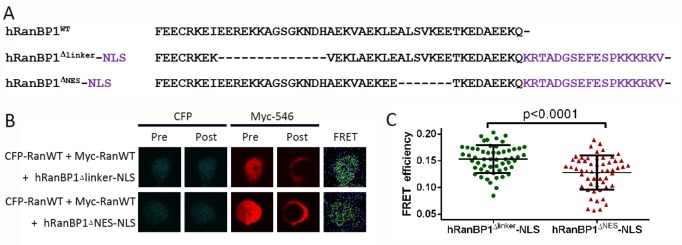
Figure 5. RanBP1 tetrameric complex exists in the cell nucleus.
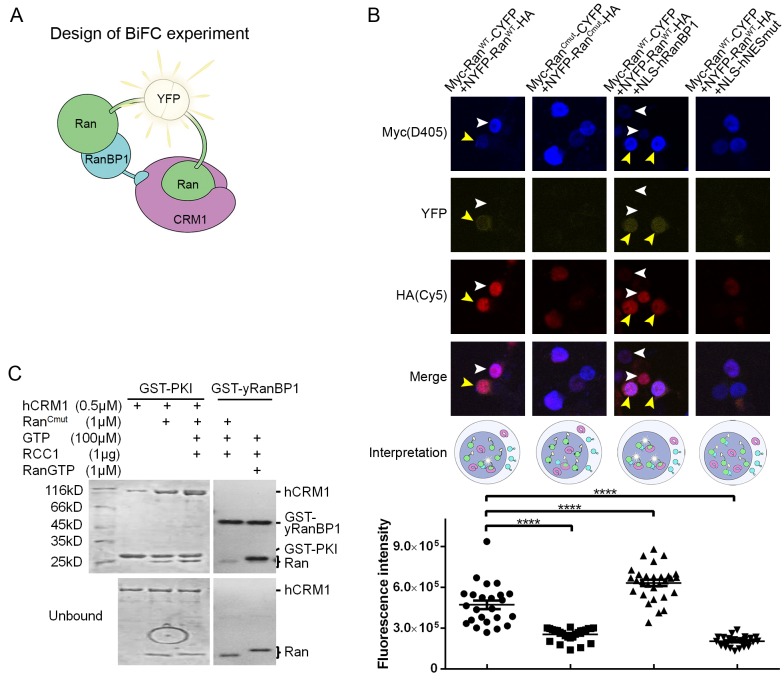
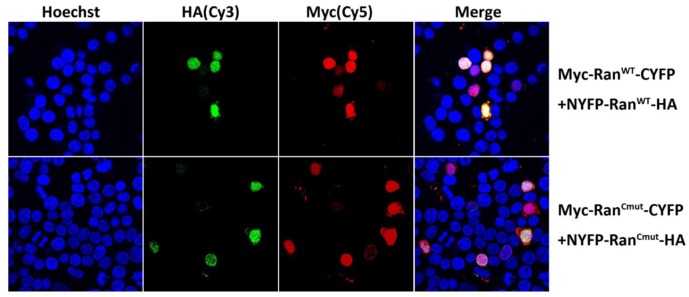
(A) Design of BiFC experiment. When the RanBP1 tetramer is formed, a YFP signal could be detected. This assay may not discriminate which Ran binds to RanBP1 (or CRM1) in the tetramer. (B) Representative immunofluorescence (HA and Myc) and fluorescence (YFP) images on cells co-transfected with fusion plasmids (labelled on top). NLS-hRanBP1 or NLS-hNESmut (hNESmut: human RanBP1 with NES mutation) was used to increase nuclear level of RanBP1 or its mutant. Nucleus was not stained so that the two transfected proteins could be stained without contaminating the YFP channel. The boundary of nucleus could be estimated by Ran fusions, which is mainly localized in the nucleus (see Figure 5—figure supplement 3). Results interpretation (middle panel) explains why the yellow fluorescence is observed or not. Bottom panel shows the nuclear YFP intensities of at least 20 cells (only florescent cells transfected with two Ran fusions) in the corresponding samples under the same level of illumination. **** denotes p<0.0001. (C) RanCmut is able to bind to CRM1 and NES (left panel) but not able to bind yRanBP1 (right panel).
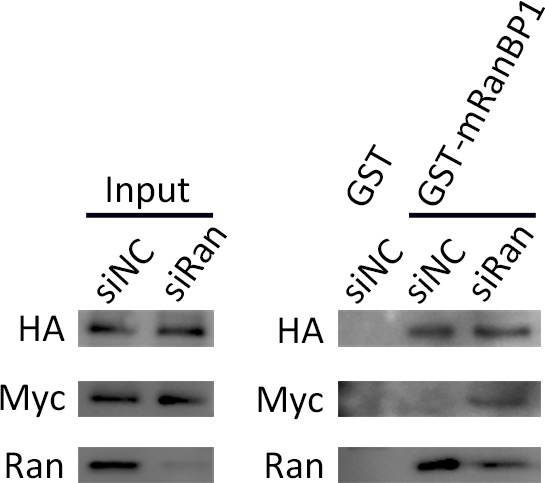
Figure 5—figure supplement 1. Pull down of 293 T cells expressed Ran fusion proteins using GST-mRanBP1 or GST, in the presence or absence of Ran knock down.