Summary
Brown adipose tissue (BAT) is rich in mitochondria and plays important roles in energy expenditure, thermogenesis and glucose homeostasis. We find that levels of mitochondrial protein succinylation and malonylation are high in BAT and subject to physiological and genetic regulation. BAT-specific deletion of Sirt5, a mitochondrial desuccinylase and demalonylase, results in dramatic increases in global protein succinylation and malonylation. Mass spectrometry-based quantification of succinylation reveals that Sirt5 regulates the key thermogenic protein in BAT, UCP1. Mutation of the two succinylated lysines in UCP1 to acyl-mimetic glutamine and glutamic acid significantly decreases its stability and activity. The reduced function of UCP1 and other proteins in Sirt5KO BAT results in impaired mitochondria respiration, defective mitophagy and metabolic inflexibility. Thus, succinylation of UCP1 and other mitochondrial proteins plays an important role in BAT and in regulation of energy homeostasis.
Keywords: Succinylation, brown fat, mitochondria, UCP1, thermogenesis
eTOC Blurb (no longer than 50 words, describing the context and significance of the findings for the broader journal readership)
Wang et. al. performed succinyl-proteomics in brown fat (BAT) of normal and Sirt5 KO mice and identified UCP1 as a new target of Sirt5 desuccinylation. UCP1 with succinyl-mimetic mutations displayed reduced activity and stability. Elevated succinylation of mitochondrial protein in Sirt5 KO BAT resulted in altered metabolic flexibility and mitophagy.
Graphical Abstract

Introduction
Brown adipose tissue (BAT) plays a central role in energy balances through its high density of mitochondria and uncoupled respiration. BAT has been shown to be present in all mammals, including humans, and can be activated to increase glucose uptake and energy expenditure (Cypess et al., 2009), making it an attractive therapeutic target for treating obesity and metabolic disease. A critical component of the regulation of BAT is through uncoupling protein 1 (UCP1) and β3-adrenergic receptor stimulation (Cypess et al., 2015), which induces transcription of UCP1 and genes involved in mitochondria biogenesis (Villarroya et al., 2017). UCP1 can also be regulated by interaction with fatty acids (Cannon and Nedergaard, 2004; Fedorenko et al., 2012), as well as by sulfenylation (Chouchani et al., 2016).
Mitochondria dysfunction is an important component in the pathophysiology of a variety of metabolic and cardiovascular diseases (Wallace, 2005). Among all tissues, brown fat relies most heavily on mitochondrial respiration to maintain its normal physiological function of thermogenesis (Cannon and Nedergaard, 2004). In addition to burning its own fat, the sympathetic nervous system drives lipolysis from white fat creating another supply of free fatty acids for BAT heat generation (Schreiber et al., 2017; Shin et al., 2017). The end product of oxidation of these endogenous and exogenous fatty acids in BAT is acetyl-CoA, which is further oxidized through the tricarboxylic acid cycle and the electron transport chain, and all of these reactions require normal mitochondrial function.
Mitochondrial protein functions are subject to multiple reversible post-translational modifications (Stram and Payne, 2016b). Examples of such modifications include acetylation, succinylation, malonylation and glutarylation (Carrico et al., 2018). These reactions are controlled by the level of acyl-donors in the cell and by the action of the mitochondrial sirtuins, Sirt3 and Sirt5, which remove acetyl and succinyl/malonyl/glutaryl groups, respectively (Giralt and Villarroya, 2012; Hirschey and Zhao, 2015; Park et al., 2013; Tan et al., 2014). Sirtuins are NAD+-dependent protein deacylases homologous to yeast silent information regulator 2 (Sir2). Surtuins require NAD+ as a cofactor, making them sensors of cellular energy status. In addition, sirtuins have been shown to play important roles in controlling metabolism in a variety of organisms (Schwer and Verdin, 2008). Sirt3 and Sirt5 are enriched in and act primarily in mitochondria (Michishita et al., 2005). Knockout of Sirt3 in liver results in increased acetylation of long-chain acyl CoA dehydrogenase (LCAD) and a decrease in its enzymatic activity (Hirschey et al., 2010). Likewise, the majority of Sirt5 substrates for desuccinylation in liver are metabolic enzymes involved in fatty acid beta-oxidation, branched chain amino acid metabolism, TCA cycle, and ketone body synthesis (Rardin et al., 2013).
In the present study, we demonstrate that there is widespread mitochondrial protein succinylation and malonylation in BAT, and that these post-translational modifications are upregulated by knockout of Sirt5. Our results demonstrate hyper-succinylation regulates three important proteins: glutamate dehydrogenase (GDH/GLUD1), succinate dehydrogenase (SDH), and most importantly, the unique BAT mitochondrial uncoupling protein UCP1. Acyl-mimetic mutation of the two major succinylation sites in UCP1 significantly decreases its stability and activity. Likewise, hypersuccinylation of GLUD1 and SDH in BAT-specific Sirt5 knockout mice decreases their activities. The cumulative consequence of increased succinylation of these proteins in Sirt5 KO BAT is altered mitochondrial homeostasis and metabolic inflexibility. Thus, succinylation of UCP1 and other Sirt5 substrates provide a new and important mechanism regulating mitochondrial function in BAT.
Results
Protein acylation level is high in BAT and subject to physiological and genetic regulation.
To explore the role of protein acylation in BAT, we performed western blot analysis of subcutaneous white adipose tissue (sWAT), visceral/epididymal white adipose tissue (eWAT), BAT and liver under random fed condition. Levels of protein succinylation and malonylation were highest in BAT, followed by liver, and much lower in sWAT and eWAT (Figure 1A). Both succinylation and malonylation in BAT were regulated by obesity, being decreased in genetically obese db/db mice (Figure 1B) and increased in obesity due to high fat diet (HFD) feeding (Figure 1C). This correlated with UCP1 expression (Figures 1 B, C) and BAT activity, which is reduced in the former and increased in the latter (Alcala et al., 2017; Goodbody and Trayhurn, 1981). More importantly, when mice were housed at 5–6°C either acutely or chronically, which increases BAT activity, levels of succinylated and malonylated proteins as detected by Western blotting increased dramatically (Figure 1D). Conversely, when mice were housed at 30°C, i.e., thermoneutral condition, where BAT activity is low, levels of succinylated/malonylated proteins were markedly reduced (Figure 1D). Interestingly, Sirt5 protein levels positively correlated with the succinylation/malonylation levels in BAT under most of these conditions (Figure 1A–D and Figure S1), indicating that the changes in acylation were not likely due to changes in desuccinylation/demalonylation, but rather reflected changes in rates of formation of these adducts. Indeed, with cold exposure, acetyl, succinyl and malonyl CoA donors increased 30–100% (Figure S1E), reflecting a state of carbon stress induced by increased mitochondrial activity (Wagner and Hirschey, 2014) where Sirt5 was induced to alleviate such stress. As expected, both Sirt5 and the acylated proteins it regulates were enriched in the mitochondrial fraction of BAT, and when normalized to mitochondrial mass, levels of succinylation, but not malonylation, were still elevated by cold acclimation (Figure 1E).
Figure 1. Protein acylation level is high in BAT and subject to physiological and genetic regulation.
Protein acylation and Sirt5 expression were assessed by Western blotting. (A)Total tissue lysates of BAT, sWAT, eWAT and liver from random-fed, 9 week-old wild type C57/B6J mice; (B) BAT from 13–14 week-old db/+ vs. db/db mice; (C) BAT from wild type mice fed high fat diet (60% fat) for 12 weeks and aged matched chow-fed mice; (D) BAT from wild type mice housed at 22°C, or acclimated to 5°C or 30°C for 10 days, and from wild type mice acutely exposed to 6°C after acclimation at 30°C for 10 days; (E) Total cell lysates and mitochondrial fractions of BAT from mice housed at 22°C or acclimated to 5°C for 10 days. n=4 to 6 per group. White dashed lines were used to separate groups in a single gel
BAT specific-Sirt5 Knockout elevates protein succinylation and malonylation levels.
To further assess the regulation of BAT by mitochondrial protein acylation, we generated BAT-specific Sirt5 KO mice (5-BKO) by breeding Sirt5 floxed mice (Yu et al., 2013) with UCP1-Cre mice (Kong et al., 2014), the latter have been well characterized to be BAT specific. No Sirt5 deletion was detected in visceral or subcutaneous WAT in 5-BKO mice (Figure S2A). Under standard housing condition (22°C, normal chow), 5-BKO mice had similar body weight and food intake as their littermate controls (Figure S2B–C). Likewise, there was no difference in weight or morphology of BAT (Figure 2A–B). As expected, there was a 90% decrease in Sirt5 mRNA in BAT from 5-BKO mice, but no differences in the expression of genes involved in browning, fatty acid synthesis or transport, lipolysis, mitochondrial oxidative phosphorylation or glucose transport (Figure 2C). However, consistent with Sirt5 being a strong desuccinylase/demalonylase, western blotting revealed two- to four-fold increases in protein succinylation and malonylation in Sirt5-deficient BAT compared to control, while protein acetylation levels were not affected (Figure 2D). As in Figure 1D, cold acclimation to 5°C for 10 days significantly increased levels of succinylated and malonylated proteins in BAT of control mice, and Sirt5 deficiency increased these levels even further. The amount of acetylated proteins was also increased by cold acclimation but were not further elevated by Sirt5 deficiency (Figure 2D). As expected, succinylated and malonylated proteins were enriched in mitochondria and were further increased with Sirt5 KO (Figure 2E).
Figure 2. BAT specific-Sirt5 deficiency elevates protein succinylation and malonylation levels.
(A) H&E staining of BAT from 11 week-old random-fed floxed and 5-BKO mice, scale bar=100 μm (B) Ratio of adipose tissue weight vs. body weight in 11 week-old chow fed mice, n=5 vs. 6. Data are represented as mean ± SEM. (C) Q-PCR of BAT from 11 week-old random-fed floxed and 5-BKO mice, n=6 per group. Data are represented as mean ± SEM, *p < 0.05, student’s t-test. (D) Western blot of BAT from 2 month-old female floxed and 5-BKO mice housed at 22°C or acclimated to 5°C for 10 days. (E) Western blot of BAT cytoplasmic (cyto) and mitochondrial fraction (mito) from 8 week-old, 24 hr-fasted floxed and 5-BKO mice.
Sirt5 deficiency leads to metabolic inflexibility in BAT.
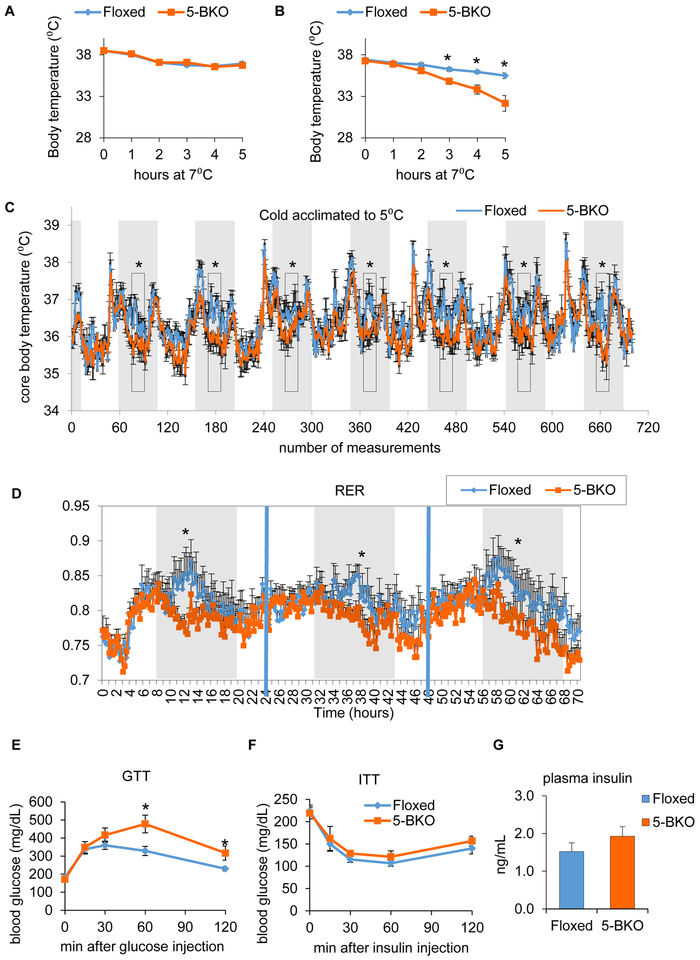
An important function of BAT is to defend against cold stress. When floxed and 5-BKO mice were subjected to acute cold exposure under ad libitum fed condition, 5-BKO mice showed similar ability to defend against cold as controls, with both groups losing ~1.5°C of body temperature over 5 hours (Figure 3A). Interestingly, after an overnight fast, which inactivates BAT but activates Sirt5 (Jokinen et al., 2017; Rothwell et al., 1984), the control mice could still maintain body temperature to acute cold exposure, whereas 5-BKO mice became much more sensitive to cold, losing almost 5° C over 5 hours (Figure 3B). This occurred despite the fact that there were no differences in the expression of genes involved in browning, respiration or lipid and glucose utilization (Figure S2D). Lipid mobilization does not appear to be impaired in Sirt5-BKO mice, as HSL phosphorylation was similar, if not higher in Sirt5 KO BAT compared to control (Figure S2E). Although Sirt5-BKO mice had no problem acclimating to 5°C on HFD, core body temperature was consistently 0.7 to 1.5°C lower in Sirt5-BKO mice than in controls during the feeding period, indicating a defect in BAT thermogenesis through fatty acid oxidation (Figure 3C). Metabolic profiling of 5-BKO and control mice using the Comprehensive Lab Animal Monitoring System (CLAMS) revealed that 5-BKO mice had similar O2 consumption and CO2 production as controls (Figure S3A–B), but their respiratory exchange ratio (RER) was significantly lower, especially in the early part of the dark period when mice are feeding (Figure 3D). This indicates that 5-BKO mice prefer to use fatty acids as fuel or are incapable of switching from FFA usage to carbohydrates. Consistent with metabolic inflexibility, 5-BKO mice showed normal glucose tolerance on a standard chow diet (Figure S3C), but developed glucose intolerance when challenged with high fat diet (Figure 3E). This occurred without a change in insulin sensitivity as measured by insulin tolerance test (Figure 3F) or any differences in fasting plasma insulin levels between genotypes (Figure 3G). The differences in glucose tolerance were greatest at later time points after glucose injection (Figure 3E) suggesting the defect in 5-BKO mice was due to decreased glucose uptake in BAT, as BAT is known to be a major site of glucose utilization in the mouse (Bartelt et al., 2011; Nedergaard et al., 2011). Thus, mice lacking Sirt5 in BAT are refractory in switching fuels from FFA to glucose after overnight fasting.
Figure 3. Sirt5 deficiency leads to metabolic inflexibility in BAT.
(A) Rectal temperature of 2 month-old floxed and 5-BKO mice during acute cold (from 22°C to 7°C) exposure. Mice were allowed free access to food and water. n=5 vs. 7. Data are represented as mean ± SEM. (B) Rectal temperature of 2.5 month-old floxed and 5-BKO mice during acute cold (from 22°C to 7°C) exposure. Mice were fasted for 18 hours. n=7 vs. 8. Data are represented as mean ± SEM. (C) Intra-abdominal temperature of HFD fed floxed and Sirt5-BKO mice after 3 days’ acclimation to 5°C, recorded at 15 min interval. Dark phase are shaded, measurements occurred between 10:00 p.m. and 2:00 a.m. were boxed. n=4 vs. 6. Data are represented as mean ± SEM. *p < 0.05. (D) Respiration exchange ratio (RER) of 5 month-old floxed and 5-BKO mice on chow diet. n=6. Data are represented as mean ± SEM. (E) Glucose tolerance test of floxed and 5-BKO mice fed with high fat (60% by calories) diet for 11 weeks. n=5 vs. 8. Data are represented as mean ± SEM. (F) Insulin tolerance tests of floxed and 5-BKO mice fed with high fat diet for 12 weeks. n=6 vs. 8, Data are represented as mean ± SEM. (F) Fasting plasma insulin levels of floxed and 5-BKO mice fed with high fat diet for 13 weeks. n=6 vs. 8. Data are represented as mean ± SEM. *p < 0.05, student’s t-test.
BAT Sirt5 desuccinylates proteins in major metabolic pathways.
To determine cold- and Sirt5-regulated succinylation sites that may contribute to the metabolic regulation of BAT, we assessed protein succinylation in whole BAT from floxed (control) and 5-BKO mice housed at room temperature and mice cold acclimated to 5° C for 10 days using succinyl-lysine affinity enrichment followed by quantitative mass spectrometry analysis (Gillet et al., 2012; Meyer et al., 2017). We identified and quantified a total of 2404 succinylation sites on 444 proteins (Supplementary Table 1). For each succinylation site we report here, the unmodified protein was also quantified in a parallel analysis in order to normalize changes in succinylation level to individual protein amount. Of these, 1387 succinylation sites from 348 proteins were regulated at least 2-fold with a false discovery rate (FDR) < 0.01 in at least one condition when normalized to unmodified protein level (Figure S4A). Among the 829 lysine sites whose relative succinylation levels were increased in Sirt5 KO BAT, only 57 were also elevated by cold acclimation (CA, Figure 4A), indicating a fine level of tuning of succinylation by CA which is not likely simply due to changes in Sirt5 activity. Likewise, when normalized to individual protein amount, only 102 lysine sites had increased relative succinylation in response to CA, indicating the global increase of relative succinylation induced by CA observed by western blotting (Figure 1D–E) was due to a combination of significant increases in the level of the mitochondrial protein and increases in the level of succinylation, and that the effects of cold acclimation were selective. Similarly, CA significantly decreased relative succinylation levels in 657 lysine sites, while only 60 sites, with 17 of them overlapping with CA, were similarly affected by Sirt5 deficiency (Figure 4A). Reactome functional term enrichment analysis of the 249 proteins whose succinylation sites increased due to Sirt5 KO revealed enrichment of several mitochondrial pathways, including complex I biogenesis, thermogenesis by uncoupling proteins, electron transport chain (ETC), mitochondrial fatty acid beta oxidation and TCA cycle (Figure 4B, S5A–B). This was observed in both room temperature (RT) and cold acclimated (CA) mice (Figure S4A, S5A–B).
Figure 4. BAT Sirt5 desuccinylates proteins in major metabolic pathways.
(A) Venn diagram showing lysine sites whose succinylation levels were increased (red) or decreased (blue) by at least two fold with FDR<0.01 comparing between Sirt5 KO vs. Floxed and cold acclimation (CA) vs. room temperature (RT) housed mice. (B) Reactome term enrichment analysis from the list of proteins containing succinylation sites that were identified by mass spectrometry and increased due to Sirt5 KO. (C-F) Succinylation fold changes (F.C.) on individual lysine sites of SDHA (C), SDHB (D), GLUD1 (E), and UCP1 (F). (G) Western blot of BAT from floxed and 5-BKO mice before and after anti-succinyl-K immunoprecipitation.
Among the most highly succinylated proteins in Sirt5 KO BAT were the mitochondrial enzymes succinate dehydrogenase A and B (SDHA and SDHB), glutamate dehydrogenase (GLUD1), and the mitochondrial uncoupling protein UCP1. Follow-up experiments to the mass spectrometry results suggested that these proteins had relatively high stoichiometries of succinylation. Thus, immunoprecipitation with anti-succinyl-lysine antibody in Sirt5 KO BAT lysates resulted in depletion of 9% of SDHA, 17% of SDHB, 24% of UCP1, and 38% of GLUD1 in the lysate (Figure S4B). In addition, mass spectrometry revealed five lysine sites on SDHA whose relative succinylation levels increased over 4-fold in response to Sirt5 loss in mice housed at room temperature (Figure 4C), and for two of these (K179 and K485), the increase was more than 10-fold. Likewise, SDHB had three lysine sites whose relative succinylation levels were increased by more than two-fold in Sirt5 KO BAT compared to control, one of which, K169, had a close to 5-fold increase (Figure 4D). GLUD1 was also a very confident target of Sirt5 in BAT; four of its lysines showed more than two-fold increase in relative succinylation in response to Sirt5 deficiency, and for two (K90, K480), the increase was more than 15-fold (Figure 4E). Mass spectrometry and immunoaffinity enrichment by anti-succinyl-lysine antibody also identified UQCRC2 of mitochondria ETC complex III as a target of Sirt5 (Supplemental table 1 and Figure 4G). In addition, NDUFB8 of complex I showed increased pulldown by anti-succinyl-K antibody, although NDUFB8 itself was not identified as a Sirt5 target by mass spectrometry, potentially because NDUFB8 is in strong association with other subunits in complex I, which are succinylated targets of Sirt5.
Perhaps the most interesting of the succinylated proteins observed in BAT was UCP1. UCP1 is unique to brown fat and critical for its thermogenic function (Cannon and Nedergaard, 2004). UCP1 has not been previously identified as being succinylated or a Sirt5 target, however, in 5-BKO mice housed at room temperature, mass spectrometry revealed 4- and 15-fold increases in UCP1 relative succinylation levels at K56 and K151 as compared to control (Figure 4F). Similar fold increases in relative succinylation on these two sites also occurred when 5-BKO mice were housed under cold acclimated condition (Figure 4F). These changes in the succinylation of UCP1, as well as SDHA/B and GLUD1, were confirmed by western blotting with protein specific antibodies after immunoprecipitation with anti-succinyl-K antibody (Figure 4G).
Hypersuccinylation due to Sirt5 deficiency impairs mitochondria respiration and enzymatic activity.
To determine if the increased succinylation/malonylation of mitochondrial OxPhos proteins altered their functions, we performed Seahorse analysis on mitochondria isolated from BAT of 5-BKO and floxed control mice. When compared to control, mitochondria from 5-BKO mice had a 30% reduction in oxygen consumption rate (OCR) when pyruvate and malate were used as substrates for complex I (Figure 5A). With addition of rotenone to block complex I and succinate as substrate for complex II, OCR was borderline significantly lower in Sirt5 deficient BAT mitochondria compared to control, indicating somewhat impaired complex II activity (Figure 5B). However, in the presence of antimycin to block complex III and N, N, N’, N’-tetramethylp-phenylenediamine (TMPD) as an electron donor for cytochrome c in complex IV, OCR only trended to be lower in BAT mitochondria lacking in Sirt5 without reaching significance (Figure 5C), consistent with the fact that complex I had the most Sirt5 targets for desuccinylation while complex IV had much fewer (Figure 4B, S5A and Supplementary table 1).
Figure 5. Hypersuccinylation due to Sirt5 deficiency impairs mitochondria respiration and enzymatic activity.
(A-C) Oxygen consumption rate (OCR) in Seahorse flux assay using isolated mitochondria of BAT from overnight fasted floxed and 5-BKO mice. n=10 wells from 5 mice per genotype. Data are represented as mean ± SEM. TMPD: N, N, N′, N′-tetramethyl-p-phenylenediamine (D) Succinate and (E) fumarate concentrations in 50% aqueous acetonitrile homogenates of BAT from random fed floxed and 5-BKO mice. n=5 vs. 6. Data are represented as mean ± SEM. (F) Succinate dehydrogenase (SDH) activity in BAT mitochondria isolated from floxed and 5-BKO mice. n=7 vs. 5. Data are represented as mean ± SEM. (G) Oil Red O staining of day 6 mature brown adipocytes. (H) Western blot of day 6 mature brown adipocytes. (I) Fatty acid oxidation assay using C14-palmitic acid in day 7 mature floxed and Sirt5 KO brown adipocytes. n=6. Data are represented as mean ± SD. ASM: acid soluble metabolite. (J) Oxygen consumption rate (OCR) in Seahorse flux assay using isolated mitochondria of BAT from overnight fasted female floxed and 5-BKO mice. n=10 wells from 5 pools (2 mice per pool) per genotype. Data are represented as mean ± SEM. (K) Glutamate oxidation assay and (L) glutamate uptake assay in the presence or absence of leucine in day 7 mature floxed and Sirt5 KO brown adipocytes. n=6. Data are represented as mean ± SD. *p < 0.05, student’s t-test with Bonferroni correction. (M) OCR and (N) ECAR of Seahorse flux assay in day 5 floxed and Sirt5 KO brown adipocytes. n=10 wells per group, normalized to protein amount. Data are represented as mean ± SEM. Glc: glucose. 2-DG: 2-deoxy-glucose. Eto: etomoxir. R/A: rotenone+ antimycin, *p < 0.05, student’s t-test.
SDH, as part of complex II, not only relays electrons in respiration chain, but also participates in the TCA cycle, converting succinate to fumarate. Metabolomic analysis of BAT extracts revealed that Sirt5 deficiency led to a significant 27% decrease in fumarate levels, with no significant change in succinate concentration (Figure 5D, E), consistent with a decrease in SDH activity. Indeed, direct assessment of SDH activity in isolated mitochondria from Sirt5 KO BAT showed a 20% reduction in SDH activity compared to control (Figure 5F).
Sirt5 deficiency impairs brown adipocyte FAO, GDH activity, and metabolic flexibility.
To determine the cell autonomous effects of Sirt5 deficiency on BAT function, we immortalized brown preadipocytes from neonatal Sirt5-floxed mice with SV40 large-T antigen (Fasshauer et al., 2000) and transduced them with GFP or GFP-Cre adenovirus to induce Sirt5 KO in vitro and allow isolation of the transduced cells by fluorescence-activated cell sorting (FACS). Sirt5 deficiency did not affect brown fat differentiation, as shown by normal oil red O staining (Figure 5G) and high levels of PPARγ protein expression following differentiation (Figure 5H). Given that many enzymes in the fatty acid oxidation pathway were substrates of Sirt5 for desuccinylation (Figures 4B, S5B), we compared the ability of Sirt5 KO and floxed brown adipocytes to oxidize 14C-palmitate. This revealed a ~30% reduction in 14CO2 release in Sirt5 KO cells vs. controls (Figure 5I). Furthermore, this was incomplete oxidation, as indicated by a lower ratio of radiolabeled CO2 to radiolabeled acid soluble metabolites (ASM) in the Sirt5 KO cells (Figure 5I). A similar fatty acid oxidation defect was also observed in the isolated mitochondria from Sirt5 KO BAT (Figure 5J).
Likewise, Sirt5 KO cells showed impaired glutamate oxidation under basal conditions (Kreb-Ringer HEPES buffer with 100 μM glutamate). Thus, Sirt5 KO brown adipocytes had a nearly 40% reduction in 14CO2 release derived from 14C-glutamic acid (Figure 5K). Interestingly, addition of leucine, an allosteric activator of GLUD1 (Tomita et al., 2011), resulted in 90% rescue of 14CO2 release from Sirt5 KO brown adipocytes compared to control, whereas glutamate oxidation in the floxed cells was not affected by addition of leucine (Figure 5K). Because glutamate uptake was similar between Sirt5 KO and control brown adipocytes (Figure 5L), the decreased glutamate oxidation in Sirt5 KO brown adipocytes is most likely due to impaired GLUD1 activity, as its allosteric activator rescued the defects.
The metabolic inflexibility and impaired mitochondria respiration of Sirt5 KO BAT was also recapitulated in vitro. Thus, in glucose- and serum-free DMEM, mimicking the fasting condition, Sirt5 KO brown adipocytes exhibited significantly lower basal OCR than control cells (Figure 5M). After addition of 10 mM glucose, OCR in control cells dropped significantly, while OCR in KO cells remained stable. This may be due to the control cells being more glycolytic under basal conditions, as reflected by higher extracellular acidification rate (ECAR) (Figure 5N), indicating that they could quickly switch to glycolysis to generate energy. In contrast, Sirt5 KO brown adipocytes failed to quickly adapt to this change (Figure 5M), despite a complete recovery of ECAR after addition of glucose (Figure 5L). Inhibiting glycolysis by 2-deoxyglucose had modest effect on OCR in both Sirt5 KO and control brown adipocytes. Finally, when etomoxir, a CPT1α inhibitor that blocks lipid utilization, was added, there was a major increase of OCR in floxed cells, but again OCR in the KO cells remained stable or decreased slightly. With both glucose and fatty acid utilization blocked, brown adipocytes rely on amino acids, such as glutamate (Yelamanchi et al., 2016). The fact that Sirt5 KO brown adipocytes had significantly lower OCR compared to control when glutamate was the major fuel further suggested that hypersuccinylation of GLUD1 due to Sirt5 deficiency impaired its activity. Taken together, in contrast to robust changes in OCR in control cells in response to different energy sources, the OCR curve in Sirt5 KO brown adipocytes remained relatively flat despite changes in substrate usage, demonstrating metabolic inflexibility of these cells.
Overacylation due to Sirt5 deficiency impairs UCP1 activity and stability.
UCP1 plays a critical role in BAT thermogenesis by allowing mitochondria to dissipate the mitochondrial proton gradient, thereby producing heat instead of ATP. As noted above, in Sirt5-KO BAT there is hypersuccinylation of two lysine residues in UCP1, K56 and K151 (Figure 4F). These two lysines in UCP1 are conserved among most placental mammals (Figure S6) and localize to unstructured loops of the molecule in the mitochondria matrix (Figure 6A). To determine whether acylation at those two sites affects UCP1 activity, acyl-mimetic K to Q mutations were made at both lysines and the wildtype (WT) or 2KQ mutant forms of UCP1 were stably expressed in 3T3-L1 cells by retroviral transduction. The expression of WT and 2KQ UCP1 were similar at mRNA levels (Figure 6B). Western blotting for protein was complicated by the fact that some antibodies to UCP1 have been raised to peptides in the region of potential acylation. Thus, the relative abundance of 2KQ-UCP1 protein appeared less than WT protein when AB10983 was used for immunoblotting, which recognizes a domain encompassing the K151 site (Figure 6C), whereas mutant and WT UCP1 protein levels appeared comparable using an antibody to other regions of UCP1 such as sc-6528 (Figure 6C), consistent with the mRNA data. Seahorse analysis of 3T3-L1 cells expressing either empty vector, WT or the 2KQ mutant form of UCP1 revealed no effect of UCP1 overexpression on basal oxygen consumption rate in the presence of 25 mM glucose (Figures 6D, E). As expected, oligomycin treatment decreased OCR in all three cell types, but the absolute OCR value remained significantly higher in cells expressing WT UCP1 compared to the other two (Figure 6D,E), indicating increased basal proton leak. Addition of 50 μM free fatty acids (FFA, oleate:palmitate=2:1), which is known to activate UCP1 (Li et al., 2014; Nicholls and Rial, 1999), had modest effect on OCR in cells transduced with empty vector, but was able to by-pass the inhibition of oligomycin and robustly activated uncoupled respiration in cells expressing WT UCP1. Cells expressing the 2KQ mutant also responded, but the OCR increase in response to FFA was only ~40% of that in cells expressing WT-UCP1. Addition of FCCP to all these cell lines further increased OCR to similar levels of maximal respiration capacity, and all cell lines showed similar non-mitochondrial respiration after rotenone/antimycin treatment (Figures 6D, E).
Figure 6. Overacylation due to sirt5 deficiency impairs UCP1 activity and stability.
(A) Mouse UCP1 structure model showing location of K56 and K151. (B) UCP1 mRNA expression in confluent 3T3-L1 pre-adipocytes stably infected with retrovirus expressing MSCV-puro vector, or wildtype or 2KQ mutant of UCP1. n=4 in each group. Data are represented as mean ± SD. (C) UCP1 protein expression in confluent 3T3-L1 preadipocytes as in Figure 6B. (D) Seahorse Flux assay using confluent 3T3-L1 preadipocytes transduced as in Figure 6B. Oligo: oligomycin, R/A: rotenone+antimycin. (E) Quantitation of OCR for Seahorse in Figure 6D. n=6 for vector, n=7 for WT and 2KQ UCP1. Data are represented as mean ± SD. *p < 0.05, Student’s t-test with Bonferroni correction. (F) UCP1 mRNA expression in confluent 3T3-L1 pre-adipocytes stably infected with retrovirus expressing MSCV-puro vector, or wildtype or 2KE mutant of UCP1. n=4 in each group. Data are represented as mean ± SD. (G) UCP1 protein expression in confluent 3T3-L1 pre-adipocytes as in Figure 6F. (H) Seahorse Flux assay using confluent 3T3-L1 pre-adipocytes transduced as in Figure 6F. (I) Quantitation of OCR for Seahorse in Figure 6H. n=6 for vector, n=7 for WT and 2KE UCP1. Data are represented as mean ± SD. *p < 0.05, Student’s t-test with Bonferroni correction. (J) Western blot of 3T3-L1 preadipocytes transduced with WT-UCP1 or the 2KQ/2KE mutant and treated with 20 μg/mL cycloheximide (CHX) for 0, 3.5 and 7 hrs. Global ubiquitination were used as positive control for CHX effect.
Similarly, reduced UCP1 activity was observed when K56 and K151 were both mutated to the succinyl-mimetic glutamic acid (E) (Figure 6H–I). However, despite similar mRNA expression (Figure 6F), the protein level of 2KE-UCP1 seemed to be significantly less than WT when either anti-UCP1 antibody (AB10983 or sc-6528) was used (Figure 6G). This could indicate that either the glutamic acid mutation is antigenically more different from lysine than glutamine, or that mutation of these lysines to glutamic acid decreased stability of the UCP1 protein. To test the latter possibility, 3T3-L1 preadipocytes expressing WT or the mutant forms of UCP1 were treated with cycloheximide, a protein synthase inhibitor, and the UCP1 turnover rate assessed by western blotting (Figure 6J). Indeed, both the 2KQ and 2KE mutated forms of UCP1 showed significantly reduced half-lives decreasing from around 24 hrs in the WT to around 3.5 hrs with the mutant protein (Figure 6J). Despite significantly shortened half-life, the steady state protein amount of 2KQ-UCP1 looked similar to WT indicating that the decreased activity in the 2KQ mutant is largely attributable to decreased function of the protein. Taken together, these results demonstrate that acylation of K56 and K151 constitutes an important layer of regulation for UCP1 affecting both protein turnover and function.
Overacylation due to Sirt5 deficiency leads to autophagy/mitophagy defect.
To determine if Sirt5 deficiency affects mitochondria homeostasis in times of changing energy availability, 5-BKO and floxed littermates were subjected to a 24-hour fast. While all animals lost similar amounts of weight when fasted and had similar changes in blood glucose and plasma insulin (Figure S7A–C), after fasting BAT weight (as a percentage of body weight) was significantly lower in 5-BKO mice versus controls (Figure S7D). Western blot analysis showed that protein succinylation was significantly decreased by fasting in control BAT, but not in 5-BKO BAT (Figure S7E). Similarly, Mito OxPhos protein levels were decreased in the control, but not in 5-BKO, BAT by fasting (Figures 7A, B). This retention of Mito OxPhos proteins in Sirt5 KO BAT during fasting was post-transcriptional, as mRNA expression of these proteins was not different between floxed and KO BAT (Figure 7C). Q-PCR analysis of genes involved in mitophagy and autophagy revealed that autophagy markers, such as Bnip3 and LC3, were significantly induced by fasting in both genotypes, but the induction was much greater in Sirt5 KO BAT (Figure 7D). Expression of mitochondrial fission 1 (Fis1) was also significantly higher in Sirt5 KO BAT compared to control after fasting, indicating more mitochondrial fission. Consistent with this, electron microscopy of BAT from fasted mice revealed that Sirt5 deficiency shifted BAT mitochondrial size distribution to smaller mitochondria (Figure 7E), without affecting mitochondrial structure (Figure 7F).
Figure 7. Overacylation due to Sirt5 deficiency leads to autophagy/mitophagy defect.
(A) Western blot of selected mitochordrial and autophagy/mitophagy markers in BAT extracts from 2.5-month-old random fed and 24 h fasted floxed and 5-BKO mice. n=4 per group. (B) Quantitation of the 24 h fasted group in Fig.7A. n=4. Data are represented as mean ± SEM. *p < 0.05, student’s t-test. (C) Q-PCR analysis of Mito Oxphos genes in BAT from 2.5-month-old random fed (same mice as Figure 2C) and 24 h fasted floxed and 5-BKO mice. n=5 to 6 per group. Data are represented as mean ± SEM. *p < 0.05, student’s t-test. (D) Q-PCR analysis of autophagy and mitophagy genes in BAT as in Fig.7C. (E) Histogram of BAT mitochondria size quantification from 24 hour fasted chow fed 2-month-old floxed and 5-BKO mice. Electron microscopic (EM) images of 600 mitochondria from 4 mice per genotype were quantified using image J. Data are represented as mean ± SEM. *p < 0.05, student’s t-test. (F) Representative EM pictures showing mitochondria size and morphology from each genotype, scale bar =500 nm. (G) Western blot of BAT from 2-month-old floxed and 5-BKO mice 3 hours after saline or leupeptin (40mg/kg) injection following 21 hour fast. n=3. (H) Quantification of LC3II protein amount in Figure 7G, normalized to Vinculin. Data are represented as mean ± SEM. *p < 0.05, student’s t-test.
LC3 is important in both autophagy and mitophagy (Tanida et al., 2008). It is synthesized as pro-LC3, cleaved into LC3I, which after conjugation with phosphatidylethanolamine becomes LC3II that inserts into the membrane of the autophagosome. When autophagosomes fuse with lysosomes, LC3II is degraded together with the inner membrane. Western blotting showed that LC3II protein levels were significantly higher in Sirt5 KO BAT compared to the controls, especially under fasting condition (Figures 7A, B). The fact that Mito OxPhos proteins accumulated more in Sirt5 KO BAT under fasting condition suggests the accumulation of LC3II was due to defects in autophagosome degradation. Consistent with this, when 5-BKO and floxed mice were fasted for 24 hrs, more LC3II accumulated in Sirt5-KO BAT than in controls (Figure 7G–H). If, however, the lysosome inhibitor leupeptin (Juhasz, 2012) was given three hours before sacrifice, LC3II accumulated to the same level in both control and Sirt5-KO BAT (Figure 7G–H), indicating that the increase in LC3II in Sirt5 KO BAT was due to a defect in autophagosome degradation. Interestingly, leupeptin treatment significantly decreased global succinylation in BAT from floxed mice but not from Sirt5 KO BAT (Figure S7F), indicating either that blockade of autophagy created a cellular signal mimicking starvation activating Sirt5 and decreasing levels of succinylation, or that autophagy blockade reduced abundance of succinyl-CoA which is required to non-enzymatically succinylate proteins.
Discussion
Protein acylation is an important post-translational modification that can regulate protein function (Stram and Payne, 2016a). This is especially true in mitochondria, where acetylation, malonylation, succinylation and glutarylation can be regulated by the mitochondrial sirtuins, Sirt3 and Sirt5 (Hirschey et al., 2010; Nishida et al., 2015; Park et al., 2013; Rardin et al., 2013; Tan et al., 2014). BAT is highly enriched in mitochondria, and mitochondria in this tissue play an especially important role in regulating energy balance. Much of the regulation of BAT occurs through the activation of UCP1, which dissipates the mitochondrial proton gradient to generate heat (Cannon and Nedergaard, 2004; Matthias et al., 2000; Nedergaard et al., 2001). In BAT, both the number of mitochondria and the level of UCP1 expression are highly regulated by both the state of differentiation of the cell and the level of β-adrenergic stimulation (Cannon and Nedergaard, 2004; Villarroya et al., 2017). UCP1 has also been shown to be regulated allosterically by association with free fatty acids (Divakaruni et al., 2012) and by sulfenylation of Cys253 in response to the ROS generated when UCP1 is activated (Chouchani et al., 2016). In this study, we show that UCP1 can also undergo succinylation, which results in a decrease in UCP1 stability, as both acyl-mimetic 2KQ and succinyl-mimetic 2KE mutant of UCP1 have markedly decreased half-lives compared to their WT counterparts. Succinylation at K56 and K151 is also important in regulating UCP1 function, as the acyl-mimetic 2KQ mutant of UCP1, which were expressed at similar levels compared to WT UCP1, has a 60% decrease in free fatty acid induced UCP1 activation.
Although UCP1 succinylation is not increased by cold acclimation when normalized to unmodified UCP1 protein amount, unpublished data from BioRxiv (http://dx.doi.org/10.1101/445718 ) showed that UCP1 acetylation at K56, K73, K151 and K67 were significantly increased by severe cold, and the acyl-mimetic 4KQ mutation significantly decreased its stability. These results suggest that decreased stability and function of acylated UCP1 may serve as a break to dampen the effect of cold-induced BAT activation, so that once the mice were transferred back to warm temperature from cold, the extra UCP1 can be quickly degraded to prevent overheating of the mice. In line with this, cold exposure has been shown to induce a major increase in succinate flux, which activates BAT (Mills et al., 2018). The increased succinyl-CoA which succinylates the newly synthesized mitochondria proteins may also negatively affect their stability and function, serving as a negative feedback in the system. When Sirt5 is knocked out, the negative feedback gets amplified, which leads to impaired BAT thermogenic function, and results in lower core body temperature in Sirt5 KO mice compared to control when acclimated to 5°C.
Succinylation of UCP1 is part of a broader program of succinylation-mediated regulation of other mitochondrial enzymes in BAT. Indeed, using mass spectrometry, we have identified a total of 2404 succinylation sites on 444 proteins in BAT, of which over one third were up-regulated by at least two-fold with Sirt5 deficiency. These proteins include a broad range of proteins involved in fatty acid oxidation, electron transport chain, and amino acid metabolism. In addition to UCP1, two enzymes identified as important functional targets of succinylation in mitochondrial of BAT are GLUD1 and SDH.
Glutamate dehydrogenase 1 (GLUD1) is heavily succinylated in Sirt5 KO BAT, as it is in the liver (Rardin et al., 2013), and this leads to a 45% decrease in its activity, as measured by 14CO2 release from 14C-glutamic acid. Interestingly, the impaired 14CO2 release in Sirt5 KO brown adipocytes is rescued by addition of leucine, a known allosteric activator of GLUD1 (Tomita et al., 2011), indicating that leucine binding to GLUD1 outweighs the inhibitory effect of increased succinylation. We also find significant succinylation of two subunits of succinate dehydrogenase in BAT of Sirt5 KO mice. This is associated with a 20% decrease in SDH activity in isolated mitochondria. In vivo, metabolic profiling reveals a decrease in SDH activity with reduced levels of fumarate in Sirt5 KO BAT, while succinate levels remain similar. Moreover, Complex II respiration is also impaired in Sirt5 KO BAT mitochondria from overnight fasted mice compared to control. The consistent effect of Sirt5 deficiency on SDH activity in this study, in contrast to the variable effects reported in the liver (Park et al., 2013; Zhang et al., 2017), may reflect the generally higher level of stoichiometry of protein succinylation observed in BAT compared to other tissues.
Consistent with other studies on acylation, the effect of elevated succinylation in response to Sirt5 deficiency in BAT is primarily inhibitory. Indeed, functions of enzyme clusters such as complexes I and II of the respiration chain and fatty acid oxidation are also impaired in Sirt5 KO BAT and brown adipocytes, and this correlates with increased succinylation on multiple lysine sites of these proteins. The cumulative effect of impaired functions of these highly acylated mitochondria proteins and enzymes is metabolic inflexibility in 5-BKO mice. In vivo, metabolic inflexibility is manifested as acute cold intolerance after overnight fast, decreased RER at the onset of dark phase, and glucose intolerance in diet-induced obesity. In vitro, metabolic inflexibility is displayed as blunted response to different energy stimuli. Metabolic inflexibility is a common feature for animals with mitochondrial sirtuin deficiency, as Sirt3 KO mice are also less metabolically flexible than their wild-type littermates and exhibited multiple features of the metabolic syndrome as they age (Hirschey et al., 2010; Jing et al., 2011; Jing et al., 2013). One interesting observation is how Sirt5 deficiency in one tissue may partially compensate for defects in other tissues. For example, while there is clear glucose intolerance in Sirt5-BKO mice when challenged with high fat diet, Sirt5 whole body KO mice tend to be more glucose tolerant than controls (Yu et al., 2013). This is likely due to decreased hepatic gluconeogenesis in Sirt5 whole body KO mice, as gluconeogenesis pathway is an important target of Sirt5 for demalonylation in liver (Nishida et al., 2015), and this may counter the effect of decreased glucose usage in Sirt5 KO BAT.
Although Sirt5 whole body KO mice display relatively mild phenotypes under normal physiological conditions (Yu et al., 2013), a number of studies have shown that Sirt5-mediated desuccinylation, as well as demalonylation and deglutarylation, can lead to increased enzymatic activity of the acylated proteins (Kumar and Lombard, 2018) and alter phenotypes of mice under stress conditions. Thus, Sirt5 demalonylates glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and other glycolytic enzymes to promote glycolytic flux in liver (Nishida et al., 2015). Sirt5 also desuccinylates and activates isocitrate dehydrogenase 2 (IDH2) (Zhou et al., 2016), and the rate-limiting ketogenic enzyme 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2) (Rardin et al., 2013), and deglutarylates carbamoyl-phosphate synthase 1 (CPS1) to enhance its activity (Tan et al., 2014). In vivo, Sirt5 KO mice display increased mortality in response to kainite-induced seizures (Li and Liu, 2016), are more susceptible to chemically-induced nigrostriatal dopaminergic degeneration (Liu et al., 2015), and display more severe cortical degeneration following ischemic injury (Morris-Blanco et al., 2016). Likewise, Sirt5 knockout in heart results in hypertrophic cardiomyopathy, with increased long-chain acyl-CoAs and decreased ATP levels in heart under fasting condition (Sadhukhan et al., 2016). Similar to heart and neuron, phenotypes of brown fat specific Sirt5 KO mice are also observed under levels of physiological stress. These poor adaptations to extreme conditions in the absence of Sirt5 are all manifestations of metabolic inflexibility.
Both fatty acid oxidation and branched chain amino acid metabolism are important pathways targeted by Sirt5 for desuccinylation, their impaired function likely made the Sirt5 KO BAT more starved during fasting, and thus led to further induction of autophagy genes such as Bnip3 and LC3. Higher Fis1 expression and smaller mitochondria are also observed in Sirt5 KO BAT compared to control BAT after fasting, and smaller mitochondria are known to be more depolarized and more susceptible to mitophagy (Gomes et al., 2011; Twig and Shirihai, 2011). Similar increases in mitochondrial fission have been shown in Sirt5 KO mouse embryonic fibroblasts (MEFs) compared to control MEFs after nutrient starvation (Guedouari et al., 2017).
Despite higher induction of autophagy genes and smaller mitochondria, fasting induced autophagy or mitophagy is defective in Sirt5 KO BAT, represented by accumulation of Mito OxPhos proteins and LC3II. Although none of the mitophagy/autophagy mediators were identified as Sirt5 targets for desuccinylation in our mass spectrometry analysis, we cannot rule out the possibility that they are Sirt5 targets for demalonylation and/or deglutarylation. Another potential explanation for the mitophagy defect is that oversuccinylation/malonylation in Sirt5 KO BAT makes the mitochondria less recognizable by the autophagy machinery.
In conclusion, mitochondrial protein succinylation and Sirt5 play important roles in regulating BAT mitochondria homeostasis. Increased mitochondrial protein succinylation in Sirt5 deficient BAT results in impaired mitochondrial enzyme activity and respiration, defects in mitophagy during nutrient deprivation and metabolic inflexibility. Reversible succinylation also serves as an important regulator of UCP1 function and stability. Malonylation and glutarylation, although not studied in this manuscript, also likely contribute to impaired mitochondrial protein function in Sirt5 KO BAT.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, C.R. Kahn (C.Ronald.Kahn@joslin.harvard.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
MICE
Sirt5fl/fl and Sirt5fl/fl; UCP1-Cre mice were maintained on C57BL/6J background. All mice were group housed at 22°C on a 12-hour light/12-hou r dark cycle with ad libitum access to food and water. In vivo metabolic parameters were measured utilizing Comprehensive Lab Animal Monitoring System (CLAMS) performed by Joslin Diabetes Center animal physiology core.
CELL LINE
3T3-L1 preadipocytes were purchased from ATCC. Brown preadipocyte were isolated in the lab as previously described(Klein et al., 2002). Briefly, SV40 T-large antigen immortalized brown preadipocytes from Sirt5 floxed neonatal pups were infected with adenovirus expressing GFP or GFP-Cre. Two days after infection, cells were sorted for GFP signal by Joslin Flow Cytometry Core.
METHOD DETAILS
Acute cold exposure
For acute cold exposure, 2.5 to 3 months old male mice on chow diet were transferred from 22°C to 7°C cold room, either with free access to food and water, or pre-fasted overnight and with free access to water only. Body temperature were measured every 1hr using a rectal probe (TH-5 thermalert monitoring thermometer).
Core body temperature measurement with implanted sensor
Subcue mini Dataloggers (http://www.subcue.com/mini.htm) were programed (delay 16 days before measurement starts and measure every 15 minutes for up to 21 days) and sealed with glass sealer two days before experiments. On the day of surgery, HFD fed Sirt5-BKO and floxed mice were given I.P. injection of 100uL/10g BW of ketamine/xylazine mix (100 mg/kg Ketamine, 16 mg/kg Xylazine). Furs next to the lower left midline of the belly were shaved and the bare skin cleaned with 70% ethanol soaked cotton ball. The skin and muscle layer were sequentially cut to create a 1cm long opening with a scalper and fine scissor, followed by insertion of the sensor into the abdominal cavity. Muscle layers were closed by suture (ethicon 639. size: 6–0) and skin layers were closed up by skin clips (Clay Adams brand MikRon Autoclip 9mm. Cat No: 427631). Mice were allowed two weeks of recovery before temperature measurement starts and the cold acclimation starts 16 days after surgery. Mice were sacrificed 10 days after being housed at 5°C.
Glucose and insulin tolerance test
For glucose tolerance test, mice were fasted overnight and intraperitoneally (i.p.) injected with 1–2 g glucose per kg of body mass. Insulin tolerance tests were performed in nonfasted mice by i.p. injection of 1.5 to 2 mU insulin per kg of body mass. Blood glucose levels were measured at 0, 15, 30, 60, and 120 minutes using a glucose meter (Infinity, US Diagnostics).
Mitochondria isolation
Mitochondria isolation was performed following published protocols (Cannon and Nedergaard, 2008; Frezza et al., 2007; Sun et al., 2014). Briefly, BAT from overnight fasted floxed and 5-BKO mice were dissected and homogenized using a glass-teflon homogenizer in the isolating buffer containing 300 mM sucrose, 1 mM EDTA, 5 mM MOPS, 5 mM KH2PO4, and 0.2% fatty acid free-BSA (pH7.2). The homogenate were filtered using 100 μm filter mesh, spin down at 800xg for 10 min. The supernatant containing mitochondria were transferred to another tube and spun down again at 8500xg for 10 min. The supernatants were then decanted and walls of the tube were wiped with paper to remove adhering fat. The pellet containing mitochondria were resuspended again in isolation buffer and transferred to a 1.5 mL eppendoff tube, spun down at 8500xg again for 10 min. The cleaned mitochondria pellet was expanded in ROS buffer containing 120 mM KCl, 10 mM HEPES, 5 mM MgCl2, and 2 mM K2HPO4 (pH7.2), spun down again at 8500xg for 10 min and resuspended in minimal amount of ROS buffer prior to determination of protein concentrations using a BCA assay (Pierce). All isolation steps were carried out in the cold room. For western blot and Succinate dehydrogenase (SDH) activity, protease inhibitor cocktails were added in isolation buffer, and the mitochondria pellet were resuspended in RIPA buffer or SDH activity assay buffer for activity measurement using a commercial kit (Sigma MAK197).
Mitochondria functional assays
The oxygen consumption rates (OCRs) were determined using the XF24 Extracellular Flux Analyzer (Seahorse Bioscience, MA, USA) following the manufacturers’ protocols. For the electron-flow (EF) measurements, isolated mitochondria were seeded at 5 μg of protein per well in XF24 V7 cell-culture microplates (Seahorse Bioscience), then pelleted by centrifugation (2,000x g for 20 min at 4°C) in 1X MAS buffer (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, and 1 mM EGTA in 0.2% FA-free BSA; pH 7.2) supplemented with 10 mM pyruvate (no pyruvate if it’s for fatty acid oxidation), 5 mM malate, with a final volume of 500 μl per well. The XF24 plate was then transferred to a temperature-controlled (37°C) Seahorse analyzer and subjected to a 12-min equilibration period and 2 assay cycles to measure the basal rate, comprising of a 1 min mix, and a 3 min measure period each; and compounds were added by automatic pneumatic injection followed by a single assay cycle after each. Concentration of drugs used in the assay for Figure 5A–C: Rotenone: 2 μM, succinate: 5 mM, antimycin: 4 μM, N, N, N′, N′-tetramethyl-p-phenylenediamine (TMPD): 100 μM in 10 mM ascorbate. For fatty acid oxidation (Figure 5J), Basal: 5 mM Malate. FFA: 100 μM Oleate:palmitate (2:1), plus 2 mM L-carnitine, plus 100 μM ATP, plus 5 μM CoA. GDP: 0.5 mM.
Brown adipocyte differentiation and functional assay.
Brown preadipocytes were cultured in DMEM with 10% FBS until two days post confluence (denoted as day 0 of differentiation). BAC differentiation was induced by adding a cocktail containing 0.5 mM IBMX, 125 μM indomethacin, 1 μM dexamethasone and 5 μM rosiglitazone to maintenance medium containing 10% FBS, 20 nM insulin and 1 nM T3. Two days after induction, cells were cultured in the maintenance medium plus 5 μM rosiglitazone. Seahorse flux assay were carried out on day 5 to day 6 differentiated brown adipocytes in XF24 V7 cell-culture microplates following manufacture’s protocol. To make the seahorse running buffer, DMEM base (Sigma D5030) were dissolved in 500 mL millipure water, 1.85 g NaCl (Sigma S3014) were separately dissolved in 500 mL water, the two were combined and 15 mg Phenol Red (Sigma P-5530) were added. 10 mL were removed from above media and 10 mL 100X GlutaMax-1 (Gibco 35050–061) was added. Assay dependent amount of glucose (G8270) were added and the media was warmed to 37°C before pH adjustment to 7.4 with 10 M NaOH from Sigma. The media was then filter sterilized and stored at 4°C. For mature brown adipocytes, basal and after port injection measurements were looped 4 times except for rotenone/antimycin injection, which is looped twice. Each loop comprises a 4 min mix, 2 min delay and 2 min measure time. OCR and ECAR results were normalized to protein content in each well. Concentration of drugs used for Figure 5M–N: Basal: glucose free DMEM running buffer. Glc: 10 mM glucose. 2-DG: 22 mM 2-deoxy-glucose. Eto: 40 μM etomoxir. R/A: 0.1 μM rotenone plus 2.5 μM antimycin.
Fatty acid oxidation
Fatty acid oxidation assay were performed on day 7 differentiated brown adipocytes following a protocol from Journal of Visualized Experiments (Akie and Cooper, 2015).
Glutamate oxidation and uptake
Glutamate oxidation assay were performed in a similar way as fatty acid oxidation. Briefly, day 7 differentiated floxed and Sirt5 KO brown adipocytes were starved in DMEM with 1 g/L glucose and 0.5% FBS for 3 hrs before switching to Kreb-Ringer HEPES buffer (10 mM NaHCO3, 120 mM NaCl, 4m M KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 30 mM HEPES, pH7.4) containing 100 μM glutamate. For half number of the wells, 1mM leucine was added 10 min before incubation with 14C labeled glutamic acid for 40 min. Media from each well were transferred to another plate and snap frozen in liquid nitrogen (Original plate with the cells were lysed with RIPA buffer for OD protein concentration). 100 μL of 70% perchloric acid were added to each well of the media plate, and the released hot CO2 were trapped in a filter paper plate pre-wetted with NaOH.
Glutamate uptake assay were performed in a separate plate, where the cells were incubated with 14C labeled glutamic acid for only 5 minutes, followed by addition of 50 μL of 200 mM cold glutamate to stop the reaction. Then the cells were washed three times with ice cold PBS, and lysed with 0.4 mL RIPA lysis buffer. 250 μL lysates were added into 4 mL scintillation fluid for counting and 4 μL lysates were used to OD protein concentration.
UCP1 KE and KQ mutation
Figure 6A of UCP1 structure showing the 2 succinyl-K residues is adapted from https://www.proteinmodelportal.org/?pid=modelDetail&provider=MODBASE&template=1okcA&pmpuid=1001082818542&range_from=1&range_to=307&ref_ac=P12242&mapped_ac=P12242&zid=async). Open reading frame of WT and 2KE/2KQ mutant UCP1 were directly synthesized from Integrated DNA Technologies and ligated into MSCVpuro vector. 3T3-L1 cell lines expressing MSCVpuro vector, WT-UCP1 and 2KE/2KQ-UCP1 were generated through retrovirus infection. UCP1 activities were measured by seahorse flux assay in XF24 V7 cell-culture microplates. For confluent 3T3-L1 preadipocytes, basal and after port injection measurements were looped 3 times except for rotenone/antimycin injection, which was looped twice. Each loop comprises a 3 min mix, 2 min delay and 3 min measure time. The 100 mM FFA (Oleate to palmitate 2:1) stock was made by dissolving 400 mg of albumin (BSA) in 10 ml of H2O, followed by adding 209 μl of Oleic acid and 85.47 mg of Palmitic acid to BSA. The suspension was mixed vigorously and heated up in water bath to 60°C for 30 min. Samples were aliquoted and stored at −20°C. Drug concentrations for Seahorse Flux Assay testing UCP1 activity in figures 6D and 6H, Basal: 25 mM glucose in DMEM running buffer. Oligomycin: 4 μM, FFA: 50 μM for the 2KQ set and 25 μM for the 2KE set. FCCP: 0.25 μM, R/A: 0.1 μM rotenone plus 2.5 μM antimycin.
Electron Microscopy
Freshly dissected BAT from 24 hour fasted floxed and 5-BKO mice were fixed in 0.2 M Cacodylate buffer containing 2.5% paraformaldehyde, 5% glutaraldehyde and 0.06% picric acid. The EM processing and imaging were carried out at Harvard EM core. Quantification of mitochondria size was performed using ImageJ (NIH).
RNA extraction and qPCR analysis
mRNA was extracted by homogenizing brown adipose tissues in TRIzol, treating with chloroform, and precipitating in 70% ethanol. cDNA was made using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, catalog 4368813). qPCR was performed utilizing C1000 Thermal Cycler (BioRad, catalog CFX384). ). Primer sequences used are listed in Supplementary Table 2.
Protein extraction, immunoblot, and immunoprecipitation
For immunoblot, tissues were homogenized in RIPA buffer with protease and phosphatase inhibitor cocktail. For immunoprecipitation, BAT were homogenized in ICK lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 5 mM NaF, 25 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 10% glycerol, 1% Triton X-100, and freshly added protease inhibitors. 2 μg of primary antibody was agitated overnight in a cold room, and protein was pulled with protein A/G Plus-Agarose. Proteins were separated using SDS-PAGE and transferred to PVDF membrane (Millipore). Quantification of immunoblots was performed using ImageJ (NIH).
Metabolomics analyses
Previously frozen BAT tissues were homogenized in 50% aqueous acetonitrile containing 0.3% formic acid (50 mg/mL). BAT amino acids, acyl CoAs, and organic acids were analyzed using stable isotope dilution techniques. Amino acid measurements were made by flow injection MS/MS using sample preparation methods described previously (An et al., 2004). The data were acquired using a Micromass Quattro Micro system equipped with a model 2777 autosampler, a model 1525 HPLC solvent delivery system, and a data system controlled by the MassLynx 4.1 operating system (Waters). Organic acids were quantified using methods described previously with Trace Ultra GC coupled to a Trace DSQ MS operating under Xcalibur 1.4 (Thermo Fisher Scientific) (Jensen et al., 2006). Acyl CoAs were measured by LC-MS/MS (where LC indicates liquid chromatography) as described previously (White et al., 2016).
Quantification and statistical analysis for non-mass spec experiments
Two tailed student’s t-test is used for statistical analysis between Sirt5 KO and floxed groups. If more than two groups are compared together, bonferroni correction is applied. All statistical details including the method, exact value of n, dispersion and precision measures can be found in the figure legend. p<0.05 is considered significant.
Data and Software Availability
Raw western images can be found in https://data.mendeley.com/datasets/djwj4vff8y/1
MASS SPECTROMETRY
Sample preparation for mass spectrometry experiments
BAT from Sirt5 floxed mice and brown fat-specific Sirt5 KO mice (5-BKO) housed at RT and cold acclimated to 5 °C were subjected to succinylation affinity enrichment and subsequent mass spectrometric analysis. For each of the 4 mouse groups, we analyzed BAT from 5 mice. We analyzed the samples using an adapted form of the automated PTM identification and quantification using exclusively DIA (PIQED) workflow as described previously (Meyer et al., 2017). Sample preparation and data collection was done blinded with anonymized labels, and unblinded before final data analysis. BAT tissue samples were homogenized in 50 mM Tris buffer pH 7.6 containing 1x HALT protease inhibitor (Pierce), 150 mM NaCl, 8 M Urea, 1 μM trichostatin A (TSA), and 3 mM Nicotinamide. Protein concentrations were determined using the BCA assay, and aliquots of lysate containing 2 mg of soluble protein from each sample were prepared in parallel. Soluble proteins were reduced with 4.5 mM dithioerytrol (DTT) for 30 minutes at 37°C, cooled to room temperature, alkylated with 10 mM iodoacetamide for 30 minutes in the dark, and then enzymatic protein hydrolysis was initiated by addition of trypsin (1:50, w:w, enzyme:substrate). The following day, enzymatically-catalyzed hydrolysis was quenched by addition of formic acid (FA) to 1% final concentration, and samples were frozen at −20°C overnight. The following day, the peptide mixtures were thawed on ice and desalted using Waters’ Oasis HLB 1cc Vac cartridges (30 mg sorbent), and peptides were eluted using 80% acetonitrile (ACN), 0.2% FA, and 19.8% water. Eluted peptides were dried completely in a speedvac and stored at −80°C until further processing.
Extra protein beyond the 2 mg per sample used for quantitative comparisons was pooled into one 2.8 mg aliquot, and one 10 mg aliquot, and those protein aliquots were processed as described above for building a BAT-specific PTM spectral library.
One-pot Immunoprecipitation of Peptides containing Acetyl-Lysine and Succinyl-Lysine
Dried peptides were resuspended in 1.4 mL IAP buffer (50 mM MOPS–NaOH, pH 7.2, 10 mM Na2HPO4, 50 mM NaCl) and dissolved by pipetting. Samples were then simultaneously enriched for both acetyl-lysine and succinyl-lysine as previously described (Basisty et al., 2018). Briefly, resuspended peptide samples were incubated with 10 μL each of antibody-bead conjugates specific for acetyl-lysine and succinyllysine (Cell Signaling Technologies, PTMScan kits #13416 and #13764, respectively). Immunoprecipitation was allowed to proceed overnight at 4°C with gentle mixing. The following day, beads were washed twice with 1 mL ice-cold IAP buffer, and then thrice with 1 mL ice-cold IAP buffer. Bound peptides were eluted sequentially with 45 μL and then 55 μL of 0.15% TFA for 5 minutes each at RT. Eluted PTM peptides were then directly loaded onto in-house made C18 StageTips, desalted with 0.2% FA in water, and eluted with solution containing 50% ACN, 49.8% water, and 0.2% FA. Eluted peptides were dried completely and stored at −80°C until further analysis.
Quantitative and qualitative peptide analysis by mass spectrometry
All mass spectrometry data was collected using a SCIEX TripleTOF 5600 system coupled to an Eksigent nanoflow liquid chromatography pump and a cHiPLC chromatography system. All online peptide separations were done using a linear gradient from 5% mobile phase B to 35% mobile phase B over 80 minutes. Mobile phase B was then ramped to 80% over 5 minutes, held at 80% B for 8 minutes before returning to 5% B for 25-minute re-equilibration.
Data-dependent acquisition was used to identify peptides. Every cycle consisted of one 250 ms precursor ion scan followed by isolating the top 10 most abundant precursor ions between 400–1,250 m/z with signal over 150 counts per second. Tandem mass spectra were accumulated for up to 100 ms collecting fragment masses between 100–2,000 m/z. Dynamic exclusion was enabled for 20 seconds.
Data-Independent acquisition (DIA) was used to quantify peptides. Every DIA cycle consisted of 250 ms precursor ion scan followed by 64 variable-width isolation windows to produce fragment ion spectra. For protein-level quantification using non-enriched peptides, variable windows were the same as recently reported (Collins et al., 2017). For PTM quantification, variable windows were determined based on the distribution of identified peptides using SWATHTuner (Zhang et al., 2015), which are available as part of the Skyline document on panorama (see location below).
To identify peptides and build spectral libraries for subsequent protein quantification, non-enriched peptides from each sample were analyzed using nanoflow liquid chromatography, data-dependent acquisition tandem mass spectrometry (nLC-DDAMS/MS). To build spectral libraries for quantification of protein acetylation and succinylation sites, peptides from separate, pooled one-pot enrichments were analyzed by nLC-DDA-MS/MS.
For quantification of proteins and PTMs each non-enriched and one-pot PTM enriched sample was analyzed by nanoflow liquid chromatography, data-independent acquisition tandem mass spectrometry (nLC-DIA-MS/MS) as described previously (Basisty et al., 2018; Meyer et al., 2017).
Mass spectrometry data analysis
Proteins and PTM-containing peptides were identified by database search against the mouse proteome (downloaded from Uniprot on August 10th, 2015) with MaxQuant. Database searches used the default parameters except for the analysis of PTM enrichments variable acetylation and succinylation were allowed. Protein identifications from MaxQuant were imported into Spectronaut to build a spectral library, which was used to quantify proteins. PTM identifications from MaxQuant were imported into Skyline to build a spectral library, and PTMs were quantified as described previously, including use of the protein quantity correction module of PIQED (1). All identifications were filtered to 1% FDR. Tables of MaxQuant identification detail output are available on massive (see link below). Further downstream analysis was done with custom code written in R available from https://github.com/jgmeyerucsd/pRoteomics.
Mass spectrometry data availability
“Raw mass spectrometry data, spectral libraries as well as tables of identifications and quantification are available on massive (https://massive.uscd.edu, ID: MSV000082491, direct link: https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=a40b6049135c4865b9aedba5f2 4bafe7). The Skyline document containing the spectral library and quantitative data is available from panorama (https://panoramaweb.org/project/Schilling/BrownAdiposeTissue/begin.view? and at https://panoramaweb.org/BAT_succinylation.url).
Supplementary Material
Supplementary table 1-succinyl-K sites and fold changes, related to Fig.4 and Fig.S4A
Supplementary table 2-qPCR primer sequence, related to STAR Methods
Highlights.
Sirt5 regulates mitochondrial protein succinylation and malonylation in brown fat Increased succinylation of UCP1 reduces its stability and function
Sirt5KO in BAT leads to metabolic inflexibility and impairs mitochondrial homeostasis These processes are altered by cold exposure and diet
Acknowledgement
We thank Joslin flow cytometry core for sorting Sirt5 floxed and KO brown preadipocytes, the Harvard Medical School Electron Microscopy Core for processing BAT for EM analysis, Histology Core for H&E staining of BAT, and all the Kahn lab members for discussion. This project was funded by R24 (5R24DK085610–09), ADA-Pfizer postdoc fellowship (9–17-CMF-016), S10 instrument grant (1S10 OD016281) and two NIH T32 grants (5T32DK007260–42 and 4T32AG000266–19).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests
References
- Akie TE, and Cooper MP (2015). Determination of Fatty Acid Oxidation and Lipogenesis in Mouse Primary Hepatocytes. J Vis Exp, e52982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala M, Calderon-Dominguez M, Bustos E, Ramos P, Casals N, Serra D, Viana M, and Herrero L (2017). Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep 7, 16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, and Newgard CB (2004). Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10, 268–274. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. (2011). Brown adipose tissue activity controls triglyceride clearance. Nat Med 17, 200–205. [DOI] [PubMed] [Google Scholar]
- Basisty N, Meyer JG, Wei L, Gibson BW, and Schilling B (2018). Simultaneous Quantification of the Acetylome and Succinylome by ‘One-Pot’ Affinity Enrichment. Proteomics 18, e1800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J (2008). Studies of thermogenesis and mitochondrial function in adipose tissues. Methods Mol Biol 456, 109–121. [DOI] [PubMed] [Google Scholar]
- Carrico C, Meyer JG, He W, Gibson BW, and Verdin E (2018). The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, and Metabolic and Disease Implications. Cell Metab 27, 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, et al. (2016). Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BC, Hunter CL, Liu Y, Schilling B, Rosenberger G, Bader SL, Chan DW, Gibson BW, Gingras AC, Held JM, et al. (2017). Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat Commun 8, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. (2009). Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, et al. (2015). Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 21, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AS, Humphrey DM, and Brand MD (2012). Fatty acids change the conformation of uncoupling protein 1 (UCP1). J Biol Chem 287, 36845–36853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Ueki K, Kriauciunas KM, Benito M, White MF, and Kahn CR (2000). Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem 275, 25494–25501. [DOI] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, and Kirichok Y (2012). Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, and Scorrano L (2007). Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2, 287–295. [DOI] [PubMed] [Google Scholar]
- Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, and Aebersold R (2012). Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics 11, O111 016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, and Villarroya F (2012). SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J 444, 1–10. [DOI] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, and Scorrano L (2011). During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbody AE, and Trayhurn P (1981). GDP binding to brown-adipose-tissue mitochondria of diabetic--obese (db/db) mice. Decreased binding in both the obese and pre-obese states. Biochem J 194, 1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedouari H, Daigle T, Scorrano L, and Hebert-Chatelain E (2017). Sirtuin 5 protects mitochondria from fragmentation and degradation during starvation. Biochim Biophys Acta 1864, 169–176. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. (2010). SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, and Zhao Y (2015). Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol Cell Proteomics 14, 2308–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, Odegaard M, Becker TC, Sherry AD, and Newgard CB (2006). Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem 281, 22342–22351. [DOI] [PubMed] [Google Scholar]
- Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, and Kahn CR (2011). Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A 108, 14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, et al. (2013). Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62, 3404–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen R, Pirnes-Karhu S, Pietilainen KH, and Pirinen E (2017). Adipose tissue NAD(+)-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health. Redox Biol 12, 246–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G (2012). Interpretation of bafilomycin, pH neutralizing or protease inhibitor treatments in autophagic flux experiments: novel considerations. Autophagy 8, 1875–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Fasshauer M, Klein HH, Benito M, and Kahn CR (2002). Novel adipocyte lines from brown fat: a model system for the study of differentiation, energy metabolism, and insulin action. Bioessays 24, 382–388. [DOI] [PubMed] [Google Scholar]
- Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, et al. (2014). IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell 158, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, and Lombard DB (2018). Functions of the sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit Rev Biochem Mol Biol 53, 311–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, and Liu L (2016). SIRT5 Deficiency Enhances Susceptibility to Kainate-Induced Seizures and Exacerbates Hippocampal Neurodegeneration not through Mitochondrial Antioxidant Enzyme SOD2. Front Cell Neurosci 10, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fromme T, Schweizer S, Schottl T, and Klingenspor M (2014). Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep 15, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Peritore C, Ginsberg J, Shih J, Arun S, and Donmez G (2015). Protective role of SIRT5 against motor deficit and dopaminergic degeneration in MPTP-induced mice model of Parkinson’s disease. Behav Brain Res 281, 215–221. [DOI] [PubMed] [Google Scholar]
- Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, and Cannon B (2000). Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem 275, 25073–25081. [DOI] [PubMed] [Google Scholar]
- Meyer JG, Mukkamalla S, Steen H, Nesvizhskii AI, Gibson BW, and Schilling B (2017). PIQED: automated identification and quantification of protein modifications from DIA-MS data. Nat Methods 14, 646–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, and Horikawa I (2005). Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16, 4623–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, et al. (2018). Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, and Perez-Pinzon MA (2016). Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci Rep 6, 29790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, and Cannon B (2011). New powers of brown fat: fighting the metabolic syndrome. Cell Metab 13, 238–240. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, and Cannon B (2001). UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504, 82–106. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, and Rial E (1999). A history of the first uncoupling protein, UCP1. J Bioenerg Biomembr 31, 399–406. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, Gut P, Najjar R, Fitch M, Hellerstein M, Gibson BW, et al. (2015). SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol Cell 59, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, et al. (2013). SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 50, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, et al. (2013). SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18, 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Saville ME, and Stock MJ (1984). Brown fat activity in fasted and refed rats. Biosci Rep 4, 351–357. [DOI] [PubMed] [Google Scholar]
- Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, et al. (2016). Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci U S A 113, 4320–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, Kolb D, Hoeks J, Kershaw EE, Sedej S, et al. (2017). Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab 26, 753–763 e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, and Verdin E (2008). Conserved metabolic regulatory functions of sirtuins. Cell Metab 7, 104–112. [DOI] [PubMed] [Google Scholar]
- Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, Jackson R, Rumore D, Xue B, Shi H, et al. (2017). Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab 26, 764–777 e765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stram AR, and Payne RM (2016a). Post-translational modifications in mitochondria: protein signaling in the powerhouse. Cell Mol Life Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stram AR, and Payne RM (2016b). Post-translational modifications in mitochondria: protein signaling in the powerhouse. Cell Mol Life Sci 73, 4063–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, and Scherer PE (2014). Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab 3, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al. (2014). Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 19, 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, and Kominami E (2008). LC3 and Autophagy. Methods Mol Biol 445, 77–88. [DOI] [PubMed] [Google Scholar]
- Tomita T, Kuzuyama T, and Nishiyama M (2011). Structural basis for leucine-induced allosteric activation of glutamate dehydrogenase. J Biol Chem 286, 37406–37413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, and Shirihai OS (2011). The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Peyrou M, and Giralt M (2017). Transcriptional regulation of the uncoupling protein-1 gene. Biochimie 134, 86–92. [DOI] [PubMed] [Google Scholar]
- Wagner GR, and Hirschey MD (2014). Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell 54, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC (2005). A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39, 359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, et al. (2016). Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab 5, 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchi SD, Jayaram S, Thomas JK, Gundimeda S, Khan AA, Singhal A, Keshava Prasad TS, Pandey A, Somani BL, and Gowda H (2016). A pathway map of glutamate metabolism. J Cell Commun Signal 10, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Sadhukhan S, Noriega LG, Moullan N, He B, Weiss RS, Lin H, Schoonjans K, and Auwerx J (2013). Metabolic characterization of a Sirt5 deficient mouse model. Sci Rep 3, 2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bharathi SS, Rardin MJ, Lu J, Maringer KV, Sims-Lucas S, Prochownik EV, Gibson BW, and Goetzman ES (2017). Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain. J Biol Chem 292, 10239–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bilbao A, Bruderer T, Luban J, Strambio-De-Castillia C, Lisacek F, Hopfgartner G, and Varesio E (2015). The Use of Variable Q1 Isolation Windows Improves Selectivity in LC-SWATH-MS Acquisition. J Proteome Res 14, 4359–4371. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang F, Sun R, Chen X, Zhang M, Xu Q, Wang Y, Wang S, Xiong Y, Guan KL, et al. (2016). SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep 17, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1-succinyl-K sites and fold changes, related to Fig.4 and Fig.S4A
Supplementary table 2-qPCR primer sequence, related to STAR Methods
Data Availability Statement
Raw western images can be found in https://data.mendeley.com/datasets/djwj4vff8y/1