Abstract
Changes in large-scale brain networks that accompany mild traumatic brain injury (mTBI) were investigated using functional magnetic resonance imaging (fMRI) during the N-back working memory task at two cognitive loads (1-back and 2-back). Thirty mTBI patients were examined during the chronic stage of injury and compared to 28 control participants. Demographics and behavioral performance were matched across groups. Due to the diffuse nature of injury, we hypothesized that there would be an imbalance in the communication between task-positive and Default Mode Network (DMN) regions in the context of effortful task execution. Specifically, a graph-theoretic measure of modularity was used to quantify the extent to which groups of brain regions tended to segregate into task-positive and DMN sub-networks. Relative to controls, mTBI patients showed reduced segregation between the DMN and task-positive networks, but increased functional connectivity within the DMN regions during the more cognitively demanding 2-back task. Together, our findings reveal that patients exhibit alterations in the communication between and within neural networks during a cognitively demanding task. These findings reveal altered processes that persist through the chronic stage of injury, highlighting the need for longitudinal research to map the neural recovery of mTBI patients.
Keywords: mild traumatic brain injury, task-positive, Default Mode Network, segregation, working memory
Introduction
Traumatic brain injury (TBI) is one of the most prevalent neurological disorders in the United States with a yearly incidence as high as 5 per 1000 people [Bazarian et al., 2005]. Mild TBI (mTBI) which is characterized by a Glasgow Coma Score (GCS) of 13–15 accounts for approximately 75% of reported cases of TBI [Faul et al., 2010]. In addition to the high incidence of civilian cases, mTBI has been referred to as the “signature injury” of the recent military conflicts in Iraq and Afghanistan due to the high number of soldiers experiencing blast-related head injuries [DePalma and S. W. Hoffman, 2016]. MTBI patients who do not present with robust radiological deficits but experience symptoms represent a unique clinical challenge, and methods that characterize functional disruptions may be particularly attractive. An even greater challenge is to understand the long-term brain sequelae for these individuals with only subtle behavioral impairments but persistent symptom complaints. Not only are post-concussive symptoms varied, including sensory, somatic, cognitive, and neuropsychological dimensions [Dischinger et al., 2009], but there is a significant overlap with symptoms associated with post-traumatic stress disorder (PTDS) [Stein and T. W. McAllister, 2009]. Therefore, the ability to objectively characterize functional brain disruptions is important in distinguishing alterations linked to head injury from those associated with comorbid neuropsychological disorders, as well for the development of personalized clinical interventions for such patients.
Brain data are increasingly investigated at the network level [Pessoa, 2014; Power et al., 2011; Rubinov and O. Sporns, 2010]. A commonly investigated network is the Default Mode Network (DMN), which comprises regions with reduced activity during task-related conditions but increased activity during “rest” conditions [Fox et al., 2005; Greicius et al., 2003; Raichle et al., 2001]. In contrast, numerous regions are recruited during externally directed behavior [Fox et al., 2005]. It has been shown that these two networks are anti-correlated at rest [Fox et al., 2005]. Reductions in functional connectivity have been reported in mTBI patients within the DMN [Iraji et al., 2014; Johnson et al., 2012; Mayer et al., 2011; Zhou et al., 2012] and within the task-positive network [Mayer et al., 2011; Shumskaya et al., 2012]. However, increased functional connectivity between the DMN and task-positive regions during resting conditions has also been observed at various stages following head injury [Mayer et al., 2011; Sours et al., 2013; Sours et al., 2015]; see [Mayer et al., 2015] for a comprehensive review of current literature). The latter findings suggest an imbalance in the communication during resting conditions between the internally directed, task-negative regions, and the externally directed task-positive regions following TBI.
With few exceptions [Bonnelle et al., 2011; Caeyenberghs et al., 2012; Caeyenberghs et al., 2013; van der Horn et al., 2015] previous studies addressing functional connectivity following TBI have relied on probing the brain during rest. Resting state data can be easily acquired in the acute stage of TBI when the clinical condition of the patient may limit the ability to obtain task-related data. However, resting state functional data likely provide researchers with a limited understanding of alterations in processing when the brain is cognitively challenged.
The interpretation of existing studies of functional connectivity in mTBI is also difficult, because several of them targeted individuals with severe TBI or included TBI patients with a range of severities [Bonnelle et al., 2011; Bonnelle et al., 2012; Caeyenberghs et al., 2012; Hillary et al., 2011; Marquez de la Plata et al., 2011; Rigon et al., 2016; Sharp et al., 2011] making inferences regarding milder injuries problematic. Furthermore, previous studies have included patients at substantially different stages of recovery after injury (e.g., 3 to 73 months) [Bonnelle et al., 2012; Sharp et al., 2011]. Finally, whereas some studies have investigated functional connectivity during task states, the behavioral performance across groups was variable, leading to potential confounds in between-group comparisons [Bonnelle et al., 2012; Caeyenberghs et al., 2012; Caeyenberghs et al., 2013; van der Horn et al., 2015].
Taken together, while advances have been made in understanding changes in brain systems following TBI through examining resting-state functional connectivity, important gaps remain in our knowledge of alterations in network-level properties during cognitive processes. In particular, since mTBI involves relatively minor and subtle injuries, it is conceivable that deficits in network-level processing only become evident when the patient is cognitively taxed. Here, we sought to investigate changes in large-scale brain networks following mTBI while addressing the problems noted above. Guided by our previous work showing alterations in between-network connectivity during resting state conditions [Sours et al., 2013; Sours et al., 2015], as well as the diffuse and heterogeneous nature of injury, we hypothesized that, relative to controls, participants with mTBI would exhibit decreased segregation between DMN and task-positive networks – i.e., reduced network antagonism – in the context of effortful task execution. Our central goal was to characterize changes in between-network segregation in chronic mTBI patients as a function of cognitive demands during a working memory task.
Materials and Methods
Participants
Thirty mTBI patients were included in the analysis (22 males, age 45.9 +/−16.9 years). MTBI patients were recruited from the R. Adam Cowley Shock Trauma Center at the University of Maryland Medical Center, and included in this study based upon an admission GCS of 13–15, and (i) a positive clinical CT or (ii) a mechanism of injury consistent with trauma and reported loss of consciousness or post-traumatic amnesia. Patients were screened and excluded for history of neurological disorders, psychological disorders (with the exception of a history of depression or anxiety disorders), seizure disorders, history of previous brain injury requiring hospitalization, and contraindications to MRI. Patients were scanned during the chronic stage of injury (on average 210 days after injury, range 141–302 days). Results were compared to a group of 28 neurologically intact controls (18 males, mean age 40.0 +/− 18.9 years) who were recruited from the local population. Mechanisms of injury included 6 motor vehicle accidents, 14 falls, 7 assaults, and 3 hits with blunt objects. Due to our inclusion criteria, our dataset consists of both complicated (positive admission CT) and uncomplicated mTBI patients (negative admission CT); twelve patients (40%) had positive admission CT scans. Patient and control demographics and clinical details are shown in Table 1. The study was approved by the University of Maryland, Baltimore, Institutional Review Board and written informed consent was obtained from all participants.
Table 1:
Demographics and Behavior
| Mild TBI Mean (SD) |
Control Mean (SD) |
P-value | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| N | 30 | 28 | NA |
| Age | 45.0 (16.9) | 40.0 (18.8) | 0.445 |
| Education | 14.3 (3.0) | 15.5 (2.2) | 0.250 |
| GCS | 14.7 (1.0) | NA | NA |
| Time since Injury (days) | 210 (37) | NA | NA |
| Hit with blunt object | 3 (10%) | ||
| Positive CT | 12 (40%) | NA | NA |
| BEHAVIOR | |||
| 1-Bk RT (ms) | 719.8 (130.3) | 687.7 (137.0) | 0.366 |
| 1-Bk ACC | 93.2 (4.1) | 92.9 (5.9) | 0.852 |
| 1-Bk d’ | 3.20 (0.48) | 3.23 (0.53) | 0.840 |
| 2-Bk RT (ms) | 900.5 (154.4) | 873.0 (168.8) | 0.518 |
| 2-Bk ACC | 83.8 (10.8) | 87.9 (8.8) | 0.121 |
| 2-Bk d’ | 2.40 (0.91) | 2.80 (0.87) | 0.093 |
| MACE | 26.2 (2.7) | 26.1 (3.0) | 0.881 |
| RPQ Total | 17.6 (16.2) | NA | NA |
Scanning Protocol and MRI Acquisition
Imaging was performed using a 3T Siemens Tim Trio Scanner (Siemens Medical Solutions; Erlangen, Germany) using a 12-channel receiver-only head coil. During each visit, the imaging session included two functional scans (T2*-weighted images using single-shot EPI: TE = 30 ms, TR =2300 ms, FOV = 230 mm, resolution = 96 × 96, 36 4-mm thick axial slices) while the participants performed a cognitive task, and a high-resolution structural scan (T1-weighted-MPRAGE: TE = 3.44 ms, TR = 2250ms, TI = 900ms, flip angle = 9°, resolution = 256 × 256 × 96, FOV = 22 cm, slice thickness= 1.5mm).
N-back Paradigm
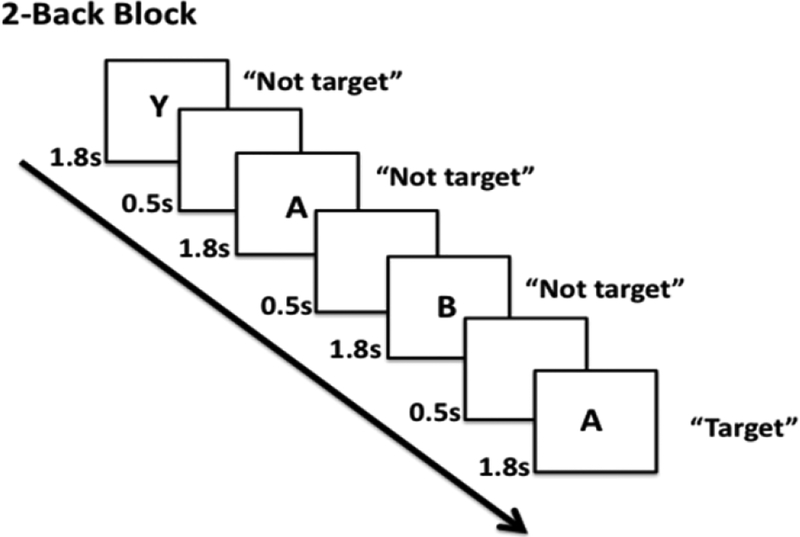
Participants were shown a stream of letters and were asked to detect if the letter matched the previously presented letter (1-back condition), or if the letter matched the one presented two letters before (2-back condition) (Figure 1). Training was performed outside of the scanner prior to scanning to ensure that all participants had a full understanding of the task. The task was presented inside the scanner using Eprime 2.0 software (Psychology Software Tools, Pittsburgh, PA). The 1-back condition was always performed prior to the 2-back condition. Each “run” consisted of 5 blocks of one of the task conditions (i.e., 1-back or 2-back); each block lasted 48.3 seconds and was followed by 23 seconds of fixation. Prior to the beginning of the first block, 11.5 seconds (5 TRs) of instructions about the task were provided to the participant. The task consisted of the presentation of a sequence of letters, each of which was presented for 1.8 seconds followed by a 0.5 second inter-stimulus interval. Participants responded with a two-button MR compatible response box. Accuracy and average reaction time for correct responses were calculated for each condition. In addition, the standard sensitivity index (d’) was calculated [Green and J. A. Swets, 1966; Macmillan and C. D. Creelman, 2005; Rossi et al., 2016].
Figure 1:
N-back working memory paradigm. Participants were shown a stream of letters and were asked to determine if the letter they saw matched the previous letter (1-back condition; not shown); or if the letter they say matched two letters before (2-back condition).
We required a minimum level of accuracy to ensure that we only analyzed data from participants who earnestly attempted to complete the task. The cutoffs were 85% for 1-back and 75% for 2-back, which were chosen as rough indicators of “attentive performance”. For instance, the 1-back task is rather easy and performance is frequently 90% or better; the 2-back task is more challenging and performance is frequently above 80% [Chen et al., 2012]. Because experimental runs contained a single task condition, to maximize the data available for analysis, the cutoffs were applied per run, not by subject (thus, for instance, a given participant could contribute data for the 2-back, but not be considered for the 1-back condition). The resulting datasets in the final analysis included 55 runs for the 1-back condition (27 control; 28 chronic mTBI), and 64 runs for the 2-back condition (27 control; 27 chronic mTBI).
Behavioral Assessment
The level of cognitive functioning was assessed by administering the Military Acute Concussion Evaluation (MACE) to each participant [McCrea et al., 2000]. Four controls and one mTBI patient did not complete the MACE. Self-reported symptoms were collected using the Modified Rivermead Post Concussive Questionnaire (RPQ) [King et al., 1995]. The RPQ asks participants to rate a series of 22 common post-concussive symptoms on a scale of 0 (not experienced at all) to 4 (a severe problem). The sum of the ratings on all of the symptoms is reported (Table 1).
Behavioral Data Analysis
Independent t-tests were used to assess the differences in demographic characteristics and behavioral scores between the control and patient groups (Table 1).
Group-Level Model
Group analysis was performed with a linear mixed model comprised of two fixed effects factors (task difficulty and group condition) and their interaction. Linear mixed models correctly account for correlated data (here, participants with data for both conditions), while not requiring that all “data cells” be filled (here, participants who only completed one of the tasks), and constitute a powerful extension of general linear models [Pinheiro and D. M. Bates, 2000]. In the analysis, subject was used as a random factor, with difficulty varying within subject. We used the lmer function of the lmerTest package [Alexandra Kuznetsova et al., 2014], which is an extension of the lme4 package [Bates et al., 2014]. As per default, factor levels were encoded as simple treatment contrasts (1-back was the reference group for task difficulty while controls were the reference group for condition). A positive estimate for the effect of difficulty would thus indicate that the dependent variable is on average greater at 2-back compared to 1-back. A positive estimate for the effect of group condition similarly indicates that the dependent variable was generally higher for the patient group compared to the control group.
Functional MRI Analysis
Preprocessing
Preprocessing was performed with tools primarily from the AFNI software package [Cox, 1996]. Skull stripping was completed using the SPM toolbox except for one dataset that required FSL’s Brain Extraction Tool (BET) [Smith, 2002] to achieve appropriate quality. Volumes were slice-time corrected using Fourier interpolation such that all slices were aligned to the first acquisition slice. Six-parameter rigid-body motion correction was performed with Fourier interpolation, spatially registering all volumes to the last functional volume which was acquired closest in time to the high-resolution anatomical scan [Cox and A. Jesmanowicz, 1999]. Each subject’s high-resolution T1-MPRAGE anatomical volume was spatially registered to the “TT_N27” template (in Talairach space, [Talairach and P. Tournoux, 1988]) using a 12-parameter affine transformation. The spatial registration for each subject was visually inspected to ensure accuracy. This same transformation was then applied to the functional data so that all data were in standard space and resampled to 2 mm isotropic voxels. All volumes were spatially smoothed using a Gaussian filter (6 mm FWHM). Finally, the signal intensity of each voxel was scaled to a mean of 100 (separately for each run), which allowed the interpretation of the estimated regression coefficients in terms of percent signal change. Excessive motion volumes (1 mm or higher frame-to-frame displacement) were censored from analysis. Only voxels with a 25% or higher likelihood of being gray matter were considered for further analysis (utilizing the “TT_N27_GW” atlas available in AFNI [Eickhoff et al., 2005]). Finally, the first 5 volumes were excluded per run (accounting for scanner equilibrium effects, and as the instruction period was of no interest).
Activation Analysis
Each participant’s fMRI data were analyzed using multiple linear regression within AFNI. The design matrix contained 19 regressors: a box-car covering the entire block period and 18 nuisance regressors: 6 motion regressors; 6 motion derivative regressors; 4 slow-drift regressors (constant, linear, quadratic, and cubic terms); and 2 transient regressors to model block onsets and block offsets (1 sec duration each). The box-car and the transient regressors were convolved with a model of the canonical hemodynamic response [Cohen, 1997]. Regression coefficients associated with the box-car regressor were tested in a voxelwise manner as described above (see Group-Level Model section).
Network Analysis
Node Definition
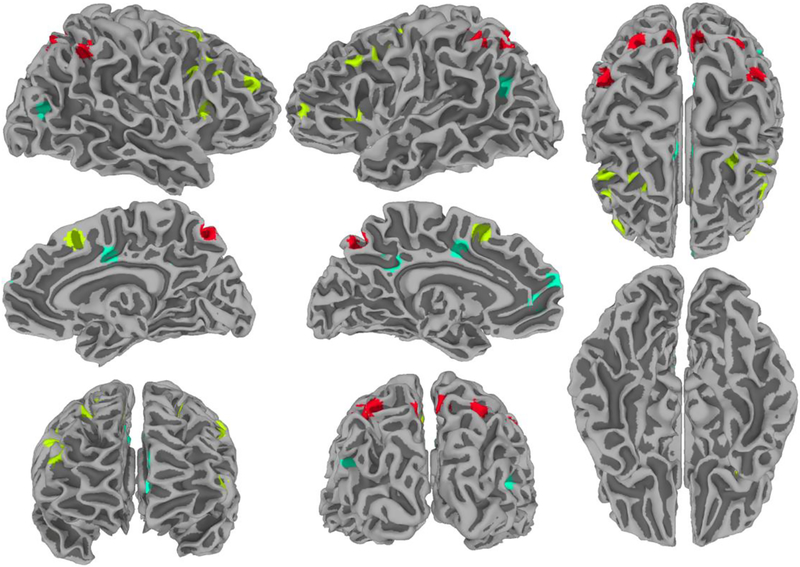
The voxelwise main effect of task difficulty including all participants (mTBI and control) was used to define a space of voxels within which peak activations and deactivations in frontal, parietal, and DMN regions (the latter based on sites exhibiting deactivation) were used to determine the center of spherical ROIs (Figure 2). Note that the procedure does not introduce circularity because activations and correlations (used for network analysis) are orthogonal measures. Each ROI was a 6-mm radius sphere comprising voxels with a 25% or higher likelihood of being gray matter (utilizing the “TT_N27_GW” atlas available in AFNI [Eickhoff et al., 2005]). We focused on frontal, parietal and DMN regions (Table 2) to test the specific hypothesis that interactions between task-positive and DMN regions engaged during effortful tasks are altered in mTBI patients. Whereas performance of working memory tasks involves a broad set of cortical regions, parietal and frontal regions may be involved in somewhat different processes [D’Esposito, 2007; Pessoa et al., 2002; Pessoa and L. G. Ungerleider, 2004]. Therefore, we opted to divide the task positive regions into frontal and parietal regions.
Figure 2:
Nodes used in the graph-theoretical analysis. Visualization of task-positive (parietal and frontal) and DMN regions of interest (ROIs) used in the modularity analysis. Parietal ROIs are shown in red, frontal ROIs are shown in yellow, and default ROIs are shown in turquoise. ROI generation was restricted to those voxels that showed a significant main effect of task difficulty. ROIs are displayed on a surface projection of the TT_N27 template of the AFNI package.
Table 2:
ROI Coordinates
| Region | Abbreviation | Group | X | Y | Z |
|---|---|---|---|---|---|
| TASK POSITIVE | |||||
| Inferior parietal sulcus left | InfParSul_L | Parietal | 29 | 65 | 48 |
| Inferior parietal lobule left | InfParLob_L | Parietal | 39 | 47 | 48 |
| Precuneus left | PreCun_L | Parietal | 5 | 67 | 46 |
| Inferior parietal sulcus right | InfParSul_R | Parietal | −27 | 67 | 48 |
| Inferior parietal lobule right | InfParLob_R | Parietal | −45 | 47 | 42 |
| Precuneus right | PreCun_R | Parietal | −7 | 67 | 50 |
| Medial premotor (SMA) left | MedPreSma_L | Frontal | 5 | −11 | 50 |
| Medial premotor (SMA) right | MedPreSma_R | Frontal | −5 | −17 | 47 |
| Frontal eye field left | FroEyeFie_L | Frontal | 23 | 1 | 51 |
| Frontal eye field right | FroEyeFie_R | Frontal | −25 | −11 | 51 |
| Anterior Insula left | AntIns_L | Frontal | 29 | −23 | 8 |
| Anterior Insula right | AntIns_R | Frontal | −35 | −17 | 12 |
| Inferior frontal junction left | InfFroJun_L | Frontal | 45 | −3 | 40 |
| Inferior frontal junction right | InfFroJun_R | Frontal | −46 | −9 | 33 |
| Middle frontal gyrus left | MidFroGyr_L | Frontal | 49 | −19 | 36 |
| Middle frontal gyrus right | MidFroGyr_R | Frontal | −49 | −21 | 34 |
| Superior frontal gyrus left | SupFroGyr_L | Frontal | 45 | −47 | 12 |
| Superior frontal gyrus right | SupFroGyr_R | Frontal | −41 | −43 | 25 |
| TASK NEGATIVE | |||||
| Posterior cingulate cortex left | PosCinCor_L | Default | 3 | 45 | 32 |
| Mid cingulate cortex left | MidCinCor_L | Default | 5 | 3 | 40 |
| Mid cingulate cortex right | MidCinCor_R | Default | −4 | 5 | 37 |
| Ventro medial prefrontal cortex | VenMedPreCor | Default | 7 | −45 | 6 |
| Medial prefrontal cortex | MedPreCor | Default | 1 | −57 | 20 |
| Middle temporal gyrus left | MidTemGyr_L | Default | 43 | 61 | 22 |
| Middle temporal gyrus right | MidTemGyr_R | Default | −39 | 69 | 10 |
Edge Definition
For each ROI, the average residual time series across voxels within the ROI was extracted. The residual time series consisted of the fMRI data once all specified regressors were accounted for. A correlation matrix was then computed and consisted of the pairwise correlations between all pairs of ROIs. Using residualized time series data is important to avoid potential correlations of no interest due to the predictable task structure. In particular, block onsets and offsets could have artifactually inflated correlations at these time points simply due to correlated large increases and decreases of activation (for related strategies, please refer to [Fox et al., 2007]). We used a robust measure of correlation to mitigate the potential impact of “extreme” points that frequently distort non-robust measures of linear association. To find the robust correlation of two time series, we first calculated their z-scores. The standardized series were then fit to a linear model via iteratively reweighted least squares, a process that down-weights potential outliers. Pearson’s correlation coefficient was finally calculated for the reweighted series. We made use of the Robust Linear Models module of the Python package “statsmodels” [Seabold and J. Perktold, 2010].
Modularity Analysis
To probe the segregation of the task-positive and DMN networks, we computed the graph theory metric called modularity (see [Newman, 2010] for a complete description of network models). For weighted networks, the modularity, Q, of a given set of group assignments for a graph is based on the total weight of connections found within the assigned communities versus the total weight of connections predicted in a random graph with equivalent degree distribution. Note that the computation of modularity employed here considered negative weights in addition to positive weights [Gomez et al., 2009; Rubinov and O. Sporns, 2011]; thus, negative weights were not set to zero. See [Balenzuela et al., 2010] for a similar application of modularity.
By considering task-positive (frontal plus parietal) ROIs and DMN ROIs as two separate communities (in other words, communities were assumed, and not computed via community detection), we computed modularity as a function of task difficulty and group. High modularity indicates clearly separable sub-networks (here, task-positive and DMN), with relatively strong functional connectivity within each sub-network and relatively weak functional connectivity between sub-networks. Statistical analysis of modularity employed the same approach as the voxelwise analysis (see Group-Level Model section). Effect sizes were calculated based on a method to obtain R2 from generalized linear mixed-effects models [Nakagawa and Holger Schielzeth, 2013]. We provide the so-called conditional effect size (reflecting overall model fit) and the marginal effect size which reflects the proportion of variance explained by the fixed factors (namely, the interaction of average connectivity with cognitive load; see Table 4). The latter refers to the fixed-effects variance divided by the total variance explained by the model and residual variance.
Table 4:
Interaction of Average Connectivity with Cognitive Load
| Estimate | Std Error | t | p-value | Conditional Effect Size | Marginal Effect Size | |
|---|---|---|---|---|---|---|
| WITHIN NETWORK | ||||||
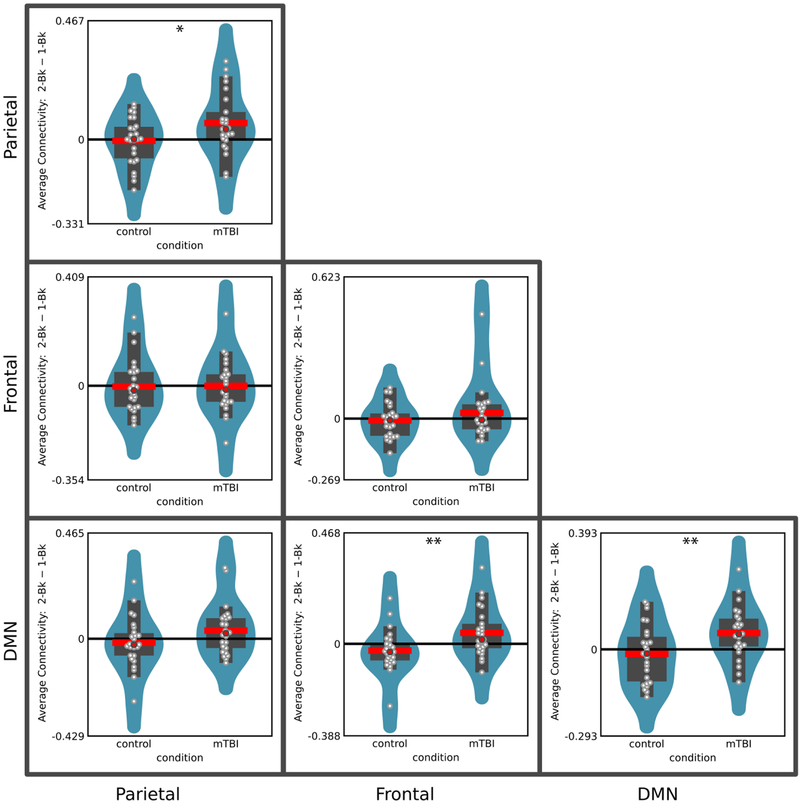
| Parietal | 0.0656 | 0.0291 | 2.256 | 0.028 | 74.8% | 2.9% |
| Frontal | 0.0365 | 0.0269 | 1.355 | 0.181 | 71.8% | 3.4% |
| DMN | 0.0716 | 0.0255 | 2.804 | 0.007 | 77.7% | 3.2% |
| BETWEEN NETWORK | ||||||
| Parietal-Frontal | 0.0019 | 0.0274 | 0.069 | 0.945 | 68.4% | 0.3% |
| Parietal - DMN | 0.0541 | 0.0299 | 1.181 | 0.076 | 76.0% | 3.8% |
| Frontal - DMN | 0.0774 | 0.0259 | 2.993 | 0.004 | 77.8% | 6.5% |
Results
Behavioral Results
No significant differences were detected between mTBI and control participants in age (p=0.445) or education (p=0.250). A summary of demographic and clinical information is provided in Table 1. Accuracy, mean reaction time of correct responses, and d’ were not significantly different between the mTBI group and the control group for both task conditions (1-back and 2-back) (p values > 0.5; Table 1). There was no significant difference in the MACE between the control group and mTBI patients (p=0.881) (Table 1).
Network Modularity Analysis
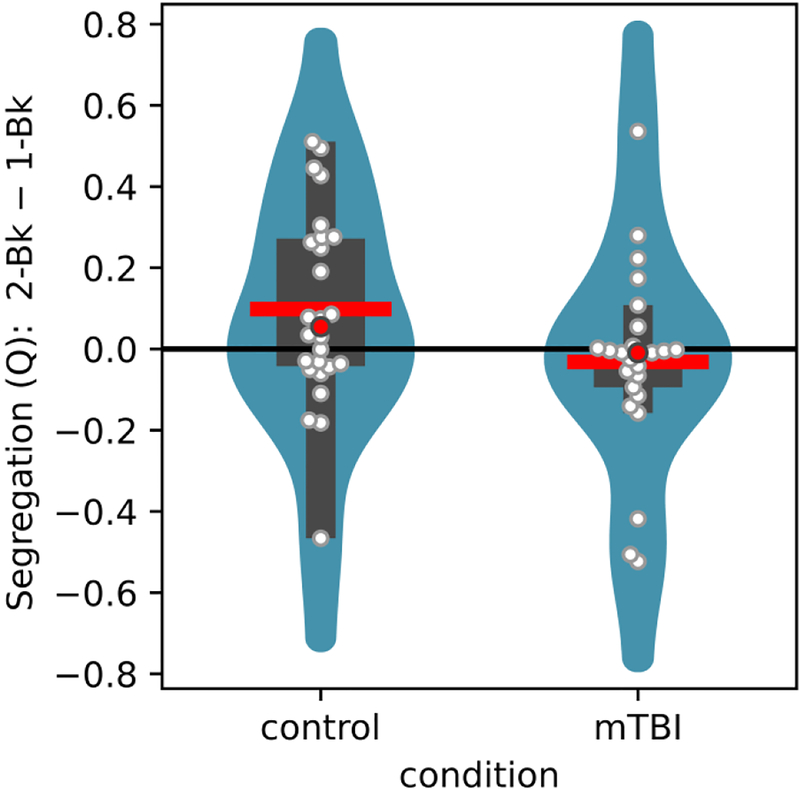
We investigated how the network-level property of modularity (Q) was altered as a function of task difficulty (1-back vs. 2-back) and group condition (control vs mTBI) by focusing on the changing relationships among task-positive (frontal and parietal) and task-negative regions. Note that our objective was to characterize potential differences in modularity by assuming two communities of brain regions (task positive and task negative); thus modularity was not used to “discover” the networks. The pairwise robust correlation between all pairs of ROIs was determined and connectivity matrices created. Higher values of Q indicated that task-positive and DMN regions tended to segregate more strongly into separate communities. We detected a significant interaction between task difficulty and group condition; in contrast to the control group, mTBI patients responded to the more demanding task with decreased modularity (Figure 3; for statistical values, see Table 3).
Figure 3:
Results from graph-theoretical analysis. Segregation values as assessed by the graph-theoretic measure of modularity (Q), illustrating the interaction pattern between task difficulty and group conditions. For each group, the difference between the 2-back and 1-back is shown by violin plots. The violin shows a smoothed estimate of the distribution; the red line shows the mean; the red circle shows the median; the wider rectangle shows the inter-quantile range; the thinner rectangle shows 1.5 times the inter-quantile range (as long as it contains data points); the white circles show the actual data points.
Table 3:
Modularity
| Estimate | Std Error | t | p-value | |
|---|---|---|---|---|
| Group | 0.019 | 0.042 | 0.328 | 0.744 |
| Difficulty | 0.097 | 0.058 | 2.189 | 0.033 |
| Interaction | −0.140 | 0.063 | −2.218 | 0.031 |
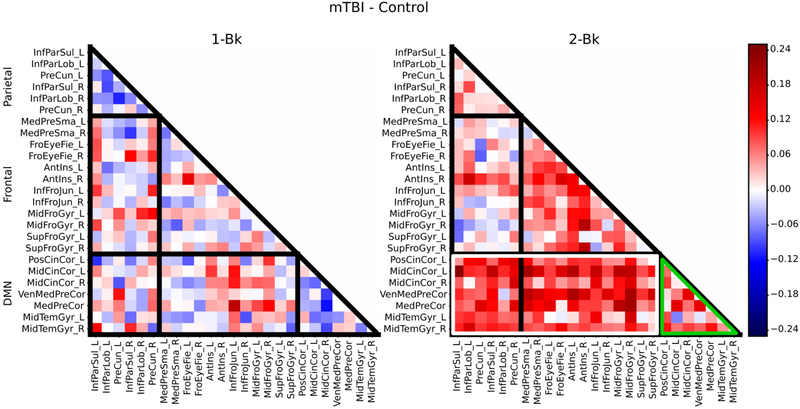
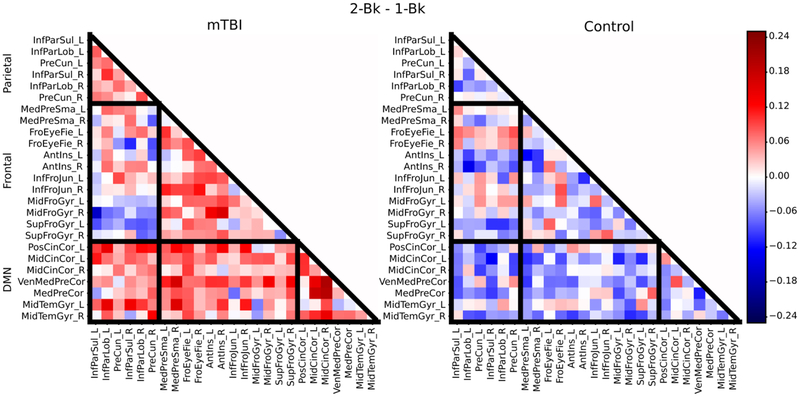
These initial findings were further characterized by inspecting the underlying changes in average connectivity (also called “strength”) within and between subdivisions of ROIs (frontal and parietal ROIs that were task-positive and the remaining DMN ROIs): increases within task-positive regions, increases within the DMN regions, or decreases between the task-positive and DMN regions may underlie the observed increase in modularity. Based on average correlation matrices for each group (mTBI and control) for the 1-back condition and the 2-back condition, subtractions of the correlation matrices were computed, including a subtraction between the mTBI and the control group for each condition to visualize differences (Figure 4), and a 2-back minus 1-back subtraction to examine the influence of cognitive load on the correlation structure (Figure 5). While some differences in within- and between-network connectivity between groups were noted during the 1-back condition (Figure 4), more substantial differences were observed during the more cognitively challenging 2-back condition (Figure 5). During the 2-back condition (Figure 4), there was greater connectivity within the DMN regions (see green outlines) as well as between DMN regions and task-positive regions in the mTBI group compared to the controls (see white outlines). With respect to the effect of task condition (Figure 5), for control participants, the 2-back minus the 1-back subtraction revealed a few strengthened connections (involving mainly frontal regions, such as the frontal eye fields and middle frontal gyrus) when difficulty increased, while decreased connectivity was found elsewhere. A markedly different pattern was observed for patients who exhibited an overall increase in both within network and between network connectivity across both DMN and task-positive regions (Figure 5).
Figure 4:
Average correlation matrices illustrating both within and between network connectivity values for the subtraction of the mTBI group minus the control group for the 1-back condition (left) and the 2-back condition (right). During the 2-back condition, there was greater connectivity within the DMN regions (green outlines) as well as between DMN regions and task-positive regions (see white outlines) in the mTBI group compared to controls.
Figure 5:
Average correlation matrices illustrating both within and between network connectivity values for the subtraction of the 2-back condition minus the 1-back condition for the mTBI group (left) and the control group (right). The black boundaries separate the parietal, frontal, and default clusters (from top of matrix to bottom of matrix respectively).
To quantify potential differences in connectivity noted in the context of Figures 4 and 5, for every participant, we determined the average pairwise connectivity within the frontal, parietal, and DMN clusters of ROIs, as well as the average pairwise connectivity between parietal and frontal, between frontal and DMN, and between parietal and DMN clusters of ROIs (Figure 6). We tested task difficulty by group interactions by using the same group-level analysis employed previously. Interactions were detected within both the parietal and the DMN clusters, as well as between frontal and DMN clusters (see Table 4 for complete results). The latter finding of increased between-network connectivity (which survived correction for multiple comparisons) likely contributed to the reduction in modularity observed in mTBI patients during the 2-back task (the higher the between-network connectivity, the lower the modularity). These findings suggest that the effect of increasing cognitive demands on both within- and between-network connectivity is altered in the mTBI participants compared to control participants.
Figure 6:
Average within- and between-cluster functional connectivity was used to illustrate the interaction pattern between task difficulty and group conditions. Interactions between task difficulty and group conditions are noted with “*” (p<0.05, uncorrected) and “**” (p<0.05, Bonferroni corrected for multiple comparisons). For each group, the difference between the 2-back and 1-back is shown by violin plots. The violin shows a smoothed estimate of the distribution; the red line shows the mean; the red circle shows the median; the wider rectangle shows the inter-quantile range; the thinner rectangle shows 1.5 times the inter-quantile range (as long as it contains data points); the white circles show the actual data points.
Discussion
In our study, mTBI patients showed marked changes in how a demanding cognitive task engages large-scale networks despite equated behavioral performance. At a broad level, healthy controls responded to higher cognitive loads with increased segregation between DMN and task-positive regions, while mTBI instead showed decreased segregation. This can be explained primarily by a distinct increase in connectivity between task-positive and DMN regions as shown by patients in response to increasing difficulty, where controls typically showed reduced communication between these two networks. At a finer subdivision of these networks, we also found differences within parietal and DMN regions. Patients demonstrated strengthening communication within these areas for increased task difficulty, whereas controls (if anything) demonstrated weakened connections. Next, we discuss the implications of our findings in the context of understanding the effect of mTBI on large-scale neural network changes.
Several groups have described alterations in functional connectivity following mTBI; however, groups investigating functional connectivity in mild TBI populations have focused on resting-state scans [Johnson et al., 2012; Mayer et al., 2011; Sours et al., 2013; Stevens et al., 2012; Zhou et al., 2012]. Probing functional connectivity during task states complements that collected during the resting-state in important ways, due to alterations in network interactions during task execution. Furthermore, the possibility of considering the functional relationship between multiple pairs of regions simultaneously is provided by graph-theoretic methods [Newman, 2010]. Recent reports have employed network analysis to investigate resting-state functional connectivity [Han et al., 2013; Messe et al., 2013; Pandit et al., 2013]. Whereas these methods have been employed in the general neuroimaging literature mostly during resting conditions, they can also reveal important network-level interactions during task execution [Kinnison et al., 2012; McMenamin et al., 2014; Najafi et al., 2016]. Specifically, here we employed modularity as a measure of the degree of segregation between DMN and task-positive networks across task conditions in a chronic mTBI population.
DMN and task-positive networks are found to be anti-correlated at rest [Fox et al., 2005], therefore exhibiting segregation. During rest, increased functional connectivity between DMN task-positive networks has been associated with reduced performance during working memory [Hampson et al., 2010; Sala-Llonch et al., 2012]. During effortful task execution, reduced anti-correlation (or increased functional connectivity) between networks is associated with increased reaction time on specific trials [Prado and D. H. Weissman, 2011] as well as increased intra-individual variability [Kelly et al., 2008]. Moreover, a direct link between working memory performance and between-network functional connectivity was shown in a control population, where increased functional connectivity between the DMN and task-positive networks during an N-back working memory task was associated with reduced task performance [Sala-Llonch et al., 2012] thus indicating evidence that the ability to adequately separate internally directed focus and externally directed cognitive demands may be important for efficient task performance. Whereas previous work in healthy participants suggests greater segregation with increasing cognitive load [Newton et al., 2011], in the present study an opposite trend was observed for chronic mTBI patients who displayed reduced segregation with increasing task difficulty (Figure 3). Perhaps due to the diffuse but subtle neuronal damage, mTBI patients are unable to enhance the segregation of internally focused attention and external goal directed processes in the face of increased cognitive demands. This altered network communication may be a contributing factor in the cognitive post-concussive symptoms that many mTBI patients experience, including those involving sustained attention [Bonnelle et al., 2011], memory [McAllister et al., 2006; McDowell et al., 1997; Miotto et al., 2010], and cognitive fatigue [Johansson et al., 2009; Ponsford, 2013].
Interestingly, in our study, we also observed that functional connectivity within the DMN regions increased with increasing task difficulty in the chronic mTBI patients but not controls. Previous studies in healthy participants have reported that increased functional connectivity within the DMN was associated with better cognitive performance during working memory tasks [Hampson et al., 2006]. While this initially appears contradictory, it should be noted that hyper-connectivity within DMN regions has been reported following severe TBI during resting conditions [Hillary et al., 2014; Sharp et al., 2011]. In addition, reduced deactivation of DMN regions has been noted during tasks in severe [Bonnelle et al., 2011; Sharp et al., 2011] and mild [Dean et al., 2015; Mayer et al., 2012] TBI populations, providing evidence for an imbalance between internally and externally directed neural networks. Furthermore, greater deactivation of the DMN has been reported in mTBI patients without persistent symptoms compared to those with persistent symptoms [van der Horn et al., 2015] suggesting this imbalance may be associated with post concussive symptom severity. Taken in this context, our findings raise the possibility that the increased connectivity within the DMN observed in the chronic mTBI patients could be viewed as an altered mechanism to partially counteract the reduced segregation between networks. In addition, previous reports have noted over-recruitment of regions associated with the task-positive network in mTBI during the N-back task at higher cognitive loads (possibly to maintain performance at the level of controls) [McAllister et al., 1999; McAllister et al., 2001; Smits et al., 2009; Wylie et al., 2015]. Therefore, increased connectivity within DMN regions may be linked to a compensatory mechanism employed by mTBI patients in order to adequately perform the task when cognitively challenged.
Overall, further work using increasingly challenging cognitive tasks and more extensive neuropsychological testing is needed to further investigate these findings and their association with behavioral performance. For example, for the 2-back condition, although we did not detect a significant group effect, mTBI patients tended to perform slightly worse than control participants. This pattern was more evident for the d’ measure, which may be a more sensitive measure of subtle cognitive deficits than accuracy in this population. It is thus possible that subtle deficits could be picked up with additional behavioral assessments.
Currently, there is controversy in the field regarding the extent of functional recovery of neural networks in mTBI patients at the chronic stage of injury. Some groups have provided evidence of recovery, whereas others have noted persistent functional alterations in the chronic stage [Han et al., 2016; Witt et al., 2010] especially in those patients with persistent symptoms [Dean et al., 2015; Sours et al., 2015]. Our results revealed changes in working memory-based network interactions persisting into the chronic stage of injury. Nevertheless, additional research is needed in the acute stage after injury to examine immediate network level changes, as well as longitudinal data to directly assess the changes in network measures throughout the course of recovery. A more comprehensive understanding of compensatory changes in neural network communication is needed in order to aid clinicians in predicting long-term patient outcomes and administering cognitive interventions to improve these outcomes.
In conclusion, our findings provide evidence of network level adaptations in the communication between brain networks in mTBI patients that are dependent on cognitive load. Specifically, the reduced segregation at the chronic stage of injury was linked to increased communication between networks in response to escalating task difficulty in mTBI patients. These results extend previous findings suggesting an imbalance in the communication between DMN and task-positive networks in mTBI patients during resting-state conditions [Mayer et al., 2011; Sours et al., 2013; Sours et al., 2015] demonstrating that this imbalance is present, if not exaggerated, during effortful task execution. Finally, our results reveal that changes in network interactions persist into the chronic stage of injury, highlighting the need of further longitudinal research to adequately map the neural recovery of mTBI patients.
Funding:
Support for this work was in part provided by the Department of Defense (W81XWH-08-1-0725 & W81XWH-12-1-0098 to R.P.G). Chandler Sours was supported by the NRSA grant from the National Institute of Neurological Disorders and Stroke (1F31NS081984)
Abbreviations:
- AFNI
Analysis of Functional NeuroImages
- fMRI
functional magnetic resonance imaging
- GCS
Glasgow Coma Score
- MACE
Military Acute Concussion Evaluation
- MFG
middle frontal gyrus
- mTBI
mild traumatic brain injury
- PCC
posterior cingulate cortex
- ROI
region of interest
- RPQ
Rivermead Post-Concussion Symptoms Questionnaire
- TBI
traumatic brain injury
Footnotes
Conflict of Interest: All of the authors included in this manuscript declare that he/she has no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Kuznetsova Alexandra, Brockhoff Per Bruun, Christensen Rune Haubo Bojesen (2014): <br />lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package).. R package version 2.0–11. [Google Scholar]
- Balenzuela P, Chernomoretz A, Fraiman D, Cifre I, Sitges C, Montoya P, Chialvo DR (2010): Modular organization of brain resting state networks in chronic back pain patients. Front Neuroinform 4: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B (2014): lme4: Linear mixed-effects models using Eigen and S4 classes.
- Bazarian JJ, McClung J, Shah MN, Cheng YT, Flesher W, Kraus J (2005): Mild traumatic brain injury in the United States, 1998−−2000. Brain Inj 19: 85–91. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ (2012): Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A 109: 4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ (2011): Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci 31: 13442–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Heitger MH, Leunissen I, Dhollander T, Sunaert S, Dupont P, Swinnen SP (2012): Graph analysis of functional brain networks for cognitive control of action in traumatic brain injury. Brain 135: 1293–1307. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Leunissen I, Michiels K, Swinnen SP (2013): Topological correlations of structural and functional networks in patients with traumatic brain injury. Front Hum Neurosci 7: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Wu CH, Liao YP, Hsu HL, Tseng YC, Liu HL, Chiu WT (2012): Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology 264: 844–851. [DOI] [PubMed] [Google Scholar]
- Cohen MS (1997): Parametric analysis of fMRI data using linear systems methods. Neuroimage 6: 93–103. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magentic resonance neuroimages. Computers and Biomedical Research 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A (1999): Real-time 3D image registration for functional MRI. Magn Reson Med 42: 1014–1018. [DOI] [PubMed] [Google Scholar]
- Dean PJ, Sato JR, Vieira G, McNamara A, Sterr A (2015): Multimodal imaging of mild traumatic brain injury and persistent postconcussion syndrome. Brain Behav 5: 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePalma RG, Hoffman SW (2016): Combat blast related traumatic brain injury (TBI): Decade of recognition; promise of progress. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- D’Esposito M (2007): From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 362: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dischinger PC, Ryb GE, Kufera JA, Auman KM (2009): Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma 66: 289–96; discussion 296–7. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald M, Coronado V (2010): Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. . Center of Disease Control. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007): Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56: 171–184. [DOI] [PubMed] [Google Scholar]
- Gomez S, Jensen P, Arenas A (2009): Analysis of community structure in networks of correlated data. Phys Rev E Stat Nonlin Soft Matter Phys 80: 016114. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. 1966. SIgnal Detection Theory and Psychophysics. New York: Wiley. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT (2010): Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging 28: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006): Brain connectivity related to working memory performance. J Neurosci 26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Chapman SB, Krawczyk DC (2016): Disrupted Intrinsic Connectivity among Default, Dorsal Attention, and Frontoparietal Control Networks in Individuals with Chronic Traumatic Brain Injury. J Int Neuropsychol Soc 22: 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Mac Donald CL, Johnson AM, Barnes Y, Wierzechowski L, Zonies D, Oh J, Flaherty S, Fang R, Raichle ME, Brody DL (2013): Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive ‘mild’ blast-related traumatic brain injury. Neuroimage 84C: 76–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Rajtmajer SM, Roman CA, Medaglia JD, Slocomb-Dluzen JE, Calhoun VD, Good DC, Wylie GR (2014): The rich get richer: brain injury elicits hyperconnectivity in core subnetworks. PLoS One 9: e104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, Good DC, Wylie GR (2011): Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol 82: 115–123. [DOI] [PubMed] [Google Scholar]
- Iraji A, Benson RR, Welch RD, O’Neil BJ, Woodard JL, Ayaz SI, Kulek A, Mika V, Medado P, Soltanian-Zadeh H, Liu T, Haacke EM, Kou Z (2014): Resting State Functional Connectivity in Mild Traumatic Brain Injury at the Acute Stage: Independent Component and Seed Based Analyses. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Berglund P, Ronnback L (2009): Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj 23: 1027–1040. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S (2012): Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 59: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39: 527–537. [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT (1995): The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242: 587–592. [DOI] [PubMed] [Google Scholar]
- Kinnison J, Padmala S, Choi JM, Pessoa L (2012): Network analysis reveals increased integration during emotional and motivational processing. J Neurosci 32: 8361–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. 2005. Detection Theory: A User’s Guide. New York: Cambridge University Press. [Google Scholar]
- Marquez de la Plata CD, Garces J, Shokri Kojori E, Grinnan J, Krishnan K, Pidikiti R, Spence J, Devous MDS, Moore C, McColl R, Madden C, Diaz-Arrastia R (2011): Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol 68: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Bellgowan PS, Hanlon FM (2015): Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci Biobehav Rev 49C: 8–18. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA (2011): Functional connectivity in mild traumatic brain injury. Hum Brain Mapp 32: 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Yang Z, Yeo RA, Pena A, Ling JM, Mannell MV, Stippler M, Mojtahed K (2012): A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain Imaging Behav 6: 343–354. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Saykin AJ (2006): Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma 23: 1450–1467. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N (1999): Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 53: 1300–1308. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ (2001): Differential working memory load effects after mild traumatic brain injury. Neuroimage 14: 1004–1012. [DOI] [PubMed] [Google Scholar]
- McCrea M, Kelly J, Randolph C (2000): Standardized Assessment of Concussion (SAC): Manual for Adminstration, Scoring, and Interpretation, Comprehensive Neuropsychological Services; 2nd Eddition. [Google Scholar]
- McDowell S, Whyte J, D’Esposito M (1997): Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia 35: 1341–1353. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L (2014): Network organization unfolds over time during periods of anxious anticipation. J Neurosci 34: 11261–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messe A, Caplain S, Pelegrino-Issac M, Blancho S, Levy R, Aghakhani N, Montreuil M, Benali H, Lehericy S (2013): Specific and Evolving Resting-State Network Alterations<br />in Post-Concussion Syndrome Following Mild Traumatic<br />Brain Injury. PloS one 8: e65470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto EC, Cinalli FZ, Serrao VT, Benute GG, Lucia MC, Scaff M (2010): Cognitive deficits in patients with mild to moderate traumatic brain injury. Arq Neuropsiquiatr 68: 862–868. [DOI] [PubMed] [Google Scholar]
- Najafi M, McMenamin BW, Simon JZ, Pessoa L (2016): Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. Neuroimage 135: 92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H (2013): A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4: 133–142. [Google Scholar]
- Newman ME (2010): Networks: an introduction. New York: Oxford UP. [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC (2011): Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum Brain Mapp 32: 1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit AS, Expert P, Lambiotte R, Bonnelle V, Leech R, Turkheimer FE, Sharp DJ (2013): Traumatic brain injury impairs small-world topology. Neurology 80: 1826–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2014): Understanding brain networks and brain organization. Phys Life Rev 11: 400–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L (2002): Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron 35: 975–987. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG (2004): Neural correlates of change detection and change blindness in a working memory task. Cereb Cortex 14: 511–520. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. 2000. <br />Mixed-effects models in S and S-PLUS. . New York, NY: Springer. [Google Scholar]
- Ponsford J (2013): Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation 32: 803–815. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011): Functional network organization of the human brain. Neuron 72: 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Weissman DH (2011): Heightened interactions between a key default-mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage 56: 2276–2282. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon A, Duff MC, McAuley E, Kramer AF, Voss MW (2016): Is Traumatic Brain Injury Associated with Reduced Inter-Hemispheric Functional Connectivity? A Study of Large-Scale Resting State Networks following Traumatic Brain Injury. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Zammit S, Button KS, Munafo MR, Lewis G, David AS (2016): Psychotic Experiences and Working Memory: A Population-Based Study Using Signal-Detection Analysis. PLoS One 11: e0153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52: 1059–1069. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2011): Weight-conserving characterization of complex functional brain networks. Neuroimage 56: 2068–2079. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Pena-Gomez C, Arenaza-Urquijo EM, Vidal-Pineiro D, Bargallo N, Junque C, Bartres-Faz D (2012): Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex 48: 1187–1196. [DOI] [PubMed] [Google Scholar]
- Seabold JS, Perktold J (2010): Statsmodels: Econometric and Statistical Moedling with Python. Proceedings of the 9th Python in Science Conference. [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R (2011): Default mode network functional and structural connectivity after traumatic brain injury. Brain 134: 2233–2247. [DOI] [PubMed] [Google Scholar]
- Shumskaya E, Andriessen TMJC, Norris DG, Vos PE (2012): Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology 79: 176–182. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, van der Lugt A (2009): Postconcussion syndrome after minor head injury: brain activation of working memory and attention. Hum Brain Mapp 30: 2789–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C, Zhuo J, Janowich J, Aarabi B, Shanmuganathan K, Gullapalli RP (2013): Default mode network interference in mild traumatic brain injury - A pilot resting state study. Brain Res 1537: 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C, Zhuo J, Roys S, Shanmuganathan K, Gullapalli RP (2015): Disruptions in Resting State Functional Connectivity and Cerebral Blood Flow in Mild Traumatic Brain Injury Patients. PLoS One 10: e0134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, McAllister TW (2009): Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry 166: 768–776. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, Witt ST (2012): Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav 6: 293–318. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- van der Horn HJ, Liemburg EJ, Scheenen ME, de Koning ME, Spikman JM, van der Naalt J (2015): Post-concussive complaints after mild traumatic brain injury associated with altered brain networks during working memory performance. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Lovejoy DW, Pearlson GD, Stevens MC (2010): Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging Behav 4: 232–247. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Freeman K, Thomas A, Shpaner M, OKeefe M, Watts R, Naylor MR (2015): Cognitive Improvement after Mild Traumatic Brain Injury Measured with Functional Neuroimaging during the Acute Period. PLoS One 10: e0126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, Grossman RI, Ge Y (2012): Default-Mode Network Disruption in Mild Traumatic Brain Injury. Radiology 265: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]