Abstract
PURPOSE
Effective treatment options are limited for patients with acute myeloid leukemia (AML) who cannot tolerate intensive chemotherapy. An international phase Ib/II study evaluated the safety and preliminary efficacy of venetoclax, a selective B-cell leukemia/lymphoma-2 inhibitor, together with low-dose cytarabine (LDAC) in older adults with AML.
PATIENTS AND METHODS
Adults 60 years or older with previously untreated AML ineligible for intensive chemotherapy were enrolled. Prior treatment of myelodysplastic syndrome, including hypomethylating agents (HMA), was permitted. Eighty-two patients were treated at the recommended phase II dose: venetoclax 600 mg per day orally in 28-day cycles, with LDAC (20 mg/m2 per day) administered subcutaneously on days 1 to 10. Key end points were tolerability, safety, response rates, duration of response (DOR), and overall survival (OS).
RESULTS
Median age was 74 years (range, 63 to 90 years), 49% had secondary AML, 29% had prior HMA treatment, and 32% had poor-risk cytogenetic features. Common grade 3 or greater adverse events were febrile neutropenia (42%), thrombocytopenia (38%), and WBC count decreased (34%). Early (30-day) mortality was 6%. Fifty-four percent achieved complete remission (CR)/CR with incomplete blood count recovery (median time to first response, 1.4 months). The median OS was 10.1 months (95% CI, 5.7 to 14.2), and median DOR was 8.1 months (95% CI, 5.3 to 14.9 months). Among patients without prior HMA exposure, CR/CR with incomplete blood count recovery was achieved in 62%, median DOR was 14.8 months (95% CI, 5.5 months to not reached), and median OS was 13.5 months (95% CI, 7.0 to 18.4 months).
CONCLUSION
Venetoclax plus LDAC has a manageable safety profile, producing rapid and durable remissions in older adults with AML ineligible for intensive chemotherapy. High remission rate and low early mortality combined with rapid and durable remission make venetoclax and LDAC an attractive and novel treatment for older adults not suitable for intensive chemotherapy.
INTRODUCTION
The median age at diagnosis of acute myeloid leukemia (AML) is 68 years. Older adults are often ineligible for intensive chemotherapy and thus have limited effective treatment options.1,2 Less-intensive approaches to treatment, such as low-dose cytarabine (LDAC), are associated with poor response rates (11% to 19%) and median survival times (< 6 months).3-5 Similarly, initial treatment with azacitidine, decitabine, or gemtuzumab ozogamicin result in complete remission (CR) plus CR with incomplete blood count recovery (CRi) rates of less than 30%.5-7 In part because of limited expectation of success, many older patients do not receive leukemia therapy.8 These factors underscore the high unmet need for more-effective and less-toxic treatment options for older adults with AML, particularly those who are ineligible for intensive chemotherapy.
B-cell leukemia/lymphoma-2 (BCL-2) family members, including BCL-2, BCL-XL, and MCL1, promote cell survival by binding and sequestering pro-apoptotic proteins in cancer cells. BCL-2 has been shown to mediate chemoresistance and enhance survival of leukemic blast and progenitor cells.9,10 Venetoclax, a selective, potent, orally bioavailable small-molecule BCL-2 inhibitor, has been studied alone and in combination with other agents in several hematologic malignancies.11-16 A phase II study reported an overall response rate (ORR) of 19% with venetoclax monotherapy in heavily pretreated patients with AML.17 Resistance to venetoclax monotherapy may be mediated by other prosurvival proteins, such as MCL1, that sequester endogenous BCL-2 homology domain 3-only proteins (eg, Bim) released by venetoclax on BCL-2 binding. Several drugs—including anthracyclines, hypomethylating agents (HMAs), and cytarabine—have demonstrated the ability to down-regulate MCL1 expression and act synergistically with venetoclax against AML cells in preclinical studies.18-20 As proof of concept, a 61% CR/CRi rate was reported for venetoclax combined with HMAs (ie, azacitidine or decitabine) in treatment-naive older adults with AML,21 exceeding previously reported response rates for HMAs alone.5,7 Here, a phase Ib/II study was conducted to determine the safety and preliminary efficacy of venetoclax in combination with LDAC in previously untreated adults with AML who were ineligible for intensive chemotherapy.
PATIENTS AND METHODS
Patients
Patients age 60 years or older with previously untreated AML and ineligible for intensive chemotherapy were enrolled (Data Supplement). Patients with secondary AML or prior treatment with HMAs for myelodysplastic syndrome (MDS) were permitted. Exclusion criteria included prior therapy for AML or any previous use of cytarabine for any indication (more details in the Treatment section). Local ethics committee approval was obtained, and patients provided written informed consent. The study was conducted in accordance with the International Conference on Harmonization, Good Clinical Practice guidelines, and the Declaration of Helsinki.
Study Design
This open-label, multicenter, multinational phase Ib/II study (ClinicalTrials.gov identifier: NCT02287233) enrolled patients between January 2015 and May 2017. Data cutoff for efficacy in this analysis was November 8, 2017; cutoff for safety was January 30, 2018. Primary objectives in the dose-escalation phase were to assess safety, pharmacokinetics (PK), maximum tolerated dose, and recommended phase II dose of venetoclax combined with LDAC. In dose expansion, the primary objectives were to obtain preliminary estimates of efficacy: ORR, including CR, CRi, and partial remission (PR); duration of response (DOR); and safety of the combination at the recommended phase II dose.22 Exploratory objectives were to identify biomarkers of efficacy and resistance.
Treatment
Patients were hospitalized and tumor lysis syndrome (TLS) prophylaxis was initiated at least 24 hours before the first dose of venetoclax and continued during a ramp-up period until the target venetoclax dose was reached. Venetoclax was administered orally, once daily, after food. Venetoclax dosing began at 50 or 100 mg and increased over 4 to 5 days to the target venetoclax dose; dosing was continued through day 28 of each cycle. In subsequent 28-day cycles, venetoclax was commenced at the target dose. LDAC (20 mg/m2) was administered by subcutaneous injection once daily, on days 1 to 10. At the completion of a 28-day cycle, if bone marrow blasts were less than 5%, venetoclax dosing was interrupted if needed to promote recovery of neutrophils and platelets. Patients could start the next cycle of therapy when the neutrophil count recovered to least 0.5 × 109/L and the platelet count to at least 25 × 109/L. Once morphologic evidence of leukemia was cleared, granulocyte colony stimulating factor was permitted at investigator’s discretion. Patients could continue receiving treatment until disease progression or until discontinuation criteria were met (Data Supplement).
Because venetoclax is a Cytochrome P450 (CYP3A) substrate, patients receiving CYP3A inhibitors (CYP3A4i) had their venetoclax dose reduced by approximately 50% for moderate CYP3A inhibitors and approximately 75% to 90% for strong inhibitors.23 If a patient was on multiple inhibitors, venetoclax dose adjustment was based on the strongest CYP3Ai.
Study Assessments
Safety.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03.24 Dose-limiting toxicities (DLTs) were determined during cycle 1 of the dose-escalation phase and defined as grade 4 toxicity, excluding AEs commonly caused by AML (eg, neutropenia, fever). Hematologic DLT was defined as failure of platelet recovery to 25 × 109/L or greater and neutrophils to 0.5 × 109/L or greater within 14 days of the last dose of venetoclax in the absence of residual AML. Clinical and laboratory TLS were considered as previously defined.25
Efficacy.
Bone marrow assessments were performed at screening, after cycles 1 and 3, and then every three cycles until two consecutive samples confirmed CR. Additional bone marrow studies were performed if there was clinical suspicion of recurrence and at final visit. Clinical responses were defined according to International Working Group response criteria for AML (Data Supplement).22
PK and exploratory biomarkers.
Pharmacokinetic assessment is described in the Data Supplement. Biomarkers that may be predictive of venetoclax activity and response were assessed during the trial (Data Supplement). Clinical outcome was correlated with the WHO 2008 classification26 and cytogenetic and molecular markers, including the chromatin-spliceosome, TP53-aneuploidy, and IDH R172 mutation subgroups, as recently proposed.27 Karyotypic abnormalities were classified using site-reported results, and centrally reported next-generation sequencing data were used to supplement molecular mutation results.
Statistical Analyses
All baseline summary statistics and analyses were based on patient characteristics obtained before initiation of venetoclax or LDAC. The safety population included patients who received at least one dose of venetoclax; the DLT-evaluable population included patients who received at least 80% of planned cycle 1 doses during dose-escalation phase. Further details of statistical analyses are contained in the Data Supplement. For the phase II portion, it was initially planned to enroll 25 patients at the recommended phase II dose to enable a 95% CI for estimation of ORR with margin of error not exceeding plus or minus 25%. The study was later amended to enroll an additional 28 patients to provide further precision regarding the observed response rate estimates, and another 21 patients were enrolled to evaluate patients allowed to receive concomitant strong CYP3Ai if indicated.
RESULTS
All patients enrolled at least 6 months before this analysis. At the time of analysis, the median treatment duration was 4.2 months (range, 0.2 to 29 months), and the median number of cycles of therapy was five. The median number of cycles of LDAC delivered to patients who achieved CR was seven (range, two to 30), and five (range, one to 16) for patients achieving CRi. Seven patients (9%) remained in the study therapy at of time of analysis.
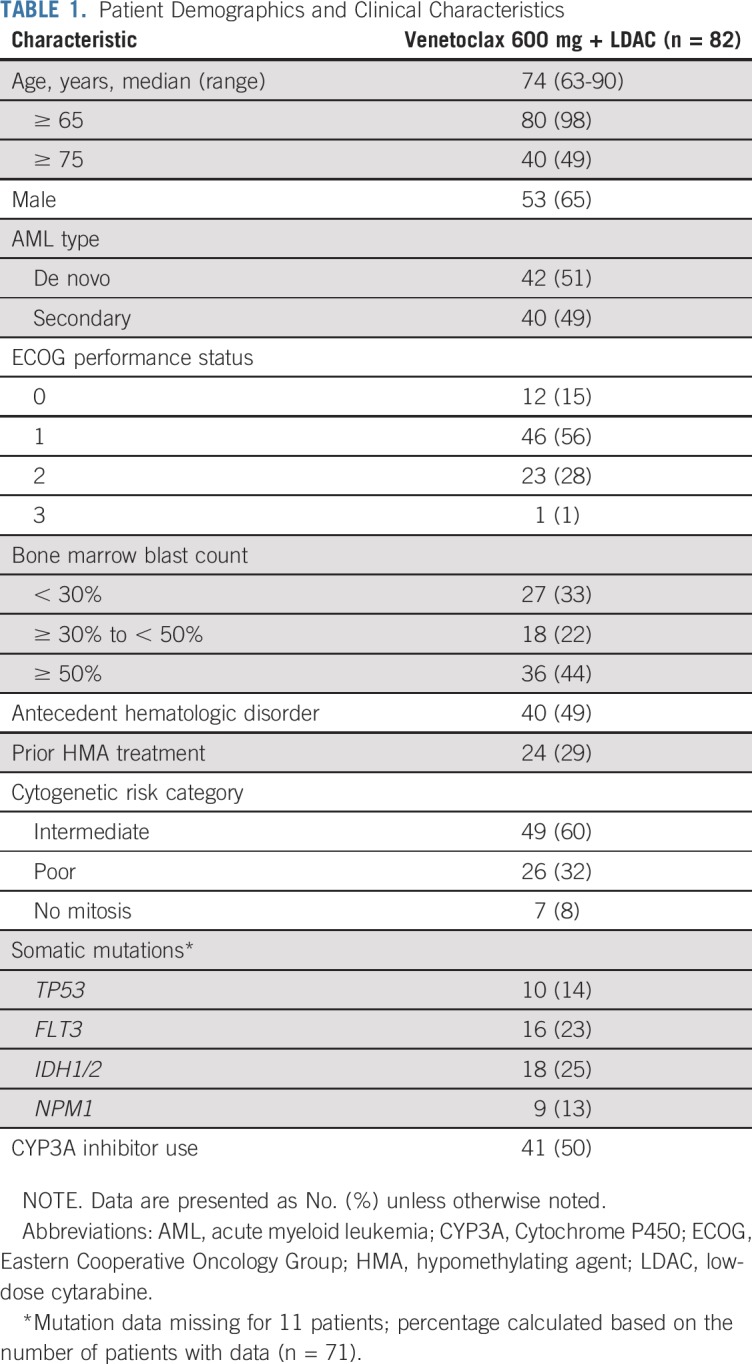
Patient Demographics and Clinical Characteristics
Eighty-two patients were enrolled to a 600 mg venetoclax cohort and received at least one dose of venetoclax. Baseline demographics and clinical characteristics for the 600 mg venetoclax cohort are shown in Table 1. The median age was 74 years (range, 63 to 90 years). Forty-nine percent of patients had secondary AML, and 50% had concomitant CYP3A inhibitor use. Baseline mutations in TP53, FLT3, IDH1/2, and NPM1 were detected in 14%, 23%, 25%, and 13% of patients, respectively.
TABLE 1.
Patient Demographics and Clinical Characteristics
DLT and Maximum Tolerated Dose
No DLTs were observed in the 600 mg venetoclax cohort during the dose-escalation phase of the study. Of the six patients in the 600 mg dose escalation cohort who proceeded to subsequent cycles, only one patient required dose interruption between cycles 1 and 2, because of thrombocytopenia without residual morphologic AML. At the 800 mg dose level (n = 10), most patients who proceeded to subsequent cycles needed dose interruption between cycles 1 and 2 to permit count recovery, and one patient experienced a hematologic dose-limiting toxicity (grade 4 thrombocytopenia lasting more than 42 days without evidence of residual leukemia in a patient with AML secondary to myeloproliferative neoplasm). Therefore, the recommended phase II dose was determined to be venetoclax 600 mg when combined with LDAC.
Safety Profile
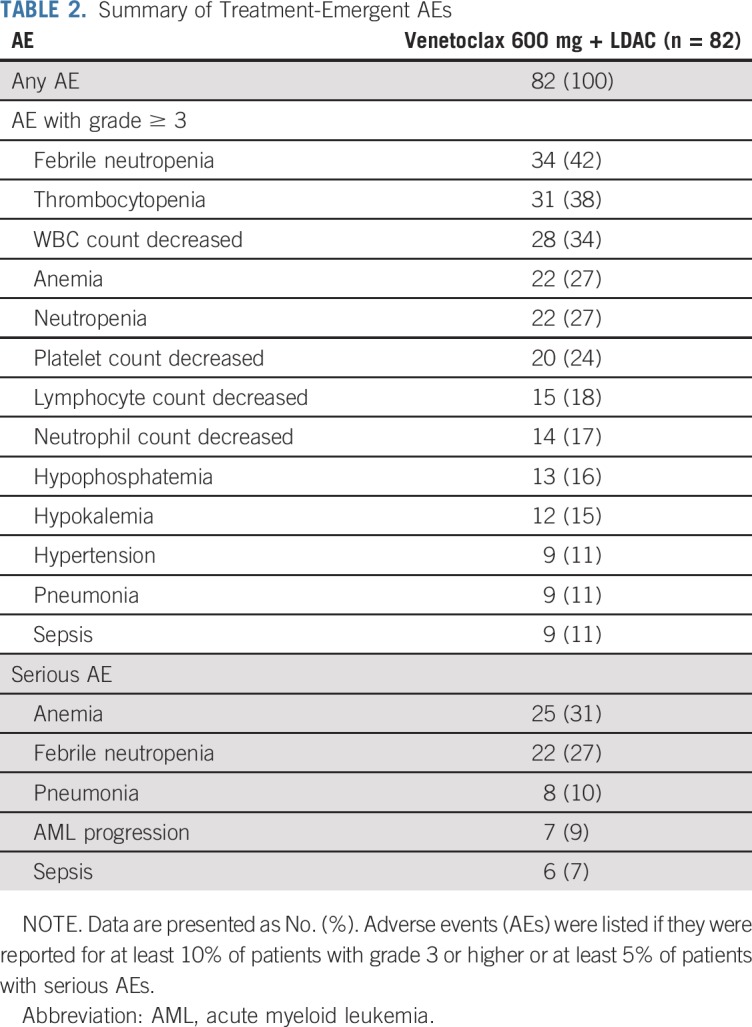
A summary of treatment-emergent AEs for the venetoclax 600 mg cohort is shown in Table 2; further breakdown is available in the Data Supplement. Consistent with expectations for AML, the common grade 3 or 4 AEs, irrespective of cause, were frequently hematologic and included febrile neutropenia (42%), thrombocytopenia (38%), neutropenia (27%), and anemia (27%). The most common nonhematologic AEs of any grade or cause were nausea (70%), diarrhea (49%), hypokalemia (48%), and fatigue (43%). Serious AEs other than AML progression, occurring in at least 5% of patients, were anemia (31%), febrile neutropenia (27%), pneumonia (10%), and sepsis (7%).
TABLE 2.
Summary of Treatment-Emergent AEs
Forty-five (55%) patients had venetoclax dose interruptions due to AEs, most commonly between subsequent 28-day cycles (due to delayed neutrophil and platelet recovery in eight and 10 patients, respectively). Dose reductions were necessary in six patients (7%), the majority due to thrombocytopenia. Reduced duration of venetoclax administration to 21 and 14 days occurred in 25 patients (30%) and 14 patients (17%), respectively. Laboratory-defined TLS was reported in two patients (elevations of potassium and phosphorus in one patient and elevations of uric acid and phosphorus in the other), although both were able to complete the venetoclax ramp-up to the intended dose. No clinical TLS was reported. The 30-day mortality rate was 6% (n = 5).
Efficacy
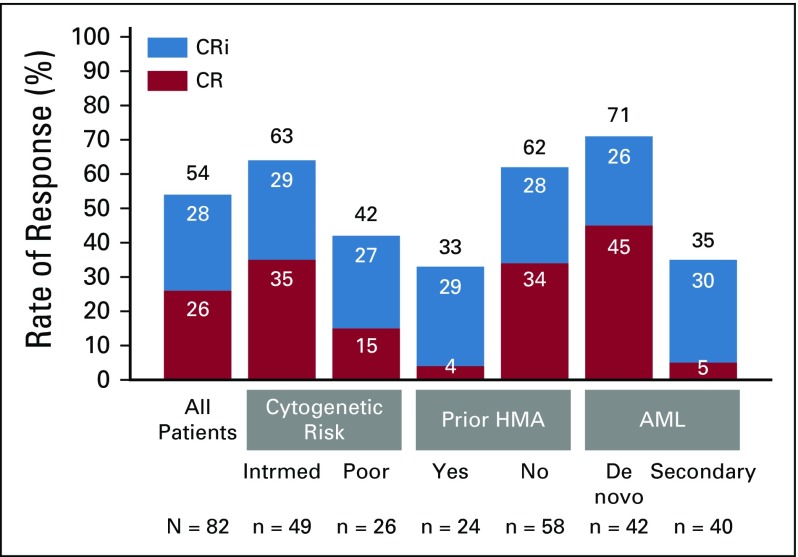
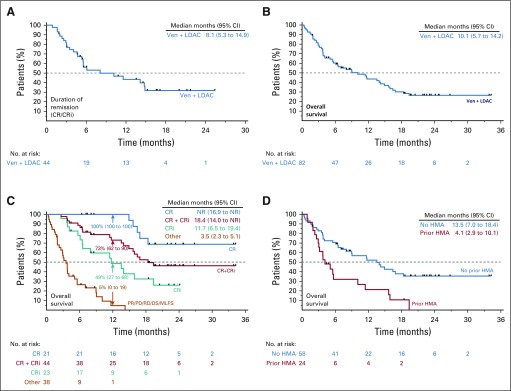
In phase I and II, a total of 82 patients were enrolled to the venetoclax 600 mg dose level. The CR/CRi rate was 54% (95% CI, 42% to 65%); CR was achieved in 26% of patients, and CRi in 28% (Fig 1). Median time to first CR/CRi was 1.4 months (range, 0.8 to 14.9 months). Patients with de novo AML and intermediate-risk cytogenetic features and without prior HMA exposure had the highest rates of CR/CRi: 71%, 63%, and 62%, respectively (Fig 1). A complete breakdown of responses by baseline patient features is shown in the Data Supplement. Among patients achieving CR/CRi after venetoclax plus LDAC, the median duration of remission was 8.1 months (95% CI, 5.3 to 14.9 months; Fig 2A). The median OS for all patients was 10.1 months (95% CI, 5.7 to 14.2 months; Fig 2B). Observed survival for the study population was better for patients achieving CR, CRi, or both (Fig 2C). The 12-month estimated survival rate for those who achieved CR, CRi, CR/CRi, or no response was 100%, 49%, 73%, and 5%, respectively (Fig 2C, arrows). Patients without prior HMA exposure had a longer median OS (13.5 months; 95% CI, 7.0 to 18.4 months) than patients with AML previously exposed to prior HMA (4.1 months; 95% CI, 2.9 to 10.1 months; Fig 2D).
FIG 1.
Complete remission (CR)/CR with incomplete blood count recovery (CRi) rates by patient subgroups. The graph shows the rates of CR and CRi in all patients, as well as key patient subgroups sorted by baseline characteristics. The numbers in the bars represent the percentage of patients with a given response, and the black number at the top of each bar is the total CR/CRi percentage in a given subgroup. Partial remissions are not shown, because only one patient had a partial remission. AML, acute myeloid leukemia; HMA, hypomethylating agent; intrmed, intermediate.
FIG 2.
Overall survival and duration of response. Kaplan-Meier curves showing (A) duration of remission for patients who had complete remission (CR)/CR with incomplete blood count recovery (CRi); (B) overall survival of all patients; (C) overall survival broken down by the patients’ best response; (D) overall survival by prior hypomethylating agent (HMA) exposure. NOTE: Numbers in parentheses are 95% CIs. DS, discontinued prior to assessment; LDAC, low-dose cytarabine; MLFS, morphologic leukemia–free state; NA, not available; NR, not reached; PD, progressive disease; PR, partial remission; RD, resistant disease; Ven, venetoclax.
Most patients who achieved remission became transfusion independent. Among patients treated with venetoclax in combination with LDAC, 46% (38 of 82) achieved independence from both RBC and platelet transfusion. Forty-eight percent (39 of 82) achieved RBC transfusion independence, and 60% (49 of 82) achieved platelet transfusion independence. Among the patients who were dependent on transfusions before enrollment, 43% (23 of 53) of those dependent on RBC transfusions and 65% (15 of 23) of those dependent on platelet transfusions became transfusion independent while in the study. The time required for platelet (≥ 50 × 109/L) and neutrophil (≥ 0.5 × 109/L) recovery was assessed among patients who achieved CR/CRi (Data Supplement). The median time to platelet recovery was 28 days, with 90% or more reaching this threshold by day 53. The median time to absolute neutrophil count recovery was 32 days, with 90% recovered by day 64.
PK
Venetoclax and cytarabine PK parameters are shown in the Data Supplement. PK concentration-time profiles of venetoclax and cytarabine are shown in the Data Supplement. These studies showed no impact on drug exposures of combining venetoclax with cytarabine, thus indicating an absence of substantial PK interaction.
Key AML Genetic Mutations and Clinical Response
There are several key genetic mutations that have been identified in driving the progression, prognosis, and outcome in AML, including mutations in TP53, FLT3, IDH1/2, and NPM1; a genomic classification system for AML has been recently developed, largely around these molecular drivers (among others).27,28 Patients with somatic mutations in NPM1 or IDH1/2 had higher than average CR/CRi rates (89% and 72%, respectively), whereas those with TP53 or FLT3 mutations had lower CR/CRi rates (30% and 44%, respectively; Data Supplement).
DISCUSSION
Optimizing treatment selection for older adults with AML remains challenging. Higher incidence of treatment-related complications is expected from intensive chemotherapy because poor performance status and comorbidities are more prevalent in the elderly.29 Poor outcomes with intensive chemotherapy are also typical for older patients with poor-risk cytogenetics, monosomal karyotype, secondary AML, or MDS after failure of prior HMA therapy.30-32 For patients older than 65 years receiving intensive chemotherapy, reported remission rates range between 45% and 57% and median OS between 5 and 12 months.33,34 For patients between 60 and 75 years of age and previously treated with an HMA, CR/CRi rates are only 28% for intensive 7 plus 3 chemotherapy and 37% for patients receiving liposomal daunorubicin and cytarabine (CPX-351).35 Accordingly, many physicians and patients elect a low-intensity approach to therapy or no active treatment at all.36
Low-intensity therapies, such as LDAC monotherapy, have historically been administered with palliative intent, with CR/CRi rates of 11% to 19% and median OS of approximately 5.5 months.4,31 Therefore, the observed CR/CRi rate of 54% and 10.1-month median survival for patients treated with venetoclax plus LDAC seems favorable. Among patients older than 75 years, the rate of CR/CRi remained 60%, with median OS of 14.9 months. Importantly, the higher clinical response rate was achieved without an increase in early mortality: the 30-day mortality rate was 6% in the first month after LDAC plus venetoclax treatment, compared with 8% to 13% with LDAC alone from literature reports.4,31 This raises the possibility of also evaluating venetoclax combined with low-intensity therapy in younger and/or more fit patients with AML, with the goal of sparing patients the greater toxicity generally observed with intensive chemotherapy.
The outcomes achieved with venetoclax plus LDAC also compare favorably with those obtained during recent studies of HMA monotherapy in older patients with AML: decitabine resulted in CR/CRi of 25.6% and median OS of 7.7 months in older adults with newly diagnosed AML5; azacitidine achieved CR/CRi of 27.8% and median OS of 10.4 months in another phase III study, which included patients either eligible or ineligible for intensive chemotherapy.7 Importantly, both historical studies excluded patients who had received prior HMA for MDS, whereas in the current study, 29% of patients had previously received an HMA. For HMA-naive participants in the current study, the CR/CRi rate was 62%, with a median duration of response 14.8 months and median OS of 13.5 months.
Historical rates of CR/CRi for patients treated with LDAC, azacitidine, or decitabine monotherapy are between 11% and 26%,5,37 with moderate difference in CR rates observed between patients with intermediate-risk versus poor-risk cytogenetics (reported differences between 3% and 13%).38,39 In the current study, rates of CR/CRi were 63% and 42% for patients with intermediate-risk and poor-risk cytogenetics, respectively. Among patients with mutant NPM1, the CR/CRi rate was 89% (eight of nine), which included two patients with coexisting FLT3 and IDH1/2 mutations and three additional patients with either mutant FLT3 or IDH1/2; all eight patients remain alive at more than 1 year. Although larger validation studies are needed, these preliminary observations suggest that such patients may be especially responsive to venetoclax plus LDAC therapy.
Responses to venetoclax monotherapy have been previously reported in patients with IDH1 or IDH2 mutations.40 In the current study, venetoclax plus LDAC resulted in a CR/CRi rate of 72% (13 of 18) and median OS of 19.4 months among patients with IDH1/2-mutant AML. Therefore, venetoclax plus LDAC may also represent a useful treatment strategy for patients with treatment-naive IDH1/2-mutant AML who are ineligible for intensive chemotherapy; further integration of IDH1/2 inhibitors into this regimen for patients with persistent IDH-positive disease may be an objective for future clinical trials. Among patients with TP53 mutation or poor cytogenetic risk, the CR/CRi rate with LDAC and venetoclax was 30% (three of 10) or 42% (11 of 26), respectively, indicating that these historically identified features of poor prognosis remain relevant for this combination.
Venetoclax plus LDAC was associated with rapid achievement of CR/CRi, with median time to first response of 1.4 months, compared with 3.1 months for LDAC alone4 and 3.5 to 4.1 months with HMA therapies.37 However, clinical vigilance and robust supportive care measures are necessary until blast clearance and hematologic recovery are achieved. At the onset of this trial, antifungal prophylaxis with azole antifungals (CYP3A inhibitors) was prohibited because of the potential of increasing venetoclax exposure.41 However, separate pharmacokinetic studies reported that venetoclax can be administered, with appropriate dose reductions, to patients with AML using concomitant moderate or strong CYP3A inhibitors (such as the antifungal azoles voriconazole or posaconazole).23 Consequently, a cohort was added to prospectively evaluate the impact of allowing CYP3A inhibitors when medically required. As such, 50% of the patients enrolled and treated had concomitant CYP3A inhibitor use; no increase in adverse events related to potential CYP3A inhibitor interaction was observed.
Because patients with prior HMA exposure were included in the current study, it is difficult to directly compare efficacy between the current study and historical results. Clinical trials of low-intensity therapies have historically excluded patients who received prior treatment with HMAs, and such patients have an especially poor prognosis. In the current study, nearly one-third (29%) of patients had prior HMA exposure. The CR/CRi rate for those patients with prior HMA exposure was 33%, comparable to rates reported for intensive chemotherapy or CPX-351 for patients experiencing disease progression after prior HMA therapy for antecedent conditions. Among patients without prior HMA treatment, the CR/CRi rate for venetoclax plus LDAC was 62%, which is similar to the reported CR/CRi rate of 67% in a recently published study that combined venetoclax with HMAs for patients with AML.21 As such, both venetoclax studies, in combination with LDAC or HMAs, support a role for venetoclax as an integral component of standard therapy, especially in older adults with AML. International randomized studies are currently underway for venetoclax or placebo combined with either LDAC (ClinicalTrials.gov identifier: NCT03069352) or azacitidine (ClinicalTrials.gov identifier: NCT02993523).
In conclusion, the combination of venetoclax and LDAC is tolerable and associated with high rates of remission in patients with previously untreated AML who are ineligible for intensive chemotherapy. The high remission rate, low early mortality, rapid induction of remission, and durable length of remission make the combination with venetoclax an attractive and novel treatment option for older adults not suitable for intensive chemotherapy.
ACKNOWLEDGMENT
We thank all investigators, study staff, patients, and their families for their participation in this study, and Mack Mabry, formerly of AbbVie, for his contributions.
AbbVie is committed to responsible data-sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan (SAP) and execution of a data-sharing agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Footnotes
Presented in part at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2016, the 58th Annual Meeting of the American Society of Hematology (ASH), San Diego, CA, December 3-6, 2016, the 22nd Congress of the European Hematology Association, Madrid, Spain, June 22-25, 2017, the 59th Annual Meeting of ASH, Atlanta, GA, December 9-12, 2017, and the 60th Annual Meeting of ASH, San Diego, CA, December 1-4, 2018.
Supported by research funding from AbbVie and Genentech (A.H.W., S.A.S., G.J.R., J.-Z.H., W.F., T.L.L., R.B.W., and A.E.), the National Health and Medical Research Council of Australia (A.H.W.), the Medical Research Future Fund (A.H.W.), the Victorian Cancer Agency (A.H.W.), and the Leukemia Lymphoma Society Specialized Centre of Research [Strasser] (A.H.W.). Medical writing and support was provided by Ryan J. Bourgo, PhD, of AbbVie, and Delyth E. Eickermann, PhD, of TRM Oncology, both funded by AbbVie. Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review, and approval of the manuscript.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT02287233
Preprint version available on bioRxiv.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew H. Wei, Jing-Zhou Hou, Tara L. Lin, Michael Savona, Ahmed Hamed Salem, Suresh Agarwal, Tu Xu, Rod Humerickhouse, Wan-Jen Hong, John Hayslip, Gail J. Roboz
Administrative support: Kaffa M. Fakouhi
Provision of study material or patients: Andrew H. Wei, Jing-Zhou Hou, Walter Fiedler, Roland B. Walter, Anoop Enjeti, Michael Savona, Kaffa M. Fakouhi
Collection and assembly of data: Andrew H. Wei, Stephen A. Strickland Jr, Jing-Zhou Hou, Walter Fiedler, Roland B. Walter, Anoop Enjeti, Michael Savona, Sangmin Lee, Relja Popovic, Suresh Agarwal, Tu Xu, Kaffa M. Fakouhi, Wan-Jen Hong, Gail J. Roboz
Data analysis and interpretation: Andrew H. Wei, Stephen A. Strickland Jr, Jing-Zhou Hou, Walter Fiedler, Tara L. Lin, Roland B. Walter, Anoop Enjeti, Ing Soo Tiong, Michael Savona, Sangmin Lee, Brenda Chyla, Relja Popovic, Ahmed Hamed Salem, Suresh Agarwal, Tu Xu, Rod Humerickhouse, Wan-Jen Hong, John Hayslip, Gail J. Roboz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Andrew H. Wei
Honoraria: Amgen, Servier, Novartis, Celgene, AbbVie/Genentech, Roche, Pfizer, Janssen Oncology
Consulting or Advisory Role: Servier, Novartis, Amgen, AbbVie/Genentech
Speakers' Bureau: AbbVie/Genentech, Novartis
Research Funding: Novartis (Inst), Celgene (Inst)
Patents, Royalties, Other Intellectual Property: A.H.W. is a former employee of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax, and is eligible for benefits related to these payments. A.H.W. receives payments from the Walter and Eliza Hall Institute related to venetoclax.
Expert Testimony: Amgen
Travel, Accommodations, Expenses: AbbVie, Amgen, Novartis, Celgene
Stephen A. Strickland Jr
Consulting or Advisory Role: Tolero Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, Novartis, Jazz Pharmaceuticals, Kite Pharma, Pfizer
Research Funding: Sunesis Pharmaceuticals (Inst), Boehringer Ingelheim (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), AbbVie (Inst), Celator Pharmaceuticals/Jazz Pharmaceuticals (Inst), Novartis (Inst), Menarini (Inst)
Walter Fiedler
Consulting or Advisory Role: Amgen, ARIAD Pharmaceuticals, Pfizer, Novartis, Jazz Pharmaceuticals
Research Funding: Amgen
Travel, Accommodations, Expenses: Amgen, Daiichi Sankyo, Jazz Pharmaceuticals
Tara L. Lin
Honoraria: Jazz Pharmaceuticals
Research Funding: Tolero Pharmaceuticals (Inst), Gilead Sciences (Inst), Prescient Therapeutics (Inst), Ono Pharmaceutical (Inst), Bio-Path Holdings (Inst), Mateon Therapeutics (Inst), Genentech (Inst), Celator Pharmaceuticals (Inst), TrovaGene (Inst), AbbVie (Inst), Pfizer (Inst), Celgene (Inst), Novartis (Inst), Imago Pharmaceuticals (Inst), Astellas Pharma (Inst), Karyopharm Therapeutics (Inst), Seattle Genetics (Inst), Incyte (Inst)
Roland B. Walter
Stock and Other Ownership Interests: Amphivena Therapeutics
Consulting or Advisory Role: Amphivena Therapeutics, Covagen, Emergent BioSolutions, Pfizer, Agios Pharmaceuticals, BioLineRx, Race Oncology, Jazz Pharmaceuticals, Tolero Pharmaceuticals, argenx, Boehringer Ingelheim, BiVictriX, Boston Biomedical, Daiichi Sankyo, Kite Pharma
Research Funding: Amgen, AbbVie, Stemline Therapeutics, Celator Pharmaceuticals, Arog Pharmaceuticals, Pharmacyclics, ADC Therapeutics, Seattle Genetics, Pfizer, BioLineRx, Agios Pharmaceuticals, Selvita, Jazz Pharmaceuticals, Aptevo Therapeutics, Covagen
Anoop Enjeti
Honoraria: Genentech, Sanofi, Novartis, AbbVie
Consulting or Advisory Role: AbbVie, Novartis
Speakers' Bureau: Sanofi, Roche
Travel, Accommodations, Expenses: Novartis
Michael Savona
Stock and Other Ownership Interests: Karyopharm Therapeutics
Consulting or Advisory Role: Karyopharm Therapeutics, Celgene, TG Therapeutics
Research Funding: Sunesis Pharmaceuticals (Inst), TG Therapeutics (Inst), Astex Pharmaceuticals (Inst), Incyte (Inst), Takeda (Inst)
Patents, Royalties, Other Intellectual Property: Product license: Boehringer Ingelheim (minority owner)
Sangmin Lee
Consulting or Advisory Role: AstraZeneca, Amgen, Alexion Pharmaceuticals, Karyopharm Therapeutics, Helsinn Therapeutics
Research Funding: LAM Therapeutics
Travel, Accommodations, Expenses: AstraZeneca, Amgen, Alexion Pharmaceuticals, Karyopharm Therapeutics, Helsinn Therapeutics, Arog Pharmaceuticals
Brenda Chyla
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Relja Popovic
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Ahmed Hamed Salem
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Travel, Accommodations, Expenses: AbbVie
Suresh Agarwal
Employment: AbbVie
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Kaffa M. Fakhoui
Employment: AbbVie/Genentech
Stock and Other Ownership Interests: AbbVie/Genentech
Research Funding: AbbVie/Genentech
Travel, Accommodations, Expenses: AbbVie/Genentech
Other Relationship: AbbVie/Genentech
Rod Humerickhouse
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Wan-Jen Hong
Employment: Genentech
Stock and Other Ownership Interests: Roche
Travel, Accommodations, Expenses: Genentech
John Hayslip
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Gail J. Roboz
Consulting or Advisory Role: Amphivena Therapeutics, Janssen, Amgen, Astex Pharmaceuticals, Celgene, Genoptix, MedImmune, Novartis, Pfizer, AbbVie, argenx, Array BioPharma, Bayer, Celltrion, Jazz Pharmaceuticals, Orsenix, Genentech, Sandoz, Actinium Pharmaceuticals, Astellas Pharma, Eisai, Bayer, Daiichi Sankyo, MEI Pharma, Otsuka Pharmaceutical, Takeda, Roche
Research Funding: AbbVie (Inst), Agios Pharmaceuticals (Inst), Astex Pharmaceuticals (Inst), Celgene (Inst), CTI (Inst), Karyopharm Therapeutics (Inst), MedImmune (Inst), MEI Pharma (Inst), Moffitt (Inst), Novartis (Inst), Onconova Therapeutics (Inst), Pfizer (Inst), Sunesis Pharmaceuticals (Inst), Tensha Therapeutics (Inst), Cellectis (Inst), Janssen (Inst), Amphivena Therapeutics (Inst)
Travel, Accommodations, Expenses: Amphivena Therapeutics, Astex Pharmaceuticals, Janssen, Pfizer, Array BioPharma, Novartis, AbbVie, Jazz Pharmaceuticals, Celgene, Celltrion, Genentech, Sandoz, Bayer, Clovis Oncology, Amgen, Sunesis Pharmaceuticals, Eisai
No other potential conflicts of interest were reported.
REFERENCES
- 1.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 2. US Department of Health and Human Services, National Institutes of Health. National Cancer Institute: Surveillance, Epidemiology, and End Results Program: Cancer Stat Facts: Leukemia – Acute Myeloid Leukemia (AML). 2018 https://seer.cancer.gov/statfacts/html/amyl.html.
- 3.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 4. Dennis M, Hills RK, Russell NH, et al: An evaluation of 17 years of low dose cytarabine as therapy for AML patients not fit for intensive treatment, including patients with adverse cytogenetics, shows improving survival, potential underutilisation and highlights the need for new therapy. Blood 2017; 130:3874.
- 5.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 7.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma E, Bonthapally V, Chawla A, et al: An evaluation of treatment patterns and outcomes in elderly patients newly diagnosed with acute myeloid leukemia: A retrospective analysis of electronic medical records from US community oncology practices. Clin Lymphoma Myeloma Leuk 16:625-636.e3, 2016. [DOI] [PubMed]
- 9.Del Poeta G, Venditti A, Del Principe MI, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML) Blood. 2003;101:2125–2131. doi: 10.1182/blood-2002-06-1714. [DOI] [PubMed] [Google Scholar]
- 10.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia. Blood. 2017;129:2702–2705. doi: 10.1182/blood-2017-01-761973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130:2401–2409. doi: 10.1182/blood-2017-06-788786. [DOI] [PubMed] [Google Scholar]
- 13.Moreau P, Chanan-Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130:2392–2400. doi: 10.1182/blood-2017-06-788323. [DOI] [PubMed] [Google Scholar]
- 14.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 16.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 17.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogenberger JM, Delman D, Hansen N, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56:226–229. doi: 10.3109/10428194.2014.910657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu X, Zhao J, Ma J, et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res. 2016;22:4440–4451. doi: 10.1158/1078-0432.CCR-15-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teh TC, Nguyen NY, Moujalled DM, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32:303–312. doi: 10.1038/leu.2017.243. [DOI] [PubMed] [Google Scholar]
- 21.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. doi: 10.1016/S1470-2045(18)30010-X. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: Evaluation of dose adjustments. Clin Ther. 2017;39:359–367. doi: 10.1016/j.clinthera.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services, National Institutes of Health , National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 2018. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
- 25.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 27.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papaemmanuil E, Döhner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med. 2016;375:900–901. doi: 10.1056/NEJMc1608739. [DOI] [PubMed] [Google Scholar]
- 29.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: A better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian H, O’brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 32.Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardin C, Turlure P, Fagot T, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: Results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109:5129–5135. doi: 10.1182/blood-2007-02-069666. [DOI] [PubMed] [Google Scholar]
- 35.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36:2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Ali HK, Jaekel N, Niederwieser D. The role of hypomethylating agents in the treatment of elderly patients with AML. J Geriatr Oncol. 2014;5:89–105. doi: 10.1016/j.jgo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Cashen AF, Schiller GJ, O’Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 39.Döhner H, Lübbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–1433. doi: 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chyla B, Daver N, Doyle K, et al. Genetic biomarkers of sensitivity and resistance to venetoclax monotherapy in patients with relapsed acute myeloid leukemia. Am J Hematol. 2018;93:E202–E205. doi: 10.1002/ajh.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal SK, Salem AH, Danilov AV, et al. Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br J Clin Pharmacol. 2017;83:846–854. doi: 10.1111/bcp.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]