Abstract
Background/Objectives:
Prothrombin complex concentrates (PCC) are increasingly administered off-label in the United States to treat bleeding in cardiovascular surgical patients and carry the potential risk for acquired thromboembolic side effects after surgery. Therefore, we hypothesized that the use of low-dose 3-factor (3F) PCC (20-30 IU/kg), as part of a transfusion algorithm, reduces bleeding without increasing postoperative thrombotic/thromboembolic complications.
Materials/Methods:
After IRB approval, we retrospectively analyzed 114 consecutive, complex cardiovascular surgical patients (age > 18 years), between February 2014-June 2015, that received low-dose 3F-PCC (Profilnine®), of which seven patients met established exclusion criteria. PCC was dosed according to an institutional perioperative algorithm. Allogeneic transfusions were recorded before and after PCC administration (n=107). The incidence of postoperative thromboembolic events was determined within 30 days of surgery, and Factor II levels were measured in a subset of patients (n=20) as a quality control measure to avoid excessive PCC dosing.
Results:
Total allogeneic blood product transfusion reached a mean of 12.4±9.9 units before PCC and 5.0±6.3 units after PCC administration (p<0.001). The mean PCC dose was 15.8±7.1 IU/kg. Four patients (3.8%) each experienced an ischemic stroke on postoperative day 1, 2, 4, and 27. Seven patients (6.5%) had acquired venous thromboembolic disease within 10 days of surgery. Median factor II level after transfusion algorithm adherence and PCC administration was 87%.
Conclusions:
3F-PCC use for refractory bleeding after cardiovascular surgery resulted in reduced transfusion of allogeneic blood and blood products. Adherence to this algorithmic approach was associated with an acceptable incidence of postoperative thrombotic/thromboembolic complications.
Introduction
Prothrombin complex concentrates (PCCs) are a form of clotting factor concentrates and are available in three-factor (3F; Factors II, IX, X) and four-factor (4F; Factors II, VII, IX, X, with select anticoagulants) formulations. In the United States, a 4F-PCC formulation is approved by the Food and Drug Administration (FDA), and for the reversal of warfarin associated bleeding and reversal of warfarin anticoagulation` in the event of urgent or emergent invasive procedures or surgery (Kcentra®, CSL Behring, Marburg, Germany).[1] There is increasing off-label usage of PCCs in cardiovascular surgery in the United States to reduce bleeding and allogeneic blood product transfusions.[2, 3] Transfusion remains common in cardiovascular surgery, although blood product shortages and transfusion-associated complications have spurred greater emphasis on use of factor concentrates in the management of acute postoperative bleeding. The European Society of Anaesthesiology recommends correction of hypofibrinogenemia with fibrinogen concentrates (cryoprecipitate is not available in mainland Europe), and the use of coagulation factors due to their high efficacy and minimal infectious risk. [4] PCCs are derived from cryoprecipitate-free plasma and undergo purification, nanofiltration, vapor-heated treatment and/or viral inactivation steps, all of which serve to significantly reduce the antigenic load to a negligible amount.[5] Furthermore, PCC administration rapidly increases vitamin K-dependent clotting factor levels, which makes usage during perioperative bleeding an attractive option for achieving hemostasis.
Thrombin (Factor IIa) generation is critical for surgical hemostasis and a near-linear association is reported for prothrombin levels (Factor II, non-activated) and thrombin generation.[6] Commercially available PCC formulations provide the procoagulant factors to replenish cofactors in both the extrinsic and intrinsic Xase complexes. Factor concentrations conventionally reported as international units (IU) per 100 IU of factor IX. These factors may replenish the prothrombinase enzyme[7] but the concentration of Factor II varies in these commercial PCC preparations. The two preparations available in the United States include a 3F-PCC (Profilnine®, Grifols Biologicals, Los Angeles, CA, USA), which contains 148 IU of Factor II per 100 IU of Factor IX,[8] and Kcentra®, which has 120 IU of Factor II per 100 IU of Factor IX.[9] The administration of high-dose PCC poses the potential risk of thrombotic/thromboembolic complications due to the long half-life of prothrombin;[10] for example, fatal intracardiac and intra-aortic thromboses have been reported after the use of 50 IU/kg of Kcentra® for warfarin reversal in preparation for emergent cardiac surgery.[11] Furthermore, by first replenishing low fibrinogen and platelets levels, low-dose PCC administration may be more effective during the management of surgical hemostasis.[12, 13] We therefore hypothesized that the use of low-dose Profilnine® (higher prothrombin levels compared with Kcentra®), administered for refractory hemorrhage as part of an institutionally-derived bleeding algorithm,[14] would be associated with decreased post-PCC blood product transfusion and minimal postoperative thromboembolic complications.
Methods
Data Collection
Following Institutional Review Board approval, a retrospective chart review of patients undergoing cardiovascular surgery at our institution between February 2014 and June 2015 was performed. Adults (age 18 years or older), that had received Profilnine® for refractory bleeding in the operating room or within the first 12 hours of postoperative ICU admission, were included. Patients under the age of 18 and parturients were excluded, as were patients with a history of disseminated intravascular coagulation (DIC) and those that received both 3F and 4F-PCC during the perioperative period. Patient-specific data from the electronic health record (Maestro Care Epic©, Verona, Wisconsin, USA) were queried and entered into a REDCap® database (Research Electronic Data Capture, Vanderbilt University, Nashville, TN).
Demographic data was obtained and included age, gender, weight, and body mass index (BMI) (Table 1). Surgical procedure, preoperative surgical diagnosis and redo sternotomy status were also documented. Other preoperative variables included medications, pre-existing coagulopathy, warfarin use, and history of thrombotic/thromboembolic events including myocardial infarction, cerebrovascular accident (CVA), pulmonary embolism (PE), deep venous thrombosis (DVT), or other thromboses. Intraoperative and postoperative transfusion data, as well as laboratory test results, were recorded (Table 2).
Table 1.
Baseline Characteristics and Perioperative Parameters
| Parameter | Study Population (N =107) |
|---|---|
| Gender | |
| Female | 39 (36.4%) |
| Male | 68 (63.6%) |
| Age, years [mean (range)]age | 58.2 (18.0-92.0) |
| BMI, kg/m2 [mean (range)] | 27.9 (16.4-49.1) |
| Baseline creatinine, mg/dl [mean (range)] | 1.6 (0.0–14.0) |
| Redo median sternotomy | 55 (51.4%) |
| CPB bypass time, minutes [mean (range)] | 203.5 (0.0–484.0) |
| Aortic cross-clamp time, minutes [mean (range)] | 117.9 (0.0–331.0) |
| PCC dose, IU/kg (mean ± SD) | 15.8 ± 7.1 |
| PCC administration timing | |
| Post-CPB (operating room) | 101 (94.4%) |
| Postoperative (ICU) | 6 (5.6%) |
| Recombinant factor VIIa dose, mcg/kg (mean ± SD) | 25.1 ± 16.4; N=56 (52.3%) |
| Recombinant factor VIIa administration | 56 (52.3%) |
| aAntifibrinolytic administration | 98 (91.6%) |
| Preoperative Thrombotic/Thromboembolic Events | |
| Cerebrovascular accident | 14 (13.1%) |
| Myocardial infarction | 9 (8.4%) |
| Deep vein thrombosis | 4 (3.7%) |
| Operation | |
| Thoracic aortic reconstruction (elective) | 61 (57.0%) |
| Thoracic aortic reconstruction (emergent dissection) | 13 (12.1% |
| bOther cardiovascular operations (elective) | 9 (8.4%) |
| Ventricular Assist Device implantation | 9 (8.4%) |
| cMulti-Valve Repair or Replacement | 6 (5.6%) |
| Heart Transplantation | 5 (4.7%) |
| Single Valve Repair or Replacement | 3 (2.8 %) |
| Coronary Artery Bypass Grafting/Multi-Valve | 1 (0.9%) |
Tranexamic acid was typically used for patients undergoing cardiac surgery on CPB. Epsilon-Aminocaproic Acid was administered in patients undergoing hypothermic circulatory arrest, history of previous seizures, or previous documented allergy to tranexamic acid.
Other operations included complex surgeries and combinations of cardiovascular procedures.
Multi-Valve refers to ≥ 2 cardiac valves.
Abbreviations: CPB=Cardiopulmonary bypass; ICU=Intensive care unit; PCC=Prothrombin complex concentrate.
Table 2.
Laboratory and Point-of-Care Hemostatic Values Before and After 3F-PCC Administration
| Parameter (units) | Before (Mean ± SD) | After (Mean ± SD) |
|---|---|---|
| Fibrinogen (mg/dl) | 245.7 ± 83.5 | 256.0 ± 61.8 |
| Platelet count (x 109/L) | 115.4 ± 62.3 | 150.5 ± 54.5 |
| PT (s) | 20.6 ± 23.3 | 12.0 ± 4.3 |
| INR | 1.8 ± 2.0 | 1.0 ± 0.4 |
| aPTT(s) | 57.8 ± 72.7 | 34.4 ± 12.8 |
| A10 FIBTEM®(mm) | 14.2 ± 6.8 | 17.1 ± 8.0 |
| A10 EXTEM®(mm) | 43.2 ± 11.7 | 51.9 ± 6.9 |
| aCT EXTEM®(s) | 84.1 ± 32.4 | 65.5 ± 13.9 |
CT EXTEM® is not sensitive to unfractionated heparin within blood up to 4U/ml.
Abbreviations: PT=Prothrombin time (seconds); aPTT=Activated partial thromboplastin time; A10=Amplitude of the tracing at 10 minutes of run time; CT=Clotting time; FIBTEM=ROTEM® fibrinogen assay; EXTEM=ROTEM® extrinsic clotting pathway assay, INR=International normalized ratio
Postoperative variables were abstracted from the electronic health record and included hourly chest tube outputs (ml) for the first 12 hours after arrival to the Cardiothoracic Intensive Care Unit (CTICU), newly diagnosed postoperative thrombotic/thromboembolic complications (MI, CVA, DVT, PE), need for hemodialysis or continuous renal replacement therapy, baseline creatinine, and peak creatinine values. Patient charts were reviewed from admission at the time of surgery until discharge or 30 days after surgery (which ever came first) in order to identify and document these postoperative complications.
The primary outcome measured was the difference in total blood product transfusion before and after PCC administration. Secondary outcomes included chest tube output after PCC dose and the association of weight-normalized PCC dose with post-dosing factor II levels.
Anesthetic Protocol
All patients underwent general anesthesia with invasive arterial/central venous pressure monitoring and transesophageal echocardiography. Cell salvage therapy was used for every operation (Haemonetics Elite®, Haemonetics, Braintree, MA, USA). All patients, which did not require hypothermic circulatory arrest and therefore were not at risk for compromise of the blood-brain barrier and lowering of the seizure threshold, received tranexamic acid (10 mg/kg bolus followed by continuous infusion of 1 mg/kg/h) as the antifibrinolytic agent.[15] Otherwise, epsilon-aminocaproic acid was administered (5g bolus following by an infusion at 1 g/hr). A combination of balanced intravenous and inhalational anesthetic agents were used to induce and maintain general anesthesia at the discretion of the attending cardiac anesthesiologist.
Cardiopulmonary Bypass Protocol
A baseline activated clotting time (ACT) was obtained after arterial line insertion prior to unfractionated heparin (UFH) administration. Per standard institutional practice, UFH was administered intravenously at 350-400 U/kg, to achieve and maintain an ACT ≥ 480 seconds during cardiopulmonary bypass (CPB). The CPB circuit prime included 10,000 U of UFH and 1,000 ml of Crystalloid (Plasmalyte-A®, Baxter Healthcare, Bloomington, IN, USA). Cooling during CPB was performed to a temperature requested by the operating surgeon and commensurate with the operation. Patients were rewarmed on CPB to 36°C, once the surgical procedure was completed on CPB.. Platelet count, Clauss fibrinogen assay, and point-of-care assessment with ROTEM® (EXTEM® and FibTEM®, TEM international, Munich, Germany) was obtained once rewarming temperature reached 34°C, according to an established, institutional algorithm (Figure 1). After separation from CPB, heparin was reversed with protamine sulfate at a dose of 0.5-1 mg/U of initial heparin dose with a goal of normalizing ACT to pre-CPB baseline. If rebound heparinization was suspected as a result of increasing ACT after correction to baseline, then a protamine infusion of 25 mg/hr was initiated and administered over 4 hours. This practice of protamine infusion was based on a quality improvement initiative at our institution.
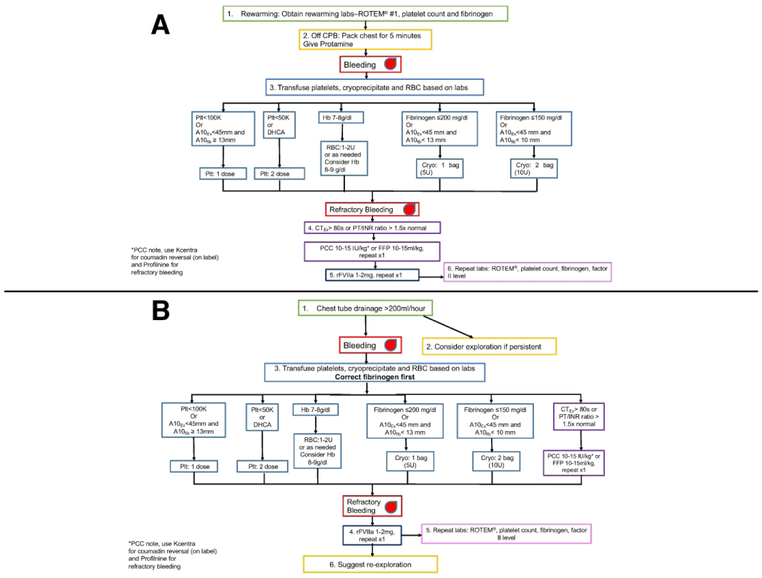
Figure 1.
A. Duke Intraoperative Transfusion Algorithm for Adult Cardiovascular Surgery based on point-of-care hemostatic testing for high-risk cardiovascular operations. B. Duke Postoperative Transfusion Algorithm for Cardiovascular Surgery for bleeding in the Cardiothoracic ICU. Abbreviations: A10fib=A10 Fibtem, A10Ex=A10 Extem, Cryo=Cryoprecipitate, CPB=Cardiopulmonary bypass, FIB=Fibrinogen level, Hb=hemoglobin, INR=International normalized ratio, PCC=Prothrombin Complex Concentrate, Plt=Platelet count, PT=Prothrombin time, ROTEM®=Rotational Thromboelastometry, RBC= Red blood cells, rFVIIA=Recombinant factor VIIa, U=Unit.
Post-CPB Transfusion Algorithm
In case of refractory bleeding after discontinuation of CPB and reversal of heparin with normalization of the ACT, the chest was packed, and coagulopathy was corrected in accordance with the Duke Intraoperative Transfusion Algorithm for Cardiovascular Surgery (Figure 1A). A target hemoglobin (Hb) between 7 and 10 g/dl was determined by the perioperative care team depending on patient comorbidities and rate of bleeding with a higher target used during rapid bleeding. There was an emphasis on initial correction of fibrinogen, as this becomes deficient before factor deficiency and thrombin generation,[16] with 5 to 10 units of cryoprecipitate transfused based on Clauss fibrinogen assay levels of ≤ 200 - 250 mg/dl and/or FIBTEM A10 values of ≤ 15 - 18mm (Figure 1). Fibrinogen concentrate could also be used for this step. Our internal quality control has shown our 5U-pack of cryoprecipitate contains between 1.5 to 2.5 grams of fibrinogen. Platelets were corrected next, then, if bleeding persisted and the International Normalized Ratio (INR) was greater than 1.5 or or EXTEM® clotting time (CT) was greater than 80s, FFP (10-15 ml/kg) or Profilnine® (10-15 IU/kg of actual body weight) was administered following approval by one of the authors (KG, JHL, or IJW), with the option of re-dosing once in the setting of continued refractory bleeding (Figure 1A). We used PCC if bleeding was refractory to FFP or when volume administration was a concern, such as in patients with congestive heart failure and acute lung injury. Notably, EXTEM® assays were sent while patients were anticoagulated with heparin during CPB and this heparin effect may prolong the CT.[17] Nevertheless, a normal CT on EXTEM® suggested absence of clotting factor deficiency; this, and the delay between testing and need for dosing, was taken under consideration by the authors that were in charge of Profilnine® approval. Clotting factor replenishment was dosed based on unpublished data, which showed that factor II levels were 70.5 ± 15.7 % and antithrombin III levels were 67.5 ± 8.9 % after administration of 4 units of FFP on cardiopulmonary bypass during aortic reconstruction surgery. Our goal was to not exceed the upper limit of the normal range of factor II (120 %) in order to avoid postoperative thrombotic/thromboembolic complications. In a hemostatic patient without ongoing consumption, 10-15 units/kg of Profilnine (~1:1.5 ratio of FIX:FII) should approximately increase the FII levels by 15 - 22.5 % after one dose, maintaining an approximate Factor II level ≤ 120% if a repeat dose is administered. PCC in this scenario, however, was administered during refractory bleeding. In fact, continued, refractory hemorrhage after PCC administration was treated with low-dose recombinant factor VIIa (rFVIIa; 10 - 20 mcg/kg dose, ~ 1-2mg in a 100 kg adult, with allowance to repeat this dose one additional time) at the discretion of the supervising anesthesiologist (~1 – 2 mg repeated once).[18] Once hemostasis was achieved, all cardiovascular surgical patients were transferred to a dedicated CTICU managed by an intensivist and intensive care team.
Postoperative Management
Upon admission to the CTICU, patients were managed according to a protocol that included continuous warming to achieve or maintain normothermia (37°C) and tracheal extubation as soon as metabolic, circulatory, and respiratory parameters were acceptable. Vitals signs, hemodynamic parameters, and chest tube outputs were recorded hourly; significant chest tube bleeding was considered to be > 200 ml/hr. Postoperative bleeding in the CTICU was managed according to a similar algorithm (Figure 1B). The decision for surgical re-exploration by the attending surgeon was based on laboratory and ROTEM® values, amount of hourly chest tube output, hemodynamic stability, and response to transfusion. Intraoperatively refractory bleeding was defined by failure to achieve visible hemostasis or clot formation in the surgical field such that chest closure was delayed In the postoperative period in the ICU, this was defined as patients that had received PCCs and continued to bleed. Postoperative refractory bleeding was managed with administration of with low-dose rFVIIa, followed by hemostastic testing and surgical re-exploration as indicated.
Statistical Analysis
Descriptive statistics were developed for demographic, clinical, and outcome variables of interest, where frequencies and percentages were computed for categorical variables, and mean, standard deviation (SD), median and interquartile range (IQR) were computed for continuous variables. Total transfused blood products, including packed red blood cells (PRBC), FFP, cryoprecipitate, and platelets, during pre- and post-PCC administration were tabulated. Paired sample Wilcoxon test was used to test the difference of blood products administered post-PCC from those given pre-PCC. That is, we obtained the difference of total blood products first for each subject and performed the Wilcoxon signed rank test. Weight-normalized PCC dose was calculated by dividing the administered PCC dose by the patient’s actual weight in kilograms. To present the early postoperative bleeding trend, we generated box plots for the chest tube output per hour for the first 12 hours after ICU arrival. The analysis was performed using SAS 9.4 (SAS Institute Inc.© Cary, NC) with figures generated by R (https://cran.r-project.org/).
Results
Of the 114 consecutive patients meeting inclusion criteria, 7 patients were excluded due to the following causes: warfarin reversal prior to a chest tube placement for a malignant effusion (n=1), esophagectomy complicated by acute liver ischemia and intraoperative death (n=1), received both 3 and 4 factor PCC (n=4), prolonged hospital admission (> 6months) following biventricular assist devices and heart transplant with limited mobility pre and post-transplant (n=1). Descriptive statistical analysis was performed for the remaining 107 patients (Table 1). Fifty-five patients (51.4%) had undergone a redo sternotomy. The most common surgical procedure requiring PCC administration was elective aortic reconstruction in 61 patients (57%). A total of 31 preoperative thrombotic/thromboembolic diagnoses were recorded (Table 1). A total of 25 patients had more than one preoperative thrombotic diagnosis.
Transfusion requirements before and after PCC
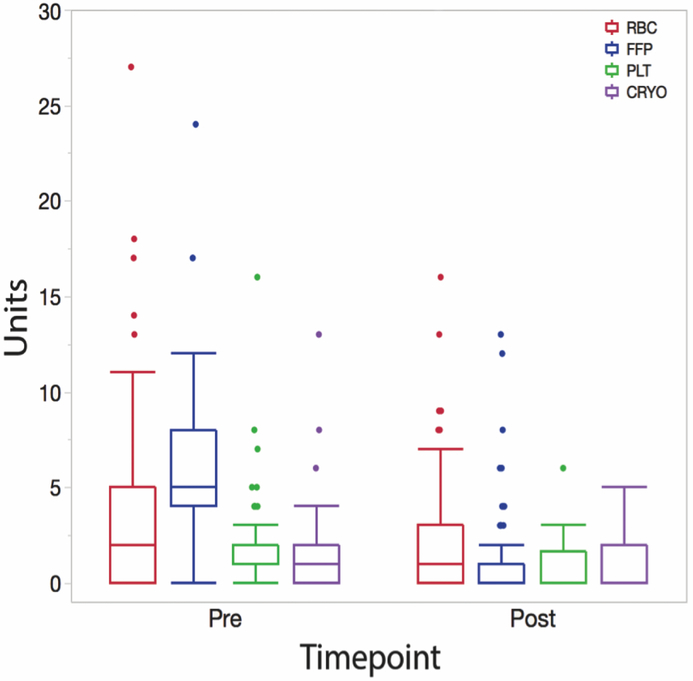
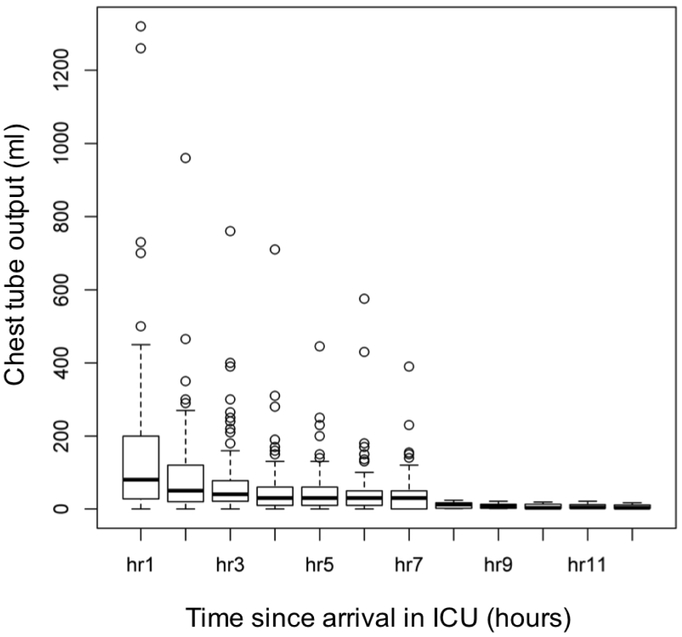
The mean total blood product transfusion was 12.4±9.9 units prior to PCC dose and 5.0±6.3 units after PCC administration (Figure 2). This reduction (mostly in FFP use) was found to be statistically significant between the two time points (p <0.0001). The mean±SD for weight-normalized PCC dose was 15.8±7.1 IU/kg. In addition, 56 of 107 patients (52.3%) received low-dose rFVIIa (25.1±16.4 mcg/kg) after PCC administration (Table 1) as denoted in our algorithm (Figure 1). The chest tube output for the first hour after arriving to the ICU was 150.8 ± 214.0 ml and quickly decreased to 89.5 ± 122.1 ml within the second hour (Figure 3). Four patients returned to the operating room for refractory hemorrhage and surgical re-exploration. Other perioperative data are summarized in table 1.
Figure 2.
Blood product transfusion (RBC, FFP, Plt, Cryo) pre- and post-PCC administration. The reduction in total allogeneic blood products transfusions after PCC administration is statistically significant (p <0.0001). Cryo=Cryoprecipitate, FFP=Fresh frozen plasma, Plt=Platelets, RBC=Packed red blood cells.
Figure 3.
Trends of chest tube output after arrival in ICU. Median chest tube output during the first hour in the ICU was 80 ml after receiving PCC. Abbreviations: ICU=Intensive care unit.
Thrombotic/Thromboembolic Outcomes
Table 1 summarizes the preoperative thrombotic/thromboembolic events in our study sample. Of these patients, 4 patients (3.7%) had a new ischemic CVA diagnosed in the postoperative period on postoperative days 1, 2, 4, and 27. Two of these patients had undergone left ventricular assist device (LVAD) implantation, one had thoracic aortic reconstruction, and one patient had central insertion of extracorporeal membrane oxygenation (ECMO) following STEMI and cardiogenic shock (cannula inserted into right atrium and ascending aorta). The patient with the aortic reconstruction received a higher dose of PCC (33.5 IU/kg) and rFVIIa (5mg or 55 mcg/kg). One patient had a pulmonary embolism diagnosed by computed tomography angiogram on postoperative day 9. This patient initially presented with a diagnosis of LVAD thrombosis, received a low dose of PCC (13.8 IU/kg) after redo LVAD surgery, did not receive rFVIIa and the post-dose factor II activity level for this patient was 64% (well below 120% as our maximum range of normal). Six patients (5.6%) had upper extremity DVT diagnosed by venous Doppler ultrasound in the postoperative period (Table 3). Four of these patients had received rFVIIa after PCC administration, three of whom had received (≥ 20 mcg/kg).
Table 3.
Postoperative Thrombotic/Thromboembolic Events after 3F-PCC usage
| Event | Diagnostic Modifier | Time-to- Event (POD) |
Operation | 3F-PCC (IU/kg) |
rFVIIa (mcg/kg) |
|---|---|---|---|---|---|
| CVA | Ischemic - R Frontoparietal cortex – CT Brain without contrast | 1 | TAA, AVR, Aortic Root Repair | 33.6 | 44.8 |
| CVA | Ischemic – R MCA territory – CT Brain Angio | 25 | BIVAD/VA ECMO | 10.8 | 0 |
| CVA | Ischemic – Diffuse hypodensities within cerebral cortex – CT Brain without contrast | 4 | BIVAD | 9.4 | 0 |
| CVA | Ischemic – R parietotemporal – CT brain without contrast | 2 | VA ECMO | 10.2 | 0 |
| PE | CT Chest Angiogram Right interlobar and bilateral lower segment arterial branches | 9 | BIVAD | 7.0 | 0 |
| DVT | Doppler US – L Axillary Vein | 4 | Heart-Lung Transplant | 38.0 | 56.9 |
| DVT | Doppler US – LIJ, Subclavian Vein – associated pacemaker wires | 9 | AVR/MVR/TVR/ASD repair | 9.8 | 0 |
| DVT | Doppler US of RUE – RIJ associated central venous catheter | 6 | AVR/Aortic Root Repair/TAA/Hemiarch | 25.9 | 13.0 |
| DVT | Doppler US of RUE – RIJ/Subclavian – associated central venous catheter | 7 | TAAA Repair | 23.1 | 22.2 |
| DVT | Doppler US of LUE – L Subclavian/Axillary associated pacemaker wires | 8 | HM2 LVAD, TV/AV Repairs, and ASD Repair | 12.1 | 0 |
| DVT | Doppler US of LUE – L IJ – no associated structures | 6 | AVR/Aortic Root/TAA | 18.3 | 0 |
Abbreviations: ASD=Atrial septal defect; AVR=Aortic valve replacement; BiVAD=Biventricular assist device include both right ventricular assist device (RVAD) and Left VAD, RVAD cannula are inserted in the right atrium and pulmonary artery; LVAD cannula are inserted in the LV apex and ascending aorta. CABG=Coronary artery bypass graft; CVA=Cerebrovascular accident; HM2=Heartmate II LVAD ; Heart-Lung transplant=combine organ transplantation with en bloc recipient cardiectomy and bilateral pneumonectomies followed by en bloc implantation of donor heart and lungs. MVR=Mitral valve replacement; POD=Postoperative day; TAAA=Thoracoabdominal aortic; TAA=Thoracic aortic aneurysm; TVR=Tricuspid valve replacement; VA ECMO=Venoarterial Extracorporeal Membrane Oxygenation, inflow inserted within right atrium and outflow inserted within the ascending aorta.
Factor II levels after Post-PCC use and Transfusion Algorithm Adherence
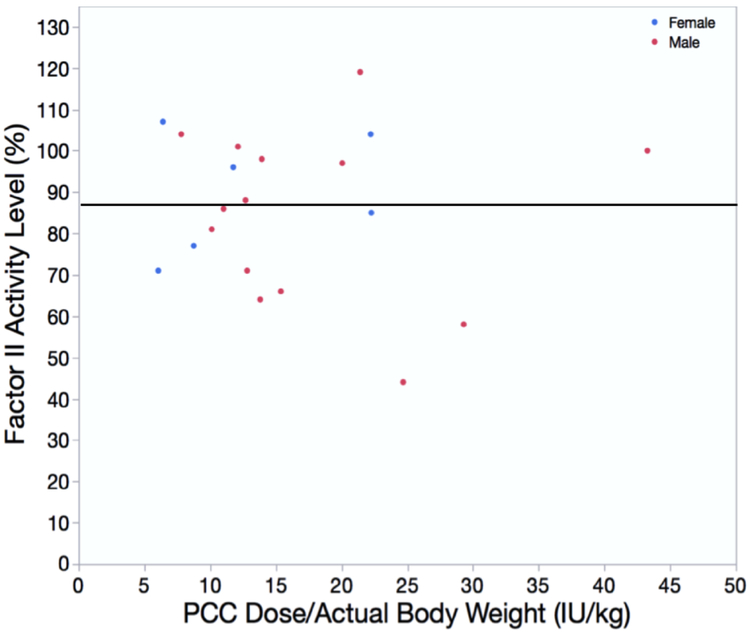
A subset of patients (n=20) had post-PCC factor II levels drawn in the ICU within the first 24 hours of ICU admission as a quality improvement initiative to guide future dose modifications if needed. We plotted the factor II activity assay level against the PCC dose given per kilogram body weight (Figure 4). The mean±SD PCC dose was 16.3±9.0 IU/kg. The median factor II level was 87%; 12 out of these 20 patients received rFVIIa as part of the algorithm, and no postoperative thromboembolic complications were reported in this subgroup during their hospital stay or within 30 days of administration. This was consistent as ~ 50% of patients required rFVIIa to achieve hemostasis and could be considered refractry to PCCs.
Figure 4.
Post PCC Factor II levels in relation to total PCC dose (intraoperative and postoperative). This chart displays factor II levels after PCC dose (dose/actual body weight in kg) within a small subgroup of patients (n=20). The median factor II level was 87% and represented by the black line. Abbreviation: IU=International units, PCC=Prothrombin complex concentrate.
Discussion
We found that low-dose 3F-PCC (15.8 ± 7.1 IU/kg; n=107) was associated with a reduction in transfusion requirements after administration for refractory bleeding as part of our bleeding algorithm for high-risk cardiovascular surgery. Low-dose Profilnine® reduced bleeding in coagulopathic patients with a relatively low incidence of adverse thromboembolic events in this high-risk surgical population, which is consistent with previous reports.[19, 20] Thromboembolic complications often occurred several days after factor concentrate administration, and these complications may have been associated with immobility and other common postoperative risk factors after high-risk cardiovascular surgery.[21]
Coagulopathy following cardiovascular surgery and CPB is due to multiple factors [22, 23] including hyperfibrinolysis,[24] platelet dysfunction, [25, 26] and dilutional changes. Our bleeding algorithm for postoperative management focuses on targeted hemostatic interventions to address acquired factor deficiencies and improve whole blood clot formation as part of a pragmatic and multimodal approach to perioperative bleeding.[14]
The majority of our patients that were treated according to our bleeding algorithms had undergone elective thoracic aortic reconstruction. Postoperative hemorrhage is common after thoracic aortic surgery and the associated hypothermia, during CPB and operation on the great vessels, which can impair platelet and coagulation factor function.[27] Additionally, in patients undergoing emergent aortic dissection repair, the second-most common index procedure in our cohort, tissue factor production and clotting cascade activation from aortic dissection begin in the preoperative period and bleeding is further exacerbated during surgery.[28] Our results showed that administration of a mean PCC dose of 15.8 ± 7.1 IU/kg as part of the algorithm resulted in a statistically significant decrease in blood product transfusion after PCC administration; however in ~50% of patients low-dose rFVIIa was also required to achieve complete hemostasis. The observation of reduced bleeding and transfusion are consistent with a previously published study of persistent microvascular bleeding following high-risk cardiovascular surgery (aortic reconstruction, valve replacement, cardiac transplantation, or ventricular assist device implantation). [29] In their report, 150 patients received either a 3F-PCC (n=50) or rFVIIa (n=100), and concluded that 3F-PCC was more effective in reducing chest tube output in the first 12 hours (Group; Median[IQR]: 3F-PCC group; 690 ml [450 - 1,030 ml] vs. recombinant factor VIIa group; 1,370 ml [745 – 2.078 ml]; p<0.001).[29]
Other studies have demonstrated similar beneficial effects of PCC administration in post-cardiotomy hemorrhage when used alone or in combination with FFP. Arnekian showed a reduction in postoperative bleeding in patients receiving 10 - 15 IU/kg of the 4F-PCC Octaplex® (Octapharma AG, Lachen, Switzerland).[2] Ortmann found a higher incidence of bleeding in patients that were previously treated with warfarin and received FFP following pulmonary endarterectomy, for chronic thromboembolic pulmonary hypertension, compared with those who received PCC despite an absence of difference in red blood cell transfusion.[3] Cappabianca evaluated the effect of PCC versus FFP in a propensity-matched analysis, and found the use of PCC alone to be associated with significantly reduced postoperative blood loss and reduced need for red blood cell (RBC) transfusion while the incidence of thrombotic/thromboembolic events such as stroke and transient ischemic attack remained similar between patient groups.[30] Their concern of renal injury should be evaluated in future prospective study.
Our algorithm also targeted fibrinogen repletion using cryoprecipitate prior to PCC administration. In a previous study, the PCC only group had less chest tube output compared to the PCC + FFP group.[2] Further, fibrinogen levels were higher in the PCC only group and this difference was statistically significant. Our mean±SD fibrinogen level prior to PCC administration was 245.7±83.5 mg/dL, which may also explain the reduction in bleeding. In a study by Ranucci,[25] fibrinogen levels of 300-350 mg/dL obviated the need for PCCs when bleeding was encountered after complex cardiovascular surgery. Although our institutional practice is not to overcorrect the fibrinogen levels owing to the large volume of multidonor-pooled cryoprecipitate required, normalization of fibrinogen levels between 200-250 mg/dL prior to factor concentrate administration aims to increase fibrin cross-linkage, optimize clot strength and facilitate the hemostatic effect of thrombin generation with lower doses of PCC or recombinant factor VIIa.[31] This rationale is also consistent with observations on failed hemostasis after recombinant factor VIIa previously reported.[32]
Postoperative Thrombotic/Thromboembolic complications
There remains a paucity of data on thromboembolic complications in the bleeding cardiovascular surgical patient following PCC administration. In our series, we documented strokes in 4 patients, venous thromboembolism (VTE) in 6 patients and a PE in 1 patient. Several groups have reported thrombosis associated with PCC administration in the context of anticoagulation reversal or hemophilia.[20, 33, 34] However, postoperative thromboembolic events following cardiovascular surgery are multifactorial in origin and should be considered in the context of other comorbidities, aortic disease, postoperative mobility, and VTE prophylaxis.[5] In a meta-analysis of 27 studies to determine thromboembolic events in patients that received PCC to reverse warfarin for urgent surgery,[35] a low incidence of thromboembolic complications were reported at 1.8% for 4F-PCC and 0.7% for 3F-PCC. Song et al. described one episode of upper extremity DVT in a 25-patient series utilizing factor VIII inhibitor bypass activity (FEIBA®, a form of activated PCC, Baxter Healthcare, Bloomington, IN, USA).[36] Due to concerns for thrombotic complications by use of FEIBA®, our group has opted to sequentially administer PCCs and then low-dose recombinant factor VIIa. Thus, we sequentially administered recombinant factor VIIa in 52.3% of cases (Table 1). This is consistent with our findings of VTE as the most common complication; the presence of indwelling central lines and permanent pacemakers may increase the likelihood of upper extremity DVT in this population.[36] More importantly, our stroke rate was similar to previously reported series in high risk cardiovascular surgical patients.[5] The rate of thrombosis was not viewed as excessive or unexpected during a multidisciplinary review of our algorithm, and was outweighed by the effectiveness of hemostasis. Other larger series have not reported any significant increase in thromboembolic events in patients that have received PCC for refractory bleeding in complex cardiovascular surgery.[2, 3] While reporting a higher incidence of thromboembolic events, our sample may be reflective of a more critically ill subgroup of patients that only received mechanical (e.g., sequential compression devices, etc.) and not pharmacologic VTE prophylaxis based on their recent refractory bleeding. Once bleeding has been abated by the management modalities we describe in our algorithm, perhaps pharmacologic VTE prophylaxis should be considered earlier in this patient group, for example guided by supranormal prothrombin levels or viscoelastic evidence of hypercoagulablity in order to counteract a prothrombotic state.
High levels of Factor II in ambulatory patients[37] or after multiple doses of PCC has been implicated in promoting thrombosis.[5] A small subgroup of our patients had factor II levels measured within 24 hours of PCC administration. There were no supratherapeutic values of factor II levels following administration (reference range: 72 - 120 % in our coagulation laboratory) or thromboembolic complications, even when low-dose recombinant factor VIIa was co-administered, although this was a small sample within our cohort. Persistence of high levels of prothrombin into the postoperative period may result in excess thrombin generation,[6, 38] logically increasing the risk of VTE, especially in an immobile population of postoperative ICU patients. That said, due to the half-life of Factor II and X, it is unlikely that thromboembolic complications are occurring more than a few days after administration are related to PCC use.
Limitations
Despite adherence to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for cohort studies, this is a retrospective review and accuracy of data depends on documentation in the electronic health record. For example, documentation of postoperative thrombotic/thromboembolic complications required new postoperative diagnoses within Epic® that was then verified by direct review of radiologic documentation, as outlined in Table 3. One important source for selection bias in this study was that the number and types of blood products administered prior to PCC administration were not predefined, but rather determined and prescribed according to clinical need and hemostatic testing. We attempted to limit this bias in our study by using patients as their controls and comparing blood products before and after PCC administration. Nevertheless, administration according to a bleeding algorithm, which allowed for clinical judgment, may have added bias for PCC use in our series. Empiric decision-making is pragmatic, however, in the operative setting and has been carefully incorporated in a randomized multicenter trial in cardiovascular surgery, which illustrated reduced postoperative bleeding and blood product usage after point-of-care hemostatic testing with a similar algorithmic approach.[39]
At our institution, the ROTEM® machine is located within our blood gas laboratory. For this reason, data may take as long as 20 minutes to become available to guide bleeding management. Therefore, our algorithm incorporates additional low-dose PCC and recombinant factor VIIa usage in response to such acute life-threatening refractory bleeding without readily available point-of-care data. Availability of point-of-care testing in the operating room or ICU may permit a strict data driven algorithm which could limit our practice of empiric factor concentrate administration during hemorrhagic shock.
Despite our efforts to define refractory bleeding, there will always be a degree of subjectivity in this definition especially when applied retrospectively. The most practically applicable published classification by Dyke et al [40] combines features that characterize escalating steps taken by clinicians to manage the bleeding as an indication of bleeding severity. These include needing to wait to close the chest due to bleeding, the need to use factor concentrates or increasing amounts of blood components and the need to re-explore the patient to stop hemorrhage. In this classification, all our patients had met, or were approaching, the criteria for Class 3, severe bleeding. Finally, while complication rates were unremarkable, safety cannot be determined by a single case series.
Conclusions
After correcting for fibrinogen and platelet loss, the use of three-factor prothrombin complex concentrates in an algorithm for refractory bleeding related to cardiovascular surgery was associated with reduced transfusion of allogeneic blood and blood products. Adherence to this algorithmic approach resulted in an acceptable incidence of postoperative thrombotic or thromboembolic complications. Larger, randomized trials are warranted to investigate these effects.
Acknowledgments
Disclosures:
KG: grant support from NIH (T32GM00860); Consultant for UpToDate®
NH, AS, YL, RR, JG, AR, YB: None declared
TLO: Research funding from Instrumentation Laboratory and Siemens, honoraria from BMS-Squibb, and the UpToDate® Board or Advisory Committee
JHL: receives fees for serving on advisory committees for CSL Behring, Grifols, Portola, Boehringer Ingelheim, Instrumentation Laboratories, and Merck.
IJW: grant support from NIH (R01HL121232-01), CSL Behring and Biomet Biologics. Consultant for UpToDate
References
- 1.Sarode R, Milling TJ Jr., Refaai MA, et al. : Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128: 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnekian V, Camous J, Fattal S, et al. : Use of prothrombin complex concentrate for excessive bleeding after cardiac surgery. Interact Cardiovasc Thorac Surg 2012; 15: 382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortmann E, Besser MW, Sharples LD, et al. : An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesth Analg 2015; 121: 26–33. [DOI] [PubMed] [Google Scholar]
- 4.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. : Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol 2017; 34: 332–95. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen B, Spahn DR, Innerhofer P, et al. : Clinical review: Prothrombin complex concentrates--evaluation of safety and thrombogenicity. Crit Care 2011; 15: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen GA, Wolberg AS, Oliver JA, et al. : Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J Thromb Haemost 2004; 2: 402–13. [DOI] [PubMed] [Google Scholar]
- 7.Xi M, Beguin S, Hemker HC: The relative importance of the factors II, VII, IX and X for the prothrombinase activity in plasma of orally anticoagulated patients. Thromb Haemost 1989; 62: 788–91. [PubMed] [Google Scholar]
- 8.Factor IX Complex Profilnine® SD. Grifols Biologicals Inc, Los Angeles, CA 90032, U.S.A, August 2010. (Last accessed. [Google Scholar]
- 9.Kcentra (R) [package insert]. CSL Behring GmbH, Marburg Germany, July 2016. (Last accessed. [Google Scholar]
- 10.Makris M, Watson HG: The management of coumarin-induced over-anticoagulation Annotation. Br J Haematol 2001; 114: 271–80. [DOI] [PubMed] [Google Scholar]
- 11.Goldhammer JE, Bakowitz MJ, Milas BL, et al. : Intracardiac Thrombosis after Emergent Prothrombin Complex Concentrate Administration for Warfarin Reversal. Anesthesiology 2015; 123: 458. [DOI] [PubMed] [Google Scholar]
- 12.Ranucci M, Baryshnikova E, Ranucci M, et al. : Fibrinogen levels compensation of thrombocytopenia-induced bleeding following cardiac surgery. Int J Cardiol 2017; 249: 96–100. [DOI] [PubMed] [Google Scholar]
- 13.Tang M, Fenger-Eriksen C, Wierup P, et al. : Rational and timely haemostatic interventions following cardiac surgery - coagulation factor concentrates or blood bank products. Thromb Res 2017; 154: 73–9. [DOI] [PubMed] [Google Scholar]
- 14.Ghadimi K, Levy JH, Welsby IJ: Perioperative management of the bleeding patient. Br J Anaesth 2016; 117: iii18–iii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma V, Katznelson R, Jerath A, et al. : The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia 2014; 69: 124–30. [DOI] [PubMed] [Google Scholar]
- 16.Solomon C, Rahe-Meyer N, Sorensen B: Fibrin formation is more impaired than thrombin generation and platelets immediately following cardiac surgery. Thromb Res 2011; 128: 277–82. [DOI] [PubMed] [Google Scholar]
- 17.Ortmann E, Rubino A, Altemimi B, et al. : Validation of viscoelastic coagulation tests during cardiopulmonary bypass. J Thromb Haemost 2015; 13: 1207–16. [DOI] [PubMed] [Google Scholar]
- 18.Andersen ND, Bhattacharya SD, Williams JB, et al. : Intraoperative use of low-dose recombinant activated factor VII during thoracic aortic operations. Ann Thorac Surg 2012; 93: 1921–8; discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White R, Rushbrook J, McGoldrick J: The dangers of prothrombin complex concentrate administration after heart surgery. Blood Coagul Fibrinolysis 2008; 19: 609–10. [PubMed] [Google Scholar]
- 20.Kohler M, Hellstern P, Lechler E, et al. : Thromboembolic complications associated with the use of prothrombin complex and factor IX concentrates. Thromb Haemost 1998; 80: 399–402. [PubMed] [Google Scholar]
- 21.Ho KM, Bham E, Pavey W: Incidence of Venous Thromboembolism and Benefits and Risks of Thromboprophylaxis After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2015; 4: e002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung JH, Gikakis N, Rao AK, et al. : Pericardial blood activates the extrinsic coagulation pathway during clinical cardiopulmonary bypass. Circulation 1996; 93: 2014–8. [DOI] [PubMed] [Google Scholar]
- 23.Boisclair MD, Lane DA, Philippou H, et al. : Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood 1993; 82: 3350–7. [PubMed] [Google Scholar]
- 24.Hunt BJ, Parratt RN, Segal HC, et al. : Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann Thorac Surg 1998; 65: 712–8. [DOI] [PubMed] [Google Scholar]
- 25.Ranucci M, Baryshnikova E, Crapelli GB, et al. : Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc 2015; 4: e002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greilich PE, Brouse CF, Beckham J, et al. : Reductions in platelet contractile force correlate with duration of cardiopulmonary bypass and blood loss in patients undergoing cardiac surgery. Thromb Res 2002; 105: 523–9. [DOI] [PubMed] [Google Scholar]
- 27.Wolberg AS, Meng ZH, Monroe DM 3rd, et al. : A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma 2004; 56: 1221–8. [DOI] [PubMed] [Google Scholar]
- 28.Guan XL, Wang XL, Liu YY, et al. : Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann Thorac Surg 2016; 101: 945–51. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka KA, Mazzeffi MA, Grube M, et al. : Three-factor prothrombin complex concentrate and hemostasis after high-risk cardiovascular surgery. Transfusion 2013; 53: 920–1. [DOI] [PubMed] [Google Scholar]
- 30.Cappabianca G, Mariscalco G, Biancari F, et al. : Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Crit Care 2016; 20: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghadimi K, Levy JH, Welsby IJ: Prothrombin Complex Concentrates for Bleeding in the Perioperative Setting. Anesth Analg 2016; 122: 1287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karkouti K, Beattie WS, Arellano R, et al. : Comprehensive Canadian review of the off-label use of recombinant activated factor VII in cardiac surgery. Circulation 2008; 118: 331–8. [DOI] [PubMed] [Google Scholar]
- 33.Lusher JM: Thrombogenicity associated with factor IX complex concentrates. Semin Hematol 1991; 28: 3–5. [PubMed] [Google Scholar]
- 34.Jablow LM, Jones CW, Carroll GG, et al. : Limb-threatening Deep Venous Thrombosis Complicating Warfarin Reversal with Three-factor Prothrombin Complex Concentrate: A Case Report. J Emerg Med 2016; 50: 28–31. [DOI] [PubMed] [Google Scholar]
- 35.Dentali F, Marchesi C, Giorgi Pierfranceschi M, et al. : Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost 2011; 106: 429–38. [DOI] [PubMed] [Google Scholar]
- 36.Song HK, Tibayan FA, Kahl EA, et al. : Safety and efficacy of prothrombin complex concentrates for the treatment of coagulopathy after cardiac surgery. J Thorac Cardiovasc Surg 2014; 147: 1036–40. [DOI] [PubMed] [Google Scholar]
- 37.Kuipers S, Cannegieter SC, Doggen CJ, et al. : Effect of elevated levels of coagulation factors on the risk of venous thrombosis in long-distance travelers. Blood 2009; 113: 2064–9. [DOI] [PubMed] [Google Scholar]
- 38.Allen GA, Hoffman M, Roberts HR, et al. : Manipulation of prothrombin concentration improves response to high-dose factor VIIa in a cell-based model of haemophilia. Br J Haematol 2006; 134: 314–9. [DOI] [PubMed] [Google Scholar]
- 39.Karkouti K, Callum J, Wijeysundera DN, et al. : Point-of-Care Hemostatic Testing in Cardiac Surgery: A Stepped-Wedge Clustered Randomized Controlled Trial. Circulation 2016; 134: 1152–62. [DOI] [PubMed] [Google Scholar]
- 40.Dyke C, Aronson S, Dietrich W, et al. : Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg 2014; 147: 1458–63 e1. [DOI] [PubMed] [Google Scholar]