Abstract
Published evidence shows correlation between several molecular markers and prostate cancer (PCa) progression including in African Americans who are disproportionately affected. Our early detection efforts led to the identification of elevated levels of anti-apoptotic protein c-FLIP and its upstream regulatory factors such as androgen receptor (AR), Recepteur d’origine nantais (RON), a receptor tyrosine kinase in human prostate tumors. Primary objective of this study was to explore whether these markers play a role in racial disparities using immunohistochemistry in prostatectomy samples from a cohort of African American (AA), Hispanic Whites (HW) and Non-Hispanic Whites (NHW). Bivariable and multivariable logistic regression analyses was used to identify statistical association between molecular markers, possible correlation with risk factors including race, obesity, prostate-specific antigen (PSA) and disease aggressiveness. Further, changes in the levels and expression of these molecular markers was also evaluated using human prostate cancer cell lines. We found significantly elevated levels of RON (p=0.0082), AR (p=0.0001), c-FLIP (p=0.0071) in AAs compared to HWs or NHWs. Furthermore, higher proportion of HW and NHWs had high Gleason score (>6) but not PSA as compared to AAs (p=0.032). In summary, our findings suggest that PSA was important in predicting aggressive disease for the cohort overall; however, high levels of RON may play a role in predisposing AA men to develop aggressive disease. Future research is needed using large datasets to confirm these findings and to explore whether all or any of these markers could aid in race-specific stratification of patients for treatment.
Keywords: Prostate cancer, Prostate Cancer Disparity, Receptor tyrosine kinase RON, c-FLIP, Androgen receptor
Introduction:
Prostate cancer (PCa) is the second leading cause of cancer related deaths in men contributing to approximately 26,000 deaths per year in the United States. Although 5-year survival of patients diagnosed with localized prostate cancer is close to 100%, the 5-year survival for metastatic prostate cancer remains poor at <30%. Epidemiological studies suggest that African American (AA) men are disproportionately affected with prostate cancer compared to Caucasian American (CA) men [1-10]. African American men are diagnosed with more aggressive disease, have poor prognosis and therapeutic response, higher risk of recurrence with low 5-year disease-free survival [1-10]. AA men with prostate cancer also have a 2.5-fold increased risk of lethal prostate cancer compared with CA men [3]. Furthermore, patients of African American descent experience more severe side effects of conventional therapies such as radiation compared with patients of European descent [11]. While the observed disparity in PCa incidence and survival is likely to be multifactorial including socioeconomic causes such as lack of access and or prohibitive treatment cost, molecular studies have implicated a role for changes in AR, inhibitor of apoptosis proteins such as survivin, and RGS12, a negative regulator of G-protein signaling [12-15]. More recently, using exome sequencing, Huang et al identified mutations or deletions of ETS transcriptional repressor namely ERF in African American cohort. That study further demonstrated potential tumor suppressor role for ERF in prostate cancer [16]. Despite these emerging data, precise pathological, biochemical and or molecular pathways that disproportionately predispose AA men to develop aggressive disease, differential therapeutic response, are yet to be defined. Therefore, understanding the underlying tumor biology and mechanisms leading to differential clinical presentations and therapeutic outcomes in each ethnic group would be critical to reduce prostate cancer disparity in these patients.
Previous studies from our laboratory showed that high-grade Gleason tumors have higher levels of the anti-apoptotic FLICE-inhibitory protein (c-FLIP) and Recepteur d’origine nantais (RON), a receptor tyrosine kinase compared with low-grade Gleason tumors (p=0.04) [2,17]. Remarkably, castrate resistant tumors had significantly elevated levels of c-FLIP. Subsequent studies recognized RON and transcription factors Sp1 and Sp3 as important regulators of c-FLIP transcriptional activity in prostate cancer cells and elevated expression of RON and Sp1 in human prostate tumors [2,18]. Further analysis of tissues from patients with recurrent and non-recurrent prostate cancer showed that the area under the receiver operating characteristic curves (AUC) for c-FLIP (0.71), Sp1 (0.66), Sp3 (0.68), and Gleason score (0.76) predicted PSA failure [19]. Remarkably, the combination of these molecular markers with Gleason and their interactions improved AUC to 0.93. These data suggest that the “biomarker signature” of c-FLIP/Sp1/Sp3 can predict disease recurrence and potential utility for patient stratification and help prevent unnecessary aggressive treatment. Furthermore, downregulation of these markers decreased prostate tumor growth both in cell culture and preclinical models. These observations clearly suggest the importance of these markers in prostate pathogenesis. However, significance of these markers in predisposing African Americans to develop aggressive and therapeutically resistant disease is unknown.
In this manuscript, we tested the hypothesis that differential levels in one or more of these molecular markers will have prognostic value to predict aggressive tumors (i.e. high Gleason score) that arise in different racial-ethnic groups. We used immunohistochemistry to evaluate differential levels of AR, RON, c-Met, c-FLIP, Sp1 and Sp3 in primary tumor tissue obtained from patients that underwent radical prostatectomy (RP) from African American (AA), Hispanic White (HW) and Non-Hispanic Whites (NHW). We observed elevated levels of RON in AAs that correlated with significantly higher Gleason Score but not PSA. In addition, higher proportion of HWs and NHWs had high Gleason score (>6) compared to AAs. PSA was important in predicting aggressive disease for overall cohort; however, high levels of RON may be important in predicting aggressive disease in African Americans specifically.
Materials and Methods:
Human tissues:
This study used banked tissues available from the IRB approved GU tissue repository at the UTHSA. Three ethnic-racial groups; African American- Blacks (AA), Hispanic-Latinos (HW) and non-Hispanic Whites Caucasians (NHW) were used to explore the expression of protein biomarkers. All patients had undergone prostatectomy as primary treatment for prostate cancer and were subsequently followed for five years by monitoring their PSA levels. GU pathologist validated tissue microarray (TMA) comprising of resected prostate tissue samples constructed by the tumor bank housed in the Department of Pathology, UT Health San Antonio.
Antibodies and immunohistochemistry:
Rabbit polyclonal antibodies specific for c-FLIP, RON, Sp1, and Sp3 were from Santa Cruz Biotechnology (Santa Cruz, CA). AR antibody was from Thermo Scientific (list and source of antibodies is provided as supplementary figure 1). The tissues were stained according to previously published protocols and appropriate negative controls were used [19]. Rabbit HRP polymer and DAB chromogen was used as the ancillary system and hematoxylin was used for counterstaining (Biocare Medical, Concord, CA and DAKO North America Inc. Carpinteria, CA). As shown in supplementary figure 1, human prostate cancer cell lines LNCaP, and 22Rv1obtained from American Type Culture Collection were grown as described previously [2,17,18].
Semiquantitative evaluation of tissue staining and biomarker score and biomarker score:
TMAs containing 30-40% tumor was chosen for pathological evaluation. A pathologist (RR) blinded to the identity of samples evaluated staining of specific proteins. Staining intensities and proportion of positive staining tumor cells were determined independently. Briefly, the proportion of positive tumor cells was scored as follows: 0, no stained cells; 1, ≤1%; 2, 1-10%; 3, 10-33%; 4, 33-66%; 5, 66-100% stained cells. The intensity score (IS) represents the average staining intensity of tumor cells: 0, no staining; 1, weak; 2, moderate; 3, strong staining [17-20]. The total (TS) ranged from 0 to 8 and was obtained from the sum of proportion score and intensity score which is biomarker score.
Quantitative real time PCR:
Total cellular RNA isolated from indicated cells using Trizol reagent (Invitrogen) was used in cDNA synthesis by using a superscript VILO cDNA synthesis kit (Invitrogen). The expressions of target genes including RON and c-MET were measured using CFX96 Touch™ Real-Time PCR Detection System with iTaq Universal SYBR Green Super mix. Relative mRNA expression was normalized to β-actin. PCR reactions were conducted with three biological replicates, each with three technical replicates as described previously [2,18]. The primer sequences used were shown in supplementary figure 1.
Western blot analysis:
Logarithmically growing cells were used for preparation of whole cell extracts as described previously [20]. The harvested cells were lysed in 2xSDS lysis buffer (0.5 M Tris-HCl, pH 6.8, 10% Glycerol, 10% SDS, 50 mM DTT, 0.5% Bromophenol blue) containing proteinase and phosphatase inhibitors. Whole cell lysates were boiled for 10 min and subjected to SDS-PAGE. After electrophoresis, the proteins were transferred to a nitrocellulose membrane. The transferred membranes were blocked in 5% (w/v) nonfat dry milk or 5% (w/v) BSA in TBS (0.5 M NaCl, 20 mM Tris-HCl, pH 7.4) with 0.1% (v/v) Tween 20 and probed for the first antibody (RON, Sc-322, Santa Cruz Biotech; c-MET, 8198, Cell Signaling), followed by incubation with a secondary antibody conjugated with horseradish peroxidase (anti-mouse, 7076, Cell Signaling; anti-rabbit, A6154, Sigma-Aldrich) with visualization by Western Lightning Plus-ECL (PerkinElmer). All the blots were stripped and reprobed with β-actin (AM1829B, Abgent) for equal protein loading. Images were captured and analyzed using G: BOX System (Syngene), and quantification was processed using Gene Tools software. Blots shown are representative of three individual experiments.
Statistical analysis:
After checking for consistency and accuracy of the data, we compared the differences in the characteristics and biomarker scores between patients of the three racial/ethnic groups (AA, HW, and NHW). The results were evaluated with summary statistics, the groups compared by T tests, Kruskal Wallis, Wilcoxon (Man Whitney U) and ANOVA, the correlations with Spearman reporting Bonferroni and Fisher’s Exact. A cutoff of 8 was selected for AR, RON, c-FLIP, Sp1 and Sp3 markers. Bivariable and multivariable logistic regression analyses were conducted to identify significant associations between aggressive prostate cancer (based on low and high Gleason scores) and biomarkers. A two-tailed P value of 0.05 was considered significant and all the analyses were performed in Stata (College Station, TX).
Results and Discussion:
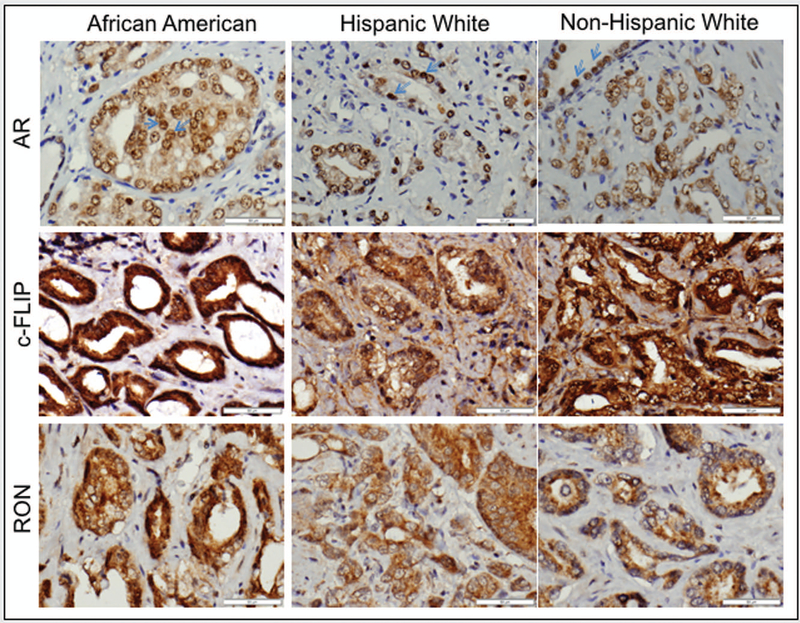
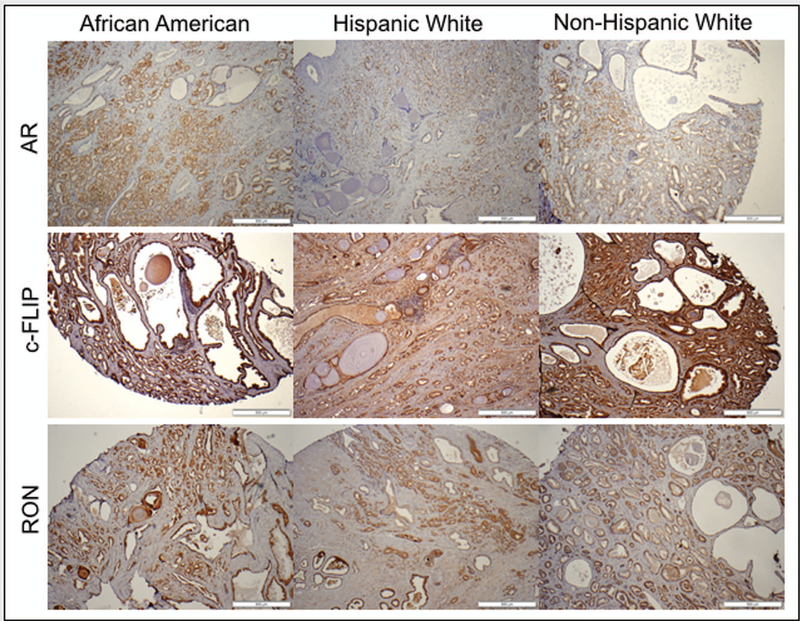
Expression changes of RON, Sp1, Sp3, c-FLIP and AR were analyzed using immunohistochemistry in a cohort of AA (n=53) and CA (n=100; HW=50 and NHW=50) patients who underwent prostatectomy. Characteristics of patients, stratified by race, are shown in Table 1. A significant increase in protein level of RON (p=0.0082), AR (p=0.0001) and c-FLIP (p=0.0071) but not Sp1 and Sp3 was observed in AA compared to HW and NHW together. A representative image depicting immunohistochemical evaluation of RON, AR, and c-FLIP is shown in figures 1A and B. Notably, we found no statistically significant correlation between changes in any of these markers with biochemical recurrence (BCR) or body mass index (BMI) using WHO classification. Furthermore, we also observed significantly higher proportion of HW and NHWs had higher Gleason score but not PSA compared to AAs (p=0.032). Only PSA showed significant association with aggressive disease in the overall cohort, after adjusting for age, race, and BMI (table 2). Remarkably, despite non-significant association of PSA in Whites (P = 0.678), our data also indicate that both AA and HW will benefit from PSA screening to predict high Gleason disease (P = 0.027 and 0.014, respectively; table 3). However, specifically in AAs, higher level of RON was significantly associated with aggressive prostate cancer.
Table 1:
Patient characteristics, stratified by race/ethnicity
| Total | African- American |
Hispanic-White | Non-Hispanic White |
P value |
|
|---|---|---|---|---|---|
| N (%) | 153 | 53 (34.6) | 50 (32.7) | 50 (32.7) | |
| Age, median (range) | 62 (41 – 80) | 59 (44 – 71) | 62 (41 – 73) | 66 (51 – 80) | 0.0001 |
| BMIa , median (range) | 28.2 (16.4 – 40.1) | 27.8 (20.8 – 40.1) | 29 (19.8 – 38.5) | 27.3 (16.4 – 38.2) | 0.2898 |
| BMI, WHO classification, n(%) | 0.559 | ||||
| Underweight/Normal (<25) | 37 (26) | 14 (27) | 7 (17) | 16 (33) | |
| Overweight (25 – 29.9) | 51 (36) | 18 (35) | 16 (39) | 17 (35) | |
| Obese (>= 30) | 53 (38) | 19 (37) | 18 (44) | 16 (33) | |
| PSAb | 5.75 (0.41 – 54.5) | 5.67 (2.17 – 53.2) | 6.03 (0.41 – 28.3) | 5.66 (2.37 – 54.5) | 0.967 |
| PSA | 0.550 | ||||
| Low (<4) | 30 (20) | 9 (17) | 12 (26) | 9 (18) | |
| High (≥4) | 118 (80) | 43 (83) | 35 (74) | 40 (82) | |
| Gleason | 0.032 | ||||
| Low | 104 (68) | 43 (81) | 29 (58) | 32 (64) | |
| High | 49 (32) | 10 (19) | 21 (42) | 18 (36) | |
| Failure | 36 (24) | 10 (19) | 11 (22) | 15 (30) | 0.393 |
| *Biomarker score (mean, standard deviation) | |||||
| ARc | 7.34 (± 1.2) | 7.7 (± 0.9) | 7.6 (± 0.9) | 6.8 (± 1.5) | 0.0001 |
| RONd | 7.6 (± 0.7) | 7.8 (± 0.5) | 7.6 (± 0.5) | 7.4 (± 0.8) | 0.0082 |
| FLIPe | 7.3 (± 1.7) | 7.4 (± 1.1) | 6.6 (± 2.5) | 7.8 (± 0.8) | 0.0071 |
| SP1b | 7.8 (± 0.9) | 7.8 (± 1.2) | 7.9 (± 0.5) | 7.8 (± 0.7) | 0.3826 |
| SP3c | 7.9 (± 0.3) | 7.9 (± 0.3) | 8 (± 0.1) | 7.9 (± 0.4) | 0.3152 |
available for 141 patients
available for 148 patients
available for 151 patients
available for 146 patients
available for 117 patients
Biomarker score is the sum of the proportion of positively stained cells (0 to 5) and intensity (0-3) of staining. Range of biomarker score varied from 0-8 and data presented in mean biomarker score±s.d.
Figure 1.
H&E staining and IHC analysis of expression of AR, c-FLIP and RON in a representative sample of African American, Hispanic White and Non-Hispanic White patients under high magnification (A) and low magnification (B).
Table 2:
Risk factors for aggressive prostate cancer
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Low Gleason |
High Gleason |
OR (95% CI) | P value |
OR (95% CI) | P value |
|
| N (%) | 104 (68%) | 49 (32%) | - | - | - | - |
| Age, median (range)b | 61 (44 – 80) | 63 (41 – 76) | 1.55 (0.95 – 2.53)a | 0.081 | 1.11 (0.55 – 2.24) | 0.772 |
| BMI | 0.740 | 0.682 | ||||
| Underweight/Normal | 26 (70) | 11 (30) | 1.00 | 1.00 | ||
| Overweight/Obese | 70 (67) | 34 (33) | 1.15 (0.51 – 2.59) | 1.25 (0.43 – 3.62) | ||
| Race | ||||||
| AA | 43 (81) | 10 (19) | 1.00 | 1.00 | ||
| HW | 29 (58) | 21 (42) | 3.11 (1.28 – 7.57) | 0.012 | 3.95 (1.21 – 12.92) | 0.023 |
| NHW | 32 (64) | 18 (36) | 2.42 (0.99 – 5.94) | 0.054 | 4.29 (1.04 – 17.71) | 0.044 |
| Biomarker | ||||||
| AR (7 or less) | 37 (73) | 12 (27) | 1.00 | 1.00 | ||
| AR (8) | 66 (66) | 35 (34) | 1.36 (0.65 – 2.86) | 0.415 | 3.06 (0.93 – 10.12) | 0.066 |
| RON (7 or less) | 30 (71) | 12 (29) | 1.00 | 1.00 | ||
| RON (8) | 69 (66) | 35 (34) | 1.27 (0.58 – 2.78) | 0.552 | 1.83 (0.53 – 6.31) | 0.338 |
| FLIP (7 or less) | 22 (76) | 7 (24) | 1.00 | 1.00 | ||
| FLIP (8) | 58 (66) | 30 (34) | 1.63 (0.62 – 4.24) | 0.320 | 1.04 (0.32 – 3.4) | 0.948 |
| SP1 (7 or less) | 9 (69) | 4 (31) | 1.00 | 1.00 | ||
| SP1 (8) | 92 (68) | 43 (32) | 1.05 (0.31 – 3.61) | 0.936 | 0.52 (.09 – 3.03) | 0.463 |
| SP3 (7 or less) | 7 (78) | 2 (22) | 1.00 | 1.00 | ||
| SP3 (8) | 96 (68) | 46 (32) | 1.68 (0.33 – 8.39) | 0.529 | 3.61 (0.35 – 37.1) | 0.281 |
| PSA (<4) | 24 (80) | 6 (20) | 1.00 | 1.00 | ||
| PSA (≥4) | 77 (65) | 41 (35) | 2.13 (0.81 – 5.63) | 0.121 | 3.93 (1.06 – 14.6) | 0.041 |
model includes data from 104 patients with complete data on all variables
for interval of 10 years
Table 3:
Association between PSA and Gleason score for each race
| Odds Ratio (95% confidence interval) |
P value | |
|---|---|---|
| African-American | 0.027 | |
| Low Gleason | 1.00 | |
| High Gleason | 1.11 (1.01 −1.22) | |
| HW | 0.014 | |
| Low Gleason | 1.00 | |
| High Gleason | 1.17 (1.03 – 1.32) | |
| NHW | 0.678 | |
| Low Gleason | 1.00 | |
| High Gleason | 1.01 (0.95 – 1.07) |
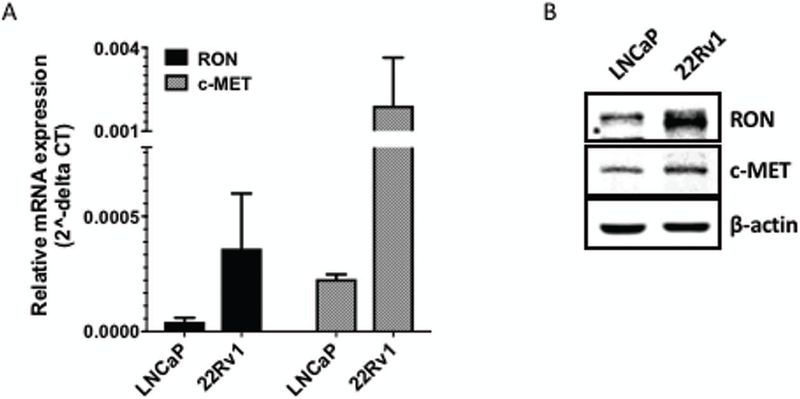
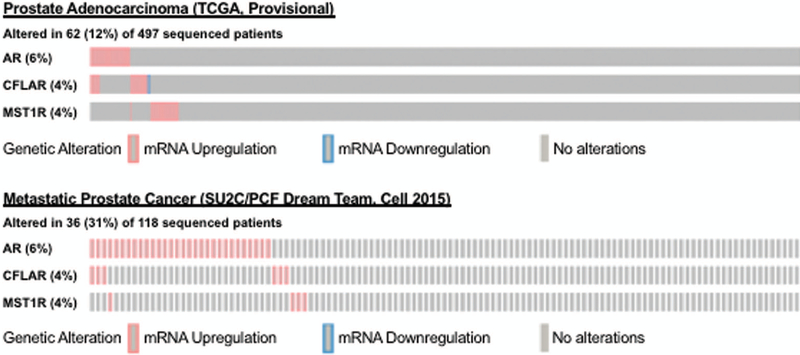
Recepteur d’origine nantais (RON) is a receptor tyrosine kinase (RTK), also known as macrophage stimulating-1 receptor (MST1R) shares structural similarities with c-MET [2]. Based on the recent report that castrate resistant prostate cancer cell line 22Rv1 is of 41% of West African origin and 42% European in origin [21], we assessed expression and levels of RON and its homolog c-MET using real-time PCR and immunoblot analysis. We found that levels and expression of RON were higher in this cell line compared to LNCaP cells (derived from a Caucasian patient; Figure 2). Interestingly, although mRNA expression of c-MET was elevated, c-MET protein could not be detected in 22Rv1. Although the reason for this is unclear, immunohistochemistry revealed negative staining for c-MET in human prostate tumors from this cohort (data not shown). Furthermore, in silico analysis of AR, c-FLIP and RON (MST1R) in human prostate tumors from TCGA (primary) and SU2C (metastatic) datasets revealed that 12% of primary and 31% of metastatic tumors have 4-6% genetic alterations in these markers (figure 3). However, it was not possible to determine the association between these markers and race due to lack of information on race/ethinicity in these datasets.
Figure 2. The differential levels of RON and c-MET in human prostate cancer cell lines.
(A) Total RNA prepared from logarithmically growing LNCaP and 22Rv1 cells was analyzed for expression of RON and c-MET using q-PCR. β-actin was used for normalization. Graph represents mean ± S.D of three individual experiments, each conducted with three technical replicates (except for data in PC-3 cells only with one single experiment). (B) Whole cell lysates from these cell lines was used determine protein levels of RON and c-MET using immunoblot analysis. β-actin was used as the loading control. Blots shown are representative of three individual experiments.
Figure 3. Genetic alterations of AR, c-FLIP and RON (MST1R) in human prostate tumors.
Primary and metastatic prostate tumor data from The Cancer Genome Atlas (TCGA) and SU2C extracted from cBioPortal presented as OncoPrint with percentage mRNA alterations. z-transformed RNA-Seq RPKM values larger than +2 or less than −2 shown in red, blue, and gray indicate overexpression, underexpression and no change respectively.
Aberrant expression of RON has been reported in many tumor types including prostate. Furthermore, multiple publications by numerous labs have found that RON indeed is a driver of tumor metastasis and potentiator of the aggressive phenotype in cancer cells [22-32]. RON undergoes autophosphorylation and dimerization in response to stimulation by macrophage stimulating protein (MSP), the ligand for RON. RON can form dimers with other receptors including its homolog c-MET, as well as EGFR, PDGFR, and IGF1R to regulate processes related to various hall marks of cancer [28,29,31]. Recent evidence also suggests that RON dimerization with EGFR and subsequent translocation to the nucleus allows it to activate genes associated with cell-stress response (p53, c-Jun, PI3K/AKT). Previously we showed that RON promotes prostate cancer cell growth by functioning as a transcription factor to activate c-FLIP [2]. We recently demonstrated that RON is activated as an alternate by-pass signaling mechanism to compensate for loss of AR under androgen-deprived conditions. These data suggest that under androgen-deprived conditions RON activates AR pathway leading to castrate-resistance [2]. Upregulation of c-FLIP not only protects cancer cells from undergoing apoptosis but also contributes to therapeutic resistance [17]. Furthermore, levels of c-FLIP are also elevated in human prostate tumors including castrate resistant tumors making it a clinically relevant target. Our findings that increased expression of AR, RON and c-FLIP in tumors is in line with published studies including our own [2,17-19]. However, the present study to the best of our knowledge is the first report showing correlation between RON/AR with ethnicity.
Published literature and emerging evidence indicate that AA men with low grade disease or Gleason score <6 are at increased risk for developing aggressive disease [3]. Furthermore, despite having identical risk factors for disease recurrence, AA with low grade Gleason 6 disease have been reported to have higher risk of seminal vesicle invasion compared to Caucasian men [33]. Additionally, AA men are not only more likely to be diagnosed with PCa at earlier age but also experience significantly higher risk of PCa-specific mortality relative to NHW men [34] and younger black men exhibit a 4.2-fold increased risk of lethal PCa compared to White men [35]. Published studies also show increased rates of progression to recurrence among men of African origin undergoing surgery [1]. Based on these published observations we propose that despite being diagnosed with low Gleason Score disease, AA tend to progress to more advanced disease. Therefore, AA men with elevated levels of RON could be at higher risk for developing aggressive PCa. However, additional studies are needed to confirm these findings to develop RON as a predictor of aggressive disease in AA men. Previously we and others have shown inverse association between RON and AR and that under androgen deprivation conditions, RON activates AR promoter activity [2,22]. Although the mechanism how RON contributes to aggressive disease in AAs is unclear, given the observed positive correlation between RON and AR, it is possible that increased levels of RON in AAs could be one of the factors that could aid progression to aggressive PCa in AA by virtue of its ability to regulate epithelial mesenchymal transition (EMT) and AR signaling under androgen deprivation. Alternatively, RON could increase the risk of progression indirectly through its ability to regulate other oncogenic pathways including NFκB and ERK [22-32]. These are clinically significant observations, as therapeutic approaches focusing on targeting elevated levels of RON will have tremendous impact in reducing PCa associated deaths in AAs. In summary, these studies encourage prospective studies to test the role of RON as a marker of patient stratification for consideration of treatment strategy. Although sample size is relatively small in our study, we hypothesize that RON may be used in the clinic to personalize patient care in AA upon further validation in a prospective cohort study. However, additional studies are needed to confirm these findings to develop RON as a predictor of aggressive disease in AA men. Furthermore, our observations from this cohort showing significant association of PSA with high Gleason disease in AA and HWs, (P = 0.027 and 0.014, respectively; table 3), argues against the United States Preventive Service Task Force (USPSTF) recommendation against PSA-screening for PCa especially for AA men [36].
Supplementary Material
Supplementary figure 1. Source of cell lines, antibodies and primer sequences used in the study.
Acknowledgements:
We acknowledge support provided by CTRC at UTHSA through the NCI support grant #2P30 CA 054174-17 (APK) and the CTRC 40th Anniversary Distinguished Professor of Oncology Endowment (APK). This work was supported in part by the funds from Veterans Affairs-Merit Award I01 BX BX003876; National Center for Complementary and Alternate Medicine 1R01 AT007448; CPRIT RP 150166 (APK) and National Cancer Institute R01 CA 149516 (RG). SH is supported in part by CPRIT Training Grant RP 170345.
Footnotes
Conflict of interest statement: Authors declare no conflicts of interest.
References
- 1.McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nature reviews Urology 2016;13(2):99–107. [DOI] [PubMed] [Google Scholar]
- 2.Batth I, Yun H, Hussain S et al. Crosstalk between RON and androgen receptor signaling in the development of castration resistant prostate cancer. Oncotarget 2016;7(12):14048–14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamoah K, Deville C, Vapiwala N et al. African American men with low-grade prostate cancer have increased disease recurrence after prostatectomy compared with Caucasian men. Urologic oncology 2015;33(2):70 e15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuart WD, Brown NE, Paluch AM, Waltz SE. Loss of Ron receptor signaling leads to reduced obesity, diabetic phenotypes and hepatic steatosis in response to high-fat diet in mice. American journal of physiology Endocrinology and metabolism 2015;308(7):E562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber D, Chhabra A, Rineer J, Weedon J, Schwartz D. A Population-Based Study of Men With Low-Volume Low-Risk Prostate Cancer: Does African-American Race Predict for More Aggressive Disease? Clinical genitourinary cancer 2015;13(4):e259–264. [DOI] [PubMed] [Google Scholar]
- 6.Obirieze AC, Moten A, Allen D, Ahaghotu CA. African-American Men with Low-Risk Prostate Cancer: Modern Treatment and Outcome Trends. Journal of racial and ethnic health disparities 2015;2(3):295–302. [DOI] [PubMed] [Google Scholar]
- 7.Barrington WE, Schenk JM, Etzioni R et al. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA oncology 2015;1(3):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorpe RJ Jr., Wilson-Frederick SM, Bowie JV et al. Health behaviors and all-cause mortality in African American men. American journal of men’s health 2013;7(4 Suppl):8S–18S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundi D, Ross AE, Humphreys EB et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013;31(24):2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy S, Shapiro M, Morton R Jr., Brawley OW Prostate cancer in black and white Americans. Cancer metastasis reviews 2003;22(1):83–86. [DOI] [PubMed] [Google Scholar]
- 11.Kerns SL, Ostrer H, Stock R et al. Genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with the development of erectile dysfunction in African-American men after radiotherapy for prostate cancer. International journal of radiation oncology, biology, physics 2010;78(5):1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wang J, Zhang L et al. RGS12 Is a Novel Tumor-Suppressor Gene in African American Prostate Cancer That Represses AKT and MNX1 Expression. Cancer research 2017;77(16):4247–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan S, Simpson J, Lynch JC et al. Racial differences in the expression of inhibitors of apoptosis (IAP) proteins in extracellular vesicles (EV) from prostate cancer patients. PloS one 2017;12(10):e0183122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Wang J, Wang Y et al. MNX1 Is Oncogenically Upregulated in African-American Prostate Cancer. Cancer research 2016;76(21):6290–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang BD, Yang Q, Ceniccola K et al. Androgen receptor-target genes in african american prostate cancer disparities. Prostate cancer 2013;2013:763569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang FW, Mosquera JM, Garofalo A et al. Exome Sequencing of African-American Prostate Cancer Reveals Loss-of-Function ERF Mutations. Cancer discovery 2017;7(9):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganapathy M, Ghosh R, Jianping X et al. Involvement of FLIP in 2-methoxyestradiol-induced tumor regression in transgenic adenocarcinoma of mouse prostate model. Clinical cancer research : an official journal of the American Association for Cancer Research 2009;15(5):1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun H, Xie J, Olumi AF, Ghosh R, Kumar AP. Activation of AKR1C1/ERbeta induces apoptosis by downregulation of c-FLIP in prostate cancer cells: A prospective therapeutic opportunity. Oncotarget 2015;6(13):11600–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedolla RG, Gong J, Prihoda TJ et al. Predictive value of Sp1/Sp3/FLIP signature for prostate cancer recurrence. PloS one 2012;7(9):e44917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J, Xie J, Bedolla R et al. Combined targeting of STAT3/NF-kappaB/COX-2/EP4 for effective management of pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2014;20(5):1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods-Burnham L, Basu A, Cajigas-Du Ross CK et al. The 22Rv1 prostate cancer cell line carries mixed genetic ancestry: Implications for prostate cancer health disparities research using pre-clinical models. The Prostate 2017;77(16):1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batth IS, Yun H, Kumar AP. Recepteur d’origine nantais (RON), more than a kinase: Role in castrate-resistant prostate cancer. Molecular carcinogenesis 2015;54(10):937–946. [DOI] [PubMed] [Google Scholar]
- 23.Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nature reviews Cancer 2013;13(7):466–481. [DOI] [PubMed] [Google Scholar]
- 24.Gurusamy D, Gray JK, Pathrose P, Kulkarni RM, Finkleman FD, Waltz SE. Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth. Cancer research 2013;73(6):1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyob H, Ekiz HA, Derose YS, Waltz SE, Williams MA, Welm AL. Inhibition of ron kinase blocks conversion of micrometastases to overt metastases by boosting antitumor immunity. Cancer discovery 2013;3(7):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benvenuti S, Lazzari L, Arnesano A, Li Chiavi G, Gentile A, Comoglio PM. Ron kinase transphosphorylation sustains MET oncogene addiction. Cancer research 2011;71(5):1945–1955. [DOI] [PubMed] [Google Scholar]
- 27.Wang MH, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta pharmacologica Sinica 2010;31(9):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu HS, Hsu PY, Lai MD et al. An unusual function of RON receptor tyrosine kinase as a transcriptional regulator in cooperation with EGFR in human cancer cells. Carcinogenesis 2010;31(8):1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi T, Furukawa Y, Kikuchi J et al. Transactivation of RON receptor tyrosine kinase by interaction with PDGF receptor beta during steady-state growth of human mesangial cells. Kidney international 2009;75(11):1173–1183. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer letters 2007;257(2):157–164. [DOI] [PubMed] [Google Scholar]
- 31.Hsu PY, Liu HS, Cheng HL et al. Collaboration of RON and epidermal growth factor receptor in human bladder carcinogenesis. The Journal of urology 2006;176(5):2262–2267. [DOI] [PubMed] [Google Scholar]
- 32.Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Annals of surgical oncology 2005;12(4):273–281. [DOI] [PubMed] [Google Scholar]
- 33.Yamoah K, Walker A, Spangler E et al. African-american race is a predictor of seminal vesicle invasion after radical prostatectomy. Clinical genitourinary cancer 2015;13(2):e65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams VL, Awasthi S, Fink AK et al. African-American men and prostate cancer-specific mortality: a competing risk analysis of a large institutional cohort, 1989-2015. Cancer medicine 2018;7(5):2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly SP, Rosenberg PS, Anderson WF et al. Trends in the Incidence of Fatal Prostate Cancer in the United States by Race. European urology 2017;71(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin K, Lipsitz R, Miller T, Janakiraman S. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Annals of internal medicine 2008; 149(3): 192–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Source of cell lines, antibodies and primer sequences used in the study.