Abstract
Several biological mechanisms linking physical activity with cancer have been proposed. However, the influence of specific components of physical activity (volume, type and intensity), and their interaction with adiposity and diet, on cancer-related biomarkers remain unclear. We used cross-sectional data on 7,219 men in the Health Professionals Follow-up Study (1992–1994) with C-reactive protein (CRP), interleukin 6 (IL6), tumor necrosis factor α receptor 2 (TNFαR2), adiponectin, C-peptide and triglycerides/high-density lipoprotein cholesterol ratio (TG/HDL). Details on physical activity, diet and adiposity were assessed by questionnaires. We used multivariable-adjusted linear regression analyses to estimate relative concentrations of biomarkers by physical activity. Total physical activity was favorably associated with all biomarkers in a fairly linear manner. Comparing the highest (63+ metabolic equivalent (MET)-h/week) to the lowest (0 to 9 MET-h/week) physical activity groups, the percent relative difference in concentration of biomarkers was −31% for CRP, −22% for IL6, −8% for TNFαR2, +9% for adiponectin, −22% for C-peptide, and −20% for TG/HDL. These differences were modestly attenuated after adjustment for adiposity. For the same total MET-hours of physical activity, the association was stronger for men engaging in both aerobic and resistance exercises compared to those engaging in aerobic only. However, no difference was found between those engaging in vigorous activities (≥20% of total MET-hours) compared to those who did smaller amount of vigorous activities. Physical activity showed similar associations for these biomarkers regardless of adiposity and dietary pattern. In conclusion, high physical activity, preferably aerobic plus resistance training, was associated with favorable cancer-related biomarkers.
Keywords: Physical activity, diet, adiposity, inflammation, insulin, interaction
Introduction
Deciphering the role of physical activity on cancer prevention is of high interest.1 Currently, convincing evidence from epidemiological studies supports the association between physical activity and cancers of the colon and breast, and possibly endometrium.2–4 Recently, findings from a pooled data from 12 prospective cohort studies including 1.44 million participants suggested a protective effect of physical activity on 13 types of cancers.5 Most of these cancers (esophageal adenocarcinoma, gallbladder, liver, kidney, small intestine, gastric cardia, endometrial, myeloid leukemia, myeloma, colon, rectum, breast, and non-hodgkin lymphoma) are known to be associated with higher body fatness,6, 7 which is the major candidate for mediating the association between physical activity and cancer risk.1, 8, 9 To explore the biological mechanisms of these associations is important to better understand effects of physical activity on cancer risk.9
Several biological mechanisms linking physical activity and cancer have been proposed. High physical activity is associated with long-term weight management and control10 and lower visceral adiposity,11 which may reduce levels of several metabolic and endocrine biomarkers involved in carcinogenesis.1, 7, 8, 12, 13 For instance, adiposity is associated with insulin resistance and elevated fasting insulin, which stimulate cell proliferation and inhibit apoptosis.7, 12–14 Obesity-associated inflammation may also promote tumorigenesis and disease progression.7, 12–14 Of note, fasting biomarkers of inflammation (e.g., C-reactive protein (CRP), interleukin-6 (IL6), adiponectin, tumor necrosis factor alpha receptor 2 (TNFαR2)) and insulin response (e.g., C-peptide, triglycerides/high-density lipoprotein cholesterol ratio (TG/HDL)) have been associated with higher risk of several cancers including colorectal, pancreatic and endometrial cancers in prospective studies.15–22
Some studies suggest that physical activity may also have direct effects on biomarkers of inflammation and insulin response, regardless of reductions in body fatness.8, 23–25 It is particularly important to determine the amount of total physical activity associated with a healthy biomarker profile, both mediated through and independent of body fatness. In addition, the importance of type and intensity of physical activity on these biomarkers remains unclear.9 Therefore, evaluating the associations of different types (aerobic vs. resistance) and intensities of physical activity (moderate vs. vigorous) with biomarkers, as well as interactions with other lifestyle risk factors, is also important to provide evidence for implementing physical activity interventions at the individual and population level.
In this study, we investigated the associations of different types and intensities of physical activity with biomarkers of inflammatory and insulin response. We also investigated whether the associations between physical activity and biomarkers of inflammatory and insulin response differed according to adiposity and dietary pattern.
Methods
Study population
The Health Professionals Follow-up Study (HPFS) is an ongoing prospective U.S. cohort which was initiated in 1986 with the enrollment of 51,529 middle-aged (40 to 75 years) male health professionals. Details of the cohort are described elsewhere.26 Briefly, participants completed questionnaires on demographic, medical, lifestyle, and other health-related information at enrollment and updated every two years. Diet was assessed using validated food frequency questionnaires at enrollment and updated every four years. The follow-up rate for the cohort exceeded over 90%.
Between 1993 and 1994, blood samples were collected from 18,225 men who were free of major diseases including cardiovascular disease, cancer, and diabetes. A blood kit was sent to each volunteered cohort member and the samples were returned to the lab in EDTA tubes via overnight courier. Details of the procedures for blood collection, handling, and storage have been previously described.27 In the current study, we included participants who were previously selected for nested case-control studies within the HPFS that measured plasma biomarkers of CRP, IL6, TNFαR2, adiponectin, C-peptide, TG, and HDL. A total of 7,219 participants who provided valid physical activity data and blood samples were included in the final analysis.
This study was approved by the Institutional Review Board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of the Harvard T.H. Chan School of Public Health.
Assessment of physical activity
Physical activity was assessed by questionnaires in 1986 and every 2 years thereafter. In each cycle, participants reported their average time spent per week in walking, jogging, running, bicycling, swimming, tennis, squash/racket ball, calisthenics/rowing, and outdoor work. In 1990 and every 2 years, participants were asked to report their average weekly amount of weight lifting/weight machine. Each activity was assigned a metabolic equivalent (MET) which represent metabolic rates for specific activity divided by metabolic rates at rest.28 All activities were summed to derive total physical activity in units of MET-hours per week. By intensity of physical activity, vigorous activities, defined as MET ≥6, were calculated by summing MET hours of activities including jogging, running, bicycling, swimming, tennis, squash/racquetball, and calisthenics/rowing. Moderate activities, defined as MET <6, included walking, heavy outdoor work, and weight lifting/weight machine. By type of physical activity, aerobic activities were calculated by including walking, stair climbing, jogging, running, bicycling, swimming, tennis, squash/racquetball, calisthenics/rowing, and heavy outdoor work.29 Resistance training included weight lifting/weight machine.30, 31 Details of the physical activity questionnaire can be found in the Supporting Information. The validity and reproducibility of the physical activity questionnaire with four 1-week activity diaries across different seasons has been previously described.32
Biomarker assessment
We assessed CRP, IL6, TNFαR2, and adiponectin as markers of inflammation. Moreover, C-peptide and TG/HDL were assessed as markers of hyperinsulinemia and insulin resistance, respectively.
The laboratory procedures were described in detail previously.33, 34 Briefly, CRP was measured by a high sensitivity immunoturbidimetric assay (Denka Seiken Co, Tokyo, Japan). IL6 and TNFαR2 were measured by enzyme-linked immunosorbent assays (R&D systems, Minneapolis, MN). Adiponectin was measured with the use of a competitive radioimmunoassay (Linco Research, St. Charles, MO). C-peptide was measured using ELISA (Diagnostic Systems Laboratories/Beckman Coulter). TG and HDL were measured using standard methods with the use of reagents from Roche Diagnostics (Indianapolis, IN) and Genzyme (Cambridge, MA). The mean intra-assay coefficients of variation were <10% for all assays.
Assessment of covariables
Self-reported questionnaires were used to collect medical and lifestyle information in 1986 and every 2 years thereafter. We calculated BMI using self-reported height and weight. Smoking status and regular aspirin/NSAID use (≥2 standard tablets of aspirin (325 mg) or NSAID per week) were collected as well. Chronic disease comorbidity score was calculated by summing the number of prevalent diseases and conditions including hypercholesterolemia, high blood pressure, diabetes, heart disease, cancer, rheumatoid/other arthritis. For this analysis, we used 1992 and 1994 questionnaires for these variables (data collected closest to blood draw). Diet was assessed using a validated semiquantitative food frequency questionnaire in 1986 and every 4 years. Participants reported their dietary intake (>130 food items) in the previous year. Using the 1994 food frequency questionnaire, we calculated three dietary pattern scores which were developed to capture the inflammatory or insulin potential of the diet: empirical dietary inflammatory pattern (EDIP), empirical dietary index for hyperinsulinemia (EDIH), and empirical dietary index for insulin resistance (EDIR). The development and validation of these dietary patterns have been previously described.35, 36 Distinct from the biennial questionnaires, participants were asked to report their waist circumference using a provided tape following the same instruction in 1987 and 1996. Lastly, we calculated predicted fat mass and percent fat using previously developed anthropometric prediction equations based on age, race, height, weight, and waist circumference. These equations were previously validated using dual-energy x-ray absorptiometry and obesity-related biomarkers in an independent dataset.37
Statistical analyses
The distribution of biomarkers was tested for normality and then natural log transformed. Descriptive statistics for continuous variable were presented as means and standard deviations, and categorical variables were presented as proportions according to total physical activity categories.
Generalized linear models were used to examine the association between physical activity and biomarker concentrations. We recalibrated all biomarkers using the method previously described by Rosner et al.38 to account for variation in sample handling and laboratory drift between batches. To reduce measurement errors in physical activity, we used average of physical activity questionnaires collected in 1992 and 1994. Total physical activity was categorized into 5 groups (i.e., 0 to 8.9, 9 to 20.9, 21 to 41.9, 42 to 62.9, and 63+ MET-h/week). The cut-offs were based on multiples of 3 MET-hours per week, which corresponds to 1 hour per week of normal walking or approximately 25 minutes per week of running for easier interpretation.31 All multivariable models adjusted for the potential confounders including age at blood draw (continuous, years), race (white or non-white), case-control status, smoking status (never, former or current), regular aspirin/NSAID use (yes or no), chronic diseases/conditions (0, 1, 2 or 3+) and dietary pattern (quintiles). Of note, we adjusted for EDIP score for biomarkers of inflammation (i.e., CRP, IL6, TNFαR2, adiponectin), EDIH score for a biomarker of hyperinsulinemia (i.e., C-peptide), and EDIR score for a biomarker of insulin resistance (i.e., TG/HDL). Since adiposity is likely a potential mediator between physical activity and biomarkers, we additionally ran a model further adjusting for BMI (18.5–24.9, 25–29.9 or ≥30 kg/m2). For a sensitivity analysis, we adjusted for waist circumference, predicted fat mass or percent fat, instead of BMI, to better adjust for adiposity. To explore whether the associations between physical activity and biomarkers vary by dietary pattern and/or adiposity, we conducted stratified analyses by dietary pattern (below or above median; healthy or poor dietary pattern) and/or BMI (below or above median; 25.4 kg/m2). We tested for interaction by including the cross-terms of physical activity (continuous) and stratification variables (binary).
For the same total MET-hours of physical activity, the association between physical activity and biomarkers may be different by type and intensity of activities. Thus, we examined the joint association of total physical activity and type (aerobic only vs. aerobic plus resistance training (i.e., resistance training, no vs. yes)) and intensity (moderate plus minimal vigorous activities (<20% of total MET-hours; median of 2 minutes per week) vs. moderate plus moderate vigorous activities (≥20% of total MET-hours; median of 149 minutes per week)) in relation to plasma biomarkers. To examine the independent association of type (aerobic vs. resistance training) and intensity (moderate vs. vigorous activity) of physical activity, we further conducted analyses mutually adjusting for each other.
All tests were two-sided and P<0.05 was considered to be statistically significant. All data analyses were performed using SAS software, version 9.4 for UNIX (SAS Institute, Inc).
Results
The characteristics of study population according to total physical activity are presented in Table 1. Participants with higher physical activity had lower BMI and healthier diets (i.e., lower inflammatory and insulinemic potential). The lowest EDIP, EDIH, and EDIR were found in the second highest physical activity group (42–62.9 MET-h/week). The proportions of current smokers and participants with 3 or more chronic disease/condition were lower with higher physical activity.
Table 1.
Characteristics of the study population by categories of total physical activity, Health Professionals Follow-up Study 1992–1994
| Characteristic | Total physical activity (MET-h/week) | ||||
|---|---|---|---|---|---|
| 0 to <9 | 9 to <21 | 21 to <42 | 42 to <63 | 63+ | |
| Participants, N | 1178 | 1603 | 2132 | 1101 | 1205 |
| Age at 1994 questionnaire return, yrs | 62.0 (8.8) | 61.4 (8.7) | 62.2 (8.8) | 61.8 (8.5) | 63.0 (8.5) |
| Plasma biomarkers | |||||
| C-reactive protein (mg/L) | 1.2 (4.5) | 0.9 (3.0) | 0.9 (3.0) | 0.7 (2.7) | 0.7 (3.0) |
| Interleukin-6 (pg/mL) | 1.6 (2.2) | 1.5 (2.2) | 1.3 (2.0) | 1.2 (5.5) | 1.2 (2.0) |
| Tumor necrosis factor alpha receptor 2 (ng/mL) | 2.7 (1.3) | 2.7 (1.3) | 2.7 (1.3) | 2.7 (1.3) | 2.7 (1.2) |
| Adiponectin (μg/mL) | 6.0 (1.6) | 6.0 (1.6) | 6.0 (1.6) | 6.7 (1.6) | 6.7 (1.6) |
| C-peptide (ng/mL) | 2.7 (1.8) | 2.2 (1.8) | 2.2 (1.8) | 2.0 (1.8) | 2.0 (1.8) |
| Triglyceride/High-density lipoprotein cholesterol ratio | 3.3 (2.2) | 3.0 (2.0) | 2.7 (2.0) | 2.5 (2.0) | 2.5 (2.2) |
| Lifestyle risk factors | |||||
| Body mass index (kg/m2) | 26.9 (4.1) | 26.2 (3.4) | 25.7 (3.2) | 25.4 (2.9) | 25.2 (2.9) |
| Empirical dietary Inflammatory pattern (% above median) |
23.9 | 20.5 | 18.9 | 17.7 | 19.8 |
| Empirical dietary index for hyperinsulinemia (% above median) |
23.0 | 20.6 | 19.0 | 17.8 | 20.3 |
| Empirical dietary index for insulin resistance (% above median) |
20.4 | 20.0 | 20.0 | 19.4 | 20.3 |
| Smoking status (%) | |||||
| Never | 39.3 | 45.0 | 47.0 | 48.3 | 47.5 |
| Former | 49.8 | 49.0 | 48.3 | 47.4 | 48.3 |
| Current | 11.0 | 6.0 | 4.7 | 4.3 | 4.2 |
| Regular aspirin/NSAID user (%) | 14.0 | 14.9 | 13.0 | 13.9 | 14.8 |
| Chronic diseases/conditions comorbidity score | |||||
| No chronic diseases/conditions | 36.3 | 38.5 | 42.5 | 45.1 | 44.2 |
| 1 chronic diseases/conditions | 31.5 | 33.3 | 31.6 | 31.3 | 31.5 |
| 2 chronic diseases/conditions | 19.3 | 18.0 | 16.4 | 15.9 | 16.6 |
| ≥3 chronic diseases/conditions | 13.0 | 10.2 | 9.5 | 7.7 | 7.7 |
Values are presented as mean (SD) for continuous variables and percentage for categorical variables.
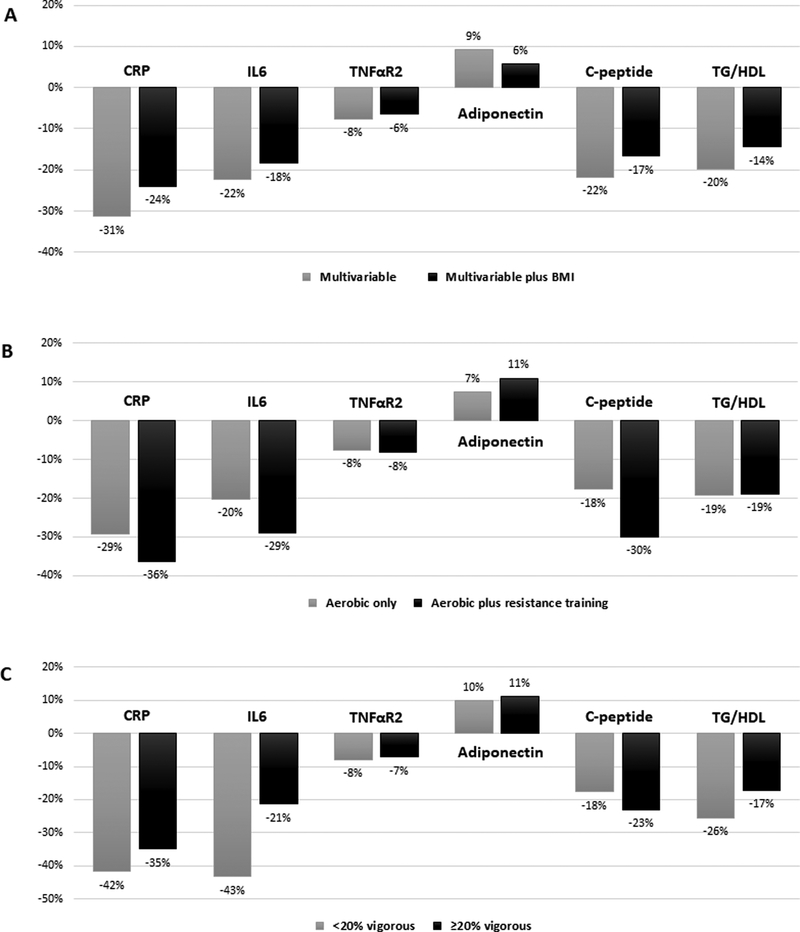
There was a decreasing trend of CRP, IL6, TNFαR2, C-peptide and TG/HDL (all Ptrend<0.001) and an increasing trend of adiponectin with higher total physical activity level (Ptrend=0.002) (Table 2). We observed better biomarker profiles among those in the highest (63+ MET-h/week) compared to lowest (0 to 9 MET-h/week) physical activity groups, though these differences were modestly attenuated after adjustment for BMI. The multivariable-adjusted percent relative difference (RD) in concentration of biomarkers unadjusted and adjusted for BMI, respectively were −31% and −24% for CRP, −22% and −18% for IL6, −8% and −6% for TNFαR2, +9% and +6% for adiponectin, −22% and −17% for C-peptide, and −20% and −14% for TG/HDL (Figure 1). Adjusting for adiposity using waist circumference, predicted fat mass or percent fat, instead of BMI, showed similar but slightly larger attenuations of the associations for all biomarkers (Supporting Information Table S1).
Table 2.
Association between total physical activity and plasma biomarkers of inflammation and insulin response, Health Professionals Follow-up Study 1992–1994
| Total physical activity (MET-h/week) | ||||||
|---|---|---|---|---|---|---|
| 0 to <9 | 9 to <21 | 21 to <42 | 42 to <63 | 63+ | Ptrend‡ | |
| C-reactive protein (mg/L) | ||||||
| Number of participants | 802 | 1033 | 1319 | 673 | 742 | |
| Multivariable† | 1.12 (1.04, 1.21) | 0.95 (0.88, 1.01)* | 0.91 (0.86, 0.97)* | 0.75 (0.69, 0.82)* | 0.77 (0.71, 0.83)* | <0.001 |
| Interleukin-6 (pg/mL) | ||||||
| Number of participants | 462 | 597 | 796 | 407 | 441 | |
| Multivariable† | 1.61 (1.48, 1.75) | 1.46 (1.36, 1.57)* | 1.31 (1.23, 1.40)* | 1.20 (1.10, 1.31)* | 1.25 (1.14, 1.36)* | <0.001 |
| Tumor necrosis factor alpha receptor 2 (ng/mL) | ||||||
| Number of participants | 647 | 808 | 1065 | 521 | 561 | |
| Multivariable† | 2.78 (2.73, 2.84) | 2.68 (2.63, 2.73)* | 2.71 (2.66, 2.75) | 2.64 (2.58, 2.70)* | 2.57 (2.52, 2.63)* | <0.001 |
| Adiponectin (μg/mL) | ||||||
| Number of participants | 705 | 882 | 1098 | 555 | 607 | |
| Multivariable† | 5.91 (5.71, 6.13) | 6.17 (5.98, 6.36)* | 6.26 (6.09, 6.44)* | 6.43 (6.18, 6.68)* | 6.46 (6.22, 6.71)* | 0.002 |
| C-peptide (ng/mL) | ||||||
| Number of participants | 540 | 745 | 1052 | 564 | 602 | |
| Multivariable† | 2.58 (2.46, 2.71) | 2.32 (2.22, 2.42)* | 2.16 (2.08, 2.24)* | 2.10 (2.00, 2.20)* | 2.02 (1.93, 2.12)* | <0.001 |
| Triglyceride/High-density lipoprotein cholesterol ratio | ||||||
| Number of participants | 572 | 723 | 916 | 471 | 484 | |
| Multivariable† | 3.04 (2.86, 3.22) | 2.84 (2.69, 2.99)* | 2.67 (2.54, 2.79)* | 2.44 (2.29, 2.61)* | 2.44 (2.28, 2.60)* | <0.001 |
Values are multivariable-adjusted absolute biomarker concentrations adjusted for: age at blood draw (continuous), smoking (never, past or current smokers), race (White or non-White), chronic diseases/conditions (0, 1, 2 or 3+), case/control status, dietary pattern (empirical dietary inflammatory pattern for c-reactive protein, interleukin-6 and tumor necrosis factor alpha receptor 2; empirical dietary index for hyperinsulinemia for C-peptide; empirical dietary index for insulin resistance for triglyceride/High-density lipoprotein cholesterol ratio).
P value for linear trend test was obtained using physical activity as a continuous variable.
P<0.05 (significant difference in plasma biomarker against the reference group (lowest total physical activity)).
Figure 1.
Multivariable-adjusted percent relative difference in biomarker concentrations comparing the highest (63+ MET-h/week) with the lowest (0 to <9 MET-h/week) physical activity groups: A) with additional adjustment for BMI (B) by type of physical activity and (C) by intensity of physical activity; Health Professionals Follow-up Study 1992–1994. Abbreviation: CRP, C-reactive protein; IL6, interleukin 6; TNFαR2, tumor necrosis factor α receptor 2; TG/HDL, triglycerides/high-density lipoprotein cholesterol ratio.
Joint associations of total physical activity and type of activity (aerobic only vs. aerobic plus resistance training) with plasma biomarkers of inflammation and insulin response are shown in Table 3, Supporting Information Table S2, and Figure 1. We found better biomarker profiles among those in the highest (63+ MET-h/week) compared to lowest (0 to 9 MET-h/week) physical activity groups. For the same total MET-hours of physical activity, these associations were stronger for men engaging in both aerobic plus resistance training compared to those engaging in aerobic only. The multivariable-adjusted percent RD in concentration of biomarkers associated with aerobic only and aerobic plus resistance training, respectively were −29% and −36% for CRP, −20% and −29% for IL6, −8% and −8% for TNFαR2, +7% and +11% for adiponectin, −18% and −30% for C-peptide, and −19% and −19% for TG/HDL. Higher aerobic activity was associated with lower levels of CRP, IL6, TNFaR2, C-peptide, and TG/HDL (all Ptrend<0.001) and higher levels of adiponectin (Ptrend=0.006), independent of resistance training. On the other hand, higher resistance training did not show a significant trend with those plasma biomarkers, independent of aerobic activity (Supporting Information Table S3).
Table 3.
Joint association of total physical activity and type of activity with plasma biomarkers of inflammation and insulin response, Health Professionals Follow-up Study 1992–1994
| Total physical activity (MET-h/week) | |||||
|---|---|---|---|---|---|
| 0 to <9 | 9 to <21 | 21 to <42 | 42 to <63 | 63+ | |
| C-reactive protein (mg/L) | |||||
| Aerobic only | 1.13 (1.05, 1.23) | 0.96 (0.89, 1.04)* | 0.93 (0.87, 1.00)* | 0.81 (0.73, 0.89)* | 0.80 (0.72, 0.88)* |
| Aerobic plus resistance training | 0.95 (0.68, 1.34)* | 0.86 (0.73, 1.02)* | 0.86 (0.76, 0.97)* | 0.65 (0.55, 0.75)* | 0.72 (0.63, 0.82)* |
| Interleukin-6 (pg/mL) | |||||
| Aerobic only | 1.63 (1.50, 1.78) | 1.48 (1.37, 1.60) | 1.32 (1.23, 1.43)* | 1.20 (1.08, 1.34)* | 1.30 (1.17, 1.45)* |
| Aerobic plus resistance training | 1.21 (0.82, 1.79) | 1.37 (1.14, 1.64) | 1.28 (1.13, 1.45)* | 1.20 (1.02, 1.41)* | 1.16 (1.00, 1.33)* |
| Tumor necrosis factor alpha receptor 2 (ng/mL) | |||||
| Aerobic only | 2.79 (2.74, 2.85) | 2.69 (2.64, 2.74) | 2.72 (2.67, 2.77) | 2.66 (2.59, 2.73)* | 2.58 (2.52, 2.65)* |
| Aerobic plus resistance training | 2.64 (2.43, 2.87) | 2.64 (2.53, 2.76) | 2.67 (2.59, 2.75) | 2.58 (2.48, 2.69)* | 2.56 (2.47, 2.65)* |
| Adiponectin (μg/mL) | |||||
| Aerobic only | 5.95 (5.73, 6.17) | 6.13 (5.92, 6.34) | 6.29 (6.09, 6.50) | 6.52 (6.21, 6.83) | 6.39 (6.09, 6.70) |
| Aerobic plus resistance training | 5.40 (4.65, 6.26) | 6.36 (5.89, 6.86) | 6.16 (5.83, 6.51) | 6.23 (5.80, 6.69) | 6.60 (6.19, 7.03)* |
| C-peptide (ng/mL) | |||||
| Aerobic only | 2.60 (2.47, 2.73) | 2.34 (2.24, 2.45)* | 2.18 (2.09, 2.27)* | 2.18 (2.05, 2.31)* | 2.14 (2.02, 2.27)* |
| Aerobic plus resistance training | 2.39 (1.95, 2.91)* | 2.20 (1.98, 2.44)* | 2.11 (1.97, 2.25)* | 1.96 (1.81, 2.13)* | 1.82 (1.68, 1.97)* |
| Triglyceride/High-density lipoprotein cholesterol ratio | |||||
| Aerobic only | 3.01 (2.83, 3.21) | 2.90 (2.73, 3.07) | 2.64 (2.50, 2.79)* | 2.33 (2.16, 2.53)* | 2.43 (2.24, 2.64)* |
| Aerobic plus resistance training | 3.38 (2.64, 4.31) | 2.60 (2.29, 2.94) | 2.73 (2.48, 3.00) | 2.70 (2.40, 3.04) | 2.44 (2.19, 2.72)* |
Values are multivariable-adjusted absolute biomarker concentrations adjusted for: age at blood draw (continuous), smoking (never, past or current smokers), race (White or non-White), chronic diseases/conditions (0, 1, 2 or 3+), case/control status, dietary pattern (empirical dietary inflammatory pattern for c-reactive protein, interleukin-6 and tumor necrosis factor alpha receptor 2; empirical dietary index for hyperinsulinemia for C-peptide; empirical dietary index for insulin resistance for triglyceride/High-density lipoprotein cholesterol ratio).
P<0.05 (significant difference in plasma biomarker against the reference group (lowest total physical activity group with aerobic only)).
Joint associations of total physical activity and intensity of activity (mostly moderate activities vs. moderate plus vigorous activities (≥20% of the total MET-hours)) with plasma biomarkers of inflammation and insulin response are shown in Table 4, Supporting Information Table S4, and Figure 1. Comparing the highest (mostly moderate activities) to lowest (mostly moderate activities) physical activity groups, the multivariable-adjusted percent RD in concentration of biomarkers was −42% for CRP, −43% for IL6, −8% for TNFαR2, +10% for adiponectin, −18% for C-peptide, and −26% for TG/HDL. For the same total MET-hours of physical activity, we did not find better biomarker profiles among those engaging in vigorous intensity as compared to those who did smaller amount of vigorous activities. Higher moderate activity was associated with lower levels of all biomarkers (all Ptrend<0.001) and higher levels of adiponectin (Ptrend=0.006), independent of vigorous activity. Moreover, higher vigorous activity was associated with lower levels of CRP, TNFαR2, C-peptide, and TG/HDL, independent of moderate activity (Supporting Information Table S5).
Table 4.
Joint association of total physical activity and intensity with plasma biomarkers of inflammation and insulin response, Health Professionals Follow-up Study 1992–1994
| Total physical activity (MET-h/week) | |||||
|---|---|---|---|---|---|
| 0 to <9 | 9 to <21 | 21 to <42 | 42 to <63 | 63+ | |
| C-reactive protein (mg/L) | |||||
| <20% vigorous | 1.20 (1.08, 1.33) | 0.94 (0.84, 1.07)* | 0.87 (0.75, 1.00)* | 0.89 (0.72, 1.10) | 0.70 (0.56, 0.88)* |
| ≥20% vigorous | 1.04 (0.92, 1.16) | 0.95 (0.87, 1.03)* | 0.92 (0.86, 0.99)* | 0.73 (0.67, 0.80)* | 0.78 (0.71, 0.85)* |
| Interleukin-6 (pg/mL) | |||||
| <20% vigorous | 1.64 (1.47, 1.84) | 1.39 (1.21, 1.60) | 1.33 (1.15, 1.54) | 0.85 (0.68, 1.06)* | 0.93 (0.72, 1.20)* |
| ≥20% vigorous | 1.56 (1.38, 1.77) | 1.49 (1.37, 1.62) | 1.31 (1.22, 1.40)* | 1.29 (1.17, 1.42)* | 1.29 (1.18, 1.42)* |
| Tumor necrosis factor alpha receptor 2 (ng/mL) | |||||
| <20% vigorous | 2.78 (2.71, 2.85) | 2.67 (2.58, 2.75) | 2.66 (2.57, 2.75) | 2.62 (2.49, 2.77) | 2.56 (2.41, 2.72) |
| ≥20% vigorous | 2.78 (2.70, 2.87) | 2.69 (2.63, 2.75) | 2.72 (2.67, 2.76) | 2.64 (2.58, 2.71)* | 2.58 (2.52, 2.64)* |
| Adiponectin (μg/mL) | |||||
| <20% vigorous | 5.82 (5.55, 6.10) | 6.15 (5.82, 6.49) | 6.33 (5.93, 6.74) | 6.38 (5.77, 7.05) | 6.40 (5.76, 7.11) |
| ≥20% vigorous | 6.03 (5.73, 6.36) | 6.18 (5.94, 6.42) | 6.24 (6.05, 6.44) | 6.43 (6.16, 6.72)* | 6.47 (6.21, 6.74)* |
| C-peptide (ng/mL) | |||||
| <20% vigorous | 2.61 (2.43, 2.80) | 2.33 (2.16, 2.52) | 2.24 (2.08, 2.42)* | 1.90 (1.68, 2.15)* | 2.15 (1.87, 2.47) |
| ≥20% vigorous | 2.55 (2.38, 2.74) | 2.31 (2.20, 2.43)* | 2.14 (2.05, 2.22)* | 2.13 (2.03, 2.25)* | 2.01 (1.91, 2.11)* |
| Triglyceride/High-density lipoprotein cholesterol ratio | |||||
| <20% vigorous | 2.98 (2.75, 3.23) | 2.90 (2.64, 3.18) | 2.63 (2.35, 2.94) | 2.51 (2.11, 2.98) | 2.22 (1.84, 2.68)* |
| ≥20% vigorous | 3.10 (2.84, 3.40) | 2.81 (2.64, 3.00) | 2.67 (2.54, 2.82) | 2.43 (2.26, 2.61)* | 2.47 (2.30, 2.65)* |
Values are multivariable-adjusted absolute biomarker concentrations adjusted for: age at blood draw (continuous), smoking (never, past or current smokers), race (White or non-White), chronic diseases/conditions (0, 1, 2 or 3+), case/control status, dietary pattern (empirical dietary inflammatory pattern for c-reactive protein, interleukin-6 and tumor necrosis factor alpha receptor 2; empirical dietary index for hyperinsulinemia for C-peptide; empirical dietary index for insulin resistance for triglyceride/High-density lipoprotein cholesterol ratio).
P<0.05 (significant difference in plasma biomarker against the reference group (lowest total physical activity group with <20% vigorous activity)).
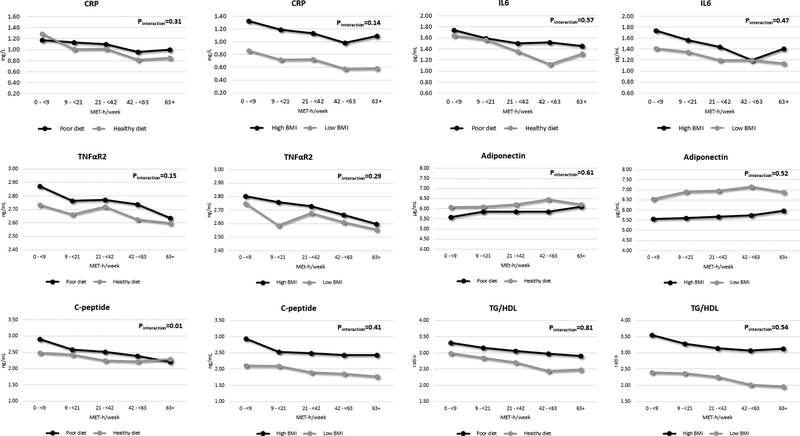
We further examined the association between total physical activity and the aforementioned plasma biomarkers stratified by diet and BMI (Figure 2). Participants with diets with high inflammatory/insulinemic potential or high BMI had higher inflammation and insulin profiles than those with diets with low inflammatory/insulinemic potential or low BMI, respectively. We generally observed greater differences in plasma biomarkers when stratified by BMI than dietary pattern. Physical activity showed similar associations for these biomarkers across dietary pattern and BMI, except for C-peptide which showed slightly lower levels among those with diets with high insulinemic potential (Pinteraction=0.01). Similar patterns for physical activity were shown when stratified by both diet and BMI (Supporting Information Figure S1).
Figure 2.
Joint association of total physical activity, diet and body mass index with plasma markers of inflammation and insulin response, Health Professionals Follow-up Study 1992–1994. Abbreviation: CRP, C-reactive protein; IL6, interleukin 6; TNFαR2, tumor necrosis factor α receptor 2; TG/HDL, triglycerides/high-density lipoprotein cholesterol ratio.
Discussion
In this large cross-sectional study, we found that high physical activity was associated with favorable plasma biomarkers of inflammation and insulin response. Given the same total MET-hours of physical activity, engaging in moderate intensity exercises or aerobic plus resistance exercises suggested stronger associations with the circulating levels of biomarkers of inflammation and insulin response compared to engaging in higher vigorous intensity exercises or aerobic exercises alone, respectively. Moreover, the combination of high physical activity, healthy diet and low adiposity may provide substantially better biomarker profiles related to cancer as well as other chronic diseases.
A number of intervention studies have shown the effect of physical activity on improving biomarkers of inflammation and insulin.23, 25, 39 These intervention studies support the causal relationship between physical activity and the biomarkers, supporting the basis of our study. However, the influence of specific components of physical activity (e.g., volume, intensity, and type) on these biomarkers remains unclear.40, 41 Moreover, the majority of previous studies were small and restricted to unhealthy populations with diseases.23, 25, 39
In the current study of 7,219 generally healthy men, we found that higher total physical activity was associated with lower levels of circulating biomarkers of inflammation and insulin response. We observed an inverse association between physical activity and biomarkers even for the low physical activity volume (+9 MET-h/week). The biomarkers of inflammation and insulin response were inversely associated with physical activity until 63 MET-hours per week, showing a linear trend. Over 63 MET-hours per week of physical activity, we consistently found the association between physical activity and biomarker profiles, although the association was not much greater than engaging in 42 to 62.9 MET-hours per week of physical activity. This finding suggests that higher physical activity is fairly linearly associated with lower levels of inflammatory and insulin-related biomarkers. Engaging in high physical activity was not associated with unfavorable biomarkers, although we found slightly increased IL6 in the most active group (63+ MET-h/week) compared to the second most active group (42–62.9 MET-h/week). Because IL6 is a sensitive cytokine that increases substantially but acutely in response to exercise,42 the most active group with very high physical activity may have reflected this acute change in our analysis.
When we adjusted for BMI, the associations between physical activity and the biomarkers of inflammation and insulin were modestly attenuated but remained statistically significant. Given that BMI is an imperfect measure of adiposity, we conducted additional analysis by adjusting for waist circumference, predicted fat mass and percent fat to better control adiposity but we found similar moderate attenuation of the estimates. If we had more precise measures of sub-compartments of fat (e.g., visceral fat), we may have observed larger attenuation of the estimates. Growing evidence suggests that high adiposity increases cancer risk through several mechanisms including upregulation of inflammation and insulin response.13, 16, 43 Physical activity may act on inflammation and insulin pathways by reducing adiposity, which is likely a mediator in the relationship between physical activity and the biomarkers of inflammation and insulin response.9 Thus, adjustment of adiposity could be an over adjustment in the aforementioned relationship to some extent. In our study, we found evidence that high physical activity has both direct (independent) and indirect (mediated through adiposity) associations with lower levels of biomarkers of inflammation and insulin response.
Type and intensity are important aspects of physical activity which may allow us to provide detailed and effective physical activity guidelines for the general population. Regarding the types of physical activity, a recent review of the literature on physical activity and insulin sensitivity in humans suggested that combination of aerobic and resistance training may be more effective to improve insulin resistance than either modality alone.44 In our study, for the same total MET-hours, aerobic plus resistance training was associated with a lower levels of CRP, IL6, and C-peptide, and higher levels of adiponectin, than aerobic training only. On the other hand, we did not find evidence of additional benefits of vigorous intensity physical activity over moderate intensity in regards to the biomarker levels. Similarly, a recent systematic review found that high-intensity interval training suggested similar benefits to moderate-intensity continuous training for body fat reduction.45
Physical activity, adiposity, and diet have complex interrelationships.8 Physical activity and diet may influence adiposity but they also have direct influence on the circulating biomarkers.8, 9 Moreover, physical activity may interact with adiposity and dietary pattern. Therefore, it is crucial to understand how these ‘triad’ of physical activity, diet, and adiposity influence the biomarkers of inflammation and insulin response. As expected, participants with high adiposity or poor dietary pattern had higher levels of inflammation and insulin response compared to those with low adiposity or healthy dietary pattern, respectively. In addition, lean people with high physical activity and healthy dietary pattern had approximately 2 to 3-fold lower CRP, IL6, C-peptide, and TG/HDL than overweight/obese people with low physical activity and poor dietary pattern. Interestingly, we found the magnitude of difference in inflammatory and insulin-related biomarkers per unit increment of physical activity to be broadly similar across adiposity level or dietary pattern. Thus, high physical activity was associated with better biomarker profiles on all groups stratified by diet and adiposity.
Our study has several strengths. First, a large sample provided sufficient power to examine the independent and joint associations of physical activity (type and intensity) and also interactions with diet and adiposity in relation to various biomarkers of inflammation and insulin response. Second, we collected detailed information on lifestyle factors and medical history which allowed us to finely control for potential confounding. Third, average of two repeated measures of physical activity with detailed information on volume, type, and intensity of activities reduced within-person measurement errors and allowed us to study the important aspects of physical activity in relation to the biomarkers.
There are several limitations to our study. Our study included predominantly white male health professionals which may limit the generalizability. However, it strengthens the internal validity and the characteristics of participants were generally similar to the large multi-ethnic cohorts in the U.S. Moreover, measurement errors from two questionnaires based physical activity and a single measure of biomarkers are inevitable but such measurement errors are likely to be non-differential which may have attenuated the associations.46 The advantage of relying on highly medically educated health professionals is that they manifest a wide range of physical activity and in general report fairly accurately. In Women’s Lifestyle validation study, objective measures of physical activity showed consistent but slightly stronger associations with cardiometabolic and endocrine biomarkers compared to questionnaire-based physical activity, though the use of two questionnaires in our study may have improved assessment of physical activity.47
Our results require confirmation but have several important implications. First, for these biomarkers of inflammation and insulin response, the association of physical activity was fairly linear, up to a level of 63 MET-hours per week. Thus, while improvement is seen at any level, a high level is required for optimization. Second, while aerobic activity constitutes the vast majority of the activity and benefits, incorporating some resistance training may have additional beneficial associations for the same level of total MET-hours. Third, the accumulated MET-hours of physical activity has similar associations with better biomarker profiles regardless of the activity being vigorous or not. Importantly, this allows some flexibility in the amount of time spent and intensity of work-out if one wants to attain a certain MET-hours. For example, if a goal is to attain 50 MET-hours per week, this can be done with 11 hours of brisk walking (moderate activity), 7 hours of running, or 8 hours of moderate and 2 hours of vigorous activity. Lastly, our results indicate that physical activity has relatively similar associations for these biomarkers across different strata of adiposity and diet, and optimal results can be found among those with high physical activity, low adiposity, and healthy dietary pattern.
Supplementary Material
Novelty and Impact: To better understand the underlying mechanism linking physical activity and cancer, the authors investigated the associations of physical activity (volume, type, and intensity), as well as the interaction with adiposity and diet, with cancer-related biomarkers. The authors found that high physical activity, preferably aerobic plus resistance training, was associated with favorable biomarkers of inflammation and insulin response. Physical activity had similar associations across adiposity and diet, and optimal results were achieved by maximizing all three.
Funding:
This work was supported by the National Institutes of Health (UM1 CA167552, R01 HL35464, K99 CA207736, and R00 CA207736). Leandro Fórnias Machado de Rezende receives a doctoral scholarship from Sao Paulo Research Foundation (FAPESP), grants #2014/25614-4 and #2016/21390-0.
Abbreviations:
- BMI
body mass index
- CRP
C-reactive protein
- EDIH
empirical dietary index for hyperinsulinemia
- EDIP
empirical dietary inflammatory pattern
- EDIR
empirical dietary index for insulin resistance
- HPFS
Health Professionals Follow-up Study
- IL6
interleukin 6
- MET
metabolic equivalent
- TG/HDL
triglycerides/high-density lipoprotein cholesterol ratio
- TNFαR2
tumor necrosis factor α receptor 2
Footnotes
Conflict of interest disclosures: The authors declared no conflicts of interest.
References
- 1.Giovannucci E An Integrative Approach for Deciphering the Causal Associations of Physical Activity and Cancer Risk: The Role of Adiposity. J Natl Cancer Inst 2018. [DOI] [PubMed] [Google Scholar]
- 2.Rezende LFM, Sa TH, Markozannes G, Rey-Lopez JP, Lee IM, Tsilidis KK, Ioannidis JPA, Eluf-Neto J. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med 2017. [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Physical activity and risk of cancer.
- 4.International Agency for Research on Cancer (IARC). Weight Control and Physical Activity IARC Handbook of Cancer Prevention Volume 6ed. Lyon: IARC press, 2002. [Google Scholar]
- 5.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med 2016;176: 816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working G. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine 2016;375: 794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund/American Institute for Cancer Research. Continous Update Project Expert Report 2018. Body fatness and weight gain and the risk of cancer.
- 8.Giovannucci E A framework to understand diet, physical activity, body weight, and cancer risk. Cancer Causes Control 2017;52: 826–33. [DOI] [PubMed] [Google Scholar]
- 9.McTiernan A Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008;8: 205–11. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports M. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;41: 459–71. [DOI] [PubMed] [Google Scholar]
- 11.Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obesity reviews : an official journal of the International Association for the Study of Obesity 2016;17: 664–90. [DOI] [PubMed] [Google Scholar]
- 12.Olson OC, Quail DF, Joyce JA. Obesity and the tumor microenvironment. Science 2017;358: 1130–1. [DOI] [PubMed] [Google Scholar]
- 13.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34: 4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Flanagan CH, Bower LW, Allott EH, Hursting SD. Molecular and metabolic mechanisms underlying the obesity–cancer link In: Romieu I, Dossus L, Willet WC. Energy Balancer and Obesity (IARC Working Group Reports; 10)ed. Lyon: International Agency for Research on Cancer, 2017. [PubMed] [Google Scholar]
- 15.Chen L, Li L, Wang Y, Li P, Luo L, Yang B, Wang H, Chen M. Circulating C-peptide level is a predictive factor for colorectal neoplasia: evidence from the meta-analysis of prospective studies. Cancer causes & control : CCC 2013;24: 1837–47. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131: 3109S–20S. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, Rifai N, Pollak MN, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst 2013;105: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Lee IM, Tworoger SS, Buring JE, Ridker PM, Rosner B, Hankinson SE. Plasma C-reactive protein and risk of breast cancer in two prospective studies and a meta-analysis. Cancer Epidemiol Biomarkers Prev 2015;24: 1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong TT, Wu QJ, Wang YL, Ma XX. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta‐analysis of epidemiologic studies. International journal of cancer 2015;137: 1967–78. [DOI] [PubMed] [Google Scholar]
- 20.Heikkilä K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer causes & control 2009;20: 15–26. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. European journal of cancer 2015;51: 2747–58. [DOI] [PubMed] [Google Scholar]
- 22.Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, Stampfer MJ, Sato K, Ma J, Buring JE. Hyperglycemia, insulin resistance, impaired pancreatic β-cell function, and risk of pancreatic cancer. Journal of the National Cancer Institute 2013;105: 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med 2017;51: 670–6. [DOI] [PubMed] [Google Scholar]
- 24.Cronin O, Keohane DM, Molloy MG, Shanahan F. The effect of exercise interventions on inflammatory biomarkers in healthy, physically inactive subjects: a systematic review. QJM 2017;110: 629–37. [DOI] [PubMed] [Google Scholar]
- 25.Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev 2017;26: 355–65. [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. The Lancet 1991;338: 464–8. [DOI] [PubMed] [Google Scholar]
- 27.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. Journal of the National Cancer Institute 2005;97: 1688–94. [DOI] [PubMed] [Google Scholar]
- 28.Ainsworth BE, Haskell WL, Leon AS, Jacobs JD, Montoye HJ, Sallis JF, Paffenbarger JR. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise 1993;25: 71–80. [DOI] [PubMed] [Google Scholar]
- 29.Plowman SA, Smith DL. Exercise physiology for health fitness and performanceed.: Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 30.Grøntved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Archives of internal medicine 2012;172: 1306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keum N, Bao Y, Smith-Warner SA, Orav J, Wu K, Fuchs CS, Giovannucci EL. Association of physical activity by type and intensity with digestive system cancer risk. JAMA oncology 2016;2: 1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996: 81–6. [DOI] [PubMed] [Google Scholar]
- 33.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ. Inflammatory markers and the risk of coronary heart disease in men and women. New England Journal of Medicine 2004;351: 2599–610. [DOI] [PubMed] [Google Scholar]
- 34.Song M, Zhang X, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer prevention research 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical Dietary Inflammatory Index. The Journal of nutrition 2016;146: 1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, Fuchs CS, Willett WC, Giovannucci EL. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. British Journal of Nutrition 2016;116: 1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Sun Q, Willett WC, Giovannucci EL. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. British Journal of Nutrition 2017;118: 858–66. [DOI] [PubMed] [Google Scholar]
- 38.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American journal of epidemiology 2008;167: 653–66. [DOI] [PubMed] [Google Scholar]
- 39.Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ open sport & exercise medicine 2017;2: e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JC, Winters‐Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Comprehensive Physiology 2012;2: 2775–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buffart L, Galvão DA, Brug J, Chinapaw M, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer treatment reviews 2014;40: 327–40. [DOI] [PubMed] [Google Scholar]
- 42.Astrom M-B, Feigh M, Pedersen BK. Persistent low-grade inflammation and regular exercise. Front Biosci (Schol Ed) 2010;2: 96–105. [DOI] [PubMed] [Google Scholar]
- 43.Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, Moore SC, Purdue MP, Wentzensen N, Hildesheim A. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiology and Prevention Biomarkers 2014: cebp. 0699.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med 2016;2: e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev 2017;18: 943–64. [DOI] [PubMed] [Google Scholar]
- 46.Mayeux R Biomarkers: potential uses and limitations. NeuroRx 2004;1: 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alessa HB, Chomistek AK, Hankinson SE, Barnett JB, Rood J, Matthews CE, Rimm EB, Willett WC, Hu FB, Tobias DK. Objective Measures of Physical Activity and Cardiometabolic and Endocrine Biomarkers. Medicine and science in sports and exercise 2017;49: 1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.