Abstract
Marine omega-3 polyunsaturated fatty acids (MO3PUFAs) have anticancer properties and may improve colon cancer survival. However, it remains unknown whether the benefit differs by tumor molecular subtype. We examined data from a phase III randomized trial of FOLFOX or FOLFOX + cetuximab among 1,735 stage III colon cancer patients who completed a dietary questionnaire at enrollment. Multivariable hazard ratios and 95% confidence intervals (CIs) were calculated for the association between MO3PUFA and disease-free survival (DFS) and overall survival according to KRAS and BRAFV600E mutations and DNA mismatch repair (MMR) status. Higher MO3PUFA intake was associated with improved 3-year DFS for KRAS-wildtype tumors (77% vs. 73%; HR, 0.84, 95% CI, 0.67-1.05), but not KRAS-mutant tumors (64% vs. 70%; HR, 1.30, 95% CI, 0.97-1.73; Pinteraction=0.02). Similar heterogeneity was found by MMR (Pinteraction=0.14): higher MO3PUFA was associated with better 3-year DFS for tumors with deficient MMR (72% vs. 67%), but not proficient MMR (72% vs. 72%). No heterogeneity was found by BRAFV600E mutation. Similar findings were obtained for overall survival. In conclusion, we found a suggestive beneficial association between higher MO3PUFA intake and improved survival among stage III colon cancer patients with wildtype KRAS and deficient MMR. Given the relatively small number of cases with tumor molecular assessments, further studies, preferably through pooled analyses of multiples cohorts, are needed to validate our findings.
Keywords: colorectal cancer, survivorship care, nutrition, inflammation, tumor microenvironment
Introduction
Colorectal cancer (CRC) is the third most common cancer and third leading cause of cancer death in the United States.1 Currently, there are more than 1.4 million Americans living with colorectal cancer.2 Substantial data support that marine ω-3 polyunsaturated fatty acids (MO3PUFAs) (i.e., eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA] and docosapentaenoic acid [DPA]) have potential anti-cancer effects and may improve CRC survival through modulation of local and systemic immune response.3 Consistent with the mechanistic data, we recently reported that higher intake of MO3PUFA after diagnosis was associated with better survival among patients with established CRC in two independent cohort studies.4, 5 Furthermore, a phase II randomized placebo-controlled trial showed that preoperative treatment with MO3PUFA conferred survival benefits among patients with CRC liver metastases.6 These data indicate the potential of MO3PUFA as an adjuvant treatment agent for improving CRC survival.
Although traditionally considered as a single disease, CRC is now known as a heterogeneous entity characterized by distinct molecular markers.7 Congruent with this notion, we found that the beneficial association between higher intake of MO3PUFA and lower incidence of CRC was restricted to a subset of tumors with microsatellite instability (MSI).8 MSI is present in about 15% of CRC cases and caused by the loss of DNA mismatch repair (MMR) activity.9 In line with our observation and the anti-inflammatory properties of MO3PUFA, growing data support the critical role of inflammation and dysregulated antitumor immune response in the development of MSI tumors.10 Immune checkpoint inhibitor therapy has been shown to be more effective for treating cancers with MSI.11, 12 These data suggest that high intake of MO3PUFA after diagnosis may primarily benefit patients with MSI tumors.
Besides MSI, other molecular markers have also been characterized in CRC. KRAS, a member of the rat sarcoma virus (ras) gene family of oncogenes, is a central signaling node that controls transcription of genes important for cell metabolism, growth and proliferation.13 Somatic mutations in KRAS codons 12 and 13 are found in approximately 40% colon cancer cases and predict the resistance to epidermal growth factor receptor (EGFR) antibody therapy.14 BRAF is another important oncogene in colon cancer that acts downstream from KRAS. A point mutation in BRAF (V600E), which is detected in approximately 8% of colon cancers, results in epigenetic silencing of the MMR pathway, thereby leading to the development of MSI and activation of the oncogenic pathway to promote cell proliferation and survival.15, 16
Given our prior data about MO3PUFA and MSI, as well as the role of KRAS and BRAF in predicting CRC treatment and survival, we examined the prognostic influence of MO3PUFA intake according to these markers in a phase III randomized adjuvant trial of stage III colon cancer patients (the Alliance N0147 trial). Our primary hypothesis was that MO3PUFA intake is associated with better survival and this beneficial association may be more evident for tumors with MSI.
Materials and Methods
Study population
The N0147 is a multicenter phase III trial led by the North Central Cancer Treatment Group (NCCTG) in which patients with resected stage III colon cancer were randomly assigned to treatment with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) with or without adjuvant cetuximab.17 NCCTG is now part of the Alliance for Clinical Trials in Oncology. Eligible patients had histologically confirmed adenocarcinoma of the colon, at least one pathologically confirmed positive lymph node, complete surgical resection performed ≤ 56 days before random assignment, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Major exclusion criteria include evidence of metastatic disease, prior or concurrent malignancies, previous EGFR therapy, age younger than 18 years, and one or more of several exclusionary comorbid conditions at the time of random assignment. A total of 2,686 patients were recruited and randomly assigned between February 10, 2004 and November 25, 2009. Given the emerging data indicating that patients with KRAS mutated tumors gained limited or no benefit from cetuximab treatment,17 the study enrollment criteria was modified in early 2008 such that all participants were subsequently tested for somatic KRAS mutations before random assignment, and only those with KRAS wildtype tumors were randomly assigned to treatment arms per the original protocol. The current analysis is limited to participants enrolled onto the primary treatment comparison arms (FOLFOX vs. FOLFOX plus cetuximab) who had completed a dietary questionnaire at the baseline visit (n = 1,735; Figure 1). Each participant signed an institutional review board-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Figure 1.
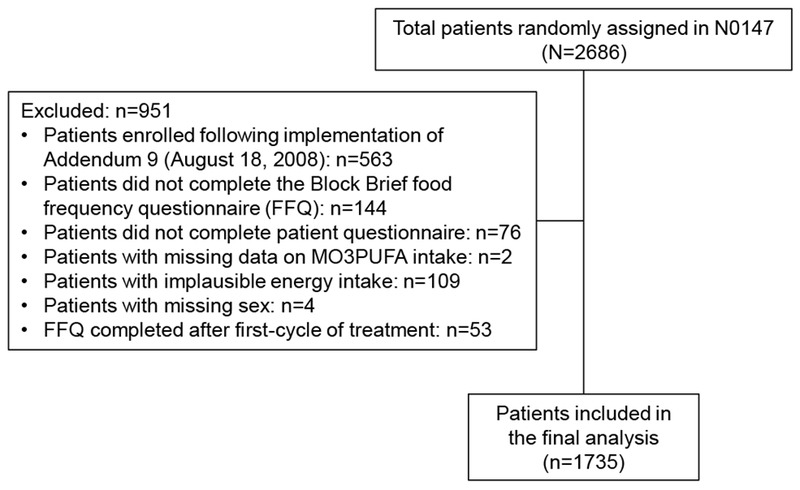
Flowchart of the analytic cohort in North Central Cancer Treatment Group Phase III Trial N0147
Exposure assessment
Until a change in protocol in 2008, participants in N0147 were asked at study enrollment to complete a 68-item modified Block Brief food frequency questionnaire (FFQ) that inquired about their usual eating habits of each listed food and beverage during the past year, including average frequency (nine options ranging from never to every day) and amount (four options) or serving size (mostly four options based on the portion size pictures provided for each food). Daily intake for each nutrient was calculated by multiplying the reported frequency and amount of consumption of each item by its nutrient content and then summing across all foods. The nutrient content of the diet was calculated by NutritionQuest using the US Department of Agriculture Food and Nutrient Database for Dietary Studies, which provides a population-weighted nutrient composition for each food item based on national dietary intake data.18
For assessment of MO3PUFA intake, two fish items were asked on the FFQ, including fried fish or fish sandwich and any other fish or shellfish not fried (including tuna). MO3PUFA included EPA, DHA, and DPA; with the mean intake of 0.020, 0.038, and 0.008 g/day, respectively. We adjusted MO3PUFA intake for total caloric intake using the nutrient residual method.19 Fish consumption in grams per week was assessed by taking the product of average frequency of each item per week, number of grams in a medium serving, and serving size (0.5 for small, 1.0 for medium, and 1.5 for large). Total fish consumption was calculated by summing across the two fish items and examined in relation to survival outcomes in the secondary analysis. The FFQ has been validated using multiple dietary records as the reference, shown reasonable validity for macronutrients,20 and used previously for fish and MO3PUFA assessment.21, 22
Covariate assessment
To control for potential confounding by other dietary factors, we also calculated total intake (including supplements) of vitamin D, calcium, fiber and processed red meat based on the FFQ. In addition, a lifestyle questionnaire was administered at the baseline study visit to collect detailed information on various potential predictors of cancer survival, including body weight, height, frequency of physical activity (at any, moderate, and vigorous level), smoking history, and use of aspirin and multivitamin. We calculated body mass index as weight in kilograms divided by height in meters squared.
Assessment of tumor molecular markers
Macrodissected formalin-fixed, paraffin-embedded tumor tissue from the original surgical resection were requested for all study participants and sent to the Mayo Clinic for centralized testing of DNA MMR status, and KRAS and BRAF mutations. All assays were performed in a Clinical Laboratory Improvement Amendments-compliant laboratory, using appropriate quality control procedures, and interpreted without knowledge of treatment, patient, and outcome information. DNA MMR status was determined by immunohistochemical assessment of three proteins: MLH-1, MSH-2, and MSH-6, as previously described.23 Patients with tumors exhibiting a loss of protein expression for any of these markers were classified as having defective MMR (dMMR); patients with no loss of expression were classified as having proficient MMR (pMMR). DNA extracted from tumor specimens was used to mutation testing. Seven mutations in codons 12 and 13 of KRAS exon 2 (Gly12Ala, Gly12Asp, Gly12Arg, Gly12Cys, Gly12Ser, Gly12Val, and Gly13Asp) were tested using the DxS mutation test kit KR-03/04 (DxS, Manchester, UK). Tumors with any of the aforementioned KRAS mutations were classified as mutant KRAS, whereas the rest were classified as wildtype KRAS. Assessment for the BRAFV600E mutation was performed using a Mayo-developed multiplex allele-specific polymerase chain reaction–based assay.24 After amplification, polymerase chain reaction products were analyzed on an ABI 3130xl instrument (Life Technologies, Applied Biosystems, Grand Island, NY) and scored for the presence or absence of the V600E variant only. Tumors with the BRAFV600E mutation were classified as mutant BRAF (vs wildtype).
Outcome ascertainment
The primary outcome of the study was disease-free survival (DFS), defined as the time from registration to the earliest occurrence of the first documented colon cancer recurrence or death as a result of any cause. The secondary endpoint was overall survival (OS), defined as the time from registration to death of any cause. Because the evaluation of recurrence and death became too sparse when the follow-up extended beyond what was specified in the protocol (5 years), we censored DFS and OS at 5 years and 8 years post-registration, respectively, on the basis of the consistency of available follow-up information.
Statistical analysis
Given the higher intake of MO3PUFA in men than women in this study (Table 1), we used sex-specific cutoffs to categorize MO3PUFA intake into quartiles. Basic characteristics of participants according to quartiles of MO3PUFA intake were compared using the Kruskal-Wallis25 and chi-square tests26 for continuous and categorical variables, respectively. We calculated the 3-year DFS and 5-year OS in each quartile of MO3PUFA intake using Kaplan-Meier method.27 We used Cox proportional hazards regression models to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) of DFS and OS according to quartiles of MO3PUFA intake.28 The linear trend of HRs across different quartiles was tested by including the median intake of each quartile in the model. Proportional hazards assumption was verified by testing for a nonzero slope of the scaled Schoenfeld residuals on ranked failure times.29 To minimize the influence of potential confounding, we considered two models: model 1 was adjusted for age at diagnosis (continuous), sex (male, female), and study arm (FOLFOX vs. FOLFOX plus cetuximab); model 2 was further adjusted for additional a priori selected predictors for CRC survival, including tumor location (distal and proximal colon, both), number of affected lymph nodes (1-3, >3), T stage (T1-T2, T3, T4), ECOG performance score (0, 1, 2), aspirin use (none, full-dose or extra-strength, low-dose or body-strength), MMR status (dMMR, pMMR), smoking status (never, past, current ≤ 20 cigarettes/day, current > 20 cigarettes/day), alcohol consumption (never, former, current), body mass index (< 18.5, 18.5 to 22.4, 22.5 to 24.9, 25 to 29.9, ≥ 30 kg/m2), frequency of vigorous physical activity (never, monthly, weekly), multivitamin use (no, yes), and total intake of vitamin D, calcium, fiber, and processed red meat (continuous). For covariates with missing data, we created and included missing indicators in the multivariable model.
Table 1.
Basic characteristics of study participants according to post-diagnostic marine ω-3 polyunsaturated fatty acid (MO3PUFA) intake*
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| No. of participants | 445 | 425 | 437 | 428 |
| Age, year | 58.2±12.0 | 57.2±11.9 | 58.5±10.6 | 58.1±10.6 |
| Sex | ||||
| Women | 216 (49) | 205 (48) | 212 (49) | 205 (48) |
| Men | 229 (51) | 220 (52) | 225 (51) | 223 (52) |
| Study arm | ||||
| FOLFOX | 219 (49) | 205 (48) | 212 (49) | 214 (50) |
| FOLFOX plus cetuximab | 226 (51) | 220 (52) | 225 (51) | 214 (50) |
| Tumor subsite | ||||
| Proximal colon | 236 (53) | 238 (56) | 220 (50) | 207 (48) |
| Distal colon | 206 (46) | 179 (42) | 205 (47) | 219 (51) |
| Both | 3 (1) | 7 (2) | 12 (3) | 2 (1) |
| No. of affected nodes | ||||
| 1-3 | 261 (59) | 249 (59) | 253 (58) | 246 (57) |
| ≥4 | 184 (41) | 176 (41) | 184 (42) | 182 (43) |
| Histology | ||||
| High | 117 (26) | 100 (24) | 118 (27) | 105 (25) |
| Low | 328 (74) | 325 (76) | 319 (73) | 323 (75) |
| MMR Status | ||||
| pMMR | 375 (88) | 351 (86) | 358 (86) | 369 (89) |
| dMMR | 49 (12) | 56 (14) | 58 (14) | 48 (11) |
| BRAF Mutation | ||||
| Mutant | 66 (16) | 51 (13) | 58 (14) | 54 (13) |
| Wildtype | 348 (84) | 342 (87) | 344 (86) | 349 (87) |
| KRAS Mutation | ||||
| Mutant | 143 (33) | 139 (34) | 155 (37) | 146 (36) |
| Wildtype | 288 (67) | 265 (66) | 259 (63) | 263 (64) |
| ECOG Performance Score | ||||
| 0 | 340 (76) | 316 (74) | 327 (75) | 340 (79) |
| 1 | 103 (23) | 103 (24) | 106 (24) | 85 (20) |
| 2 | 2 (1) | 6 (2) | 4 (1) | 3 (1) |
| Aspirin Use | ||||
| None | 295 (73) | 259 (68) | 256 (65) | 254 (68) |
| Low-dose | 67 (17) | 77 (20) | 88 (22) | 81 (22) |
| Full dose | 40 (10) | 47 (12) | 49 (13) | 39 (10) |
| Regular Multivitamin Use | ||||
| 0=NO | 231 (55) | 223 (57) | 201 (50) | 209 (52) |
| 1=YES | 187 (45) | 168 (43) | 204 (50) | 190 (48) |
| Smoking Frequency | ||||
| Never | 198 (45) | 213 (50) | 215 (49) | 208 (49) |
| Past | 200 (45) | 185 (44) | 193 (44) | 195 (46) |
| Current, ≤20 cigs/day | 29 (7) | 18 (4) | 24 (6) | 19 (4) |
| Current, >20 cigs/day | 15 (3) | 9 (2) | 3 (1) | 5 (1) |
| Alcohol Consumption | ||||
| Never | 132 (30) | 120 (28) | 134 (31) | 128 (30) |
| Past | 144 (33) | 120 (28) | 126 (29) | 123 (29) |
| Current | 167 (37) | 185 (44) | 176 (40) | 176 (41) |
| Vigorous physical activity | ||||
| Never | 327 (74) | 278 (66) | 296 (68) | 254 (60) |
| Monthly | 91 (21) | 115 (27) | 100 (23) | 127 (30) |
| Weekly | 23 (5) | 29 (7) | 37 (9) | 46 (10) |
| BMI, kg/m2 | ||||
| <18.5 | 6 (1) | 3 (1) | 9 (2) | 3 (1) |
| 18.5-22.4 | 55 (12) | 49 (12) | 59 (14) | 47 (11) |
| 22.5-24.9 | 72 (16) | 68 (16) | 63 (14) | 59 (14) |
| 25.0-29.9 | 166 (37) | 160 (38) | 152 (35) | 165 (39) |
| ≥30 | 145 (33) | 142 (33) | 154 (35) | 152 (35) |
| Total MO3PUFA, g/day | 0.025±0.008 | 0.045±0.007 | 0.067±0.01 | 0.13±0.006 |
| Women | 0.021±0.007 | 0.039±0.005 | 0.063±0.009 | 0.12±0.005 |
| Men | 0.028±0.009 | 0.049±0.006 | 0.071±0.008 | 0.14±0.006 |
| EPA, mg/day | 6±3 | 12±4 | 20±5 | 44±22 |
| DHA, mg/day | 15±5 | 27±5 | 39±7 | 72±29 |
| DPA, mg/day | 3±2 | 6±2 | 9±3 | 16±7 |
| Total omega-3 PUFA, g/day | 1.1±0.6 | 1.1±0.4 | 1.2±0.4 | 1.3±0.4 |
| Total omega-6 PUFA, g/day | 10.3±3.1 | 10.3±2.7 | 10.8±2.6 | 10.9±2.9 |
| Vitamin D, IU/day | 334±251 | 301±233 | 326±229 | 334±226 |
| Calcium, mg/day | 870±483 | 871±467 | 850±450 | 863±470 |
| Total Fiber, g/day | 23.4±9.9 | 25.1±8.7 | 25.7±8.6 | 28.0±10.0 |
| Fish, g/day | 3.5±2.4 | 8.1±4.1 | 14.4±5.6 | 35.7±22.0 |
| Processed red meat, g/day | 31.7±33.1 | 29.6±28.1 | 29.7±26.2 | 26.3±29.3 |
Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid.
Mean±SD is shown for continuous variables. N (%) is shown for categorical variables. For some variables, the number of participants across all categories does not sum to the total due to missing data.
To examine whether the MO3PUFA-survival association differs by tumor molecular markers, we performed stratified analysis according to MMR status (dMMR, pMMR), and KRAS and BRAF mutations (wildtype, mutant). In light of the relation between BRAF mutation and MSI, we also stratified by the combined status of MMR and BRAF. Given the limited sample size in each stratum, we used the binary MO3PUFA intake (low and high intake groups) dichotomized at the sex-specific median levels. Multiplicative interaction was assessed using the Wald test for the product term between the stratified variable and MO3PUFA intake. To facilitate clinical interpretation, we also calculated the 3-year DFS and 5-year OS in each of the patient strata characterized jointly by MO3PUFA intake and the status of molecular markers.
As a secondary analysis, we examined the association of fish intake with DFS and OS using similar analytic approaches.
Analyses were based on follow-up time through August 5, 2015, and were performed by using SAS version 9.4 (SAS Institute, Cary, NC). Two-sided P values less than 0.05 were considered statistically significant. All data collection and statistical analyses were performed by the Alliance Statistics and Data Center.
Results
Median follow-up time was 6.9 years among the 1,735 patients included in this analysis. The median consumption of MO3PUFAs was 0.05 g/day in women [interquartile range (IQR): 0.03-0.08] and 0.06 g/day in men (IQR: 0.04-0.09). Table 1 presents the basic characteristics of the 1,735 patients according to quartiles of MO3PUFA intake. Compared to patients with lower MO3PUFA intake, those with higher intake were less likely to have proximal colon cancer, dMMR and BRAF mutation, and were more likely to have KRAS mutation. They also tended to use low-dose aspirin and multivitamins, to exercise, to consume more fiber and less processed red meat, and to refrain from smoking.
Table 2 shows the association of MO3PUFA intake with DFS and OS. No statistically significant association was found between MO3PUFA intake and overall DFS or OS (P for trend=0.84 and 0.73 for the multivariable analysis, respectively).
Table 2.
Post-diagnostic marine ω-3 polyunsaturated fatty acid (MO3PUFA) intake and disease-free survival and overall survival among patients with stage III colon cancer
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend* | |
|---|---|---|---|---|---|
| Median intake (interquartile range), g/day | 0.03 (0.02-0.03) | 0.04 (0.04-0.05) | 0.07 (0.06-0.08) | 0.12 (0.10-0.14) | |
| Disease-free survival (DFS) | |||||
| No. of events/patients | 121/363 | 113/344 | 116/353 | 114/351 | |
| 3-year survival (%) | 70.7 | 72.8 | 71.8 | 73.3 | |
| Model 1, HR (95% CI) † | 1 (reference) | 0.97 (0.75-1.25) | 0.96 (0.74-1.24) | 0.93 (0.72-1.20) | 0.60 |
| Model 2, HR (95% CI) ‡ | 1 (reference) | 0.99 (0.76-1.29) | 0.99 (0.76-1.29) | 0.97 (0.75-1.27) | 0.84 |
| Overall survival (OS) | |||||
| No. of events/patients | 109/363 | 95/344 | 92/353 | 96/351 | |
| 5-year survival (%) | 77.0 | 77.8 | 79.0 | 80.0 | |
| Model 1, HR (95% CI) † | 1 (reference) | 0.92 (0.70-1.22) | 0.83 (0.63-1.10) | 0.89 (0.68-1.17) | 0.43 |
| Model 2, HR (95% CI) ‡ | 1 (reference) | 0.88 (0.67-1.17) | 0.83 (0.62-1.10) | 0.93 (0.70-1.23) | 0.73 |
Abbreviation: CI, confidence interval; HR, hazard ratio.
P for trend was calculated for overall colon cancer.
Cox proportional hazards regression model adjusted for sex, age, and study arm (FOLFOX vs. FOLFOX plus cetuximab).
Further adjusted for tumor subsite (proximal colon, distal colon, both), No. of affected nodes (1-3, >3), T stage (T1-T2, T3, T4), ECOG performance score (0, 1, 2), aspirin use (none, full dose or extra-strength, low-dose or body-strength), MMR Status (pMMR, dMMR), smoking status (never, past, current (≤20 cigs/day), current (>20 cigs/day)), alcohol consumption (never, past, current), BMI (<18.5, 18.5-22.4, 22.5-24.9, 25.0-29.9, ≥30), frequency of vigorous physical activity (never, monthly, weekly), multivitamin use (no, yes), and total intake of vitamin D, calcium, fiber, and processed red meat (continuous).
We then examined the stratified association of dichotomized MO3PUFA intake with survival according to tumor molecular markers (Table 3). We observed a statistically significant interaction with KRAS status (P for interaction=0.02 for DFS); MO3PUFA intake above the median was associated with better 3-year DFS among patients with KRAS-wildtype tumors (77% vs. 73%; multivariable HR=0.84, 95% CI, 0.67-1.05), whereas no beneficial association was found for those with KRAS-mutant tumors (64% vs. 70%; HR=1.30, 95% CI, 0.97-1.73). Similar heterogeneity was found by MMR status: higher MO3PUFA intake was associated with better DFS for tumors with dMMR (72% vs. 67%; HR=0.69, 95% CI, 0.41-1.16), but not for those with proficient MMR (72% vs. 72%; HR=1.08, 95% CI, 0.89-1.30), although the formal test for interaction was not statistically significant (P for interaction=0.14). No difference in the MO3PUFA-survival association was observed by BRAF mutation. When tumors were classified jointly according to MMR and BRAF mutation status, higher MO3PUFA intake was suggestively associated with improved DFS for tumors with dMMR, regardless of BRAF mutation status (HR=0.47 [95% CI, 0.20-1.12] for dMMR/BRAF mutant tumors; 0.59 [95% CI, 0.25-1.38] for dMMR/BRAF wildtype tumors), although the associations did not achieve statistical significance due to the sparse data. Similar findings were obtained for OS.
Table 3.
Association between post-diagnostic marine ω-3 polyunsaturated fatty acid (MO3PUFA) intake and disease-free survival and overall survival according to tumor subsite and molecular markers among patients with stage III colon cancer*
| Disease-free survival (DFS) |
Overall survival (OS) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of events/patients | 3-year survival (%) | HR (95% CI) † | P for interaction‡ | No. of events/patients | 5-year survival (%) | HR (95% CI) † | P for interaction‡ | |
| KRAS mutation | ||||||||
| Mutant | ||||||||
| Low intake | 91/282 | 70 | 1 (reference) | 0.02 | 79/282 | 78 | 1 (reference) | 0.04 |
| High intake | 123/301 | 64 | 1.30 (0.97–1.73) | 103/301 | 72 | 1.26 (0.92–1.71) | ||
| Wildtype | ||||||||
| Low intake | 181/553 | 73 | 1 (reference) | 154/553 | 77 | 1 (reference) | ||
| High intake | 146/522 | 77 | 0.84 (0.67–1.05) | 122/522 | 84 | 0.85 (0.67–1.09) | ||
| MMR status | ||||||||
| pMMR | ||||||||
| Low intake | 231/726 | 72 | 1 (reference) | 0.14 | 200/726 | 78 | 1 (reference) | 0.47 |
| High intake | 243/727 | 72 | 1.08 (0.89–1.30) | 196/727 | 80 | 1.03 (0.84–1.27) | ||
| dMMR | ||||||||
| Low intake | 40/105 | 67 | 1 (reference) | 35/105 | 68 | 1 (reference) | ||
| High intake | 31/106 | 72 | 0.69 (0.41-1.16) | 31/106 | 75 | 0.78 (0.46–1.34) | ||
| BRAF mutation | ||||||||
| Mutant | ||||||||
| Low intake | 44/117 | 68 | 1 (reference) | 0.73 | 46/117 | 64 | 1 (reference) | 0.90 |
| High intake | 42/112 | 70 | 1.01 (0.64–1.58) | 42/112 | 68 | 1.00 (0.64–1.57) | ||
| Wildtype | ||||||||
| Low intake | 219/690 | 73 | 1 (reference) | 179/690 | 80 | 1 (reference) | ||
| High intake | 222/693 | 73 | 1.02 (0.84–1.23) | 179/693 | 81 | 1.04 (0.84–1.29) | ||
| MMR/BRAF status | ||||||||
| pMMR/Mutant | ||||||||
| Low intake | 21/64 | 70 | 1 (reference) | 0.12 | 22/64 | 68 | 1 (reference) | 0.07 |
| High intake | 29/60 | 63 | 1.74 (0.90–3.35) | 29/60 | 60 | 1.65(0.87–3.16) | ||
| dMMR/Mutant | ||||||||
| Low intake | 23/51 | 63 | 1 (reference) | 24/51 | 55 | 1 (reference) | ||
| High intake | 13/50 | 76 | 0.47 (0.20–1.12) | 13/50 | 75 | 0.42(0.17–1.02) | ||
| pMMR/Wildtype | ||||||||
| Low intake | 201/629 | 72 | 1 (reference) | 168/629 | 79 | 1 (reference) | ||
| High intake | 204/633 | 73 | 1.02 (0.84–1.25) | 161/633 | 81 | 1.01(0.81–1.27) | ||
| dMMR/Wildtype | ||||||||
| Low intake | 14/48 | 72 | 1 (reference) | 9/48 | 80 | 1 (reference) | ||
| High intake | 17/54 | 68 | 0.59 (0.25–1.38) | 17/54 | 74 | 0.93(0.34–2.58) | ||
Abbreviation: dMMR, deficient mismatch repair; CI, confidence interval; HR, hazard ratio; pMMR, proficient mismatch repair.
Only patients who had available data on stratified variables (i.e., tumor subsite and molecular markers) were included in the analysis. Low intake was defined as below the median in each sex, and high intake as equal to or above the median intake. The median intake of MO3PUFA was 0.05 g/day in women and 0.06 g/day in men.
Multivariable HRs (95% CIs) were derived from model 2 described in Table 2.
Wald test was used to calculate the p value for the product term between the stratified variable and MO3PUFA intake (both binary) except for the analysis by the joint MMR/BRAF status, for which likelihood ratio test with 3 degrees of freedom was used to compare the models with and without the product terms between MO3PUFA intake and MMR/BRAF status (4 categories).
In a sensitivity analysis, we examined the interactions between MO3PUFA intake and molecular markers with MO3PUFA intake categorized in quartiles instead of binary classification. Although the number of events was limited in some strata, the interaction pattern remained essentially unchanged. For example, the multivariable HRs of DFS comparing extreme quartiles of MO3PUFA intake were 0.83 (95% CI, 0.61-1.14) for KRAS-wildtype tumors and 1.43 (95% CI, 0.95-2.16) for KRAS-mutant tumors (data only shown in the text).
For the secondary analysis on fish intake, we observed similar patterns of interaction for DFS (P for interaction=0.01 for KRAS mutation, 0.40 for MMR). Higher fish intake was associated with better DFS in patients with KRAS-wildtype (HR=0.80, 95% CI, 0.64-1.00) and dMMR tumors (HR=0.85, 95% CI, 0.50-1.43), whereas no beneficial association was found for KRAS-mutant (HR=1.34, 95% CI, 1.00-1.80) and pMMR tumors (HR=1.02, 95% CI, 0.85-1.23) (Supplementary Table 1).
Discussion
In this prospective study of stage III colon cancer patients, we noted a suggestive beneficial association between higher MO3PUFA intake and improved DFS and OS among patients with wildtype KRAS and dMMR tumors. These data appear to be consistent with our previous findings that MO3PUFA was preferably associated with lower risk of MSI tumors, and together suggest that MO3PUFA may improve colon cancer patients’ survival by modulation of the unique microenvironment in tumors that lack KRAS mutation and arise from the MSI pathway.
Increasing data support that there are at least two pathways for colorectal tumorigenesis16: the conventional pathway that originates from loss of adenomatous polyposis coli function followed by mutations in the oncogene KRAS, activation of prostaglandin-endoperoxide synthase 2 and loss of heterozygosity of P53; and the serrated pathway that is characterized by methylation-induced transcriptional silencing of the DNA MMR gene MLH1 and the resultant development of MSI tumors. Therefore, KRAS mutation and MSI may represent two largely distinct etiological pathways underlying CRC development, although a small subset of tumors demonstrates both features.
Chronic inflammation has been proposed to constitute risk factors for the development of MSI cancers,9 which demonstrate a vigorous immune microenvironment characterized by high infiltration of activated cytotoxic T lymphocytes and upregulation of counter-inhibitory checkpoint molecules.10, 30 Therefore, MSI tumors are more responsive to immune checkpoint-targeted therapy.31 Mechanistically, chronic inflammation induces oxidative stress, a state in which reactive oxygen species are produced in excess abundance and modify DNA structures.32 Moreover, key mediators in the inflammatory process, such as prostaglandin E2, may promote MSI development by inducing transcriptional silencing of DNA-repair genes through DNA methylation-mediated mechanisms.33
In line with these clinical and mechanistic data, our previous study indicated that higher intake of MO3PUFA, a potent anti-inflammatory agent, might preferably protect against the development of MSI tumors.8 We further showed that this benefit may be related to the improvement in the regulatory T cell-mediated immunosuppression through the anti-inflammatory activity of MO3PUFA.34 The current study extends these findings and suggests that, in patients with established MSI tumors, high intake of MO3PUFA after diagnosis may also be beneficial and contributes to improved survival. Several clinical trials have indicated that MO3PUFA supplementation reduces circulating and colonic mucosal levels of pro-inflammatory mediators, including prostaglandin E2 and chemokine C-C motif ligand 2,35–38 improves immune response,39 and delay tumor progression.40 These findings support our results and may have important clinical implications. While newly approved immunotherapeutic agents for treating MSI tumors has shown great promise, the clinical efficacy remains suboptimal, with a response rate of 30-50% in the recent phase II trials.11, 12 Therefore, given the well-known immunomodulatory activity and the observed benefit of MO3PUFA for MSI tumors in our studies, it is possible that integrating MO3PUFA into the current immunotherapeutic regimen may help improve treatment outcome.41
For KRAS mutation, we found that the protective association of MO3PUFA intake with colon cancer survival was restricted to KRAS-wildtype tumors. This is not surprising, since increasing data suggest that KRAS-mutant tumors are largely driven by metabolic dysregulation rather than inflammation.42 Activation of oncogenic KRAS plays an important role in metabolic reprogramming of tumor cells and drives the shift toward an anabolic metabolism necessary to produce biomass to support unconstrained proliferation.43–45 Consistent with the crucial role of KRAS in metabolic regulation,42, 46 colorectal tumor subtype with overrepresentation of KRAS mutation (the so-called consensus molecular subtype 3) is found to be enriched with multiple metabolism signatures in an international consortium for gene expression profiling of colon cancer.7 Therefore, it is possible that the predominance of the immunomodulatory activities of MO3PUFA may make it more effective for improving the tumor microenvironment and clinical outcomes of KRAS-wildtype tumors that have fewer metabolic dependencies.
In contrast with the positive findings in the tumor marker-stratified analysis, we did not find any association between higher MO3PUFA intake and survival in the overall cohort. This observation contrasts with our previous reports that higher intake of MO3PUFA after diagnosis was associated with better survival in two independent cohorts that include stage I-III CRC4 and stage III colon cancer only,5 respectively. One of the reasons may be related to the brief FFQ used in the current study, which included only two questions about fish intake and did not specifically inquire about dark-meat fish (e.g., mackerel, salmon, and sardines) that contains much higher MO3PUFA and is the major source of MO3PUFA intake in the US.47, 48 As a consequence, MO3PUFA intake assessed in the current study was much lower than in our prior study that is also based on a chemotherapy trial of stage III colon cancer patients5 (median intake in the highest quartile: 0.13 vs. 0.40 g/day). Also, as diet was assessed at baseline only, we were unable to capture any change in patients’ diet during or after treatment. The resultant measurement error from these sources may have attenuated any association between MO3PUFA intake and patients’ survival in the overall cohort, highlighting the need for better assessment of MO3PUFA status in future studies (e.g., using the plasma samples).
Another limitation of the current study is the increased likelihood for chance findings due to multiple hypothesis testing and limited sample size in some of the strata. However, all the tested hypotheses were developed a priori based on the previous evidence. Furthermore, consistent findings obtained in the current and prior studies and the supporting mechanistic data indicate the plausibility of our observations. In addition, given that this analysis is a secondary analysis of a clinical trial, residual confounding cannot be excluded. However, the standardized treatment in the context of a chemotherapy trial minimized any confounding by treatment, especially considering that the parent study reported a null effect. Moreover, we adjusted for a variety of covariates that may influence cancer survivorship and the results appeared to be robust. Nonetheless, further larger studies are needed to confirm the findings.
Our study has several strengths. It represents one of the first efforts to prospectively characterize the prognostic influence of a dietary factor according to tumor molecular profiles. The findings not only provide insight into the biological mechanisms underlying the anti-tumor effect of MO3PUFA, but also have clinical implications for development of precision therapy.49 Moreover, systematic collection of tumor tissues and centralized assessment of tumor markers using standard protocols ensure the data quality and minimize any outcome misclassification. Finally, the availability of detailed information on potential confounders with detailed outcome follow-up allows for robust confounding control and minimizes selection bias.
In conclusion, higher intake of MO3PUFA may be associated with better survival in stage III colon cancer patients with wildtype KRAS. A suggestive beneficial association is also found for tumors with deficient MMR. The collective findings of the current and prior studies suggest a potential benefit of MO3PUFA in modulation of the inflammatory microenvironment in tumors arising from the MSI pathway. However, given that individual studies like ours are limited by the relatively small number of cases with tumor molecular assessments, further studies, preferably through pooled analyses of multiples cohorts, are needed to further validate our findings. Also, future studies using circulating MO3PUFA levels would provide important data to help elucidate the role of MO3PUFA in colon cancer survival.
Supplementary Material
Novelty & Impact Statements.
In by far the largest study, we found a suggestive association between higher consumption of marine omega-3 polyunsaturated fatty acid (MO3PUFA) and better colon cancer survival among patients with wild-type KRAS and deficient DNA mismatch repair. Taken together with our previous findings that higher MO3PUFA might lower risk of microsatellite instable colon cancer, these results suggest a benefit of MO3PUFAs for tumors arising from the microsatellite instability pathway, and have implications for improving patient care.
Acknowledgements:
We acknowledge the accrual of patients by Balkrishna Jahagirdar, Metro-Minnesota National Cancer Institute Community Oncology Research Program, supported by Grant UG1CA189863.
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology), K99CA215314 and R00CA215314 (to M.S.), U10CA180790 (to S.R.A.), and U10CA180867 (to Dana-Farber/Partners CancerCare, Boston, MA, Harold J. Burstein); by the American Cancer Society (MRSG-17-220-01 – NEC to M.S.); and by the 2017 AACR-AstraZeneca Fellowship in Immuno-oncology Research (17-40-12-SONG to M.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethics approval and consent to participate
The study was approved by Mayo Clinic Institutional Review Board and NCCTG (now part of Alliance for Clinical Trials in Oncology). Each participant of the study signed an institutional review board-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. The study was performed in accordance with the Declaration of Helsinki.
Abbreviations:
- CI
confidence interval
- CRC
colorectal cancer
- DFS
disease-free survival
- DHA
docosahexaenoic acid
- dMMR
defective mismatch repair
- DPA
docosapentaenoic acid
- ECOG
Eastern Cooperative Oncology Group
- EGFR
epidermal growth factor receptor
- EPA
eicosapentaenoic acid
- FFQ
food frequency questionnaire
- HR
hazard ratio
- IQR
interquartile range
- MMR
mismatch repair
- MO3PUFA
marine omega-3 polyunsaturated fatty acid
- MSI
microsatellite instability
- NCCTG
North Central Cancer Treatment Group
- OS
overall survival
- pMMR
proficient mismatch repair.
Footnotes
Availability of data and materials
The data will be uploaded to National Clinical Trials Network (NCTN) Data Archive within six months of the publication date (earliest of online or print publication date).
Conflict of interest: Dr. Limburg serves as co-Chief Medical Officer for Exact Sciences through a contracted services agreement with Mayo Clinic. Dr. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement. Dr. Limburg also holds an NCI contract to conduct cancer prevention clinical trials, with recent support from Ironwood Pharmaceuticals, Inovio Pharmaceuticals, Astellas Pharma, and Synergy Pharmaceuticals.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66: 271–89. [DOI] [PubMed] [Google Scholar]
- 3.Song M, Chan AT. The Potential Role of Exercise and Nutrition in Harnessing the Immune System to Improve Colorectal Cancer Survival. Gastroenterology 2018;155: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song M, Zhang X, Meyerhardt JA, Giovannucci EL, Ogino S, Fuchs CS, Chan AT. Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 2017;66: 1790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Blarigan EL, Fuchs CS, Niedzwiecki D, Ye X, Zhang S, Song M, Saltz L, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, et al. Marine omega-3 polyunsaturated fatty acid and fish intake after colon cancer diagnosis and survival: CALGB 89803 (Alliance). Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A, Loadman PM, Toogood GJ, Hull MA. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014;63: 1760–8. [DOI] [PubMed] [Google Scholar]
- 7.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21: 1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song M, Nishihara R, Wu K, Qian ZR, Kim SA, Sukawa Y, Mima K, Inamura K, Masuda A, Yang J, Fuchs CS, Giovannucci EL, et al. Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138: 2073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011;6: 479–507. [DOI] [PubMed] [Google Scholar]
- 14.Chan DLH, Segelov E, Wong RS, Smith A, Herbertson RA, Li BT, Tebbutt N, Price T, Pavlakis N. Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst Rev 2017;6: CD007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang M, Ou J, Hutchinson L, Green MR. The BRAF oncoprotein functions through the transcriptional repressor MAFG to mediate the CpG Island Methylator phenotype. Mol Cell 2014;55: 904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138: 2088–100. [DOI] [PubMed] [Google Scholar]
- 17.Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, Kahlenberg MS, Shields AF, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307: 1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NutritionQuest. Assessment and Analysis Services Assessment Tools and Analysis Services https://nutritionquestcom/assessment/.
- 19.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65: 1220S–8S; discussion 9S-31S. [DOI] [PubMed] [Google Scholar]
- 20.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology 1990;1: 58–64. [DOI] [PubMed] [Google Scholar]
- 21.Nkondjock A, Krewski D, Johnson KC, Ghadirian P, Canadian Cancer Registries Epidemiology Research G. Specific fatty acid intake and the risk of pancreatic cancer in Canada. Br J Cancer 2005;92: 971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA 2005;293: 172–82. [DOI] [PubMed] [Google Scholar]
- 23.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20: 1043–8. [DOI] [PubMed] [Google Scholar]
- 24.Domingo E, Laiho P, Ollikainen M, Pinto M, Wang L, French AJ, Westra J, Frebourg T, Espin E, Armengol M, Hamelin R, Yamamoto H, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet 2004;41: 664–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance Analysis. Journal of the American Statistical Association 1952;47: 583–621. [Google Scholar]
- 26.Agresti A An Introduction to Categorical Data Analysised. NJ: John Wiley & Sons Hoboken,, 2007. [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 1958;53: 457–81. [Google Scholar]
- 28.Cox DR. Regression models and life tables (with discussion). J R Statist Soc B 1972;34: 187–220. [Google Scholar]
- 29.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika 1982;69: 239–41. [Google Scholar]
- 30.Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, Angell HK, Fredriksen T, Elie N, Fauquembergue E, Drouet A, Leprince J, et al. Correlation between Density of CD8+ T-cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy. Cancer Res 2015;75: 3446–55. [DOI] [PubMed] [Google Scholar]
- 31.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22: 813–20. [DOI] [PubMed] [Google Scholar]
- 32.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis 2000;21: 361–70. [DOI] [PubMed] [Google Scholar]
- 33.Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med 2012;18: 224–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, Inamura K, Masugi Y, Nowak JA, Nosho K, Wu K, Wang M, et al. Marine omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA oncology 2016;2: 1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpato M, Perry SL, Marston G, Ingram N, Cockbain AJ, Burghel H, Mann J, Lowes D, Wilson E, Droop A, Randerson-Moor J, Coletta PL, et al. Changes in plasma chemokine C-C motif ligand 2 levels during treatment with eicosapentaenoic acid predict outcome in patients undergoing surgery for colorectal cancer liver metastasis. Oncotarget 2016;7: 28139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murff HJ, Shrubsole MJ, Cai Q, Su T, Martin SM, Motley S, Milne G, Zheng W, Dai D: A randomized, controlled trial of fish oil supplementation on eicosanoid production in patients at risk for colorectal cancer AACR Special Conference: Colorectal Cancer: From Initiation to Outcomes 2016. [Google Scholar]
- 37.Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, Richter F, Richter A, Kasper H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology 1993;105: 1317–22. [DOI] [PubMed] [Google Scholar]
- 38.Hillier K, Jewell R, Dorrell L, Smith CL. Incorporation of fatty acids from fish oil and olive oil into colonic mucosal lipids and effects upon eicosanoid synthesis in inflammatory bowel disease. Gut 1991;32: 1151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braga M, Gianotti L, Radaelli G, Vignali A, Mari G, Gentilini O, Di Carlo V. Perioperative immunonutrition in patients undergoing cancer surgery: results of a randomized double-blind phase 3 trial. Arch Surg 1999;134: 428–33. [DOI] [PubMed] [Google Scholar]
- 40.Camargo Cde Q, Mocellin MC, Pastore Silva Jde A, Fabre ME, Nunes EA, Trindade EB. Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer. Nutr Cancer 2016;68: 70–6. [DOI] [PubMed] [Google Scholar]
- 41.Miccadei S, Masella R, Mileo AM, Gessani S. omega3 Polyunsaturated Fatty Acids as Immunomodulators in Colorectal Cancer: New Potential Role in Adjuvant Therapies. Front Immunol 2016;7: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimmelman AC. Metabolic Dependencies in RAS-Driven Cancers. Clin Cancer Res 2015;21: 1828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496: 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149: 656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A 2013;110: 8882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011;11: 761–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver KL, Ivester P, Chilton JA, Wilson MD, Pandey P, Chilton FH. The content of favorable and unfavorable polyunsaturated fatty acids found in commonly eaten fish. J Am Diet Assoc 2008;108: 1178–85. [DOI] [PubMed] [Google Scholar]
- 48.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 2011;93: 950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogino S, Nowak JA, Hamada T, Phipps AI, Peters U, Milner DA Jr., Giovannucci EL, Nishihara R, Giannakis M, Garrett WS, Song M Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut 2018;67: 1168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


