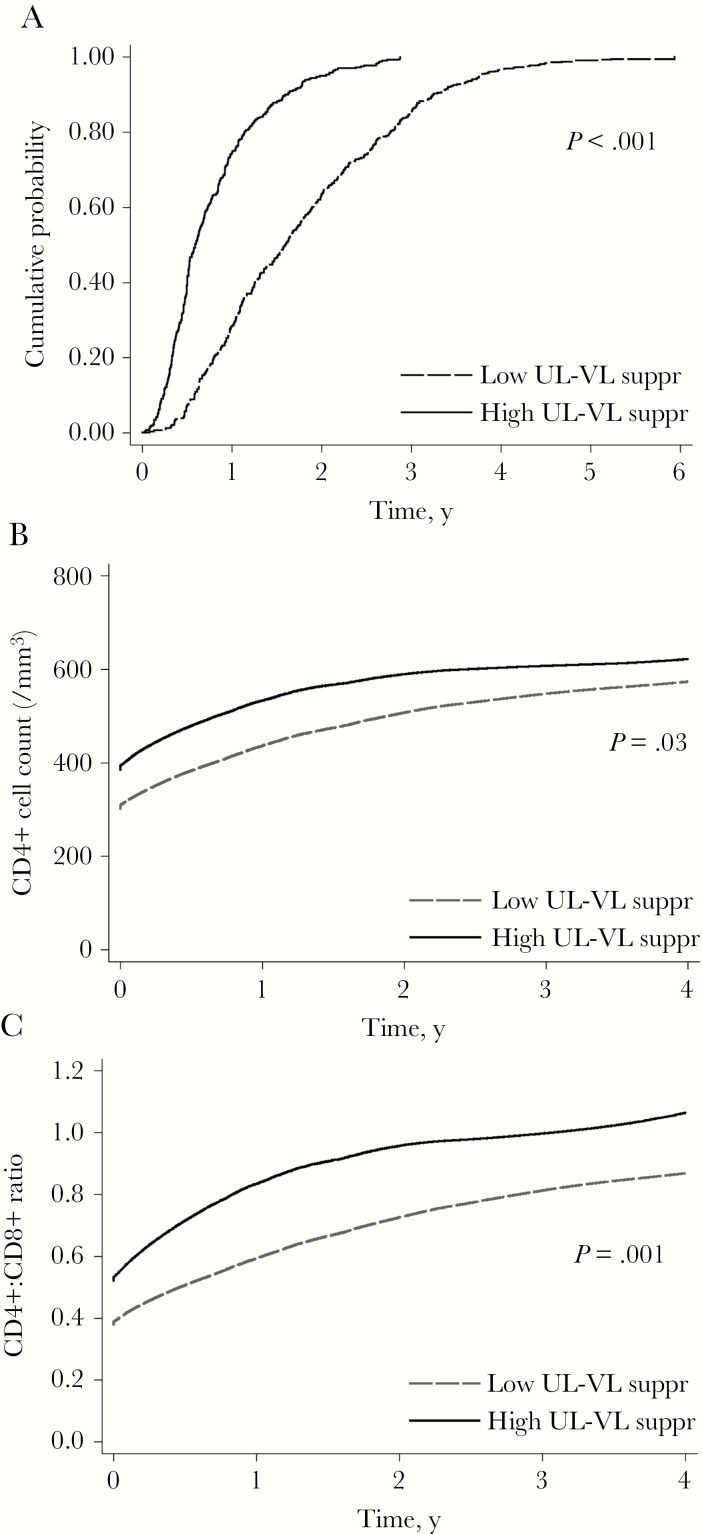
Patients with “high” UL HIV-RNA suppression (UL-VL not detected occurring for ≥50% of the total follow-up duration, n = 290) had a significantly shorter time until achieving UL-VL not detected (P < .001) (Figure 1A), a faster increase in CD4+ T-cell count (P = .03) (Figure 1B), and a faster increase in CD4/CD8 ratio (P = .001) (Figure 1C) than patients with low UL HIV-RNA suppression (UL-VL not detected occurring for <50% of the total follow-up duration, n = 427).
Figure 1.
Time to reach ultralow viral load (UL-VL) not detected and changes in immunological parameters after initiating antiretroviral therapy according to residual viremia and virological rebound. Cumulative proportion of patients reaching an undetectable UL-VL with detection threshold at ultrasensitive levels is depicted in (A), adjusted for baseline HIV-RNA viral load. Locally weighted scatterplot smoothing lines are depicted for CD4+ T-cell count (B) and CD4+:CD8+ ratio (C) during follow-up. All figures are stratified on patients with low vs high HIV-RNA suppression (defined as <50% vs ≥50%, respectively, of follow-up spent with a UL-VL not detected). Abbreviation: UL-VL, ultralow viral load.
Virological Rebound During Follow-up
VR occurred 237 times during follow-up and was defined by 2 consecutive HIV-RNA VLs >50 copies/mL for 102 (43.0%) VRs, 1 HIV-RNA VL >200 copies/mL for 124 (52.3%) VRs, or both criteria for 11 (4.6%) VRs. Overall, VR was observed in 131 (18.3%) patients during follow-up, of whom 68 (51.9%) exhibited VR once, 41 (31.3%) twice, and 22 (16.8%) 3 or more times. The median time until VR (IQR) was 1.6 (0.9–2.2) years, only considering the first VR for those with multiple rebounds.
In multivariable analysis accounting for conditional risk sets (Table 3, Model 1), VR was associated with having a CD4/CD8 <0.8 during follow-up (P = .01) and a higher number of HIV-RNA VL tests per year (P = .001). Of note, NNRTI-containing ART was associated with a significantly reduced risk of VR (P = .003), and other combinations with a significantly higher risk of VR (P = .02) in univariable analysis, yet this was not the case in multivariable analysis. When replacing cumulative duration with UL-VL not detected with ART regimen in the final multivariable model (given the collinearity between these 2 variables), longer periods of UL-VL suppression were significantly and inversely associated with VR (Table 3, Model 2). Analysis restricted to firstline therapy among individuals with virological response to their firstline regimen again gave similar results (Supplementary Table 4).
Table 3.
Determinants of Virological Rebound After Antiretroviral Therapy Initiation
| Univariablea | Multivariableb Model 1 | Multivariablec Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (n = 716), per 10 y | 0.95 (0.80–1.14) | .6 | ||||
| Gender | ||||||
| Male | 1.00 | |||||
| Female | 0.72 (0.46–1.11) | .14 | ||||
| Baseline HIV-RNA | ||||||
| <105 copies/mL | 1.00 | 1.00 | 1.00 | |||
| ≥105 copies/mL | 1.43 (0.99–2.09) | .06 | 1.45 (0.96–2.19) | .08 | 1.18 (0.78–1.79) | .4 |
| Cumulative duration with UL-VL not detected | 0.41 (0.29–0.58) | <.001 | 0.45 (0.30–0.67) | <.001 | ||
| Baseline CD4+ T-cell count (n = 666) | ||||||
| >500 cells/mm3 | 1.00 | |||||
| 350–500 cells/mm3 | 1.51 (0.83–2.74) | .18 | ||||
| <350 cells/mm3 | 1.25 (0.71–2.19) | .4 | ||||
| Baseline CD4+ T-cell nadir | ||||||
| ≥250 cells/mm3 | 1.00 | |||||
| <250 cells/mm3 | 0.86 (0.57–1.30) | .5 | ||||
| Time-varying CD4:CD8 ratio (n = 681) | ||||||
| ≥0.8 | 1.00 | 1.00 | 1.00 | |||
| <0.8 | 2.04 (1.32–3.18) | .001 | 1.95 (1.17–3.24) | .01 | 1.87 (1.15–3.05) | .01 |
| Anchor drug of ART during follow-upe | ||||||
| NNRTI | 0.60 (0.43–0.84) | .003 | 0.76 (0.50–1.15) | .2 | - | |
| PI | 1.20 (0.88–1.64) | .2 | 1.00 (0.69–1.43) | .9 | - | |
| Integrase inhibitor | 0.82 (0.48–1.40) | .5 | 1.23 (0.64–2.33) | .5 | - | |
| Other combination | 1.68 (1.11–2.56) | .02 | 1.08 (0.66–1.75) | .8 | - | |
| Concomitant treatment switch | ||||||
| No | 1.00 | |||||
| Yes | 1.48 (0.94–2.32) | .09 | ||||
| No. of HIV-RNA tests per year | ||||||
| ≤3 | 0.33 (0.18–0.62) | <.001 | 0.29 (0.14–0.63) | .002 | 0.24 (0.12–0.49) | <.001 |
| 3–4 | 0.78 (0.39–1.58) | .5 | 0.83 (0.36–1.93) | .7 | 0.66 (0.31–1.40) | .3 |
| 4–6 | 1.25 (0.63–2.50) | .5 | 1.29 (0.61–2.71) | .5 | 0.99 (0.50–1.95) | .9 |
| >6 | 1.00 | 1.00 | 1.00 |
Analysis includes 717 HIV-infected patients initiating antiretroviral therapy and accounts for subsequent lines of ART after switching regimens. Hazard ratios and 95% confidence intervals were estimated from a conditional risk set Cox proportional hazards model (using time from the previous event).
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HCV, hepatitis C virus; HR, hazard ratio; NNRTI, non–nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; UL-VL, ultralow viral load.
aAdjusted for number of HIV-RNA tests per year to account for individuals with more frequent testing (hence, higher possibility of detecting virological rebound).
bIn the multivariable model, treatment switch was excluded from the model as its associated P value was below the prespecified threshold (P = .157). A total of 36 patients had missing data on CD4:CD8 ratio and hence were not included in the multivariable model.
cReplacing cumulative duration with UL-VL not detected as a time-varying variable with ART regimen in Model 1 (given the collinearity between these 2 variables).
eIndividual antiretroviral classes were compared with all classes combined as the reference group.
DISCUSSION
With advances in ART capable of providing more potent, convenient, and tolerable regimens, virological suppression can be achieved in most HIV-infected patients regardless of initial firstline regimen. The main focus has now shifted toward maintaining long-term virological response and measuring the level at which suppression has been achieved. This longitudinal analysis, conducted in 717 treatment-naïve patients starting combined ART who were able to achieve virological response, demonstrated the effect of ART regimen and immune status not only on residual viremia but also subsequent VR during a median follow-up of more than 3 years.
Indeed, some of the determinants more commonly associated with HIV-RNA VL suppression at higher detection thresholds were found with respect to UL-VL not detected: younger age, female gender, lower baseline VL, and higher baseline CD4+ cell count. Lower baseline plasma VLs before ART initiation and faster time to plasma virologic suppression during ART have already been shown in females compared with males [12]. The association between pre-ART VL and UL-VL not detected during treatment has also been previously highlighted in several short-term studies [8, 9, 13–17], suggesting a link between extensive viral spread with higher levels of circulating virus before ART initiation and the infection of long-lived reservoirs [1].
One of the more important findings of our study was that ART containing an INSTI was associated with a UL-VL not detected during the course of follow-up. Our results mirror those of Gianotti et al., over a shorter time period, in which a summary statistic of percent time with residual viremia was lower among patients undergoing treatment with an INSTI [13], yet their patient population and definition of low-level viremia were divergent from our study. Other studies have demonstrated that HIV-RNA plasma concentrations decrease faster in the first weeks after initiating treatment with INSTI-containing ART compared with PI-class antiretrovirals [6, 18–20] or EFV [21]. As the vast majority of patients were receiving raltegravir (RAL), further investigation on other INSTI agents, such as dolutegravir (DTG), are needed. In contrast to several previous studies [13, 14, 22, 23], no effect of NNRTI on residual viremia emerged in our study. This discrepancy could be explained by differences in NNRTI agents, as patients in our cohort were more commonly treated with rilpivirine (n = 216) and efavirenz (n = 188) and less so with nevirapine (n = 4). Based on ultralow virological suppression, our findings tend to support recommendations of current treatment guidelines to start combined ART with an INSTI-based regimen, independent of CD4+ count and HIV-RNA VL [3, 4].
As persistent residual viremia appears to impact immune activation, inflammation, and microbial translocation [24, 25], VLs at ultralow levels could bear some clinical importance. The ongoing presence of virus despite low viral concentrations could prevent some systemic inflammatory markers from returning to normal levels; however, the association between residual inflammation and residual HIV-1 replication is probably bi-directional and complex [26]. Indeed, as shown in several studies among patients undergoing treatment intensification [27–31] or effective ART for 7 years [27], residual viremia under ART has been associated with low-grade immune activation. Another study has shown that immune activation still persists despite UL-VL not detected after 10 years of treatment [28], but this was assessed with only 1 cross-sectional measure of UL-VL.
Several studies have shown that restoration of the CD4/CD8 ratio is associated with faster virological control after ART initiation (VL <50 copies/mL) [29, 30]. At the same time, CD4 decline appears to be accelerated in studies investigating immunologic progression among elite HIV controllers without ART [29–31]. In our study, we observed that patients with more extensive viral suppression below ultrasensitive detection levels during ART, defined as UL-VL not detected for ≥50% of follow-up, have faster increases of CD4 cell count and CD4/CD8 ratio. Interestingly, CD4/CD8 ratio is inversely correlated to levels of inflammation and immune activation in patients with virological suppression [32]. Whether the immunological effects linked to increased UL-VL suppression also correspond to improvement in inflammation remains to be fully elucidated.
The relationship between HIV-RNA replication and VR has been examined in several studies using assays with higher detection thresholds, but this association with respect to residual viremia is unclear. Past research has shown that patients with a slower time to achieving <50 copies/mL are more likely to continue treatment with residual viremia [13], and when <50 copies/mL occurs >6 months after initiating ART, they have an almost 2-fold risk of subsequent VR [6]. Reports with clearer evidence have stated that the inability to achieve UL-VL during firstline therapy could lead to a higher risk of VR in certain settings [33, 34], whereas others failed to observe similar results [35]. In our study, we observed that patients without VR were able to suppress UL-VL not detected at a significantly faster rate, similar to patients with “high” suppression of UL HIV-RNA during the course of treatment. Furthermore, we demonstrated that the longer UL-VL not detected occurred during treatment duration, the lower the risk of VR. These results suggest the importance of extensive long-term suppression in order to avoid subsequent VR.
Our study has several noteworthy advantages. The large number of patients with many repeated samples, including HIV-RNA VL measures, is one of the most extensive to date. These data allow more flexibility in analytical options, which is why we decided to examine not only the proportion of viral suppression during follow-up, as others have done [13], but, more appropriately, the risk factors associated with the rate of ultralow viral suppression across all visits over time. This is an important distinction as the proportion of viral suppression can be biased by length of follow-up; most studies have evaluated this statistic at a single time point (ie, last follow-up visit). Furthermore, we did not focus only on firstline ART regimens or time until first VR, but rather the overall therapeutic strategies adapted throughout follow-up (by including time-varying treatment switches in the models) and multiple rounds of VR (via conditional risk set proportional hazards models). Including this information provides a more accurate representation of the dynamic processes occurring during routine follow-up.
Despite these advantages, our study has some limitations. First, this study lacks information on adherence, pharmacological data, and ART-associated adverse events, which could play a role in UL-VL detection [36, 37]. Second, we did not analyze data on genotypic resistance at the start of treatment or at the time of rebound. Third, HIV-RNA VLs were measured using a commercially available assay as part of clinical follow-up. Single-copy assays are more adapted to measuring ultrasensitive detection; nevertheless, this time- and cost-prohibitive assay requires large amounts of blood to be collected and cannot be readily used in routine practice [34]. The major limitation of routine PCR is that when HIV RNA VLs are extremely low, within-assay variation is large and could have an impact on reproducibility when evaluating detection or within-patient variation [38]. It should be mentioned, however, that other studies investigating residual viremia have also used real-time PCR assays with fairly reproducible conclusions [10, 13, 17, 33]. Finally, although estimates were adjusted for the number of tests per year, we cannot completely exclude missed virological rebounds. Finally, PI-containing regimens were more often given to individuals with high HIV-RNA VL and low CD4+ T-cell count at ART initiation, and this bias could have influenced our results. Although we did adjust for these factors in multivariable analysis, residual confounding cannot be fully excluded.
In conclusion, notwithstanding the nonrandomized allocation of treatment combinations, we showed that diverse ART regimens have different effects on residual viremia after achieving <50 HIV-RNA copies/mL. In particular, higher rates of virological suppression with UL-VL not detected are more common in individuals undergoing INSTI-based ART regimens. Moreover, given the association between residual viremia and immune activation, INSTI-class antiretrovirals, a preferred third agent for firstline ART according to current recommendations, could also lead to improvement in immunological parameters and possibly reduced inflammation. Further evidence would be needed, however, on other INSTI-class agents to confirm this hypothesis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales.
Potential conflicts of interest. S.L. has received travel grants from Gilead Sciences and ViiV Healthcare. J.L.M. has received travel grants from Abbvie Labs, Gilead Sciences, Merck Laboratories MSD, Janssen Pharmaceuticals, and ViiV Healthcare and has received honoraria for invited talks from Gilead Sciences, Merck Laboratories MSD, and ViiV Healthcare. P.M.G. has received grant support from BMS and Janssen Pharmaceuticals, personal fees as a member of symposia faculty from BMS, Abbvie Labs, and Mylan, and honoraria for participation on international boards from Gilead Sciences and ViiV Healthcare. C.K. has received grant support from Merck Laboratories MSD, Janssen Pharmaceuticals, and ViiV Healthcare. A.G.M. has received honoraria for advisories or invited talks or conferences and research grants from Abbvie Labs, Gilead Sciences, Merck Laboratories MSD, Janssen Pharmaceuticals, and ViiV Healthcare. L.M.J. has received honoraria from BMS, Mylan, Gilead Sciences, Merck Laboratories MSD, Janssen Pharmaceuticals, and ViiV Healthcare. The other authors have no conflicts to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Palmer S, Maldarelli F, Wiegand A, et al. . Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008; 105:3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol 2008; 122:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 13 March 2017. [Google Scholar]
- 4. European AIDS Clinical Society. Guidelines. Version 8.2. January 2017. English. http://www.eacsociety.org/files/guidelines_8.2-english.pdf. Accessed 24 September 2018. [Google Scholar]
- 5. Di Biagio A, Rusconi S, Marzocchetti A, et al. . ARCA Collaborative Group The role of baseline HIV-1 RNA, drug resistance, and regimen type as determinants of response to first-line antiretroviral therapy. J Med Virol 2014; 86:1648–55. [DOI] [PubMed] [Google Scholar]
- 6. Raffi F, Hanf M, Ferry T, et al. . Dat’AIDS Study Group Impact of baseline plasma HIV-1 RNA and time to virological suppression on virological rebound according to first-line antiretroviral regimen. J Antimicrob Chemother 2017; 72:3502. [DOI] [PubMed] [Google Scholar]
- 7. Chaix ML, Seng R, Frange P, et al. . ANRS PRIMO Cohort Study Group Increasing HIV-1 non-B subtype primary infections in patients in France and effect of HIV subtypes on virological and immunological responses to combined antiretroviral therapy. Clin Infect Dis 2013; 56:880–7. [DOI] [PubMed] [Google Scholar]
- 8. Sarmati L, Parisi SG, Montano M, et al. . Nevirapine use, prolonged antiretroviral therapy and high CD4 nadir values are strongly correlated with undetectable HIV-DNA and -RNA levels and CD4 cell gain. J Antimicrob Chemother 2012; 67:2932–8. [DOI] [PubMed] [Google Scholar]
- 9. Martin-Blondel G, Sauné K, Vu Hai V, et al. . Factors associated with a strictly undetectable viral load in HIV-1-infected patients. HIV Med 2012; 13:568–73. [DOI] [PubMed] [Google Scholar]
- 10. Doyle T, Smith C, Vitiello P, et al. . Plasma HIV-1 RNA detection below 50 copies/mL and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis 2012; 54:724–32. [DOI] [PubMed] [Google Scholar]
- 11. Grennan JT, Loutfy MR, Su D, et al. . CANOC Collaboration Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012; 205:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gandhi M, Bacchetti P, Miotti P, et al. . Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 2002; 35:313–22. [DOI] [PubMed] [Google Scholar]
- 13. Gianotti N, Galli L, Galizzi N, et al. . Time spent with residual viraemia after virological suppression below 50 HIV-RNA copies/mL according to type of first-line antiretroviral regimen. Int J Antimicrob Agents 2018; 52:492–9. [DOI] [PubMed] [Google Scholar]
- 14. McKinnon E, Castley A, Payne L, et al. . Determinants of residual viraemia during combination HIV treatment: impacts of baseline HIV RNA levels and treatment choice. HIV Med 2016; 17:495–504. [DOI] [PubMed] [Google Scholar]
- 15. Ripamonti D, Hill A, Lauthouwers E, et al. . Time to HIV-1 RNA suppression below 5 copies/ml during first-line protease inhibitor-based antiretroviral treatment - any impact of residual viremia on treatment success? AIDS Rev 2013; 15:230–6. [PubMed] [Google Scholar]
- 16. Charpentier C, Choquet M, Joly V, et al. . Virological outcome at week 48 of three recommended first-line regimens using ultrasensitive viral load and plasma drug assay. J Antimicrob Chemother 2014; 69:2819–25. [DOI] [PubMed] [Google Scholar]
- 17. Maggiolo F, Callegaro A, Cologni G, et al. . Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr 2012; 60:473–82. [DOI] [PubMed] [Google Scholar]
- 18. Squires K, Kityo C, Hodder S, et al. . Integrase inhibitor versus protease inhibitor based regimen for HIV-1 infected women (WAVES): a randomised, controlled, double-blind, phase 3 study. Lancet HIV 2016; 3:e410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walmsley SL, Antela A, Clumeck N, et al. . SINGLE Investigators Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 20. Clotet B, Feinberg J, van Lunzen J, et al. . ING114915 Study Team Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 21. Lennox JL, DeJesus E, Lazzarin A, et al. . STARTMRK Investigators Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 22. Bonora S, Nicastri E, Calcagno A, et al. . Ultrasensitive assessment of residual HIV viraemia in HAART-treated patients with persistently undetectable plasma HIV-RNA: a cross-sectional evaluation. J Med Virol 2009; 81:400–5. [DOI] [PubMed] [Google Scholar]
- 23. Haïm-Boukobza S, Morand-Joubert L, Flandre P, et al. . Higher efficacy of nevirapine than efavirenz to achieve HIV-1 plasma viral load below 1 copy/ml. AIDS 2011; 25:341–4. [DOI] [PubMed] [Google Scholar]
- 24. Boyd A, Meynard J-L, Morand-Joubert L, et al. . Association of residual plasma viremia and intima-media thickness in antiretroviral-treated patients with controlled human immunodeficiency virus infection. PLoS One 2014; 9(11):e113876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastard JP, Soulié C, Fellahi S, et al. . Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther 2012; 17:915–9. [DOI] [PubMed] [Google Scholar]
- 26. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS 2016; 11:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mavigner M, Delobel P, Cazabat M, et al. . HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One 2009; 4(10):e7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guihot A, Dentone C, Assoumou L, et al. . Residual immune activation in combined antiretroviral therapy-treated patients with maximally suppressed viremia. AIDS 2016; 30:327–30. [DOI] [PubMed] [Google Scholar]
- 29. Noel N, Lerolle N, Lécuroux C, et al. . ANRS C021 CODEX Study Group Immunologic and virologic progression in HIV controllers: the role of viral “Blips” and immune activation in the ANRS CO21 CODEX study. PLoS One 2015; 10:e0131922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boufassa F, Saez-Cirion A, Lechenadec J, et al. . ANRS EP36 HIV Controllers Study Group CD4 dynamics over a 15 year-period among HIV controllers enrolled in the ANRS French observatory. PLoS One 2011; 6:e18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereyra F, Palmer S, Miura T, et al. . Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis 2009; 200:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng L, Taiwo B, Gandhi RT, et al. . Factors associated with CD8+ T-cell activation in HIV-1-infected patients on long-term antiretroviral therapy. J Acquir Immune Defic Syndr 1999 2014; 67:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gianotti N, Galli L, Salpietro S, et al. . Virological rebound in human immunodeficiency virus-infected patients with or without residual viraemia: results from an extended follow-up. Clin Microbiol Infect 2013; 19:E542–4. [DOI] [PubMed] [Google Scholar]
- 34. Calcagno A, Motta I, Ghisetti V, et al. . HIV-1 very low level viremia is associated with virological failure in highly active antiretroviral treatment-treated patients. AIDS Res Hum Retroviruses 2015; 31:999–1008. [DOI] [PubMed] [Google Scholar]
- 35. Helou E, Shenoi S, Kyriakides T, et al. . Characterizing patients with very-low-level HIV viremia: a community-based study. J Int Assoc Provid AIDS Care 2017; 16:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. . Austrian HIV Cohort Study Group Factors associated with low-level viraemia and virological failure: results from the Austrian HIV Cohort Study. PLoS One 2015; 10:e0142923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pasternak AO, de Bruin M, Jurriaans S, et al. . Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis 2012; 206:1443–52. [DOI] [PubMed] [Google Scholar]
- 38. Ruelle J, Debaisieux L, Vancutsem E, et al. . HIV-1 low-level viraemia assessed with 3 commercial real-time PCR assays show high variability. BMC Infect Dis 2012; 12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.