Abstract
Activation of vagal C-fibers is likely involved in some types of pathological coughing, especially coughing that is associated with airway inflammation. This is because stimulation of vagal C-fibers leads to strong urge to cough sensations, and because C-fiber terminals can be strongly activated by mediators associated with airway inflammation. The most direct manner in which a given mediator can activate a C-fiber terminal is through interacting with its receptor expressed in the terminal membrane. The agonist-receptor interaction then must lead to the opening (or potentially closing) of ion channels that lead to a membrane depolarization. This depolarization is referred to as a generator potential. If, and only if, the generator potential reaches the voltage necessary to activate voltage-gated sodium channels, action potentials are initiated and conducted to the central terminals within the CNS. Therefore, there are three target areas to block the inflammatory mediator induced activation of C-fiber terminals. First, at the level of the mediator-receptor interaction, secondly at the level of the generator potential, and third at the level of the voltage-gated sodium channels. Here we provide a brief overview of each of these therapeutic strategies.
I. Introduction
Coughing associated with inflammation is most likely secondary to vagal sensory C-fiber activation. This assumption is based on the finding that experimentally evoked cough is dependent on sensory nerves carried by the vagi [1]. The sensory nerves in the vagi comprise both fast conducting A-fibers and slow conducting C-fibers, but inflammatory mediators, in general, are rather selective activators of the C-fiber subpopulation. Vagal C-fibers can also be stimulated by mechanical events associated with secretions and edema that may accompany airways inflammation.
Vagal sensory C-fibers richly innervate the larynx, trachea, extrapulmonary and intrapulmonary bronchi, and parenchymal tissues. Based on a classification scheme from elegant studies carried out by the Coleridge’s and their many colleagues, the fibers innervating the large airways are often referred to as “bronchial” C-fibers whereas those in the deep lung are referred to as “pulmonary” C-fibers [2]. Pulmonary C-fibers are also included in nerve fibers referred to as J-receptors by Paintal et al, based on their presumed juxta-capillary terminations [3]. Vagal C-fibers in the respiratory tract have also been categorized based on the ganglion in which their cell bodies reside: the vagal nodose ganglion or the vagal jugular ganglion. The neurons in the nodose ganglia have a placodal embryonic origin, whereas the jugular neurons, similar to neurons in the dorsal root ganglia, have a neural crest origin [4]. In guinea pigs, the C-fibers innervating the extrapulmonary airways are disproportionately jugular C-fibers, whereas the C-fibers in the intrapulmonary tissue comprise both nodose and jugular C-fibers [5]. The distinction between placodal (nodose) C-fibers and neural crest (jugular, DRG) C-fibers is important because they have distinct activation profiles, neuropeptide content, growth factor dependency, and central terminations [6]. The distinctions in phenotype between jugular and nodose C-fibers would appear to be more dependent on their ganglionic origin of the cell bodies than the location of their peripheral terminations. Jugular C-fibers in the trachea have a similar phenotype to those in the lungs and even those in the esophagus; likewise, nodose C-fiber phenotype is relatively constant among those in extrapulmonary airway, intrapulmonary airways, and esophagus [7]. It is likely that neural crest vs placodal vagal C-fibers have distinct roles in cough and other visceral reflexes; precisely what these roles are in human disease remains an important unknown question.
A given inflammatory mediator activates C-fibers (evokes action potential discharge) by first binding to its receptor expressed at the C-fiber terminations. This then leads to membrane depolarization by causing a net inward depolarizing current that is due to the activation of some type of non-selective cation channel, chloride channel, or potentially inhibition of a potassium channel. This depolarization is referred to as the generator potential. The generator potential is all but irrelevant if it is not large and fast enough to reach the voltage threshold for activation of voltage-gated sodium channels (NaV), which in turn are responsible for the induction and conduction of the action potential.
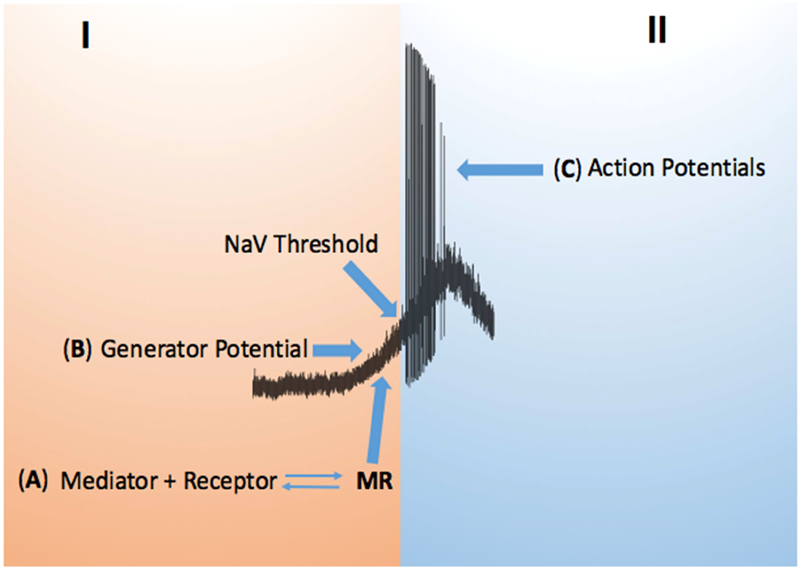
There are, therefore, three general approaches to peripherally block C-fiber activation by inflammatory mediators; antagonizing the mediator receptors, interfering with the ion channels that cause the generator potential, and by blocking the NaV channels—this is schematized in figure 1.
Figure 1:
Activation of a C-fiber by an inflammatory mediator consists of 2 parts, part I where an inflammatory mediator (M) binds to its receptor (R) on the C-fiber afferent terminals (A). This, MR interaction, leads to a terminal membrane depolarization referred to as a generator potential (B) that may reach the voltage threshold for NaV channels that in turn cause the second part (II) of action potential generation and conduction to the CNS (C). Without a large and fast depolarization in part I, the generator potential will electronically fade back to resting potential and there will not be a part II. The frequency of action potential discharge is a function of the rate and amplitude of the generator potential.
II. Blocking Inflammatory Mediator Receptors
Antagonizing a specific inflammatory mediator has the advantage of being a relatively specific treatment reducing the likelihood of unwanted on-target side-effects. A disadvantage of this strategy is that it will only benefit those suffering from coughs that are driven by activation of a particular mediator. In airways inflammation there are literally hundreds of disparate inflammatory mediators present, so it might seem unlikely that blocking the action of one mediator would be of much use. It should be kept in mind though that most inflammatory mediators do not directly activate either jugular or nodose C-fibers.
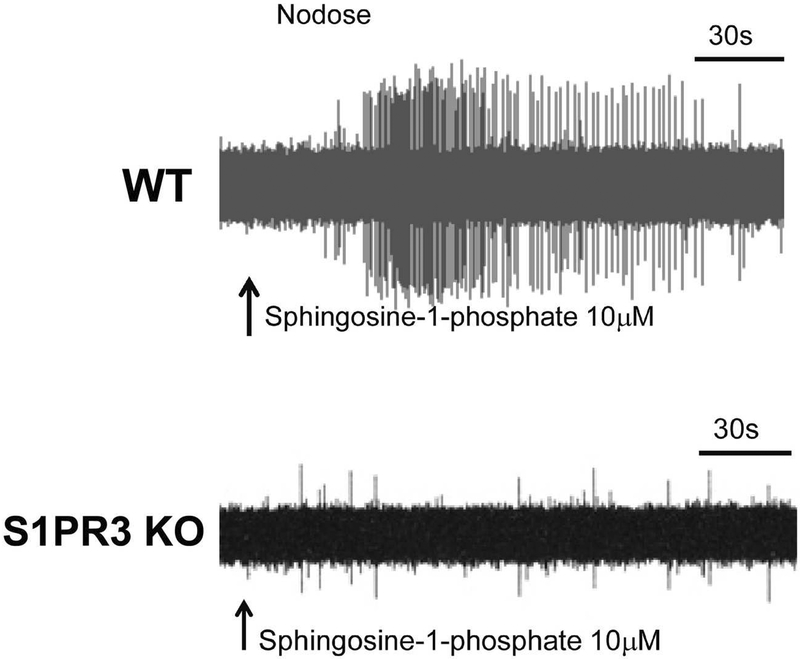
For a mediator to directly activate a C-fiber, at a minimum, the C-fiber neurons must express the receptor for the mediator. To get a handle on the nature of mediators most likely to activate C-fibers we carried out an extensive RNAseq (transcriptome) analysis of jugular and nodose vagal sensory neurons [8]. Most C-fibers in the respiratory tract are activated when the capsaicin receptor TRPV1 is stimulated, thus we focused our attention on C-fiber neurons by first determining whether the neuron in question expressed the capsaicin receptor, or in another strategy, by first determining whether it was stimulated by capsaicin. The data revealed that only a relatively select few mediators have receptors that are strongly expressed by the mouse vagal C-fiber neurons, as illustrated in table 3 of the Wang et al paper; (see entire data set in the supplement of the paper) [8]. When evaluating these data, three qualifications are useful to keep in mind. First, these data pertain only to the mouse and there is likely to be variation amongst species. Second, the data refers to mRNA expression, and so it is not known whether a functional receptor is expressed on the terminal membrane. Moreover, one cannot de facto assume that stimulation of the receptor by the cognate mediator will lead to activation. Chemicals may inhibit nerve excitability, or even enhance excitability without evoking action potentials. We have found, however, that most often activation of a mediator receptor leads to overt activation of C-fiber terminals [9]. A recent example is found with sphingosine 1 phosphate (S1P). The transcriptome data showed that among the 5 S1P receptors (S1PR1–5) S1PR3 is the major S1P receptor subtype expressed by vagal C-fibers. Accordingly, we found that S1P evokes strong action potential discharge in mouse lung C-fibers and this response is absent in S1PR3 knockout mice (see figure 2), [10]. Similarly, we were surprised to see the strong expression of type 1 and type 2 interferon receptors in vagal C-fibers and we found that type 1 (α and β) and type 2 interferon (γ) interferons directly stimulated the neuronal cell bodies isolated from the ganglia [8]. More recently, we have found that both type 1 and type 2 interferons evoked action potential discharge at the terminals of 60–80% of capsaicin-sensitive vagal C-fibers in mouse lungs (unpublished). Consistent with this, interferon γ stimulates vagal neurons in guinea pigs where it has been linked to enhanced cough [11].
Figure 2:
Top, and example of a nodose C-fiber in the mouse lung responding to application of shingosine-1-phosphate with strong action potential discharge. Bottom, a tracing showing the when the nodose C-fiber under study is in an S1PR3 knockout mouse, shingosine-1-phosphate is without effect. These data are taken Mayur et al. [10].
Blocking the receptor for a given mediator has recently proven effective in inhibiting chronic idiopathic cough in humans. In this case the mediator was ATP which strongly activates nodose C-fibers by stimulating its receptor P2X. The P2X receptors are cationic ionotropic receptors. Stimulating P2X receptors with ATP leads to an opening of the receptor-channel that then leads to the generator potential. There are seven P2X receptor subtypes. The nodose C-fiber neurons innervating the airways express P2X3 and P2X2 mRNA [12, 13]. The P2X receptors are trimeric receptors, and in theory the nodose neurons can form homomeric P2X2,2,2 and P2X3,3,3 receptors, as well as the heteromeric P2X3,2,3 receptor. The activation of nodose C-fibers by ATP is mimicked by α,β, methylene ATP and this agonist activates P2X3,3,3 and P2X3,2,3 receptors but not homomeric P2X2,2,2 receptors. The available evidence supports the hypothesis that ATP causes action potential discharge in nodose C-fibers via the P2X3,2,3 receptors [12].
Afferent Pharmaceuticals was the first company to evaluate the potential anti-tussive efficacy of an antagonist that selectively blocks P2X3 (and P2X2,3) receptors. In a phase two clinical trial this antagonist proved to be remarkably effective in inhibiting the cough of people suffering with chronic coughing for an average of 9 years [14]. This drug is now being further developed by Merck. Knowledge regarding the extent to which blocking the homomeric P2X3 vs the heteromeric P2X2,3 receptors causes the anti-tussive efficacy of Gefapixant will depend on information gained from future clinical trials with more selective agents.
III. Blocking the generator potential
In the case of ionotropic receptors like P2X, blocking the receptor also blocks the ion channel responsible for the generator potential. Other examples of generator potential-evoking ionotropic receptors that are known to activate vagal C-fibers include the serotonin 5-HT3 receptor and cholinergic nicotinic receptors [15, 16].
Transient receptor potential (TRP) channels can also act as ionotropic receptors. Among the numerous TRP channels, TRPV1 and TRPA1 are highly expressed by vagal nodose and jugular C-fibers [8] and their activation leads to strong action potential discharge. Inhalation of TRPV1 or TRPA1 stimulants leads to coughing in human volunteers[17, 18]. An anti-tussive strategy of blocking TRPs-mediated generator potentials is based on the inference that there are stimuli in the airways that bind and activate these channels. TRPV1 can be activated by heat, and Lee and colleagues have shown that elevation in airway temperature leads to TRPV1 dependent C-fiber activation [19]. The levels of heat required though makes it an unlikely mechanism of driving cough under most circumstances. Acid can also stimulate vagal C-fibers at least partially via TRPV1 channels [20], and there is evidence that the airway mucosa may be acidified in airway inflammatory disease [21]. There are also numerous endogenous lipid mediators that can bind to and open TRPV1 including certain endocannabinoids and eicosanoids [22].
TRPA1 is perhaps more likely to be activated by chemicals that may present to the airway mucosa [23]. Airway C-fibers are activated by environmental irritants via TRPA1; e.g. ozone, acrolein, saturated aldehydes and isocyanates [24]. Airway C-fibers are also activated via TRPA1 via autacoids known to be present in inflamed airways including oxidative metabolites of PGD2 and other prostanoids [25], nitrated fatty acids [26], and electrophilic alkynals, in particular 4-oxononenal [27].
Many inflammatory mediators stimulate G-protein coupled receptors (GPCRs) and cytokine receptors. These stimuli must evoke signal transduction events that open (or in some cases close) ion channels to cause a generator potential. It seems likely that many different GPCRs that evoke action potentials in vagal afferent C-fibers signal to the same channels, so an understanding of these events may allow for the development of drugs that interfere with these “choke points” to reduce the activity of many different mediators.
Bradykinin, adenosine, prostanoids, histamine, thrombin, among other mediators, stimulate C-fibers via GPCR activation. Many GPCRs that activate C-fibers are linked to Gq-PLC signaling. It is the β isoform of PLC that is linked to GPCRs. There are four β isoforms of PLC, but the nodose C-fibers selectively express PLCβ3 [8]. Consistent with this finding we recently found that activation of nodose C-fibers via a GPCR (protease activated receptor 1-PAR1) is substantially inhibited in mice in which PLCβ3 was genetically deleted (unpublished). A similar observation was found in the GPCR activation of itch C-fibers in mouse skin, and itch C-fibers may have some similarities to vagal C-fibers causing cough[28, 29].
TRPV1 and TRPA1 opening has been linked to GPCR activation [30]. In DRG and in jugular neurons, activation of nociceptors by bradykinin B2 receptors is inhibited by blocking TRPV1 [31] [32]. This may not be the case in nodose C-fibers in the lungs. Pulmonary nodose C-fibers in mice lacking TRPV1 respond to bradykinin with the same peak intensity as wild type mice [33]. Our more recent unpublished data has shown that the pattern and intensity of action potential discharge evoked by PAR1 or bradykinin B2 receptors is the same in wild-type and TRPA1/TRPV1 double knockout mice. This raises the question of what are the ion channels that subserve the generator potential if TRP channels are not involved in the GPCR activation of nodose C-fiber terminals?
One possibility is that it involves Kv7 channels. Kv7 channels are formed by the products of KCNQ genes. Kv7 channels underlie an extensively studied ionic current called the M-current [34]. The M-current is a potassium current that is activated at or near the resting membrane potential. The M-current got its name because it was found to be inhibited by activation of cholinergic muscarinic GPCRs. If the M-current is prominent at the resting membrane potential, blocking this current will depolarize the membrane, and in some cases, this can lead to action potential discharge. Airway specific nodose C-fiber neurons express KCNQ genes, notably KCNQ3 and KCNQ2 [35]. The corresponding KV7.3 and 7.2 ion channels that mediate the M-current in nodose C-fibers were inhibited by GPCR (PAR1) activation. This led to membrane depolarization, but not sufficient enough to account for PAR1 activation of the nerve terminals [35].
A drug may target the generator potential by stimulating an inhibitory current. The M-current provides an example of this strategy. Retigabine, a synthetic stimulator of M-current, causes a substantial membrane hyperpolarization, and inhibition of an excitatory stimulant to evoke an effective generator potential at the C-fiber terminals. Accordingly, this drug substantially inhibited S02-evoked coughing in awake mice [35].
The GPCR-evoked generator potential may also involve anionic rather than cationic channels. Unlike most neurons in the CNS, opening chloride channels in primary adult sensory neurons leads to an efflux of chloride and a membrane depolarization due to a higher concentration of intracellular Cl−. In both nodose and jugular C-fiber neuron, bradykinin B2 receptors leads to an activation of certain calcium-activated chloride channels, and this contributes to the net inward depolarizing current [36, 37]. With respect to terminals within the airways, pharmacologically blocking chloride channels inhibited the peak action potential discharge of guinea pigs jugular C-fibers in response to bradykinin [37].
IV. Blocking NaVs
The surest way to block coughs, that are initiated in the periphery, is to block the sodium channel responsible for action potential initiation or conduction to the central terminals of the primary sensory nerve fibers. In effect this would be the chemical equivalent of severing the vagus nerves.
To date there have been no clinical studies of potent sodium channel blocking drugs in treating cough. The NaV blockers available for use in humans such as lidocaine have low affinity for the sodium channels such that the blockade at the terminals requires concentrations of 0.1 – 1mM. Although benzonatate (Tesslon Perles) is an NaV blocker commonly prescribed to treat cough, like lidocaine it has low potency and the NaV blockade actually achieved is likely incomplete as well as transient. A non-selective NaV blocker, GSK2339345, failed in a clinical trial for chronic idiopathic cough. At the doses studied, however, the drug did not inhibit capsaicin evoked cough all but proving that the drug was not potent enough to effectively block the NaVs in capsaicin-sensitive vagal C-fibers [38]. Fortunately, over the past decade there has been substantive progress made in the development of new NaV blockers that are addressing both the issue of potency (NaV blockers with IC50s in the nanomolar range), and safety [39].
The key advancement with respect to safety was in the unraveling of the NaV subtypes. There are nine distinct NaV1 channels (NaV1.1 – NaV1.9). These subtypes have distinct biophysical characteristics and importantly also have a distinct expression profile. For example, the heart expresses predominantly NaV1.5 and striated muscle expresses NaV1.4. The modest chemical distinctions among most of the nine NaV1 channels makes drug selectivity difficult but possible. Nature has, in fact, already provided very potent NaV1 blockers with some selectivity. For example, tetrodotoxin (TTX) is a lethal toxin that blocks NaV 1.1, 1.2, 1.3, 1.4, 1.6, and 1.7—but not NaV 1.5, 1.8, or 1.9 [40]. Tetrodotoxin has long been known to virtually silence action potential conduction in most vagus nerves. In common with nociceptors in the somatosensory system, the major TTX-sensitive NaV1 expressed by jugular and nodose C-fibers is NaV1.7 [41] [42]. When NaV1.7 gene expression was silenced in guinea pig nodose and jugular neurons, conduction of action potentials in vagal C-fibers was largely inhibited. These animals behaved normally but failed to cough when exposed to mechanical or chemical stimuli that normally cause strong cough responses [41].
All nodose and jugular C-fiber neurons in mice and guinea pigs express NaV 1.7, 1.8 and 1.9 channels [42]. Blocking NaV1.7 alone inhibits conduction of action potential in vagal nerves, but the role of NaVs at the terminals within the airways may be more complex. We have found that blocking the NaV1.7 channels prevented action potential conduction in most jugular C-fibers in most vagus nerve axons, but did not block the action potential discharge when the blocker was delimited to the airways where the jugular C-fiber terminals reside [42]. In other words, an inhaled NaV1.7 blocker would not inhibit jugular C-fiber activity in guinea pigs. To inhibit action potential discharge at the terminals, blocking NaV1.7 and NaV1.8 was the most effective strategy. NaV1.9 is unique amongst the NaV1 channels, in that its biophysical characteristics precludes it from being a major participant in the fast upstroke of an action potential. It is more likely that this channel contributes to C-fiber excitability by amplifying generator potentials. We have found that vagal C-fibers in lungs of NaV1.9 −/− mice respond only weakly to a chemical stimulus (ATP) (unpublished observation). These data indicate that NaV1.9 blockers may provide a generalized inhibition of chemical activation of vagal C-fibers, and as such may prove to be effective anti-tussive agents.
V. Conclusions
Inhibiting the activity of vagal nociceptors (C-fibers) represents a rational approach to developing new peripherally acting anti-tussive drugs. This can be accomplished by blocking the stimulus, blocking the generator potential, or by blocking the NaV channels. Blocking the action of a specific stimulus (e.g. blocking a mediator receptor) has the advantage of being a safe approach that will unlikely to by encumbered with many on-target side-effects. The disadvantage of this approach is that its therapeutic scope may be narrow, affecting only a subtype of cough that is evoked by that specific mediator/stimulus. A promising outcome of this strategy is in the development of P2X3 receptor antagonists. On the other end of the spectrum, one may chemically silence all C-fibers by blocking the sodium channels involved in the induction and conduction of action potentials. This has the advantage of efficacy; it would block all evoked cough irrespective of the stimulus. The disadvantage is that by silencing the nerves it may also block beneficial sensations and neuronal reflex behaviors. In between these approaches would be a strategy aimed at blocking the generator potentials. These strategies are all in play within pharmaceutical companies aiming to find orally active novel non-opioid analgesics. Those trying to develop new anti-tussive drugs have the advantage of targeting airway nociceptors topically with an inhaled drug delivery approach. This may be of particular value for those drugs aimed at causing a general inhibition of the generator potentials or action potentials.
Funding
This work was supported by NIH RO1HL137807 and R01HL122228 to BJU. MJP was partly also supported by Dr. David Marsh Symposium Research Award, Johns Hopkins University (Asthma and allergy Division).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klassen KP, Morton DR, and Curtis GM, The clinical physiology of the human bronchi. III. The effect of vagus section on the cough reflex, bronchial caliber, and clearance of bronchial secretions. Surgery, 1951. 29(4): p. 483–90. [PubMed] [Google Scholar]
- 2.Coleridge HM CJ, Schultz HD, Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther, 1989. 42(1): p. 1–63. [DOI] [PubMed] [Google Scholar]
- 3.Paintal AS, Mechanism of stimulation of type J pulmonary receptors. J Physiol, 1969. 203(3): p. 511–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschuler SM, et al. , Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol, 1989. 283(2): p. 248–68. [DOI] [PubMed] [Google Scholar]
- 5.Undem BJ CB, Lee MG, Weinreich D, Myers AC, Kollarik M, Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol, 2004. 556 (pt 3): p. 905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzone SB and Undem BJ, Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev, 2016. 96(3): p. 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu S UB, Kollarik M, Vagal afferent nerves with nociceptive properties in guinea-pig oesohagus. J Physiol, 2005. 563(Pt 3): p. 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. , Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One, 2017. 12(10): p. e0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor-Clark T UB, Transduction mechanisms in airway sensory nerves. J Appl Physiol, 2006. 101(3): p. 950–959. [DOI] [PubMed] [Google Scholar]
- 10.Patil MJ, et al. , Sphingosine-1-phosphate activates mouse vagal airway afferent C-fibres via S1PR3 receptors. J Physiol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Z, et al. , IFN-gamma Enhances the Cough Reflex Sensitivity via Calcium Influx in Vagal Sensory Neurons. Am J Respir Crit Care Med, 2018. 198(7): p. 868–879. [DOI] [PubMed] [Google Scholar]
- 12.Kwong K KM, Nassenstein C, Ru F, Undem BJ, P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol, 2008. 295(5): p. L858–L865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassenstein C T-CT, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ, Phenotypic distinctions between neural crest and placodal derived vagal C-fibers in mouse lungs. J Physiol, 2010. 588(23): p. 4769–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdulqawi R, et al. , P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet, 2015. 385(9974): p. 1198–205. [DOI] [PubMed] [Google Scholar]
- 15.Lee LY and Gu Q, Cough sensors. IV. Nicotinic membrane receptors on cough sensors. Handb Exp Pharmacol, 2009(187): p. 77–98. [DOI] [PubMed] [Google Scholar]
- 16.Chuaychoo B LM, Kollarik M, Undem BJ, Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther, 2005. 18: p. 355–360. [DOI] [PubMed] [Google Scholar]
- 17.Collier JG and Fuller RW, Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol, 1984. 81(1): p. 113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birrell MA, et al. , TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med, 2009. 180(11): p. 1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin RL, Hayes D Jr., and Lee LY, Bronchoconstriction induced by hyperventilation with humidified hot air: role of TRPV1-expressing airway afferents. J Appl Physiol (1985), 2009. 106(6): p. 1917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollarik M UB, Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol, 2002. 543: p. 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt JF, et al. , Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med, 2000. 161(3 Pt 1): p. 694–9. [DOI] [PubMed] [Google Scholar]
- 22.Sisignano M, et al. , TRP-channels as key integrators of lipid pathways in nociceptive neurons. Prog Lipid Res, 2014. 53: p. 93–107. [DOI] [PubMed] [Google Scholar]
- 23.Taylor-Clark TE and Undem BJ, Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol. 178(3): p. 406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geppetti P, Patacchini R, and Nassini R, Transient receptor potential channels and occupational exposure. Curr Opin Allergy Clin Immunol, 2014. 14(2): p. 77–83. [DOI] [PubMed] [Google Scholar]
- 25.Taylor-Clark TE, et al. , Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol, 2008. 73(2): p. 274–81. [DOI] [PubMed] [Google Scholar]
- 26.Taylor-Clark TE, et al. , Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol, 2009. 75(4): p. 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor-Clark TE, et al. , Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol, 2008. 586(14): p. 3447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ru F, et al. , Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol, 2017. 595(11): p. 3651–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han L, et al. , Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nat Neurosci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldhuis NA, et al. , The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev, 2015. 67(1): p. 36–73. [DOI] [PubMed] [Google Scholar]
- 31.Katanosaka K, et al. , Contribution of TRPV1 to the bradykinin-evoked nociceptive behavior and excitation of cutaneous sensory neurons. Neurosci Res, 2008. 62(3): p. 168–75. [DOI] [PubMed] [Google Scholar]
- 32.Carr MJ KM, Meeker SN, Undem BJ, A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther, 2003. 304: p. 1275–1279. [DOI] [PubMed] [Google Scholar]
- 33.Kollarik M UB, Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. J Physiol, 2004. 555: p. 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez CC, et al. , Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol, 2008. 586(7): p. 1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H, et al. , KCNQ/M-channels regulate mouse vagal bronchopulmonary C-fiber excitability and cough sensitivity. JCI Insight, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh EJ WD, Bradykinin decreases K(+) and increases CL(−) conductances in vagal afferent neurones of the guinea pig. J Physiol, 2004. 558(Pt 2): p. 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MG MDJ, Undem BJ, Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol, 2005. 566: p. 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JA, et al. , Effects of a novel sodium channel blocker, GSK2339345, in patients with refractory chronic cough. Int J Clin Pharmacol Ther, 2017. 55(9): p. 712–719. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand D, et al. , Functional Studies of Sodium Channels: From Target to Compound Identification. Curr Protoc Pharmacol, 2016. 75: p. 9 21 1–9 21 35. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, et al. , Selective Voltage-Gated Sodium Channel Peptide Toxins from Animal Venom: Pharmacological Probes and Analgesic Drug Development. ACS Chem Neurosci, 2018. 9(2): p. 187–197. [DOI] [PubMed] [Google Scholar]
- 41.Muroi Y RF, Kollarik M, Canning BJ, Hughes SA, Walsh S, Sigg M, Carr MJ, Undem BJ, Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol, 2011. 589(Pt 23): p. 5663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollarik M, et al. , Different role of tetrodotoxin-sensitive voltage-gated sodium channel (NaV1) subtypes in action potential initiation and conduction in vagal airway nociceptors. J Physiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]