Abstract
PURPOSE
This randomized clinical trial compared a personally tailored, automated telephone symptom management intervention to improve self-management among long-term survivors of prostate cancer with usual care enhanced with a nontailored newsletter about symptom management. We hypothesized that intervention-group participants would have more confident symptom self-management and reduced symptom burden.
METHODS
A total of 556 prostate cancer survivors who, more than 1 year after treatment, were experiencing symptom burden were recruited from April 2015 to February 2017 across four Veterans Affairs sites. Participants were randomly assigned to intervention (n = 278) or usual care (n = 278) groups. We compared differences in the primary (symptom burden according to Expanded Prostate Cancer Index Composite-26 [EPIC], confidence in self-management) and secondary outcomes between groups using intent-to-treat analyses. We compared domain-specific changes in symptom burden from baseline to 5 and 12 months among the intervention group according to the primary symptom focus area (urinary, bowel, sexual, general) of participants.
RESULTS
Most of the prostate cancer survivors in this study were married (54.3%), were white (69.2%), were retired (62.4%), and underwent radiation therapy (56.7% v 46.2% who underwent surgery), and the mean age was 67 years. There were no baseline differences in urinary, bowel, sexual, or hormonal domain EPIC scores across groups. We observed higher EPIC scores in the intervention arm in all domain areas at 5 months, though differences were not statistically significant. No differences were found in secondary outcomes; however, coping appraisal was higher (2.8 v 2.6; P = .02) in intervention-arm patients at 5 months. In subgroup analyses, intervention participants reported improvement from baseline at 5 and 12 months in their symptom focus area domains.
CONCLUSION
This intervention was well received among veterans who were long-term survivors of prostate cancer. Although overall outcome differences were not observed across groups, the intervention tailored to symptom area of choice may hold promise to improve associated burden.
INTRODUCTION
Although the adverse effect profiles for prostate cancer treatments continue to improve, surgery and radiation therapy still result in adverse consequences that include incontinence, impotence, and bowel issues.1-14 Many of the 3 million US survivors of prostate cancer deal with long-term symptom burden that reduces their quality of life.3,4,7,12,15-22 They also face psychosocial consequences, which include fear of cancer recurrence, limited confidence in dealing with the cancer and its adverse effects, and partner distress.23-31 Persistence of symptoms is particularly unfortunate, because many symptoms can be ameliorated or even eliminated through self-management or clinical intervention. Moreover, there have been no efforts to identify those survivors for whom better symptom self-management would translate into measurable quality-of-life improvements. Although some interventions to improve symptom burden have targeted patients with prostate cancer at early post-treatment times, there are virtually no interventions focused on long-term survivors who deal with symptoms for months and years after treatment.
To address this gap, we conducted a randomized clinical trial of an automated self-management support intervention for long-term survivors of prostate cancer compared against a nontailored newsletter that discusses self-management. Our intervention, called Building Your New Normal, assessed ongoing prostate cancer–related symptoms using automated telephone technology and delivered self-management guidance through a series of tailored newsletters. Compared with a single nontailored newsletter, we hypothesized that intervention participants would have improved and more confident symptom self-management as well as reduced symptom burden at 5 and 12 months after enrollment.
METHODS
This study was based on the conceptual framework of self-management after cancer treatment32 as well as the theoretical foundations of social cognitive theory and the transactional model of stress and coping.33,34 Details of the study, recruitment, random assignment, intervention, and follow-up were published previously.35
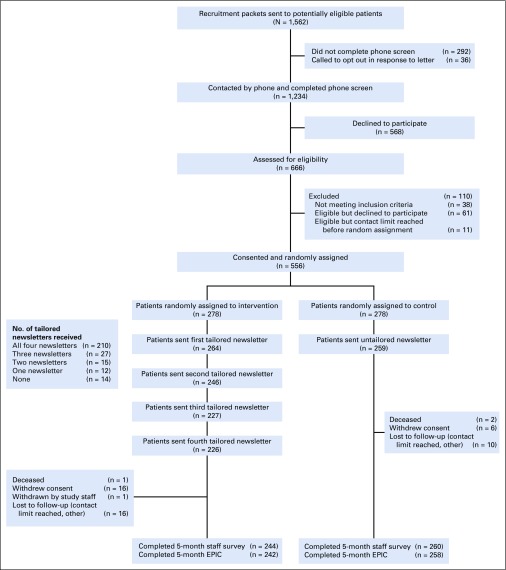
This two-armed, randomized, controlled trial enrolled 556 prostate cancer survivors from April 2015 through February 2017, and follow-up continued through February 2018 (Fig 1). We recruited men from four Department of Veterans Affairs sites (Ann Arbor, Cleveland, Pittsburgh, St Louis). The study protocol received approval from the Veterans Affairs Central Institutional Review Board. The study was considered minimal risk, and verbal informed consent was approved.
FIG 1.
Study flow. EPIC, Expanded Prostate Cancer Index Composite-26.
Eligibility, Recruitment, and Randomization
We identified men treated for prostate cancer within the last 1 to 10 years using the Veterans Affairs Corporate Data Warehouse and Central Cancer Registry data files. To be eligible, patients had to be between 40 and 80 years of age and have a working telephone. Patients were ineligible if they were undergoing treatment for a separate cancer, had dementia, or had other significant mental impairment in their medical record.
A recruitment packet with introductory letter, information sheet, and opt-out number was sent to potential participants. A research coordinator called those who did not opt out to determine interest and eligibility. A brief screening question was used to assess symptoms that veterans wanted to improve (urinary, sexual, bowel, and/or general). Those interested and eligible were offered enrollment.
Once enrolled, participants completed a baseline telephone survey to collect demographic details and confirm prostate cancer diagnosis date and treatment type. Participants were then randomly assigned by computer to the Building Your New Normal intervention (automated telephone assessments plus tailored newsletters) or control. Random assignment was stratified by original treatment (ie, surgery, radiation therapy) to ensure equal proportions in both arms, given the distinct long-term symptoms across treatments. After random assignment, the automated telephone system was activated and delivered a standardized instrument to assess prostate cancer symptom burden and quality of life—the Expanded Prostate Cancer Index Composite-26 (EPIC)36—to participants in both arms. The automated system attempted eight calls during 4 days after random assignment. Participants remained in the study even if EPIC was not administered during that window.
Follow-up assessments were completed at 5 months (primary end point) and 12 months (secondary end point) after enrollment for both groups. Follow-up surveys were divided into two parts: the first part was administered by a research coordinator, and the second part was administered by the automated telephone system (including EPIC) to ensure standardized quality-of-life assessment across groups.
Intervention and Control Arms
The intervention was the multimodal Building Your New Normal intervention to improve prostate cancer symptom self-management. As described previously,35 the program was developed and pilot tested in collaboration with the Center for Health Communications Research, designated by the National Cancer Institute as a Center of Excellence in Cancer Communications, alongside clinical experts in prostate cancer survivorship care who included urologic oncologists, nurses, sexual and mental health experts, and advanced practice providers. As part of pilot testing, we demonstrated that monitoring of quality of life among prostate cancer survivors using the automated telephone system was feasible and consistent with written assessments.37 Intervention participants were contacted by the automated system each month for 4 months after enrollment to assess symptoms using EPIC, and they were offered the opportunity to choose a symptom area for tailored self-management print materials (urinary, sexual, bowel, general). The tailored newsletter content, which included more information about the chosen symptom and self-management strategy suggestions38 and which incorporated a cognitive behavioral therapy framework, was then generated, printed, and mailed to the participant address (Appendix Fig A1, online only). Intervention participants could switch their symptom focus area each month across the four EPIC domains and could receive different tailored newsletters each time. If they chose to focus on the same area more than once, they continued to receive information about that symptom and associated self-management information, but each newsletter had different and more detailed information. The control arm received enhanced usual care, which consisted of one nontailored newsletter that described self-management approaches to address prostate cancer symptoms.
Measures
We selected outcomes that were based on our conceptual framework and hypotheses. The primary outcomes analyses were conducted using 5- and 12-month follow-up assessments.
Primary outcomes.
The primary outcome was symptom burden for each of the four EPIC domains (urinary, incontinence and irritative/obstructive; bowel; sexual; and general). Each domain was scored from 0 to 100, and higher scores equated to lower burden.12,21,36 We defined scores of 70 or greater as clinically meaningful indications of low symptom burden for each domain. The second primary outcome was confidence in symptom self-management, measured using a five-item scale developed from pilot work.
Secondary outcomes.
We had four secondary outcomes. Three were assessed at 5 and 12 months: cancer control and outlook (by a validated scale of three cancer control items and two cancer outlook items),24-26 the perceived efficacy in patient-physician interactions (with the PEPPI instrument), and coping (appraised with six items from the validated Brief Cope instrument). We assessed 12-month subjective health using the validated veteran quality-of-life scale (the Veterans RAND 12-item health survey).39
Covariables.
Covariables included veteran-reported age, race/ethnicity, education, marital status, employment, initial prostate cancer treatment, and study site.
Sample Size and Statistical Power
We designed the study to enroll 550 participants for 90% power to detect a 0.33 standardized mean difference as a minimal, clinically important between-group difference in each of the four EPIC domains.40 The calculation was based on a regression analysis that adjusted for baseline values with an α of .0125, an assumed correlation of 0.5 between baseline and follow-up scores, and assumed 15% attrition at each follow-up assessment to have sufficient power to detect differences between groups at both 5- and 12-month assessment points.
Analysis
Primary analyses.
The primary analysis was based on the intent-to-treat principle41 and included all patients regardless of intervention engagement. Baseline analyses included descriptive statistics by arm of patient characteristics, baseline brief screener response, and each EPIC domain.
The a priori primary end point was based on the 5-month assessment, because it was closest to intervention completion. To evaluate the hypothesis that intervention participants would have higher (ie, better) mean scores on each EPIC domain at 5 months relative to controls, we used multiple linear regression analysis for each domain in two stages. First, we obtained between-group differences using an indicator for intervention group as the primary independent variable and adjusted analysis for site and treatment type indicators. Second, we adjusted for additional baseline variables that were potential outcome predictors (baseline outcome measure, age, education) and baseline variables predictive of missing 5-month outcomes. We conducted similar regression analyses for confidence in self-management 5 months after enrollment. Assumptions were checked for all models using residual analyses. All analyses were repeated for EPIC and confidence outcomes at 12 months. We evaluated secondary outcomes using the same approach. We reported mean differences between intervention and control groups; although an α of .0125 was used for sample size calculation to adjust for multiple comparisons of four primary outcomes, 95% CIs are reported throughout for consistency.
Intervention-arm subgroup analyses.
We were specifically interested in assessing change in symptom burden from baseline to 5 and 12 months among intervention-group participants who received tailored content, anchored to the initial symptom area each participant chose to work on. We categorized intervention participants into groups according to their initial symptom focus areas and estimated improvement (change) in both 5-month and time-averaged (across 5 and 12 months) EPIC scores from baseline across each domain. The time-averaged improvement was obtained using a mixed-effects model with both 5- and 12-month data as response variables. We repeated analyses by categorizing intervention participants into groups according to symptom focus areas chosen any time during intervention.
Sensitivity analyses.
We assessed associations between time since diagnosis and primary outcomes in the intervention-arm analyses. We also conducted analyses to understand dose-response effects on symptom burden changes from baseline to 5 months.
Participant experience.
We evaluated the overall reported satisfaction of intervention participants with the study and with materials they received through postintervention qualitative telephone interviews among 26 purposively sampled patients to be reported as a separate process evaluation manuscript (Data Supplement).
RESULTS
As shown in Figure 1, a total of 1,234 potential participants completed the phone screen, and 556 (45.1%) consented and were randomly assigned (n = 278 to intervention, n = 278 to control). Most (90.7%) provided the 5-month primary outcomes (and 81.7% provided the 12-month data). More participants missed the 5-month primary outcome assessment in the intervention than control group (12.2% v 6.5%; P = .02), but no attrition differences existed for 12-month assessments (18.7% v 18.0%; P = .83).
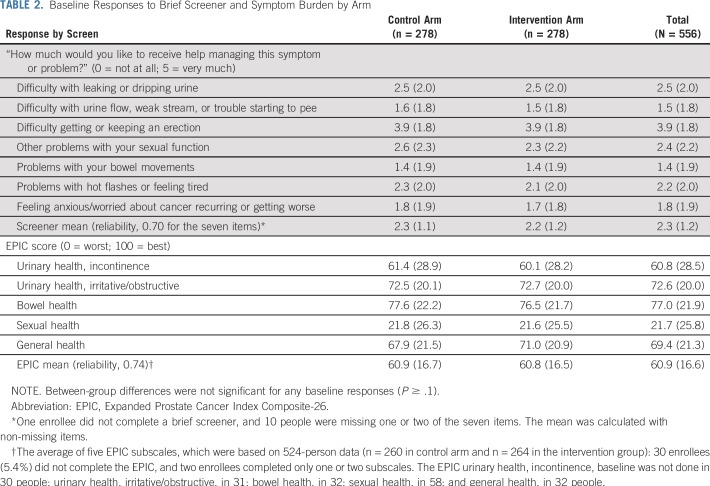
There were no significant group differences in baseline demographic factors except education (P = .01; Table 1). The average participant age was 66.7 years (range, 49 to 83 years); most were married (54.3%), were retired (62.4%), and were earning less than $50,000 annually (79.3%); more than one quarter identified as black. The average time since diagnosis was 4.1 years (range, 1.1 to 8.0 years). Just less than half (46.2%) received surgery; 56.7%, radiation treatment; and 24.8%, androgen deprivation therapy. There were no differences across groups in any baseline quality-of-life domain scores for screening question(s) or EPIC (Table 2). Of 278 intervention participants, 210 received all four newsletters (75.5%). Most participants chose one (n = 93) or two (n = 92) focus areas, but 62 and 16 choose three and all four focus areas, respectively. The most common initial symptom focus area was sexual health, followed by urinary, bowel, and general. Sexual health was chosen at least once by three quarters of intervention participants, whereas nearly 80% of intervention participants never chose bowel health.
TABLE 1.
Baseline Characteristics by Randomization Status
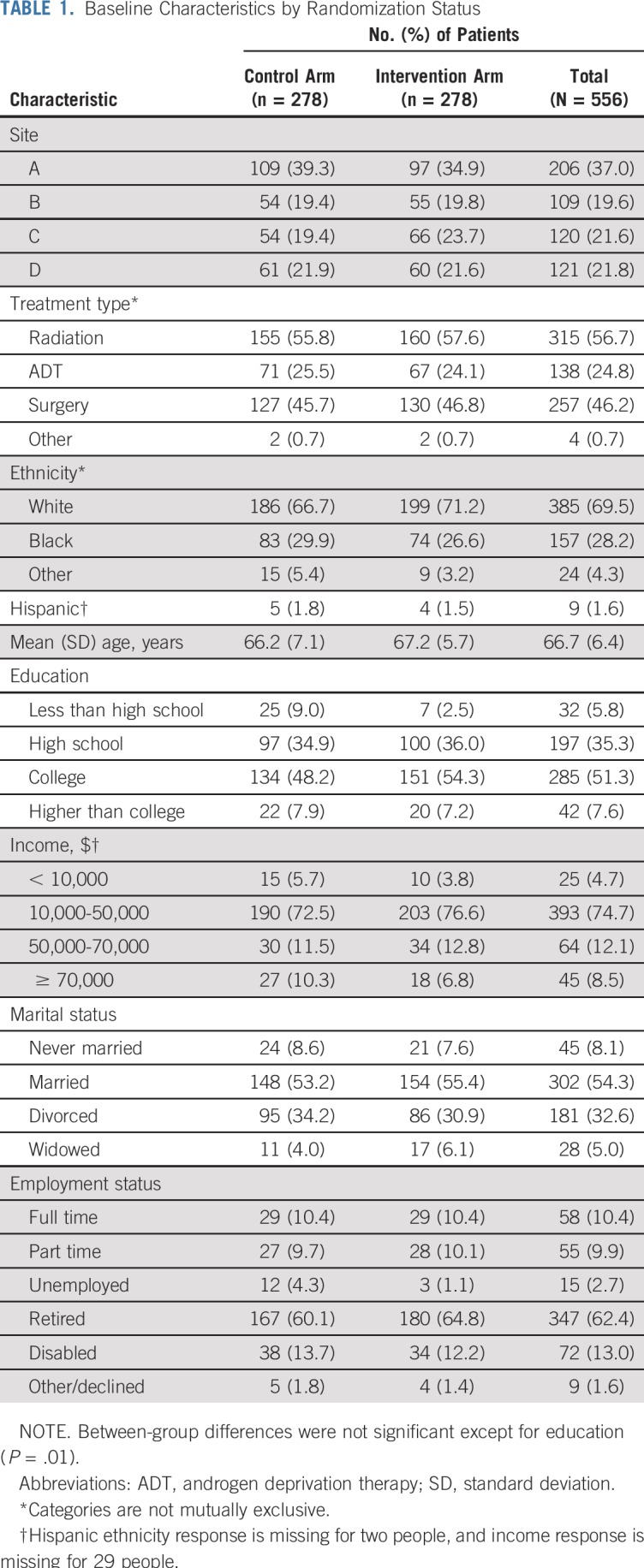
TABLE 2.
Baseline Responses to Brief Screener and Symptom Burden by Arm
Overall, there were 25,777 outbound and 8,888 inbound automated call minutes used during the study. Total estimated call costs for the study were $531, and the average control and intervention participant call costs were $0.65 and $1.40, respectively.
Primary Analyses
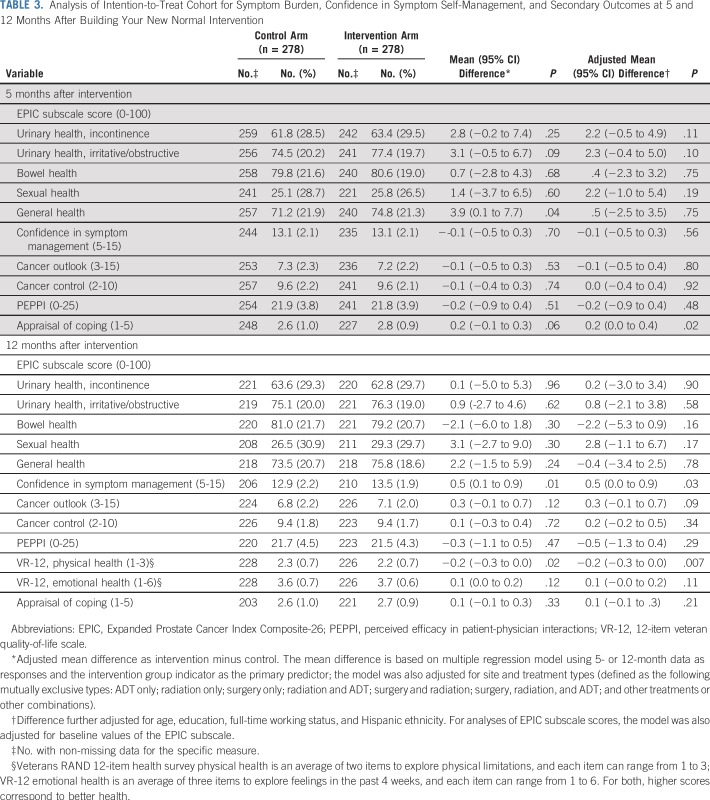
At the 5-month follow-up, mean EPIC scores were slightly higher (ie, lower burden) in each domain in the intervention group, compared with the control group, though none of the adjusted mean differences were statistically significant at the .0125 significance level (Table 3). We found no differences in confidence in symptom self-management, cancer control and outlook, or perceived efficacy in patient-physician interactions at 5 months. At 5 months, the mean appraisal of coping score was higher in the intervention arm by 0.2, which was not a meaningfully large difference. These overall results were similar at 12 months, with exception of higher mean score in the intervention arm in confidence in symptom self-management. At 12 months, subjective physical health was lower in the intervention than in the control arm, but no differences were seen in subjective emotional health (Table 3). We did observe significant differences by arm in proportions of participants with EPIC domain scores 70 or greater for urinary, incontinence (P = .02), and urinary, irritative/obstructive (P = .05), domains (data not shown).
TABLE 3.
Analysis of Intention-to-Treat Cohort for Symptom Burden, Confidence in Symptom Self-Management, and Secondary Outcomes at 5 and 12 Months After Building Your New Normal Intervention
Intervention-Arm Subgroup Analyses
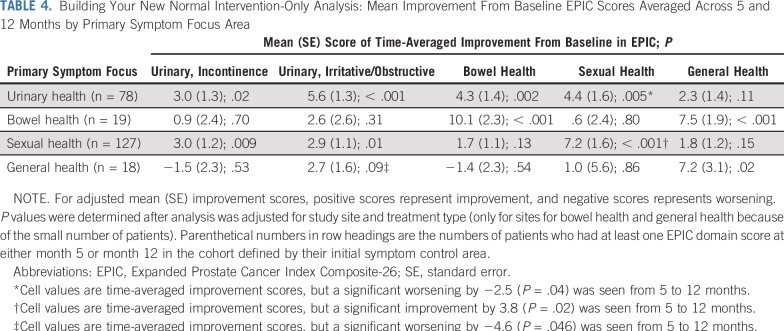
When we evaluated EPIC score changes from baseline to 5 and 12 months according to primary symptom domains, as an a priori analysis, we found subsequent improvement in corresponding domains averaged across 5 and 12 months. Veterans who focused on urinary health saw improvements of +3.0 points for incontinence (P = .02), and +5.6 points for irritative/obstructive (P < .001) domains. For those who focused on the bowel domain, improvements were +10.1 points (P < .001); the sexual domain, +7.2 points (P < .001); and the general domain, +7.2 points (P = .02; Table 4). We found similar results upon evaluation of EPIC score changes from baseline to 5 and 12 months according to symptom domains chosen at least once by intervention participants (data not shown).
TABLE 4.
Building Your New Normal Intervention-Only Analysis: Mean Improvement From Baseline EPIC Scores Averaged Across 5 and 12 Months by Primary Symptom Focus Area
Time since diagnosis was not associated with improvement in any EPIC domains, nor were varying degrees of dose (ie, whether intervention participants chose the same area one or more times) for sexual or urinary health (the most common areas chosen). However, bowel health symptoms did improve with each additional content dose; the estimated improvements were +6.5 (95% CI, 3.3 to 9.6; P < .001) and +5.7 (95% CI, 2.2 to 9.1; P = .001) points per dose at 5 and 12 months, respectively. We found high intervention satisfaction: 63% of intervention and 67% of control arm participants reported being very satisfied with the program. Many positive comments about the intervention were obtained from the process evaluation and are being reported separately.
DISCUSSION
Prostate cancer treatments continue to adversely affect quality of life for many prostate cancer survivors. Programs to help survivors manage these adverse effects have generally been delivered within the first few months after treatment, although symptoms often persist for months or years.7-9,15,19,42-46 In addition, survivorship programs tend to be confined to cancer centers and not available to survivors who have returned to community providers. This is true inside and outside of the Veterans Health Administration national health care system. As demonstrated in this study, the systematic, automated collection and use of patient-reported outcomes across multiple sites to support cancer survivors is feasible and has broad relevance that ranges from clinical trial administration to population-based symptom management.47 To our knowledge, this is the first randomized trial that compared an easily scaled and personally tailored intervention for veterans who are long-term survivors of prostate cancer with standard information to improve overall symptom burden and confidence in symptom self-management after prostate cancer treatment.
Despite trends in the right direction, we did not observe statistically significant differences in our primary outcomes (overall symptom burden assessed using EPIC or confidence in symptom self-management) between intervention and control groups in this large, multisite trial. However, for patients who chose urinary and bowel symptoms in the intervention group, the mean change from baseline in subgroup analyses did approach minimally important differences (Table 4).40 Although randomized trials of symptom self-management interventions are lacking for this population, our findings suggest opportunities to improve symptom burden and quality of life among long-term survivors of prostate cancer through intervention tailoring.42,48,49 Possible explanations for the lack of significance include the possibility that many long-term survivors have become so familiar with symptom coping that a light-touch intervention like this one was not sufficient to measurably change symptom burden. Arguably, these long-term survivors were already fairly confident in their abilities to manage symptoms, because they had been doing so for years.
This study was successful in the deployment of population-based, patient-reported outcome assessments and the generation of tailored self-management content. Despite the relatively high burden of the Building Your New Normal intervention—four 30-minute calls during 4 months—retention was high: more than 80% remained in the study and completed the 5-month assessment. In fact, these findings are consistent with retention rates for other automated chronic disease management programs (eg, diabetes, heart failure).50 The continued engagement in this program indicates that scalable opportunities exist to not only understand population-based symptom burdens among cancer survivors but also give back through low-cost, tailored aural and written support materials. Indeed, the low costs for the automated telephone calls highlight the scalability of this method, as seen in other studies.51,52 Second, we obtained a high participation rate: roughly half of those invited agreeing to participate in the study. Hence, we rapidly enrolled more than 500 survivors across four sites and completed this large study within 2 years. This indicates a notable unmet need among prostate cancer survivors and willingness to engage in self-management for symptoms that men might not otherwise feel comfortable speaking about with providers (eg, sexual, urinary, bowel problems). Moreover, more than 80% of men were satisfied with the program and would recommend that others participate. Process interviews with participants were also strongly positive.
Importantly, intervention-arm subgroup analyses revealed improvements in symptom burden from baseline to follow-up when evaluated according to the initial symptom on which participants chose to focus. These subgroup findings suggest that a tailored intervention according to chosen symptoms42 has real potential to have positive impacts in this population and to reach clinically meaningful EPIC score changes. Indeed, the majority of participants chose to focus on sexual and urinary health, and fewer focused on bowel and general health. Longitudinal, automated tailored engagement with self-management support across domains, as in this 4-month program, coupled with support from clinicians to engage in self-management53 might provide the integration and boosts necessary to improve symptom burden for survivors.
Study strengths include the large sample size from multiple sites, high participation and retention, and validated measures of symptom burden and patient-reported outcomes. Yet, limitations must be noted. Although we achieved diversity of participants from Veterans Health Administration sites, there are limitations to generalization across all racial/ethnic and demographic groups. Veterans without telephones or the ability to use automated telephone systems could not enroll; however, this limitation is increasingly uncommon. We cannot fully know the characteristics of veterans who did not enroll; however, we did not see differences in enrollment by site, years since diagnosis, or treatment type.
The Building Your New Normal intervention focused on helping long-term survivors of prostate cancer manage a symptom area of importance to them. Intervention-group results suggest promise for such an intervention to improve symptom burden, and this study highlighted the opportunity for this easily-scaled, low-cost intervention. The collection of patient-reported outcomes in this survivor population of veterans may provide much needed information to inform initial treatment decision making and aid long-term symptom management.54 Such information can support survivors and their clinicians to optimize prostate cancer care both inside and outside of the Veterans Health Administration. Additional study of this intervention on health care delivery system utilization, and modification to be more effective, seems warranted, because engagement and unmet needs seem strong.
ACKNOWLEDGMENT
We thank Elizabeth Garcia, Karen Smith, Katiuscia O’Brian, and Mirela Grabic for helping coordinate the project; Keosha Corder, Laura Poma, and Ryan Blake for helping with recruitment; Rodney Dunn for his assistance with EPIC score analysis; and Jennifer Burns and Leah Gillon for data management. We thank Viji Ramaswami, Dennis O’Reilly, Ian Moore, and Shannon Considine (supported by University of Michigan Rogel Cancer Center Health Communications Shared Resource Grant No. P30 CA46592) for intervention and newsletter development. We also thank all of the veterans who participated in this research.
Appendix
FIG A1.
Building Your New Normal, a tailored self-management newsletter.
Footnotes
Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
Supported in part by the Veterans Affairs Health Services Research and Development Service Grant No. IIR 12-116 and by the Veterans Affairs Health Services Research and Development Service Career Development Award No. CDA 12-171 (T.A.S.) and National Cancer Institute Grant No. R37-CA-222885 (T.A.S.). Funding from VA Health Services Research and Development was received for Optimizing Veteran-Centered Prostate Cancer Survivorship Care (T.A.S., T.M., S.H., S.T.H.).
The funding source did not play any role in the design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Veterans Affairs Health Services Research and Development Service.
Clinical trial information: NCT01900561.
AUTHOR CONTRIBUTIONS
Conception and design: Ted A. Skolarus, Hyungjin Myra Kim, Robert L. Grubb III, Hui Zhu, John D. Piette, Sarah T. Hawley
Collection and assembly of data: Ted A. Skolarus, Tabitha Metreger, Soohyun Hwang, Robert L. Grubb III, Jeffrey R. Gingrich, Hui Zhu, Sarah T. Hawley
Provision of study material or patients: Ted A. Skolarus, Robert L. Grubb III, Jeffrey R. Gingrich, Hui Zhu
Data analysis and interpretation: Ted A. Skolarus, Daniela Wittmann, Hyungjin Myra Kim, Robert L. Grubb III, Hui Zhu, John D. Piette, Sarah T. Hawley
Administrative support: Tabitha Metreger, Soohyun Hwang
Financial support: Sarah T. Hawley
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Self-Management in Long-Term Prostate Cancer Survivors: A Randomized, Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ted A. Skolarus
Patents, Royalties, Other Intellectual Property: UpToDate royalties for prostate cancer survivorship chapter
Robert L. Grubb III
Employment: Anthem Foundation (I)
Research Funding: FKD Therapies, GlaxoSmithKline
Travel, Accommodations, Expenses: Blue Earth Diagnostics
Hui Zhu
Leadership: Molecular Theranostics
Stock and Other Ownership Interests: Molecular Theranostics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–1214. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 3.Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-stage prostate cancer. Semin Radiat Oncol. 2008;18:67–72. doi: 10.1016/j.semradonc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrer M, Suárez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Gore JL, Kwan L, Lee SP, et al. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101:888–892. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 6.Johansson E, Bill-Axelson A, Holmberg L, et al. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: The Randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol. 2009;55:422–430. doi: 10.1016/j.eururo.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 7.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: The Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–899. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 8.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 9.Miller DC, Wei JT, Dunn RL, et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: Potential influence of sexual motivation and/or indifference. Urology. 2006;68:166–171. doi: 10.1016/j.urology.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 10.Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep. 2003;4:185–195. doi: 10.1007/s11934-003-0068-1. [DOI] [PubMed] [Google Scholar]
- 11.Reeve BB, Stover AM, Jensen RE, et al. Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans: A population-based study. Cancer. 2012;118:5679–5687. doi: 10.1002/cncr.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 13.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 14.Wilt TJ, Shamliyan TA, Taylor BC, et al. Association between hospital and surgeon radical prostatectomy volume and patient outcomes: A systematic review. J Urol. 2008;180:820–828. doi: 10.1016/j.juro.2008.05.010. discussion 828-829. [DOI] [PubMed] [Google Scholar]
- 15.Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: Long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 16.Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 17.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 18.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92:1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 19.Taylor KL, Luta G, Miller AB, et al. Long-term disease-specific functioning among prostate cancer survivors and noncancer controls in the prostate, lung, colorectal, and ovarian cancer screening trial. J Clin Oncol. 2012;30:2768–2775. doi: 10.1200/JCO.2011.41.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei JT, Dunn RL, Marcovich R, et al. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164:744–748. doi: 10.1097/00005392-200009010-00029. [DOI] [PubMed] [Google Scholar]
- 21.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 22.Yassine MD, Copeland G, Wei JT, et al. Common and persistent adverse outcomes following prostate cancer treatment: Findings from the Michigan Prostate Cancer Survivor Study. Cancer Prev Res. 2011;4 (suppl; abstr B24) [Google Scholar]
- 23.Bruun P, Pedersen BD, Osther PJ, et al. The lonely female partner: A central aspect of prostate cancer. Urol Nurs. 2011;31:294–299. [PubMed] [Google Scholar]
- 24.Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol. 2003;21:3777–3784. doi: 10.1200/JCO.2003.02.115. [DOI] [PubMed] [Google Scholar]
- 25.Clark JA, Rieker P, Propert KJ, et al. Changes in quality of life following treatment for early prostate cancer. Urology. 1999;53:161–168. doi: 10.1016/s0090-4295(98)00457-9. [DOI] [PubMed] [Google Scholar]
- 26.Clark JA, Talcott JA. Confidence and uncertainty long after initial treatment for early prostate cancer: Survivors’ views of cancer control and the treatment decisions they made. J Clin Oncol. 2006;24:4457–4463. doi: 10.1200/JCO.2006.06.2893. [DOI] [PubMed] [Google Scholar]
- 27.Couper J, Bloch S, Love A, et al. Psychosocial adjustment of female partners of men with prostate cancer: A review of the literature. Psychooncology. 2006;15:937–953. doi: 10.1002/pon.1031. [DOI] [PubMed] [Google Scholar]
- 28.Ervik B, Nordøy T, Asplund K. In the middle and on the sideline: The experience of spouses of men with prostate cancer. Cancer Nurs. 2013;36:E7–E14. doi: 10.1097/NCC.0b013e31824fe1ef. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: A longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10:3135–3143. doi: 10.1111/jsm.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner T, Galbraith M, Hays L. From a woman’s perspective: Life as a partner of a prostate cancer survivor. J Midwifery Womens Health. 2011;56:154–160. doi: 10.1111/j.1542-2011.2010.00017.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas KS, Bower JE, Williamson TJ, et al. Post-traumatic disorder symptoms and blunted diurnal cortisol production in partners of prostate cancer patients. Psychoneuroendocrinology. 2012;37:1181–1190. doi: 10.1016/j.psyneuen.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster C, Fenlon D. Recovery and self-management support following primary cancer treatment. Br J Cancer. 2011;105:S21–S28. doi: 10.1038/bjc.2011.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- 34.Bandura A. Self-efficacy mechanism in human agency. Am Psychol. 1982;37:122–147. [Google Scholar]
- 35.Skolarus TA, Metreger T, Hwang S, et al. Optimizing veteran-centered prostate cancer survivorship care: Study protocol for a randomized controlled trial. Trials. 2017;18:181. doi: 10.1186/s13063-017-1925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szymanski KM, Wei JT, Dunn RL, et al. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76:1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skolarus TA, Holmes-Rovner M, Hawley ST, et al. Monitoring quality of life among prostate cancer survivors: The feasibility of automated telephone assessment. Urology. 2012;80:1021–1026. doi: 10.1016/j.urology.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Skolarus TA, Wittmann D, Northouse L, et al. Recommendations for prostate cancer survivorship care: An update to the 2009 Michigan Cancer Consortium guidelines for the primary care management of prostate cancer post-treatment sequelae. J Mens Health. 2014;11:95–107. [Google Scholar]
- 39.Usman Iqbal S, Rogers W, Selim A, et al. The Veterans RAND 12 item health survey (VR-12): What it is and how it is used. https://www.hosonline.org/globalassets/hos-online/publications/veterans_rand_12_item_health_survey_vr-12_2007.pdf
- 40.Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite short form. Urology. 2015;85:101–105. doi: 10.1016/j.urology.2014.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials. 2000;21:167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 42.Bernat JK, Wittman DA, Hawley ST, et al. Symptom burden and information needs in prostate cancer survivors: A case for tailored long-term survivorship care. BJU Int. 2016;118:372–378. doi: 10.1111/bju.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darwish-Yassine M, Berenji M, Wing D, et al. Evaluating long-term patient-centered outcomes following prostate cancer treatment: Findings from the Michigan Prostate Cancer Survivor study. J Cancer Surviv. 2014;8:121–130. doi: 10.1007/s11764-013-0312-8. [DOI] [PubMed] [Google Scholar]
- 44.Gavin AT, Drummond FJ, Donnelly C, et al. Patient-reported ‘ever had’ and ‘current’ long-term physical symptoms after prostate cancer treatments. BJU Int. 2015;116:397–406. doi: 10.1111/bju.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Punnen S, Cowan JE, Chan JM, et al. Long-term health-related quality of life after primary treatment for localized prostate cancer: Results from the CaPSURE registry. Eur Urol. 2015;68:600–608. doi: 10.1016/j.eururo.2014.08.074. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro LH, Prota C, Gomes CM, et al. Long-term effect of early postoperative pelvic floor biofeedback on continence in men undergoing radical prostatectomy: A prospective, randomized, controlled trial. J Urol. 2010;184:1034–1039. doi: 10.1016/j.juro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 47.Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 2017;3:1043–1050. doi: 10.1001/jamaoncol.2016.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adam S, Feller A, Rohrmann S, et al. Health-related quality of life among long-term (≥5 years) prostate cancer survivors by primary intervention: A systematic review. Health Qual Life Outcomes. 2018;16:22. doi: 10.1186/s12955-017-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parahoo K, McDonough S, McCaughan E, et al. Psychosocial interventions for men with prostate cancer. Cochrane Database Syst Rev. 2013;(12):CD008529. doi: 10.1002/14651858.CD008529.pub3. [DOI] [PubMed] [Google Scholar]
- 50.Piette JD, Rosland AM, Marinec NS, et al. Engagement with automated patient monitoring and self-management support calls: Experience with a thousand chronically ill patients. Med Care. 2013;51:216–223. doi: 10.1097/MLR.0b013e318277ebf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith DH, O’Keeffe-Rosetti M, Owen-Smith AA, et al. Improving adherence to cardiovascular therapies: An economic evaluation of a randomized pragmatic trial. Value Health. 2016;19:176–184. doi: 10.1016/j.jval.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berner ES, Burkhardt JH, Panjamapirom A, et al. Cost implications of human and automated follow-up in ambulatory care. Am J Manag Care. 2014;20:SP531–SP540. [PubMed] [Google Scholar]
- 53.Cunningham P. Patient perceptions of clinician self-management support for chronic conditions. Am J Manag Care. 2016;22:e125–e133. [PubMed] [Google Scholar]
- 54.Drummond FJ, Kinnear H, O’Leary E, et al. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment: Results from the PiCTure (prostate cancer treatment, your experience) study. J Cancer Surviv. 2015;9:361–372. doi: 10.1007/s11764-014-0419-6. [DOI] [PubMed] [Google Scholar]