Abstract
The implication of RNA in multiple cellular processes beyond protein coding has revitalized interest in the development of small molecules for therapeutically targeting RNA and for further probing its cellular biology. However, the process of rationally designing such small molecule probes is hampered by the paucity of information about fundamental molecular recognition principles of RNA. In this Review, we summarize two important and often underappreciated aspects of RNA–small molecule recognition: RNA conformational dynamics and the biophysical properties of interactions of small molecules with RNA, specifically thermodynamics and kinetics. While conformational flexibility is often said to impede RNA ligand development, the ability of small molecules to influence the RNA conformational landscape can have a significant effect on the cellular functions of RNA. An analysis of the conformational landscape of RNA and the interactions of individual conformations with ligands can thus guide the development of new small molecule probes, which needs to be investigated further. Additionally, while it is common practice to quantify the binding affinities (Ka or Kd) of small molecules for biomacromolecules as a measure of their activity, further biophysical characterization of their interaction can provide a deeper understanding. Studies that focus on the thermodynamic and kinetic parameters for interaction between RNA and ligands are next discussed. Finally, this Review provides the reader with a perspective on how such in-depth analysis of biophysical characteristics of the interaction of RNA and small molecules can impact our understanding of these interactions and how they will benefit the future design of small molecule probes.
Graphical Abstract
Previously considered to act mainly as a messenger, RNA has received a growing level of attention in recent years as evidence of its direct participation in cellular processes continues to accumulate.1–3 In addition to the well-characterized rRNA, tRNA, microRNAs, and other small silencing RNAs, several other noncoding transcripts that directly participate in the progression of various cancers, cardiovascular disease, and neurological disorders have been discovered.4–9 Furthermore, many bacterial and viral diseases extensively rely on RNA-mediated processes for survival and proliferation.10–13 Disease-mediating RNAs thus offer an opportunity to develop therapies for challenging diseases such as prostate cancer, breast cancer, myotonic dystrophies, Huntington’s disease, HIV/AIDS, etc. As a result, there has been an increased level of interest in targeting RNA with druglike small molecules.14–19
Notwithstanding a few promising examples, the path toward fully harnessing the therapeutic potential of RNA with small molecules remains arduous.14,20,21 A major challenge is the limited understanding of the principles underlying the recognition of RNA by its cellular binding partners.22–26 Because these recognition events are ultimately responsible for RNA function, the paucity of biophysical information in this area has hampered efforts to design small molecules to modulate RNA function. A similar lack of complete biophysical characterization is apparent for the small molecules that have been shown to interact with RNA, because these complexes are rarely evaluated beyond the measurement of the equilibrium dissociation constant (Kd). Overemphasis on quantifying the binding efficiency in terms of Kd may preclude the potential benefits of a broader characterization process that includes consideration of the small molecule modulation of RNA dynamics as well as the detailed study of other thermodynamic and kinetic properties of RNA–small molecule interactions. As evidenced by studies that use these techniques, some which are discussed below, such analyses are likely to provide more insights into the molecular basis of RNA–small molecule recognition. These studies help reveal the fundamental principles of these interactions that are necessary for further development of small molecule probes that target RNA effectively in the cell.
From a ligand development perspective, RNA flexibility has been raised as a significant challenge to structure-based approaches.27,28 However, this flexibility can also offer opportunities for modulation of RNA function by altering its conformational landscape.29–31 For example, binding of a ligand to the internal ribosome entry site (IRES) of various RNA viruses inhibits RNA translation by perturbing local RNA flexibility.29 Similarly, gene regulation by some riboswitches proceeds via a mechanism in which the ligand induces a conformation unsuitable for carrying out the normal RNA function.32 As such, detailed characterization of the conformational outcomes of ligand binding and its downstream effects may lead to increased success in RNA ligand development.
Similarly, in-depth thermodynamic studies of RNA–small molecule interactions, while often challenging, are likely to aid in RNA ligand development. Detailed thermodynamic studies often provide insight into how given functional groups contribute to the enthalpy and entropy of RNA–small molecule interactions as well as how these energetic terms contribute to binding selectivity. These molecular and energy details are essential for future rational design of selective RNA-targeting probes.
Lastly, we consider the role of investigating the kinetics of RNA–small molecule interactions. For protein targets, it has become apparent that using Kd alone is not sufficient to predict biological activity especially in cases in which the duration of the target–ligand complex is more important for function modulation than for the rate at which the complex forms.33 The same phenomenon is likely to apply to RNA targets. Additionally, for RNAs such as bacterial riboswitches that are under kinetic control,34,35 an efficient regulatory ligand is likely to be one with fast association, leading to effective competition with the endogenous ligand of the target. Studies considering kinetics are thus likely to be more efficient than those that focus solely on optimizing Kd’s.
Herein, we briefly summarize representative studies that highlight the importance of conformational dynamics along with the kinetics and thermodynamics of RNA–small molecule interactions and provide a perspective on how such studies can be beneficial for the future development of RNA-targeted small molecule probes. We will first discuss examples of small molecule-induced changes in the RNA conformational landscape and the derived insight into RNA recognition. Insights from select thermodynamic and kinetic studies of RNA–small molecule interactions will then be presented. While other recent reviews have focused on the discovery, design, and bioactivity of RNA ligands,14,17,19,20 we propose that an increased emphasis on the fundamental biophysical aspects of RNA recognition will be integral to the future design and development of small molecule probes for successfully targeting RNA in disease.
MODULATION OF THE RNA CONFORMATIONAL LANDSCAPE
The highly dynamic nature of RNA allows it to undergo conformational changes upon interaction with binding partners and other cellular stimuli.36–38 This ability to adopt several conformations is foundational to the large number of RNA functions despite the low chemical diversity of RNA compared to that of proteins.38 Given the importance of RNA conformational states and the associated conformational transitions,39,40 it is plausible that small molecules could modulate RNA function by altering the RNA’s conformational landscape or the rates of transition between two or more conformations. In the first scenario, the small molecule can stabilize and enrich a preexisting RNA conformation41 or induce a new conformation,42 though these two paths can be difficult to distinguish. In the second scenario, the small molecule can interact with key positions on the RNA, affecting the rates of RNA dynamics, which are responsible for the conformational transition.36 Riboswitches are an excellent example of ligand-induced conformational changes. Upon binding of specific ligands, these bacterial mRNA domains undergo a large conformational switch resulting in changes in gene expression.43–46 While modulation of RNA dynamics and conformational landscapes by small molecules is widely accepted as a regulatory mechanism, outside of riboswitches, we currently have limited examples of studies specifically analyzing the effect of ligand binding on RNA conformation. Such investigations are important in the study of RNA–small molecule interactions as they provide insight into the ligand’s binding mode and mechanism of modulation, both of which can be useful in the future design of ligands with improved efficacy. Several studies have focused on understanding the conformational dynamics in RNA structure caused by small molecule interactions. In this section, we will discuss representative examples of such studies using different techniques and present an overview of the insights gleaned from them.
Example Studies.
NMR studies of conformational dynamics have significantly enhanced the current understanding of this complex subject. An RNA commonly used as a model system for studying RNA dynamics is HIV-1 TAR (Figure 1a). For example, Al-Hashimi and co-workers used various nuclear magnetic resonance (NMR) techniques to evaluate the effect of argininamide (Figure 1c) binding to a TAR analogue with a modified loop and found that binding led to a decreased average interhelical angle (Figure 1b).47 This change in global conformation was similar to that induced by magnesium but was achieved through different local conformations of the bulge and neighboring residues. Similarly, Varani and co-workers have evaluated the binding of argininamide and two other peptide ligands to HIV-1 TAR using 13C NMR to measure motion-sensitive relaxation parameters.48 While the bulge and apical loops in unbound TAR were highly dynamic,48,49 ligand binding rigidified most loop bases, U23 in the bulge, as well as the lower stem (Figure 1a). Ligand binding, however, did not globally rigidify the RNA as the remaining two bulge bases were more flexible in the bound form. This complexity of ligand-induced RNA conformational changes is likely an indication of the rich mechanisms available for RNA function modulation with peptide and small molecule ligands. The Varani group also observed the effect of ligand binding on TAR conformation in a fragment-based study.50 The authors identified a TAR binding arginine derivative (Figure 1d) that could be used as a probe for fragment screening via interligand nuclear Overhauser effect (ILOE) NMR. Interaction of each fragment with the RNA was tested by irradiating proton nuclei on the fragment and then checking for nuclear Overhauser enrichment on the RNA-bound probe. The Overhauser effect thus signals RNA binding as it is expected to occur only if the fragment is near the RNA-bound probe. It was found that in addition to reporting on fragments that interact with the RNA, the probe also preorganized TAR into a conformation capable of binding a subset of fragments in the screening library. These observations suggest that for ligands with multiple binding moieties, initial binding of one moiety may induce conformational changes in the RNA that then allow more favorable binding of the remaining moieties. On the basis of these insights, the Varani lab developed cyclic peptides containing arginine residues that bind to TAR RNA and induce a non-Tat binding conformation, which in turn is shown to inhibit reverse transcription and gene expression in HIV-1 virus strains.51
Figure 1.
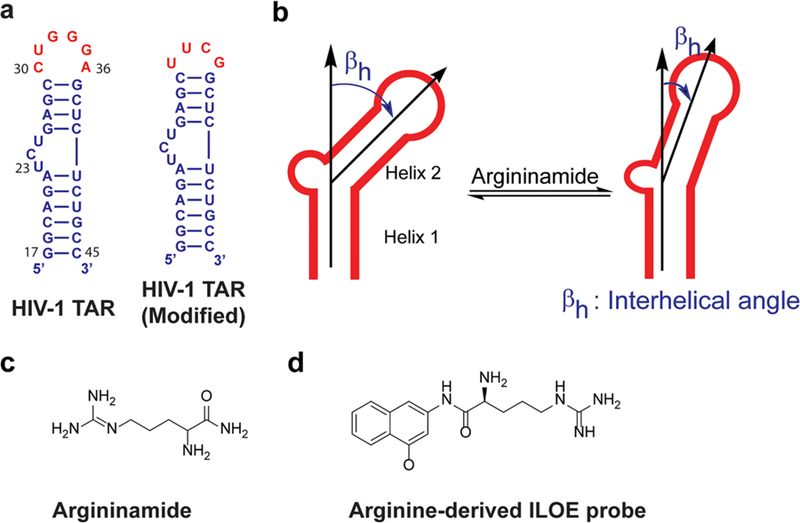
Ligand-induced conformational changes in HIV-1 TAR. (a) Wild type HIV-TAR48,50 and HIV-1 TAR with a modified loop.47 Differences are colored red. (b) Reduction of interhelical angles in TAR RNA upon argininamide binding.47 (c) Structure of argininamide. (d) Structure of the arginine-derived probe.50
Another commonly used technique for analysis of the conformational landscape in RNA is fluorescence spectroscopy. In a recent study, Baird and co-workers investigated the conformational landscape of the class I cyclic diguanylate (c-di-GMP-I) riboswitch aptamer domain using a Foörster resonance energy transfer (FRET) assay in which the FRET efficiency reported on the average proximity of labeled domains.42 They observed that at low magnesium concentrations, kanamycin B (Figure 2a) stabilized a conformation that was not observed in the native RNA either in the presence or in the absence of its cognate ligand c-di-GMP. While the resolution afforded by fluorescence measurements cannot provide conclusive evidence about whether the stabilized conformation was populated in the conformational ensemble of unbound RNA, this study shows that ligand binding can induce completely new conformations or shift the equilibrium to a previously poorly populated state. It was also found that preincubation of the RNA with kanamycin B slowed equilibration with c-di-GMP, most likely indicating a need for RNA structural rearrangement prior to binding. Following observation of the significant effect of magnesium concentration on the conformational outcome of kanamycin B binding, another important conclusion of this study was the fact that using a single set of experimental conditions in the evaluation of RNA–ligand interactions could yield misleading results. Similar observations were made by Soto and co-workers, namely that cation-induced conformational changes may have implications for small molecule binding depending on how the cations affect the binding site.52 These sensitivities to environmental conditions also underscore the potential drawbacks of studying small molecule–RNA binding under non-biologically relevant conditions.
Figure 2.
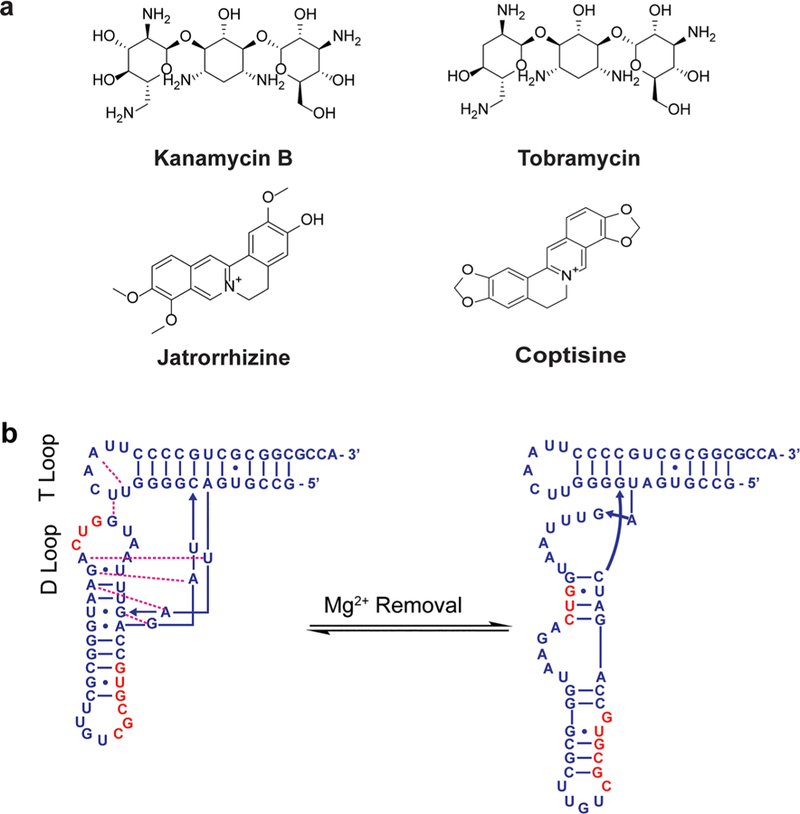
Conformational spaces of diverse RNAs are affected by ligand binding. (a) Structures of small molecules that induce conformational changes in c-di-GMP-I riboswitch (kanamycin B), tRNAAsp (tobramycin), and poly(A) (jatrorrhizine and coptisine).42,53,64 (b) Unfolding of tRNAAsp upon removal of magnesium. Nucleotides in the D-loop and anticodon stem bulge are colored red. Figure adapted from ref 53.
Similarly, the chemical probing technique selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) provides information about RNA secondary structure. Significant effects of ligand binding on RNA folding were observed by Weeks and co-workers in a study of Saccharomyces cerevisiae tRNAAsp binding to magnesium and tobramycin (Figure 2a) using SHAPE.53 Removal of magnesium resulted in a structural rearrangement through the formation of stable G-C pairs over the less stable pairs that formed when magnesium stabilized the tertiary structure (Figure 2b). Similarly, addition of tobramycin induced unfolding of the magnesium-stabilized native structure by disruption of T- and D-loop interactions followed by complete unfolding of the D-stem. Given the complexity of structural rearrangements induced by removal of magnesium on one hand and the addition of tobramycin on the other, it was hypothesized that RNA folding might not have a nature as hierarchical as commonly assumed. Indeed, other studies have supported the hypothesis that some RNA secondary and tertiary structures fold cooperatively rather than purely hierarchically.54,55 These findings are likely to improve the accuracy of RNA structure prediction programs, which may in turn improve probe development through in silico prescreening techniques. It is important to note that although this study was performed in vitro, the same probing method has been used in a cellular context.56–61 Additionally, other methods such as icSHAPE62 (in vivo click selective 2′-hydroxyl acylation and profiling experiment) and PARIS63 (psoralen analysis of RNA interactions and structures) have also been developed for probing RNA structure in cells. Such cellular applications are likely to yield more biologically relevant results.
Suresh Kumar and Chatterjee used ultraviolet (UV) melting and differential scanning calorimetry (DSC) to show that binding of small molecules jatrorrhizine and coptisine (Figure 2a) to single-stranded poly(A) at neutral pH induces the RNA to form a double-stranded structure similar to that induced at acidic pH.64 These findings further emphasize that small molecule effects are not limited to minor changes in the overall conformational space; small molecules can induce folding of even homopolymeric single-stranded RNAs.
Restructuring of RNA upon small molecule binding has also been demonstrated through a design approach. Bong and co-workers modified the type I hammerhead ribozyme by replacing bases in critical structural elements with poly(U).65 Binding of melamine-functionalized tris(2-aminoethyl)amine [t4M (Figure 3a)] restored the ribozyme secondary and tertiary structure as demonstrated in enzymatic activity measurements (Figure 3b). Similarly, Nakatani and co-workers used a naphthyridine carbamate tetramer [Z-NCTS (Figure 2c)] to activate the hammerhead ribozyme by stabilizing tertiary contacts that are essential for enzyme activity.66 The authors used this mechanism to engineer a Z-NCTS-dependent gene regulatory switch in mammalian cells. In another study, the Nakatani laboratory demonstrated ligand-induced −1 ribosomal frameshifting via stabilization of a pseudoknot structure in mRNA upon ligand binding (Figure 3d).67,68 These studies demonstrated that it is feasible to design ligands that modulate RNA function through the induction of a predefined change in RNA structure.
Figure 3.
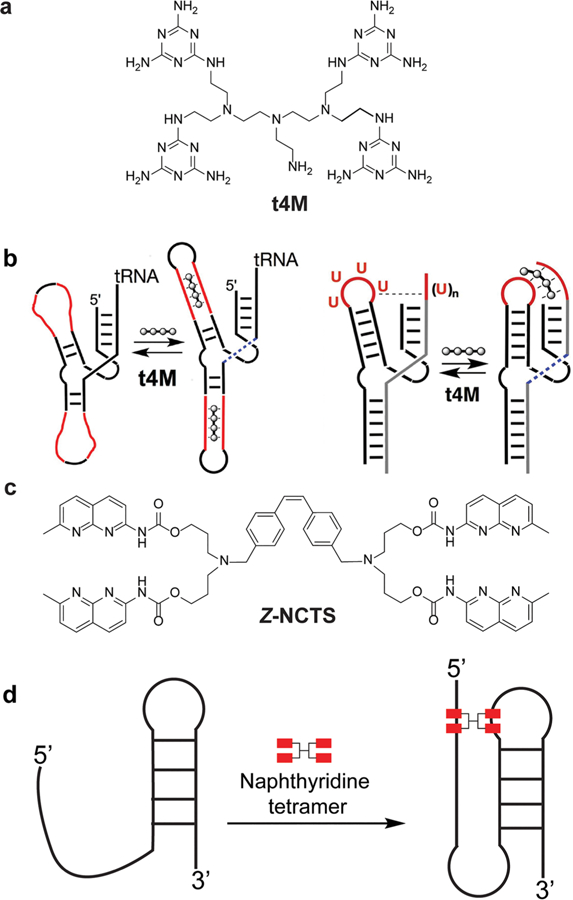
Ligand-induced conformational changes in a ribozyme and a pseudoknot. (a) Melamine-functionalized derivative of tris(2-aminoethyl)amine (t4M). (b) Restoration of ribozyme secondary structure (left) and tertiary contacts (right) upon t4M binding. Figure adapted from ref 69. (c) Naphthyridine carbamate tetramer (Z-NCTS). (d) Stabilization of a pseudoknot by Z-NCTS.67,68
Our laboratory recently reported a fluorescence-based technique for classifying site-specifically labeled RNA constructs via small molecule interactions that provided further insight into the importance of dynamics. The RNAs contained a solvatochromatic fluorophore in canonical secondary structures (bulge, internal loop, hairpin, and stem). The high accuracy with which the assay initially differentiated the canonical structures indicated that small molecule binding may accentuate the dynamic differences between these structural categories.70 In a subsequent study, we showed that individual RNAs within a secondary structure class could be further differentiated at 37 °C and in the presence of the osmolyte polyethylene glycol (PEG-12000).71 These destabilizing conditions allowed increased secondary structure dynamics, which led to more unique small molecule binding patterns for each RNA construct. These results suggested that in vitro evaluation of RNA binding under cell-like temperature and buffer conditions might expedite identification of selective small molecules.
The importance of RNA dynamics in ligand recognition has also been demonstrated in virtual screening studies.41 In collaboration with the Al-Hashimi lab, we studied the docking of an in-house-developed amiloride derivative library to an ensemble of HIV-1 TAR RNA structures constructed from molecular dynamics simulations based on NMR residual dipolar coupling data.72 Amiloride derivatives with the best docking scores bound to four of the 20 conformations within the ensemble, and the ligand with the most significant docking score also demonstrated the tightest and most specific TAR binding. Recently, a virtual screening experiment based on docking against a new RDC-informed ensemble of HIV-1 TAR structures was employed by the Al-Hashimi lab for screening a large virtual library consisting of ∼100000 small molecules.27 Specific conformations were again preferred by molecules with better docking scores, and conformations preferred by low-scoring molecules were also identified. The ability of different RNA conformations to preferentially bind different small molecules thus underscores the importance of RNA dynamics and its influence on ligand recognition. Because a high correlation was observed between the docking scores and the observed TAR binding efficiency (area under the curve = 88% for receiver operator character curve analysis), examination of the RNA conformations that accommodate these high-scoring compounds along with the structures of these compounds can be proposed to guide better probe development.
Opportunities and Challenges for Studies of Conformation and Dynamics.
The studies discussed above demonstrate that various techniques, including NMR, fluorescence spectroscopy, SHAPE, mobility shift assays, UV melting, and DSC, can provide insight into how small molecules modulate the RNA conformational landscape at various scales. Ligand binding can alter local single-base dynamics, affect the formation and stability of secondary structure even for homopolymeric RNAs, and induce the formation of tertiary structure by facilitating interaction of distal regions. This aspect of RNA–small molecule interaction can greatly expand the avenues available to researchers for modulating RNA function in cells as demonstrated by example studies that led to bioactive ligands allowing conditional gene expression in mammalian cells and modulating translation of viral RNA.29,66,73,74 In particular, interactions of RNA with biomacromolecules can be controlled by inducing a desired conformation in an RNA,74 as seen for translation inhibitors targeting the hepatitis C virus internal ribosome entry site. One can even envision the use of small molecules to deliberately induce complex formation between two RNA molecules as demonstrated in a proof-of-concept study involving two DNA hairpins.75 However, challenges that hinder the realization of this promise remain. First, designing a ligand that can induce a predefined RNA secondary or tertiary structure is feasible only in cases in which the folding of the RNA has been previously studied. In addition, the assessment of conformational change still faces some technical challenges. The use of NMR spectroscopy, for example, requires large amounts of sample and tends to be low-throughput. On the other hand, installation of an RNA label for high-throughput fluorescence-based experiments could potentially affect the native dynamics of the RNA. These concerns can be addressed in some cases by combining these techniques with methods such as SHAPE, mobility shift assays, UV melting, and DSC as described above.53,64,68
THERMODYNAMIC ANALYSIS OF RNA–SMALL MOLECULE INTERACTIONS
Successful targeting of RNA with small molecules will likely be made more efficient by the detailed study of the thermodynamics of RNA–small molecule interactions. For proteins, retrospective analysis of a class of compounds developed for a given target often shows improvement of the change in enthalpy with increased activity, while the entropic term remains the same or becomes even more unfavorable.76 While the interaction of small molecules with RNAs is different from that with proteins due to the chemical and structural differences between the two biomolecules, favorable enthalpy is likely to play an important role in the development of selective RNA ligands.
The assessment of enthalpic and entropic contributions to the total free energy of ligand–target interactions can contribute to the discovery of the most effective ligands in two ways. First, detailed thermodynamic studies can help in compound prioritization for further optimization. Because it is often more difficult to improve the enthalpic component of an interaction than the entropic component,77 the compound with the more favorable enthalpic change can be chosen for further optimization. Second, in addition to guiding compound prioritization, calorimetric data for ligand analogues can provide insight into the molecular forces that are essential for RNA–small molecule interactions, especially when structural information is available.78 Below we discuss some example calorimetric studies of RNA–small molecule interactions and focus on what was learned from the experiments. The examples discussed here can generally be classified into two groups: (1) enthalpy-driven interactions and (2) entropy-driven interactions. Finally, we will discuss the role of the heat capacity change in the RNA–small molecule interactions. These examples have generally focused on hydrophobic ligands such as alkaloids, highly charged ligands such as aminoglycosides, or native riboswitch ligands and are described in roughly chronological order within each section. In all cases, researchers used isothermal titration calorimetry (ITC) to evaluate the enthalpic and entropic contributions.
Enthalpy-driven interactions.
In a thermodynamic study of the alkaloid coralyne (Figure 4) binding to double stranded poly(A) RNA, Giri and Suresh Kumar observed that the interaction was favorable both enthalpically and entropically.79 This interaction was enthalpy-driven, with enthalpy change accounting for 60% of the total free energy. The high enthalpy term was indicative of intercalative binding common to planar aromatic compounds. The 40% entropic contribution was attributed to water release from both the RNA and the small molecule upon binding. ITC and Job’s plot analyses were used to estimate the size of the binding site as four nucleotides, indicating that coralyne intercalates in every other internucleobase space. These observations begin to establish the principles for targeting double-stranded RNAs with intercalating molecules.
Figure 4.
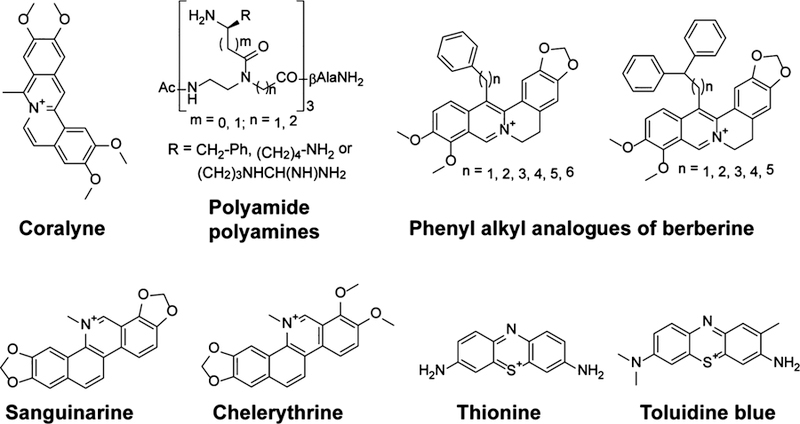
Select compounds for which calorimetric studies were reported by Suresh Kumar and co-workers as well as Patino and co-workers.79,89–92
Similarly, Arya and co-workers analyzed the binding of neomycin to oligo(A)30 using ITC and found the interaction to be enthalpically driven.80 Two enthalpically favorable binding events were observed; however, the first was entropically favorable, and the second unfavorable, possibly due to differences in water release. The favorable enthalpy change for both binding events suggests that polycationic molecules such as neomycin have the potential to interact with RNA through specific noncovalent interactions and can therefore serve as a good starting point for the development of selective probes.
Ferré-D’Amaré, Rueda, and co-workers reported calorimetric data of binding of c-di-GMP to the aptamer domain of the class I cyclic-di-GMP riboswitch.81 The interaction of c-di-GMP with wild type and mutant aptamer domains of class I c-di-GMP riboswitches was found to be enthalpy-driven, with highly unfavorable entropic terms. In an earlier study, Batey and co-workers measured the thermodynamic parameters of purine ligands binding to the aptamer domain of the purine riboswitch.82 These interactions also were enthalpically driven with large unfavorable entropic terms. The unfavorable entropy change was thought to correspond to the RNA conformational changes associated with ligand binding. This trend might be a general trait of riboswitch ligand binding, although the presence of dications in some cases stabilizes the RNA, leading to a favorable entropy change.83–88 Ligand design for riboswitch targets may therefore benefit from optimizing molecular properties that give an enthalpy change that is large enough to offset the unfavorable entropy change.
The dominance of the enthalpy change was also reported by Patino and co-workers in a study of polyamide polyamines (Figure 4) binding to HIV-1 TAR RNA.91 The entropic term was unfavorable for most of these compounds. In this study, the free energy associated with binding of each polyamide polyamine to TAR was further dissected into an electrostatic component and a non-electrostatic component by measuring the dependence of Kd on buffer ionic strength. For all compounds, the Kd increased with an increase in KCl concentration, consistent with the binding mode of positively charged molecules; these molecules may compete with cations for the negatively charged RNA. Detailed analysis of the impact of KCl concentration on Kd showed that at most two positive counterions were released from the RNA upon binding, indicating that the charged groups on the ligands interact with TAR through specific hydrogen bonding and π-cation interactions rather than nonspecific electrostatic interactions with the backbone. This observation was consistent with the observed predominance of the enthalpy change, as displacement of several cations would result in a highly favorable entropic term due to an increased level of disorder.
Suresh Kumar and Basu recently published a review of the use of ITC and DSC (Figure 5a) in the biophysical characterization of interaction of RNA with alkaloid compounds.89 The RNA targets included tRNA, single-stranded RNA, double-stranded RNA, poly(A), and triplex RNA. Several thermodynamic aspects were discussed, including enthalpic and entropic contributions to the free energy of binding, the temperature dependence of free energy, and the partitioning of the free energy into electrostatic and non-electrostatic components. The interactions of seven alkaloids with tRNAPhe were enthalpically driven, but they were still entropically favorable. The free energy was independent of temperature because both the enthalpy and the entropy change strongly as a function of temperature, resulting in enthalpy–entropy compensation. Dissection of the free energy accompanying binding of tRNA to berberine analogues with phenyl alkyl substituents (Figure 4) showed a considerably smaller electrostatic component, indicating that these compounds interact with the RNA via interactions other than nonspecific electrostatic interactions. Consistent with the extended nonpolar surface of these analogues, the substantial involvement of the hydrophobic effect in their interactions with tRNA was shown by evaluating ΔH as a function of temperature. DSC studies showed that ethidium caused a stronger stabilization of tRNA (21 °C) compared to berberine and palmatine (6.5 and 5.5 °C, respectively), likely due to differences in binding modes.
Figure 5.
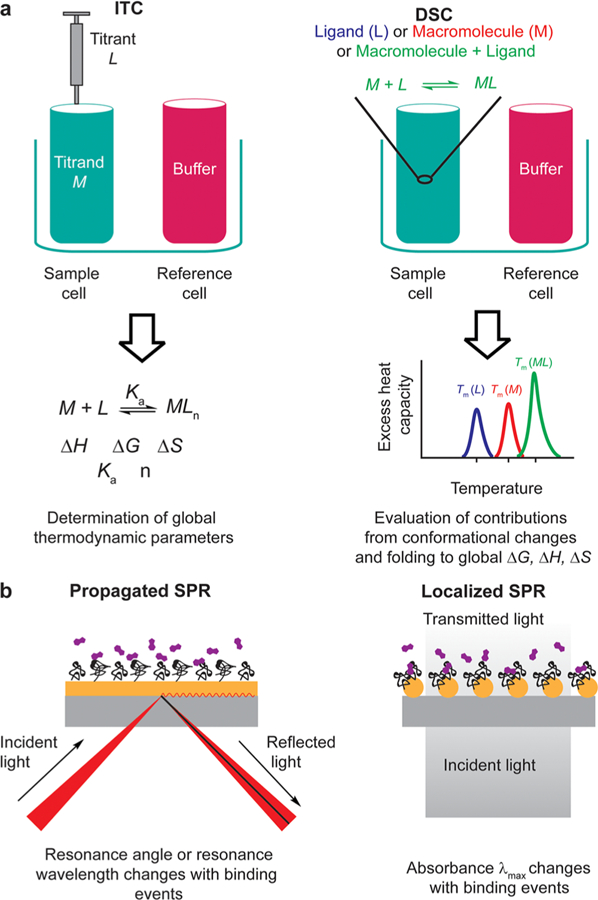
Schematic illustration of techniques discussed herein. (a) Comparison between ITC and DSC.93 The two methods yield complementary information allowing full thermodynamic characterization of an interaction. Tm is the melting temperature. (b) Comparison between propagated and localized SPR. Localized SPR uses metal nanoparticles instead of metal film (kaki). In this format, free small molecules (purple) bind to immobilized biomolecules (black).94,95
In a study of benzophenanthridine alkaloids sanguinarine and chelerythrine (Figure 4) binding to single-stranded poly(I), poly(G), and poly(C), Suresh Kumar and Basu showed that the energetics of a small molecule’s binding to homopolymeric RNAs can differ on the basis of the RNA sequence.92 While all interactions with sanguinarine were enthalpy-driven, interactions with chelerythrine were enthalpy-driven for poly(I) and entropy-driven for poly(C), and the two energetic terms were comparable for poly(G). This observation showed that the limited chemical diversity of nucleobases is not necessarily an impediment to RNA ligand development; the homopolymeric RNAs were different enough to lead to different thermodynamic profiles with the same ligand.
Finally, Suresh Kumar and Saha recently investigated the binding of the phenothiazinium dyes thionine and toluidine blue O (Figure 4) with double-stranded RNAs using ITC.90 All interactions were enthalpy-driven. The favorable enthalpy changes suggested that the two dyes interact with double-stranded RNAs via intercalative modes.
Entropy-Driven Interactions.
One of the earliest thermodynamic studies of aminoglycosides binding to RNA was reported by Pilch and co-workers in 2003.96 All four aminoglycosides (Figure 6a) investigated for binding to ribosomal A-site RNA using ITC had favorable entropies of interaction.96 Indeed, the binding energies for all aminoglycosides except lividomycin were dominated by the entropic term. Molecular dynamics simulations showed that all hydroxyl groups on the additional ring in lividomycin form hydrogen bonds with the RNA, thus leading to a more favorable enthalpy. Another interesting observation was that the free energy of binding of lividomycin to the A site was similar to that of the paromomycin complex, yet paromomycin had a much larger entropy term. Because salt- and osmolyte-dependent studies showed no differences in water or counterion release upon ligand binding, it was posited that the difference in entropy resulted from different configurational entropies of the two RNA–small molecule complexes. The additional ring in lividomycin compared to paromomycin can lead to the entropic difference because lividomycin may suffer a greater reduction in configurational entropy upon complexation. This study also found that neomycin gave an increased free energy of binding via a more favorable enthalpic term. This outcome suggested that the protonated amino group at position 6′ in neomycin makes stronger interactions with RNA than the hydroxyl group at the same position in paromomycin. This study exemplifies how thermodynamic studies can be used to gain insight into the energetic impact of subtle structural differences in a series of similar compounds.
Figure 6.
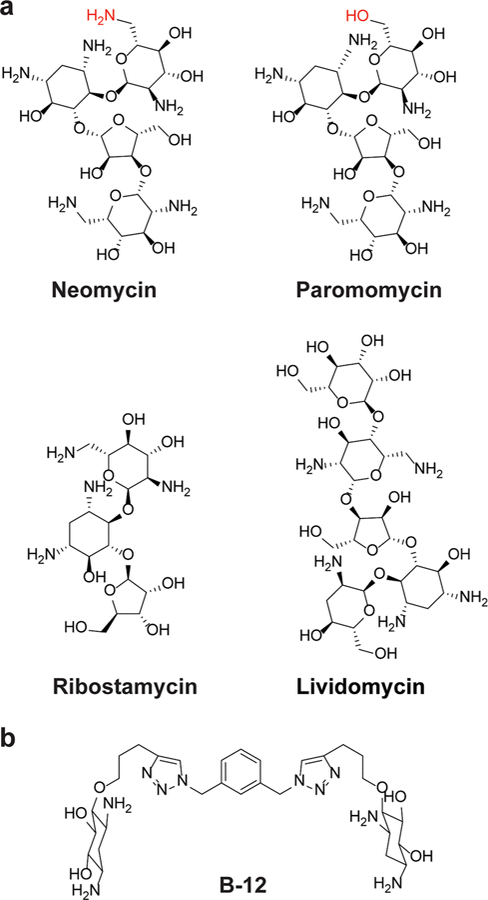
Compounds for which calorimetric studies were reported by (a) Pilch and co-workers and (b) Hergenrother and co-workers.96,97 Functional groups that differ between neomycin and paromomycin are colored red.
In another study, Hergenrother and co-workers reported detailed biophysical characterization of dimers of 2-deoxystreptamine interacting with a series of RNA hairpin loops of different sizes.97 Compound B-12 (Figure 6b) had shown selectivity for octaloops in a prior study.98 To investigate the origin of this selectivity, calorimetric studies were carried out on B-12 and its related analogues. Binding of these compounds to the hairpin loops was entropy-driven in most cases. Of note, the selectivity of B-12 for octaloops stemmed from a much larger entropic component. The analogues of B-12 also exhibited the same trend; an increase in free energy of binding coincided with an increase in the entropic component. Because the compounds contain basic amines, the favorable entropy change likely stems from cation release when the positively charged compound forms electrostatic contacts with the RNA.97
Along with the conformational analyses described in the previous section, Suresh Kumar and Chatterjee also evaluated the energetics of binding of jatrorrhizine and coptisine (Figure 2a) to poly(A).64 The interactions were favorable both enthalpically and entropically, but the entropic term dominated the binding energy. The large favorable entropy change was interpreted as resulting from the release of water molecules and ions from the single-stranded poly(A) upon small molecule binding.
Heat Capacity Change.
In addition to the enthalpy and entropy change, molecular interactions are often accompanied by a change in the constant-pressure heat capacity (ΔCp), which can be estimated by evaluating ΔH as a function of temperature. Changes in heat capacity upon complex formation and biomolecular folding are often described as originating from differential hydration of the molecules before and after the binding or folding process.99–101 Because hydration of nonpolar groups increases the heat capacity of the solution through strengthened water hydrogen bonds,102 an RNA–small molecule interaction that decreases the total solvent-accessible nonpolar surface area may lead to a negative ΔCp. Indeed, several studies have reported negative ΔCp values for RNA–small molecule and RNA–peptide interactions in which the complex is thought to undergo a conformational change resulting in an increased level of burial of nonpolar surface area.87,89–91,96,103–106 Due to the several factors that may contribute to ΔCp, however, it is important to note that interpretation of ΔCp data is not always straightforward. In addition to hydration, ΔCp calculations include contributions from vibrational motion, from the conformational entropy of the biomolecule, and from the mere coupling of conformational equilibria to binding equilibria.99,100 While definite conclusions about the meaning of ΔCp data often require additional experimentation, large changes can provide insight into the importance or degree of the conformational change in ligand binding.
Opportunities and Challenges for Thermodynamic Studies.
The example studies described above demonstrate the feasibility of thermodynamic analyses and, most importantly, their potential to provide insight into the determinants of RNA–small molecule recognition. For example, the functional groups critical for selective RNA binding can be identified by evaluating closely related small molecules. Additionally, the binding mode can in some cases be inferred from thermodynamic data in the absence of structural information. Lastly, thermodynamic studies have allowed assessment of phenomena accompanying small molecule binding such as cation and water displacement, which have in turn been used to identify small molecules that bind RNA via specific interactions rather than general nonspecific electrostatic interactions.
Detailed thermodynamic studies have unfortunately not been the norm in the biophysical characterization of RNA–small molecule interactions, in large part due to technical challenges associated with calorimetric studies of RNA. For example, small molecules in the discovery stage often exhibit weak interactions with RNA. As a result, high concentrations of the small molecule are often required for a measurable change in heat, and these conditions often lead to aggregation of small molecules. Furthermore, the amount of dimethyl sulfoxide (DMSO) used is known to profoundly affect RNA structure and ligand binding.107 This puts further restrictions on accessible concentrations of small molecules as the majority of organic molecules require DMSO as a co-solvent. Methods for circumventing the limitations of direct calorimetric measurements include using circular dichroism for thermodynamic analysis108 and using kinetic data to calculate thermodynamic parameters via Eyring analysis.109 However, the former has not been sufficiently explored, and the latter is not applicable when accurate kinetic data cannot be obtained, usually due to interactions that are too slow for accurate determination of rate constants using traditional methods. Direct calorimetric studies thus often remain the method of choice for evaluating the thermodynamics of binding.
As others have stated, it is important to note that caution must be exercised when using calorimetric data, as they are heavily dependent on experimental conditions. The measured enthalpy may encompass other global factors in addition to compound–target interaction.110 For example, the buffer dependence of enthalpy change has been observed when binding is coupled with protonation of the small molecules; the observed ΔH deviates from the actual ΔH by the heat of ionization of the buffer.96,111
KINETIC ANALYSIS OF RNA–SMALL MOLECULE INTERACTIONS
Alongside the conformational and thermodynamic analysis of RNA–small molecule interactions, detailed kinetic analysis will likely be integral to RNA-targeted drug discovery. For example, as in the case of some RNA riboswitches that are under kinetic control,34,35 an effective ligand for a given RNA might not be one that has a small Kd; it might be one that has a faster kon and can kinetically compete with the endogenous ligands of the RNA target. Moreover, ligand selectivity for an RNA target might not always be achieved through a low Kd; even in the absence of thermodynamic selectivity, one could still achieve kinetic selectivity.112 For example, the ligand might have a significantly slow koff for the target of interest compared to those of other RNAs despite having comparable Kd’s, thereby increasing the relative biological activity. A recent analysis of association rate constants of RNA binding and protein binding ligands revealed that RNA ligands tend to have slower on rates compared to those of protein ligands.113 Among other factors, the authors suggested that the flexible backbone of RNA compared to proteins may reduce association rates by increasing the RNA conformational sampling time before interacting with the ligand. The same phenomenon also applies to the induced-fit binding mechanism in which the RNA–small molecule complex takes longer to reach the optimal conformational states.82 While this analysis does not imply that RNA always binds ligands with slow association rates, its outcome suggests that decreasing the dissociation rate might lead to the discovery of efficacious RNA binding ligands when the association rate cannot be increased.
Below, we will discuss example studies in which the binding kinetics of RNA–small molecule recognition were analyzed. While there is a limited number of such studies, the information we currently have underscores the importance of such studies because they often explain inconsistencies between activity trends and Kd trends in a series of compounds. In each case discussed here, surface plasmon resonance (SPR) was used to measure the on and off rates of RNA–ligand interactions.
Example Kinetic Studies.
In early studies, Wong and co-workers used SPR to evaluate the kinetics of binding of aminoglycosides to domain II of the HIV-1 Rev response element (RRE) (Figure 7a).114 They observed that the Kd values of neomycin and paromomycin could not explain the large difference in the inhibition of binding of RRE to the Rev protein. They posited that the significantly slower koff of neomycin compared to that of paromomycin might be responsible for the 100-fold difference in inhibitory potency.
Figure 7.
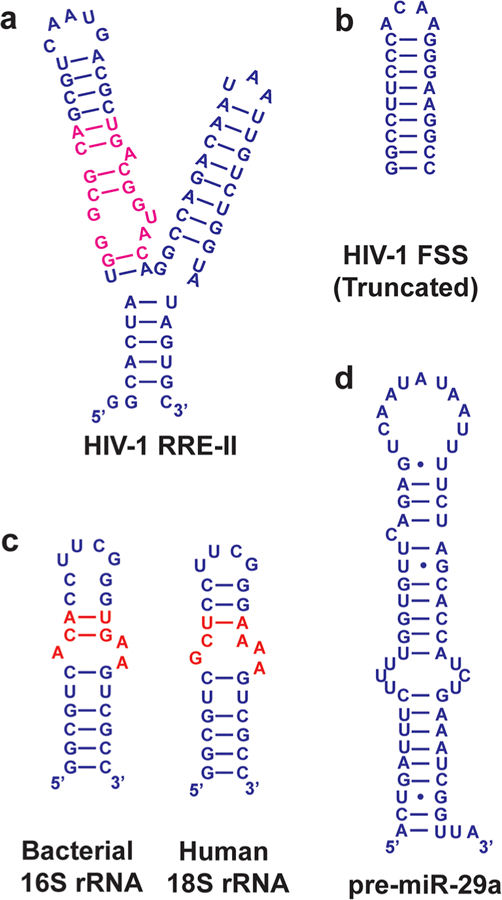
Select RNA constructs used in kinetics studies. (a) Domain II of HIV-1 RRE. The Rev binding site is colored magenta.114 (b) Upper stem-loop of the HIV-1 frameshift stimulatory signal.122 (c) Bacterial 16S and human 18S rRNA. Differences are colored red. Both RNAs were capped with the stable UUCG loop.115 (d) miR-29a precursor.123
Tor, Vaskevich, Rubinstein, and co-workers analyzed the interaction of neomycin B with the 16S bacterial ribosomal decoding A site, human ribosomal 18S RNA, and two other constructs using localized SPR (Figures 5b and 7c).115 Neomycin B had a 10-fold faster kon for bacterial A-site RNA than for human 18S RNA. Authors suggested that this difference in kon might be the basis for preferential binding of neomycin to the bacterial A site over the eukaryotic A site.
Disney and co-workers observed that the small molecule 2H-4 targeting the r(CUG) trinucleotide repeat RNA in myotonic dystrophy type 1 bound r(CUG)10 with a kon higher than that of the alternative splicing regulator muscleblind-like 1 protein (MBNL1), indicating that the cellular inhibition of RNA–protein interactions by the small molecule 2H-4 may occur through an increased kon that leads to effective competition.116 Previous structural studies indicate that MBNL1 induces a significant conformational change in bound RNA by binding to distant sites in single-stranded RNA.117,118 The low kon observed by SPR is consistent with this hypothesis, because double-stranded r(CUG)10 likely does not display optimal molecular properties for fast association with MBNL1. The observed kon would therefore be low regardless of whether the RNA conformational change occurs before (conformational selection) or after (induced-fit) initial MBNL1 binding.119–121
In a recent study, Miller and co-workers sought to enhance the selectivity of a nanomolar binder (1Z) of the HIV-1 frameshift stimulatory signal (FSS) (Figure 7b) RNA by methylating the nitrogen atoms in the amide bonds of the compound.122 It was hypothesized that N-methylation would bias the conformational ensemble of the compound toward favorable conformations for FSS binding, thus improving the affinity and selectivity. SPR analysis of two methylated analogues of 1Z showed that both compounds had kon values an order of magnitude higher than that of the original compound, while koff values remained similar. Importantly, methylated analogues also had antiviral activity higher than that of 1Z in HEK293T cells. However, this increased activity cannot be fully attributed to the improved association rates because methylation also led to higher cell permeability.
In the analysis of compounds binding to pre-miR-29 (Figure 7d), Nakatani and co-workers found that conformational restriction can lead to rapid rates of association and dissociation.123 Of the 21 compounds analyzed, 12 had rapid association and dissociation constants, while nine had slow association and/or dissociation constants. Compounds with rapid kinetics were more planar and more rigid than those with slow kinetics. The authors suggested that the rapid compounds might bind to the secondary structures of pre-miR-29, leading to selectivity over double-stranded RNA (dsRNA), while the slow compounds bind to the major groove of both pre-miR-29 and dsRNA. Conformational restriction and the planarity of compounds can therefore be used to impact the kinetics of binding and the selectivity.
Opportunities and Challenges for Kinetic Studies.
Analysis of RNA–small molecule kinetics so far has led to several lessons that are likely to expedite the development of RNA probes. For example, in some cases compounds with similar equilibrium constants have drastically different activities stemming from the differences in the kinetics of binding. For targeting RNA–protein complexes, small molecules with a higher association rate can be more fit to compete with the protein ligand, ultimately leading to bioactivity. The few studies discussed here have also begun to elucidate molecular properties that affect binding kinetics, including conformational restriction and planarity. Consideration of such properties may lead to effective and selective compounds with activity in the cell.
Although kinetic analyses of ligand–target interactions have the potential to expedite drug discovery, however, some challenges remain. As Copeland noted in the 10-year retrospective of the drug-target residence model, a short residence time might be sufficient for a drug effect in some cases, and long residence times might even result in adverse effects.124 According to this hypothesis, ligand discovery efforts toward RNA targets should not simply optimize for long residence times; each system should be studied mechanistically to evaluate the optimal kinetic profile. These efforts are also hindered by the limited knowledge of the molecular basis of favorable kinetic parameters.78 Additionally, although several methods such as biolayer interferometry, surface acoustic wave, NMR, waveguide-based grating-coupled interferometry, and total internal reflection fluorescence16,78,119,125–132 can potentially be used to measure kinetics, the most common label-free method for analyzing RNA–small molecule interactions has been SPR biosensors. This technology, however, still faces several challenges that may make it difficult to obtain accurate quantitative data in some cases.
First, molecular interactions are often assumed to follow pseudo-first-order kinetics, due to the relative simplicity of the calculations involved relative to higher-order kinetics.133 However, establishing true pseudo-first-order conditions is nontrivial.133 For SPR kinetic data to be meaningful, it is therefore imperative to ascertain that the collected data exhibit the qualitative aspects of pseudo-first-order kinetics (e.g., the association and dissociation phases are single exponentials, the dissociation phase has one rate constant that is independent of analyte concentration, and steady-state responses can be estimated from the association phase at long association times).133 Reliable analysis of kinetic data thus requires that sensorgrams have high-quality shape with clear curvature, which may be difficult to achieve for processes with very fast kinetics such as binding of neomycin to HIV-1 FSS.134 Slowly dissociating processes may also be problematic especially when the observation time is not long enough.133 However, a single-cycle method and corresponding binding models have been described that can be used for such systems.135,136 Additionally, standard multicycle experiments can potentially account for incomplete dissociation by adding a corresponding term in the dissociation model.137
A second challenge facing SPR analysis of RNA–small molecule interactions is related to the requirement to immobilize on the binding partners. The most common strategy of immobilizing the RNA may sometimes lead to low signal-to-noise ratios due to the low molecular weight of the small molecule. In this case, the signal can be improved by immobilizing the small molecule.115,137 However, immobilization of the smaller partner may lead to increased mass-transport limitation if the association rate is higher than the diffusion rate of the high-molecular weight RNA on the chip surface. Binding models that account for mass-transport limitation should therefore be used during data analysis.133,137 Mass-transport limitation can also be mitigated by adjusting the flow rate.136 When possible, it may be good practice to evaluate RNA–small molecule interactions in both immobilization formats to check for the consistency of data.
The select challenges described above indicate that using SPR technology, the most commonly used label-free method, can lead to inaccurate data if experiments are not carefully designed. However, even in the absence of accurate quantitative data, qualitative SPR measurements can provide valuable information about the relative rates of binding of a small molecule to an RNA target in real time. Additionally, increased use of alternative methods is likely to address some of the challenges specific to SPR techniques.16,78,119,125–132
CONCLUSIONS AND PERSPECTIVES
The past decade has seen promising progress in targeting RNA with small molecules. However, RNA ligands with enough selectivity and potency to advance to the clinic are yet to be discovered. In this Review, we have discussed how consideration of the RNA conformational landscape, as well as detailed thermodynamic and kinetic analyses of RNA–small molecule interactions, can provide insights into corresponding selectivity mechanisms and thus expedite RNA drug discovery.
While the large conformational landscape of RNA poses a significant challenge to ligand development, especially to structure-guided strategies, it also offers increased opportunities for efficient RNA targeting through modulation of RNA conformational ensembles. As a result, competitive binding may become less of a requirement. Modulation of the RNA conformational landscape may thus fill the gap of competitive modulation of RNA–protein interfaces, which have not been sufficiently characterized to guide discovery of competitive RNA binding ligands. Consequently, a detailed study of conformational changes should be incorporated into the small molecule ligand development process. In particular, the types of RNA conformational changes induced should be characterized and correlated to structural and chemical properties of ligands to aid in future rational development of new small molecule scaffolds. This information will allow purposeful induction of specific RNA conformational changes at the secondary and tertiary structure level, which so far has proven to be a viable approach for modulating and understanding RNA activity in cells.66,74
From detailed thermodynamic studies, information about the importance of certain functional groups and the relative contribution of specific and nonspecific electrostatic interactions can be obtained.91,96 In some cases, the mode of binding of an interaction can be inferred from the energy profile when structural information is unavailable. Additionally, thermodynamic studies show that RNA–small molecule interactions can be enthalpy- or entropy-driven and that selectivity among RNAs can be entropy-driven, although the available evidence is too limited to make any general conclusions about the thermodynamic profiles of selective RNA–small molecule interactions. As our brief survey indicates, thermodynamic measurements have focused mainly on two classes of compounds: the highly charged aminoglycosides and the highly intercalating alkaloids. General conclusions about RNA binding molecules will be possible as more researchers incorporate detailed thermodynamic evaluation in RNA ligand development campaigns. While the prospective use of thermodynamic data still faces challenges,76,77,110 it is worth considering in RNA ligand development efforts, particularly in structure–activity relationship studies when evaluating the impact of functional group choice on affinity and selectivity.
More frequent kinetic analysis of RNA–small molecule interactions can also often provide insight into inhibition mechanisms, allowing rationalization of differences in ligand activity that cannot be explained by Kd values.115 These findings underscore the fact that inhibition of the complex biological interactions in vivo often cannot be adequately modeled by equilibrium measurements in in vitro closed systems lacking consideration of kinetic control of the interaction of interest and of modulation through kinetic competition.33,124 A second benefit of kinetic studies has been the identification of molecular determinants of fast kinetic parameters (e.g., conformational restriction and planarity),122,123 which will be important for the prospective design of ligands with a desired kinetic profile. These prospective studies will depend on the extensive accumulation of structure–kinetics relationship data, which will be possible only if more RNA small molecule ligand development campaigns incorporate analysis of kinetics as a core feature of biophysical characterization of RNA–small molecule interactions.
With a few notable exceptions, it is worth mentioning that most molecules discussed herein have not been assessed for engagement of the target RNA in a cell and/or for exerting biological activity. Therefore, the use of molecular recognition insights gained from conformational, kinetics, and thermodynamics studies to design a molecule with specific biological activity is still limited. Nonetheless, the lessons learned here demonstrate the value these considerations are likely to have in RNA probe development.
Finally, we emphasize that ample opportunities remain for further exploration of the molecular details of RNA interactions responsible for its cellular functions and about corresponding strategies to ablate these interactions with druglike small molecules. For example, targeting large RNAs has not been sufficiently explored, in part due to the difficulties in determining their three-dimensional structures as well as in identifying the minimal substructures that can be targeted to modulate the function of the whole RNA. In cases in which a large construct is needed to adequately model an RNA in vitro, biophysical analyses discussed herein might prove to be difficult to achieve. First, the increased RNA complexity could render analysis of dynamics challenging. Second, analysis of thermodynamics of binding using calorimetric methods might suffer from the increased cost of making large amounts of long RNAs. Finally, an increased molecular weight disparity between small molecule ligand and the RNA target could hinder kinetics analyses with biosensor methods that rely on ligand-induced changes in the refractive index. These technical challenges, however, are likely to be addressed in the near future as researchers make improvements to current analytical methods. In conclusion, while the conformational, thermodynamics, and kinetics analyses of RNA–ligand interactions may currently be met with significant challenges, studies that move beyond Kd measurements offer distinct and important opportunities for advances in small molecule targeting as we expand the “druggable” space of biomolecules.
ACKNOWLEDGMENTS
The authors thank all members of the Hargrove lab for providing valuable feedback on the manuscript. The authors acknowledge financial support for this work from Duke University, National Institutes of Health Grant U54GM10329, and National Institute of General Medical Sciences Maximizing Investigator’s Research Award (MIRA) Grant R35GM124785. A.U.J. acknowledges additional support from the Lews Siegel Fellowship from the Pratt School of Engineering, Duke University.
KEYWORDS
- RNA targeting with small molecules
modulation of RNA structure and/or function using chemical probes
- RNA–small molecule interactions
study of noncovalent interactions between RNA and small molecules at the molecular level
- RNA conformational dynamics
phenomenon of switching between different RNA conformations
- Binding kinetics
association and dissociation rates characteristic of an RNA–small molecule interaction
- Binding thermodynamics
changes in enthalpy, entropy, and/or heat capacity of a system accompanying interactions between RNA and small molecules
- Isothermal titration calorimetry (ITC)
experimental technique used to study the thermodynamics of the interactions between RNA and small molecules
- Surface plasmon resonance (SPR)
optical and label-free method commonly used for measuring the kinetics of binding of RNA and small molecules
- Fluorescence resonance energy transfer (FRET)
experimental technique based on energy transfer between two chromophores often used for studying RNA conformational dynamics and small molecule interactions
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Cech TR, and Steitz JA (2014) The Noncoding RNA Revolution-Trashing Old Rules to Forge New Ones. Cell 157, 77–94. [DOI] [PubMed] [Google Scholar]
- (2).Morris KV, and Mattick JS (2014) The rise of regulatory RNA. Nat. Rev. Genet 15, 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lee TI, and Young RA (2013) Transcriptional Regulation and Its Misregulation in Disease. Cell 152, 1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Schmitt AM, and Chang HY (2016) Long Noncoding RNAs in Cancer Pathways. Cancer Cell 29, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Maass PG, Luft FC, and Bahring S (2014) Long non-coding RNA in health and disease. J. Mol. Med. (Heidelberg, Ger.) 92, 337–346. [DOI] [PubMed] [Google Scholar]
- (6).Qiu MT, Hu JW, Yin R, and Xu L (2013) Long noncoding RNA: an emerging paradigm of cancer research. Tumor Biol 34, 613–620. [DOI] [PubMed] [Google Scholar]
- (7).Karapetyan AR, Buiting C, Kuiper RA, and Coolen MW (2013) Regulatory Roles for Long ncRNA and mRNA. Cancers 5, 462–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ling H, Fabbri M, and Calin GA (2013) MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discovery 12, 847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Esteller M (2011) Non-coding RNAs in human disease. Nat. Rev. Genet 12, 861–874. [DOI] [PubMed] [Google Scholar]
- (10).Dibrov SM, and Hermann T (2016) Structure of the HCV Internal Ribosome Entry Site Subdomain IIa RNA in Complex with a Viral Translation Inhibitor. Methods Mol. Biol 1320, 329–335. [DOI] [PubMed] [Google Scholar]
- (11).Hermann T (2016) Small molecules targeting viral RNA. Wiley Interdiscip. Rev. RNA 7, 726–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).van Bel N, Das AT, Cornelissen M, Abbink TE, and Berkhout B (2014) A short sequence motif in the 5′ leader of the HIV-1 genome modulates extended RNA dimer formation and virus replication. J. Biol. Chem 289, 35061–35074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Davis DR, and Seth PP (2011) Therapeutic targeting of HCV internal ribosomal entry site RNA. Antivir. Chem. Chemother 21, 117–128. [DOI] [PubMed] [Google Scholar]
- (14).Morgan BS, Forte JE, and Hargrove AE (2018) Insights into the development of chemical probes for RNA. Nucleic Acids Res 46, 8025–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Velagapudi SP, Luo Y, Tran T, Haniff HS, Nakai Y, Fallahi M, Martinez GJ, Childs-Disney JL, and Disney MD (2017) Defining RNA–Small Molecule Affinity Landscapes Enables Design of a Small Molecule Inhibitor of an Oncogenic Noncoding RNA. ACS Cent. Sci 3, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yang WY, He F, Strack RL, Oh SY, Frazer M, Jaffrey SR, Todd PK, and Disney MD (2016) Small Molecule Recognition and Tools to Study Modulation of r(CGG)(exp) in Fragile X-Associated Tremor Ataxia Syndrome. ACS Chem. Biol 11, 2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Connelly CM, Moon MH, and Schneekloth JS Jr. (2016) The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chem. Biol 23, 1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Thomas JR, and Hergenrother PJ (2008) Targeting RNA with Small Molecules. Chem. Rev 108, 1171–1224. [DOI] [PubMed] [Google Scholar]
- (19).Guan L, and Disney MD (2012) Recent advances in developing small molecules targeting RNA. ACS Chem. Biol 7, 73–86. [DOI] [PubMed] [Google Scholar]
- (20).Donlic A, and Hargrove AE (2018) Targeting RNA in mammalian systems with small molecules. Wiley Interdiscip. Rev. RNA 9, No. e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Warner KD, Hajdin CE, and Weeks KM (2018) Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug Discovery 17, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Marz M, and Stadler PF (2011) RNA Interactions. In RNA Infrastructure and Networks (Collins LJ, Ed.) pp 20–38, Springer, New York. [Google Scholar]
- (23).Blakeley BD, DePorter SM, Mohan U, Burai R, Tolbert BS, and McNaughton BR (2012) Methods for identifying and characterizing interactions involving RNA. Tetrahedron 68, 8837–8855. [Google Scholar]
- (24).McHugh CA, Russell P, and Guttman M (2014) Methods for comprehensive experimental identification of RNA-protein interactions. Genome Biology 15, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Shortridge MD, and Varani G (2015) Structure based approaches for targeting non-coding RNAs with small molecules. Curr. Opin. Struct. Biol 30, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).McFadden EJ, and Hargrove AE (2016) Biochemical Methods To Investigate lncRNA and the Influence of lncRNA:Protein Complexes on Chromatin. Biochemistry 55, 1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ganser LR, Lee J, Rangadurai A, Merriman DK, Kelly ML, Kansal AD, Sathyamoorthy B, and Al-Hashimi HM (2018) High-performance virtual screening by targeting a high-resolution RNA dynamic ensemble. Nat. Struct. Mol. Biol 25, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Chen L, Calin GA, and Zhang S (2012) Novel Insights of Structure-Based Modeling for RNA-Targeted Drug Discovery. J. Chem. Inf. Model 52, 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Lozano G, Trapote A, Ramajo J, Elduque X, Grandas A, Robles J, Pedroso E, and Martinez-Salas E (2015) Local RNA flexibility perturbation of the IRES element induced by a novel ligand inhibits viral RNA translation. RNA Biol 12, 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Parsons J, Castaldi MP, Dutta S, Dibrov SM, Wyles DL, and Hermann T (2009) Conformational inhibition of the hepatitis C virus internal ribosome entry site RNA. Nat. Chem. Biol 5, 823–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hermann T (2002) Rational ligand design for RNA: the role of static structure and conformational flexibility in target recognition. Biochimie 84, 869–875. [DOI] [PubMed] [Google Scholar]
- (32).Serganov A, and Nudler E (2013) A Decade of Riboswitches. Cell 152, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Copeland RA, Pompliano DL, and Meek TD (2006) Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discovery 5, 730–739. [DOI] [PubMed] [Google Scholar]
- (34).Wickiser JK, Cheah MT, Breaker RR, and Crothers DM (2005) The kinetics of ligand binding by an adenine-sensing riboswitch. Biochemistry 44, 13404–13414. [DOI] [PubMed] [Google Scholar]
- (35).Wickiser JK, Winkler WC, Breaker RR, and Crothers DM (2005) The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol. Cell 18, 49–60. [DOI] [PubMed] [Google Scholar]
- (36).Mustoe AM, Brooks CL, and Al-Hashimi HM (2014) Hierarchy of RNA functional dynamics. Annu. Rev. Biochem 83, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Shajani Z, and Varani G (2007) NMR studies of dynamics in RNA and DNA by 13C relaxation. Biopolymers 86, 348–359. [DOI] [PubMed] [Google Scholar]
- (38).Al-Hashimi HM (2007) Beyond static structures of RNA by NMR: Folding, refolding, and dynamics at atomic resolution. Biopolymers 86, 345–347. [DOI] [PubMed] [Google Scholar]
- (39).Sherpa C, Rausch JW, Le Grice SF, Hammarskjold ML, and Rekosh D (2015) The HIV-1 Rev response element (RRE) adopts alternative conformations that promote different rates of virus replication. Nucleic Acids Res 43, 4676–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Al-Hashimi HM, and Walter NG (2008) RNA dynamics: it is about time. Curr. Opin. Struct. Biol 18, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Stelzer AC, Kratz JD, Zhang Q, and Al-Hashimi HM (2010) RNA Dynamics by Design: Biasing Ensembles Towards the Ligand-Bound State. Angew. Chem., Int. Ed 49, 5731–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Baird NJ, Inglese J, and Ferré-D’Amaré AR (2015) Rapid RNA-ligand interaction analysis through high-information content conformational and stability landscapes. Nat. Commun 6, 8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).McCown PJ, Corbino KA, Stav S, Sherlock ME, and Breaker RR (2017) Riboswitch diversity and distribution. RNA 23, 995–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Deigan KE, and Ferré-D’Amaré AR (2011) Riboswitches: discovery of drugs that target bacterial gene-regulatory RNAs. Acc. Chem. Res 44, 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Haller A, Souliere MF, and Micura R (2011) The dynamic nature of RNA as key to understanding riboswitch mechanisms. Acc. Chem. Res 44, 1339–1348. [DOI] [PubMed] [Google Scholar]
- (46).Edwards TE, Klein DJ, and Ferré-D’Amaré AR (2007) Riboswitches: small-molecule recognition by gene regulatory RNAs. Curr. Opin. Struct. Biol 17, 273–279. [DOI] [PubMed] [Google Scholar]
- (47).Pitt SW, Majumdar A, Serganov A, Patel DJ, and Al-Hashimi HM (2004) Argininamide Binding Arrests Global Motions in HIV-1 TAR RNA: Comparison with Mg2+-induced Conformational Stabilization. J. Mol. Biol 338, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Bardaro JMF, Shajani Z, Patora-Komisarska K, Robinson JA, and Varani G (2009) How binding of small molecule and peptide ligands to HIV-1 TAR alters the RNA motional landscape. Nucleic Acids Res 37, 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Merriman DK, Xue Y, Yang S, Kimsey IJ, Shakya A, Clay M, and Al-Hashimi HM (2016) Shortening the HIV-1 TAR RNA Bulge by a Single Nucleotide Preserves Motional Modes over a Broad Range of Time Scales. Biochemistry 55, 4445–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Davidson A, Begley DW, Lau C, and Varani G (2011) A small-molecule probe induces a conformation in HIV TAR RNA capable of binding drug-like fragments. J. Mol. Biol 410, 984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lalonde MS, Lobritz MA, Ratcliff A, Chamanian M, Athanassiou Z, Tyagi M, Wong J, Robinson JA, Karn J, Varani G, and Arts EJ (2011) Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA. PLoS Pathog 7, No. e1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Smith AL, Kassman J, Srour KJ, and Soto AM (2011) Effect of Salt Concentration on the Conformation of TAR RNA and Its Association with Aminoglycoside Antibiotics. Biochemistry 50, 9434–9445. [DOI] [PubMed] [Google Scholar]
- (53).Wang B, Wilkinson KA, and Weeks KM (2008) Complex ligand-induced conformational changes in tRNA(Asp) revealed by single-nucleotide resolution SHAPE chemistry. Biochemistry 47, 3454–3461. [DOI] [PubMed] [Google Scholar]
- (54).Schlick T, and Pyle AM (2017) Opportunities and Challenges in RNA Structural Modeling and Design. Biophys. J 113, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Behrouzi R, Roh JH, Kilburn D, Briber RM, and Woodson SA (2012) Cooperative Tertiary Interaction Network Guides RNA Folding. Cell 149, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Smola MJ, Calabrese JM, and Weeks KM (2015) Detection of RNA–Protein Interactions in Living Cells with SHAPE. Biochemistry 54, 6867–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).McGinnis JL, and Weeks KM (2014) Ribosome RNA Assembly Intermediates Visualized in Living Cells. Biochemistry 53, 3237–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Tyrrell J, McGinnis JL, Weeks KM, and Pielak GJ (2013) The Cellular Environment Stabilizes Adenine Riboswitch RNA Structure. Biochemistry 52, 8777–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).McGinnis JL, Liu Q, Lavender CA, Devaraj A, McClory SP, Fredrick K, and Weeks KM (2015) In-cell SHAPE reveals that free 30S ribosome subunits are in the inactive state. Proc. Natl. Acad. Sci. U. S. A 112, 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Watters KE, Abbott TR, and Lucks JB (2016) Simultaneous characterization of cellular RNA structure and function with in-cell SHAPE-Seq. Nucleic Acids Res 44, e12–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Spitale RC, Crisalli P, Flynn RA, Torre EA, Kool ET, and Chang HY (2013) RNA SHAPE analysis in living cells. Nat. Chem. Biol 9, 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Spitale RC, Flynn RA, Zhang QC, Crisalli P, Lee B, Jung J-W, Kuchelmeister HY, Batista PJ, Torre EA, Kool ET, and Chang HY (2015) Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Lu Z, Zhang QC, Lee B, Flynn RA, Smith MA, Robinson JT, Davidovich C, Gooding AR, Goodrich KJ, Mattick JS, Mesirov JP, Cech TR, and Chang HY (2016) RNA Duplex Map in Living Cells Reveals Higher-Order Transcriptome Structure. Cell 165, 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Chatterjee S, and Suresh Kumar G (2017) Small molecule induced poly(A) single strand to self-structure conformational switching: evidence for the prominent role of H-bonding interactions. Mol. BioSyst 13, 1000–1009. [DOI] [PubMed] [Google Scholar]
- (65).Mao J, DeSantis C, and Bong D (2017) Small Molecule Recognition Triggers Secondary and Tertiary Interactions in DNA Folding and Hammerhead Ribozyme Catalysis. J. Am. Chem. Soc 139, 9815–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Dohno C, Kimura M, and Nakatani K (2018) Restoration of Ribozyme Tertiary Contact and Function by Using a Molecular Glue for RNA. Angew. Chem., Int. Ed 57, 506–510. [DOI] [PubMed] [Google Scholar]
- (67).Matsumoto S, Caliskan N, Rodnina MV, Murata A, and Nakatani K (2018) Small synthetic molecule-stabilized RNA pseudoknot as an activator for – 1 ribosomal frameshifting. Nucleic Acids Res 46, 8079–8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Matsumoto S, Hong C, Otabe T, Murata A, and Nakatani K (2013) Ligand-inducible formation of RNA pseudoknot. Bioorg. Med. Chem. Lett 23, 3539–3541. [DOI] [PubMed] [Google Scholar]
- (69).Mao J, DeSantis C, and Bong D (2017) Small Molecule Recognition Triggers Secondary and Tertiary Interactions in DNA Folding and Hammerhead Ribozyme Catalysis. J. Am. Chem. Soc 139, 9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Eubanks CS, Forte JE, Kapral GJ, and Hargrove AE (2017) Small Molecule-Based Pattern Recognition To Classify RNA Structure. J. Am. Chem. Soc 139, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Eubanks CS, and Hargrove AE (2017) Sensing the impact of environment on small molecule differentiation of RNA sequences. Chem. Commun 53, 13363–13366. [DOI] [PubMed] [Google Scholar]
- (72).Patwardhan NN, Ganser LR, Kapral GJ, Eubanks CS, Lee J, Sathyamoorthy B, Al-Hashimi HM, and Hargrove AE (2017) Amiloride as a new RNA-binding scaffold with activity against HIV-1 TAR. MedChemComm 8, 1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Dohno C, Kimura M, and Nakatani K (2018) Restoration of Ribozyme Tertiary Contact and Function by Using a Molecular Glue for RNA. Angew. Chem., Int. Ed 57, 506–510. [DOI] [PubMed] [Google Scholar]
- (74).Dibrov SM, Ding K, Brunn ND, Parker MA, Bergdahl BM, Wyles DL, and Hermann T (2012) Structure of a hepatitis C virus RNA domain in complex with a translation inhibitor reveals a binding mode reminiscent of riboswitches. Proc. Natl. Acad. Sci. U. S. A 109, 5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Hong C, Hagihara M, and Nakatani K (2011) Ligand-Assisted Complex Formation of Two DNA Hairpin Loops. Angew. Chem., Int. Ed 50, 4390–4393. [DOI] [PubMed] [Google Scholar]
- (76).Ladbury JE, Klebe G, and Freire E (2010) Adding calorimetric data to decision making in lead discovery: a hot tip. Nat. Rev. Drug Discovery 9, 23–27. [DOI] [PubMed] [Google Scholar]
- (77).Ferenczy GG, and Keserű GM (2010) Thermodynamics guided lead discovery and optimization. Drug Discovery Today 15, 919–932. [DOI] [PubMed] [Google Scholar]
- (78).Renaud J-P, Chung C.-w., Danielson UH, Egner U, Hennig M, Hubbard RE, and Nar H (2016) Biophysics in drug discovery: impact, challenges and opportunities. Nat. Rev. Drug Discovery 15, 679–698. [DOI] [PubMed] [Google Scholar]
- (79).Giri P, and Suresh Kumar G (2008) Binding of protoberberine alkaloid coralyne with double stranded poly(A): a biophysical study. Mol. BioSyst 4, 341–348. [DOI] [PubMed] [Google Scholar]
- (80).Xi H, Gray D, Kumar S, and Arya DP (2009) Molecular recognition of single-stranded RNA: Neomycin binding to poly(A). FEBS Lett 583, 2269–2275. [DOI] [PubMed] [Google Scholar]
- (81).Wood S, Ferré-D’Amaré AR, and Rueda D (2012) Allosteric tertiary interactions preorganize the c-di-GMP riboswitch and accelerate ligand binding. ACS Chem. Biol 7, 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Gilbert SD, Stoddard CD, Wise SJ, and Batey RT (2006) Thermodynamic and Kinetic Characterization of Ligand Binding to the Purine Riboswitch Aptamer Domain. J. Mol. Biol 359, 754–768. [DOI] [PubMed] [Google Scholar]
- (83).Liberman JA, Bogue JT, Jenkins JL, Salim M, and Wedekind JE (2014) Chapter Eighteen - ITC Analysis of Ligand Binding to PreQ1 Riboswitches. InMethods in Enzymology (Burke-Aguero DH, Ed.) pp 435–450, Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Ren A, and Patel DJ (2014) c-di-AMP binds the ydaO riboswitch in two pseudo-symmetry-related pockets. Nat. Chem. Biol 10, 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Schroeder KT, Daldrop P, and Lilley DM (2011) RNA tertiary interactions in a riboswitch stabilize the structure of a kink turn. Structure 19, 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Trausch JJ, Ceres P, Reyes FE, and Batey RT (2011) The Structure of a Tetrahydrofolate-Sensing Riboswitch Reveals Two Ligand Binding Sites in a Single Aptamer. Structure 19, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Palou-Mir J, Musiari A, Sigel RKO, and Barceló-Oliver M (2016) Characterization of the full-length btuB riboswitch from Klebsiella pneumoniae. J. Inorg. Biochem 160, 106–113. [DOI] [PubMed] [Google Scholar]
- (88).Zhang J, Jones CP, and Ferré-D’Amaré AR (2014) Global analysis of riboswitches by small-angle X-ray scattering and calorimetry. Biochim. Biophys. Acta, Gene Regul. Mech 1839, 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Suresh Kumar G, and Basu A (2016) The use of calorimetry in the biophysical characterization of small molecule alkaloids binding to RNA structures. Biochim. Biophys. Acta, Gen. Subj 1860, 930–944. [DOI] [PubMed] [Google Scholar]
- (90).Saha B, and Suresh Kumar G (2017) Binding interaction of phenothiazinium dyes with double stranded RNAs: Spectroscopic and calorimetric investigation. J. Photochem. Photobiol., B 167, 99–110. [DOI] [PubMed] [Google Scholar]
- (91).Pascale L, Azoulay S, Di Giorgio A, Zenacker L, Gaysinski M, Clayette P, and Patino N (2013) Thermodynamic studies of a series of homologous HIV-1 TAR RNA ligands reveal that loose binders are stronger Tat competitors than tight ones. Nucleic Acids Res 41, 5851–5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Basu P, and Suresh Kumar G (2017) Small molecule–RNA recognition: Binding of the benzophenanthridine alkaloids sanguinarine and chelerythrine to single stranded polyribonucleotides. J. Photochem. Photobiol., B 174, 173–181. [DOI] [PubMed] [Google Scholar]
- (93).Jelesarov I, and Bosshard HR (1999) Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J. Mol. Recognit 12, 3–18. [DOI] [PubMed] [Google Scholar]
- (94).Jatschka J, Dathe A, Csáki A, Fritzsche W, and Stranik O (2016) Propagating and localized surface plasmon resonance sensing – A critical comparison based on measurements and theory. Sensing and Bio-Sensing Research 7, 62–70. [Google Scholar]
- (95).Willets KA, and Van Duyne RP (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem 58, 267–297. [DOI] [PubMed] [Google Scholar]
- (96).Pilch DS, Kaul M, Barbieri CM, and Kerrigan JE (2003) Thermodynamics of aminoglycoside–rRNA recognition. Biopolymers 70, 58–79. [DOI] [PubMed] [Google Scholar]
- (97).Thomas JR, Liu X, and Hergenrother PJ (2006) Biochemical and thermodynamic characterization of compounds that bind to RNA hairpin loops: toward an understanding of selectivity. Biochemistry 45, 10928–10938. [DOI] [PubMed] [Google Scholar]
- (98).Thomas JR, Liu X, and Hergenrother PJ (2005) Size-Specific Ligands for RNA Hairpin Loops. J. Am. Chem. Soc 127, 12434–12435. [DOI] [PubMed] [Google Scholar]
- (99).Prabhu NV, and Sharp KA (2005) HEAT CAPACITY IN PROTEINS. Annu. Rev. Phys. Chem 56, 521–548. [DOI] [PubMed] [Google Scholar]
- (100).Mikulecky PJ, and Feig AL (2006) Heat Capacity Changes Associated with DNA Duplex Formation: Salt- and Sequence-Dependent Effects. Biochemistry 45, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Takach JC, Mikulecky PJ, and Feig AL (2004) Salt-Dependent Heat Capacity Changes for RNA Duplex Formation. J. Am. Chem. Soc 126, 6530–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Madan B, and Sharp K (1997) Molecular Origin of Hydration Heat Capacity Changes of Hydrophobic Solutes: Perturbation of Water Structure around Alkanes. J. Phys. Chem. B 101, 11237–11242. [Google Scholar]
- (103).Sokoloski JE, Dombrowski SE, and Bevilacqua PC (2012) Thermodynamics of Ligand Binding to a Heterogeneous RNA Population in the Malachite Green Aptamer. Biochemistry 51, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Islam MM, and Kumar GS (2009) Small molecule–RNA interaction: Spectroscopic and calorimetric studies on the binding by the cytotoxic protoberberine alkaloid coralyne to single stranded polyribonucleotides. Biochim. Biophys. Acta, Gen. Subj 1790, 829–839. [DOI] [PubMed] [Google Scholar]
- (105).Xi H, Davis E, Ranjan N, Xue L, Hyde-Volpe D, and Arya DP (2011) Thermodynamics of Nucleic Acid “Shape Readout” by an Aminosugar. Biochemistry 50, 9088–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Jayaraman B, Mavor D, Gross JD, and Frankel AD (2015) Thermodynamics of Rev–RNA Interactions in HIV-1 Rev–RRE Assembly. Biochemistry 54, 6545–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Lee J, Vogt CE, McBrairty M, and Al-Hashimi HM (2013) Influence of Dimethylsulfoxide on RNA Structure and Ligand Binding. Anal. Chem 85, 9692–9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Mayhood TW, and Windsor WT (2005) Ligand binding affinity determined by temperature-dependent circular dichroism: Cyclin-dependent kinase 2 inhibitors. Anal. Biochem 345, 187–197. [DOI] [PubMed] [Google Scholar]
- (109).Eyring H (1935) The Activated Complex in Chemical Reactions. J. Chem. Phys 3, 107–115. [Google Scholar]
- (110).Geschwindner S, Ulander J, and Johansson P (2015) Ligand Binding Thermodynamics in Drug Discovery: Still a Hot Tip? J. Med. Chem 58, 6321–6335. [DOI] [PubMed] [Google Scholar]
- (111).Barbieri CM, Kaul M, Bozza-Hingos M, Zhao F, Tor Y, Hermann T, and Pilch DS (2007) Defining the Molecular Forces That Determine the Impact of Neomycin on Bacterial Protein Synthesis: Importance of the 2′-Amino Functionality. Antimicrob. Agents Chemother 51, 1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Tonge PJ (2018) Drug–Target Kinetics in Drug Discovery. ACS Chem. Neurosci 9, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Gleitsman KR, Sengupta RN, and Herschlag D (2017) Slow Molecular Recognition by RNA. RNA 23, 1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Hendrix M, Priestley ES, Joyce GF, and Wong C-H (1997) Direct Observation of Aminoglycoside–RNA Interactions by Surface Plasmon Resonance. J. Am. Chem. Soc 119, 3641–3648. [DOI] [PubMed] [Google Scholar]
- (115).Frolov L, Dix A, Tor Y, Tesler AB, Chaikin Y, Vaskevich A, and Rubinstein I (2013) Direct Observation of Aminoglycoside–RNA Binding by Localized Surface Plasmon Resonance Spectroscopy. Anal. Chem 85, 2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Guan L, and Disney MD (2013) Small-Molecule-Mediated Cleavage of RNA in Living Cells. Angew. Chem., Int. Ed 52, 1462–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Teplova M, and Patel DJ (2008) Structural insights into RNA recognition by the alternative-splicing regulator muscleblind-like MBNL1. Nat. Struct. Mol. Biol 15, 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).deLorimier E, Hinman MN, Copperman J, Datta K, Guenza M, and Berglund JA (2017) Pseudouridine Modification Inhibits Muscleblind-like 1 (MBNL1) Binding to CCUG Repeats and Minimally Structured RNA through Reduced RNA Flexibility. J. Biol. Chem 292, 4350–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Latham MP, Zimmermann GR, and Pardi A (2009) NMR Chemical Exchange as a Probe for Ligand-Binding Kinetics in a Theophylline-Binding RNA Aptamer. J. Am. Chem. Soc 131, 5052–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).de Mol NJ, Dekker FJ, Broutin I, Fischer MJE, and Liskamp RMJ (2005) Surface Plasmon Resonance Thermodynamic and Kinetic Analysis as a Strategic Tool in Drug Design. Distinct Ways for Phosphopeptides to Plug into Src- and Grb2 SH2 Domains. J. Med. Chem 48, 753–763. [DOI] [PubMed] [Google Scholar]
- (121).Leulliot N, and Varani G (2001) Current Topics in RNA–Protein Recognition: Control of Specificity and Biological Function through Induced Fit and Conformational Capture. Biochemistry 40, 7947–7956. [DOI] [PubMed] [Google Scholar]
- (122).Hilimire TA, Bennett RP, Stewart RA, Garcia-Miranda P, Blume A, Becker J, Sherer N, Helms ED, Butcher SE, Smith HC, and Miller BL (2016) N-Methylation as a Strategy for Enhancing the Affinity and Selectivity of RNA-binding Peptides: Application to the HIV-1 Frameshift-Stimulating RNA. ACS Chem. Biol 11, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Fukuzumi T, Murata A, Aikawa H, Harada Y, and Nakatani K (2015) Exploratory Study on the RNA-Binding Structural Motifs by Library Screening Targeting pre-miRNA-29 a. Chem. - Eur. J 21, 16859–16867. [DOI] [PubMed] [Google Scholar]
- (124).Copeland RA (2016) The drug-target residence time model: a 10-year retrospective. Nat. Rev. Drug Discovery 15, 87–95. [DOI] [PubMed] [Google Scholar]
- (125).Heym RG, Hornberger WB, Lakics V, and Terstappen GC (2015) Label-free detection of small-molecule binding to a GPCR in the membrane environment. Biochim. Biophys. Acta, Proteins Proteomics 1854, 979–986. [DOI] [PubMed] [Google Scholar]
- (126).Shah NB, and Duncan TM (2014) Bio-layer Interferometry for Measuring Kinetics of Protein-protein Interactions and Allosteric Ligand Effects. J. Visualized Exp, No. e51383. [DOI] [PMC free article] [PubMed]
- (127).Kozma P, Kehl F, Ehrentreich-Förster E, Stamm C, and Bier FF (2014) Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron 58, 287–307. [DOI] [PubMed] [Google Scholar]
- (128).Wartchow CA, Podlaski F, Li S, Rowan K, Zhang X, Mark D, and Huang K-S (2011) Biosensor-based small molecule fragment screening with biolayer interferometry. J. Comput.-Aided Mol. Des 25, 669. [DOI] [PubMed] [Google Scholar]
- (129).Kozma P, Hámori A, Kurunczi S, Cottier K, and Horvath R (2011) Grating coupled optical waveguide interferometer for label-free biosensing. Sens. Actuators, B 155, 446–450. [Google Scholar]
- (130).Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, Wei J, Li P, Heidecker B, Ma W, Varma R, Zhao L-S, Perillat D, Carricato G, Recknor M, Du K, Ho H, Ellis T, Gamez J, Howes M, Phi-Wilson J, Lockard S, Zuk R, and Tan H (2009) Label-Free Detection of Biomolecular Interactions Using BioLayer Interferometry for Kinetic Characterization. Comb. Chem. High Throughput Screening 12, 791–800. [DOI] [PubMed] [Google Scholar]
- (131).Kozma P, Hamori A, Cottier K, Kurunczi S, and Horvath R (2009) Grating coupled interferometry for optical sensing. Appl. Phys. B: Lasers Opt 97, 5–8. [Google Scholar]
- (132).Gronewold TMA (2007) Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta 603, 119–128. [DOI] [PubMed] [Google Scholar]
- (133).Schuck P, and Zhao H (2010) The Role of Mass Transport Limitation and Surface Heterogeneity in the Biophysical Characterization of Macromolecular Binding Processes by SPR Biosensing. Methods Mol. Biol 627, 15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Palde PB, Ofori LO, Gareiss PC, Lerea J, and Miller BL (2010) Strategies for Recognition of Stem–Loop RNA Structures by Synthetic Ligands: Application to the HIV-1 Frameshift Stimulatory Sequence. J. Med. Chem 53, 6018–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Karlsson R, Katsamba PS, Nordin H, Pol E, and Myszka DG (2006) Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem 349, 136–147. [DOI] [PubMed] [Google Scholar]
- (136).Li J, Sakata A, He H, Bai L-P, Murata A, Dohno C, and Nakatani K (2016) Naphthyridine-Benzoazaquinolone: Evaluation of a Tricyclic System for the Binding to (CAG)n Repeat DNA and RNA. Chem. - Asian J 11, 1971–1981. [DOI] [PubMed] [Google Scholar]
- (137).González-Fernández E, de-los-Santos-Álvarez N, Miranda-Ordieres AJ, and Lobo-Castañón MJ (2012) SPR evaluation of binding kinetics and affinity study of modified RNA aptamers towards small molecules. Talanta 99, 767–773. [DOI] [PubMed] [Google Scholar]


