Abstract
PURPOSE
S-1 is a standard postoperative adjuvant chemotherapy for patients with stage II or III gastric cancer in Asia. Neoadjuvant or perioperative strategies dominate in Western countries, and docetaxel has recently shown significant survival benefits when combined with other standard regimens in advanced cancer and perioperative settings.
PATIENTS AND METHODS
This randomized phase III study was designed to prove the superiority of postoperative S-1 plus docetaxel over S-1 alone for R0 resection of pathologic stage III gastric cancer. The sample size of 1,100 patients was necessary to detect a 7% increase in 3-year relapse-free survival as the primary end point (hazard ratio, 0.78; 2-sided α = .05; β = .2).
RESULTS
The second interim analysis was conducted when the number of events reached 216 among 915 enrolled patients (median follow-up, 12.5 months). Analysis demonstrated the superiority of S-1 plus docetaxel (66%) to S-1 (50%) for 3-year relapse-free survival (hazard ratio, 0.632; 99.99% CI, 0.400 to 0.998; stratified log-rank test, P < .001), and enrollment was terminated as recommended by the independent data and safety monitoring committee. Incidences of grade 3 or greater adverse events, particularly neutropenia and leukopenia, were higher in the S-1 plus docetaxel group, but all events were manageable.
CONCLUSION
Addition of docetaxel to S-1 is effective with few safety concerns in patients with stage III gastric cancer. The present findings may also be applicable in countries in which perioperative adjuvant chemotherapy or chemoradiation is not standard.
INTRODUCTION
Gastric cancer is the third most common cause of cancer-related death worldwide.1 Although surgical resection is the only potentially curative treatment currently available, adjuvant therapies using chemotherapy2 and chemoradiation3 in the perioperative period have been developed and tested in numerous clinical studies worldwide.
In Asia, postoperative therapy with chemotherapy alone had been the focus of adjuvant trials because of the higher incidence of initially resectable cancers and excellent local control achieved by extended lymph node dissection (D2 gastrectomy).4 In 2007, the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) demonstrated that 12 months of postoperative adjuvant chemotherapy with S-1 significantly improved overall survival (OS) and relapse-fee survival (RFS) compared with surgery alone for patients with stage II or III gastric cancer.5,6 More recently, the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer trial demonstrated a similar survival benefit with the capecitabine-oxaliplatin doublet for 6 months compared with surgery alone in terms of hazard ratio (HR) for death within 5 years7,8; thus, Asian patients currently have two standard treatments with similar clinical efficacy.9 With regard to S-1 monotherapy, the need for improvement has consistently been pointed out because of the relatively poor outcome for patients with stage III gastric cancer and a lack of reduction in relapse rate at hematogenous sites.5,6
Docetaxel has shown efficacy not only as a monotherapy,10 but also in combination with S-1 or fluorouracil plus cisplatin.11-16 To test the superiority of S-1 plus docetaxel to S-1 alone in a postoperative adjuvant setting, we conducted a randomized phase III study to compare these regimens for patients with pathologic stage III gastric cancer (JACCRO GC-07).
PATIENTS AND METHODS
Systemic Design and Patients
The current study was designed by the steering committee members, including the principal investigators (K.Y. and Ya.K.) and the sponsor (Japan Clinical Cancer Research Organization [JACCRO]). The institutional review board at each study site approved the study protocol. The study was conducted in accordance with the principles of the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects. The steering committee and an independent data and safety monitoring committee oversaw the study conduct. All participating patients provided written informed consent before study enrollment. Data were maintained by the independent JACCRO GC-07 data center and analyzed by the JACCRO Statistical Analysis Department. Data and analyses were verified and assured by all academic members of the steering committee. The protocol, amendments, and statistical analysis plan are available in the Data Supplement.
This study involved patients age 20 to 80 years who underwent R0 resection by D2 or more extensive gastrectomy and were determined by pathologic examination to belong to one of the following subsets of patients with stage III gastric cancer defined by the 3rd English edition of the Japanese Classification of Gastric Carcinoma whose staging is identical to that for stomach cancer in the 7th edition of the TNM classification: stage IIIA (T2N3, T3N2, T4aN1), stage IIIB (T3N3, T4aN2, T4bN0, T4bN1), or stage IIIC (T4aN3, T4bN2, T4bN3).17,18
Procedures
Treatment was to be started within 42 days postoperatively. Daily S-1 dose was determined by body surface area (< 1.25 m2, 80 mg; ≥ 1.25 to < 1.5 m2, 100 mg; ≥ 1.5 m2, 120 mg) and orally administered two times per day after breakfast and dinner. Patients who were assigned to the S-1/docetaxel group were treated with S-1 on days 1 to 14 of a 3-week cycle during the first course. During the second to seventh courses, patients received intravenous infusion of docetaxel (40 mg/m2 body surface area) on day 1 of each cycle and S-1 on days 1 to 14 of a 3-week cycle. After the eighth course, treatment with S-1 continued on days 1 to 28 of 6-week cycles for up to 1 year. In the S-1 group, patients were treated with S-1 on days 1 to 28 of 6-week cycles for up to 1 year. If patients experienced grade 3 or 4 hematologic toxicity or grade 2, 3, or 4 nonhematologic toxicity, the dose was reduced in accordance with a predetermined dose-reduction procedure for each study drug. Patients were observed for 5 years after surgery.
Outcomes
The primary end point was 3-year RFS. Secondary end points were 3-year OS, 5-year OS, 5-year RFS, and adverse events. We assessed disease stage, extent of lymph node dissection, and histologic type in accordance with the standards defined by the Japanese Gastric Cancer Association.17 Relapse was judged by imaging diagnosis and/or clinical relapse on the basis of nonimaging exacerbation of the disease condition. Imaging examinations using ultrasonography, computed tomography, upper GI X-ray, or endoscopy was performed within 14 days before enrollment. Ultrasonography or computed tomography was performed every 6 months, and endoscopy was performed every 12 months until the end of the follow-up period or recurrence/metastasis. We carried out hematologic tests and clinical symptom assessments during adjuvant chemotherapy at 3-week intervals for the S-1 plus docetaxel group and at 2-week intervals for the S-1 group. Adverse events were monitored throughout treatment courses until the end of treatment and were graded according to the Common Terminology Criteria for Adverse Events (version 4.0), Japanese edition, Japan Clinical Oncology Group version. We assessed the severity of adverse events and the causal relationship to study drugs.
Statistical Analysis
The sample size was planned on the basis of the results of the ACTS-GC study6 in which 3-year RFS in the S-1 group was estimated to be 68% for stage IIIA disease, 50% for stage IIIB disease, and 62% for stage III disease (stage IIIA plus stage IIIB) as defined by the 2nd English edition of the Japanese Classification of Gastric Carcinoma.19 Accordingly, 3-year RFS in the present S-1 group was estimated to be 62%. As no previous study was available for use as a basis to estimate 3-year RFS in the S-1 plus docetaxel group, it was determined that S-1 plus docetaxel could be regarded as a standard therapy if 3-year RFS was 7% higher than that in the S-1 group, with acceptable safety. The sample size for each treatment group was estimated at 530 on assumptions of a 3-year follow-up period, two-sided α = .05, and β = .2. After consideration of patient withdrawal, we estimated that 1,100 patients (550 per group) must be recruited. The estimated cumulative number of primary outcome events after 3 years of follow-up was 507.
Enrolled patients were stratified by disease stage (IIIA, IIIB, or IIIC), histologic type (differentiated or undifferentiated), and trial site and were randomly assigned to the S-1 plus docetaxel group or the S-1 group at a 1:1 ratio by the minimization method using a centralized patient registration system at the JACCRO GC-07 data center.
Efficacy endpoints were evaluated in the intention-to-treat (ITT) analysis set, which consisted of all patients who met eligibility criteria but not exclusion criteria. The safety end point was evaluated in the safety analysis set, which consisted of all patients who received at least one study drug treatment. Treatment compliance and adverse events were evaluated in patients who received the study drugs for more than 1 year postoperatively in the safety analysis set.
RFS was analyzed by a stratified log-rank test with allocation adjustment factors in the ITT analysis set. We estimated cumulative survival curves and annual survival rates using Kaplan-Meier curves. Between-group analyses were performed with the stratified log-rank test with allocation adjustment factors, except for study sites. For between-group efficacy comparisons, the HR and two-sided 95% CI were estimated using the Cox proportional hazards regression model. The same analyses were applied to OS. In all cases, the significance level was set at a two-sided α of .05.
Two interim analyses for the primary end point were planned in this study. The first interim analysis took place at 25 months after study initiation, with 100 events—one fifth of the planned number of events. Early study termination was planned on the basis of the efficacy or futility at the second interim analysis with 170 events—two thirds of the plan—approximately 34 months after study initiation. Interim analyses were conducted by the independent JACCRO Statistical Analysis Department in accordance with the interim analysis plan. No study-associated personnel were notified of the analyzed results until study termination. For the O’Brien and Fleming type of interim analysis, Lan-Demets α-spending functions were applied.20 Detailed interim analysis methods can be found in the Data Supplement.
RESULTS
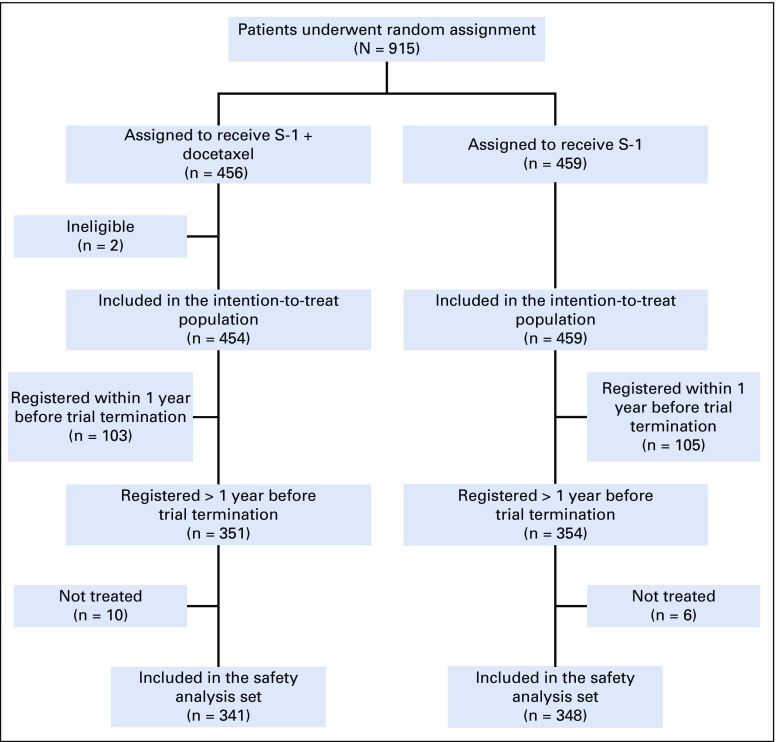
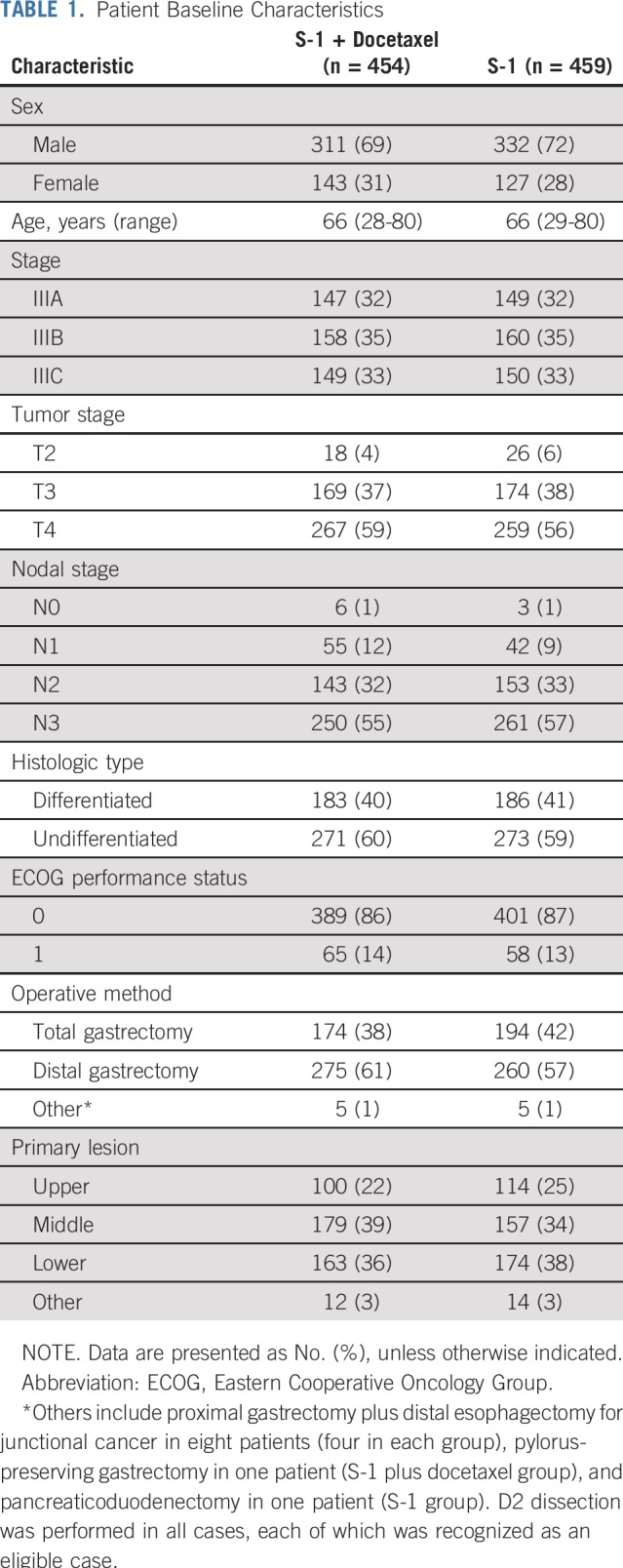
Of 915 randomly assigned patients, two patients in the S-1 plus docetaxel group were excluded (stage IV and double registration); therefore, 913 patients (n = 454 in S-1 plus docetaxel group; n = 459 in S-1 group) were included in the ITT analysis set. Among the 705 patients who registered before April 30, 2016, being 1 year before registration termination, 10 and six untreated patients in the S-1 plus docetaxel group and S-1 group, respectively, were excluded from the safety analysis set. Therefore, 341 patients in the S-1 plus docetaxel group and 348 patients in the S-1 group were included in treatment compliance and adverse event analyses (Fig 1). Overall, patient baseline characteristics were well balanced between the two groups (Table 1).
FIG 1.
CONSORT diagram.
TABLE 1.
Patient Baseline Characteristics
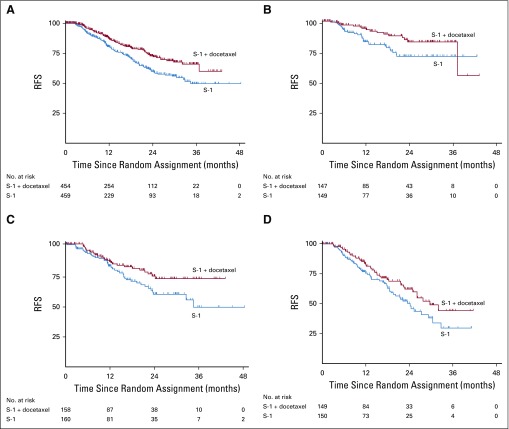
In this study, 1,100 patients were planned to be enrolled from April 2013 to December 2017. We carried out the second interim analysis in April 2017 when the number of events reached 216 in 915 patients at 138 study sites (median follow-up, 12.5 months). The second interim analysis of RFS demonstrated the superiority of the S-1 plus docetaxel group (HR, 0.632; 99.99% CI, 0.400 to 0.998; P < .001), with a 3-year RFS of 66% (95% CI, 59% to 73%) in the S-1 plus docetaxel group and 50% (95% CI, 41% to 58%) in the S-1 group (Fig 2). The study was terminated in September 2017 as recommended by the independent data and safety monitoring committee. Median RFS was not reached in the S-1 plus docetaxel group, whereas median RFS in the S-1 group was 34.5 months (95% CI, 29.5 months to not reached). At this stage, actual 3-year RFS was available only in 179 patients—67% in the S-1 plus docetaxel group and 53% in the S-1 group (HR, 0.708; 95% CI, 0.437 to 1.148).
FIG 2.
Relapse-free survival (RFS). Kaplan-Meier estimates of RFS in all patients (A) and in those with stage IIIA (B), IIIB (C), and IIIC disease (D). The second interim analysis was performed after 216 events had occurred (median follow-up, 12.5 months) and demonstrated superiority of S-1 plus docetaxel (65.9%) over S-1 (49.5%) for 3-year RFS (hazard ratio, 0.632; 99.99% CI, 0.400 to 0.998; P < .001). Therefore, the study was terminated.
As of April 2017, 44 and 60 deaths had occurred in the S-1 plus docetaxel and S-1 alone groups, respectively, but because of the small numbers of events, no significant difference was obtained for OS (P = .13).
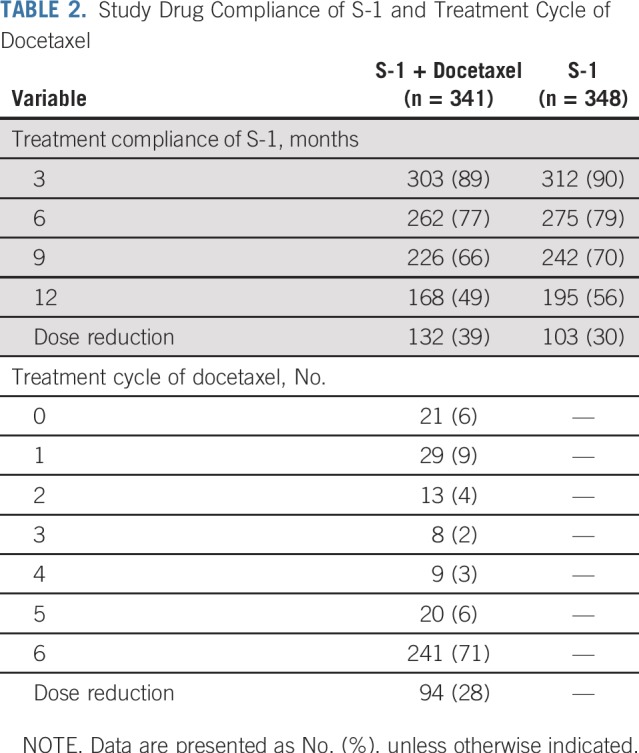
Of the 341 patients in the S-1 plus docetaxel group, 21 (6%) did not receive docetaxel. Of the 320 patients who received docetaxel, 234 (69%) received all six doses, and dose reduction was applied in 94 patients (28%). With regard to patient compliance with S-1, similar results were obtained in the two groups. In the S-1 plus docetaxel group, 303 patients (89%) continued S-1 for 3 months, 262 patients (77%) for 6 months, 226 patients (66%) for 9 months, and 168 patients (49%) for 12 months. In the S-1 alone group, 312 patients (90%) continued S-1 for 3 months, 275 patients (79%) for 6 months, 242 patients (70%) for 9 months, and 195 patients (56%) for 12 months (Table 2). S-1 dose reduction was necessary in 132 patients (39%) in the S-1 plus docetaxel group and 103 patients (30%) in the S-1 alone group. Drug administration was delayed at least once in 66% (95% CI, 61% to 71%) of patients who underwent S-1 plus docetaxel and 57% (95% CI, 52% to 62%) of patients who underwent S-1 monotherapy. The delay was more often observed during the first 3 months (46% [95% CI, 41% to 51%] v 31% [95% CI, 26% to 35%) and the subsequent 3 months (48% [95% CI, 42% to 54%] v 30% [95% CI, 25% to 35%]).
TABLE 2.
Study Drug Compliance of S-1 and Treatment Cycle of Docetaxel
Common reasons for treatment discontinuation with more than 5% incidences were the same in both groups—patient request, adverse event that lasted more than 28 days, physicians’ decision, and recurrence.
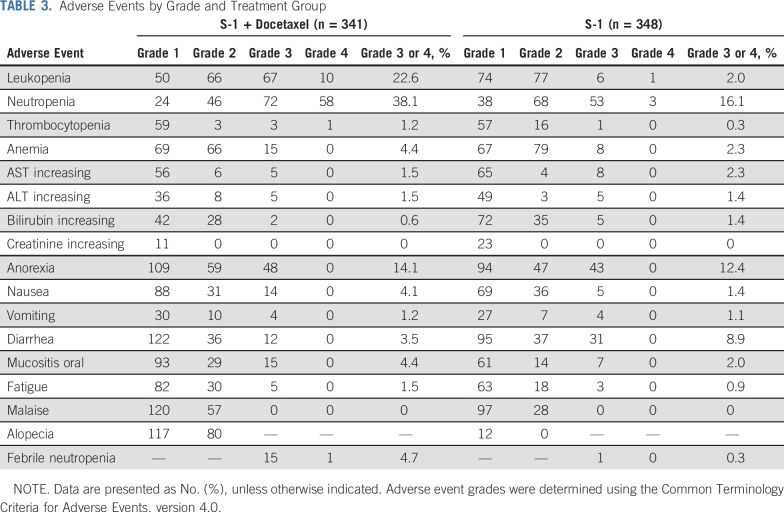
Common adverse events (≥ 20% incidences) of all grades in the S-1 plus docetaxel group included anorexia, anemia, alopecia, skin hyperpigmentation, leukopenia, fatigue, lacrimation, peripheral sensory neuropathy, nausea, neutropenia, mucositis oral, malaise, weight loss, diarrhea, and hyperbilirubinemia. Grade 3 or greater adverse events were observed in 198 (58%) of 341 patients (95% CI, 53% to 63%) in the S-1 plus docetaxel group and 147 (42%) of 348 patients (95% CI, 37% to 47%) in the S-1 alone group. Common adverse events of grade 3 or greater (> 4% incidences) in the S-1 plus docetaxel group were neutropenia, leukopenia, anorexia, febrile neutropenia, mucositis oral, anemia, and nausea.
Higher incidences were observed for increased serum bilirubin and diarrhea of grade 3 or greater in the S-1 group. One patient in the S-1 group died of respiratory failure, which was considered to be an adverse drug reaction (Table 3). Hospitalization as a result of severe adverse events was observed in 47 patients (14%; 95% CI, 10% to 17%) in the S-1 plus docetaxel group (anorexia: n = 8; bowel obstruction: n = 7; febrile neutropenia: n = 5; thrombosis: n = 4) and 41 patients (12%; 95% CI, 8.4% to 15%) in the S-1 group (anorexia: n = 8; diarrhea: n = 7; bowel obstruction: n = 5; colitis: n = 4).
TABLE 3.
Adverse Events by Grade and Treatment Group
Common first relapse sites were peritoneal sites, hematogenous sites, and lymph nodes. Significantly lower relapse rates in the S-1 plus docetaxel group compared with the S-1 group were found for hematogenous sites (5.3% [95% CI, 3.2% to 7.3%] v 9.8% [95% CI, 7.2% to 12.7%]; P = .012) and lymph nodes (4.8% [95% CI, 2.8% to 6.7%] v 11.3% [95% CI, 8.5% to 14.4%]; P < .001). In contrast, we observed no difference in the incidence of local recurrence (0.4% [95% CI, 0% to 1%] v 0.4% [95% CI, 0% to 0.9%]; P = 1.0) and peritoneal surface (9.3% [95% CI, 6.5% to 11.8%] v 12.9% [95% CI, 9.9% to 16.1%]; P = .092).
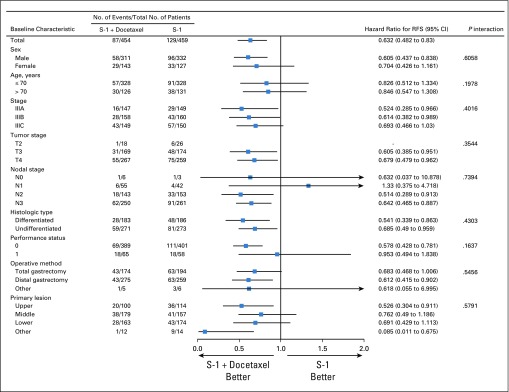
We analyzed the RFS of eligible patients according to sex, age, cancer stage, tumor stage, nodal stage, histologic type, performance status, operative method, and primary lesion (Fig 3). There were no significant interactions between treatment groups and these variables.
FIG 3.
Forest plot of relapse-free survival (RFS). Subgroup analyses of RFS were performed using patient baseline characteristics.
DISCUSSION
Since the era of comparisons with surgery alone, no pivotal trials that explore the superiority of more intensive postoperative chemotherapy over S-1 monotherapy have been conducted, the current study being an exception. Because a combination of S-1 and cisplatin (standards of care for advanced/metastatic cancer in Japan)21 was found to be poorly tolerated in the postoperative adjuvant setting,22 a combination of S-1 and docetaxel attracted attention, as a phase III comparison with S-1 monotherapy in patients with advanced/metastatic gastric cancer revealed significant improvement in time to progression.14 Furthermore, feasibility studies have indicated that S-1 plus docetaxel was well tolerated after gastrectomy.23,24 Consequently, this combination met the predetermined hypothesis at the second interim analysis for more than 15% improvement in 3-year RFS in the current study. Thus, S-1 plus docetaxel could replace not only S-1 monotherapy but also the capecitabine-oxaliplatin doublet (CAPOX) (observed to be equivalent to S-1 in terms of the reduction in HR compared with surgery alone) as an adjuvant chemotherapeutic regimen of choice for stage III gastric cancer. CAPOX could still be selected for those who wish to shorten the duration of treatment to 6 months and those who wish to avoid adverse events specific to docetaxel, such as alopecia.
Fortunately, the RFS benefit was accompanied by a favorable safety profile and good compliance. More than two thirds of patients in the S-1 plus docetaxel group received docetaxel six times as planned, despite its well-documented toxicities, including leukopenia, neutropenia, anorexia, malaise, and alopecia.12-14 All adverse events, including febrile neutropenia (2%), were manageable and well tolerated by patients with no treatment-related deaths. Furthermore, docetaxel did not interfere with S-1 chemotherapy, as the two groups had similar S-1 compliance rates. Compliance of S-1 in the S-1 alone arm was almost identical to that in the previous phase III trial in which 87%, 78%, 71%, and 66% of patients continued S-1 for 3 months, 6 months, 9 months, and 12 months, respectively.5,6 A steep decline of the continuation rate at 12 months in the current study could be a result of cancer recurrence, which was more frequent because of accrual only of patients with stage III cancer.
There were several limitations to the current study. Data related to OS were not presented in detail because the number of deaths at the time of the interim analysis remained too small. Thus, additional follow-up will be conducted for future evaluation of secondary end points that include OS, although the surrogacy of 3-year RFS in adjuvant chemotherapy trial for gastric cancer has been well established in a meta-analysis25 and another pivotal trial.8 Analyses of the sites of relapse will also need to be recalculated at such timepoints for more robust conclusions. In addition, early termination of the study at the interim analysis with median follow-up time of 12.5 months, although deemed necessary as a result of the overt difference in the RFS, remains a matter of concern. The number of patients at risk at 3 years did not reach the desired number as proposed by Gebski et al26 in the S-1 plus docetaxel group, and the interpretation of survival estimates at this timepoint will have to be made with caution. As the HRs for S-1 plus docetaxel observed in the strata of patients who were observed for up to 1 year (n = 208), approximately 1 to 2 years (n = 258), and approximately 2 to 3 years (n = 268) were consistently in favor of S-1 plus docetaxel at 0.789 (95% CI, 0.212 to 2.943), 0.658 (95% CI, 0.393 to 1.104), and 0.546 (95% CI, 0.347 to 0.857), we estimate that more definitive conclusions will be reached pending additional follow-up. There is additional concern that the significant survival difference between the two groups may have been a result of the poor 3-year RFS of patients in the S-1 group (50%), which was far lower than the pretrial estimation (62%). To avoid such overestimation, greater attention should have been paid to the fact that the staging system used in the current study had been substantially altered from the edition used at the time of ACTS-GC study. In addition, it is possible that patients who participated in the current study had more advanced disease than those in the ACTS-GC. Nevertheless, patients were randomly assigned in good balance, and our result—that there was a difference in outcomes between the two groups—seems to be solid.
In conclusion, the present results demonstrate a significant clinical benefit of adding docetaxel to S-1. Therefore, this combination can be recommended as a standard postoperative adjuvant chemotherapy for patients with stage III gastric cancer.
Footnotes
Presented at the 2018 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 30-June 3, 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Kazuhiro Yoshida, Yasuhiro Kodera, Mitsugu Kochi, Wataru Ichikawa, Yoshihiro Kakeji, Takeshi Sano, Masashi Fujii
Collection and assembly of data: Kazuhiro Yoshida, Yasuhiro Kodera, Mitsugu Kochi, Yoshihiro Kakeji, Takeshi Sano, Narutoshi Nagao, Masazumi Takahashi, Akinori Takagane, Takuya Watanabe, Masahide Kaji, Hiroshi Okitsu, Takashi Nomura, Takanori Matsui, Takaki Yoshikawa, Jin Matsuyama, Makoto Yamada, Seiji Ito
Data analysis and interpretation: Yasuhiro Kodera, Kazuhiro Yoshida, Masahiro Takeuchi, Masashi Fujii
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, A Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kazuhiro Yoshida
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Eli Lilly, Daiichi Sankyo, Merck Serono, Johnson & Johnson, Covidien, Bayer Yakuhin, Olympus, Terumo, Otsuka, Sanofi, Denka, Nippon Kayaku, MSD, Yakult Honsha, Tsumura, Pfizer, Intuitive Surgical, Ono Pharmaceutical, Asahi Kasei
Research Funding: Sanofi (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Takeda (Inst), Eli Lilly (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Johnson & Johnson (Inst), Covidien (Inst), Otsuka (Inst), Nippon Kayaku (Inst), Tsumura (Inst), Eisai (Inst), Kyowa Hakko Kirin (Inst), Astellas Pharma (Inst), Toyama Chemical (Inst), KCI (Inst), Abbott (Inst), Toray Medical (Inst), sAsahi Kasei (Inst)
Yasuhiro Kodera
Honoraria: Olympus, Chugai Pharma, Eli Lilly, Johnson & Johnson, Yakult Honsha, Taiho Pharmaceutical, Otsuka, Kaken Pharmaceutical, Ono Pharmaceutical, Asahi Kasei, Covidien, Medtronic, MSD, AbbVie, Dainippon Sumitomo Pharma, Intuitive Surgical, Bristol-Myers Squibb, Hisamitsu Pharmaceutical, Tsumura
Research Funding: Chugai Pharma (Inst), Daiichi Sankyo (Inst), Bristol-Myers Squibb (Inst), Otsuka (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Abbott (Inst), AbbVie (Inst), Sanofi (Inst), Yakult (Inst), Eli Lilly (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Kaken Pharmaceutical (Inst), Tsumura (Inst), Merck (Inst), Covidien (Inst), Japan Blood Products Organization (Inst), Novartis (Inst), Maruho (Inst), EA Pharma (Inst), Otsuka (Inst), Nippon Kayaku (Inst), MSD (Inst)
Wataru Ichikawa
Honoraria: Merck Serono, Taiho Pharmaceutical, Chugai Pharma
Consulting or Advisory Role: Ono Pharmaceutical
Research Funding: Takeda (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Merck Serono (Inst), Shionogi (Inst), Ono Pharmaceutical (Inst)
Yoshihiro Kakeji
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Eli Lilly, Ono Pharmaceutical, Takeda, Merck Serono
Research Funding: Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Takeda (Inst), Yakult Honsha (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst)
Takeshi Sano
Speakers' Bureau: Taiho Pharmaceutical, Chugai Pharma, Eli Lilly, Ono Pharmaceutical, MSD, Johnson & Johnson, Medtronic
Masazumi Takahashi
Honoraria: Taiho Pharmaceutical, Takeda
Travel, Accommodations, Expenses: Takeda
Takaki Yoshikawa
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Abbott, Daiichi Sankyo, Ajinomoto, Ono Pharmaceutical, Nihonkayaku, Johnson & Johnson, Medtronic, Olympus, Eli Lilly, MSD, Terumo, Miyarisan Pharmaceutical
Consulting or Advisory Role: Novartis, MSD Oncology, Chugai Pharma
Research Funding: Novartis, Chugai Pharma, Taiho Pharmaceutical, Yakult, Eli Lilly
Seiji Ito
Honoraria: Taiho Pharmaceutical, Olympus Medical Systems, Chugai Pharma, Yakult Honsha, Eli Lilly, Otsuka
Research Funding: Ono Pharmaceutical (Inst), Merck Sharp & Dohme (Inst)
Masahiro Takeuchi
Consulting or Advisory Role: Hisamitsu Pharmaceutical, Kowa, Shionogi, AbbVie
Masashi Fujii
Travel, Accommodations, Expenses: Japan Clinical Cancer Research Organization, Taiho Pharmaceutical
No other potential conflicts of interest were reported.
REFERENCES
- 1.International Agency for Research on Cancer Cancer Today: Population fact sheets. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 4.Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: Resource-stratified guidelines. Lancet Oncol. 2013;14:e535–e547. doi: 10.1016/S1470-2045(13)70436-4. [DOI] [PubMed] [Google Scholar]
- 5.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 6.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 8.Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 9.Nelen SD, Heuthorst L, Verhoeven RHA, et al. Impact of centralizing gastric cancer surgery on treatment, morbidity, and mortality. J Gastrointest Surg. 2017;21:2000–2008. doi: 10.1007/s11605-017-3531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi T, Sakata Y, Kanamaru R, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent gastric cancer: A Japanese Cooperative Study Group trial (group A) [in Japanese] Gan To Kagaku Ryoho. 1998;25:1915–1924. [PubMed] [Google Scholar]
- 12.Yoshida K, Hirabayashi N, Takiyama W, et al. Phase I study of combination therapy with S-1 and docetaxel (TXT) for advanced or recurrent gastric cancer. Anticancer Res. 2004;24:1843–1851. [PubMed] [Google Scholar]
- 13.Yoshida K, Ninomiya M, Takakura N, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402–3407. doi: 10.1158/1078-0432.CCR-05-2425. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: A randomized study (START) J Cancer Res Clin Oncol. 2014;140:319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wada Y, Yoshida K, Suzuki T, et al. Synergistic effects of docetaxel and S-1 by modulating the expression of metabolic enzymes of 5-fluorouracil in human gastric cancer cell lines. Int J Cancer. 2006;119:783–791. doi: 10.1002/ijc.21879. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 18.American Joint Committee on Cancer . AJCC Cancer Staging Handbook. ed 7. New York, NY: Springer Verlag; 2010. [Google Scholar]
- 19.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 20.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 21.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 22.Kodera Y, Ishiyama A, Yoshikawa T, et al. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703) Gastric Cancer. 2010;13:197–203. doi: 10.1007/s10120-010-0559-y. [DOI] [PubMed] [Google Scholar]
- 23.Fujitani K, Tamura S, Kimura Y, et al. Three-year outcomes of a phase II study of adjuvant chemotherapy with S-1 plus docetaxel for stage III gastric cancer after curative D2 gastrectomy. Gastric Cancer. 2014;17:348–353. doi: 10.1007/s10120-013-0273-7. [DOI] [PubMed] [Google Scholar]
- 24.Emi Y, Orita H, Yamamoto M, et al. Feasibility of adjuvant S-1 plus docetaxel against stage II-III gastric cancer following R0 resection in gastrectomy. EJC Suppl. 2009;7(abstr 6536):372. [Google Scholar]
- 25.Oba K, Paoletti X, Alberts S, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: A meta-analysis. J Natl Cancer Inst. 2013;105:1600–1607. doi: 10.1093/jnci/djt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebski V, Garès V, Gibbs E, et al. Data maturity and follow-up in time-to-event analyses. Int J Epidemiol. 2018;47:850–859. doi: 10.1093/ije/dyy013. [DOI] [PubMed] [Google Scholar]