Abstract
Background The IMPROVE score is a validated venous thromboembolism (VTE) assessment tool to risk stratify hospitalized, medically ill patients based on clinical variables. It was hypothesized that addition of D-dimer measurement to derive a new IMPROVEDD score would improve identification of at risk of VTE.
Methods The association of the IMPROVE score and D-dimer ≥ 2 × the upper limit of normal (ULN) with the risk of symptomatic deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was evaluated in 7,441 hospitalized, medically ill patients randomized in the APEX trial. Based on the Cox regression analysis, the IMPROVEDD score was derived by adding two points to the IMPROVE score if the D-dimer was ≥ 2 × ULN.
Results Baseline D-dimer was independently associated with symptomatic VTE through 77 days (adjusted HR: 2.22 [95% CI: 1.38–1.58], p = 0.001). Incorporation of D-dimer into the IMPROVE score improved VTE risk discrimination (ΔAUC: 0.06 [95% CI: 0.02–0.09], p = 0.0006) and reclassification (continuous NRI: 0.34 [95% CI: 0.17–0.51], p = 0.001; categorical NRI: 0.13 [95% CI: 0.03–0.23], p = 0.0159). Patients with an IMPROVEDD score of ≥2 had a greater VTE risk compared with those with an IMPROVEDD score of 0 to 1 (HR: 2.73 [95% CI: 1.52–4.90], p = 0.0007).
Conclusion Incorporation of D-dimer into the IMPROVE VTE risk assessment model further improves risk stratification in hospitalized, medically ill patients who received thromboprophylaxis. An IMPROVEDD score of ≥2 identifies hospitalized, medically ill patients with a heightened risk for VTE through 77 days.
Keywords: venous thromboembolism, D-dimer, risk assessment model, thromboprophylaxis
Introduction
Venous thromboembolism (VTE) is a major contributor to the global disease burden with an estimated incidence of 3.0 to 3.3 cases per 100 hospitalizations per year. 1 Hospitalized, medically ill patients represent a population with heterogeneous predisposition to VTE for which risk assessment is recommended prior to thromboprophylaxis. 2 3 4 However, existing risk assessment models (RAMs) may not adequately identify at-risk subsets, and risk stratification remains an ongoing challenge and imprecise science. 5 6 D-dimer, a biomarker for fibrinolysis, has been associated with heightened VTE risk among patients hospitalized for an acute medical illness. 7 8 9 The International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE RAM has undergone extensive external validation in the medically ill population. 6 10 11 In this article, D-dimer was incorporated into the IMPROVE VTE RAM to derive the IMPROVEDD VTE risk score. 12 The model-based performance was tested in a cohort of hospitalized medical patients receiving primary pharmacologic prophylaxis. It was hypothesized that incorporation of the biomarker D-dimer would provide incremental prognostic value to the IMPROVE RAM in identifying patients at risk of developing symptomatic deep vein thrombosis, nonfatal pulmonary embolism, and VTE-related death.
Methods
Study Design
The Acute Medically Ill VTE (Venous Thromboembolism) Prevention with Extended Duration Betrixaban Trial (APEX; ClinicalTrials.gov identifier: NCT01583218) was a randomized, double-blind, multinational clinical trial that compared extended-duration betrixaban (80 mg once daily for 35–42 days) to standard-duration enoxaparin (40 mg once daily for 10 ± 4 days) among hospitalized medical patients. 9 13 Four principal enrollment criteria were as follows: (1) hospitalization for acute medical illness, including heart failure, respiratory failure, infection, ischemic stroke, or rheumatic disorder; (2) age ≥75 years, age 60 to 74 years with D-dimer ≥ 2 × the upper limit of normal (ULN), or age 40 to 59 years with D-dimer ≥ 2 × ULN and history of VTE or cancer; (3) anticipated severe immobilization for ≥ 24 hours followed by moderate or severe immobilization for 3 or more days; and (4) anticipated hospitalization for 3 or more days. Serum samples for D-dimer were obtained at the time of screening and sent to the central laboratory for analysis using the quantitative STA Liatest D-Di immunoturbidimetric assay (Diagnostica Stago, Asnières-sur-Seine, France). Endpoints were assessed at 42 and 77 days after randomization to approximate the 90-day endpoint from the original IMPROVE VTE RAM. All VTE events were adjudicated by an independent clinical events committee blinded to thromboprophylaxis allocation based on the documentation from the case report form, the narratives prepared by the study sites, and any other available supporting source documentation. The process of events adjudication occurred in two phases (Phase I and Phase II). Phase I review was conducted by two independent physicians. If the Phase I reviewers agreed in the adjudication of the event, the process was complete. If the Phase I reviewers did not agree, the event was submitted for adjudication by Phase II physician committee. In the Phase II meeting, each case was decided by majority rule of the Phase II reviewers. The enrollment period was from March 2012 to October 2015 and the follow-up of the last patient was completed in January 2016.
Statistical Analysis
All randomized patients fulfilling the enrollment criteria were included in the analyses. Baseline characteristics among patients with and without events were compared using the χ 2 test for categorical variables and the one-way ANOVA or Kruskal-Wallis test for continuous variables, as appropriate. Cumulative incidence of symptomatic VTE from randomization to 42 and 77 days was estimated by the Kaplan–Meier method. To test the association between D-dimer and VTE-related events, univariate and multivariate regression analyses evaluated variables included in the IMPROVE associative model (i.e., previous VTE, known thrombophilia, current lower-limb paralysis, current cancer, immobilized ≥7 days, intensive care unit (ICU) or coronary care unit (CCU) stay, and age >60 years). An additional sensitivity analysis that considered the confounding effect of thromboprophylaxis on this association was performed. To determine the appropriate weight for D-dimer, the risk estimate of D-dimer relative to the per point increase in the IMPROVE score was calculated from the adjusted Cox proportional hazards model. Consequently, the IMPROVEDD score was calculated by adding two points to the IMPROVE score if the D-dimer level was ≥ 2 × ULN.
The model-based probability for the IMPROVE and IMPROVEDD score was estimated using logistic regression analysis. Metrics of model discrimination and reclassification were computed to assess the improvement in VTE predictability by the IMPROVEDD score, including area under the receiver–operating–characteristic curve (AUC), integrated discrimination improvement (IDI), and net reclassification improvement (NRI). 14 Model calibration was assessed by comparing the observed and predicted risk for each IMPROVEDD score category. In addition, decision curve analysis was performed to compare the net benefit of the IMPROVE and IMPROVEDD score in VTE prediction. 15 A cutoff for the IMPROVEDD score corresponding to an event rate of ≥ 1%, as recommended for warranting pharmacologic prophylaxis by the American College of Chest Physicians (ACCP) guidelines, was used to dichotomize patients as at-risk versus low-risk category. Accordingly, the risk for symptomatic VTE was compared between the at-risk warranting prophylaxis (≥ 2 points) and low-risk (0–1 points) categories. Analyses were performed independently by an academic research organization, Percutaneous-Pharmacologic Endoluminal Revascularization for Unstable Syndromes Evaluation (PERFUSE) Study Group, using SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina, United States).
Results
Baseline Characteristics
Patients who developed symptomatic VTE at 77 days were hospitalized longer and were more likely to have had a previous VTE, ICU or CCU stay, higher IMPROVE score, and D-dimer ≥2 × ULN ( Table 1 ). Age, sex, race, weight, height, body mass index, creatinine clearance, and acute medical condition were balanced between patients with events and without events.
Table 1. Patient characteristics in the study population with and without symptomatic VTE.
| Characteristic | With symptomatic VTE ( N = 104) | Without symptomatic VTE ( N = 7,337) |
|---|---|---|
| Age, mean (SD)—y | 77.0 (9.0) | 76.4 (8.4) |
| Male sex, n (%) | 43 (41.3) | 3,349 (45.6) |
| Race, n (%) | ||
| White | 94 (90.4) | 6,868 (93.6) |
| Black/African American | 3 (2.9) | 137 (1.9) |
| Asian | 0 (0.0) | 16 (0.2) |
| Others | 7 (6.7) | 316 (4.3) |
| Weight, mean (SD)—kg | 81.8 (19.8) | 80.3 (19.3) |
| Height, mean (SD)—cm | 166.4 (8.4) | 165.3 (9.1) |
| Body mass index, mean (SD)—kg/m 2 | 29.5 (6.9) | 29.4 (6.6) |
| Creatinine clearance, n (%) | ||
| < 30 mL/min | 7 (6.7) | 316 (4.3) |
| ≥ 30 to < 60 mL/min | 46 (44.2) | 3,055 (41.7) |
| ≥ 60 to < 90 mL/min | 32 (30.8) | 2,595 (35.5) |
| ≥ 90 mL/min | 19 (18.3) | 1,352 (18.5) |
| Duration of hospitalization, median (Q1, Q3) a | 12.0 (7.0, 17.0) | 10.0 (8.0, 14.0) |
| Acute medical condition, n (%) | ||
| Heart failure | 34 (32.7) | 3,304 (45.0) |
| Respiratory failure | 20 (19.2) | 885 (12.1) |
| Infection | 33 (31.7) | 2,103 (28.7) |
| Rheumatic disorder | 3 (2.9) | 219 (3.0) |
| Ischemic stroke | 14 (13.5) | 824 (11.2) |
| IMPROVE VTE risk factor, n (%) | ||
| Previous VTE a | 17 (16.3) | 581 (7.9) |
| Known thrombophilia b | 0 (0.0) | 8 (0.1) |
| Current lower-limb paralysis | 7 (6.7) | 559 (7.6) |
| Current cancer | 3 (2.9) | 284 (3.9) |
| Immobilized ≥7 d | 0 (0.0) | 0 (0.0) |
| ICU or CCU stay a | 21 (20.2) | 682 (9.3) |
| Age >60 y | 99 (95.2) | 7,037 (95.9) |
| IMPROVE score, n (%) a | ||
| 0 | 1 (1.0) | 119 (1.6) |
| 1 | 63 (60.6) | 5,322 (72.5) |
| 2 | 14 (13.5) | 561 (7.6) |
| 3 | 8 (7.7) | 777 (10.6) |
| 4 | 15 (14.4) | 463 (6.3) |
| ≥ 5 | 3 (2.9) | 95 (1.3) |
| IMPROVE score, median (Q1, Q3) a | 1.0 (1.0, 2.5) | 1.0 (1.0, 2.0) |
| D-dimer ≥ 2 × ULN, n (%) a | 75 (77.3) | 4,315 (60.5) |
Abbreviations: CCU, coronary care unit; ICU, intensive care unit; ULN, upper limit of normal; VTE, venous thromboembolism.
p < 0.05.
Defined as inherited or acquired disorder of hemostasis including antithrombin III deficiency, protein C deficiency, and protein S deficiency.
Risk Stratification by D-Dimer
Baseline D-dimer levels were measured in 7,235 patients. The risk for symptomatic VTE was significantly higher among patients with D-dimer ≥ 2 × ULN compared with < 2 × ULN, with respective rates of 1.11 versus 0.57% at 42 days and 2.37 versus 1.00% at 77 days (HR: 2.26 [95% CI: 1.41–3.64], p = 0.0008; Fig. 1 ). Of the seven risk factors identified in the IMPROVE RAM study, previous VTE and ICU or CCU stay were statistically associated with symptomatic VTE in the study population ( Table 2 ). Multivariate analysis confirmed that D-dimer was independently associated with symptomatic VTE at 42 days (adjusted HR: 2.28 [95% CI: 1.35–3.85], p = 0.0020) and at 77 days (adjusted HR: 2.22 [95% CI: 1.38–3.58], p = 0.0010). When thromboprophylaxis allocation was considered in the model, estimates for the VTE risk factors do not alter substantially ( Table S1 , supplementary table available in the online version only).
Fig. 1.
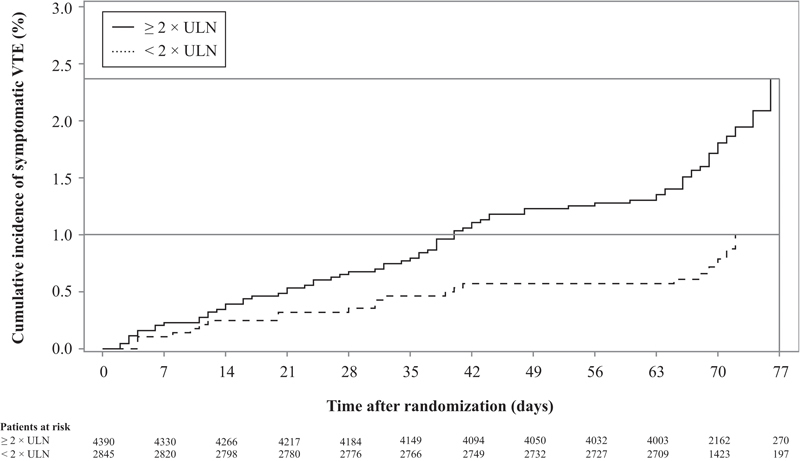
Kaplan–Meier curves for symptomatic VTE stratified by D-dimer concentration.
Table 2. Univariable and multivariable regression analysis of VTE risk factors a .
| Variable | Comparison | Univariable model | Multivariable model | ||
|---|---|---|---|---|---|
| HR (95% CI) | p -Value | Adjusted HR (95% CI) | p -Value | ||
| At 42 d | |||||
| D-dimer | ≥ 2 × ULN vs. < 2 × ULN | 2.33 (1.38–3.94) | 0.0015 | 2.28 (1.35–3.85) | 0.0020 |
| IMPROVE VTE risk factor | |||||
| Previous VTE | Yes vs. no | 2.31 (1.32–4.02) | 0.0032 | 2.20 (1.16–4.17) | 0.0155 |
| Known thrombophilia | Yes vs. no | – | – | – | – |
| Current lower-limb paralysis | Yes vs. no | 0.58 (0.21–1.59) | 0.29 | 0.66 (0.24–1.79) | 0.41 |
| Current cancer | Yes vs. no | 0.58 (0.14–2.35) | 0.44 | 0.63 (0.16–2.57) | 0.52 |
| Immobilization | ≥ 7 d vs. < 7 d | – | – | – | – |
| ICU or CCU stay | Yes vs. no | 2.60 (1.55–4.37) | 0.0003 | 2.95 (1.75–4.99) | < 0.0001 |
| Age | > 60 y vs. ≤ 60 y | 0.72 (0.29–1.78) | 0.48 | 0.99 (0.37–2.62) | 0.98 |
| At 77 d | |||||
| D-dimer | ≥ 2 × ULN vs. < 2 × ULN | 2.26 (1.41–3.64) | 0.0008 | 2.22 (1.38–5.38) | 0.0010 |
| IMPROVE VTE risk factor | |||||
| Previous VTE | Yes vs. no | 2.21 (1.32–3.72) | 0.0028 | 2.20 (1.22–3.97) | 0.0084 |
| Known thrombophilia | Yes vs. no | – | – | – | – |
| Current lower-limb paralysis | Yes vs. no | 0.91 (0.42–1.96) | 0.81 | 1.01 (0.47–2.18) | 0.98 |
| Current cancer | Yes vs. no | 0.75 (0.24–2.36) | 0.62 | 0.82 (0.26–2.59) | 0.73 |
| Immobilization | ≥ 7 vs. < 7 d | – | – | – | – |
| ICU or CCU stay | Yes vs. no | 2.69 (1.67–4.34) | <0.0001 | 2.98 (1.84–4.84) | < 0.0001 |
| Age | > 60 vs. ≤ 60 y | 0.86 (0.35–2.11) | 0.74 | 1.20 (0.46–3.14) | 0.71 |
Abbreviations: CCU, coronary care unit; HR, hazard ratio; ICU, intensive care unit; ULN, upper limit of normal; VTE, venous thromboembolism.
A total of 206 patients with incomplete covariate information were dropped from the model.
Derivation of the IMPROVEDD VTE Risk Score
In the Cox proportional hazards model, symptomatic VTE risk was approximately 2.26 to 2.33 times greater among patients with D-dimer ≥ 2 × ULN compared with < 2 × ULN ( Table 3 ). The risk was approximately 1.22 to 1.26 times greater for each point increase in the IMPROVE score. There was no significant interaction between D-dimer and the IMPROVE score in the model ( p = 0.41 at 42 days and p = 0.61 at 77 days). Based on the relative size of the risk estimates, the IMPROVEDD VTE risk score was derived by adding two points to the IMPROVE score for patients with D-dimer ≥ 2 × ULN ( Table 4 ).
Table 3. Cox proportional hazards model for symptomatic VTE.
| Variable | Comparison | HR (95% CI) | p -Value |
|---|---|---|---|
| At 42 d | |||
| D-dimer | ≥ 2 × ULN vs. < 2 × ULN | 2.33 (1.38–3.94) | 0.0015 |
| IMPROVE score | Per point increase | 1.22 (1.03–1.45) | 0.0223 |
| At 77 d | |||
| D-dimer | ≥ 2 × ULN vs. < 2 × ULN | 2.26 (1.41–3.64) | 0.0008 |
| IMPROVE score | Per point increase | 1.26 (1.09–1.47) | 0.0026 |
Abbreviations: HR, hazard ratio; ULN, upper limit of normal.
Table 4. Improvement in model performance by the IMPROVEDD VTE risk score.
| Metric | Value | p -Value |
|---|---|---|
| At 42 d | ||
| ΔAUC | 0.061 (0.026–0.097) | 0.0008 |
| IDI | ||
| Absolute | 0.0012 (0.0005–0.0019) | 0.0004 |
| Relative | 1.71 | |
| NRI, continuous | ||
| Overall | 0.346 (0.162–0.530) | 0.0018 |
| Events correctly reclassified | 54% | < 0.0001 |
| Nonevents correctly reclassified | −19% | < 0.0001 |
| NRI, categorical | ||
| Overall | 0.215 (0.111–0.319) | < 0.0001 |
| Events correctly reclassified | −6% | 0.25 |
| Nonevents correctly reclassified | 28% | < 0.0001 |
| At 77 d | ||
| ΔAUC | 0.057 (0.024–0.090) | 0.0006 |
| IDI | ||
| Absolute | 0.0015 (0.0007–0.0023) | 0.0002 |
| Relative | 1.25 | |
| NRI, continuous | ||
| Overall | 0.337 (0.169–0.506) | 0.0010 |
| Events correctly reclassified | 55% | < 0.0001 |
| Nonevents correctly reclassified | −21% | < 0.0001 |
| NRI, categorical | ||
| Overall | 0.125 (0.026–0.225) | 0.0159 |
| Events correctly reclassified | −11% | 0.0278 |
| Nonevents correctly reclassified | 24% | < 0.0001 |
Abbreviations: ΔAUC, improvement in the area under ROC curve; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
Risk Discrimination and Reclassification by the IMPROVEDD Score
Metrics of model discrimination and reclassification are summarized in Tables 4 and 5 . The AUC of the D-dimer, IMPROVE score, and IMPROVEDD score were 0.588, 0.560, and 0.621 at 42 days and 0.584, 0.568, and 0.625 at 77 days, respectively ( Fig. 2 ). Addition of D-dimer to the IMPROVE score significantly improved the risk discrimination and reclassification at 42 and 77 days.
Table 5. Reclassification by the IMPROVEDD VTE risk score a .
| Estimated risk by the IMPROVE score | Estimated risk by the IMPROVEDD score | Total | ||
|---|---|---|---|---|
| < 1% | ≥ 1% to < 2% | ≥ 2% | ||
| At 42 d | ||||
| Overall | ||||
| < 1% | 117 (100.0) | 0 (0.0) | 0 (0.0) | 117 |
| ≥ 1% to < 2% | 2,263 (32.2) | 4,461 (63.5) | 301 (4.3) | 7,025 |
| ≥ 2% | 0 (0.0) | 15 (16.1) | 78 (83.9) | 93 |
| Total | 2,380 | 4,476 | 379 | 7,235 |
| Events | ||||
| < 1% | 1 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| ≥ 1% to < 2% | 12 (15.2) | 60 (75.9) | 7 (8.9) | 79 |
| ≥ 2% | 0 (0.0) | 0 (0.0) | 2 (100.0) | 2 |
| Total | 13 | 60 | 9 | 82 |
| Nonevents | ||||
| < 1% | 116 (100.0) | 0 (0.0) | 0 (0.0) | 116 |
| ≥ 1% to < 2% | 2,251 (32.4) | 4,401 (63.4) | 294 (4.2) | 6,946 |
| ≥ 2% | 0 (0.0) | 15 (16.5) | 76 (83.5) | 91 |
| Total | 2,367 | 4,416 | 370 | 7,153 |
| At 77 d | ||||
| Overall | ||||
| < 1% | 48 (41.0) | 69 (59.0) | 0 (0.0) | 117 |
| ≥ 1% to < 2% | 2,053 (31.3) | 4,077 (62.1) | 431 (6.6) | 6,561 |
| ≥ 2% | 0 (0.0) | 163 (29.3) | 394 (70.7) | 557 |
| Total | 2,101 | 4,309 | 825 | 7,235 |
| Events | ||||
| < 1% | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 |
| ≥ 1% to < 2% | 13 (16.3) | 61 (76.3) | 6 (7.5) | 80 |
| ≥ 2% | 0 (0.0) | 5 (31.3) | 11 (68.8) | 16 |
| Total | 13 | 67 | 17 | 97 |
| Nonevents | ||||
| < 1% | 48 (41.4) | 68 (58.6) | 0 (0.0) | 116 |
| ≥ 1% to < 2% | 2,040 (31.5) | 4,016 (62.0) | 425 (6.6) | 6,481 |
| ≥ 2% | 0 (0.0) | 158 (29.2) | 383 (70.8) | 541 |
| Total | 2,088 | 4,242 | 808 | 7,138 |
Values expressed as number of patients (row percentage).
Fig. 2.
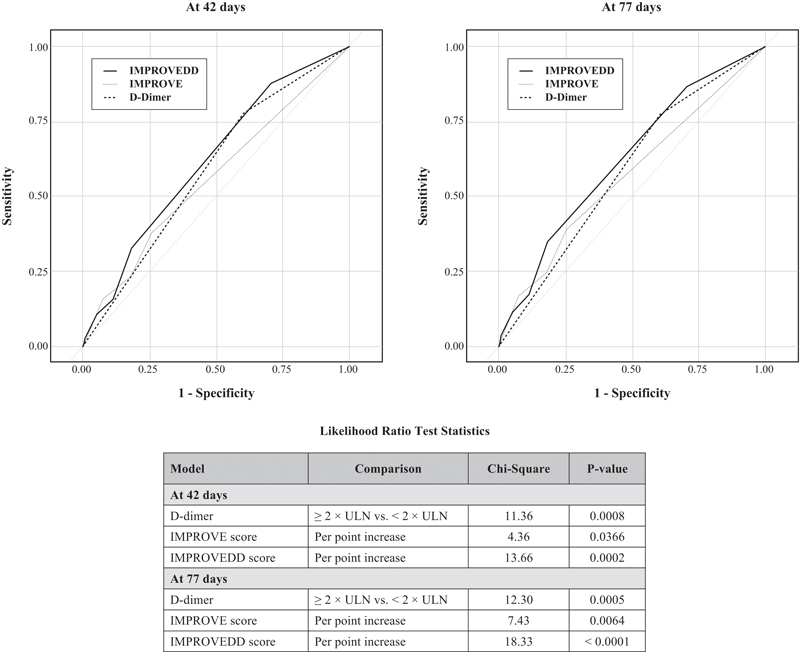
Receiver–operating–characteristic (ROC) curves for D-dimer, IMPROVE, and IMPROVEDD models in predicting symptomatic VTE.
The observed and predicted risk for each IMPROVEDD score category is provided in Table 6 . The rates were generally comparable in the two categories that comprise the majority of patients: IMPROVEDD scores of 1 (28.4%) and 3 (48.7%). Calibration of the IMPROVEDD score was suboptimal in the other categories: overestimation was noted in the score of 0 and ≥ 5, whereas underestimation was noted in the scores of 2 and 4. Decision curves of the IMPROVE and IMPROVEDD scores in VTE prediction at 42 and 77 days were shown in Figs. S1 and S2 (supplementary figures available in the online version only). With the VTE threshold of 1% that corresponds to the cutoff warranting pharmacologic prophylaxis, IMPROVE score is associated with minimal net benefit (0.002 at both 42 and 77 days) against the “treat all” strategy, whereas the use of IMPROVEDD score would avoid undue thromboprophylaxis in 15 and 11 per 100 patients, respectively.
Table 6. Observed and predicted risk by the IMPROVEDD VTE risk score.
| IMPROVEDD score | Patients, n (%) | At 42 d | At 77 d | ||||
|---|---|---|---|---|---|---|---|
| Event, n | Observed risk, % | Predicted risk, % | Event, n | Observed risk, % | Predicted risk, % | ||
| 0 | 48 (0.7) | 0 | 0.0 | 0.4 | 0 | 0.0 | 0.5 |
| 1 | 2,053 (28.4) | 10 | 0.5 | 0.6 | 13 | 0.6 | 0.7 |
| 2 | 279 (3.9) | 3 | 1.1 | 0.8 | 3 | 1.1 | 1.0 |
| 3 | 3,520 (48.7) | 42 | 1.2 | 1.2 | 47 | 1.3 | 1.4 |
| 4 | 510 (7.0) | 14 | 2.7 | 1.6 | 17 | 3.3 | 1.9 |
| ≥ 5 | 825 (11.4) | 13 | 1.6 | 2.2 | 17 | 2.1 | 2.7 |
Identifying At-Risk Patients by the IMPROVEDD Score
The American College of Chest Physicians 2 selected a cutoff corresponding to an event rate of ≥ 1% to dichotomize individuals as at-risk and in need of prophylaxis versus low-risk. Consequently, patients with an IMPROVEDD score of ≥ 2 were deemed as at-risk, whereas those with a score of 0 to 1 were deemed to be at low-risk ( Table 7 ). Compared with low-risk patients, at-risk patients had a higher rate of symptomatic VTE at 42 and 77 days. The Kaplan–Meier rates of symptomatic VTE were 1.11 versus 0.39% at 42 days and 2.22 versus 0.91% at 77 days (HR: 2.73 [95% CI: 1.52–4.90], p = 0.0007; Fig. 3 ).
Table 7. Risk stratification by the IMPROVEDD VTE risk category.
| Risk category | Event rate (95% CI) | Odds ratio (95% CI) | p -Value |
|---|---|---|---|
| At 42 d | |||
| At-risk (≥2 points) | 1.40% (1.08–1.72%) | 2.97 (1.53–5.77) | 0.0002 |
| Low-risk (0–1 points) | 0.48% (0.18–0.77%) | Reference | |
| At 77 d | |||
| At-risk (≥ 2 points) | 1.64% (1.29–1.98%) | 2.67 (1.49–4.80) | 0.0002 |
| Low-risk (0–1 points) | 0.62% (0.28–0.95%) | Reference | |
Fig. 3.
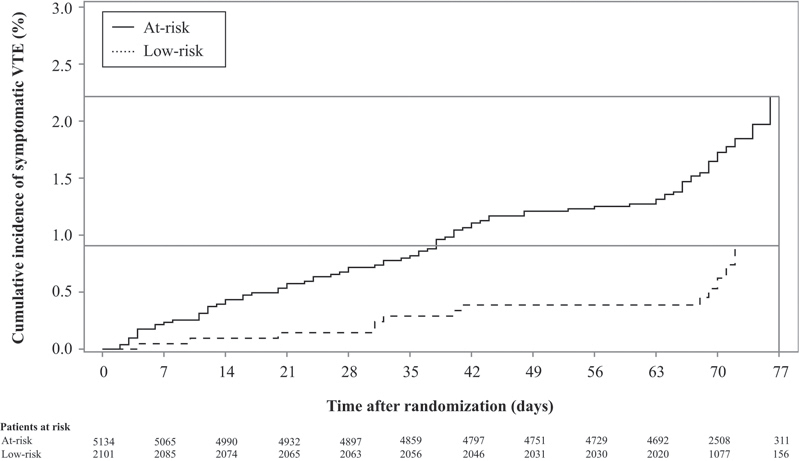
Kaplan–Meier curves for symptomatic VTE stratified by the IMPROVEDD risk category.
Discussion
Incorporation of the quantitative D-dimer level, which increases as overall hypercoagulability increases, provides incremental prognostic value to the IMPROVE VTE RAM at 42 and 77 days. Incorporating D-dimer into the IMPROVE VTE risk score significantly improves VTE risk discrimination and reclassification. An IMPROVEDD score of ≥2 identified a subset of hospitalized, medically ill patients at a sustained, heightened symptomatic VTE risk through 77 days.
VTE risk assessment for hospitalized patients has been associated with reduced morbidity, mortality, and incidence of hospital-acquired thrombosis. 16 17 18 To identify the at-risk population and to guide appropriate thromboprophylaxis among hospitalized patients, several RAMs have been developed using clinical parameters. 12 19 20 21 Research efforts have focused on exploring biomarkers associated with VTE in various populations. D-dimer has been considered as one of the most promising, well-validated, readily available markers for VTE prediction, particularly among cancer patients. 22 D-dimer has also been used in combination with gender and the location of VTE to predict recurrence after discontinuing anticoagulation. 23 This is the first study to demonstrate the complementary nature of combining a biomarker such as D-dimer with clinical variables for the risk stratification of hospitalized, medically ill patients. Results from this study demonstrate that there is a spectrum of thromboembolic risk across the IMPROVEDD score categories, with a higher score indicating a greater VTE risk that persists beyond the course of hospitalization. At-risk patients, as identified by the IMPROVEDD score, may be appropriate candidates for extended thromboprophylaxis to minimize the risk of symptomatic VTE.
With the intent of maintaining generalizability from the IMPROVE derivation cohort, the weights for each VTE risk factor were unchanged, and two points were added to the original score for patients with D-dimer ≥ 2 × ULN. It is notable that adding D-dimer measurement to the IMPROVE score has been implemented in the enrollment criteria of an ongoing trial for preventing hospital-associated VTE (Medically Ill Patient Assessment of Rivaroxaban Versus Placebo in Reducing Post-Discharge Venous Thrombo-Embolism Risk [MARINER]). 24 In the MARINER study, eligible patients (i.e., “high-risk” patients) must have a total modified IMPROVE VTE risk score of ≥4, or a risk score of 2 or 3 with a plasma D-dimer level of more than twice the ULN. The relative weight for D-dimer of ≥ 2 × ULN is equivalent to two points when taken together with the IMPROVE VTE risk score and matches the rationale in this analysis. Furthermore, it supports the use of D-dimer measurement in conjunction with a standardized RAM for optimizing VTE risk assessment among acutely ill, hospitalized medical patients. However, although the incorporation of biomarker may enhance the performance of clinical RAM, it remains uncertain whether this refinement is clinically meaningful to warrant the additional complexity and expense. Future studies are therefore required to evaluate the practicability of IMPROVEDD score in the “real-world” setting.
Limitations
VTE risk factors encompassed in the analyses were not all inclusive. First, other previously described risk factors (e.g., myocardial infarction, chronic obstructive pulmonary disease, chronic kidney disease, and dyslipidemia) and biomarkers (e.g., prothrombin fragment 1 + 2, soluble P-selectin, clotting factor VIII, and thrombin generation potential) may offer additional refinement in risk stratification among acutely ill, hospitalized medical patients. Second, small numbers of events in certain score categories may preclude an accurate calibration of the IMPROVEDD model. Also the lack of patients who were immobilized ≥7 days as a risk factor in the IMPROVE model may have altered the model characteristics. Nonetheless, the cutoff of an IMPROVEDD score of ≥ 2 demonstrated excellent discriminatory capacity in this population. Third, asymptomatic DVT was not included as a component of the outcome in the analysis. Fourth, it should be noted that D-dimer measurement could be influenced by the analytical methods and reporting standards from different laboratories. 25 Finally, the data were derived from a population that agreed to participate in a clinical trial. The results are applicable to patients who received primary prophylaxis with either standard-duration enoxaparin or extended-duration betrixaban and may not be generalizable to other settings.
Conclusion
Baseline D-dimer level demonstrated a robust, incremental prognostic value to the IMPROVE RAM in VTE risk stratification for medical patients. Strategies to improve VTE risk stratification should consider incorporation of D-dimer measurement into standard RAMs. An IMPROVEDD VTE risk score of two or greater identifies a subset of hospitalized, medically ill patients receiving thromboprophylaxis at a sustained, heightened risk for symptomatic VTE through 77 days. This population may potentially benefit from an extended course of thromboprophylaxis. Independent validation and impact analysis should be undertaken before employing the IMPROVEDD VTE risk score in clinical practice.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov . Unique identifier: NCT01583218.
Conflict of Interest None declared.
Funding
The study was funded by Portola Pharmaceuticals, Inc.
Supplementary Material
References
- 1.Jha A K, Larizgoitia I, Audera-Lopez C, Prasopa-Plaizier N, Waters H, Bates D W. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf. 2013;22(10):809–815. doi: 10.1136/bmjqs-2012-001748. [DOI] [PubMed] [Google Scholar]
- 2.Kahn S R, Lim W, Dunn A Set al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012141(2, Suppl):e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Chou R, Humphrey L L, Starkey M, Shekelle P; Clinical Guidelines Committee of the American College of Physicians.Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians Ann Intern Med 201115509625–632. [DOI] [PubMed] [Google Scholar]
- 4.ISTH Steering Committee for World Thrombosis Day.Venous thromboembolism: A Call for risk assessment in all hospitalised patients Thromb Haemost 201611605777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanders S A, Greene M T, Grant P et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584. doi: 10.1001/jamainternmed.2014.3384. [DOI] [PubMed] [Google Scholar]
- 6.Greene M T, Spyropoulos A C, Chopra V et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(09):1.001E12–1.001E21. doi: 10.1016/j.amjmed.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins L, Bara L, Boutitie F et al. Correlation of plasma coagulation parameters with thromboprophylaxis, patient characteristics, and outcome in the MEDENOX study. Arch Pathol Lab Med. 2004;128(05):519–526. doi: 10.5858/2004-128-519-COPCPW. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Li X, Cheng Y, Yao C, Zhong N; Investigators Group.Measurement of D-dimer as aid in risk evaluation of VTE in elderly patients hospitalized for acute illness: a prospective, multicenter study in China Clin Invest Med 20113402E96–E104. [DOI] [PubMed] [Google Scholar]
- 9.Cohen A T, Harrington R, Goldhaber S Z et al. The design and rationale for the Acute Medically Ill Venous Thromboembolism Prevention with Extended Duration Betrixaban (APEX) study. Am Heart J. 2014;167(03):335–341. doi: 10.1016/j.ahj.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Mahan C E, Liu Y, Turpie A G et al. External validation of a risk assessment model for venous thromboembolism in the hospitalised acutely-ill medical patient (VTE-VALOURR) Thromb Haemost. 2014;112(04):692–699. doi: 10.1160/TH14-03-0239. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg D, Eichorn A, Alarcon M, McCullagh L, McGinn T, Spyropoulos A C. External validation of the risk assessment model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for medical patients in a tertiary health system. J Am Heart Assoc. 2014;3(06):e001152. doi: 10.1161/JAHA.114.001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spyropoulos A C, Anderson F A, Jr, FitzGerald G et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(03):706–714. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 13.Cohen A T, Harrington R A, Goldhaber S Z et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375(06):534–544. doi: 10.1056/NEJMoa1601747. [DOI] [PubMed] [Google Scholar]
- 14.Pencina M J, D'Agostino R B, Sr, D'Agostino R B, Jr, Vasan R S.Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond Stat Med 20082702157–172., discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 15.Vickers A J, Elkin E B. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(06):565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lester W, Freemantle N, Begaj I, Ray D, Wood J, Pagano D. Fatal venous thromboembolism associated with hospital admission: a cohort study to assess the impact of a national risk assessment target. Heart. 2013;99(23):1734–1739. doi: 10.1136/heartjnl-2013-304479. [DOI] [PubMed] [Google Scholar]
- 17.Roberts L N, Porter G, Barker R D et al. Comprehensive VTE prevention program incorporating mandatory risk assessment reduces the incidence of hospital-associated thrombosis. Chest. 2013;144(04):1276–1281. doi: 10.1378/chest.13-0267. [DOI] [PubMed] [Google Scholar]
- 18.Catterick D, Hunt B J. Impact of the national venous thromboembolism risk assessment tool in secondary care in England: retrospective population-based database study. Blood Coagul Fibrinolysis. 2014;25(06):571–576. doi: 10.1097/MBC.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbar S, Noventa F, Rossetto V et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 20.Kucher N, Koo S, Quiroz R et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 21.Woller S C, Stevens S M, Jones J P et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–95400. doi: 10.1016/j.amjmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122(12):2011–2018. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 23.Eichinger S, Heinze G, Jandeck L M, Kyrle P A. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121(14):1630–1636. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 24.Raskob G E, Spyropoulos A C, Zrubek J et al. The MARINER trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. Thromb Haemost. 2016;115(06):1240–1248. doi: 10.1160/TH15-09-0756. [DOI] [PubMed] [Google Scholar]
- 25.Lippi G, Tripodi A, Simundic A M, Favaloro E J. International survey on D-dimer test reporting: a call for standardization. Semin Thromb Hemost. 2015;41(03):287–293. doi: 10.1055/s-0035-1549092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


