Abstract
Sterol structure influences liquid ordered domains in membranes, and the dependence of biological functions on sterol structure can help identify processes dependent on ordered domains. In this study we compared the effect of sterol structure on ordered domain formation in symmetric vesicles composed of mixtures of sphingomyelin, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and cholesterol, and in asymmetric vesicles in which sphingomyelin was introduced into the outer leaflet of vesicles composed of DOPC and cholesterol. In most cases, sterol behavior was similar in symmetric and asymmetric vesicles, with ordered domains most strongly stabilized by 7-dehydrocholesterol (7DHC) and cholesterol, stabilized to a moderate degree by lanosterol, epicholesterol and desmosterol, and very little if at all by 4-cholesten-3-one. However, in asymmetric vesicles desmosterol stabilized ordered domain almost as well as cholesterol, and to a much greater degree than epicholesterol, so that the ability to support ordered domains decreased in the order 7-DHC > cholesterol > desmosterol > lanosterol > epicholesterol > 4-cholesten-3-one. This contrasts with values for intermediate stabilizing sterols in symmetric vesicles in which the ranking was cholesterol > lanosterol ~ desmosterol ~ epicholesterol or prior studies in which the ranking was cholesterol ~ epicholesterol > lanosterol ~ desmosterol. The reasons for these differences are discussed. Based on these results, we re-evaluated our prior studies in cells and conclude that endocytosis levels and bacterial uptake are even more closely correlated with the ability of sterols to form ordered domains than previously thought, and do not necessarily require that a sterol have a 3β-OH group.
Keywords: Sterol, Steroid, Liquid Ordered, Lipid Asymmetry, FRET
Graphical abstract
1. Introduction
Model biomembrane vesicles have long been used to investigate the principles of membrane assembly and organization. However, the information that can be gained is sometimes limited because they do not usually possess transbilayer lipid asymmetry, the difference between lipid composition of the outer (exoplasmic, exofacial) leaflet and the inner (cytoplasmic, cytofacial) leaflet of a membrane [1, 2]. Many natural biomembranes possess lipid asymmetry [3, 4], and it has been established that lipid asymmetry is vital to cellular processes such as phagocytosis [5], blood clotting [6], and host cell invasion/infection [7, 8]
One area in which the role of lipid asymmetry has only begun to be explored is its influence on bilayer physical properties. In a lipid bilayer composed of only phospholipids and sphingolipids there are two common physical states: the solid-like gel state and the liquid disordered (Ld) state. The lipids in the Ld state are more loosely packed than those in the gel state. In a pure lipid bilayer each individual lipid has its own characteristic gel to Ld melting temperature (Tm). Tm values are generally higher for sphingolipids, which have saturated acyl chains that promote tight lipid packing. In the presence of cholesterol, and other natural sterols, the liquid ordered (Lo) state can form, especially when mixed with membrane lipids with saturated acyl chains [9-13].
In lipid vesicles containing mixtures of high Tm lipids, low Tm lipids and cholesterol, Lo and Ld phases can co-exist [9, 10, 14, 15]. The working hypothesis for lipid domains in cell membranes is that there are sphingolipid and cholesterol rich Lo domains that co-exist with Ld domains [16, 17]. Lo domains in natural membranes are often called lipid rafts [18]. Lipid rafts have been proposed to play important roles in many cellular processes such as endocytosis, amyloid formation, protein and lipid sorting, cell signal transduction, and pathogen invasion [19-27].
Recent advances in our lab have made it possible to carry out studies using asymmetric vesicles (AUV) prepared by cyclodextrin-catalyzed exchange of phospholipids and sphingolipids between vesicles with different lipid compositions. Recently, we developed the use of two 6-sugar-ring alpha cyclodextrins for lipid exchange: hydroxy-propyl α-cyclodextrin (HPαCD) [28-31] and methyl-α-cyclodextrin (MαCD) [32]. These cyclodextrins can efficiently exchange phospholipids and sphingolipids into lipid vesicles (or even living mammalian cells), but due to their small hydrophobic cavity size, do not transfer sterols [33]. Studies using such asymmetric vesicles [28, 31, 34-37], and earlier studies with cholesterol-containing planar asymmetric bilayers [38, 39] have begun to provide insights into how asymmetry influences membrane domain formation. These studies show that under some conditions, the coupling between the inner and outer leaflet can induce or destroy domain formation in each leaflet, while under other conditions coupling appears to be weak enough to allow domain formation in only a single leaflet. Interdigitation and membrane curvature are two parameters than can stabilize or destabilize interleaflet coupling [28, 31, 35, 37, 40, 41]. In addition, the difference in composition in the inner and outer leaflet requires that the composition of ordered domains must differ in each leaflet, and this means that their properties might differ from each other or from that in symmetric membranes, as has been noted in some studies [28, 36].
Sterol structure also impacts domain formation. Studies have examined the effect of sterol structure on both symmetric model membrane vesicles and living cells. It is well established that cholesterol supports ordered domain formation by packing tightly with sphingolipids [42, 43]. Some of this is due to the inability of the small hydroxyl group at carbon 3 to shield the cholesterol sterol rings from water, and the alleviation of the resulting unfavorable hydrophobic effect by shielding arising from closely packed phospholipid or sphingolipid head groups [44]. It is thought that the other structural components of cholesterol which may contribute to its packing ability are its relatively flat fused rings, lack of protruding methyl groups on the rings, and the carbon 17 isooctyl alkyl tail [45-47].
These structural features vary considerably among natural and synthetic sterols, and as a result sterols exhibit a wide range of abilities to support or inhibit ordered domain formation [12, 43, 45, 46, 48, 49]. As we suggested in an early study [43] the possible role of membrane domains in a biological function can be probed by defining the extent to which the ability of a sterol to support ordered domain formation (measured in vesicles with mixtures of high Tm lipid, low Tm lipid and sterol) is correlated with the ability of that sterol to support that biological function [50-53]. This is more informative than simply studying the effect of removing cholesterol from cell membranes, which can have pleiotropic effects, including those resulting from a large change in the total amount of lipid in the membrane or due to loss of specific cholesterol-protein interactions. Examining cholesterol function by sterol substitution has been used in a number of studies [54-58] including from our lab [27, 59, 60].
However, studies have not examined the influence of various sterols upon domain formation in asymmetric vesicles. The ability of sterols to support ordered domain formation in the appropriate asymmetric vesicles is likely to lead to a more accurate estimate of sterol raftsupporting ability in asymmetric cell membranes. In particular, we were interested in whether differences between sterol effects upon symmetric and asymmetric membranes might explain the anomalous results in our previous studies [12, 43, 48] which had found that, unlike other sterols, the ability of epicholesterol and desmosterol to support ordered domain formation did not correlate well with endocytosis or bacterial uptake. Epicholesterol did not support these processes, despite a good ability to support ordered domain formation, while desmosterol supported function despite apparently only having a modest ability to support ordered domain formation [52]. Another issue was that the estimates of domain-supporting ability in symmetric vesicles came from combining the results of multiple studies which differed from one another in terms of the vesicle type used (in terms of vesicle size), the type of ordered domain forming lipid (DPPC or SM), and type of the disordered domain forming lipid (DOPC or the nitroxide-labeled lipid 12SLPC) [12, 43, 48, 49].
Therefore, we decided to compare how changes in sterol structure affect Lo domain formation in symmetric vesicles and asymmetric vesicles. For these studies, AUV were prepared by exchanging SM into outer leaflets of vesicles composed of DOPC and sterol. By also introducing FRET acceptors into the AUV outer leaflet during exchange, we could assay the presence and thermal stability of ordered domains, including nanodomains that would be missed by light microscopy methods. We found that epicholesterol stabilized ordered domains to a lesser degree than expected in both symmetric and asymmetric vesicles, while desmosterol supported ordered domain formation in asymmetric vesicles more strongly than in symmetric vesicles. Re-evaluating our prior results, we now find an even stronger correlation between the ability of sterols to form ordered domains and both endocytosis and bacterial uptake. The revised ranking of sterol propensities to support ordered domain formation resulting from these studies should help improve predictions regarding the functional importance of ordered domains in other biological processes.
2. Materials and Methods
2.1. Materials
Porcine brain sphingomyelin (SM); 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC); cholesterol; 1,2-dioleoylphosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (rhodamine-DOPE) were purchased from Avanti Polar Lipids (Alabaster, AL); Desmosterol, epicholesterol, 7-dehydrocholesterol (7DHC), pregnenolone), lanosterol, 4-cholesten-3-one, were purchased from Steraloids, Inc. (Newport, Rhode Island). 1,6-diphenyl-1,3,5-hexatriene (DPH) was purchased from Sigma-Aldrich (St. Louis, MO). Lipids and probes were dissolved in chloroform (with the exception of DPH, which was dissolved to 10 μM in ethanol) and stored at −20°C. The concentrations of lipids were determined by dry weight and that of fluorescent lipids by absorbance using εRhodamine-DOPE 88,000 M−1cm−1 at 560 nm, εDPH 84,800 M−1cm−1 at 352 nm. High performance thin layer chromatography (HP-TLC) plates (Silica Gel 60) were purchased from VWR International (Batavia, IL). Methyl-alpha cyclodextrin (MαCD) was purchased from AraChem Cyclodextrin Shop (Tilburg, the Netherlands), dissolved in distilled water at close to 100mM, and then filtered through a Sarstedt (Numbrecht, Germany) 0.2μm pore syringe filter. At the high MαCD concentrations used, its exact concentration was most conveniently assayed by comparing the refractive index of the solutions to that of a standard curve with a known amount of cyclodextrin dissolved in a known final volume of solution. Absorbance was measured using a Beckman 640 spectrophotometer (Beckman Instruments, Fullerton, CA) with quartz cuvettes. Fluorescence was measured on a SPEX Fluorolog 3 spectrofluorometer (Jobin-Yvon, Edison, NJ) using quartz semimicro cuvettes (excitation path length 10 mm and emission path length 4 mm). Sterols exhibited a single band on HP-TLC. Pictures of sterol structures were generated using ChemDraw (CambridgeSoft, Waltham, MA).
2.2. Methods
2.2.1. Preparation of Symmetric LUV:
Lipids dissolved in chloroform were combined in glass tubes, dried under a warm nitrogen stream and subjected to high vacuum for 1 h. The dried lipid mixtures were placed in a 70°C water bath and dispersed in phosphate buffered saline (10 mM Na phosphate, 150 mM NaCl), pH 7.4 (PBS) or 25% (w/w) sucrose in distilled water to a final concentration of 500 μl at 2mM lipid concentration. The samples were vortexed briefly and then agitated at 55°C for 15 min using a VWR Multitube Vortexer (Westchester, PA) placed within a convection oven (GCA Corp, Precision Scientific, Chicago, IL). The lipid mixtures were then cooled to room temperature and subjected to seven cycles of freeze thaw in either a dry ice/acetone bath or in a liquid nitrogen bath, in either case alternating with a 37°C water bath. To form LUVs of uniform vesicle size, the lipid mixtures were then extruded 11 times through 100 nm-pore polycarbonate membranes (Avanti Polar Lipids, Alabaster, AL). To wash away external sucrose in the case of sucrose-entrapped LUV, 333 μl aliquots of LUV formed in sucrose were mixed with 3.7 ml PBS and pelleted by ultracentrifugation at 190,000 × g for 25 min at 23°C using a Beckman L8-80 M ultracentrifuge with a SW-60 rotor. Following pelleting, the supernatant was removed, the LUV pellet dispersed in 333 μl PBS, covered with aluminum foil, and reserved for use. Unless otherwise noted samples were used within 2 h of preparation. For FRET measurements 50 μl aliquots of LUV lipid mixtures were dispersed in cuvettes containing 940 μl of either PBS or 26.7% (w/w) sucrose and 10 μl of 10 μM DPH dissolved in ethanol was added. The resulting lipid and DPH concentrations for FRET measurements was 100 μM and .1 μM, respectively.
2.2.2. Preparation of Donor Lipid-Loaded MαCD for Lipid Exchange Experiments:
Desired ratios of porcine brain SM and DOPC dissolved in chloroform were combined in glass tubes, dried under a warm nitrogen stream and then subjected to high vacuum for 1h. To prepare F samples for FRET measurements, to the unlabeled lipids an additional 13 mol% rhodamine-DOPE dissolved in chloroform was added to the glass tubes before drying. The dried lipids were placed in a 70°C water bath and dispersed at 70°C with an aliquot of pre-warmed PBS and then an aliquot of pre-warmed MαCD to give a final concentration of 40mM MαCD and 16 mM lipid. The samples were vortexed briefly, agitated at 55°C as above for 15 min, cooled to room temperature, covered in foil, and reserved for further use.
2.2.3. Preparation of Acceptor LUV for Lipid Exchange Experiments:
DOPC and one of a variety of sterols (25 mol%) dissolved in chloroform were combined in glass tubes. The mixtures were dried under warm nitrogen stream and subjected to high vacuum for 1 h. They were then placed in a 70°C water bath and dispersed to 8 mM lipid with 25% (w/w) sucrose in distilled water. The samples were vortexed briefly and agitated at 55°C for 15 min as above. LUV were then prepared from the lipid mixtures as described above for symmetric vesicles.
2.2.4. Outer Leaflet Lipid Exchange:
To wash away untrapped sucrose from acceptor LUVs, 333 μl aliquots of acceptor lipid in sucrose were diluted with 3.7 ml 1× PBS and subjected to ultracentrifugation at 190,000 × g for 25 min at 23°C as above. The supernatant was discarded, the LUV pellets were resuspended to 8 mM lipid concentration with 1× PBS and used immediately. To exchange the outer leaflet of acceptor LUV, 333 μl of the donor lipid-MαCD mixture and 333 μl of the acceptor lipid mixtures were combined, covered in foil, and shaken for 30 min at room temperature. These lipid-exchange mixtures were layered over 3.3 ml 9% (w/w) sucrose dissolved in water and subjected to ultracentrifugation at 190,000 × g for 40 min at 23°C. (We switched from doing exchange at 37°C and centrifugation over 10-11% (w/w) sucrose as in our prior study to further minimize lipid flip and maximize vesicle yield [32]. Following centrifugation, most of the supernatant was carefully removed, leaving approximately 750 μl sucrose and loosely pelleted AUV in the bottom of the centrifuge tube. The upper portion of the tube was swabbed with a clean, dry cotton-tipped applicator to remove residual adhering donor lipids and cyclodextrin. Approximately 3.25 ml PBS was then added to the tube and thoroughly mixed with the sucrose and AUV. This mixture was centrifuged a second time as above for 25 min. Following centrifugation, all remaining supernatant was removed and the pellet was dispersed in up to 333 μl PBS for immediate use. (In some cases, samples were dispersed in as little as 200 μl PBS based on visual assessment of AUV pellet size.) Exact AUV lipid concentration was determined by HP-TLC and was 1.05 ± 0.52 mM (~13% of theoretical maximal yield). Samples were then further diluted twenty-fold (to ~25-75 μM lipid) by adding 50 μl sample aliquots to quartz semi-micro cuvettes containing 940 μl of either PBS or 26.7% (w/w) sucrose in distilled water.
2.2.5. FRET Assay to Detect Outer Leaflet Domains:
Two types of samples were prepared: AUV Fo samples of desired lipid combination and AUV F samples of the same lipid composition, except prepared from donor lipid containing an extra 13 mol% rhodamine-DOPE (FRET acceptor) to incorporate the rhodamine-DOPE in the outer leaflet of the AUV. Before the DPH (FRET donor) was added to the samples, background (lipid-only) measurements of fluorescence intensity at 427nm were made using an excitation wavelength of 358nm on a SPEX Fluorolog 3 spectrofluorimeter (Jobin-Yvon, Edison, NJ). The slits were set to 1 mm (about 2 nm bandpass) for excitation and 4 mm (about 8 nm bandpass) for emission. Next, 10 μl aliquots of 10 μM DPH dissolved in ethanol were added to each cuvette and samples vortexed vigorously several times over a period of 20 min. Samples were cooled to 16°C and DPH fluorescence intensity at 427 nm (excitation 358 nm) was measured as samples were heated, with readings every four degrees (after temperature stabilized), up to 64°C. Sample temperature was measured with a probe thermometer (a traceable digital thermometer with a YSI microprobe; Fisher Scientific) inserted inside a sample cuvette before each measurement. Background fluorescence values, generally <1% of sample fluorescence, were subtracted before computing F/Fo values. When desired, F/Fo ratios were normalized by dividing the F/Fo ratio calculated at each temperature to the F/Fo ratio observed at 64°C.
2.2.6. Estimation of Tmid and Tend:
Normalized F/Fo results were analyzed to calculate Lo melting midpoints (Tmid ) and endpoints (Tend). Tmid was estimated by finding the point of the maximal slope of a sigmoidal fit of the F/Fo curve, using Slide Write Plus software (Advanced Graphics Software, Encinitas, CA). Tend was estimated by finding the minimum value of a polynomial fit applied to the normalized F/Fo data from 16°C-20°C below to 16°C-20°C above F/Fo minimum, using Excel software (Microsoft Corporation, Redmond, WA) and utilities available at http://www.wolframalpha.com/.
2.2.7. High-Performance TLC (HP-TLC):
Aliquots of samples and lipid standards were dissolved in 1:1 (v/v) chloroform/methanol. Dissolved lipids were applied to HP-TLC (Silica Gel 60) plates (Merck) and chromatographed to within 20% of full plate height in 65:25:4 chloroform:methanol :distilled water (v/v/v). After chromatography, the TLC plates were air-dried and rechromatographed (to separate sterols from solvent front) to near full plate height using 3:2 hexanes:ethyl acetate. The plates were again air dried, saturated with 3% (w/v) cupric-acetate-8% (v/v) phosphoric acid by spraying, and then air-dried again. Plates were then charred on a hot place at ~180 °C to develop lipid bands. Lipid band intensity was measured using ImageJ software (National Institutes of Health). Lipids in samples were quantified by comparing background-subtracted band intensity with that of various standard amounts of each lipid chromatographed on the same TLC plate. The intensity in the standard bands was fit to a linear intensity vs. lipid quantity curve using Excel software (Microsoft Corporation, Redmond, WA).
2.2.8. C6-NBD-PC Assay of Lipid Flip:
To estimate the stability of lipid asymmetry after lipid exchange, changes in the leaflet distribution of C6-NBD-PC were measured [30, 61, 62]. For initial outer leaflet localization, 10 mol% C6-NBD-PC was added to donor lipids during vesicle formation (after exchange this gave about 2 mol% C6-NBD-PC in the AUV). For symmetric LUV and inner leaflet localization in AUV, 2mol% C6-NBD-PC was added to lipids during LUV formation. In the case of AUV, most of the C6-NBD-PC in the outer leaflet of the acceptor vesicles was removed during the subsequent lipid exchange step. For all samples, vesicles were pre-incubated for up to 48h at room temperature and diluted to approximately 100 μM lipid concentration in 1M Tris buffer, pH 10 just prior to measuring fluorescence. The fluorescence intensity of C6-NBD-PC in each type of vesicle was measured before and after the addition of sodium dithionite (NaDt) dissolved in 1mM Tris buffer, pH 10 (final NaDt concentration 5mM) and the fraction of C6-NBD-PC fluorescence destroyed was calculated. Fluorescence measurements were made at room temperature as above but using an excitation wavelength of 464 nm and emission at 531 nm. The slit bandwidths during fluorescence measurements were set to 1 mm (about 2 nm bandpass) for excitation and 4 mm (about 8 nm bandpass) for emission.
3. Results
3.1. Using FRET to Detect Ordered Membrane Domains with Different Sterols.
To reinvestigate the effect of sterol structure on ordered domain formation, we compared sterol behavior in symmetric and asymmetric LUV composed of mixtures of SM, DOPC and sterol. This combination of lipids shows distinct segregation of the bilayer into Ld and ordered domains [63] and facilitated comparison to most of our previous studies. Previous studies indicated that in the presence of sterols, bilayers tend to form Lo state ordered domains, although some form gel domains [28, 45, 64]. We use the term ordered domains to refer to domains that are either in the Lo or gel state.
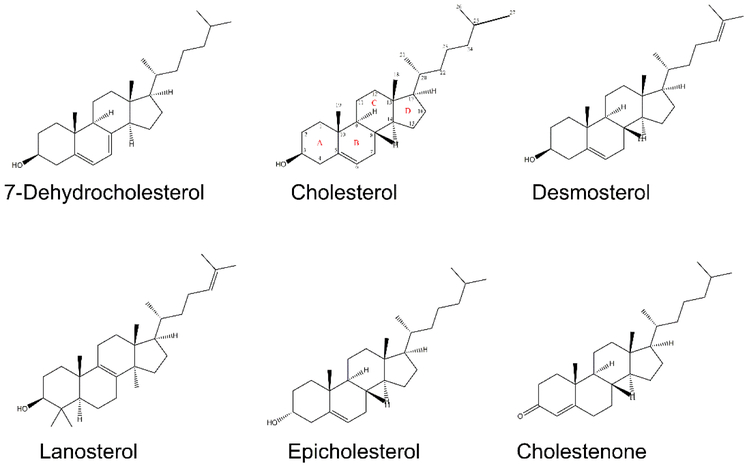
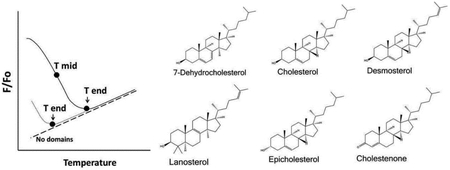
In these experiments, the effects of desmosterol and epicholesterol on ordered domain formation/stability was compared to that of cholesterol. In addition, we reexamined three other sterols/steroids with varying abilities to support ordered domain formation: 7-dehydrocholesterol (7DHC), which supports ordered domain formation even better than cholesterol [65], lanosterol, which has a modest ability to support ordered domain formation [66], and 4-cholesten-3-one, which destabilizes ordered domain formation [45]. (Although 4-cholesten-3-one is a steroid, not a sterol, we will refer to all cholesterol analogs as sterols for convenience.) The structures of these sterols are shown in Figure 1. It should be noted that we also tried to use pregnenolone as an example of a sterol that does not support ordered domain formation [46], but found that it was largely lost from the lipid bilayers during LUV preparation (data not shown).
Figure 1.
Sterols used in this study.
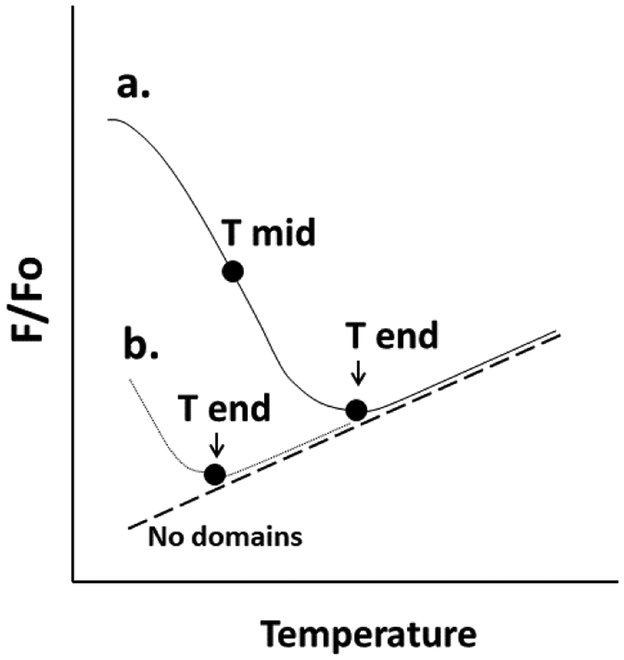
Domain formation was assayed using FRET. Unlike ordinary light microscopy, in which domain detection is limited to 200 nanometers, FRET can detect domains as small as or smaller than 5 nm, depending on the FRET donors and acceptors selected [67]. To measure the presence of co-existing ordered and disordered domains with FRET samples of vesicles were prepared with FRET donor, DPH (Fo samples), or both FRET donor and FRET acceptor, rhodamine-DOPE (F samples). Rhodamine-DOPE preferentially localizes in disordered domains [63, 68-70], while DPH tends to partition more-or-less equally between ordered and disordered lipids [71, 72]. Partial segregation of DPH from rhodamine-DOPE in bilayers containing co-existing ordered and disordered lipid domains results in weak FRET (weak quenching of DPH fluorescence), and thus higher F/Fo, where F/Fo is the ratio of DPH fluorescence in the presence of FRET acceptor to that in its absence (the level of FRET is equal to 1-F/Fo) [68]. Upon heating, ordered domains melt, and their lipids become disordered, mixing homogenously with the other lipids in the bilayer. This leads to a decreased average distance between FRET donor and FRET acceptor, and thus an increase in the FRET-induced quenching of DPH fluorescence by rhodamine-DOPE. The thermal stability of ordered domains can be defined from F/Fo versus temperature [63, 68, 73]. A parameter we have used in the past as a measure of ordered domain thermal stability is Tmid, or the apparent midpoint temperature for the transition between a bilayer with co-existing ordered and Ld domains, and a homogeneous Ld bilayer [63, 68, 74]. One can also estimate Tend, or the endpoint temperature for this transition. Figure 2 shows a schematic illustration of these two parameters. Tend is particularly useful when a transition occurs at too low a temperature for estimation of Tmid. For the sterols examined the order of Tend values was closely correlated with Tmid values (Table 1), indicating they are both good measures of relative ordered domain stability.
Figure 2.
Schematic illustration of melting parameters. The fraction of FRET donor fluorescence unquenched by FRET acceptor (F/Fo) vs. temperature is shown for two samples containing ordered domains with different melting/mixing temperatures. The definitions of Tmid (apparent midpoint of the melting transition) and Tend (the approximate end of the melting transition) are schematically illustrated. Sample “a” has a higher melting temperature than sample “b”. For sample b most ordered domain melting has already occurred at temperatures below the measured range. Notice that F/Fo can be temperature-dependent for a sample with no ordered domains (dashed line). See Methods and text for details.
Table 1.
Tmid and Tend values in symmetric LUV with different sterols. Samples were prepared as described in Methods and Figure 3. Tmid and Tend were calculated as described in Methods. Mean and standard deviation from three separate calculations are shown.
| Sterol | Symmetric LUV Tmid (°C) | Symmetric LUV Tend (°C) | ||||
|---|---|---|---|---|---|---|
| PBS In PBS Out |
Sucrose In PBS Out |
Sucrose In Sucrose Out |
PBS In PBS Out |
Sucrose In PBS Out |
Sucrose In Sucrose Out |
|
| 7DHC | 31.4 ± 0.1 | 31.0 ± 1.0 | 25.8 ± 0.2 | 56.0 ± 1.2 | 56.1 ± 1.5 | 59.8 ± 0.3 |
| Cholesterol | 26.0 ± 0.5 | 28.5 ± 0.6 | 23.5 ± 0.3 | 54.7 ± 0.7 | 54.9 ± 0.4 | 53.9 ± 0.8 |
| Desmosterol | 15.7 ± 4.4 | 22.8 ± 0.4 | 14.8 ± 2.5 | 43.9 ± 0.2 | 43.7 ± 1.7 | 52.9 ± 0.2 |
| Lanosterol | 20.6 ± 3.0 | 22.5 ± 0.6 | 21.1 ± 0.8 | 47.1 ± 1.6 | 43.6 ± 1.0 | 43.4 ± 1.0 |
| Epicholesterol | 20.2 ± 4.1 | 16.4 ± 9.5 | 22.0 ± 2.7 | 43.7 ± 0.6 | 41.1 ± 0.4 | 46.0 ± 0.5 |
| 4-cholesten-3-one | << 16°C | << 16°C | << 16°C | 28.1 ± 1.9 | 21.1 ± 1.6 | 26.0 ± 0.4 |
3.2. The Effect of Sterol Structure on Ordered Domain Stability in Symmetric Vesicles.
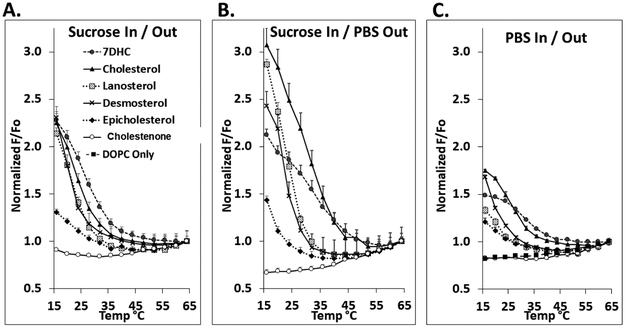
Figure 3 shows normalized F/Fo vs. temperature in symmetric vesicles containing mixtures of 1:1 mol:mol SM:DOPC with 25 mol% of various sterols. (Unnormalized F/Fo values are shown in Supplemental Figure 1.) Control vesicles composed of DOPC with 25 mol% cholesterol, which does not form ordered domains, were also prepared. Table 1 summarizes Tmid and Tend values for vesicles with different sterols. The significance (p-values) for differences in Tmid and Tend values from Table 1 for symmetric LUV with different sterols is given in Supplemental Table 1. On average, Tend values were 26.6 ± 1.4 °C higher than Tmid (Supplemental Table 2). It should be noted that prior studies have shown the thermal melting of ordered lipid domains is reversible in both symmetric [48, 63, 68, 74] and asymmetric vesicles [31].
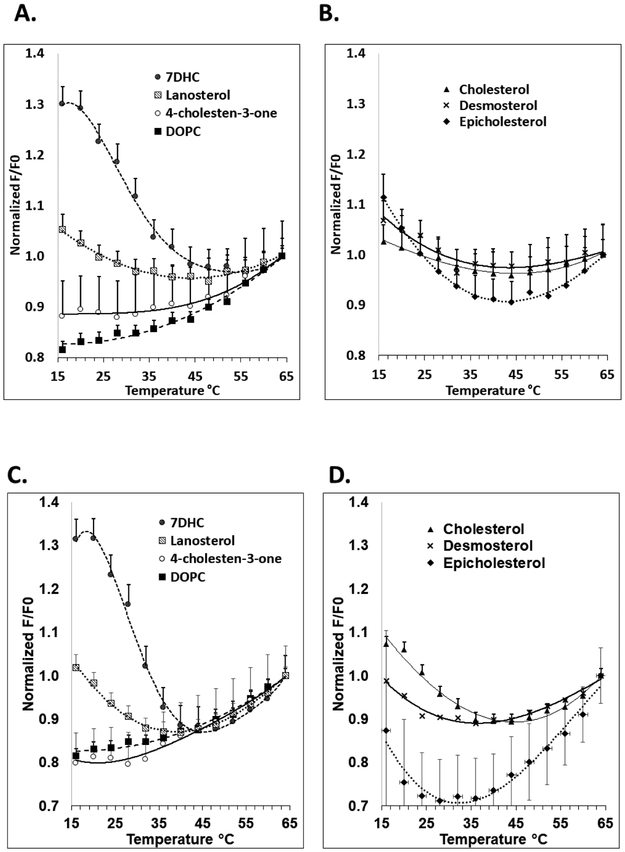
Figure 3.
Domain melting curves in symmetric vesicles assayed by FRET. The fraction of DPH fluorescence unquenched by rhodamine lipid (F/Fo) versus temperature shown after normalization to F/Fo at high temperature (64°C). Samples contained LUV with 100 μM lipid composed of 1:1 mol:mol DOPC:SM with 25 mol% sterol. Samples contained 0.01 μM DPH, and ‘F samples’ also contained 2 mol% rhodamine-DOPE. In panels A. and B., vesicles were formed with entrapped sucrose and dispersed in either sucrose (A.) or PBS (B.). In C., vesicles were formed with entrapped PBS and dispersed in PBS. Mean and standard deviation from three separate experiments are shown. Symbols: Filled circles, 7DHC; triangles, cholesterol; shaded squares, lanosterol; crosses, desmosterol; diamonds, epicholesterol; open circles; 4-cholesten-3-one; filled squares, no sterol.
Studies of sterol effects upon ordered domain formation in symmetric vesicles were previously carried out in PBS [12, 43]. However, asymmetric vesicles are generally prepared with sucrose entrapped in the aqueous lumen and then dispersed in PBS. To compare results in symmetric vesicles with those in asymmetric vesicles (see below), we prepared symmetric vesicles with the same solutions as for the asymmetric vesicles, i.e. sucrose entrapped in their aqueous lumen and dispersed in PBS. In addition, to determine the extent to which domain forming properties of the lipids were sensitive to aqueous environment, symmetric vesicles were prepared with PBS or sucrose in both the aqueous lumen and external solution.
In vesicles dispersed in PBS, both Tmid and Tend show that ordered domains were most stable in the presence of 7DHC and cholesterol. Ordered domains were stable to a lesser extent in the presence of lanosterol, epicholesterol and desmosterol, and least stable in the presence of 4-cholesten-3-one, which showed FRET behavior very similar to the control samples lacking ordered domains. This order is in agreement with previous studies, except that in the present study epicholesterol generally did not support ordered domain formation more strongly than lanosterol [43] (See Discussion for details). Sucrose-containing symmetric vesicles were prepared for comparison to asymmetric vesicle preparations (see below). FRET results similar to those in PBS were obtained with vesicles containing entrapped sucrose and sucrose in the external solution, and in vesicles containing entrapped sucrose and PBS in the external solution (Figure 3 and Table 1).
Note that in most cases Figure 3 shows a gradual increase in F/Fo at high temperatures under conditions in which ordered domains are absent/have melted. This may reflect a thermally-induced decrease in the fluorescence lifetime of the donor, which would reduce is vulnerability to FRET, or a thermally-induced change in the orientation of the FRET donor and acceptor which could increase FRET. Another factor is some preferential bleaching of DPH in the Fo samples relative to F samples as temperature is increased (data not shown).
3.3. Preparation of Asymmetric Vesicles with Different Sterols and Sphingomyelin Levels and Measurement of Ordered Domain Stability in Symmetric Vesicles with FRET.
Next, we measured ordered domain stability in asymmetric vesicles containing the same sterols used in symmetric vesicles. Large AUV were prepared using MαCD-mediated lipid exchange [32]. Exchange replaces the outer leaflet lipids in a population of vesicles (acceptor vesicles) with phospholipids and/or sphingolipids from a second population of vesicles (the donor vesicles). Sterols do not interact with MαCD and so are not disturbed by exchange [75]. In the experiments, DOPC and SM were exchanged into the outer leaflet of acceptor LUV containing DOPC and 25 mol% sterol. Since lipid flip-flop is slow and MαCD-mediated lipid exchange delivers lipids to only the outer leaflet, lipids introduced into acceptor vesicles by MαCD locate in the AUV outer leaflet ([61] and see below).
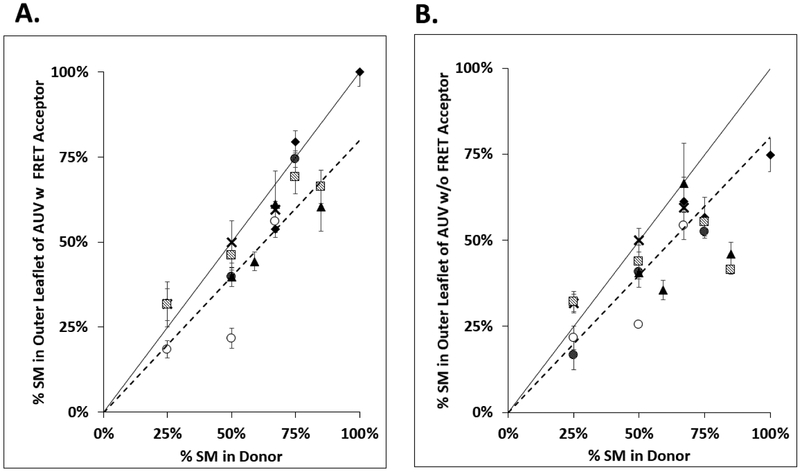
Outer leaflet composition of AUV was varied by using a range of donor lipid DOPC:SM ratios. Figure 4 shows the correlation between the % SM in donor phospholipids+sphingolipids (i.e. % SM in the non-sterol lipid) and the calculated % SM in the outer leaflet of resulting AUV measured by HP-TLC (Supplemental Tables 3 and 4). In general, the % SM in AUV was close to what was expected if the exchange equilibrated all of donor lipid with the outer leaflet lipids of acceptor vesicles (dashed line in Figure 4).
Figure 4.
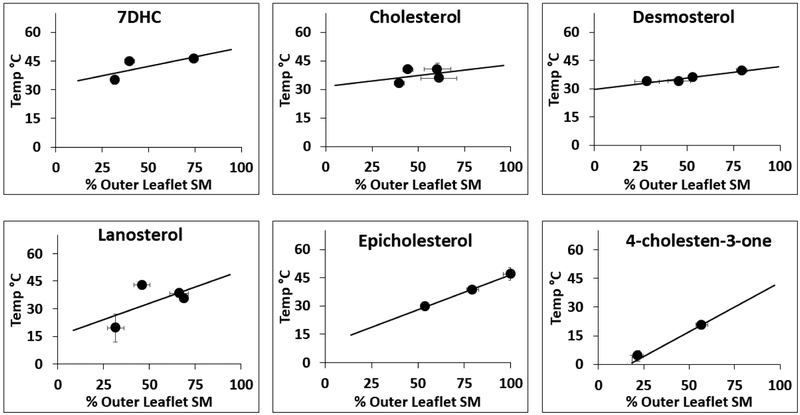
Correlation between donor lipid composition and outer leaflet SM composition in AUV. The percent outer leaflet SM in AUV vs. %SM in donor lipids used for lipid exchange is shown. Final samples consist of AUV with a variety of sterols, outer leaflet containing DOPC:SM and inner leaflet containing DOPC. % SM in donor = 100% × [(SM/(SM+DOPC)) in donor lipid]. The % outer leaflet SM = [(SM/(SM+DOPC))/0.52) × 100%] calculated from the total lipid composition of AUV. It assumes SM is transferred into the outer leaflet, and that outer leaflet contains 52% of the total AUV lipid. The dashed line represents outer leaflet SM composition if donor lipid and acceptor outer leaflet lipids are equilibrated by exchange. The solid line represents expected composition of outer leaflet if donor lipid simply replaced acceptor lipid. Panel A. shows AUV with rhodamine-DOPE. Panel B. shows AUV without rhodamine-DOPE. Mean and standard deviation from three separate experiments are shown. Symbol definitions as in Figure 3.
Next, FRET experiments were carried out upon the asymmetric vesicles. The AUV had sucrose in their internal lumen to aid in isolation. Ordered domain stability was measured with AUV dispersed in sucrose or PBS. Representative normalized F/Fo values vs. temperature are shown in Figure 5. Because it is impossible to precisely control the amount of SM introduced into AUV to get exactly 50 mol% outer leaflet SM, this data cannot be directly compared to that for symmetric vesicles with 50 mol% SM. To circumvent this issue, as noted above we prepared a series of AUV with different levels of SM in their outer leaflets by varying the donor lipid DOPC:SM ratio. Thermal stability of ordered domains in each preparation was then measured. To determine the thermal stability of ordered domains AUV with 50% outer leaflet SM, Tend values were graphed vs. % outer leaflet SM (Figure 6). This revealed a roughly linear relationship (Figure 6 and Supplemental Figure 3) which was used to estimate Tend at 50% outer leaflet SM (Table 2). The significance (p-values) for differences in mean Tend values for AUV with different sterols and different solution conditions is given in Supplemental Table 5. It should be noted that we observed a small difference in SM exchange efficiency when preparing F samples (which have rhodamine-DOPE) vs. Fo samples (which lack rhodamine-DOPE) even when using identical DOPC:SM donor lipid ratios (Supplemental Tables 3 and 4). However, because Fo fluorescence curves are not significantly dependent upon SM content (Supplemental Figure 4) this did not affect the shape of the F/Fo curves, and thus Tend values.
Figure 5.
Effect of sterol structure upon outer leaflet ordered-domain thermal stability in AUV. The fraction of DPH fluorescence unquenched by rhodamine lipid (F/Fo) versus temperature curves are shown after normalization to 1 at high temperature (64°C). AUV were dispersed either in sucrose (A. and B.) or PBS (C. and D.). Data is shown for compositions with outer leaflets close to 1:1 DOPC:SM, with inner leaflets containing DOPC, and with 25 mol% sterol. AUV F samples contain rhodamine-DOPE that was exchanged into the outer leaflet by mixing with donor lipids. Mean and standard deviation from three separate experiments are shown. Symbol definitions as in Figure 3.
Figure 6.
Effect of SM level upon Tend for AUV. Tend vs. % outer leaflet SM for AUV dispersed in PBS shown. For each sterol, three or four sets of AUV with varying DOPC:SM ratios in their outer leaflet were prepared. Mean Tend, mean % outer leaflet SM and standard deviation of both Tend (y-axis error bars) and of % outer leaflet SM (x axis error bars) from three separate experiments were calculated. Note: error bars are only visible when larger than symbol size.
Table 2.
Tend in AUV with 1:1 DOPC:SM outer leaflets. Tend was calculated from Tend vs. % outer leaflet SM as shown in Figure 6. Samples were dispersed in PBS (left column) or sucrose (right column). Mean and standard deviation from three separate vesicle preparations are shown.
| Sterol | Asymmetric LUV Tend (°C) | |
|---|---|---|
| Sucrose In PBS Out |
Sucrose In Sucrose Out |
|
| 7DHC | 42.4 ± 1.6 | 48.6 ± 2.9 |
| Cholesterol | 37.5 ± 0.7 | 43.8 ± 3.5 |
| Desmosterol | 36.1 ± 1.1 | 41.1 ± 1.1 |
| Lanosterol | 33.0 ± 2.3 | 40.4 ± 0.4 |
| Epicholesterol | 28.2 ± 0.1 | 30.7 ± 1.0 |
| 4-cholesten-3-one | 17.2 ± 1.7 | 21.4 ± 1.8 |
It is also noteworthy that the slope of the Tend vs. % outer leaflet SM line was similar for samples with different sterols. The slope was 0.28 ± 0.13°C per % outer leaflet SM for AUV dispersed in PBS and 0.35 ± 0.10°C per % outer leaflet SM for AUV dispersed in sucrose.
Another issue encountered was that the amount of rhodamine-DOPE transferred into the outer leaflet was low and somewhat variable. To compensate for the former, a high amount of rhodamine-DOPE was included in the donor lipid mixture. With regard to the latter, the variability of rhodamine-DOPE levels did not pose a problem for estimation of Tend. This is because although varying rhodamine-DOPE levels affects (unnormalized) F/Fo values (Supplemental Figure 5), control experiments showed that Tend was independent of F/Fo (Supplemental Figure 6).
3.4. The Effect of Sterol Structure on Ordered Domain Stability in Asymmetric Vesicles.
The data in Table 2 shows the effect of sterol structure on ordered domain stability in AUV. As in symmetric LUV, in AUV 7DHC and cholesterol most strongly stabilized ordered domains and 4-cholesten-3-one showed the least stable ordered domain formation. Desmosterol, lanosterol, and epicholesterol and were significantly less stabilizing than 7DHC, also as observed in symmetric vesicles. Both epicholesterol and (likely) lanosterol were also less stabilizing than cholesterol. However, in AUV desmosterol stabilized ordered domain formation almost as well as cholesterol. This contrasts with symmetric vesicles, in which cholesterol generally stabilizes ordered domains significantly more than desmosterol. Another difference between symmetric vesicles and AUV is that ordered domain stability in symmetric vesicles with epicholesterol was similar to that in vesicles with desmosterol and lanosterol, while in AUV epicholesterol stabilized ordered domains significantly less than desmosterol and lanosterol.
Another striking result was that Tend averaged about 10 °C higher for symmetric LUV than in the corresponding AUV (compare Tables 1 and 2). This difference likely reflects lower lipid ordering in AUV relative to symmetric vesicles due to interleaflet coupling (see Discussion).
It should also be noted that Tend was higher (5.3 ± 1.6 °C) for AUV dispersed in sucrose vs. those dispersed in PBS (Table 2). This might reflect the difference in osmotic pressure with these two different external solutions (see Discussion). Finally, it should be noted that there was a greater difference between F/Fo values at low and high temperatures in symmetric vesicles than in AUV (compare Figures 3 and 5). This likely reflects factors that decrease segregation between DPH and rhodamine-DOPE in AUV relative to that in symmetric vesicles (see Discussion).
3.5. Asymmetry in Asymmetric Vesicles is Stable.
An important parameter for asymmetric vesicles is the stability of asymmetry. Previous studies using various approaches have shown that lipid asymmetry is stable in vesicles containing SM and DOPC, with and without cholesterol present [28, 34, 61, 76]. One approach uses a fluorescently-labeled lipid (C6-NBD-PC) which flips between leaflets at a rate similar to corresponding unlabeled lipids [77]. NBD is destroyed, and its fluorescence abolished, by addition of the membrane-impermeable reagent sodium dithionite (NaDt). For this reason, when NaDt is added to the external solution, the fraction of NBD fluorescence abolished equals the fraction of NBD lipid in the vesicle outer leaflet. The rate of lipid flip can be measured by introducing C6-NBD-PC into only one leaflet and measuring the amount of NBD fluorescence abolished vs. incubation time prior to NaDt addition. Here, these measurements were carried out using symmetric vesicles and AUV containing SM, DOPC and cholesterol or 4-cholesten-3-one. We chose 4-cholesten-3-one, which can loosen lipid packing [43], as the steroid most likely to increase phospholipid flip relative to vesicles with cholesterol. We found that both in AUV with cholesterol and 4-cholsten-3-one flip was slow, indicating that phospholipid asymmetry remained largely intact for at least 48 h (see Supplemental Results and Supplemental Figure 7).
4. Discussion
4.1. Reevaluation of the Effect of Sterol Structure Upon Ordered Domain Formation and Stability.
In this study, the effect of sterol structure on membrane domain formation was both reevaluated in symmetric vesicles, and then measured in asymmetric vesicles. The results from symmetric vesicles and asymmetric vesicles were then compared. In general terms, for symmetric vesicles dispersed in PBS the ability of sterols to support ordered domain formation was similar to that observed in our prior studies [12, 43, 48, 49]. Ordered domain formation was most strongly promoted by 7DHC, somewhat less by cholesterol, even less by epicholesterol, lanosterol and desmosterol, and least by 4-cholsten-3-one. However, there was one noteworthy difference in that we found here that epicholesterol supported ordered domain formation to a significantly lesser degree than in prior studies, in which epicholesterol supported ordered domain formation only slightly less than cholesterol. As noted above, a possible explanation is that the prior studies involved a different vesicle type and lipid composition, specifically symmetric multilamellar vesicles containing of DPPC in place of SM, and 12SLPC, a nitroxide-labeled lipid, in place of DOPC [43].
The effect of sterol structure on ordered domain formation in symmetric and asymmetric vesicles was similar, following the same general ranking noted above. However, one difference was that in AUV desmosterol stabilized ordered domain formation almost as well as cholesterol. The present study, prior studies by our lab, and investigations by others [52] all found that in symmetric vesicles, desmosterol supports ordered domain formation to a much lesser degree than cholesterol. The physical explanation for this difference is not obvious, but presumably involves interleaflet interactions. In symmetric vesicles, ordered domains in each leaflet are spatially coupled, with inner and outer ordered domains showing up in register with one another [78]. This is indicative of ordered domains in one leaflet stabilizing the formation of ordered domains in the opposite leaflet. It is possible that desmosterol, which differs from cholesterol by having a double bond it is aliphatic tail, somehow reduces the coupling between the inner and outer leaflets, and thus the extent to which ordered domains in different leaflets can stabilize each other in symmetric vesicles.
It should be noted that additional factors may alter that effects of sterols in vivo. One is the distribution of sterols in each leaflet. The question of sterol leaflet localization is very controversial. Some groups believe it is mainly located in the inner leaflet, others believe it is mainly in the outer leaflet.
4.2. Reasons for Differences in Domain Stability and FRET in Symmetric and Asymmetric Vesicles.
There were other differences between the properties of asymmetric and symmetric vesicles. First, the stability of ordered domains was less in asymmetric vesicles than in symmetric vesicles, as shown by lower Tend values in asymmetric vesicles. This agrees with results from our very recent studies [31], and indicates that the interaction of the outer leaflet with the disordered state favoring lipids in the inner leaflet destabilizes outer leaflet ordered domain formation.
Another difference was that the difference between FRET (i.e. F/Fo) in the presence of ordered domains at low temperature and F/Fo in their absence at higher temperatures was much smaller in asymmetric vesicles than in symmetric vesicles. One factor is the lesser thermal stability of ordered domains in AUV. Because of this, the fraction of the bilayer in an ordered state would be less in the AUV relative to symmetric vesicles at the same temperature. An additional factor could be the reduced segregation of FRET donor and acceptor in asymmetric vs. symmetric vesicles due to lesser preferential partition into specific domains. In this regard it has been found that coupling between ordered and disordered domains in opposite leaflets can loosen packing in the ordered domains [36]. If lipid packing in the outer leaflet ordered domains is looser in AUV than in symmetric vesicles, then rhodamine-DOPE might partition a bit more into the ordered domains in AUV, leading to increased FRET (lower F/Fo) when domains are present in AUV relative to that in symmetric vesicles.
Finally, a smaller domain size is another factor that could reduce the effective segregation of donor and acceptor. If domains are small, then a significant amount of FRET donors and acceptors will be located near domain boundaries, such that FRET acceptor in one domain could frequently accept fluorescence from a FRET donor in another domain. This would result in increased FRET relative to when domains are large, and could explain the difference in FRET between symmetric and asymmetric vesicles if domains are smaller in AUV than in symmetric vesicles.
4.3. Effect of Aqueous Solution Composition Upon Ordered Domain Stability.
We did not observe any consistent effect of sterol structure on ordered domain stability in symmetric vesicles with sucrose vs. PBS in the external solution. However, in AUV ordered domain stability was greater in samples containing sucrose in the external solution than in samples containing external PBS. A possible explanation arises from the fact that the samples with PBS outside were under a net osmotic pressure that would tend to slightly stretch the bilayer (25% (w/w) sucrose ~800 mOsm, 1× PBS ~325 mOsm), while those with sucrose outside were not under a net osmotic pressure. Loosely packed disordered domains stretch under osmotic pressure, while tightly packed ordered domains do not, as demonstrated by osmotic swelling of lipid vesicles when in the disordered state, but not when in an ordered state [79-81]. In an asymmetric bilayer, if the inner leaflet is in a disordered state and stretches it should become more disordered. To the extent that coupling occurs between the inner and outer leaflet, the ordered state in the outer leaflet should be destabilized. This is interesting, because it suggests that conditions that stretch asymmetric membranes in vivo might tend to destabilize ordered domain formation. In symmetric vesicles with co-existing ordered and disordered domains, osmotic pressure would not be expected to greatly destabilize ordered domains which are in register (i.e. span the bilayer) and are resistant to stretching. Instead, the disordered domains should stretch.
4.4. Reevaluating Proposed Roles of the Raft-forming Properties of Sterols in Endocytosis and Bacterial Uptake.
The differences between symmetric model membrane vesicles and natural membranes are so large in terms of asymmetry, the complexity of membrane lipid composition, and the presence or absence of membrane proteins, that it is difficult to extrapolate results from simple symmetric vesicles to predict the properties of natural membranes. The present study narrows this gap by taking lipid asymmetry into account. The difference in sterol behavior in symmetric and asymmetric vesicles we find has important functional implications, and allows us to make more accurate predictions about for what processes ordered membrane domains are or are not of functional importance in living cells. For example, the revised estimates of the influence of desmosterol and epicholesterol on ordered domain formation from our study help explain results from sterol substitution studies examining the relationship between sterol structure and endocytosis. We previously concluded that the level of endocytosis (both clathrin-mediated and clathrin-independent) showed a strong correlation with the Lo domain (raft) forming properties of a sterol, but that desmosterol and epicholesterol were exceptions, in that the former did support a high level of endocytosis despite a modest ability to support ordered domain formation, while the latter did not support endocytosis, despite a structure that strongly supported ordered domain formation [27] [59]. We proposed that the presence of the 3-alpha configuration OH group in epicholesterol (it is 3-beta in cholesterol) somehow interfered with endocytosis. We now revise these conclusions. Based on the fact that the plasma membrane is highly asymmetric with SM concentrated in the exofacial leaflet, we would say the scale of sterol raft-stabilizing ability in asymmetric membranes is more relevant to that in cells than raft-stabilizing ability in symmetric vesicles. On this basis it appear the correlation of endocytosis with raftforming ability of a sterol is very strong, even stronger than we had previously proposed. Also, we cannot conclude that there is an endocytosis-inhibiting effect when a sterol has a 3 alpha configuration OH group because we now have found that epicholesterol is only modestly raft-stabilizing. Similarly, we would revise the conclusions of a follow-up study examining the ability of Yersinia pseudotuberculosis to enter host cells after a variety of sterols was substituted for cholesterol [59]. Only a subset of the sterols we had classified as able to strongly support ordered domain formation supported uptake of Yersinia. In addition, desmosterol supported uptake. Based on our prior estimate of desmosterol’s weak raft-forming ability, we suggested that the ability to strongly form ordered domains was neither necessary nor sufficient to support uptake of Yersinia. We would now say the ability to strongly support ordered domain formation is necessary, but not sufficient, to support uptake.
Overall, the revised estimates of the abilities of different sterols to stabilize the formation of ordered domains strengthens the possibility that the ability of a sterol to support ordered domain formation is a crucial property in endocytosis and bacterial uptake. However, it should be emphasized that this does not by itself directly demonstrate that ordered domains are involved in these processes. The reason for this is that other properties that are closely related to the ability of a sterol to stabilize the formation of ordered domains, such as overall membrane order, or the tightness of sphingolipid-sterol interactions could be involved rather than membrane domains themselves.
We hope the revised ranking of sterol propensities to support ordered domain formation resulting from these studies in asymmetric vesicles will help improve future predictions regarding the functional importance of ordered domains in other biological processes. Of course, additional factors such as the exact sphingolipid/phospholipid composition of each leaflet and presence of membrane proteins in natural membranes could also influence how sterols stabilize ordered domains. These will have to be addressed in future studies.
Supplementary Material
Highlights.
Sterol structure effects on rafts differ in symmetric and asymmetric vesicles.
Epicholesterol was modestly raft stabilizing in symmetric and asymmetric vesicles.
Desmosterol was strongly raft stabilizing in asymmetric vesicles only.
Effect of sterol properties on endocytosis and bacterial uptake was re-evaluated.
These processes were found to be closely linked to sterol raft supporting ability.
Acknowledgments
This work was supported by NIH Grant GM 122493
Abbreviations:
- AUV
asymmetric unilamellar vesicles
- C6-NBD-PC
1-palmitoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl) amino] hexanoyl]-sn-glycero-3-phosphocholine
- Chol
cholesterol
- Desm
desmosterol
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- rhodamine-DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine- N-(lissamine rhodamine B sulfonyl)
- DPH
diphenylhexatriene
- Epi
epicholesterol
- 4-chol
4-cholesten-3-one
- FRET
Förster resonance energy transfer
- HPαCD
hydroxy propyl-alpha-cyclodextrin
- HP-TLC
high-performance thin-layer chromatography
- Lan
lanosterol
- LUV
large unilamellar vesicles
- Ld
liquid disordered state
- Lo
liquid ordered state
- MαCD
methyl-alpha-cyclodextrin
- PBS
phosphate buffered saline
- 7DHC
7-dehydrocholesterol
- SM
sphingomyelin
- Tend
ordered domain melting endpoint
- Tmid
ordered domain melting midpoint
- 12SLPC
1-palmitoyl-2-(12-doxyl)stearoyl-sn-glycero-3-phosphocholine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mondal M, Mesmin B, Mukherjee S, Maxfield FR, Sterols Are Mainly in the Cytoplasmic Leaflet of the Plasma Membrane and the Endocytic Recycling Compartment in CHO Cells, Molecular Biology of the Cell, 20 (2009) 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu SL, Sheng R, Jung JH, Wang L, Stec E, O'Connor MJ, Song S, Bikkavilli RK, Winn RA, Lee D, Baek K, Ueda K, Levitan I, Kim KP, Cho W, Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol, Nature Chemical Biology, 13 (2017) 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bretscher MS, Asymmetrical lipid bilayer structure for biological membranes, Nature: New Biology, 236 (1972) 11–12. [DOI] [PubMed] [Google Scholar]
- [4].Verkleij A, Zwaal R, Roelofsen B, Comfurius P, Kastelijn D, Van Deenen L, The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy, Biochimica et Biophysica Acta (BBA)-Biomembranes, 323 (1973) 178–193. [DOI] [PubMed] [Google Scholar]
- [5].Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA, Phosphatidylserine receptor is required for clearance of apoptotic cells, Science, 302 (2003) 1560–1563. [DOI] [PubMed] [Google Scholar]
- [6].Lentz BR, Exposure of platelet membrane phosphatidylserine regulates blood coagulation, Progress in Lipid Research, 42 (2003) 423–438. [DOI] [PubMed] [Google Scholar]
- [7].Morizono K, Chen IS, Role of phosphatidylserine receptors in enveloped virus infection, Journal of Virology, 88 (2014) 4275–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mercer J, Helenius A, Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells, Science, 320 (2008) 531–535. [DOI] [PubMed] [Google Scholar]
- [9].Brown DA, London E, Functions of lipid rafts in biological membranes, Annual Review of Cell and Developmental Biology, 14 (1998) 111–136. [DOI] [PubMed] [Google Scholar]
- [10].Brown DA, London E, Structure and origin of ordered lipid domains in biological membranes, Journal of Membrane Biology, 164 (1998) 103–114. [DOI] [PubMed] [Google Scholar]
- [11].Grosjean K, Mongrand S, Beney L, Simon-Plas F, Gerbeau-Pissot P, Differential effect of plant lipids on membrane organization: hot features and specificities of phytosphingolipids and phytosterols, Journal of Biological Chemistry, (2015) jbc. M114. 598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E, Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide, Journal of Biological Chemistry, 276 (2001) 33540–33546. [DOI] [PubMed] [Google Scholar]
- [13].Ramstedt B, Slotte JP, Sphingolipids and the formation of sterol-enriched ordered membrane domains, Biochimica et Biophysica Acta, 1758 (2006) 1945–1956. [DOI] [PubMed] [Google Scholar]
- [14].Korlach J, Schwille P, Webb WW, Feigenson GW, Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy, Proceedings of the National Academy of Sciences of the United States of America, 96 (1999) 8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levental I, Grzybek M, Simons K, Raft domains of variable properties and compositions in plasma membrane vesicles, Proceedings of the National Academy of Sciences of the United States of America, 108 (2011) 11411–11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schroeder R, London E, Brown D, Interactions between Saturated Acyl Chains Confer Detergent Resistance on Lipids and Glycosylphosphatidylinositol (Gpi)-Anchored Proteins - Gpi-Anchored Proteins in Liposomes and Cells Show Similar Behavior, Proceedings of the National Academy of Sciences of the United States of America, 91 (1994) 12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brown DA, London E, Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes?, Biochemical and Biophysical Research Communications, 240 (1997) 1–7. [DOI] [PubMed] [Google Scholar]
- [18].Simons K, Ikonen E, Functional rafts in cell membranes, Nature, 387 (1997) 569–572. [DOI] [PubMed] [Google Scholar]
- [19].Taylor DR, Hooper NM, Role of lipid rafts in the processing of the pathogenic prion and Alzheimer's amyloid-beta proteins, Seminars in Cell and Developmental Biology, 18 (2007) 638–648. [DOI] [PubMed] [Google Scholar]
- [20].Williamson R, Usardi A, Hanger DP, Anderton BH, Membrane-bound beta-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism, FASEB Journal, 22 (2008) 1552–1559. [DOI] [PubMed] [Google Scholar]
- [21].Cuadras MA, Greenberg HB, Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo, Virology, 313 (2003) 308–321. [DOI] [PubMed] [Google Scholar]
- [22].Gulbins E, Kolesnick R, Raft ceramide in molecular medicine, Oncogene, 22 (2003) 7070–7077. [DOI] [PubMed] [Google Scholar]
- [23].Lyman MG, Curanovic D, Enquist LW, Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts, PLoS Pathogens,4 (2008) e1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murphy SC, Hiller NL, Harrison T, Lomasney JW, Mohandas N, Haldar K, Lipid rafts and malaria parasite infection of erythrocytes, Molecular Membrane Biology, 23 (2006) 81–88. [DOI] [PubMed] [Google Scholar]
- [25].Riethmuller J, Riehle A, Grassme H, Gulbins E, Membrane rafts in host-pathogen interactions, Biochimica et Biophysica Acta, 1758 (2006) 2139–2147. [DOI] [PubMed] [Google Scholar]
- [26].Korade Z, Kenworthy AK, Lipid rafts, cholesterol, and the brain, Neuropharmacology, 55 (2008) 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim JH, Singh A, Del Poeta M, Brown DA, London E, The effect of sterol structure upon clathrin-mediated and clathrin-independent endocytosis, Journal of Cell Science, 130 (2017) 2682–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin Q, London E, Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles, Biophysical Journal, 108 (2015) 2212–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin Q, London E, The influence of natural lipid asymmetry upon the conformation of a membrane-inserted protein (perfringolysin O), Journal of Biological Chemistry, 289 (2014) 5467–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lin Q, London E, Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry, PloS One, 9 (2014) e87903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang Q, London E, Lipid Structure and Composition Control Consequences of Interleaflet Coupling in Asymmetric Vesicles, Biophysical Journal, 115 (2018) 664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li G, Kim J, Huang Z, St Clair JR, Brown DA, London E, Efficient replacement of plasma membrane outer leaflet phospholipids and sphingolipids in cells with exogenous lipids, Proceedings of the National Academy of Sciences of the United States of America, 113 (2016) 14025–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Uekama K, Hirayama F, Irie T, Cyclodextrin Drug Carrier Systems, Chemical Reviews, 98 (1998) 2045–2076. [DOI] [PubMed] [Google Scholar]
- [34].Cheng HT, Megha, London E , Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation, Journal of Biological Chemistry, 284 (2009) 6079–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chiantia S, London E, Acyl chain length and saturation modulate interleaflet coupling in asymmetric bilayers: effects on dynamics and structural order, Biophysical Journal, 103 (2012) 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heberle FA, Marquardt D, Doktorova M, Geier B, Standaert RF, Heftberger P, Kollmitzer B, Nickels JD, Dick RA, Feigenson GW, Katsaras J, London E, Pabst G, Subnanometer Structure of an Asymmetric Model Membrane: Interleaflet Coupling Influences Domain Properties, Langmuir, 32 (2016) 5195–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eicher B, Marquardt D, Heberle FA, Letofsky-Papst I, Rechberger GN, Appavou MS, Katsaras J, Pabst G, Intrinsic Curvature-Mediated Transbilayer Coupling in Asymmetric Lipid Vesicles, Biophysical Journal, 114 (2018) 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wan C, Kiessling V, Tamm LK, Coupling of cholesterol-rich lipid phases in asymmetric bilayers, Biochemistry, 47 (2008) 2190–2198. [DOI] [PubMed] [Google Scholar]
- [39].Collins MD, Keller SL, Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chiantia S, Schwille P, Klymchenko AS, London E, Asymmetric GUVs prepared by MbetaCD-mediated lipid exchange: an FCS study, Biophysical Journal, 100 (2011) L1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fujimoto T, Parmryd I, Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation, Front Cell Dev Biol, 4 (2016) 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Silvius JR, Role of cholesterol in lipid raft formation: lessons from lipid model systems, Biochimica et Biophysica Acta, 1610 (2003) 174–183. [DOI] [PubMed] [Google Scholar]
- [43].Xu XL, London E, The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation, Biochemistry, 39 (2000) 843–849. [DOI] [PubMed] [Google Scholar]
- [44].Huang J, Feigenson GW, A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers, Biophysical Journal, 76 (1999) 2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Beattie ME, Veatch SL, Stottrup BL, Keller SL, Sterol structure determines miscibility versus melting transitions in lipid vesicles, Biophysical Journal, 89 (2005) 1760–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wenz JJ, Barrantes FJ, Steroid structural requirements for stabilizing or disrupting lipid domains, Biochemistry, 42 (2003) 14267–14276. [DOI] [PubMed] [Google Scholar]
- [47].Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP, Cholesterol interactions with phospholipids in membranes, Progress in Lipid Research, 41 (2002) 66–97. [DOI] [PubMed] [Google Scholar]
- [48].Megha, Bakht O, London E, Cholesterol precursors stabilize ordinary and ceramide-rich ordered lipid domains (lipid rafts) to different degrees. Implications for the Bloch hypothesis and sterol biosynthesis disorders, Journal of Biological Chemistry, 281 (2006) 21903–21913. [DOI] [PubMed] [Google Scholar]
- [49].Wang J, Megha, London E, Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): implications for lipid raft structure and function, Biochemistry, 43 (2004) 1010–1018. [DOI] [PubMed] [Google Scholar]
- [50].Rohanizadegan M, Sacharow S, Desmosterolosis presenting with multiple congenital anomalies, European Journal of Medical Genetics, 61 (2018) 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Porter FD, Herman GE, Malformation syndromes caused by disorders of cholesterol synthesis, Journal of Lipid Research, 52 (2011) 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vainio S, Jansen M, Koivusalo M, Rog T, Karttunen M, Vattulainen I, Ikonen E, Significance of sterol structural specificity. Desmosterol cannot replace cholesterol in lipid rafts, Journal of Biological Chemistry, 281 (2006) 348–355. [DOI] [PubMed] [Google Scholar]
- [53].Andersson HC, Kratz L, Kelley R, Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay, American Journal of Medical Genetics, 113 (2002) 315–319. [DOI] [PubMed] [Google Scholar]
- [54].Romanenko VG, Rothblat GH, Levitan I, Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol, Biophysical Journal, 83 (2002) 3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Romanenko VG, Rothblat GH, Levitan I, Sensitivity of volume-regulated anion current to cholesterol structural analogues, Journal of General Physiology, 123 (2004) 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang J, Wu F, Shi C, Substitution of membrane cholesterol with beta-sitosterol promotes nonamyloidogenic cleavage of endogenous amyloid precursor protein, Neuroscience, 247 (2013) 227–233. [DOI] [PubMed] [Google Scholar]
- [57].Jafurulla M, Chattopadhyay A, Structural stringency of cholesterol for membrane protein function utilizing stereoisomers as novel tools: a review, in: Gelissen I BA. (Ed.) Cholesterol Homeostasis, Humana Press, New York, New York, 2017, pp. 21–39. [DOI] [PubMed] [Google Scholar]
- [58].Jafurulla M, Rao BD, Sreedevi S, Ruysschaert JM, Covey DF, Chattopadhyay A, Stereospecific requirement of cholesterol in the function of the serotonin1A receptor, Biochimica et Biophysica Acta, 1838 (2014) 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kim J, Fukuto HS, Brown DA, Bliska JB, London E, Effects of host cell sterol composition upon internalization of Yersinia pseudotuberculosis and clustered β1 integrin, Journal of Biological Chemistry, 293 (2018) 1466–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].LaRocca TJ, Pathak P, Chiantia S, Toledo A, Silvius JR, Benach JL, London E, Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts, PLoS Pathogens, 9 (2013) e1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Son M, London E, The dependence of lipid asymmetry upon polar headgroup structure, Journal of Lipid Research, 54 (2013) 3385–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].LeBarron J, London E, Effect of lipid composition and amino acid sequence upon transmembrane peptide-accelerated lipid transleaflet diffusion (flip-flop), Biochimica Et Biophysica Acta-Biomembranes, 1858 (2016) 1812–1820. [DOI] [PubMed] [Google Scholar]
- [63].Pathak P, London E, The Effect of Membrane Lipid Composition on the Formation of Lipid Ultrananodomains, Biophysical Journal, 109 (2015) 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lin QQ, London E, Transmembrane Protein (Perfringolysin O) Association with Ordered Membrane Domains (Rafts) Depends Upon the Raft-Associating Properties of Protein-Bound Sterol, Biophysical Journal, 105 (2013) 2733–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Keller RK, Arnold TP, Fliesler SJ, Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome, Journal of Lipid Research, 45 (2004) 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bacia K, Schwille P, Kurzchalia T, Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes, Proceedings of the National Academy of Sciences of the United States of America, 102 (2005) 3272–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Heberle FA, Wu J, Goh SL, Petruzielo RS, Feigenson GW, Comparison of three ternary lipid bilayer mixtures: FRET and ESR reveal nanodomains, Biophysical Journal, 99 (2010) 3309–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pathak P, London E, Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation, Biophysical Journal, 101 (2011) 2417–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Loura LMS, Fedorov A, Prieto M, Fluid–Fluid Membrane Microheterogeneity: A Fluorescence Resonance Energy Transfer Study, Biophysical Journal, 80 (2001) 776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Baumgart T, Hess ST, Webb WW, Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension, Nature, 425 (2003) 821–824. [DOI] [PubMed] [Google Scholar]
- [71].Ahmed SN, Brown DA, London E, On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes, Biochemistry, 36 (1997) 10944–10953. [DOI] [PubMed] [Google Scholar]
- [72].Lentz BR, Barenholz Y, Thompson TE, Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes, Biochemistry, 15 (1976) 4521–4528. [DOI] [PubMed] [Google Scholar]
- [73].Bakht O, Pathak P, London E, Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms, Biophysical Journal, 93 (2007) 4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fastenberg ME, Shogomori H, Xu X, Brown DA, London E, Exclusion of a transmembrane-type peptide from ordered-lipid domains (rafts) detected by fluorescence quenching: extension of quenching analysis to account for the effects of domain size and domain boundaries, Biochemistry, 42 (2003) 12376–12390. [DOI] [PubMed] [Google Scholar]
- [75].Huang Z, London E, Effect of cyclodextrin and membrane lipid structure upon cyclodextrin–lipid interaction, Langmuir, 29 (2013) 14631–14638. [DOI] [PubMed] [Google Scholar]
- [76].Cheng HT, London E, Preparation and properties of asymmetric large unilamellar vesicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature, Biophysical Journal, 100 (2011) 2671–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].John K, Schreiber S, Kubelt J, Herrmann A, Muller P, Transbilayer movement of phospholipids at the main phase transition of lipid membranes: Implications for rapid flip-flop in biological membranes, Biophysical Journal, 83 (2002) 3315–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Garg S, Ruhe J, Ludtke K, Jordan R, Naumann CA, Domain registration in raft-mimicking lipid mixtures studied using polymer-tethered lipid bilayers, Biophysical Journal, 92 (2007) 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].London E, Feigenson GW, Fluorescence quenching in model membranes. 1. Characterization of quenching caused by a spin-labeled phospholipid, Biochemistry, 20 (1981) 1932–1938. [DOI] [PubMed] [Google Scholar]
- [80].Blok M, Van Deenen L, De Gier J, Effect of the gel to liquid crystalline phase transition on the osmotic behaviour of phosphatidycholine liposomes, Biochimica et Biophysica Acta (BBA)-Biomembranes, 433 (1976) 1–12. [DOI] [PubMed] [Google Scholar]
- [81].Boss WF, Kelley CJ, Landsberger FR, A novel synthesis of spin label derivatives of phosphatidylcholine, Analytical Biochemistry, 64 (1975) 289–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.