Abstract
Benign breast disease (BBD) is an established breast cancer (BC) risk factor, but it is unclear whether the magnitude of the association applies to women at familial or genetic risk. This information is needed to improve BC risk assessment in clinical settings. Using the Prospective Family Study Cohort, we used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of BBD with BC risk. We also examined whether the association with BBD differed by underlying familial risk profile (FRP), calculated using absolute risk estimates from the Breast Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) model. During 176,756 person-years of follow-up (median: 10.9 years, maximum: 23.7) of 17,154 women unaffected with BC at baseline, we observed 968 incident cases of BC. A total of 4,704 (27%) women reported a history of BBD diagnosis at baseline. A history of BBD was associated with a greater risk of BC: HR = 1.31 (95% CI: 1.14–1.50), and did not differ by underlying FRP, with HRs of 1.35 (95% CI: 1.11–1.65), 1.26 (95% CI:1.00–1.60), and 1.40 (95% CI: 1.01–1.93), for categories of full-lifetime BOADICEA score <20%, 20 to <35%, ≥35%, respectively. There was no difference in the association for women with BRCA1 mutations (HR: 1.64; 95% CI: 1.04–2.58), women with BRCA2 mutations (HR: 1.34; 95% CI: 0.78–2.3) or for women without a known BRCA1 or BRCA2 mutation (HR: 1.31; 95% CI: 1.13–1.53) (pinteraction = 0.95). Women with a history of BBD have an increased risk of BC that is independent of, and multiplies, their underlying familial and genetic risk.
Keywords: breast cancer, benign breast disease, familial risk, Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm
Introduction
An estimated 1.6 million breast biopsies occur annually in the United States, and 75% of these return a diagnosis of benign breast disease (BBD).1,2 Approximately 30% of breast cancers diagnosed occur in women with a history of BBD.2 BBD falls generally into three broad histologic groups: nonproliferative disease (NP), proliferative disease without atypia (PDWA), and proliferative disease with atypical hyperplasia (AH)3 with estimated frequencies of approximately 66%, 30%, and 4%, respectively.4 The increase in breast cancer risk after BBD varies by histologic group and is highest for AH.5–12 A recent metaanalysis confirmed that AH and PDWA are associated with increased breast cancer risk, but that NP, the most common histologic group, is not significantly associated with risk.12
Whether the risk associated with BBD varies by breast cancer family history (BCFH) is less clear. With the exception of three previous reports by Dupont and Page of a higher risk for women with a first-degree family history and AH compared to women who had AH but no BCFH,3,13,14 other studies that have examined the association of BBD with breast cancer risk by BCFH have not found statistically significant interactions between BCFH and AH.4,8,15–18 The evidence for other BBD types is limited and less clear, with two studies reporting no BCFH interaction with PDWA,8,15 but the Mayo Clinic Study reported a 62% increased breast cancer risk in women with NP lesions and a strong BCFH (defined as having at least one first-degree relative with breast cancer before the age of 50 years or having two or more relatives with breast cancer).4 With the exception of the Mayo Clinic Study,4,16 these previous studies of BBD have usually assessed BCFH as yes/no.3,8,13–15,17,18 Additionally, some previous studies had BCFH information for only a subset of study participants, resulting in small numbers of women having both BBD and a positive BCFH.
Given the limited data on whether the risk of BBD varies by BCFH, we examined whether BBD was associated with breast cancer risk for women across the spectrum of underlying predicted absolute familial risk, using a prospective cohort enriched for women at familial or genetic risk. We examined whether having any BBD, the majority of which is NP and associated with low risk of breast cancer for the general population, is associated with breast cancer risk for women at high predicted absolute risk based on BCFH.
Materials and Methods
Study sample
The Prospective Family Study Cohort (ProF-SC)19 includes the Breast Cancer Family Registry (BCFR),20 and the Kathleen Cuningham Foundation Consortium for research into Familial Breast cancer Follow-Up Project (kConFab).21,22 All probands and their family members were followed prospectively from baseline for cancer and other health outcomes. We confirmed reported breast cancer diagnosis through pathology reports or cancer registry linkages for 81% of incident cases. For the BCFR, systematic follow-ups were conducted 10 years and 15 years after the first round of recruitment to the BCFR, while the kConFab participants have been followed-up every 3 years. Screening for germline BRCA1 and BRCA2 mutations was conducted by the BCFR and kConFab, as previously described.20,23,24 Ethics approval for the six sites of the BCFR and for kConFab was granted by the applicable human research ethics committees at the participating institutions. All participants in the BCFR and kConFab provided written informed consent before participation.
At baseline, there were 18,856 women unaffected with breast cancer and eligible for our study.19 We applied the following additional eligibility criteria sequentially: no bilateral risk-reducing mastectomy (n = 18,722, 99.3%); at least 2 months of follow-up (either actively participating in follow-up themselves or having a family member update their cancer and vital status) (n = 18,235, 96.7%); age at baseline between 18 and 79 years (n = 17,741, 94.1%); sufficient pedigree data to allow calculation of a full-lifetime breast cancer risk score using the Breast Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) (n = 17,646, 93.6%); and answered the question about BBD in the baseline questionnaire (n = 17,154, 91% from 6,842 families).
Baseline data
Using the same core questionnaire at baseline, the BCFR and kConFab captured participants’ demographic characteristics, education, race/ethnicity, height and weight, menstrual and reproductive history including age at menarche, parity, breastfeeding, age at first birth, age at menopause, hormonal birth control use, menopausal hormone therapy (HT), history of screening mammography, personal medical history including previous breast and ovarian surgeries, and behavioral/lifestyle factors such as cigarette smoking and alcohol consumption. Participants also completed a family history questionnaire that captured their personal history of breast and other cancers, as well as cancers in their first-degree and second-degree relatives. The cohort consisted of the family probands and their relatives, and all were studied using the same protocols and questionnaires.
Definition of BBD
The baseline questionnaire asked about any previous diagnosis BBD and the age at first BBD diagnosis. We classified women as having BBD if they answered “yes” to the question: “Has a doctor ever told you that you had a benign breast disease, such as a non-cancerous cyst or breast lump?.” We reviewed pathology reports for a subset of study participants at the New York BCFR site and found high (93.5%) agreement with self-reported BBD. Given the window of vulnerability of the breast to carcinogenic influences before tissue differentiation driven by the first pregnancy, we also considered BBD relative to the timing of the first pregnancy. We determined if BBD was first diagnosed before the first pregnancy by subtracting age at first birth from age at first BBD for parous women.
Familial risk profile
For each participant, we calculated both the 1-year risk of invasive breast cancer and the lifetime risk (risk from birth to age 80 years) from multigenerational pedigree data on breast and ovarian cancer in relatives using the BOADICEA.25,26
Statistical methods
We used Cox proportional hazard regression models with age as the time scale to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for breast cancer associated with familial risk profile (FRP) and BBD. To examine the association of BBD with breast cancer for younger women, we censored follow-up time at age 45 years. We evaluated BBD as a binary (yes, no) variable and relative to the timing of the first pregnancy. Additionally, we evaluated time since first BBD diagnosis as a time-dependent variable, categorized as: no BBD (reference), 0–5 years, 6–10 years, 11–20 years, and ≥21 years. For all analyses, the referent group was women who did not report a personal history of BBD.
We calculated person-years from 2 months after baseline questionnaire completion to the diagnosis of breast cancer or the earliest of the following events: risk-reducing mastectomy, death, age 80 years, or loss to follow-up. We used a robust variance estimator to account for the family structure of the cohort. We incorporated left-truncation in all models to avoid potential survivor bias. All models were stratified by birth cohort in 10-year categories and adjusted for race/ethnicity and study center. We considered the following variables as potential confounders: age at baseline, body mass index, education, age at menarche, parity, breastfeeding, age at first birth, hormonal birth control use, HT use, screening mammography, menopausal status, cigarette smoking, and alcohol consumption. Any variable that brought about at least a 10% change in the BBD parameter estimate was retained in the model as a potential confounder. We also considered history of tamoxifen use as a potential confounder for the subset of women who had completed the follow-up questionnaire that asked about tamoxifen use, but inclusion of tamoxifen did not alter the association of BBD and breast cancer risk. We estimated multiplicative interactions between BBD and both full-lifetime and 1-year BOADICEA score using the Wald test. To address the potential impact of age on 1-year BOADICEA score, we utilized a residual method where the 1-year BOADICEA was regressed on baseline age as a quadratic and the residuals used in subsequent models. As the same inference was found when using the residual method and the 1-year BOADICEA score, we only present models using the 1-year risk score.
We plotted the predicted age-specific absolute cumulative risk for women with different familial risks based on BOADICEA and underlying age-specific incidences from the Surveillance, Epidemiology, and End Results Program.27–30 We performed sensitivity analyses by including only those with confirmed invasive breast cancer based on pathology reports (81% of all cases were confirmed invasive) as cases, as well as including only women with available information on tamoxifen use. We assessed the proportional hazards assumption by evaluating Schoenfeld residuals. All statistical tests were two sided and p-values <0.05 where considered statistically significant. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Results
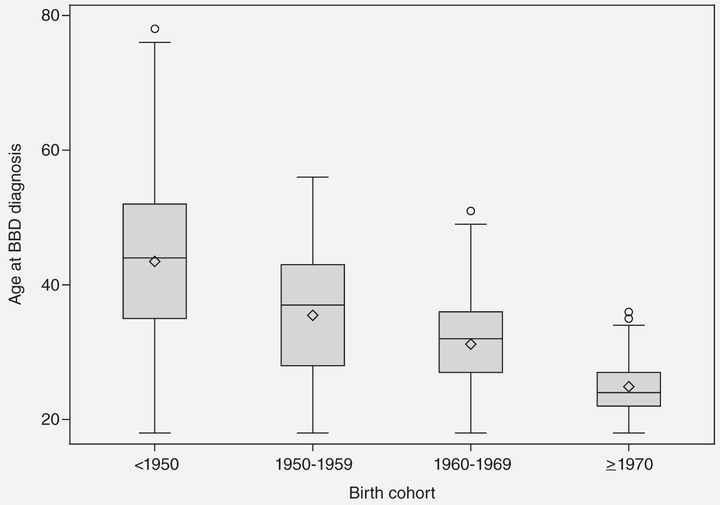
During 176,756 person-years of follow-up (median 10.9 years, maximum 23.7 years), 968 incident cases of invasive or in situ breast cancer were reported at an average age at diagnosis of 55.8 years and average age at enrollment into the cohort of 46.8 years. The baseline characteristics of the unaffected cohort by BBD status are summarized in Table 1. Of all women included in our study, 27% (n = 4,704) reported having a previous diagnosis of BBD and, for these, the self-reported average age at BBD diagnosis was 37.9 years. The age at first BBD diagnosis decreased by birth cohort (Fig. 1). The median time from BBD diagnosis to diagnosis of breast cancer was 17.7 years.
Table 1.
Characteristics of women according to personal history of benign breast disease (BBD)1 status in the Breast Cancer Prospective Family Study Cohort (ProF-SC)
| Characteristic at baseline | BBD (n = 4,704) | No BBD (n = 12,450) | p-value2 |
|---|---|---|---|
| Mean values (SD) | |||
| Age at baseline (years) | 49.80 (12.80) | 45.70 (15.80) | <0.0001 |
| Body mass index (kg/m2) | 25.70 (5.60) | 25.90 (5.70) | 0.07 |
| Age at menarche (years) | 12.80 (1.60) | 12.90 (1.60) | 0.001 |
| Age at first BBD diagnosis (years) | 37.9 (12) | NA | |
| Age at breast cancer diagnosis (years) | 57.40 (11.40) | 54.90 (12.70) | 0.002 |
| BOADICEA full-lifetime score (%) | 23.80 (16.20) | 23.30 (16.70) | <0.0001 |
| BOADICEA 1-year risk score (%) | 0.57 (0.70) | 0.43 (0.59) | <0.0001 |
| N (%) | |||
| BBD relative to pregnancy (in parous women) | |||
| BBD before first pregnancy | 585 (15.6) | NA | |
| BBD after first pregnancy | 3,177 (84.5) | NA | |
| Race/ethnicity | <0.0001 | ||
| Non-Hispanic white | 3,968 (84.4) | 9,772 (78.5) | |
| Non-Hispanic black | 163 (3.5) | 639 (5.1) | |
| Hispanic | 293 (6.2) | 1,129 (9.1) | |
| Asian | 123 (2.6) | 532 (4.3) | |
| Other | 148 (3.2) | 319 (2.6) | |
| Missing | 9 (0.2) | 59 (0.5) | |
| Education | 0.003 | ||
| High school graduate or less | 1,478 (31.4) | 4,225 (33.9) | |
| Some college/vocational school | 1,818 (38.7) | 4,720 (37.9) | |
| Bachelor’s/graduate degree | 1,393 (29.6) | 3,444 (27.7) | |
| Missing | 15 (0.3) | 61 (0.5) | |
| Parity (number of full-term pregnancies)/breastfeeding history | <0.0001 | ||
| Nulliparous | 808 (17.2) | 3,286 (26.4) | |
| Parous 1-2/no breastfeeding | 637 (13.5) | 1,372 (11.0) | |
| Parous 1-2/breastfed | 1,406 (29.9) | 3,205 (25.7) | |
| Parous 3+/no breastfeeding | 395 (8.4) | 988 (7.9) | |
| Parous 3+/breastfed | 1,444 (30.7) | 3,514 (28.2) | |
| Missing | 14 (0.3) | 85 (0.7) | |
| Hormonal birth control use | <0.0001 | ||
| Never | 1,024 (21.8) | 3,127 (25.1) | |
| Former | 3,296 (70.1) | 7,323 (58.8) | |
| Current | 370 (7.9) | 1,937 (15.6) | |
| Missing | 14 (0.3) | 63 (0.5) | |
| Menopausal status | |||
| Premenopausal | 2,132 (45.3) | 7,025 (56.4) | <0.0001 |
| Postmenopausal | 2,159 (45.9) | 4,726 (38.0) | |
| Missing | 413 (8.8) | 699 (5.6) | |
| Hormone therapy use | <0.0001 | ||
| Never | 3,141 (66.8) | 9,799 (78.7) | |
| Former | 710 (15.1) | 1,235 (9.9) | |
| Current | 754 (16.0) | 1,228 (9.9) | |
| Missing | 99 (2.1) | 188 (1.5) | |
| Cigarette smoking history3 | <0.0001 | ||
| Nonsmoker | 2,542 (54.0) | 7,419 (59.6) | |
| Former smoker | 1,345 (28.6) | 2,932 (23.6) | |
| Current smoker | 607 (12.9) | 1,712 (13.8) | |
| Missing | 210 (4.5) | 387 (3.1) | |
| Alcohol consumption4 | 0.002 | ||
| Non-regular drinker | 2,273 (48.3) | 6,372 (51.2) | |
| Former drinker | 711 (15.1) | 1,832 (14.7) | |
| Current drinker | 1,707 (36.3) | 4,189 (33.7) | |
| Missing | 13 (0.3) | 57 (0.5) | |
| Ever had a mammogram | <0.0001 | ||
| Yes | 4,334 (92.1) | 8,227 (66.1) | |
| No | 364 (7.7) | 4,200 (33.7) | |
| Missing | 6 (0.1) | 23 (0.2) | |
| BRCA1/2 mutation carriers | |||
| BRCA1/2 carrier | 302 (6.42) | 906 (7.28) | 0.05 |
| Noncarrier or not tested | 4,402 (93.58) | 11,544 (92.72) |
Women were classified as having BBD if they answered “yes” to the question:“Has a doctor ever told you that you had a benign breast disease, such as a noncancerous cyst or breast lump?.”
p-values from Wilcoxon–Mann–Whitney test, t-test, or χ2 test where appropriate.
Smokers are defined as having smoked at least one cigarette per day for 3 months or longer.
Regular drinkers are defined as consuming one alcoholic beverage at least once a week for 6 months or longer.
Figure 1.
Age at BBD diagnosis by birth cohort.
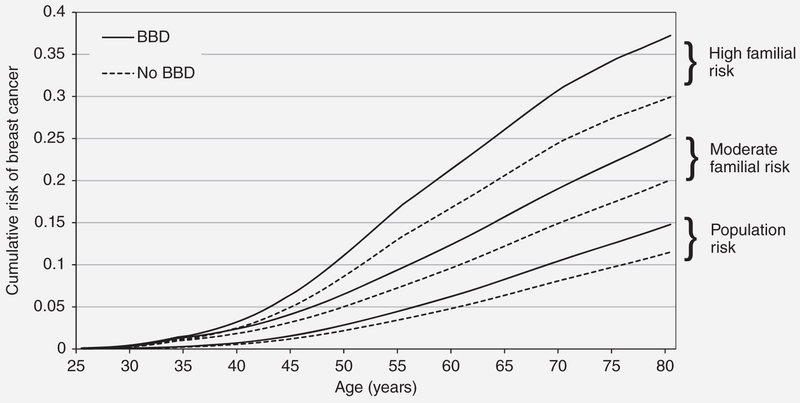
Table 2 presents results from the Cox proportional hazards analysis of BBD. We found an approximately 30% increased risk of breast cancer associated with a reported history of BBD (HR: 1.31, 95% CI: 1.14–1.5), but no evidence of a multiplicative interaction for either measure of FRP (1-year risk p-value: 0.34; lifetime risk p-value: 0.51). Although there was no evidence of statistically significant multiplicative interaction, Figure 2 illustrates the implications of the present study for predicted age-specific cumulative risks of breast cancer. In terms of absolute risk, the risk difference when comparing women with a history of BBD with those without is greater for women with higher underlying familial risk. For example, for cumulative risk to age 80 years, the risk difference was 7.3% for women with high familial risk (lifetime risk >30%) compared to 3.3% for women at general population risk (lifetime risk ~12%).
Table 2.
Adjusted hazard ratios (HR) and 95% confidence intervals (CI) for breast cancer risk and history of benign breast disease (BBD)
| Person-years | Number of breast cancers1 | Adjusted model2 |
|
|---|---|---|---|
| HR (95% CI) | |||
| Benign breast disease (BBD) | |||
| No BBD | 128,062.0 | 592 | Reference |
| Yes BBD | 48,694.5 | 370 | 1.31 (1.14–1.50) |
| Benign breast disease (BBD) and pregnancy | |||
| No BBD, nulliparous | 34,242.6 | 116 | Reference |
| BBD before first pregnancy | 6,152.0 | 42 | 1.34 (0.93–1.94) |
| BBD in nulliparous | 8,005.7 | 55 | 1.49 (1.07–2.06) |
| BBD after first pregnancy | 32,532.6 | 256 | 1.33 (1.04–1.69) |
| No BBD, parous | 93,797.1 | 481 | 1.02 (0.82–1.27) |
| Recency of benign breast disease (BBD) | |||
| No BBD | 116,882.8 | 539 | Reference |
| 0–5 years | 642.8 | 29 | 1.31 (0.89–1.91) |
| 6–10 years | 3,142.0 | 47 | 1.12 (0.83–1.52) |
| 11–20 years | 17,013.9 | 104 | 1.30 (1.05–1.61) |
| ≥21 years | 21,697.5 | 125 | 1.35 (1.10–1.66) |
Abbreviations: HR, hazard ratio; CI, confidence interval.
The total number of breast cancer cases do not add up to total due to missing information on covariates.
All models are adjusted for full-lifetime BOADICEA risk score, race/ethnicity, and study site and stratified by birth cohort. BBD models are also adjusted for screening mammography, recency of BBD models are also adjusted for age at baseline.
Figure 2.
Cumulative risk of breast cancer by history of benign breast disease and underlying familial risk profile. Predicted age-specific cumulative risk (from birth) of breast cancer, by history of benign breast disease and familial risk at baseline, where 12% lifetime risk is approximately the population risk of breast cancer by age 80 years, where moderate familial risk (>20% full-lifetime BOADICEA) is equivalent to having one affected first-degree relative, and high familial risk is equivalent to having two affected first-degree relatives (>30% BOADICE).
For women with a reported history of BBD, 17.2% were nulliparous, 12.9% had BBD before their first pregnancy, while the remainder had BBD after their first pregnancy (69.9%). We found similar associations for BBD regardless of when the BBD was first diagnosed relative to the timing of first pregnancy (BBD before pregnancy HR: 1.34, BBD after pregnancy HR: 1.33, Table 2). The risk associated with BBD was slightly higher for nulliparous, compared to parous women (HR for nulliparous: 1.49; 95% CI: 1.07–2.06).
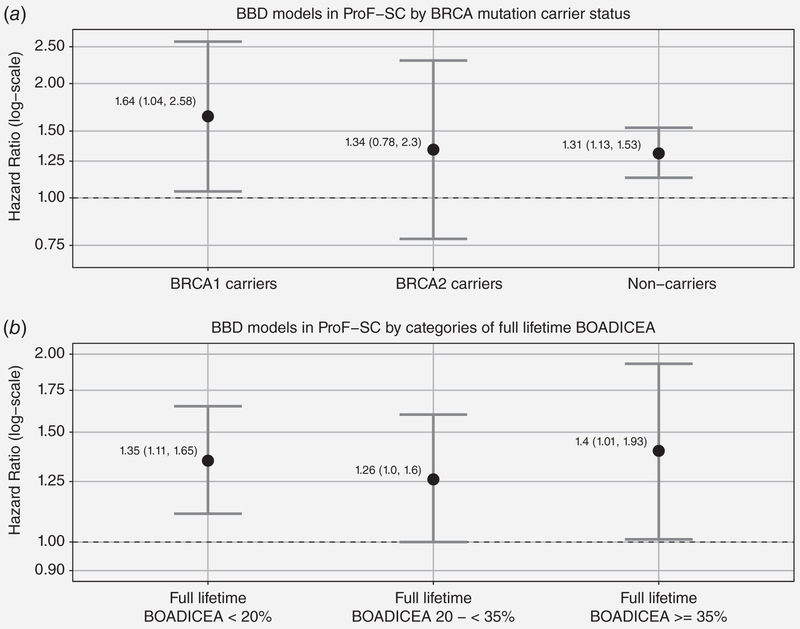
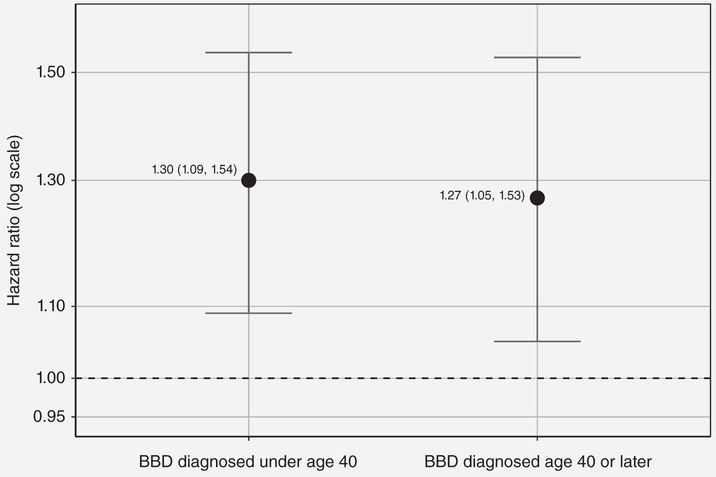
When we stratified the analyses by BRCA1 and BRCA2 mutation carrier status (Fig. 3, panel A), we found a 31% increased breast cancer risk associated with BBD for women not known to be carriers (either true negative or not tested) (HR: 1.31, 95% CI: 1.13–1.53). For BRCA1 mutation carriers, BBD was associated with a 64% increased risk (HR: 1.64, 95% CI: 1.04–2.58), while for BRCA2 mutation carriers, BBD was associated with a 34% increased risk that was not statistically significant (HR: 1.34, 95% CI: 0.78–2.3). There was no difference in the association for women with BRCA1 mutations (HR: 1.64; 95% CI: 1.04–2.58), women with BRCA2 mutations (HR: 1.34; 95% CI: 0.78–2.3), or for women without a known BRCA1 or BRCA2 mutation (HR: 1.31; 95% CI: 1.13–1.53) (p-value for interaction: 0.95). BBD was associated with an increased risk for women in each category of FRP based on fulllifetime BOADICEA (Fig. 3, panel B). Figure 4 shows that we found similar BBD associations of ~30% increased risk of breast cancer regardless of the age BBD was first diagnosed (over or under age 40 years). An increased risk of breast cancer associated with BBD was also found from the sensitivity analyses which (i) excluded nonpathologically confirmed breast cancers and ductal carcinoma in situ cases, (ii) excluded women with a personal history of other cancers at baseline, and (iii) adjusted for tamoxifen (data not shown).
Figure 3.
BBD models by BRCA mutation carrier status and underlying FRP in ProF-SC unaffected cohort.
Figure 4.
BBD models by age at BBD diagnosis in ProF-SC unaffected cohort.
Discussion
By analyzing this large, prospective cohort of women enriched for family history of breast cancer or carrying a known mutation in a major breast cancer susceptibility gene, we detected an increased risk of breast cancer associated with having a personal history of BBD, consistent with previous epidemiological studies of women unselected for familial or genetic risk.4,6,11,12,15,17,18,31 We found that the increased risk associated with having a history of BBD did not vary by extent of family history or BRCA1 or BRCA2 mutation status. This finding is consistent with previous reports of no interaction with BCFH and BBD,4,8,15–18 suggesting that the more limited statistical power for examining BCFH at the higher end of the risk spectrum was not the main reason for a lack of interaction with BCFH reported by previous studies.
The lack of multiplicative interaction means that, on an absolute risk scale, women with a greater underlying FRP will have a higher absolute risk from BBD as the underlying absolute risk increases based on extent of family history. This is critically important, as it may affect clinical recommendations at an individual level. For example, the predicted cumulative risk of breast cancer to age 80 years based on our HR estimates is 14.8% for women with BBD at average population risk (12% lifetime risk) and increases to 37.2% for women with both BBD and high familial risk (30% lifetime risk) (Fig. 2). Considering both factors could mean the difference between recommendations for early and intensified screening and/or initiation of chemoprevention.
The average age at first BBD diagnosis in our study (37.9 years) was lower than previously reported ages at BBD biopsy that ranged from 42.5 to 57.8 years4,15,18 but those studies included women from older birth cohorts than ours. As seen in Figure 1, BBD was diagnosed at earlier ages in the younger birth cohorts, and the mean age of BBD diagnosis in our older birth cohort (<1,950) was 43.5 years, which is similar to previous reports. While we did not have information on BBD histologic subtypes, studies have reported that increasing ages at BBD correlate with increasing aggressiveness of the BBD subtypes; with nonproliferative lesions typically being reported earlier (ages 42.5–49.9 years) than AH (age 49.4–57.8 years).4,15,18
Our study found that the increased risk associated with BBD remained for several decades (Table 2). This is consistent with reports from the Mayo Clinic cohort of an elevated risk of breast cancer for at least 25 years after the initial biopsy.4 Chemoprevention with selective estrogen receptor modulators and aromatase inhibitors has not been optimally implemented for women with BBD, and one of the cited barriers to implementation has been inaccurate estimation of breast cancer risk, particularly underestimation of risk.32,33 This is despite clear effectiveness of chemoprevention in reducing breast cancer risk in two different trials of women with different histological BBD subtypes.32 In our study, tamoxifen was not a confounder in a sensitivity analysis that included women for whom we had information on prior tamoxifen use.
Breast cancer risk varies by BBD subtype, and a limitation of our study is that we did not confirm self-reported BBD with pathology review to determine subtypes. Previous studies have reported high agreement between self-reported BBD and biopsy-confirmed BBD.34 While we found no evidence of multiplicative interaction with FRP and BBD, we cannot rule out potential effect modification by FRP and specific BBD subtype. DuPont and Page reported a significant interaction between BCFH and AH.3,13 Some other studies have also suggested a possible interaction with BBD subtypes and BCFH from stratified analyses by BCFH, although formal test of multiplicative interactions were not statistically significant.4,8,15,17,18 For example, a nested case–control study of over 1,200 women with BBD subtype information combined all proliferative lesions into one category due to the small number of women with AH and a BCFH (n = 13 from 192 women with a positive BCFH).18 Our study found no significant overall interaction (p = 0.2), but a twofold increase in breast cancer risk for women with any proliferative disease and no BCFH (OR = 2.00, 95% CI: 1.42–2.80), and no association in women with any proliferative disease and a BCFH (OR = 1.05, 95% CI: 0.69–1.61). Compared to women without a first-degree BCFH, women with BCFH were estimated to have an increased breast cancer risk for all histological subtypes of BBD (NP, proliferative disease, and PDWA), except for AH. Despite the major limitation of our study not having BBD histology, we consider our results to be important to clinical risk validation models because most large cohorts and cohorts that have served for risk model validation have only self-reported BBD and/or have simply used prior biopsy as an indicator of higher risk.
Additionally, while most risk models include both BBD and BCFH as independent risk factors, most previous studies have had limited statistical power to formally evaluate whether these factors interact. If these major drivers of breast cancer risk prediction were synergistic, that would support changing the way they are considered in risk models. The most widely used risk prediction model, Breast Cancer Risk Assessment Tool, does incorporate information on number of biopsies and whether it was AH, but does not include extensive information on family history such as ages at diagnosis and total number of affected relatives (it asks only if there are one or more than one affected relative). In our study, we found no evidence that women with BRCA1 or BRCA2 mutations nor women at very high familial risk (having two or more affected first-degree relatives) differed in their increased risk associated with BBD than did women without a familial or genetic risk. Risk prediction can be enhanced with more detailed pathological subtype information, as illustrated by Frank et al.35 Even in the absence of multiplicative interactions, relative risk factors will always have a larger absolute impact on those at the higher end of the familial risk spectrum (as illustrated in Fig. 2).
Strengths of our study were the comprehensive definition of family history that incorporates multigenerational pedigree information and ages at diagnosis of the relatives, extending beyond the conventional binary variable, which enabled us to classify women across a wide range of familial and genetic risk. This heterogeneity of the cohort with respect to FRP allowed us to evaluate associations for women across a wide spectrum of risk. Additionally, the family design enriched for familial risk of breast cancer provided us with a well-powered study and a relatively large number of prospective breast cancer cases, for which a large proportion (81%) were pathologically confirmed. Moreover, the extensive epidemiological information on breast cancer risk factors considered for confounding and the prospective design reduced the effect of bias.
In conclusion, our large prospective family-based cohort study found that BBD is associated with an increased risk of breast cancer that is independent of, and multiplies, women’s underlying familial and genetic risk. For example, the difference in cumulative incidence of breast cancer to age 80 years for women who had BBD compared to those without BBD is 7.3% if they have a high underlying familial risk (30% lifetime risk) compared to 3.3% for those at average population risk (12% lifetime risk). Therefore, a diagnosis of BBD has a larger impact on changing absolute risk of breast cancer in women with a higher underlying familial risk than in women without.
What’s new?
Benign breast disease (BBD) is an established breast cancer (BC) risk factor, but it is unclear whether the magnitude of the association applies to women at familial or genetic risk. To find out, the authors used a family-based prospective cohort of women enriched for breast cancer family history (BCFH) to examine the association of BBD with BC risk. They observed a 30% increased BC risk associated with BBD, independent of BCFH. Previous studies have been underpowered to examine this interaction with extent of family history; this study confirms the importance of both BBD and BCFH when performing clinical risk assessment.
Acknowledgements
We thank Heather Thorne, Eveline Niedermayr, Lucy Stanhope, Yasmin Rady, Sandra Picken, all the BCFR and kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the many families who contribute to the BCFR and kConFab for their contributions to this resource.
Grant sponsor: Australian National Breast Cancer Foundation; Grant numbers: IF 17 kConFab, PRAC-17-004; Grant sponsor: Cancer Australia; Grant number: 809195; Grant sponsor: National Cancer Institute, NIH; Grant numbers: T32-CA009529, UM1 CA164920 ; Grant sponsor: National Center for Advancing Translational Sciences; Grant number: TL1 TR001875; Grant sponsor: National Health and Medical Research Council; Grant numbers: 145684, 288704, 454508 ; Grant sponsor: National Institute of Health USA; Grant number: 1RO1CA159868
Abbreviations:
- AH
atypical hyperplasia
- BBD
benign breast disease
- BC
breast cancer
- BCFH
breast cancer family history
- BCFR
Breast Cancer Family Registry
- BOADICEA
Breast Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm
- CI
confidence interval
- FRP
familial risk profile
- HR
hazard ratio
- HT
hormone therapy
- kConFab
Kathleen Cuningham Foundation Consortium for research into Familial Breast cancer
- NP
nonproliferative disease
- PDWA
proliferative disease without atypical hyperplasia
- ProF-SC
Prospective Family Study Cohort
Footnotes
Conflict of interest
Dr. Dite receives funding from Genetic Technologies Ltd. to undertake work on breast cancer risk that is not related to this study. Dr. Friedlander serves on an advisory board and receives honoraria at Astra Zeneca and MSD, has received lecture fees from Astra Zeneca, and serves as a noncompensated consultant for AbbVie. The other authors have no conflicts of interests to disclose.
References
- 1.Silverstein M Where’s the outrage? J Am Coll Surg 2009;208:78–9. [DOI] [PubMed] [Google Scholar]
- 2.Visscher DW, Frost MH, Hartmann LC, et al. Clinicopathologic features of breast cancers that develop in women with previous benign breast disease. Cancer 2016;122:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985;312:146–51. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005;353:229–37. [DOI] [PubMed] [Google Scholar]
- 5.Bodian CA, Perzin KH, Lattes R, et al. Prognostic significance of benign proliferative breast disease. Cancer 1993;71:3896–907. [DOI] [PubMed] [Google Scholar]
- 6.Carter CL, Corle DK, Micozzi MS, et al. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol 1988;128:467–77. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JT, Hartmann LC, Vierkant RA, et al. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am J Surg Pathol 2006;30:665–72. [DOI] [PubMed] [Google Scholar]
- 8.McDivitt RW, Stevensm JA, Lee NC, et al. Histologic types of benign breast disease and the risk for breast cancer. Cancer 1992;69:1408–14. [DOI] [PubMed] [Google Scholar]
- 9.Minami Y, Ohuchi N, Taeda Y, et al. Risk of breast cancer in Japanese women with benign breast disease. Jpn J Cancer Res 1999;90:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaaban AM, Sloane JP, West CR, et al. Histopathologic types of benign breast lesions and the risk of breast cancer: case-control study. Am J Surg Pathol 2002;26:421–30. [DOI] [PubMed] [Google Scholar]
- 11.Dupont WD, Page DL. Breast cancer risk associated with proliferative disease, age at first birth, and a family history of breast cancer. Am J Epidemiol 1987;125:769–79. [DOI] [PubMed] [Google Scholar]
- 12.Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat 2015;149:569–75. [DOI] [PubMed] [Google Scholar]
- 13.Dupont WD, Parl FF, Hartmann WH, et al. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 1993;71:1258–65. [DOI] [PubMed] [Google Scholar]
- 14.Page DL, Dupont WD, Rogers LW, et al. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 1985;55:2698–708. [DOI] [PubMed] [Google Scholar]
- 15.Collins LC, Baer HJ, Tamimi RM, et al. The influence of family history on breast cancer risk in women with biopsy-confirmed benign breast disease. Cancer 2006;107:1240–7. [DOI] [PubMed] [Google Scholar]
- 16.Degnim AC, Visscher DW, Berman HK, et al. Stratification of breast cancer risk in women with atypia:a Mayo cohort study. J Clin Oncol 2007;25:2671–7. [DOI] [PubMed] [Google Scholar]
- 17.London SJ, Connolly JL, Schnitt SJ, et al. A prospective study of benign breast disease and the risk of breast cancer [Erratum appears in JAMA 1992 Apr 1;267(13):1780]. JAMA 1992;267:941–4. [PubMed] [Google Scholar]
- 18.Kabat GC, Jones JG, Olson N, et al. A multicenter prospective cohort study of benign breast disease and risk of subsequent breast cancer. Cancer Causes Control 2010;21:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry MB, Phillips K-A, Daly MB, et al. Cohort profile: the breast cancer prospective family study cohort (ProF-SC). Int J Epidemiol 2015;45:683–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John EM, Hopper JL, Beck JC, et al. The breast cancer family registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 2004;6:R375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne RHR. kConFab: a research resource of Australasian breast cancer families. Kathleen Cuningham foundation consortium for research into familial breast cancer. Med J Aust 2000;172:463–4. [DOI] [PubMed] [Google Scholar]
- 22.Phillips K-A. Predictors of participation in clinical and psychosocial follow-up of the kConFab breast cancer family cohort. Fam Cancer 2005;4:105–13. [DOI] [PubMed] [Google Scholar]
- 23.Mann GJ, Thorne H, Balleine RL, et al. Analysis of cancer risk and BRCA1 and BRCA2 mutation prevalence in the kConFab familial breast cancer resource. Breast Cancer Res 2006;8:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhausen SL, Ozcelik H, Southey MC, et al. BRCA1 and BRCA2 mutation carriers in the breast cancer family registry: an open resource for collaborative research. Breast Cancer Res Treat 2009;116:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 2008;98:1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 2004;91:1580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 13 Regs Research Data, Nov 2011 Sub (1992-2009) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.Sed., 2011.
- 28.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 18 Regs Research Data, Nov 2011 Sub (2000-2009) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.Sed., 2011.
- 29.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Research Data, Nov 2011 Sub (1973-2009) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.Sed., 2011.
- 30.Lee AJ, Cunningham AP, Kuchenbaecker KB, et al. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer 2014;110:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J 2007;13:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuzick J, Sestak I, Thorat MA. Impact of preventive therapy on the risk of breast cancer among women with benign breast disease. Breast 2015;24 (Supplement 2):S51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann LC, Degnim AC, Santen RJ, et al. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015;372:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su X, Colditz GA, Willett WC, et al. Genetic variation and circulating levels of IGF-I and IGFBP-3 in relation to risk of proliferative benign breast disease. Int J Cancer 2010;126:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank RD, Winham SJ, Vierkant RA, et al. Evaluation of 2 breast cancer risk models in a benign breast disease cohort. Cancer 2018;124:3319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]