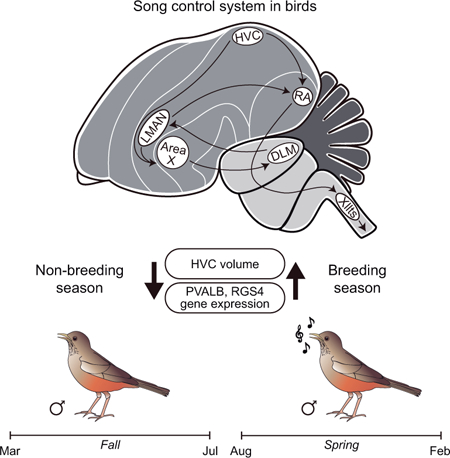
Abstract
In vocal learning birds, memorization and song production rely on a set of telencephalic nuclei referred to as the song control system. Seasonal changes in song production are correlated with changes in the volume of the song control nuclei and are influenced by photoperiodic conditions and hormonal cues. The seasonal volume changes in the avian brain that controls singing are thought to involve regulation of neuronal replacement, which are a striking example of neuronal plasticity. The Rufous-bellied Thrush (Turdus rufiventris) is a seasonally breeding bird that actively sings during the spring and summer (breeding season) and is relatively silent in the fall, yet possible mechanisms behind the periodic changes in song production remain unknown. Here, we have examined two song control nuclei: HVC (used as a proper noun) and RA (robust nucleus of arcopallium) in fall males, spring males and fall females of Rufous-bellied Thrush. The cytoarchitectonic organization was analyzed and quantified from Nissl-stained sections, and gene expression of song nuclei markers was examined by in situ hybridization during breeding and non-breeding seasons. We observed a reduction in HVC volume, and reductions in parvalbumin and RGS4 expression in HVC and RA in males during the non-breeding season. These findings provide evidence of seasonal changes in the song system of a representative tropical-breeding Turdidae species that does not maintain territories or mate bonding, setting the histological and molecular groundwork for future studies aimed at better understanding of song nuclei changes in seasonally breeding songbirds.
Keywords: vocal learning, thrush, pvalb, in situ hybridization, brain, bird
Graphical Abstract
INTRODUCTION
Vocal learning is a trait present in three groups of birds: hummingbirds, parrots and songbirds. Vocal learning and song production rely on a set of specialized brain nuclei known as the song control system, which shares important similarities with vocal control systems in humans. Two main pathways connect the song nuclei and control vocal learning and production in vocal learning birds: the posterior and anterior pathways. The posterior vocal motor pathway is involved in song production and includes two song nuclei: HVC and RA (Figure 1). The anterior pathway, implicated in song learning and plasticity, starts with a projection from HVC to striatal area X and also includes the lateral part of the magnocellular nucleus of the nidopallium (LMAN) (Figure 1) (Nottebohm et al., 1976, Doupe, 1993, Bottjer et al., 1998, Bolhuis et al., 2010).
Figure 1.
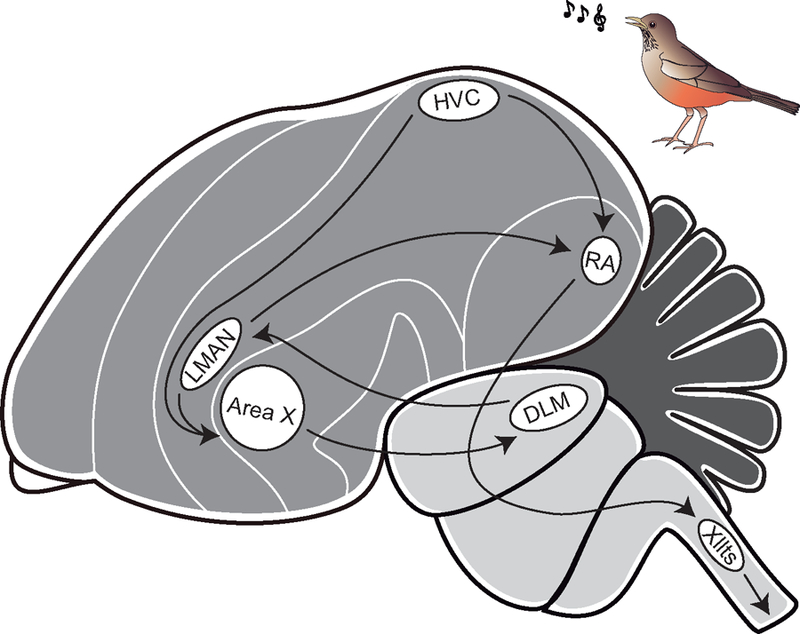
The song control system of the Rufous-bellied Thrush, Turdus rufiventris. Schematic representation of the song control system showing the song control pathways.
Seasonal reproductive behaviors are associated with photoperiodic changes and are triggered by increases in circulating sex steroids such as testosterone (Brenowitz et al., 1991, Buttemer et al., 1991, Tsutsui et al., 1991). Day length is the primary cue for the onset of the breeding season, and its influence on testosterone levels correlates with increases in song nuclei volume and singing behavior (Bernard and Ball, 1997, Smith et al., 1997a, Wennstrom et al., 2001, Riters et al., 2002, Sartor et al., 2005, Boseret et al., 2006, Small et al., 2015). Increases in the size of HVC have been observed during the breeding season in several songbird species, but the mechanisms underlying this change are unclear. Although increased plasma levels of testosterone are tightly associated with increased HVC volume (Nottebohm, 1981), this correlation has not always been observed. For example, castrated males of Spizella arborea show increased HVC and RA volumes in a conditioned long-day environment, despite the lack of high serum levels of testosterone (Bernard et al., 1997). Further studies of seasonal changes of vocal nuclei in seasonal breeders where the photoperiod is less dramatic would be helpful to further clarify these relationships.
The Rufous-bellied Thrush is a tropical thrush and a seasonal singing songbird found in South America. In Brazil, its breeding is generally from August to January (spring and summer). During the breeding season, males defends territories and sing loud, continuous, and varied songs. These songs are substitute by few descending notes used in non-breeding territorial defense (Sick et al., 1997). Its melodious and complex songs are characteristic of the spring season and accompany changes in reproductive behavior. The seasonal control of singing in this species has not yet been studied, thus the mechanisms that govern its singing behavior are largely unknown. Most of the endocrine studies on the relationship between tropical species reveal that they have relatively low levels of circulating steroid hormones, yet are able to show seasonality and will increase aggression if confronted by a conspecific male (Hau et al., 2000). Although we still do not know the hormone profiles of Rufous-bellied Thrush, it represents a possible comparison where there are a number of other closely related species that occur in both the tropics and temperate latitudes. Tropical species, including the species of the present study exhibit similar patterns of seasonality and aggressive behaviors, thus Rufous-bellied Thrush is a very suitable model to understand seasonality in the hormonal status, singing behavior and neurobiology of a tropical bird.
Here we used histology,, and in situ hybridization to examine the song nuclei of wild-captured Rufous-bellied Thrushes during the breeding and non-breeding seasons. Our main focus was the HVC, since this nucleus connects the anterior and posterior pathways and is known to undergo major seasonal morphological changes, based on studies in other seasonal breeders (Nottebohm, Stokes and Leonard, 1976, Brenowitz, Nalls, Wingfield and Kroodsma, 1991, Wingfield et al., 1992, Brenowitz and Larson, 2015). We found that HVC volume changes substantially between the breeding (spring and summer) and non-breeding seasons (fall and winter), and is accompanied by changes in the expression of the markers parvalbumin (PVALB) and RGS4.
MATERIAL AND METHODS
Animals and tissue collection
A total of 10 specimens of Turdus rufiventris were captured at the Instituto de Pesquisas Veterinárias Desidério Finamor (IPVDF) (30°03' S 51°18' W), in Rio Grande do Sul-Brazil. Males and females were collected in two seasons: fall (June and July: non-breeding season) and spring (October: breeding season). Birds were sacrificed by decapitation and the brains were immediately dissected out and flash frozen in Tissue-Tek OCT compound (Sakura) on dry ice and 100% isopropanol. The brains were stored at −80°C and later transferred to the Universidade Federal do Pará (UFPA) on dry ice. This study was approved by the Ethics Committee at the Instituto de Ciências Biológicas – UFPA, and performed in accordance to the special license 20902–1 issued to MPDS by IBAMA/SISBIO.
Riboprobe synthesis
The cDNA clones for PVALB and for the regulator of G-protein signaling 4 (RGS4) were from the zebra finch brain cDNA ESTIMA collection, cloned in psBluescript II KS plasmids. Polymerase chain reaction (PCR) amplification was performed using forward and reverse primers with a T3 overhang (Reverse: 5’ - GTA AAA CGA CGG CCA GTG AG - 3' and Forward: 5’ - ATG ACC ATG ATT ACG CCA AG – 3’) to amplify a fragment containing the cDNA insert and T3 polymerase promoter, using the vector as template. PCR was performed in 50 µL reaction volumes containing 39.2 µL of RNase free water, 1.5 µL of 10 mM MgCl2, 5 µL of 10x buffer, 1 µL of each primer (0.5 µM), 1 µL of dNTP mix (10 mM), 0.3 µL of Taq DNA polymerase, and 1 µL of DNA template. The temperature profile consisted of preheating at 94 °C for 3 min, 32 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 30 s, and extension at 72 °C for 90s, followed by a final extension step at 72 °C for 10 min. Then, antisense riboprobes were synthesized using T3 RNA polymerase (Lide Technologies) and DIG-labeling mix (Roche). The riboprobe reaction (Life Technologies) was performed in 20 µL reaction volumes containing 0.5 µL of RNAse inhibitor, 2 µL of DTT, 2 µL of DIG, 2 µL of 10x reaction buffer, 5 µL of Template, 2 µL of T3 enzyme mix, and 6.5 µL of nuclease-free water.
Brain sectioning and Nissl staining
The brains were cut (20µm) on the parasagittal plane on a Leica CM1850 UV cryostat. Sections were mounted onto colorfrost plus microscope slides (Fisher Scientific), fixed in 3% paraformaldehyde, and stored at −80°C until further use. Selected serial sections were stained in an aqueous solution of 0.1 % cresyl violet. After staining, the slides were dehydrated in absolute ethanol for 2 min and cleared with two washes in xylene for 2 min each. The sections were then cover-slipped using CytosealXYL (Richard-Allan Scientific) mounting medium.
In situ hybridization
In situ hybridization was performed according to a previously established protocol, with modifications (Lovell et al., 2013, Carleton et al., 2014). Serial brain sections from three fall males, four spring males and three fall females were acetylated for 10 min in a solution of 0.33% acetic anhydride in water, rinsed twice with 2x SSPE buffer (0.02 M EDTA and 2.98 M NaCl in 0.2 M phosphate buffer) and dehydrated in 70%, 95% and 100% RNAse-free ethanol. Each section was then hybridized with a solution (16 µl) containing 50% formamide, 2x SSPE, 1 µg/µl BSA, 1 µg/µl poly-A (SIGMA) in DEPC-treated water, and 1 µl of DIG-labeled riboprobe. Slides were cover-slipped, sealed by immersion in mineral oil, and incubated overnight at 62°C. On the following day oil was removed with chloroform and slides were de-coverslipped in 2x SSPE, followed by incubation for 1 hr and 10 min at 62°C in 2x SSPE containing 50% formamide, and then twice in 0.1x SSPE for 30 min at 62°C. Sections were then rinsed briefly in TNT (TN: 100mM Tris, pH 7.5; 150mM NaCl/ TNT: TN + 0.3% Triton-X 100), demarcated using a pap pen (Life Technologies) and blocked for 30 min at RT in 200 µL of TNB buffer (0.1 M Tris, 150 mM NaCl, bovine serum albumin) blocking buffer (200 µl TNB/slide, 1% skim milk) and incubated overnight in TNB with an alkaline phosphatase conjugated anti-DIG antibody (Roche) (TNB + 0,3% skim milk + anti-DIG-AP; 1:600 dil.). Then, the slides were washed twice for 15 min in TMN (100mM Tris, pH 9.5; 150mM NaCl, 0.05M MgCl2), and incubated for 1–3 days in a detection solution containing the alkaline phosphatase substrates Nitro-Blue Tetrazolium Chloride (NBT) (Roche) and 5-Bromo-4-Chloro-3-Indolyl-phosphate p-Toluidine Salt (BCIP) (Roche) in a TMN solution with 1:1 chromogenic proportion. Slides were washed overnight in distilled water to remove salts, fixed in 4% paraformaldehyde, washed twice in distilled water, and cover-slipped with Cytoseal (Thermo Scientific). Slides were imaged using a Nikon SMZ1500 microscope and processed on NIS-Elements D program (v. 4.10.01, Nikon). For qualitative assessment of mRNA expression in brain tissue we compared the neuroanatomical expression of these two selected genes with data retrieved from the ZEBrA database (www.zebrafinchatlas.org).
Statistical analysis and quantification of HVC
The area of HVC from three non-breeding and three breeding males was estimated using ImageJ (Schneider et al., 2012). The border of HVC was outlined in each Nissl-stained section containing HVC (viewed at 40x magnification). The total volume of HVC was estimated by multiplying the outlined HVC area in one brain section by the interval between measured sections (400 µm), and then adding all partial volumes, as in Olson et al. 2011. Student’s t-test (unpaired, parametric, two-tailed test) was performed using GraphPad Prism version 7.05 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. Statistical significance was set at an alpha of 0.05 (Supporting Information Tables S1 and S2).
HVC reconstruction
Images were captured with NIKON-DS Ri1 and NIS elements program and ImageJ (NIH image program) was used to analyze the images. Image files in TIFF format were opened in the stack mode and scaled based on the scale bar in each image (210 px = 0.25mm). HVC was outlined in each section and the area was measured. HVC volume was estimated by calculating the area of each section and multiplying by the distance to the next section. Adobe Illustrator was used to generate serial reconstructions of HVC. Scale bars represent 0.25 mm.
RESULTS
HVC reconstruction reveals seasonal changes in volume
The nuclei comprising the song control system of the Rufous-bellied Thrush show conserved position and organization relative to that seen in canary and zebra finch atlases. To examine seasonal changes in HVC volume, we performed Nissl staining on brain sections of females, males during non-breeding and breeding seasons. Females showed a discrete HVC, consisting of a small nucleus at the expected position in the dorsal-caudal nidopallium, displaying the large cells characteristic of neurons in this nucleus. In males the HVC was significantly larger in males during the breeding season compared to the non-breeding season (Figure 2). Whole-HVC volumetric reconstructions corroborate this trend with a P value of 0.0125 (Supporting Information Tables S1 and S2). In summary, the Rufous-bellied Thrush shows strong seasonal plasticity of HVC, a forebrain structure associated with seasonal singing behaviors in temperate species.
Figure 2.
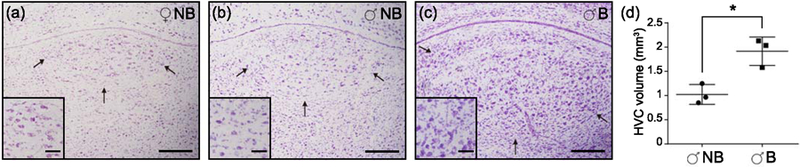
The song control nucleus HVC of Turdus rufiventris. Nissl stained brain sections of T. rufiventris showing the HVC nuclei of female (a), and male during non-breeding (b) and breeding (c) seasons. The average volumes of the HVC were compared between non-breeding and breeding males. A single asterisk denotes a significant difference (P value of 0.0125) between designated groups. Error bars represent the standard error of the mean (d). Arrows denote the HVC nuclei. Scale bars are 0.2mm (a-c) and 0.05mm on close-up squares at the bottom left of each image.
PVALB and RGS4 expression in the HVC and RA
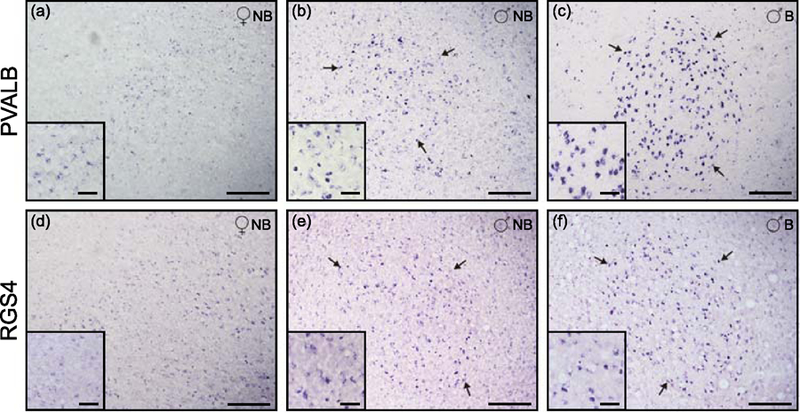
The reduced volume of HVC in males during the non-breeding season that was observed by Nissl staining led us to investigate the expression pattern of PVALB and RGS4, two genes that are robust markers of several song nuclei in other songbird species, particularly of HVC in zebra finches (Mello et al., 1995, Mello, 2004, Lovell et al., 2008). These confirmed molecular markers allowed for a detailed examination of this nucleus in serial sections, thus providing a strong evidence to identify these brains structures in this thrush. In females, expression for both markers was very low or undetected (Figures 3a and 3d). In males during the breeding season the increase in HVC volume is accompanied by an increased expression of these two markers within the HVC area (Figures 3b–c and 3e–f).
Figure 3.
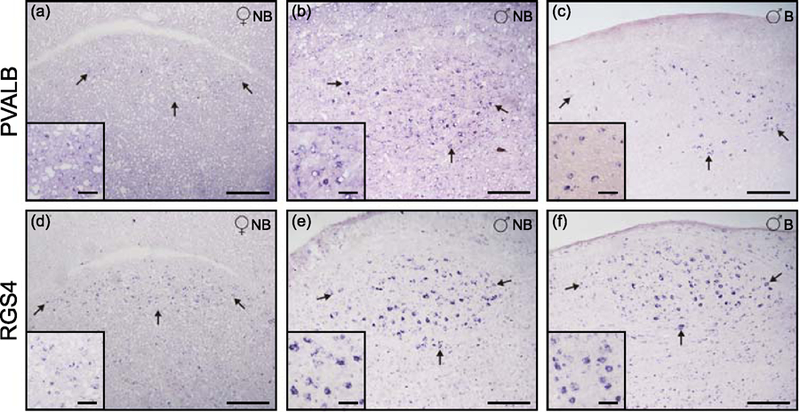
Expression pattern of the HVC song nuclei markers PVALB and RGS4. HVC of female (a,d), and males in non-breeding (b,e) and breeding (c,f) seasons. Arrows highlight the HVC nuclei. Scale bars are 0.25mm (a-f) and 0.05mm on close-up squares at the bottom left of each image.
We also examined RGS4 and PVALB expression in RA nucleus, where we observed less cells expressing these markers from the non-breeding season (Figures 4b and 4e), and more cells are expressing RGS4 and PVALB in males during breeding season (Figures 4c and 4f). Cells expressing these genes were not observed in females (Figures 4a and 4d). Despite the apparent seasonal difference in gene expression in males, the cross-sectional area of the RA was very similar between males collected during the breeding and non-breeding seasons.
Figure 4.
Expression pattern of the RA song nuclei markers PVALB and RGS4. RA nuclei within the arcopallium of female (a,d), and males in non-breeding (b,e) and breeding (a,f) seasons. Arrows indicate the RA nuclei. Scale bars are 0.2mm (a-c) and 0.05mm on close-up squares at the bottom left of each image.
DISCUSSION
In this study, we report the cytoarchitectonic organization of the song control system of a tropical new-world Turdus thrush. While this species is representative of low-latitude neotropical forest ecosystems (Sick et al., 1997), it is also a member of a large genus with a cosmopolitan distribution, including thrush species that breed in arctic ecosystems as wells as those that are island endemics (Gill and Donsker, 2018). The reproductive biology of this species, and perhaps the vocal neuroanatomy, is potentially influenced by selective forces characteristic of tropical ecosystems, yet it is a migratory thrush within the tropics in a genus with a global representation. In addition to representing the first brain examination of this exceptional singer, it adds to the few studies that examine the song system or brain of a member of the Turdidae family (Iwaniuk et al., 2006, de Lima et al., 2015). Our Nissl-stained sections demonstrated seasonal plasticity of song nucleus HVC, namely a significant decline in volume in males from the breeding to the non-breeding season. HVC nucleus reconstruction analysis provided further confirmation of difference in volume between males in both seasons. The seasonal and sex differences in song circuit neuroanatomy are consentient with patterns of seasonal and sex-specific singing behavior.
Previous studies have linked increased HVC volume during the breeding season to high levels of testosterone in the blood (Nottebohm 1981), and indeed many populations of songbirds have been shown to have vocal nuclei associated with seasonal fluctuations in steroid levels. However tropical songbirds generally lack the surges in circulating testosterone that are associated with reproductive behaviors such as territory maintenance (Hau et al., 2000, Hau et al., 2004), or the surges are disassociated from the onset of song. One putative mechanism to stimulate increases in vocal nuclei of equatorial species is to increase the androgen sensitivity of cells within the vocal nuclei by the up-regulation of androgen receptors (Quispe et al. 2016, Frankl-Vilches and Gahr 2017), thus allowing for the development and maintenance of song year-round, the dissociation of singing behavior from territoriality, and possibly even between sexes, allowing both males and females to sing. Furthermore, the relative contributions of hormonal signal versus tissue-level sensitivity may vary greatly among species, and depend in part on other hormone-dependent behaviors that make up the species life-history strategy. Although we cannot directly infer the physiological mechanism underlying the difference in HVC volume we observed in the Rufous-bellied Thrush, its lack of territorial behavior in the non-breeding season, and male-only singing behavior suggest that these observed changes in HVC volume may be due more to increases in hormone signal rather than sensitivity, similar to what we know occurs in high-latitude songbirds. Furthermore, the small HVC volumes found in females, which do not sing, suggests that male sex hormones (ergo the signal) are directly responsible for the increased HVC volume in this species.
Using in situ hybridization, we analyzed the expression of two song nuclei markers: RGS4 and PVALB. RGS4 is an intracellular regulator of G protein signaling known to control the expression of NMDA-receptors in response to elevated glutamate signaling (Saugstad et al., 1998) and previously found to be expressed in the forebrain of songbirds with high enrichment in HVC and RA (Lovell et., al., 2008). PVALB is also a marker of these song system nuclei (Braun et al., 1991) and plays a critical role in neuroprotection. PVALB is a marker of vocal circuitry in male but not female zebra finches, undergoes developmental increases during song learning (Olson et al. 2011) and its expression is positively related to the amount of singing that occur (Zengin-Toktas and Wooley 2017). We found enriched mRNA expression of RGS4 and PVALB within the HVC and RA in breeding males compared to non-breeding males, showing that the seasonal effects on the vocal circuit differ in a molecular-functional basis as well as in volume. Although we do not have singing data for the thrushes in this study, the reduction of PVALB during the non-breeding season suggests a reduced neuroprotective capacity in the vocal circuitry, and reduced RGS4 suggests a reduced degree of glutamate signaling in the vocal circuit during the non-breeding season associated with less singing behavior. Similarly, females had low expression of these markers within HVC and RA.
CONCLUSIONS
This work provides a seasonal study of the structural and molecular changes in song control nuclei in T. rufiventris, demonstrating significant plasticity of the brain nuclei in males between the breeding and non breeding seasons. More specifically, we have shown that gene expression in song nuclei changes between these periods, and two song nuclei, HVC and RA are significantly reduced during the non-breeding season of this tropical species. We suggest that this reduction in vocal brain structures is related to the lack of territorial behavior and the increase in migratory or semi-nomadic behaviors during the non-breeding season during which song is not exhibited.
Supplementary Material
Supplementary Figure 1. Reconstruction of HVC in parasagittal section series based on Nissl stained sections. The drawings on the left depict brain areas shown on the right for female (a), and males during non-breeding (b) and breeding (c) seasons. The medial-to-lateral progression values are shown below the drawings for each section. Scale bars are 0.25mm.
ACKNOWLEDGEMENTS
The authors want to thank Igor Schneider for critical input on the manuscript, Fabricio Angioletti and Marcio Repenning for assistance in collecting specimens, and Peter Lovell for preparing and providing the cDNAs from the ESTIMA collection. This work was funded by the Brazilian Avian Genome Consortium (CNPq/FAPESPA-SISBIO Aves), and benefitted from funding for the Mello lab through an R24 grant from the NIH/NIGMS.
Grant sponsor:
Brazilian Avian Genome Consortium (CNPq/FAPESPA-SISBIO Aves), Federal University of Para, Belem, Para, Brazil
Footnotes
COMPETING INTEREST
The authors declare that they have no competing interest.
REFERENCES
- Bernard DJ, Ball GF. 1997. Photoperiodic condition modulates the effects of testosterone on song control nuclei volumes in male European starlings. Gen Comp Endocrinol 105:276–283. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Wilson FE, Ball GF. 1997. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea). Brain Res 760:163–169. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. 2010. Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci 11:747–759. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. 2006. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria). J Neurobiol 66:1044–1060. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Brady JD, Walsh JP. 1998. Intrinsic and synaptic properties of neurons in the vocal-control nucleus IMAN from in vitro slice preparations of juvenile and adult zebra finches. J Neurobiol 37:642–658. [PubMed] [Google Scholar]
- Braun K, Scheich H, Heizmann CW, Hunziker W. 1991. Parvalbumin and calbindin-D28K immunoreactivity as developmental markers of auditory and vocal motor nuclei of the zebra finch. Neuroscience 40:853–869. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Larson TA. 2015. Neurogenesis in the adult avian song-control system. Cold Spring Harb Perspect Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Nalls B, Wingfield JC, Kroodsma DE. 1991. Seasonal changes in avian song nuclei without seasonal changes in song repertoire. J Neurosci 11:1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttemer WA, Astheimer LB, Wingfield JC. 1991. The effect of corticosterone on standard metabolic rates of small passerine birds. J Comp Physiol B 161:427–431. [DOI] [PubMed] [Google Scholar]
- Carleton JB, Lovell PV, McHugh A, Marzulla T, Horback KL, Mello CV. 2014. An optimized protocol for high-throughput in situ hybridization of zebra finch brain. Cold Spring Harb Protoc 2014:pdb prot084582. [DOI] [PMC free article] [PubMed]
- Gill F, Donsker D (Eds). 2018. IOC World Bird List (v8.2) 10.14344/IOC.ML.8.2. [DOI]
- Hau M, Stoddard ST, Soma KK. 2004. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm and Behav 45:40–49. [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Soma KK, Wingfield JC. 2000. Testosterone and year-round territorial aggression in a tropical bird. Gen Comp Endocrinol 117:20–33. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Koperski DT, Cheng KM, Elliott JE, Smith LK, Wilson LK, Wylie DRW. 2006. The effects of environmental exposure to DDT on the brain of a songbird: Changes in structures associated with mating and song. Behav Brain Res 173:1–10. [DOI] [PubMed] [Google Scholar]
- de Lima JL, Soares FA, Remedios AC, Thom G, Wirthlin M, Aleixo A, Schneider MP, Mello CV, Schneider PN. 2015. A putative RA-like region in the brain of the scale-backed antbird, Willisornis poecilinotus (Furnariides, Suboscines, Passeriformes, Thamnophilidae). Genet Mol Biol 38:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell PV, Carleton JB, Mello CV. 2013. Genomics analysis of potassium channel genes in songbirds reveals molecular specializations of brain circuits for the maintenance and production of learned vocalizations. BMC Genomics 14:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell PV, Clayton DF, Replogle KL, Mello CV. 2008. Birdsong “transcriptomics”: neurochemical specializations of the oscine song system. PLoS One 3:e3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. 1995. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J Neurosci 15:6919–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV. 2004. Gene regulation by song in the auditory telencephalon of songbirds. Front Biosci 9:63–73. [DOI] [PubMed] [Google Scholar]
- Nottebohm F 1981. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science 214:1368–1370. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. 1976. Central control of song in the canary, Serinus canarius. J Comp Neurol 165:457–486. [DOI] [PubMed] [Google Scholar]
- Olson CR, Rodrigues PV, Jeong JK, Prahl DJ, Mello CV. 2011. Organization and development of zebra finch HVC and paraHVC based on expression of zRalDH, an enzyme associated with retinoic acid production. J Comp Neurol 519:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. 2002. Seasonal changes in the densities of alpha(2) noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol 444:63–74. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. 2005. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria). Horm Behav 47:467–476. [DOI] [PubMed] [Google Scholar]
- Robertson G, et al. 2010. De novo assembly and analysis of RNA-seq data, Nat. Methods, vol. 7: 909–912. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. 1998. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J Neurosci 18:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick H, Haffer J, Alvarenga HF, Pacheco JF, Barruel P. 1997. Ornitologia Brasileira Editora Nova Fronteira, Rio de Janeiro [In Portuguese] ISBN: 85-209-0816-0. [Google Scholar]
- Small TW, Brenowitz EA, Wojtenek W, Moore IT. 2015. Testosterone Mediates Seasonal Growth of the Song Control Nuclei in a Tropical Bird. Brain Behav Evol 86:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. 1997a. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci 17:6001–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Wingfield JC, Bottjer SW. 1991. Adenohypophysectomy in the zebra finch. Gen Comp Endocrinol 81:163–173. [DOI] [PubMed] [Google Scholar]
- Wennstrom KL, Reeves BJ, Brenowitz EA. 2001. Testosterone treatment increases the metabolic capacity of adult avian song control nuclei. J Neurobiol 48:256–264. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Lewis DM. 1992. Hormonal responses to removal of a breeding male in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Horm Behav 26:145–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Reconstruction of HVC in parasagittal section series based on Nissl stained sections. The drawings on the left depict brain areas shown on the right for female (a), and males during non-breeding (b) and breeding (c) seasons. The medial-to-lateral progression values are shown below the drawings for each section. Scale bars are 0.25mm.


