Abstract
Imatinib, the first-in-class BCR-ABL tyrosine kinase inhibitor (TKI), had been a revolution for the treatment of chronic myeloid leukemia (CML) and had greatly enhanced patient survival. Second- (dasatinib, nilotinib, and bosutinib) and third-generation (ponatinib) TKIs have been developed to be effective against BCR-ABL mutations making imatinib less effective. However, these treatments have been associated with arterial occlusive events. This review gathers clinical data and experiments about the pathophysiology of these arterial occlusive events with BCR-ABL TKIs. Imatinib is associated with very low rates of thrombosis, suggesting a potentially protecting cardiovascular effect of this treatment in patients with BCR-ABL CML. This protective effect might be mediated by decreased platelet secretion and activation, decreased leukocyte recruitment, and anti-inflammatory or antifibrotic effects. Clinical data have guided mechanistic studies toward alteration of platelet functions and atherosclerosis development, which might be secondary to metabolism impairment. Dasatinib, nilotinib, and ponatinib affect endothelial cells and might induce atherogenesis through increased vascular permeability. Nilotinib also impairs platelet functions and induces hyperglycemia and dyslipidemia that might contribute to atherosclerosis development. Description of the pathophysiology of arterial thrombotic events is necessary to implement risk minimization strategies.
Keywords: BCR-ABL, arterial thrombotic events, tyrosine kinase inhibitors, chronic myeloid leukemia
Introduction
In 2001, the approval of imatinib , the first-in-class tyrosine kinase inhibitor (TKI) targeting BCR-ABL, transformed the prognosis of patients with chronic-phase (CP) chronic myeloid leukemia (CML) from a life-threatening condition to a manageable and chronic disease. 1 Yet, despite satisfactory outcomes, 33% of patients did not achieved optimal response because of treatment resistance or intolerance. 1 The identification of the predominant resistance mechanism (i.e., point mutations in the kinase domain of Bcr-Abl) led to the development of second-generation BCR-ABL TKIs (dasatinib, nilotinib, and bosutinib, respectively, approved in 2006, 2007, and 2012) active against most of the BCR-ABL mutated forms. 2 3 Second-generation TKIs demonstrated no or little improvement of the overall survival compared with imatinib, 4 5 6 but two of these (i.e., dasatinib and nilotinib) improve surrogate outcomes and permit quicker and deeper achievement of molecular response, which is criteria to try treatment cessation (i.e., MR 4 or higher molecular response stable for at least 2 years). 7 Based on these results, dasatinib and nilotinib were approved in 2010 for frontline management of CML, whereas bosutinib is used only after failure or intolerance of first-line BCR-ABL TKIs. Unfortunately, these treatments were ineffective against a common mutation (14% of all mutations) in the gatekeeper residue of BCR-ABL (i.e., the T315I a mutation), 8 9 10 requiring the development of a third-generation TKI (ponatinib), efficient against this mutation. Ponatinib is currently the only treatment active against the T315I mutation and is therefore reserved for patients with this mutation or for patients resistant to frontline treatments. 11
Since its approval, the first-generation TKI, imatinib, has demonstrated reassuring safety profile, with low rate of grade 3/4 adverse events and excellent tolerability. 12 13 Conversely, new-generation BCR-ABL TKIs—nilotinib, dasatinib, bosutinib, and ponatinib—are more recent and display different safety profile. Dasatinib, nilotinib, and ponatinib are largely associated with fluid retention and dasatinib specifically induces high rate of pleural effusions. 14 15 16 17 18 Nilotinib induces metabolic disorders such as dyslipidemia and hyperglycemia, whereas bosutinib safety profile is mainly characterized by gastrointestinal events (i.e., diarrhea, nausea, vomiting). 19 20 Finally, ponatinib has been rapidly associated with high rate of vascular occlusion. 21
Recently, meta-analyses of randomized clinical trials established that ponatinib is not the only new-generation TKI that increases the cardiovascular risk. 22 23 The four new-generation BCR-ABL TKIs increase the risk of vascular occlusive events compared with imatinib, especially arterial occlusive diseases, and this is in accordance with clinical trial data. 22 23 24 25 However, this cardiovascular risk is controversy for dasatinib because of the low incidence (1.1 per 100 patient-year) of cardiovascular events in clinical trials. 26 27 Recently, a large retrospective analysis of CP-CML patients treated with BCR-ABL TKIs at the MD Anderson Cancer Center confirmed the increased risk of vascular occlusive events with dasatinib. 28 Another controversial point is the effect of imatinib on the cardiovascular system. Indeed, imatinib is associated with low risk of cardiovascular events and it was therefore hypothesized that imatinib prevents their occurrence. 29 30 Clinical data indicate that most patients developing arterial occlusive events with new-generation BCR-ABL TKIs are high-risk patients, but cardiovascular events also occurred in young and healthy patients. Additional information on clinical safety of BCR-ABL TKIs is described in the Supplementary Material ( Table S1 ). We assume that the mechanism underlying arterial thrombosis with BCR-ABL TKIs might be multiple. The predominance of arterial events raised concerns about the impact of BCR-ABL TKIs on platelet functions, atherosclerosis, and metabolism, and precluded prothrombotic states to be responsible of these events. 31
Table 1. In vitro and ex vivo investigations of the effects of BCR-ABL TKIs on platelet production and functions.
| Endpoints | Methods | Models | TKIs | Findings | Ref. |
|---|---|---|---|---|---|
| Platelet production | Platelet count | Murine whole blood | Dasatinib | Thrombocytopenia platelet production
platelet production
|
33 |
| Flow cytometry (DNA ploidy) Migration assay (Dunn chamber) |
Megakaryocyte primary culture | Dasatinib |
 megakaryocyte differentiation
megakaryocyte differentiation
 megakaryocyte migration
megakaryocyte migration
 proplatelet formation
proplatelet formation
|
33 | |
| Platelet aggregation | Born aggregometry; Light transmission aggregometry | Washed human platelet | Imatinib | = CRP-, collagen- and thrombin-induced platelet aggregation | 38 39 42 |
| Light transmission aggregometry | Human platelet (PRP) | Imatinib |
 ADP-induced platelet aggregation
ADP-induced platelet aggregation
 collagen- and CRP-induced platelet aggregation
collagen- and CRP-induced platelet aggregation
|
34 | |
| Light transmission aggregometry, immunostaining (PAC-1) | Human platelet (PRP); patient blood | Dasatinib |
 ADP-, collagen-, thrombin- and CRP-induced platelet aggregation
ADP-, collagen-, thrombin- and CRP-induced platelet aggregation
|
34 35 38 | |
| Light transmission aggregometry; Born aggregometry | Human platelet (PRP); Washed human platelet | Nilotinib | = platelet aggregation | 34 39 42 | |
| Born aggregometry | Washed human platelet | Ponatinib |
 CRP-induced platelet aggregation
CRP-induced platelet aggregation
= thrombin-induced platelet aggregation |
42 | |
| Platelet activation | Immunostaining (PS) | Washed human platelet | Imatinib | = PS exposure | 42 |
| Western blot | Human platelet lysate | Imatinib | = Src, Lyn, LAT, and BTK activation | 42 | |
| Immunostaining (PS) | Patient blood | Dasatinib |
 PS exposure
PS exposure
|
35 | |
| Immunostaining (PS) | Washed human platelet | Nilotinib | = PS exposure | 42 | |
| Immunostaining (PS) | Patient blood | Nilotinib |
 PS exposure
PS exposure
|
35 | |
| Western blot | Human platelet lysate | Nilotinib | = Src, Lyn, LAT and BTK activation | 42 | |
| Immunostaining (PS) | Patient blood | Bosutinib |
 PS exposure
PS exposure
|
35 | |
| Immunostaining (PS) | Washed human platelet, patient blood | Ponatinib |
 PS exposure
PS exposure
|
35 42 | |
| Western blot | Human platelet lysate | Ponatinib |
 Src, Lyn, LAT and BTK activation
Src, Lyn, LAT and BTK activation
|
42 | |
| Granule release | Immunostaining (P-selectin) | Human platelet | Imatinib |
 thrombin-, PAR-1- and CRP-mediated α-granule release
thrombin-, PAR-1- and CRP-mediated α-granule release
= PAR-4-mediated α-granule release |
34 |
| Immunostaining (P-selectin) | Washed human platelet | Imatinib | = α-granule release | 42 | |
| Immunostaining (P-selectin) | Human platelet | Dasatinib |
 thrombin-, PAR-1-, PAR-4- and CRP-mediated α-granule release
thrombin-, PAR-1-, PAR-4- and CRP-mediated α-granule release
|
34 | |
| Immunostaining (P-selectin) | Washed human platelet | Nilotinib | = PAR-4-, CRP- and thrombin-mediated α-granule release | 34 42 | |
| Immunostaining (P-selectin) | Murine platelet | Nilotinib |
 CRP-, PAR-4- and thrombin-mediated α-granule release
CRP-, PAR-4- and thrombin-mediated α-granule release
|
34 | |
| Immunostaining (P-selectin) | Human platelet | Nilotinib |
 PAR-1-mediated α-granule release
PAR-1-mediated α-granule release
|
34 | |
| Immunostaining (P-selectin) | Washed human platelet | Ponatinib |
 α-granule release
α-granule release
|
42 | |
| Platelet spreading | Microscopy (platelet spreading) | Washed human platelet | Imatinib | = platelet spreading and lamellipodia formation | 42 |
| Microscopy (platelet spreading) | Washed human platelet | Nilotinib | = platelet spreading and lamellipodia formation | 42 | |
| Microscopy (platelet spreading) | Washed human platelet | Ponatinib |
 platelet spreading and lamellipodia formation
platelet spreading and lamellipodia formation
|
42 | |
| Thrombus formation | In vitro flow study, PFA-100 | Human blood, murine whole blood | Imatinib | = platelet deposition and thrombus volume = closure time |
34 36 44 |
| Ex vivo and in vitro flow study | Murine whole blood, human whole blood | Imatinib |
 thrombus volume and aggregate formation
thrombus volume and aggregate formation
|
34 42 | |
| In vitro and ex vivo flow study | Human blood, murine whole blood, patient whole blood | Dasatinib |
 thrombus volume and platelet deposition
thrombus volume and platelet deposition
|
34 35 36 | |
| PFA-100 | Human whole blood | Dasatinib |
 closure time (collagen/epinephrine activation)
closure time (collagen/epinephrine activation)
= closure time (collagen/ADP activation) |
44 | |
| Ex vivo flow study | Murine whole blood, patient whole blood | Nilotinib |
 thrombus volume (growth and stability)
thrombus volume (growth and stability)
|
34 | |
| In vitro flow study | Human whole blood, murine whole blood | Nilotinib | = platelet deposition and thrombus volume | 34 36 42 | |
| In vitro flow study | Human blood | Bosutinib |
 platelet deposition (late)
platelet deposition (late)
|
36 | |
| PFA-100 | Patient blood | Ponatinib |
 closure time
closure time
|
41 | |
| In vitro flow study | Human whole blood | Ponatinib |
 aggregate formation
aggregate formation
|
42 |
Abbreviations: ADP, adenosine diphosphate; BTK, Bruton's tyrosine kinase; CRP, C-reactive protein; DNA, deoxyribonucleic acid; LAT, linker for activation of T-cells; PAR, protease-activated receptor; PFA, platelet function assay; PRP, platelet-rich plasma; PS, phosphatidyl serine.
This review particularly focuses on the contribution of glucose and lipid metabolism, atherosclerosis, and platelets in the occurrence of cardiovascular events with new-generation TKIs. The last section discusses relevant off-targets that might be implicated in the cardiovascular toxicity. The discovery of the mechanism(s) by which arterial occlusive events arose in CML patients would help in the management of patients treated with BCR-ABL TKIs and implement risk minimization measures. Discovery of the pathophysiology of these events in CML patients might also led to the development of predictive biomarkers or to the development of new therapies with no or reduced cardiovascular toxicity profile while keeping an unaltered efficacy.
Impact on Platelet Functions
BCR-ABL TKIs are associated with both bleeding and thrombotic complications. Table 1 describes experiments assessing the impact of BCR-ABL TKIs on platelet production and functions. Imatinib and dasatinib induce hemorrhagic events in patients with CML. Interestingly, dasatinib-associated hemorrhages occurred both in patients with and without thrombocytopenia. 32 In vitro and in vivo investigations demonstrated that dasatinib affects both platelet functions (i.e., platelet aggregation, secretion, and activation) and platelet formation by impairment of megakaryocyte migration. 33 34 35 36 Furthermore, dasatinib decreases thrombus formation in vitro, in vivo, and ex vivo, 34 and decreases the number of procoagulant platelets (i.e., phosphatidylserine-exposing platelets). 35 Several dasatinib off-targets are implicated in platelet signaling and functions including members of the SFKs (e.g., Src, Lyn, Fyn, Lck, and Yes) ( Fig. 1 ). 37 38 However, SFKs are also inhibited by bosutinib without disturbance of platelet aggregation and adhesion. Dasatinib also inhibits Syk, BTK, and members of the ephrin family b (e.g., EphA2), all known to be involved in platelet functions.
Fig. 1.
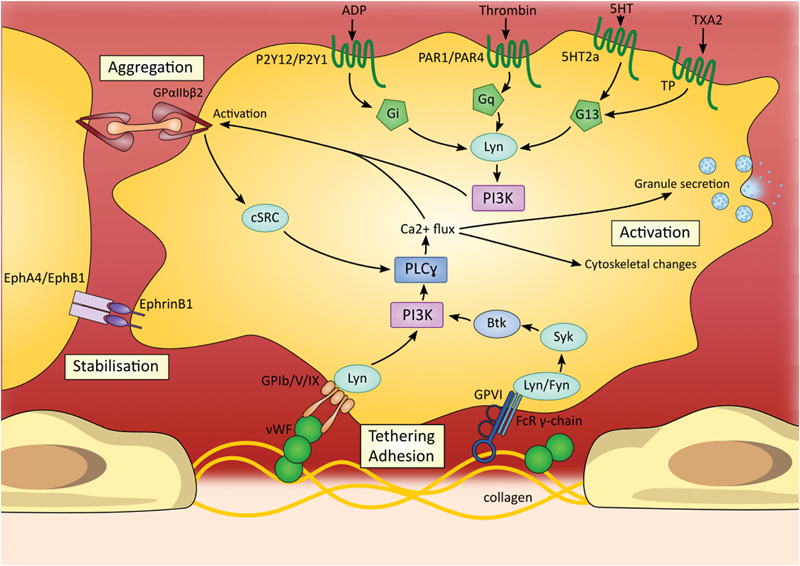
Signaling pathways supporting platelet adhesion, activation, and aggregation. Tyrosine kinases are involved in several pathways and contribute to platelet adhesion, aggregation, and activation. Important players in platelet signaling are members of the Src family kinases; particularly Lyn, Fyn, and cSRC. These three tyrosine kinases are inhibited by dasatinib which might explain platelet dysfunction encountered with this treatment. Additionally, dasatinib also inhibits BTK, Syk, EphA4, and EphB1—four tyrosine kinases involved in platelet activation and aggregate stabilization. 5HT, 5-hydroxytryptamine; ADP, adenosine diphosphate; Btk, Bruton's tyrosine kinase; Ca, calcium; Eph, ephrin; FcR, Fc receptor; GP, glycoprotein; PAR, protease-activated receptor; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; TXA2, thromboxane A2; vWF, Von Willebrand factor.
Experimental assessments of platelet functions with imatinib demonstrate less pronounced effects on platelets. Imatinib inhibits platelet aggregation only at high doses, 34 and does not interfere with platelet aggregation in vivo. 39 However, in vitro studies also indicate decreased platelet secretion and activation by imatinib. 34 The mechanism by which imatinib inhibits platelet functions is unknown. Oppositely to dasatinib, imatinib does not inhibit SFKs, ephrins, BTK, and Syk. A hypothesis also suggests that imatinib induces bleeding disorders because of BCR-ABL rearrangements in megakaryocytic cell lines, leading to clonal expansion of dysfunctional megakaryocytes. 40
Even if ponatinib induces very few bleeding disorders, assessment of primary hemostasis in CML patients demonstrated that ponatinib induces defect in platelet aggregation. This impairment was found at all ponatinib dosage, in patients with or without low platelet counts. 41 These results were in accordance with in vitro studies which previously demonstrated similar characteristics than dasatinib (i.e., decrease of platelet spreading, aggregation, P-selectin secretion, and phosphatidylserine exposure). 35 42 However, in vitro assays tested ponatinib at 1 µM, a dose far higher than the concentration observed in patients on treatment. 43 Nilotinib and bosutinib are not associated with bleeding disorders in CML patients. First in vitro studies demonstrated little or no effect on platelet aggregation and activation with these two TKIs. 36 39 44 However, recent experiments described prothrombotic phenotype of platelets induced by nilotinib, with increase of PAR-1 c –mediated platelet secretion, adhesion, and activation, without disturbing platelet aggregation. 34 Additional studies demonstrated that nilotinib increases secretion of adhesive molecules as well as thrombus formation and stability ex vivo. 34
To summarize, dasatinib and imatinib induce hemorrhagic events through alteration of platelet functions, but the molecular mechanism needs to be better determined. Ponatinib also impairs platelet functions. Therefore, no current data involve platelets in the pathogenesis of arterial thrombosis occurring with dasatinib and ponatinib. Oppositely, nilotinib might induce arterial thrombosis through alteration of platelet secretion, adhesion, and activation.
Metabolic Dysregulation
Glucose Metabolism
BCR-ABL TKIs have contradictory effect on glucose metabolism. Imatinib and dasatinib improve glucose metabolism and type 2 diabetes management in CML patients (i.e., decrease of antidiabetic drug dosage and reversal of type 2 diabetes). 14 45 46 47 48 49 This clinical profile is in accordance with in vivo studies in which imatinib is effective to prevent the development of type 1 diabetes in prediabetic mice, without impacting the adaptive immune system. 50 Therefore, imatinib is currently tested in clinical trials for patients suffering from type 1 diabetes mellitus (NCT01781975). The mechanism(s) by which dasatinib and imatinib improve glucose metabolism remains unknown. Global hypotheses suggest that imatinib increases peripheral insulin sensitivity, promotes β-cell survival, or decreases hepatic glucose production ( Fig. 2 ). 51 52 53 54 This latter hypothesis (i.e., decreased hepatic glucose production by imatinib) is not currently the preferred theory, whereas it was demonstrated that imatinib weakly affects hepatic glucose production. 51 Several targets might be involved in this metabolic effect. PDGFR has already been linked with type 1 diabetes reversal. 50 Hägerkvist et al hypothesized that c-Abl inhibition by imatinib promotes β-cell survival through activation of NF-κB signaling and inhibition of proapoptotic pathways ( Fig. 2 ). 53 54 Inhibition of c-Abl in β-cells might also increase insulin production and contribute to the glucose regulation by imatinib. 55 It was also speculated that imatinib decreases insulin resistance in peripheral tissues due to c-Abl-dependent JNK inactivation. d 51 Similar hypotheses might be translated to dasatinib because of the similar off-target inhibitory profile (i.e., dasatinib also inhibits c-Abl and PDGFR). It was hypothesized that imatinib and dasatinib impact glucose metabolism through reduced adipose mass. 51 56 However, clinical data do not demonstrate weight loss in CML patients and do not favor this hypothesis. In both imatinib- and dasatinib-treated patients, increased circulating adiponectin e level correlates with decreased insulin resistance. 57 58 This correlation might be explained by the translocation of the glucose transporter GLUT4 f from the cytoplasm to the cell membrane following adiponectin signaling. 59 Additionally, adiponectin has been related to decreased hepatic glucose production which could be an additional mechanism by which imatinib and dasatinib improve glucose metabolism. 60 It was speculated that the raise of adiponectin level with imatinib and dasatinib is the consequence of increased adipogenesis subsequent to PDGFR inhibition. 61
Fig. 2.
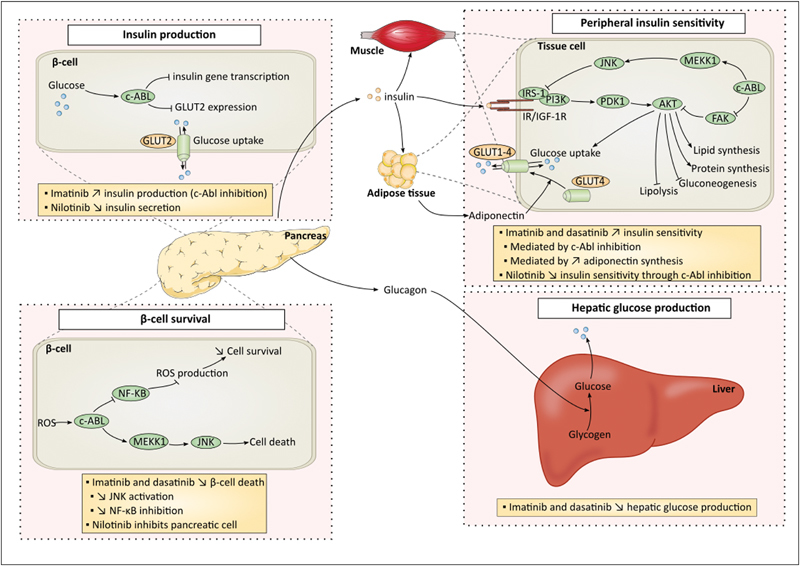
Effects of BCR-ABL TKIs on glucose metabolism. Imatinib and dasatinib possess hypoglycemic effects, whereas nilotinib increases blood glucose level and diabetes development. The figure describes glucose metabolism and boxes contain emitted hypotheses for effects of imatinib, dasatinib, and nilotinib on glucose metabolism. Four major hypotheses have been emitted including impact on insulin production by β-cells, β-cell survival, peripheral insulin sensitivity, and hepatic glucose production. ABL, Abelson; FAK, focal adhesion kinase; GLUT, glucose transporter; IRS-1, insulin receptor substrate 1; JNK, c-Jun N-terminal kinases; MEKK1, MAPK/ERK kinase kinase 1; NF-κB, nuclear factor-kappa B; PDK1, pyruvate dehydrogenase kinase 1; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species.
Oppositely to imatinib and dasatinib, case reports and clinical trials indicate that nilotinib increases blood glucose level and promotes diabetes mellitus. 62 63 64 65 Indeed, 20% of nilotinib-treated patients developed diabetes after 3 years of treatment, 65 whereas 29% of patients suffer from increase of fasting glucose after 1 year of therapy. 64 However, no variations of glycated hemoglobin were reported. 64 65 Clinical data indicate no direct effect of nilotinib on β-cells, but suggest fasting insulin increase, fasting C-peptide decrease, and an increase of HOMA-IR values (i.e., a model to assess insulin resistance). 64 66 67 Therefore, the preferred hypothesis to explain the development of hyperglycemia is the manifestation of insulin resistance. Weakened insulin secretion occurred sometimes, but it is likely that this impairment is the consequence of β-cell exhaustion. 68 However, in vitro experiments demonstrated inhibitory effect of nilotinib on pancreatic cell growth. 69 Breccia et al proposed an additional hypothesis linking development of hyperglycemia and body mass index. They suggested that the development of hyperglycemia might be the consequence of increase fat level tissue resulting in decrease peripheral insulin sensitivity. 70 However, dietetic measures to restrict glucose exogenous uptake in patients who developed hyperglycemia were not successful, 63 and nilotinib does not induce changes in patient body weight. 71 Little is known regarding the mechanism by which nilotinib induces insulin resistance. Racil et al suggested that peripheral insulin resistance is mediated by c-Abl inhibition which is involved in insulin receptor signaling ( Fig. 2 ). 67 This hypothesis is contrary to the hypothesis described with dasatinib and imatinib in which c-Abl enhances insulin sensitivity through c-Abl inhibition. These two hypotheses describe different pathways involving c-Abl but with opposite outcomes. To date, no hypothesis is preferred and additional studies are required to understand the opposite effect on glucose metabolism between TKIs, whereas both have been attributed to c-Abl inhibition. Interestingly, Frasca et al described opposite role of c-Abl in insulin signaling depending on the receptor involved, the signaling pathway, and the cell context. 72 Similar investigations should be performed in the context of c-Abl inhibition by BCR-ABL TKIs. For bosutinib and ponatinib, little is known regarding their impact on glucose metabolism, but no drastic changes in glucose profile has been reported during clinical trials.
Lipid Metabolism
Similarly with glucose metabolism, effects on lipid metabolism are conflicting between TKIs. Oppositely to in vivo study which demonstrated no impact of imatinib on total cholesterol and triglycerides levels in diabetic mice, 29 imatinib is associated in CML patients with a rapid and progressive decrease of cholesterol and triglycerides levels. 66 73 74 75 First hypothesis relates the inhibition of PDGFR by imatinib ( Fig. 3 ). PDGFR is involved in the synthesis of the lipoprotein lipase (LPL) and in the regulation of the lipoprotein receptor-related protein (LRP). 73 74 However, all BCR-ABL TKIs possess inhibitory activity against PDGFR but do not share this positive impact on lipid profile. Recently, Ellis et al described that imatinib impairs gene expression of proteins involved in plasma lipid regulation. Indeed, in in vitro model of CML cells, imatinib affects gene expression of four genes implicated in lipid synthesis (HMG-CoA reductase g gene and apobec1 h ), lipid clearance (LDLR gene i ) and in exchange of lipids from very low-density lipoprotein (VLDL) or low-density lipoprotein (LDL) to high-density lipoprotein (HDL) (CETP j gene). However, these studies were performed in a model of CML cells and need to be confirmed in more relevant models (e.g., primary cell lines, hepatocytes). 76 Franceschino et al suggested that imatinib decreases diarrhea-related lipid absorption due to inhibition of c-kit in interstitial Cajal cells (i.e., c-kit signaling is critical for the survival and development of these cells). 73 However, this hypothesis is unlikely, few patients (3.3%) developed grade 3/4 diarrhea, and patients treated with interferon-α and cytarabine developed diarrhea at a same rate and do not present lipid level reduction in the phase 3 clinical trial (NCT00333840).
Fig. 3.
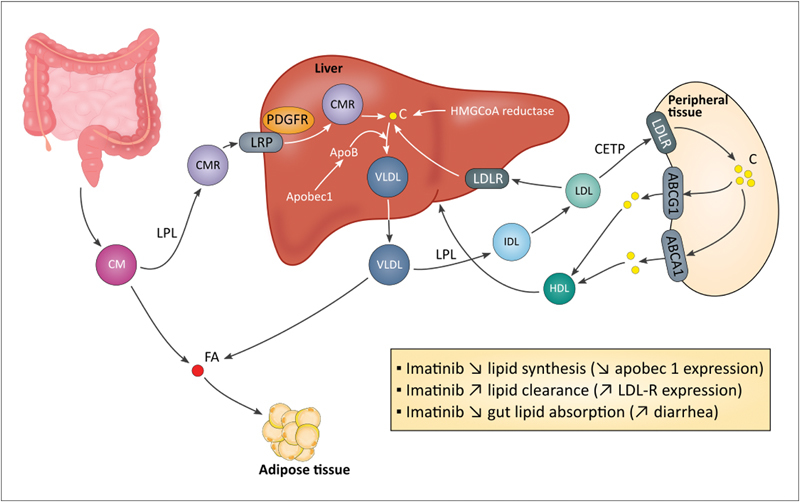
Effects of BCR-ABL TKIs on lipid metabolism. Several hypotheses have been emitted to explain the imatinib-induced hypolipidemic effect. Imatinib regulates expression of genes involved in lipid metabolism: Apobec1 that regulates ApoB expression through the introduction of a stop codon into ApoB mRNA (ApoB is essential for VLDL production), and LDLR that is implicated in lipid clearance. Imatinib-induced PDGFR inhibition influences LPL synthesis and dysregulates LRP. Dasatinib and nilotinib increase cholesterol plasma level through an unknown mechanism. Global hypotheses can be emitted and include increased hepatic lipid synthesis (possibly related to hyperinsulinemia) and decreased lipid clearance through LDLR functional defect or decreased LPL synthesis. ABC, ATP-binding cassette; C, cholesterol; CETP, cholesteryl ester transfer protein; CM, chylomicron; FA, fatty acid; HMGCoA reductase, hydroxymethylglutaryl-CoA reductase; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; LRP, lipoprotein receptor-related protein; PDGFR, platelet-derived growth factor receptor; VLDL, very low-density lipoprotein.
Oppositely, dasatinib and mostly nilotinib are associated with an increase of cholesterol level. 26 66 77 Nilotinib induces quick rise of total cholesterol, HDL, and LDL (i.e., within 3 months). Nilotinib-induced dyslipidemia are responsive to statin and lipid level normalized after nilotinib discontinuation. 78 To date, the mechanism by which dasatinib and nilotinib impact lipid metabolism is unknown. Future researches should determine how these treatments induce dyslipidemia. Global hypotheses could be formulated and include an increase of lipid synthesis that might be secondary to insulin resistance and hyperinsulinemia. This hypothesis is particularly relevant with nilotinib and it is also associated with hyperglycemia. Dasatinib and nilotinib might also decrease blood lipid clearance (e.g., disturbance of LDLR and LPL synthesis). The development of dyslipidemia might contribute to the occurrence of arterial occlusive events that occurred with nilotinib and dasatinib. However, the relationship between impaired lipid metabolism and cardiovascular occlusive events is unknown with BCR-ABL TKIs, and there is no indication that correct management of lipid metabolism can prevent arterial thrombosis (e.g., stenosis occurred in a nilotinib-treated patient despite the management of its hyperlipidemia through statin treatment). 79 On their side, bosutinib and ponatinib do not disturb lipid metabolism. 78 80
Effects on Atherosclerosis
Endothelial Dysfunction
Fig. S1 in the Supplementary Material details the role of endothelial cells (ECs) in atherosclerosis. Several in vitro and in vivo experiments assess the impact of imatinib on EC viability and functions ( Table 2 ). These studies demonstrate that imatinib does not affect EC viability nor induce apoptosis but increases EC proliferation. 39 81 82 83 84 Only one study reports a proapoptotic effect of imatinib on ECs, but their experiments were performed on a cell line (i.e., EA.hy926 cells), 85 a model less reliable than primary cultures (e.g., HUVEC, k HCAEC l ). In vitro studies also assessed the effect of imatinib on EC functions. In these studies, imatinib does not influence adhesion molecule expressions (i.e., ICAM-1 m and VCAM-1 n ), EC migration, reactive oxygen species (ROS) production, nor angiogenesis. 81 82 85 86 87 Letsiou et al suggested that imatinib decreases EC inflammation by decreasing the secretion of proinflammatory mediators. 86 The impact of imatinib on endothelial permeability is not clear. Indeed, in vitro studies demonstrate that imatinib increases endothelial permeability by decreasing the level of plasma membrane VE-cadherin, o 85 86 whereas in vivo experiments indicate decreased vascular leak following imatinib treatment in a murine model of acute lung injury. 88 Additionally, imatinib has been tested in patients suffering from acute lung injury, a disease characterized by vascular leakage, and demonstrate promising clinical efficacy. Therefore, imatinib might positively affect atherogenesis by decreasing endothelial inflammation and reducing vascular leakage.
Table 2. In vivo and in vitro investigations of the effects of BCR-ABL TKIs on endothelial cell viability and major functions.
| Endpoints | Methods | Models | TKIs | Findings | Ref. |
|---|---|---|---|---|---|
| EC proliferation/survival | Cell counting; trypan blue staining | EA.hy 926 cell; HCAEC | Imatinib | = EC viability <10µM | 84 85 |
| Caspase assay; Annexin V staining; Hoechst staining; TUNEL assay | HMEC-1; HUVEC; Human pulmonary EC; Mouse EC | Imatinib | = EC apoptosis | 81 82 87 | |
| TUNEL assay; Annexin V staining | EA.hy 926 cell | Imatinib |
 EC apoptosis
EC apoptosis
|
85 | |
| MTT cell proliferation assay; 3 H-thymidine incorporation; WST-1 assay; cell counting | HMEC-1; HUVEC; HCAEC | Imatinib | = EC proliferation | 39 81 82 84 | |
| Resazurin proliferation assay; PCNA expression | HUVEC; BAEC | Imatinib |
 EC proliferation (≥1.2 µM)
EC proliferation (≥1.2 µM)
|
83 | |
| Caspase assay; Hoechst staining; Annexin V staining; TUNEL assay | Human pulmonary EC | Dasatinib |
 EC apoptosis
EC apoptosis
|
87 | |
| 3 H-thymidine incorporation; WST-1 assay; MTT assay | HUVEC; HCAEC; HMEC-1; HCtAEC | Nilotinib |
 EC proliferation
EC proliferation
|
39 82 89 | |
| Annexin V staining | HUVEC | Nilotinib | = EC apoptosis | 82 | |
| Caspase assay; Annexin V staining | HCAEC; HUVEC | Ponatinib |
 EC apoptosis
EC apoptosis
|
82 90 | |
| 3 H-thymidine incorporation; WST-1 assay | HUVEC; HMEC-1; EPC | Ponatinib |
 EC proliferation
EC proliferation
|
82 90 | |
| Oxidative stress | Fluorescent ROS detection; Immunofluorescence (8-oxo-dG) | Human Pulmonary EC; Rat lung | Imatinib | = endothelial ROS | 87 |
| Fluorescent ROS detection; Immunofluorescence (8-oxo-dG) | Human Pulmonary EC; Rat lung | Dasatinib |
 endothelial ROS
endothelial ROS
|
87 | |
| EC migration | Wound scratch assay; Microchemotaxis assay; Transwell migration assay | HMEC-1; HUVEC; EA.hy 926 cell; HCAEC | Imatinib | = EC migration | 81 82 84 85 |
| Wound scratch assay | HUVEC; HCAEC; HMEC-1 | Nilotinib |
 EC migration
EC migration
|
39 | |
| Transwell migration assay | HUVEC | Nilotinib | = EC migration | 82 | |
| Transwell migration assay | HUVEC | Ponatinib |
 EC migration
EC migration
|
82 | |
| Angiogenesis | Tube-formation assay | HMEC-1; HUVEC | Imatinib | = angiogenesis | 81 82 |
| Tube-formation assay | HUVEC; HCAEC; HMEC-1 | Nilotinib |
 angiogenesis
angiogenesis
|
39 | |
| Tube-formation assay | HUVEC | Nilotinib | = angiogenesis | 82 | |
| Tube-formation assay | HUVEC | Ponatinib |
 angiogenesis
angiogenesis
|
82 | |
| Permeability | Permeability to albumin | EA.hy 926 cell | Imatinib |
 endothelial permeability (10 µM)
endothelial permeability (10 µM)
|
85 |
| Immunofluorescence (VE-cadherin) | EA.hy 926 cell; HPAEC | Imatinib |
 membrane VE-cadherin (10 µM)
membrane VE-cadherin (10 µM)
|
85 86 | |
| BAL protein levels | Mice (2-hit model of ALI) | Imatinib |
 BAL protein levels
BAL protein levels
|
86 88 | |
| Permeability to FITC-Dextran; permeability to HRP | HMEC-1; HUVEC; Human lung microvascular EC | Imatinib | = endothelial permeability | 94 147 | |
| Immunostaining | HUVEC | Imatinib |
 intercellular gaps
intercellular gaps
|
147 | |
| Evans blue/albumin extravasation | Mice | Imatinib |
 Evans blue extravasation
Evans blue extravasation
|
147 | |
| Pulmonary microvascular permeability assay; permeability assay (FITC-Dextran) | Mice; HMEC-1; HPAEC | Dasatinib |
 endothelial permeability
endothelial permeability
|
94 | |
| Permeability assay (FITC-Dextran) | HRMEC | Dasatinib |
 VEGF-induced permeability
VEGF-induced permeability
|
148 | |
| CAM expression | Confocal microscopy; ELISA; qRT-PCR; flow cytometry | HMEC-1; Pulmonary EC (rat lung); EA.hy926 | Imatinib | = ICAM-1, VCAM-1 and E-selectin expression = soluble ICAM-1, VCAM-1 and E-selectin |
81 87 149 |
| Immunoblotting (VCAM-1) | Human lung EC | Imatinib |
 VCAM-1 expression
VCAM-1 expression
|
86 | |
| Confocal microscopy | Pulmonary EC (rat lung) | Dasatinib |
 ICAM-1, VCAM-1 and E-selectin expression
ICAM-1, VCAM-1 and E-selectin expression
|
87 | |
| ELISA | Rat | Dasatinib |
 soluble ICAM-1, VCAM-1 and E-selectin
soluble ICAM-1, VCAM-1 and E-selectin
|
87 | |
| qRT-PCR; flow cytometry | EA.hy926 | Dasatinib | = ICAM-1, VCAM-1 and E-selectin expression | 149 | |
| Unknown | HUVEC | Nilotinib |
 ICAM-1, VCAM-1 and E-selectin expression (≥1 µM)
ICAM-1, VCAM-1 and E-selectin expression (≥1 µM)
|
39 | |
| qRT-PCR; flow cytometry | EA.hy926 | Nilotinib |
 ICAM-1, VCAM-1 and E-selectin expression
ICAM-1, VCAM-1 and E-selectin expression
|
149 | |
| Secretory | ELISA (IL-6; IL-8) | Stimulated HPAEC | Imatinib |
 IL-8 and IL-6 (LPS induced)
IL-8 and IL-6 (LPS induced)
|
86 |
| qRT-PCR ; ELISA (IL-1β; IL-6; TNF-α) | EA.hy926 cell ; HUVEC | Imatinib | = IL-1β, IL-6 and TNF-α expression and production | 149 | |
| qRT-PCR ; ELISA (IL-1β; IL-6; TNF-α) | EA.hy926 cell ; HUVEC | Dasatinib | = IL-1β, IL-6 and TNF-α expression and production | 149 | |
| qRT-PCR ; ELISA (IL-1β; IL-6; TNF-α) | EA.hy926 cell ; HUVEC | Nilotinib | = IL-6 and TNF-α expression and production IL-1β expression and production
IL-1β expression and production
|
149 | |
| ELISA (t-PA; PAI-1; ET-1; vWF; total NO) | HCtAEC | Nilotinib |
 t-PA
t-PA
 PAI-1, ET-1, vWF and total NO
PAI-1, ET-1, vWF and total NO
|
89 | |
| Adhesion | Unknown | HUVEC | Ponatinib |
 adhesion to plastic surface at 1 µM
adhesion to plastic surface at 1 µM
|
90 |
Abbreviations: 8-oxo-dG, 8-hydroxy-2′-deoxyguanosine; ALI, acute lung injury; BAEC, bovine aortic endothelial cell; BAL, bronchoalveolar level ; EC, endothelial cell; ELISA, enzyme-linked immunosorbent assay; EPC, endothelial progenitor cell; ET-1, endothelin 1; FITC, fluorescein isothiocyanate; HCAEC, human coronary artery endothelial cell; HCtAEC, human carotid artery endothelial cell; HMEC-1, human microvascular endothelial cell; HPAEC, human pulmonary artery endothelial cell; HRMEC, human retinal microvascular endothelial cells; HUVEC, human umbilical vein endothelial cell; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; LPS, lipopolysaccharide; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; ROS, reactive oxygen species; t-PA, tissue plasminogen activator; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; VCAM-1, vascular cell adhesion molecule 1; VE-cadherin, vascular endothelial cadherin; vWF, Von Willebrand factor.
Nilotinib and ponatinib reduce EC proliferation and might impaired endothelial regeneration. 39 82 89 90 Additionally, ponatinib induces EC apoptosis, although it is well recognized that high glucose concentration induces EC death, 91 suggesting that nilotinib might, by this intermediary, affect EC viability. Moreover, clinical data indicate that dasatinib induces pulmonary arterial hypertension, whereas imatinib is possibly beneficial in this disease. 92 93 This pathology is initiated by dysfunction or injury of pulmonary ECs. 87 Therefore, in vivo and in vitro studies investigated effect of imatinib and dasatinib on pulmonary ECs and demonstrate that dasatinib induces apoptosis on pulmonary ECs mediated by increased mitochondrial ROS production. 87 Future researches should assess if this effect is also found in arterial ECs and ROS production should also be tested with other new-generation BCR-ABL TKIs.
In addition to their effect on EC viability, nilotinib and ponatinib also influence EC functions, inhibit their migration, and decrease angiogenesis. 39 82 It was suggested that the antiangiogenic effect of ponatinib is the consequence of VEGFR p inhibition, but this hypothesis cannot explain the antiangiogenic effect of nilotinib (i.e., nilotinib does not inhibit VEGFR). 82 Nilotinib also increases adhesion molecule expressions (i.e., ICAM-1, VCAM-1, and E-selectin) in vitro, 39 suggesting that nilotinib might increase leukocyte recruitment. However, further experiments are needed to validate this hypothesis (e.g., assessment of endothelium permeability and transendothelial migration). Dasatinib also induces endothelium leakage in vitro, and the RhoA-ROCK q pathway is involved in this phenomenon. 94 It was demonstrated that RhoA activation induces the phosphorylation of myosin light chain that increases the actomyosin contractibility and disrupt endothelial barrier. 94 Therefore, increased endothelium permeability is a potential mechanism by which dasatinib and nilotinib promote atherosclerosis development and arterial thrombosis. Likewise, it is plausible that ponatinib affects endothelium integrity because of its inhibitory activity against VEGFR, which is recognized as a permeability-inducing agent. Additional hypotheses suggest that inhibition of Abl kinase (i.e., Arg r and c-Abl) and PDGFR might also be implicated in vascular leakage. 85 Finally, Guignabert et al demonstrated that both in rats and in CML patients taking dasatinib, there is an increase of soluble adhesion molecules, which are well-known markers of endothelial dysfunction. 87
Inflammation
Fig. S2 in the Supplementary Material describes the role of immune cells and inflammation process during atherosclerosis. Table 3 summarizes in vitro studies that investigate impacts of BCR-ABL TKIs on survival, proliferation, and major functions of monocytes, macrophages, and T-lymphocytes. Globally, in vitro studies demonstrate that imatinib inhibits the development and maturation of monocytes and alters monocyte functions. 95 96 Imatinib decreases production of proinflammatory cytokines (i.e., TNF-α s and IL-6 t ) and diminishes the potential of monocytes to phagocytose. 97 98 These impacts on monocyte functions are possibly related to c-fms u inhibition. 99 Imatinib also inhibits macrophage functions in vitro. Imatinib decreases lipid uptake without impacting the lipid efflux and decreases activity and secretion of two matrix metalloproteinases (MMPs; i.e., MMP-2 and MMP-9 v ) on a posttranscriptional level. 100 Additionally, imatinib inhibits T-lymphocyte activation and proliferation and decreases proinflammatory cytokines secretion (i.e., IFN-γ w ). 101 The inhibition of monocyte, macrophage, and T-cell functions by imatinib might prevent the development of atherosclerosis or reduce the risk of atherosclerotic plaque rupture.
Table 3. In vitro studies on effects of BCR-ABL TKIs on proliferation, survival, and major functions of monocytes, macrophages, and T-lymphocytes.
| Endpoints | Methods | Models | TKIs | Findings | Ref. |
|---|---|---|---|---|---|
| Monocytes/Macrophages | |||||
| Proliferation/survival | Propidium iodide staining | PBMC | Imatinib | = viability | 150 |
| Cell counting | Ovarian tumor ascites samples | Imatinib |
 macrophage production
macrophage production
|
96 | |
| Cell counting | Ovarian tumor ascites samples | Dasatinib |
 macrophage production
macrophage production
|
96 | |
| WST-1 assay | Human macrophages | Ponatinib | = macrophage viability | 82 | |
| Monocyte differentiation | Morphology assessment | Human monocyte | Imatinib |
 differentiation into macrophages
differentiation into macrophages
|
95 |
| Secretion | ELISA; qPCR | Human monocyte and macrophage; PBMC | Imatinib |
 TNF-α, IL-6 and IL-8 production
TNF-α, IL-6 and IL-8 production
|
97 150 |
| ELISA | PBMC; Human monocyte and macrophage | Imatinib | = IL-10 production | 150 | |
| ELISA; Bioplex system; nitrite assay | Raw 264.7; bone-marrow derived macrophage | Dasatinib |
 TNF-α, IL-6, IL-12p40 and NO production
TNF-α, IL-6, IL-12p40 and NO production
|
103 151 | |
| qPCR; Bioplex system | Primary macrophage (mice) | Dasatinib |
 IL-10 production
IL-10 production
|
103 | |
| Bioplex system | Bone-marrow derived macrophage | Bosutinib |
 IL-6, IL-12p40 and TNF-α production
IL-6, IL-12p40 and TNF-α production
|
103 | |
| qPCR; Bioplex system | Primary macrophage (mice) | Bosutinib |
 IL-10 production
IL-10 production
|
103 | |
| Phagocytosis | Antigen-uptake assay | Human monocyte | Imatinib |
 phagocytosis
phagocytosis
|
97 |
| Cholesterol uptake | Cholesterol uptake assay | THP-1; PBMC | Imatinib |
 LDL uptake
LDL uptake
|
100 |
| Cholesterol uptake assay | THP-1 | Bosutinib |
 LDL uptake
LDL uptake
|
100 | |
| MMP production/activity | Zymography | THP-1 | Imatinib |
 MMP-2 and MMP-9 secretion and activity
MMP-2 and MMP-9 secretion and activity
|
100 |
| T Lymphocytes | |||||
| Proliferation/survival | 3 H-TdR incorporation; CFSE staining; titrated thymidine | Naïve CD4 + T cell; Human T cell | Imatinib |
 T-cell proliferation
T-cell proliferation
|
101 152 153 |
| Annexin V staining; Caspase assay | Human T cell | Imatinib | = T-cell apoptosis | 101 152 153 | |
| Annexin V staining | Human T cell | Imatinib | = T cell apoptosis | ||
| CFSE dye | Human T cell | Dasatinib |
 T-cell proliferation
T-cell proliferation
|
107 | |
| Annexin V staining | PBMC; Human T cell | Dasatinib | = T cell viability | 105 107 | |
| CFSE dye | CD8 + T cell; PBMC | Nilotinib |
 T cell proliferation
T cell proliferation
|
106 154 | |
| Secretion | ELISA | Human T cell; CD8 + and CD4 + T cell | Imatinib |
 IFN-γ production
IFN-γ production
|
101 107 |
| ELISA; proteome profile array | Human T cell; PBMC | Dasatinib |
 TNF-α, IFN-γ, IL-2, IL-6, IL-17 production
TNF-α, IFN-γ, IL-2, IL-6, IL-17 production
|
105 107 | |
| Proteome profile array | PBMC | Dasatinib |
 chemotactic factors secretion
chemotactic factors secretion
(SDF-1, MIP-1α, MIP-1β, MCP-1, CXCL-1) |
105 | |
| ELISPOT assay | CD8 + T cell | Nilotinib |
 IFN-γ production
IFN-γ production
|
154 | |
| Activation | Immunofluorescence | Human T cell | Imatinib |
 T cell activation
T cell activation
|
101 |
| Flow cytometry (CD25, CD69) | Human T cell | Imatinib | = T cell activation | 153 | |
| Flow cytometry (CD25, CD69) | Human T cell; PBMC | Dasatinib |
 T cell activation
T cell activation
|
105 107 | |
| Flow cytometry (CD25, CD69) | Human T cell | Nilotinib |
 T cell activation
T cell activation
|
154 | |
Abbreviations: CFSE, carboxyfluorescein succinimidyl ester; CXCL1, (C-X-C motif) ligand 1; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunospot; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein-1; MIP-1, macrophage inflammatory protein 1; NO, nitric oxide; PBMC, peripheral blood mononuclear cell; qPCR, quantitative polymerase chain reaction; SDF-1, stromal cell-derived factor 1; TNF, tumor necrosis factor.
Effects of new-generation TKIs on inflammatory cells were less studied, but first experiments indicate similarities with imatinib about its impact on monocytes and macrophages. Both dasatinib and nilotinib have similar inhibitory profile on macrophage-colony formation that has been linked to CSFR inhibition. 96 102 Dasatinib also possesses anti-inflammatory functions by attenuating proinflammatory cytokines production (i.e., TNF-α, IL-6, and IL-12 x ) by macrophages and increasing production of anti-inflammatory mediator (i.e., IL-10 y ). 103 These effects are thought to be mediated by SIK z inhibition, a subfamily of three serine/threonine kinases that regulate macrophage polarization. 103 104 Finally, dasatinib is associated with decreased T-cell functions and particularly it decreases the production of proinflammatory cytokines (e.g., TNF-α, IFN-γ) and chemotactic mediators. 105 Nilotinib and bosutinib also possess anti-inflammatory activity and decrease cytokine production and T-cell activation. 103 106 Inhibition of Lck, ai a tyrosine kinase implicated in T-cell receptor signaling, is implicated in the impairment of T-cell functions by dasatinib and nilotinib. 107 108 It has been hypothesized that nilotinib decreases mast cell activity through c-kit inhibition, 62 109 which might result in a decrease of the vascular repair system. 39 62 Clinical profile of nilotinib in patients with CML consolidates this hypothesis and demonstrates a decreased of mast cell level. 39 However, similar decreased of mast cell is also reported with imatinib without high rate of arterial thrombosis. 110
Globally, BCR-ABL TKIs possess reassuring profile on inflammatory cells. However, impact of new-generation TKIs on several functions of macrophages have not been assessed (e.g., MMP secretion and activity, lipid uptake, and foam cell formation), whereas effect of ponatinib on inflammatory cells is unknown. The assessment of lipid uptake and foam cell formation is particularly relevant with new-generation TKIs because there are numerous interactions between TKIs and ABC transporters. aii 111 112
Fibrous Cap Thickness
Fig. S3 in the Supplementary Material describes the mechanism by which atherosclerotic plaque ruptures and induces arterial thrombosis. Table 4 summarizes in vitro and in vivo experiments performed on VSMCs and fibroblasts. Imatinib decreases VSMC proliferation and growth but results are conflicting about its impact on apoptosis. Some studies demonstrate no impact on SMC apoptosis, whereas others indicate increased SMC death. 83 113 114 115 116 Imatinib also affects VSMC functions and decreases their migration and LDL binding, inducing decreased LDL retention by the sub-endothelium. 113 117 Imatinib also exerts negative effect on the synthesis of major ECM components (type I collagen and fibronectin A) by fibroblasts, correlating to decreased ECM accumulation in vivo. 118 The impact of imatinib on SMCs is thought to be mediated by PDGFR inhibition, 114 which is involved in several VSMC functions including VSMC survival and plasticity. 113 Subsequent to the hypothesis that imatinib inhibits PDGFR signaling, prevents abundant SMC and fibroblast proliferation, and inhibits abundant ECM accumulation, imatinib has been tested for the management of several fibrotic diseases (e.g., dermal and liver pulmonary fibrosis, systemic sclerosis). 30 118 119 Imatinib successfully acts on pulmonary fibrosis and pulmonary arterial hypertension (i.e., a disease involving vascular remodeling mediated by pulmonary SMC proliferation), 93 114 and has beneficial activity in sclerotic chronic graft-versus-host disease. 120 Finally, imatinib was tested in vivo for the prevention of cardiovascular diseases and demonstrates efficacy for the treatment of myocardial fibrosis by reducing ECM component synthesis (i.e., procollagen I and III). 30 In a rat model, imatinib successfully inhibits stenosis after balloon injury and presents interest in intimal hyperplasia and stenosis after bypass grafts. 115 116 121 122 123 Imatinib also successfully prevents arterial thrombosis following microvascular surgery in rabbits. 124 Imatinib was also encompassed in a stent but do not demonstrate efficacy in restenosis prevention. 84
Table 4. In vitro and in vivo studies on effects of BCR-ABL TKIs on proliferation, survival, and major functions of smooth muscle cells and fibroblasts.
| Endpoints | Methods | Models | TKIs | Findings | Ref. |
|---|---|---|---|---|---|
| Proliferation/survival | Resazurin assay; immunofluorescence; 3 H-thymidine incorporation; BrdU incorporation; MTT assay | HVSMC; BAoSMC; PASMC; ASMC; VSMC; HAoSMC; HCASMC; Rabbit | Imatinib |
 SMC proliferation
SMC proliferation
|
83 84 114 115 116 123 155 |
| Caspase assay; PARP (Western blot); JC-1 dye; Annexin V staining | BAoSMC; Dermal fibroblast; PASMC | Imatinib | = SMC/fibroblast apoptosis | 83 118 155 | |
| TUNEL; caspase assay | PASMC; HAoSMC; Rabbit | Imatinib |
 SMC apoptosis (PDGF-stimulated)
SMC apoptosis (PDGF-stimulated)
|
114 116 123 | |
| Trypan blue exclusion | HCASMC; A10 cell line | Imatinib | = SMC viability | 84 | |
| Cell counting; Propidium iodide staining | A10 cell line, HAoSMC | Dasatinib |
 SMC proliferation
SMC proliferation
|
113 125 | |
| Migration | Transwell cell migration assay | HAoSMC; PASMC; HCASMC; A10 cell | Imatinib |
 SMC migration
SMC migration
|
84 116 155 |
| Transwell cell migration assay | HAoSMC; A10 cell | Dasatinib |
 SMC migration
SMC migration
|
113 125 | |
| Secretion/synthesis | Radiolabel incorporation | Human VSMC | Imatinib |
 proteoglycan synthesis
proteoglycan synthesis
|
117 |
| RT-PCR; Western blot; Sircol collagen assay | Dermal fibroblast | Imatinib |
 COL1A1, COL1A2, fibronectin 1 synthesis
COL1A1, COL1A2, fibronectin 1 synthesis
 collagen synthesis
collagen synthesis
|
118 | |
| RT-PCR | Dermal fibroblast | Imatinib | = MMP-1, MMP-2, TIMP-1, TIMP-2, TIMP-3 and TIMP-4 | 118 | |
| qRT-PCR | Human fibroblast | Nilotinib | Decreases COL1A1 and COL1A2 synthesis | 127 | |
| Fibrosis | Sirius red staining | Rat | Imatinib |
 myocardial fibrosis, liver fibrosis
myocardial fibrosis, liver fibrosis
|
30 119 |
| Intima/media ratio | Rat (Balloon injury model) | Imatinib |
 stenosis
stenosis
|
121 122 | |
| Intima/media ratio | Rabbit | Imatinib |
 intimal thickness
intimal thickness
|
124 | |
| Hydroxyproline, collagen content | Rat liver | Imatinib |
 hydroxyproline and collagen content
hydroxyproline and collagen content
|
128 | |
| Hydroxyproline, collagen content | Rat liver | Nilotinib |
 hydroxyproline and collagen content
hydroxyproline and collagen content
|
128 | |
| Sirius red staining | Rat liver | Nilotinib |
 liver fibrosis
liver fibrosis
|
128 |
Abbreviations: ASMC, arterial smooth muscle cell; BAoSMC, bovine aortic smooth muscle cell; BrdU, bromodeoxyuridine; COL, collagen; HaOSMC, human aortic smooth muscle cell; HCASMC, human coronary artery smooth muscle cell; HVSMC, human vascular smooth muscle cell; MMP, matrix metalloproteinase; PARP, poly(ADP-ribose) polymerase; PASMC, pulmonary smooth muscle cell; PDGF, platelet-derived growth factor; qRT-PCR, quantitative reverse transcription polymerase chain reaction; SMC, smooth muscle cell; TIMP, tissue inhibitor of metalloproteinase; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; VSMC, vascular smooth muscle cell.
Impact of new-generation TKIs on fibrosis was less studied but demonstrate similar inhibitory effect on VSMCs and fibroblasts. Indeed, dasatinib inhibits PDGFR more potently than imatinib, 113 and the hypothesis that dasatinib prevents restenosis similarly with imatinib was emitted. Therefore, a patent has been filed claiming the use of dasatinib for the prevention of stenosis and restenosis. 125 Compared with imatinib, dasatinib has additional off-targets and is able to inhibit Src, aiii a kinase involved in dermal fibrosis in addition to PDGFR. 126 Therefore, dasatinib was tested in patients with scleroderma-like chronic graft-versus-host disease, a disease resulting from inflammation and progressive fibrosis of the dermis and subcutaneous tissues, and first results are encouraging. 126 Nilotinib also appears to be clinically efficient in scleroderma-like graft-versus-host disease by reducing collagen expression. 127 Finally, nilotinib was tested in vivo for the treatment of liver fibrosis and demonstrates decreased fibrotic markers and inflammatory cytokines (IL-1α, IL-1β, IFN-γ, IL-6). 128 However, only low-dose nilotinib was found to be efficient against fibrosis and normalized collagen content. 128 This lack of antifibrotic effect at higher doses might be explained by inhibition of additional off-targets by nilotinib that affect the benefit of low-dose nilotinib against fibrosis. Arterial thrombosis occurring with dasatinib and nilotinib are probably not the consequence of VSMC impairment, but investigations should be performed on VSMCs rather than on fibroblasts. Additional investigations are warranted to complete impact of BCR-ABL TKIs on VSMC functions (e.g., VSMC apoptosis, proliferation, and migration) and confirm their safety toward VSMCs.
Off-targets
BCR-ABL TKIs bind the highly conserved ATP binding site and are therefore not very specific to BCR-ABL and possess multiple cellular targets (kinases and nonkinase proteins). 129 130 This allowed the possibility to exploit them in other indications (e.g., PDGFR inhibition by imatinib is used in BCR-ABL-negative chronic myeloid disorders), 131 but this may also induce toxicities and side effects. 129 The development of arterial thrombotic events with new-generation BCR-ABL TKIs is likely to be related to inhibition of off-targets, as described throughout this review. Fig. 4 describes inhibitory profiles of imatinib, dasatinib, nilotinib, bosutinib, and ponatinib. Globally, imatinib is the most selective BCR-ABL TKIs, whereas dasatinib and ponatinib inhibit numerous off-targets.
Fig. 4.
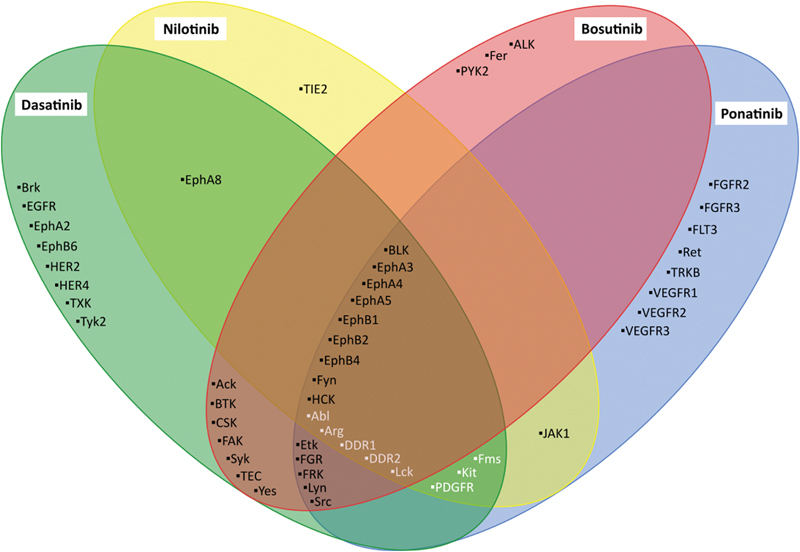
Specificity of imatinib, dasatinib, nilotinib, and ponatinib toward tyrosine kinases. Green, yellow, red, and blue circles contain tyrosine kinase inhibited by dasatinib, nilotinib, bosutinib, and ponatinib, respectively. Tyrosine kinases in white represent imatinib off-targets. This figure summarizes results from 13 experiments. 39 43 130 132 133 134 135 136 137 156 157 158 159 In case of conflictual results between studies, a conservative approach has been applied. Additional information is provided in the Supplementary Material .
However, inhibitory profiles are difficult to determine and several researches published discrepancies. For conflicting results, a conservative approach has been applied in Fig. 4 , but supplementary information ( Table S2 ) describes the tyrosine kinase selectivity profile of the five BCR-ABL TKIs and indicates divergences between studies. 43 130 132 133 134 These discrepancies can be explained by the difference in drug concentration and methodologies. To date, several methods have been used to determine inhibitory profile of BCR-ABL TKIs including in vitro kinase assay, 133 134 135 kinase expression in bacteriophages, 136 and affinity purification methods combined with mass spectrophotometry. 130 132 However, all these methods suffer from caveats, including the incompatibility to perform live-cell studies. A cell-permeable kinase probe was developed to figure out this problem, but this assay is still limited by the number of off-target tested (i.e., it requires to predefine tested off-targets) and therefore, the missing of targets is possible. 137 For this reason, the inhibitory activity of each TKI has not been tested toward all tyrosine kinase and Fig. 4 includes only off-targets for which at least one of the five BCR-ABL TKI has been tested. Thus, inhibitory profiles need to be carefully considered and it has to keep in mind that BCR-ABL TKI metabolites may possess activity against supplemental off-targets.
As described over this review, PDGF signaling has countless effects on several cells and tissues and is involved in several proatherogenic mechanisms (e.g., adipogenesis, vascular leakage, VSMC viability, and functions) and vascular homeostasis, which led to the suggestion of its implication in the potential beneficial cardiovascular effect of imatinib. 116 123 138 However, dasatinib, nilotinib, and ponatinib also inhibit PDGFR but increase the risk of arterial occlusive events. This difference of clinical outcome might be explained by the concentration of BCR-ABL TKIs necessary to obtain a same degree of PDGFR inhibition. 43 Indeed, Rivera et al reported that when adjusted to the maximum serum concentration, imatinib inhibits more profoundly PDGFR than dasatinib, nilotinib, and ponatinib. 43 Therefore, at effective concentration, it is probable that the degree of PDGFR inhibition is too low with dasatinib, nilotinib, and ponatinib to obtain the beneficial effect of PDGFR inhibition on atherosclerosis. Another possible hypothesis concerns the less conclusive specificity of new-generation TKIs which leads to inhibition of additional off-targets that might counterbalance the positive effect of PDGFR inhibition.
Other tyrosine kinases have been incriminated in the occurrence of arterial thrombosis with new-generation TKIs. DDR-1 aiv possesses functions in vascular homeostasis, atherogenesis, and is expressed in pancreatic islet cells. However, and similarly with PDGFR, it is inhibited by all BCR-ABL TKIs. 26 62 Other hypotheses include impairment of VEGF signaling by ponatinib 43 90 or the inhibition of several ephrin receptors by new-generation TKIs but not by imatinib which might inhibit monocyte recruitment. 139 Finally, it has been suggested that the inhibition of c-Abl itself is implicated in the increase of the cardiovascular risk. Indeed, imatinib possesses lower inhibitory effect on c-Abl than new-generation TKIs, which might further explain the difference in cardiovascular safety. 43 Additionally, c-Abl modulates Tie-2, av a tyrosine kinase that possesses important effect on endothelial cell function, angiogenesis, and inflammation. 140 141
Perspectives and Conclusions
This review summarizes the data underlying the potential preventive effect of imatinib on the occurrence of arterial thrombosis. Globally, in vitro and in vivo experiments demonstrate that imatinib possesses antiplatelet activity, hypolipidemic and hypoglycemic effects, and inhibits inflammation and atherosclerosis development in several cell types (i.e., decreases of inflammatory cell and VSMC functions and increased vascular permeability). These benefits were largely attributed to PDGFR inhibition. It is currently unknown why new-generation TKIs that also inhibit PDGFR present opposite cardiovascular safety profile and this point needs to be elucidated.
New-generation BCR-ABL TKIs increase the risk of arterial thromboembolism with different clinical features (e.g., time-to-event and absolute rate) and are associated with different safety profiles, suggesting different pathways to explain the pathophysiology. The safety profile of nilotinib is mostly characterized by impaired glucose and lipid metabolism. However, both the molecular mechanism of these alterations and their impact on the occurrence of arterial thrombosis are unknown. Both dasatinib and ponatinib exhibit antiplatelet effect, whereas it was recently suggested that nilotinib potentially induces prothrombotic phenotype of platelets. Based on the clinical characteristics and case reports, atherosclerosis appears the most plausible mechanisms by which new-generation TKIs induce arterial thrombosis. However, in vitro and in vivo studies of viability and functions of SMCs and inflammatory cells demonstrate reassuring impact of dasatinib and nilotinib, even if additional studies are required to complete this evaluation. However, first experiments indicate that dasatinib, nilotinib, and ponatinib influence EC survival and/or endothelium integrity, suggesting a reasonable hypothesis by which new-generation TKIs induce atherosclerosis development and, subsequently, arterial thrombosis. Additional studies on the shedding of functional extracellular vesicles by endothelial cells might be interesting regarding their important role in coronary artery diseases. 142 Finally, the impact of new-generation TKIs on human blood coagulation and fibrinolysis has never been studied and should be addressed.
To conclude, new-generation TKIs increase the risk of arterial thrombosis in patients with CML, whereas imatinib, the first-generation TKI, might prevent the development of cardiovascular events. To date, the cellular events and signaling pathways by which these events occurred are unknown and researches are extremely limited focusing mainly on imatinib and nilotinib. Researches need to be extended to all new-generation BCR-ABL TKIs (i.e., dasatinib, bosutinib, and ponatinib). The understanding of the mechanisms by which new-generation BCR-ABL TKIs induce or promote arterial occlusive events will improve the clinical uses of these therapies. To date, only general risk minimization measures have been proposed (e.g., management of dyslipidemia, diabetes, arterial hypertension following standard of care). 14 22 23 143 144 145 146 The understanding of the pathophysiology is required to implement the most appropriate risk minimization strategies for thrombotic events and to select patients to whom the prescription of these drugs should be avoided when applicable. Finally, the understanding of the pathophysiology will help in the design of new BCR-ABL inhibitors sparing the toxic targets.
Review Criteria
Relevant articles published from the database inception to July 11, 2017, were identified from an electronic database (PubMed) using the keywords “vascular,” “thrombosis,” “atherosclerosis,” “arteriosclerosis,” “venous,” “arterial,” “hemostasis,” “metabolic,” “metabolism,” “glycemia,” “glycaemia,” “cholesterol,” “triglycerides,” and “platelet” combined with the five approved BCR-ABL TKIs. The search strategy is presented in supplementary files. Articles published in languages other than English were excluded from the analysis. Primary criteria were pathophysiological explanation of arterial thrombotic events. Abstracts and full-text articles were reviewed with a focus on atherogenesis, plaque rupture, platelet functions, and their link with the development of arterial thrombosis with BCR-ABL TKIs. The reference section of identified articles was also examined.
Authors' Contributions
H.H. was responsible for the first draft of the manuscript. F.M., C.C., C.G., J.M.D., and J.D. contributed to the final draft of the manuscript.
Conflicts of Interest J.D. reports personal fees from Roche Diagnostics, Stago Diagnostica, Bayer Healthcare, and Daiichi-Sankyo; travel grants from Bayer Healthcare, Boehringer Ingelheim, CSL Behring, and Stago Diagnostica outside the submitted work.
F.M. reports personal fees from Boehringer Ingelheim, Bayer Healthcare, and Bristol-Myers Squibb-Pfizer outside the submitted work.
C.G. reports personal fees from Novartis, Celgene, and Amgen outside the submitted work.
The other authors have no conflicts of interest to disclose.
T315I: Substitution at position 315 in BCR-ABL from a threonine to an isoleucine. This substitution alters the structure of the ATP-binding pocket and eliminates a crucial hydrogen bond required for binding of first- and second-generation TKIs.
Ephrin family: Members of this family are involved in platelet spreading, adhesion to fibrinogen and platelet secretion.
PAR-1: protease-activated receptor 1. PAR receptors mediate cellular effects of thrombin in platelets and endothelial cells.
JNK: c-Jun N-terminal kinases. JNK is responsive to stress stimuli and mediates insulin resistance through inhibition of insulin receptor substrate.
Adiponectin is a protein regulating glucose metabolism. Adiponectin increases peripheral insulin sensitivity by improving glucose uptake.
GLUT4: Glucose transporter type 4. GLUT4 is an insulin-regulated glucose transporter expressed in peripheral tissues.
HMGCoA reductase: 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. HMGCoA reductase catalysis is the conversion of HMG-CoA to mevalonic acid, an essential step in cholesterol synthesis.
Apobec1: Apolipoprotein B mRNA editing enzyme catalytic subunit 1. Apobec1 introduces a stop codon into ApoB mRNA.
LDLR: Low-density lipoprotein receptor. This cell surface receptor mediates LDL endocytosis.
CETP: Cholesteryl ester transfer protein.
HUVEC: Human umbilical vein endothelial cells.
HCAEC: Human coronary artery endothelial cells.
ICAM-1: Intercellular adhesion molecule 1. ICAM-1 stabilizes leukocyte-endothelial cell adhesion and facilitates leukocyte transmigration.
VCAM-1: Vascular cell adhesion molecule 1. VCAM-1 mediates rolling-type and firm adhesion of leukocyte.
VE-cadherin: Vascular endothelial cadherin. VE-cadherin is a cell–cell adhesion molecules and implies in endothelial junctions.
VEGFR: Vascular endothelial growth factor. This protein plays major roles in vasculogenesis and angiogenesis.
RhoA-ROCK: Ras homolog gene family, member A—Rho-associated protein kinase. Rho-kinase regulates cytoskeletal reorganization, cell migration, cell proliferation, and survival.
Arg: Abelson-related gene (also known as ABL2). Arg possesses cytoskeletal-remodeling functions.
TNF-α: Tumor necrosis factor alpha. This cytokine is mainly involved in systemic inflammation and regulates immune cells.
IL-6: Interleukin 6. IL-6 is a proinflammatory cytokine secreted by T-cells and macrophages to stimulate immune response.
CSFR: Colony-stimulating factor receptor. CSFR drives growth and development of monocytes.
MMP-2 and MMP-9 are two proteases capable of degrading extracellular matrix components. These two MMPs are the main proteases involved in atherogenesis.
IFN-γ: Interferon gamma. IFN-γ is involved in innate and adaptive immunity and activates macrophages.
IL-12: Interleukin 12. IL-12 is involved in T-cell differentiation and functions.
IL-10: Interleukin 10. IL-10 exerts immunoregulation and regulates inflammation.
SIK: Salt-inducible kinase. SIKs regulate production of anti- and proinflammatory cytokines.
Lck: Lymphocyte-specific protein tyrosine kinase. Lck is mostly involved in T-cell maturation.
ABC transporter: ATP-binding cassette transporters. ABCG1 and ABCA1 are implicated in macrophage reverse cholesterol transport. Their deficiency leads to foam cell formation and atherosclerosis development.
Src is involved in angiogenesis and cell survival and proliferation.
DDR-1: Discoidin domain receptor tyrosine kinase 1. DDR1 is involved in the regulation of cell growth, differentiation, and metabolism.
Tie-2: Tunica interna endothelial cell kinase. Tie-2 regulates angiogenesis, endothelial cell survival, proliferation, migration, adhesion and cell spreading, cytoskeleton reorganization, and vascular quiescence. Tie-2 also possesses anti-inflammatory functions by preventing the leakage of proinflammatory mediators and leukocytes.
Supplementary Material
References
- 1.Bhamidipati P K, Kantarjian H, Cortes J, Cornelison A M, Jabbour E. Management of imatinib-resistant patients with chronic myeloid leukemia. Ther Adv Hematol. 2013;4(02):103–117. doi: 10.1177/2040620712468289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorre M E, Mohammed M, Ellwood Ket al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification Science 2001293(5531):876–880. [DOI] [PubMed] [Google Scholar]
- 3.Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology (Am Soc Hematol Educ Program) 2009;2009(01):461–476. doi: 10.1182/asheducation-2009.1.461. [DOI] [PubMed] [Google Scholar]
- 4.Cortes J E, Saglio G, Kantarjian H M et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34(20):2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochhaus A, Saglio G, Hughes T P et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(05):1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brümmendorf T H, Cortes J E, de Souza C A et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015;168(01):69–81. doi: 10.1111/bjh.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mealing S, Barcena L, Hawkins N et al. The relative efficacy of imatinib, dasatinib and nilotinib for newly diagnosed chronic myeloid leukemia: a systematic review and network meta-analysis. Exp Hematol Oncol. 2013;2(01):5. doi: 10.1186/2162-3619-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamontanara A J, Gencer E B, Kuzyk O, Hantschel O. Mechanisms of resistance to BCR-ABL and other kinase inhibitors. Biochim Biophys Acta. 2013;1834(07):1449–1459. doi: 10.1016/j.bbapap.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Pagnano K B, Bendit I, Boquimpani C et al. BCR-ABL mutations in chronic myeloid leukemia treated with tyrosine kinase inhibitors and impact on survival. Cancer Invest. 2015;33(09):451–458. doi: 10.3109/07357907.2015.1065499. [DOI] [PubMed] [Google Scholar]
- 10.Ursan I D, Jiang R, Pickard E M, Lee T A, Ng D, Pickard A S. Emergence of BCR-ABL kinase domain mutations associated with newly diagnosed chronic myeloid leukemia: a meta-analysis of clinical trials of tyrosine kinase inhibitors. J Manag Care Spec Pharm. 2015;21(02):114–122. doi: 10.18553/jmcp.2015.21.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network.NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid LeukemiaVersion 1.2017. 2016
- 12.Thanopoulou E, Judson I. The safety profile of imatinib in CML and GIST: long-term considerations. Arch Toxicol. 2012;86(01):1–12. doi: 10.1007/s00204-011-0729-7. [DOI] [PubMed] [Google Scholar]
- 13.Kalmanti L, Saussele S, Lauseker M et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29(05):1123–1132. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 14.Moslehi J J, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33(35):4210–4218. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breccia M, Alimena G. Pleural/pericardic effusions during dasatinib treatment: incidence, management and risk factors associated to their development. Expert Opin Drug Saf. 2010;9(05):713–721. doi: 10.1517/14740331003742935. [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency.Sprycel - Summary of Product Characteristics 2017Available at:http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000709/WC500056998.pdf. Accessed December 22, 2017
- 17.European Medicines Agency.Iclusig - Summary of Product Characteristics 2017Available at:http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002695/WC500145646.pdf. Accessed December 22, 2017
- 18.European Medicines Agency.Tasigna - Summary of Product Characteristics 2017Available at:http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000798/WC500034394.pdf. Accessed December 22, 2017
- 19.Kantarjian H M, Cortes J E, Kim D W et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2014;123(09):1309–1318. doi: 10.1182/blood-2013-07-513937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saglio G, Kim D-W, Issaragrisil S et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 21.Poch Martell M, Sibai H, Deotare U, Lipton J H. Ponatinib in the therapy of chronic myeloid leukemia. Expert Rev Hematol. 2016;9(10):923–932. doi: 10.1080/17474086.2016.1232163. [DOI] [PubMed] [Google Scholar]
- 22.Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné J M. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 23.Haguet H, Douxfils J, Mullier F, Chatelain C, Graux C, Dogné J M. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: a systematic review and meta-analysis. Expert Opin Drug Saf. 2017;16(01):5–12. doi: 10.1080/14740338.2017.1261824. [DOI] [PubMed] [Google Scholar]
- 24.Cortes J, Kim D-W, Pinilla-Ibarz Jet al. Ponatinib efficacy and safety in heavily pretreated leukemia patients: 3-year results of the PACE trial20th Congress of EHA; Copenhagen, Denmark, June 12, 2015
- 25.Pasvolsky O, Leader A, Iakobishvili Z, Wasserstrum Y, Kornowski R, Raanani P. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardio-Oncology. 2015;1(01):5. doi: 10.1186/s40959-015-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gora-Tybor J, Medras E, Calbecka M et al. Real-life comparison of severe vascular events and other non-hematological complications in patients with chronic myeloid leukemia undergoing second-line nilotinib or dasatinib treatment. Leuk Lymphoma. 2015;56(08):2309–2314. doi: 10.3109/10428194.2014.994205. [DOI] [PubMed] [Google Scholar]
- 27.le Coutre P D, Hughes T P, Mahon F X et al. Low incidence of peripheral arterial disease in patients receiving dasatinib in clinical trials. Leukemia. 2016;30(07):1593–1596. doi: 10.1038/leu.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sam P Y, Ahaneku H, Nogueras-Gonzalez G M et al. Cardiovascular events among patients with chronic myeloid leukemia (CML) treated with tyrosine kinase inhibitors (TKIs) Blood. 2016;128(22):1919. [Google Scholar]
- 29.Lassila M, Allen T J, Cao Z et al. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(05):935–942. doi: 10.1161/01.ATV.0000124105.39900.db. [DOI] [PubMed] [Google Scholar]
- 30.Ma L K, Li Q, He L F et al. Imatinib attenuates myocardial fibrosis in association with inhibition of the PDGFRα activity. Arq Bras Cardiol. 2012;99(06):1082–1091. doi: 10.1590/s0066-782x2012005000109. [DOI] [PubMed] [Google Scholar]
- 31.Fossard G, Blond E, Balsat M et al. Hyperhomocysteinemia and high doses of nilotinib favor cardiovascular events in chronic phase chronic myelogenous leukemia patients. Haematologica. 2016;101(03):e86–e90. doi: 10.3324/haematol.2015.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kostos L, Burbury K, Srivastava G, Prince H M. Gastrointestinal bleeding in a chronic myeloid leukaemia patient precipitated by dasatinib-induced platelet dysfunction: case report. Platelets. 2015;26(08):809–811. doi: 10.3109/09537104.2015.1049138. [DOI] [PubMed] [Google Scholar]
- 33.Mazharian A, Ghevaert C, Zhang L, Massberg S, Watson S P. Dasatinib enhances megakaryocyte differentiation but inhibits platelet formation. Blood. 2011;117(19):5198–5206. doi: 10.1182/blood-2010-12-326850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alhawiti N, Burbury K L, Kwa F A et al. The tyrosine kinase inhibitor, nilotinib potentiates a prothrombotic state. Thromb Res. 2016;145:54–64. doi: 10.1016/j.thromres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Lotfi K, Deb S, Sjöström C, Tharmakulanathan A, Boknäs N, Ramström S. Individual variation in hemostatic alterations caused by tyrosine kinase inhibitors: a way to improve personalized cancer therapy? Blood. 2016;128(22):1908. doi: 10.1016/S0049-3848(16)30188-8. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Grosser T, Diamond S L. Microfluidic whole blood testing of platelet response to pharmacological agents. Platelets. 2017;28(05):457–462. doi: 10.1080/09537104.2016.1268254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senis Y A, Mazharian A, Mori J. Src family kinases: at the forefront of platelet activation. Blood. 2014;124(13):2013–2024. doi: 10.1182/blood-2014-01-453134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gratacap M P, Martin V, Valéra M C et al. The new tyrosine-kinase inhibitor and anticancer drug dasatinib reversibly affects platelet activation in vitro and in vivo. Blood. 2009;114(09):1884–1892. doi: 10.1182/blood-2009-02-205328. [DOI] [PubMed] [Google Scholar]
- 39.Albrecht-Schgoer K, Huber K, Grebien F et al. Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on vascular endothelial cells: a potential explanation for drug-induced vasculopathy in CML. Blood. 2013;122(21):257. [Google Scholar]
- 40.Shimabukuro-Vornhagen A, Rothe A, Nogova L, Kochanek M, Scheid C, von Bergwelt-Baildon M. Improvement of platelet dysfunction in chronic myelogenous leukemia following treatment with imatinib: a case report. J Med Case Reports. 2011;5:215. doi: 10.1186/1752-1947-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neelakantan P, Marin D, Laffan M, Goldman J, Apperley J, Milojkovic D. Platelet dysfunction associated with ponatinib, a new pan BCR-ABL inhibitor with efficacy for chronic myeloid leukemia resistant to multiple tyrosine kinase inhibitor therapy. Haematologica. 2012;97(09):1444. doi: 10.3324/haematol.2012.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loren C P, Aslan J E, Rigg R A et al. The BCR-ABL inhibitor ponatinib inhibits platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling, platelet activation and aggregate formation under shear. Thromb Res. 2015;135(01):155–160. doi: 10.1016/j.thromres.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera V M, Pritchard J R, Gonzalvez F, Baker T, Gozgit J M, Hodgson G. Comparative TKI profiling analyses to explore potential mechanisms of Ponatinib-associated arterial thrombotic events. Blood. 2014;124(21):1783. [Google Scholar]
- 44.Quintás-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114(02):261–263. doi: 10.1182/blood-2008-09-180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breccia M, Muscaritoli M, Aversa Z, Mandelli F, Alimena G. Imatinib mesylate may improve fasting blood glucose in diabetic Ph+ chronic myelogenous leukemia patients responsive to treatment. J Clin Oncol. 2004;22(22):4653–4655. doi: 10.1200/JCO.2004.04.217. [DOI] [PubMed] [Google Scholar]
- 46.Breccia M, Alimena G. The metabolic consequences of imatinib mesylate: changes on glucose, lypidic and bone metabolism. Leuk Res. 2009;33(07):871–875. doi: 10.1016/j.leukres.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Agostino N M, Chinchilli V M, Lynch C J et al. Effect of the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib, and imatinib) on blood glucose levels in diabetic and nondiabetic patients in general clinical practice. J Oncol Pharm Pract. 2011;17(03):197–202. doi: 10.1177/1078155210378913. [DOI] [PubMed] [Google Scholar]
- 48.Breccia M, Muscaritoli M, Cannella L, Stefanizzi C, Frustaci A, Alimena G. Fasting glucose improvement under dasatinib treatment in an accelerated phase chronic myeloid leukemia patient unresponsive to imatinib and nilotinib. Leuk Res. 2008;32(10):1626–1628. doi: 10.1016/j.leukres.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Ono K, Suzushima H, Watanabe Y et al. Rapid amelioration of hyperglycemia facilitated by dasatinib in a chronic myeloid leukemia patient with type 2 diabetes mellitus. Intern Med. 2012;51(19):2763–2766. doi: 10.2169/internalmedicine.51.8314. [DOI] [PubMed] [Google Scholar]
- 50.Louvet C, Szot G L, Lang J et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105(48):18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hägerkvist R, Jansson L, Welsh N. Imatinib mesylate improves insulin sensitivity and glucose disposal rates in rats fed a high-fat diet. Clin Sci (Lond) 2008;114(01):65–71. doi: 10.1042/CS20070122. [DOI] [PubMed] [Google Scholar]
- 52.Han M S, Chung K W, Cheon H G et al. Imatinib mesylate reduces endoplasmic reticulum stress and induces remission of diabetes in db/db mice. Diabetes. 2009;58(02):329–336. doi: 10.2337/db08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hägerkvist R, Makeeva N, Elliman S, Welsh N. Imatinib mesylate (Gleevec) protects against streptozotocin-induced diabetes and islet cell death in vitro. Cell Biol Int. 2006;30(12):1013–1017. doi: 10.1016/j.cellbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Hägerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. FASEB J. 2007;21(02):618–628. doi: 10.1096/fj.06-6910com. [DOI] [PubMed] [Google Scholar]
- 55.Xia C Q, Zhang P, Li S et al. C-Abl inhibitor imatinib enhances insulin production by β cells: c-Abl negatively regulates insulin production via interfering with the expression of NKx2.2 and GLUT-2. PLoS One. 2014;9(05):e97694. doi: 10.1371/journal.pone.0097694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadiq S, Owen E, Foster Tet al. Nilotinib-induced metabolic dysfunction: insights from a translational pilot study using in vitro adipocyte models and patient cohorts22nd EHA congress; June 22–25, 2017; Madrid, Spain [DOI] [PMC free article] [PubMed]
- 57.Iizuka K, Niwa H, Kato T, Takeda J. Dasatinib improves insulin sensitivity and affects lipid metabolism in a patient with chronic myeloid leukaemia. BMJ Case Rep. 2016;2016:201. doi: 10.1136/bcr-2015-214284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitter S, Vandyke K, Schultz C G, White D, Hughes T P, Zannettino A C. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: a mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J Clin Endocrinol Metab. 2010;95(08):3763–3767. doi: 10.1210/jc.2010-0086. [DOI] [PubMed] [Google Scholar]
- 59.Hosch S E, Olefsky J M, Kim J J. APPLied mechanics: uncovering how adiponectin modulates insulin action. Cell Metab. 2006;4(01):5–6. doi: 10.1016/j.cmet.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Lihn A S, Pedersen S B, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6(01):13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 61.Fitter S, Vandyke K, Gronthos S, Zannettino A C. Suppression of PDGF-induced PI3 kinase activity by imatinib promotes adipogenesis and adiponectin secretion. J Mol Endocrinol. 2012;48(03):229–240. doi: 10.1530/JME-12-0003. [DOI] [PubMed] [Google Scholar]
- 62.Aichberger K J, Herndlhofer S, Schernthaner G H et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(07):533–539. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 63.Breccia M, Muscaritoli M, Gentilini F et al. Impaired fasting glucose level as metabolic side effect of nilotinib in non-diabetic chronic myeloid leukemia patients resistant to imatinib. Leuk Res. 2007;31(12):1770–1772. doi: 10.1016/j.leukres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 64.Castagnetti F, Breccia M, Gugliotta G et al. Nilotinib 300 mg twice daily: an academic single-arm study of newly diagnosed chronic phase chronic myeloid leukemia patients. Haematologica. 2016;101(10):1200–1207. doi: 10.3324/haematol.2016.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delphine R, Gautier J-f, Breccia M et al. Incidence of hyperglycemia by 3 years in patients (Pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with Nilotinib (NIL) or Imatinib (IM) in ENESTnd. Blood. 2012;120(21):1686. [Google Scholar]
- 66.Iurlo A, Orsi E, Cattaneo D et al. Effects of first- and second-generation tyrosine kinase inhibitor therapy on glucose and lipid metabolism in chronic myeloid leukemia patients: a real clinical problem? Oncotarget. 2015;6(32):33944–33951. doi: 10.18632/oncotarget.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Racil Z, Razga F, Drapalova J et al. Mechanism of impaired glucose metabolism during nilotinib therapy in patients with chronic myelogenous leukemia. Haematologica. 2013;98(10):e124–e126. doi: 10.3324/haematol.2013.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito Y, Miyamoto T, Chong Y, Maki T, Akashi K, Kamimura T. Nilotinib exacerbates diabetes mellitus by decreasing secretion of endogenous insulin. Int J Hematol. 2013;97(01):135–138. doi: 10.1007/s12185-012-1222-7. [DOI] [PubMed] [Google Scholar]
- 69.Hadzijusufovic E, Herndlhofer S, Aichberger K J et al. Nilotinib exerts direct effects on vascular endothelial cells and may act as a co-trigger of atherosclerosis in patients with Ph+ CML. Blood. 2011;118(21):2753. [Google Scholar]
- 70.Breccia M, Loglisci G, Salaroli A, Serrao A, Alimena G. Nilotinib-mediated increase in fasting glucose level is reversible, does not convert to type 2 diabetes and is likely correlated with increased body mass index. Leuk Res. 2012;36(04):e66–e67. doi: 10.1016/j.leukres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Saglio G, Larson R A, Hughes T P et al. Efficacy and safety of Nilotinib in chronic phase (CP) chronic myeloid leukemia (CML) patients (Pts) with type 2 diabetes in the ENESTnd trial. Blood. 2010;116(21):3430. [Google Scholar]
- 72.Frasca F, Pandini G, Malaguarnera R et al. Role of c-Abl in directing metabolic versus mitogenic effects in insulin receptor signaling. J Biol Chem. 2007;282(36):26077–26088. doi: 10.1074/jbc.M705008200. [DOI] [PubMed] [Google Scholar]
- 73.Franceschino A, Tornaghi L, Benemacher V, Assouline S, Gambacorti-Passerini C. Alterations in creatine kinase, phosphate and lipid values in patients with chronic myeloid leukemia during treatment with imatinib. Haematologica. 2008;93(02):317–318. doi: 10.3324/haematol.11680. [DOI] [PubMed] [Google Scholar]
- 74.Gottardi M, Manzato E, Gherlinzoni F. Imatinib and hyperlipidemia. N Engl J Med. 2005;353(25):2722–2723. doi: 10.1056/NEJMc052500. [DOI] [PubMed] [Google Scholar]
- 75.Gologan R, Constantinescu G, Georgescu D et al. Hypolipemiant besides antileukemic effect of imatinib mesylate. Leuk Res. 2009;33(09):1285–1287. doi: 10.1016/j.leukres.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 76.Ellis M, Krashin E, Hamburger-Avnery O, Gan S, Elis A, Ashur-Fabian O. The anti-leukemic and lipid lowering effects of imatinib are not hindered by statins in CML: a retrospective clinical study and in vitro assessment of lipid-genes transcription. Leuk Lymphoma. 2017;58(05):1172–1177. doi: 10.1080/10428194.2016.1228928. [DOI] [PubMed] [Google Scholar]
- 77.Franklin M, Burns L, Perez S, Yerragolam D, Makenbaeva D. Incidence of type II diabetes mellitus and hyperlipidemia in patients prescribed Dasatinib or Nilotinib as first or second line therapy for chronic myelogenous leukemia (CML) Blood. 2016;128(22):4766. doi: 10.1080/03007995.2017.1399870. [DOI] [PubMed] [Google Scholar]
- 78.Rea D, Mirault T, Cluzeau T et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica. 2014;99(07):1197–1203. doi: 10.3324/haematol.2014.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maurizot A, Beressi J P, Manéglier B et al. Rapid clinical improvement of peripheral artery occlusive disease symptoms after nilotinib discontinuation despite persisting vascular occlusion. Blood Cancer J. 2014;4:e247. doi: 10.1038/bcj.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Breccia M, Molica M, Alimena G. How tyrosine kinase inhibitors impair metabolism and endocrine system function: a systematic updated review. Leuk Res. 2014;38(12):1392–1398. doi: 10.1016/j.leukres.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 81.Venalis P, Maurer B, Akhmetshina A et al. Lack of inhibitory effects of the anti-fibrotic drug imatinib on endothelial cell functions in vitro and in vivo. J Cell Mol Med. 2009;13(10):4185–4191. doi: 10.1111/j.1582-4934.2008.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gover-Proaktor A, Granot G, Shapira S et al. Ponatinib reduces viability, migration, and functionality of human endothelial cells. Leuk Lymphoma. 2017;58(06):1455–1467. doi: 10.1080/10428194.2016.1239258. [DOI] [PubMed] [Google Scholar]
- 83.Vallières K, Petitclerc E, Laroche G. On the ability of imatinib mesylate to inhibit smooth muscle cell proliferation without delaying endothelialization: an in vitro study. Vascul Pharmacol. 2009;51(01):50–56. doi: 10.1016/j.vph.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Hacker T A, Griffin M O, Guttormsen B, Stoker S, Wolff M R. Platelet-derived growth factor receptor antagonist STI571 (imatinib mesylate) inhibits human vascular smooth muscle proliferation and migration in vitro but not in vivo. J Invasive Cardiol. 2007;19(06):269–274. [PubMed] [Google Scholar]
- 85.Vrekoussis T, Stathopoulos E N, De Giorgi U et al. Modulation of vascular endothelium by imatinib: a study on the EA.hy 926 endothelial cell line. J Chemother. 2006;18(01):56–65. doi: 10.1179/joc.2006.18.1.56. [DOI] [PubMed] [Google Scholar]
- 86.Letsiou E, Rizzo A N, Sammani S et al. Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308(03):L259–L269. doi: 10.1152/ajplung.00323.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guignabert C, Phan C, Seferian A et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126(09):3207–3218. doi: 10.1172/JCI86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rizzo A N, Sammani S, Esquinca A E et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1294–L1304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katgı A, Sevindik ÖG, Gökbulut A A et al. Nilotinib does not alter the secretory functions of carotid artery endothelial cells in a prothrombotic or antithrombotic fashion. Clin Appl Thromb Hemost. 2015;21(07):678–683. doi: 10.1177/1076029614550817. [DOI] [PubMed] [Google Scholar]
- 90.Hadzijusufovic E, Kirchmair R, Theurl M et al. Ponatinib exerts multiple effects on vascular endothelial cells: possible mechanisms and explanations for the adverse vascular events seen in CML patients treated with Ponatinib. Blood. 2016;128(22):1883. [Google Scholar]
- 91.Lorenzi M, Cagliero E, Toledo S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes. 1985;34(07):621–627. doi: 10.2337/diab.34.7.621. [DOI] [PubMed] [Google Scholar]
- 92.Shah N P, Wallis N, Farber H W et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol. 2015;90(11):1060–1064. doi: 10.1002/ajh.24174. [DOI] [PubMed] [Google Scholar]
- 93.Frost A E, Barst R J, Hoeper M M et al. Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J Heart Lung Transplant. 2015;34(11):1366–1375. doi: 10.1016/j.healun.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 94.Dasgupta S K, Le A, Vijayan K V, Thiagarajan P. Dasatinib inhibits actin fiber reorganization and promotes endothelial cell permeability through RhoA-ROCK pathway. Cancer Med. 2017;6(04):809–818. doi: 10.1002/cam4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dewar A L, Domaschenz R M, Doherty K V, Hughes T P, Lyons A B. Imatinib inhibits the in vitro development of the monocyte/macrophage lineage from normal human bone marrow progenitors. Leukemia. 2003;17(09):1713–1721. doi: 10.1038/sj.leu.2403071. [DOI] [PubMed] [Google Scholar]
- 96.Brownlow N, Mol C, Hayford C, Ghaem-Maghami S, Dibb N J. Dasatinib is a potent inhibitor of tumour-associated macrophages, osteoclasts and the FMS receptor. Leukemia. 2009;23(03):590–594. doi: 10.1038/leu.2008.237. [DOI] [PubMed] [Google Scholar]
- 97.Dewar A L, Doherty K V, Hughes T P, Lyons A B. Imatinib inhibits the functional capacity of cultured human monocytes. Immunol Cell Biol. 2005;83(01):48–56. doi: 10.1111/j.1440-1711.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 98.Dewar A L, Cambareri A C, Zannettino A CW et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105(08):3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 99.Metharom P, Martin K, Kumar A H et al. Pleiotropic role for monocyte C-fms protein in response to vascular injury: potential therapeutic target. Atherosclerosis. 2011;216(01):74–82. doi: 10.1016/j.atherosclerosis.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 100.Gacic J, Vorkapic E, Olsen R S et al. Imatinib reduces cholesterol uptake and matrix metalloproteinase activity in human THP-1 macrophages. Pharmacol Rep. 2016;68(01):1–6. doi: 10.1016/j.pharep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 101.Cwynarski K, Laylor R, Macchiarulo E et al. Imatinib inhibits the activation and proliferation of normal T lymphocytes in vitro. Leukemia. 2004;18(08):1332–1339. doi: 10.1038/sj.leu.2403401. [DOI] [PubMed] [Google Scholar]
- 102.Brownlow N, Russell A E, Saravanapavan H et al. Comparison of nilotinib and imatinib inhibition of FMS receptor signaling, macrophage production and osteoclastogenesis. Leukemia. 2008;22(03):649–652. doi: 10.1038/sj.leu.2404944. [DOI] [PubMed] [Google Scholar]
- 103.Ozanne J, Prescott A R, Clark K. The clinically approved drugs dasatinib and bosutinib induce anti-inflammatory macrophages by inhibiting the salt-inducible kinases. Biochem J. 2015;465(02):271–279. doi: 10.1042/BJ20141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Darling N J, Toth R, Arthur J S, Clark K. Inhibition of SIK2 and SIK3 during differentiation enhances the anti-inflammatory phenotype of macrophages. Biochem J. 2017;474(04):521–537. doi: 10.1042/BCJ20160646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schade A E, Schieven G L, Townsend R et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111(03):1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blake S J, Lyons A B, Hughes T P. Nilotinib inhibits the Src-family kinase LCK and T-cell function in vitro. J Cell Mol Med. 2009;13(03):599–601. doi: 10.1111/j.1582-4934.2009.00500_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blake S, Hughes T P, Mayrhofer G, Lyons A B. The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol. 2008;127(03):330–339. doi: 10.1016/j.clim.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 108.Lee K C, Ouwehand I, Giannini A L, Thomas N S, Dibb N J, Bijlmakers M J. Lck is a key target of imatinib and dasatinib in T-cell activation. Leukemia. 2010;24(04):896–900. doi: 10.1038/leu.2010.11. [DOI] [PubMed] [Google Scholar]
- 109.Farha S, Dweik R, Rahaghi F et al. Imatinib in pulmonary arterial hypertension: c-Kit inhibition. Pulm Circ. 2014;4(03):452–455. doi: 10.1086/677359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Juurikivi A, Sandler C, Lindstedt K A et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Ann Rheum Dis. 2005;64(08):1126–1131. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun Y L, Kumar P, Sodani K et al. Ponatinib enhances anticancer drug sensitivity in MRP7-overexpressing cells. Oncol Rep. 2014;31(04):1605–1612. doi: 10.3892/or.2014.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yvan-Charvet L, Wang N, Tall A R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30(02):139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Z, Lee F Y, Bhalla K N, Wu J. Potent inhibition of platelet-derived growth factor-induced responses in vascular smooth muscle cells by BMS-354825 (dasatinib) Mol Pharmacol. 2006;69(05):1527–1533. doi: 10.1124/mol.105.020172. [DOI] [PubMed] [Google Scholar]
- 114.Nakamura K, Akagi S, Ogawa A et al. Pro-apoptotic effects of imatinib on PDGF-stimulated pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2012;159(02):100–106. doi: 10.1016/j.ijcard.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 115.Li L, Blumenthal D K, Masaki T, Terry C M, Cheung A K. Differential effects of imatinib on PDGF-induced proliferation and PDGF receptor signaling in human arterial and venous smooth muscle cells. J Cell Biochem. 2006;99(06):1553–1563. doi: 10.1002/jcb.20993. [DOI] [PubMed] [Google Scholar]
- 116.Rocha A, Azevedo I, Soares R. Anti-angiogenic effects of imatinib target smooth muscle cells but not endothelial cells. Angiogenesis. 2007;10(04):279–286. doi: 10.1007/s10456-007-9080-2. [DOI] [PubMed] [Google Scholar]
- 117.Ballinger M L, Osman N, Hashimura Ket al. Imatinib inhibits vascular smooth muscle proteoglycan synthesis and reduces LDL binding in vitro and aortic lipid deposition in vivo J Cell Mol Med 201014(6B):1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Distler J H, Jüngel A, Huber L C et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56(01):311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 119.Yoshiji H, Noguchi R, Kuriyama S et al. Imatinib mesylate (STI-571) attenuates liver fibrosis development in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288(05):G907–G913. doi: 10.1152/ajpgi.00420.2004. [DOI] [PubMed] [Google Scholar]
- 120.Salhotra A, Tsai N, Thomas S H et al. Sclerodermatous chronic GVHD in patients receiving tyrosine kinase inhibitors after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(01):139–141. doi: 10.1038/bmt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Makiyama Y, Toba K, Kato K et al. Imatinib mesilate inhibits neointimal hyperplasia via growth inhibition of vascular smooth muscle cells in a rat model of balloon injury. Tohoku J Exp Med. 2008;215(04):299–306. doi: 10.1620/tjem.215.299. [DOI] [PubMed] [Google Scholar]
- 122.Park D, Kim S M, Min S I, Ha J, Kim I G, Min S K. Inhibition of intimal hyperplasia by local perivascular application of rapamycin and imatinib mesilate after carotid balloon injury. J Korean Surg Soc. 2013;85(06):296–301. doi: 10.4174/jkss.2013.85.6.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leppänen O, Rutanen J, Hiltunen M O et al. Oral imatinib mesylate (STI571/Gleevec) improves the efficacy of local intravascular vascular endothelial growth factor-C gene transfer in reducing neointimal growth in hypercholesterolemic rabbits. Circulation. 2004;109(09):1140–1146. doi: 10.1161/01.CIR.0000117234.08626.7C. [DOI] [PubMed] [Google Scholar]
- 124.Sevim K Z, Silistreli O, Gorgu M, Sevim O, Ergur B. Short-term vasculoprotective effects of imatinib mesylate on intimal hyperplasia of arterial anastomosis: An experimental study using a rabbit model. Can J Plast Surg. 2012;20(04):223–228. doi: 10.1177/229255031202000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu J, Chen Z, Bhalla K N.Treatment of restenosis and stenosis with dasatinib (2014)USF Patents. 152
- 126.Sánchez-Ortega I, Servitje O, Arnan M et al. Dasatinib as salvage therapy for steroid refractory and imatinib resistant or intolerant sclerotic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(02):318–323. doi: 10.1016/j.bbmt.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 127.Marinelli Busilacchi E, Olivieri J, Viola N et al. The exaggerated collagen expression in Gvhd-fibroblasts is effectively inhibited by therapeutic concentration of Nilotinib. Blood. 2016;128(22):4597. [Google Scholar]
- 128.Shiha G E, Abu-Elsaad N M, Zalata K R, Ibrahim T M. Tracking anti-fibrotic pathways of nilotinib and imatinib in experimentally induced liver fibrosis: an insight. Clin Exp Pharmacol Physiol. 2014;41(10):788–797. doi: 10.1111/1440-1681.12286. [DOI] [PubMed] [Google Scholar]
- 129.Giles F J, O'Dwyer M, Swords R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2009;23(10):1698–1707. doi: 10.1038/leu.2009.111. [DOI] [PubMed] [Google Scholar]
- 130.Rix U, Hantschel O, Dürnberger G et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110(12):4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 131.Pardanani A, Tefferi A. Imatinib targets other than bcr/abl and their clinical relevance in myeloid disorders. Blood. 2004;104(07):1931–1939. doi: 10.1182/blood-2004-01-0246. [DOI] [PubMed] [Google Scholar]
- 132.Bantscheff M, Eberhard D, Abraham Y et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25(09):1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 133.Weisberg E, Manley P W, Breitenstein W et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(02):129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 134.Buchdunger E, Cioffi C L, Law N et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295(01):139–145. [PubMed] [Google Scholar]
- 135.Manley P W, Drueckes P, Fendrich G et al. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta. 2010;1804(03):445–453. doi: 10.1016/j.bbapap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 136.Fabian M A, Biggs W H, III, Treiber D K et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(03):329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 137.Shi H, Zhang C J, Chen G Y, Yao S Q. Cell-based proteome profiling of potential dasatinib targets by use of affinity-based probes. J Am Chem Soc. 2012;134(06):3001–3014. doi: 10.1021/ja208518u. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Wang Z, Kwong S Q et al. Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55(03):612–625. doi: 10.1016/j.jhep.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 139.Sakamoto A, Sugamoto Y, Tokunaga Y et al. Expression profiling of the ephrin (EFN) and Eph receptor (EPH) family of genes in atherosclerosis-related human cells. J Int Med Res. 2011;39(02):522–527. doi: 10.1177/147323001103900220. [DOI] [PubMed] [Google Scholar]
- 140.Khan A A, Sandhya V K, Singh P et al. Signaling network map of endothelial TEK tyrosine kinase. J Signal Transduct. 2014;2014:173026. doi: 10.1155/2014/173026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chislock E M, Ring C, Pendergast A M. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival. Proc Natl Acad Sci U S A. 2013;110(30):12432–12437. doi: 10.1073/pnas.1304188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boulanger C M, Loyer X, Rautou P E, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14(05):259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 143.Aghel N, Delgado D H, Lipton J H. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc Health Risk Manag. 2017;13:293–303. doi: 10.2147/VHRM.S108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Damrongwatanasuk R, Fradley M G. Cardiovascular complications of targeted therapies for chronic myeloid leukemia. Curr Treat Options Cardiovasc Med. 2017;19(04):24. doi: 10.1007/s11936-017-0524-8. [DOI] [PubMed] [Google Scholar]
- 145.Valent P, Hadzijusufovic E, Hoermann G et al. Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res. 2017;59:47–54. doi: 10.1016/j.leukres.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.García-Gutiérrez V, Jiménez-Velasco A, Gómez-Casares M T, Sánchez-Guijo F, López-Sendón J L, Steegmann Olmedillas J L. [Cardiovascular management of patients with chronic myeloid leukemia from a multidisciplinary perspective, and proposing action protocol by consensus meeting] Med Clin (Barc) 2016;146(12):5610–5.61E10. doi: 10.1016/j.medcli.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 147.Aman J, van Bezu J, Damanafshan A et al. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126(23):2728–2738. doi: 10.1161/CIRCULATIONAHA.112.134304. [DOI] [PubMed] [Google Scholar]
- 148.Kim S R, Suh W. Beneficial effects of the Src inhibitor, dasatinib, on breakdown of the blood-retinal barrier. Arch Pharm Res. 2017;40(02):197–203. doi: 10.1007/s12272-016-0872-z. [DOI] [PubMed] [Google Scholar]
- 149.Sukegawa M, Wang X, Nishioka C et al. The BCR/ABL tyrosine kinase inhibitor, nilotinib, stimulates expression of IL-1β in vascular endothelium in association with downregulation of miR-3p. Leuk Res. 2017;58:83–90. doi: 10.1016/j.leukres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 150.Wolf A M, Wolf D, Rumpold H et al. The kinase inhibitor imatinib mesylate inhibits TNF-alpha production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci U S A. 2005;102(38):13622–13627. doi: 10.1073/pnas.0501758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fraser C K, Lousberg E L, Kumar R, Hughes T P, Diener K R, Hayball J D. Dasatinib inhibits the secretion of TNF-alpha following TLR stimulation in vitro and in vivo. Exp Hematol. 2009;37(12):1435–1444. doi: 10.1016/j.exphem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 152.Seggewiss R, Loré K, Greiner E et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105(06):2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 153.Dietz A B, Souan L, Knutson G J, Bulur P A, Litzow M R, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104(04):1094–1099. doi: 10.1182/blood-2003-12-4266. [DOI] [PubMed] [Google Scholar]
- 154.Chen J, Schmitt A, Chen Bet al. Nilotinib hampers the proliferation and function of CD8+ T lymphocytes through inhibition of T cell receptor signalling J Cell Mol Med 200812(5B):2107–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perros F, Montani D, Dorfmüller P et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(01):81–88. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 156.Canning P, Tan L, Chu K, Lee S W, Gray N S, Bullock A N. Structural mechanisms determining inhibition of the collagen receptor DDR1 by selective and multi-targeted type II kinase inhibitors. J Mol Biol. 2014;426(13):2457–2470. doi: 10.1016/j.jmb.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma. 2008;49(04):615–619. doi: 10.1080/10428190801896103. [DOI] [PubMed] [Google Scholar]
- 158.Remsing Rix L L, Rix U, Colinge J et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia. 2009;23(03):477–485. doi: 10.1038/leu.2008.334. [DOI] [PubMed] [Google Scholar]
- 159.Green M R, Newton M D, Fancher K M. Off-Target Effects of BCR-ABL and JAK2 Inhibitors. Am J Clin Oncol. 2016;39(01):76–84. doi: 10.1097/COC.0000000000000023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


