Abstract
Introduction:
Subsequent to the 2006 FDA approval of cetuximab, a variety of molecular targeting agents have been evaluated in head and neck squamous cell carcinoma (HNSCC). The treatment outcomes of recurrent and/or metastatic (R/M) HNSCC, in particular, remain dismal. The 2016 FDA approval of PD-1 immune checkpoint inhibitors has expanded the treatment options for R/M HNSCC, and highlights the potential for immune-based therapies.
Areas covered:
We will review the clinical application of EGFR-targeted agents, alone and in combination with other drugs. Molecular targeting agents directed against the IL6/PI3K/STAT3 signaling pathway will be covered. In addition, evaluation of immune checkpoint inhibitors in HNSCC, along with ongoing combination trials incorporating these agents, will be discussed. The expanded indications of emerging drugs and the potential clinical benefit of new drugs and treatment combinations will be summarized.
Expert opinion:
In recent years, there has been a major shift towards immunotherapy-based approaches for the treatment of HNSCC, leading to significant improvements in outcomes for a subset of patients. Leveraging the increased understanding of the genetic alterations that characterize individual HNSCC tumors will facilitate precision medicine approaches using targeted agents, immunotherapies, as well as standard chemotherapy and radiation.
Keywords: chemotherapy, epidermal growth factor receptor, HPV, immunotherapy, molecular targeted therapy, programmed cell death 1 receptor, programmed cell death ligand 1, head and neck squamous cell carcinoma, The Cancer Genome Atlas
1. Background
Head and neck cancer represents the 6th most common malignancy worldwide, accounting for 6% of cancer incidence and 2% of cancer-related deaths [1]. Head and neck squamous cell carcinoma (HNSCC) originates from the mucosa of the oral cavity, pharynx, and larynx. The incidence of HNSCC is closely linked to consumption of tobacco and alcohol, which induce molecular changes in mucosal epithelial cells leading to malignant transformation [2]. Infection with human papillomavirus (HPV) is also recognized as a risk factor for HNSCC, particularly oropharyngeal cancer [3]. The incidence of HPV (+) HNSCC is increasing at an alarming rate, and the pathophysiology and clinical course of HPV-related oropharyngeal cancer is distinct from HPV (−) disease [4]. HPV (+) oropharyngeal cancer is more common among young patients and is associated with a more favorable prognosis compared to HPV (−) oropharyngeal cancer [5–7]. In light of the increased understanding of HPV (+) oropharyngeal cancer, the classical tumor-node-metastasis (TNM) staging system for HNSCC has been amended in 8th American Joint Committee on Cancer (AJCC) staging system, with HPV status becoming one of the key criteria to describe oropharyngeal cancer stage [8]. The HPV-related tumors account for more than 70% of oropharyngeal cancer and 54% of tonsillar cancer [9]. In view of the enhanced responsiveness of HPV (+) HNSCC to radiation therapy, dose de-escalation is under evaluation in phase II testing [10]. However, roughly 25% of HPV (+) cases are lethal and therapeutic agents specific for HPV (+) disease are not currently available [11].
In addition to greater understanding of HPV (+) oropharyngeal cancer, several notable advancements have been made in the analysis and treatment of HNSCC tumors since our previous review of emerging drugs in 2010 [12]. Key among these advances has been large-scale determination of the mutational and expression profiles of HNSCC tumors. In 2010, the International Cancer Genome Consortium (ICGC) and the Cancer Genome Atlas (TCGA) programs characterized the genomes of human cancers of all major types and subtypes, including HNSCC [13]. In 2011, two academic consortia reported the first comprehensive mutational profiling efforts in HNSCC, which noted an unexpected frequency of Notch1 mutations [14,15]. In 2015, The Cancer Genome Atlas (TCGA) consortium reported the comprehensive genomic characteristics of 279 primary HNSCC tumors [16]. To date, the TCGA has completed genomic characterization of 530 HNSCC tumors. In addition to elucidation of mutational profiles via whole exome sequencing (WES) of tumor DNA, work by the TCGA has also determined copy number alterations (CNAs), differential gene and protein expression patterns, and patterns of epigenetic modifications. These studies have generally revealed that mutational burden and chromosomal alterations are higher in HPV (−) HNSCC tumors compared to HPV (+) tumors [16]. Aberrant activation of specific signaling pathways (described below) have been revealed in both HPV (+) and HPV (−) HNSCC, pointing towards potential new targets for therapeutic intervention [17].
Combination chemotherapy regimens and postoperative concurrent chemoradiation therapy (CCRT) have not consistently resulted in notable improvements in the survival rate of HNSCC patients [18,19]. Moreover, the combination of chemotherapeutic agents may increase the rate of adverse events, including myelosuppression, mucositis, hair loss, and general weakness. The development and application of molecular targeting agents is aimed at minimizing off-target effects and adverse toxicities. Cumulative evidence supports EGFR as a therapeutic target in HNSCC leading to the 2006 FDA approval of the monoclonal antibody cetuximab. EGFR is a transmembrane tyrosine kinase receptor that activates the Ras and PI3K pathways and induces cellular proliferation, angiogenesis, invasion, and metastasis [20]. Treatment with cetuximab resulted in improved response rates, relative to conventional treatment, in patients with platinum-refractory metastatic or recurrent HNSCC [21]. However, despite widespread EGFR expression in HNSCC tumors, only a subset of HNSCC patients benefit from cetuximab therapy and studies to date have failed to identify predictive biomarkers to guide treatment selection.
More recently, clinical investigations have focused on the application of immuno-oncology approaches. The interactions between tumor cells and immune cells have profound effects on tumor growth. Activation of checkpoint proteins such as programmed cell death protein-1 (PD-1) or cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) on the surface of effector T cells normally serves to attenuate the activity of cytotoxic T cells and induce antigen tolerance [22]. Tumor cells, however, can also activate checkpoint proteins on T cells via expression of ligands such as PD-L1, leading to suppression of anti-tumor immunity in the tumor microenvironment (TME). The monoclonal antibodies pembrolizumab and nivolumab bind to PD-1 on effector T cells, thereby blocking PD-L1/PD-1 interactions and preventing suppression of anti-tumor immunity [23]. Treatment with nivolumab or pembrolizumab, has been shown to improve survival outcomes in solid tumors, including R/M HNSCC [24–26]. In view of the success of these agents, several other immunotherapy drugs are currently under development and numerous clinical trials are investigating opportunities for expanding the indications for immune-oncology approaches in HNSCC.
2. Medical need
The anatomy of the head and neck region encompasses organs that are critical for swallowing, phonation, and respiration. HNSCC treatment often impairs these functions and thus treatment-related morbidities should be considered alongside determinations of survival outcomes. Multidisciplinary approaches, including surgery, radiation therapy, and/or chemotherapy have been applied to improve survival and functional results for locally advanced or R/M HNSCC. To facilitate organ preservation, chemotherapy is often utilized to avoid radical surgical resection. In localized HNSCC, CCRT has achieved 5-year survival rates of 80%, while only 10–40% survival rates are seen with CCRT in the setting of advanced disease [27,28]. Two trials demonstrated that the addition of docetaxel as a chemotherapy induction regimen with cisplatin and 5-FU improved disease-free survival (DFS) and overall survival (OS) in unresectable, locoregionally advanced HNSCC [29,30]. By contrast, induction chemotherapy (docetaxel/5-FU/cisplatin) followed by CCRT failed to show superior survival benefit in subsequent trials in advanced HNSCC with high risk for local or distant metastasis [31,32]. Induction chemotherapy and other modifications are now used in limited cases, depending on the general condition and prior treatment status of the patient. Postoperative CCRT for patients with high-risk features (positive lymph nodes, positive margins, extracapsular spread, perineural/vascular invasion) improved survival rates survival rates by about 13%. A post-hoc analysis of the European Organization for Research and Treatment of Cancer (EORTC #22931) and Radiation Therapy Oncology Group (RTOG #9501) trials demonstrated that positive margin and extracapsular spread (ECS) of metastatic lymph node are the most significant indications for administration of postoperative CCRT [33]. Cisplatin-based CCRT is still considered to be the first-line treatment for locally advanced HNSCC [34]. Although various targeted agents have been developed, only cetuximab has been recommended for primary systemic therapy. However, cetuximab has not replaced platinum-based treatment due to inconsistent therapeutic results. Meta-analyses have not shown a survival benefit or improvement in quality of life for patients receiving long-term treatment with cetuximab plus radiation therapy compared to treatment with conventional cisplatin plus radiation therapy. Only patients with HPV (+) or oropharyngeal cancer exhibited a marginal survival benefit from cetuximab plus radiation therapy [35].
A substantial proportion of patients diagnosed with HNSCC initially present with advanced stage disease. Aggressive and multidisciplinary approaches fail to prevent local (60%) and/or distant failure (30%) [1,36]. In R/M HNSCC resistant to platinum-based chemotherapy, life expectancy is less than 1 year [37]. Patients with lymph node metastasis constitute about two-thirds of newly diagnosed patients [38]. Less than half (45%) of patients with R/M HNSCC are eligible to receive a second line systemic therapy. Overall, the response rates and survival outcomes of R/M HNSCC remain unacceptably low. Furthermore, treatment options are limited, particularly for individuals who exhibit resistance to prior regimens, underscoring the need to develop new treatment approaches.
3. Existing chemotherapy
Treatment modalities are divided into definitive, salvage, and palliative options, depending on the clinical objective. Definitive or curative therapy aims to eradicate the tumor leading to no evidence of disease. Salvage treatment involves cases that have failed or recurred following definitive therapy, and also has the goal of reaching no evidence of disease. The aims of palliative treatments are to relieve symptoms and improve the quality of life.
3.1. EGFR targeting drugs
Signaling via EGFR acts to promote the proliferation and survival of cancer cells, while also stimulating angiogenesis and metastasis [39]. Despite the molecular heterogeneity of HNSCC tumors, EGFR expression is found in 50–90% of HNSCC, where expression levels are associated with poor prognosis due to progressive tumor growth, low sensitivity to radiation, and high risk of recurrence [40]. EGFR targeting drugs can be divided into two main categories; monoclonal antibodies (cetuximab, panitumumab, zalutumumab, and nimotuzumab) and tyrosine kinase inhibitors (gefitinib, erlotinib, afatinib, and lapatinib). Monoclonal antibodies bind the extracellular domain of EGFR and exert their therapeutic effects by blocking ligand binding to the receptor and by promoting ligand-independent internalization and downregulation of EGFR. Additionally, binding of cetuximab to cell surface EGFR has been shown to promote antibody-dependent cell-mediated cytotoxicity (ADCC) [41]. TKIs inhibit ATP binding to the intracellular domain of EGFR, thereby inhibiting the tyrosine kinase activity of the receptor and subsequent downstream signaling.
Early diagnosis is key to improving the treatment outcomes of all cancers including HNSCC. Early stage HNSCC is usually treated by either local resection or radiation therapy alone with good oncologic and functional outcomes. For locally advanced HNSCC, a multinational phase III trial evaluating the use of cetuximab with radiation therapy showed that combination treatment increased median overall survival rate by about 20 months relative to radiation alone [21]. A follow-up study confirmed an improved 5-year overall survival rate in the combination group (45.6%) compared to radiation alone group (36.4%) [42]. Based on these results, the addition of cetuximab to radiation therapy was approved for use in locally advanced HNSCC in 2006. Subsequently, the clinical application of cetuximab was extended to R/M HNSCC.
For R/M tumors, cisplatin was the primary treatment option before the development of cetuximab. A placebo-controlled, randomized study for R/M HNSCC, performed by Eastern Cooperative Oncology Group (ECOG), showed that patients treated with cetuximab plus cisplatin showed a better response rate (26%) than patients treated with cisplatin alone (10%) [43]. Median progression-free survival (PFS) and OS were also better in the cetuximab group but the differences were not statistically significant. A single-arm study evaluating cetuximab for patients with cisplatin-refractory tumors demonstrated that the disease control rate (DCR; partial remission, complete remission, and stable disease) and response rate (RR) were 46% and 13%, respectively [44]. Median time to progression was 70 days. The efficacy of cetuximab plus platinum-based therapy (cisplatin or carboplatin and 5-FU) for R/M HNSCC was evaluated in the EXTREME trial [43]. This trial demonstrated that the addition of cetuximab to platinum/5-FU chemotherapy improved median overall survival from 7.4 to 10.1 months and PFS from 3.3 to 5.6 months. RR was increased from 20% to 36% with the addition of cetuximab [45]. The FDA and European Medicine Agency (EMA) approved the addition of cetuximab to platinum-based therapy for R/M HNSCC in 2011. Following this approval, other EGFR targeting drugs, including blocking antibodies and small molecule inhibitors, have been developed and are undergoing clinical evaluation (Table 1).
Table 1.
Emerging targeted agents currently active, under investigation, or recruiting in head and neck squamous cell carcinoma
| Compound | Company | Mechanism of action | Stage of development | Setting |
|---|---|---|---|---|
| Cetuximab | Eli Lilly, Bristol-Myers Squibb, Merck KGaA | EGFR inhibitor | III | Combined with chemotherapy and/or radiation therapy or other targeted agents |
| Afatinib | Boehringer Ingelheim | ErbB family inhibitor | I/III | Maintenance after RT / monotherapy |
| Buparlisib (BKM-120) | Adlai Nortye, Norvatis | PI3K inhibitor | I/II | Combined with cetuximab |
| Alpelisib (BYL-719) | Norvatis | Ib/II | Neoadjuvant setting | |
| Copenlisib | Bayer | Ib/II | Combined with cetuximab | |
| Taselisib | Hoffmann-La Roche | I | Monotheraphy | |
| Everolimus | Norvatis | mTOR inhibitor | I/II | Adjuvant monotherapy or neoadjuvant Combined with cisplatin/paclitaxel/cetuximab |
| Temsirolimus | Pfizer | I/II | Combined with paclitaxel/carboplatin | |
| Ruxolitinib | Incyte | JAK1/2 inhibitor | II | Neoadjuvant setting for operable patients |
| AZD9150 | AstraZeneca | STAT3 inhibitor | I/II | Combined with durvalumab |
| C188-9 | Tvardi Therapeutics | I | ||
| Bevacizumab | Genetech (Roche) | Angiogenesis inhibitor | I/II/III | Combined with cetuximab or CCRT |
| Pazopanib | Norvatis | I | Combined with cetuximab | |
| Axitinib | Pfizer | II | Monotherapy | |
| Palbociclib | Pfizer | CDK 4/6 inhibitor | I/II | Combined with cetuximab or carboplatin/cisplatin or RT |
| Ribociclib | Norvatis | I | Combined with platinum, cetuximab with/without RT, or gedatolisib | |
| Abemaciclib | Eli Lilly | II | Combined with platinum | |
| Ficlatuzumab (AV-299) | AVEO oncology | HGF inhibitor | Ib | Combined with cetuximab |
RT; radiation therapy
CCRT; concurrent chemoradiation therapy
Panitumumab is a fully human IgG2 antibody targeting EGFR. A phase III randomized study was performed to evaluate the treatment efficacy of panitumumab in R/M HNSCC. Cisplatin and 5-FU with or without panitumumab was assigned to each group [46]. PFS was 5.8 months and 4.6 months in the groups with and without panitumumab, respectively. This difference did not achieve statistical significance. High-grade (≥3) toxicities, including diarrhea, cardiac arrhythmias, hypomagnesemia, and ocular and skin toxicity, were more frequently reported in the panitumumab group. In another trial, the addition of panitumumab to docetaxel/cisplatin resulted modestly improved PFS and overall response rate (ORR) (6.9 months and 44%, respectively) compared to treatment with docetaxel/cisplatin alone (5.5 months and 37%, respectively) [47]. However, adverse toxicities were greater in the panitumumab-treated group, thus limiting the clinical application of panitumumab to advanced HNSCC. It is noteworthy that panitumumab showed comparable treatment response to conventional chemotherapy.
EGFR is a member of the ErbB family of transmembrane receptors, which includes EGFR/ErbB1/human epidermal growth factor receptor (HER)-1, ErbB2/HER-2/neu, ErbB3/HER3, and ErbB4/HER-4 [48]. A number of small molecule tyrosine kinase inhibitors (TKI) targeting the ErbB family have been or are currently being evaluated in clinical trials for head and neck cancer, and include afatinib, dacomitinib, gefitinib, erlotinib, lapatinib, and vandetanib (Table 1). Afatinib acts to irreversibly inhibit EGFR, ErbB2/HER-2, and ErbB4/HER-4, and has been investigated in patients with R/M HNSCC. Patients receiving afatinib exhibited a PFS of 2.6 months and an ORR of 10% compared to 1.7 months PFS and 6% ORR in patients receiving methotrexate. Non-oropharyngeal cancer and absence of p16 expression were favorable indicators of response. factors. Other good response indicators were high levels of phosphatase tensin homolog (PTEN), low levels of HER-3, or amplified EGFR [49]. Afatinib efficacy in R/M HNSCC has also been compared to cetuximab [50]. The primary endpoint was tumor shrinkage before crossover assessed by investigator (IR) and independent central review (ICR). While cetuximab treatment resulted in ORR of 6.5% (IR) and 9.7% (ICR), afatinib treatment demonstrated an ORR of 16.1% (IR) and 8.1% (ICR) before crossover (stage I). The DCR of afatinib (50%) was also comparable to that of cetuximab (56.5%) as measured by IR, while the incidence of drug-related adverse effects was higher for afatinib than cetuximab. After crossover (stage II), DCR (IR/ICR) was 38.9%/33/3% with afatinib and 18.8%/18.8% with cetuximab. This study also indicated a lack of cross-resistance to afatinib and cetuximab. The tolerability of afatinib followed by platinum and taxane-based chemotherapy as induction chemotherapy in unresectable, locally advanced HNSCC is a potential concern (NCT01732640). The combination of afatinib with carboplatin and paclitaxel should be administered with caution due to paclitaxel clearance-related toxicities [51]. In addition, ongoing trials are investigating the impact of afatinib treatment following CCRT in patients at high risk of recurrence (NCT01427478) and the efficacy of co-targeting EGFR with afatinib and cetuximab (NCT02979977). This study will also examine afatinib in the context of EGFR amplification/mutation, high levels of PTEN, or HER2 mutation.
The TKI dacomitinib irreversibly inhibits EGFR, ErbB2/HER-2, and ErbB4/HER-4. Monotherapy with dacomitinib in R/M HNSCC exhibited a 12.7% RR and a median PFS and OS of 12.1 and 34.6 weeks, respectively [52]. Investigation of candidate predictive biomarkers of dacomitinib demonstrated that mutations in PIK3CA and high levels of inflammatory cytokines (IL6, IL-8, IL-1A, IL-1B, IL-4, TNF) in the tumor tissue were found to be negative predictors of response to dacomitinib treatment [53].
Erlotinib, a reversible EGFR TKI, has been evaluated in combination therapy with cisplatin or bevacizumab for locally advanced HNSCC [54,55]. While phase III trials failed to demonstrate a benefit of erlotinib therapy in unselected HNSCC patients, we previously reported an exceptional responder who was treated with a brief course of neoadjuvant erlotinib [56]. WES of pretreatment tumor and blood demonstrated that the tumor harbored an activating MAPK1 E322k mutation without EGFR alterations. While near 10% of cervical cancers harbor this mutation, it is only detected in 1–2% of HNSCC, underscoring the challenges of delivering precision medicine. Gefitinib, another reversible EGFR TKI, has demonstrated negligible activity compared with standard chemotherapy for R/M HNSCC [57]. Lapatinib, a reversible EGFR and ErbB2/HER-2 TKI, was found to be inactive in R/M patients [58]. Vandetinib is a multi-targeted TKI blocking both EGFR and VEGFR-2. Addition of vandetanib to docetaxel was not beneficial to patients with R/M HNSCC [59]. These cumulative negative results have halted further investigation of gefitnib, erlotinib, or lapatinib for HNSCC therapy and point out the disconnect between target expression and target inhibition.
3.2. Immunotherapeutic agents
The HNSCC microenvironment is characterized by a high degree of immunosuppression [60]. Immune-based therapies offer considerable promise and are expected to generate systemic, durable anti-tumor responses. Collectively, cisplatin, carboplatin, paclitaxel, docetaxel, 5-FU, methotrexate, cetuximab (non-nasopharyngeal), gemcitabine (nasopharyngeal), and capecitabine are used as first-line single-agent options.
Nivolumab is a fully human IgG4 monoclonal antibody targeting PD-1, while pembrolizumab is a humanized IgG4 monoclonal antibody targeting PD-1 consisting of a high affinity mouse anti-PD-1 derived variable region attached to a human IgG4 immunoglobulin molecule with an engineered Fc region for stabilization. Their amino acid sequence are identical apart from the regions binding to the epitope of the antigen. Binding of nivolumab and pembrolizumab is dominated by interaction with PD-1 N-loop and C-loop, respectively [61]. Each agent was tested in a phase III studies in comparison with standard chemotherapy with similar results. While neither agent demonstrated improved PFS compared with chemotherapy (about 2 months), both drugs showed an increase in overall survival (OS) (7.7 months for nivolumab vs 5.1 months for chemotherapy and 8.4 months for pembrolizumab vs. 7.1 months for chemotherapy) [62,63]. In the KEYNOTE-055 trial, patients with R/M HNSCC refractory to platinum and cetuximab were enrolled [64]. This single-arm study showed that the ORR for pembrolizumab was 16% with a median duration of response of 8 months. Three-quarters of the responses were ongoing at the time of analysis. Response rate was not affected by HPV or PD-L1 tumor expression. Median PFS and OS for pembrolizumab were 2.1 months and 8 months, respectively [60].
The KEYNOTE-012 study was the first published clinical trial demonstrating the activity of pembrolizumab in HNSCC. The initial cohort consisted of 60 patients with PD-L1 (+) HNSCC and was subsequently expanded to enroll 132 patients with R/M HNSCC, regardless of PD-L1 expression status. In the initial cohort, the observed ORR was 18% in all patients (25% and 14% in HPV (+) and HPV (−) cases, respectively) with one case of complete remission. The duration of response was about 53 weeks [65]. Among the responders, 82% had responses lasting 6 months or longer, which was not affected by HPV status. The median duration of response and OS was 12 months and 13 months, respectively. In the expansion study, the observed ORR was 18% (32% and 14% in HPV (+) and HPV (−) cases, respectively with four cases of complete remission. The ORR of the PD-L1 (+) group (22%) was better than the PD-L1 (−) group (4%) [66]. Serious adverse reactions that occurred in ≥2% of subjects included pneumonia, dyspnea, confusion state, vomiting, pleural effusion, or respiratory failure. Patients without disease progression were treated for up to 24 months. This favorable result from patients with heavily pre-treated R/M HNSCC led to accelerated FDA approval of pembrolizumab for second-line therapy for R/M HNSCC on August 5, 2016. In the follow-on KEYNOTE-040 trial, patients were assigned to pembrolizumab treatment or other standard of care (SOC) with single chemotherapeutic agents (methotrexate, docetaxel, or cetuximab). While there was no significant difference observed in PFS, ORR was higher in the pembrolizumab group (14.6%) than the SOC group (10.1%). Patients with PD-L1 (+) tumors exhibited better response to pembrolizumab than PD-L1 (−) patients. While pembrolizumab provided a 19% reduction in risk of death compared to SOC in these patients with R/M HNSCC, it failed to meet its prespecified efficacy endpoint of OS and PFS. The grade 3–4 adverse events occurred less frequently in the pembrolizumab group (13.4%) than in the SOC group (36.3%) [62]. In the ongoing KEYNOTE-048 trial, the efficacy and safety of pembrolizumab as monotherapy or in combination with chemotherapy (platinum plus 5-FU) relative to standard of care (cetuximab with platinum plus 5-FU) will be compared in patients with R/M SCC (NCT02358031) [67].
The phase III CheckMate-141 trial evaluated nivolumab safety and efficacy in R/M HNSCC [68]. Nivolumab increase OS by 2.4 months when compared with SOC based on investigator’s choice, including docetaxel, methotrexate, or cetuximab. OS at 12 and 18 months was better in the nivolumab group (34% and 21.5%, respectively) than the SOC arm (19.7% and 8.3%, respectively). An improved response rate was more evident in HPV (+) patients. Based on these results, the FDA (2016) and the European Medicines Agency approved nivolumab as second-line therapy for platinum-refractory R/M HNSCC [68]. This trial subsequently allowed for crossover from patients in the SOC arm to the nivolumab arm. PFS curves were similar at 3 months for nivolumab and the crossover arm, indicating that nivolumab treatment improves survival. The objective response rate in the nivolumab arm (13.3%) was better than that seen in the SOC arm (5.8%) [63,68]. The rate of adverse events grade 3 to 4 was lower in the nivolumab (13.1%) than the SOC arm (35%). Fatigue, nausea, rash, loss of appetite, and pruritus were the most commonly encountered adverse effects with nivolumab treatment, although quality of life (QOL) was better with nivolumab treatment [69]. Both clinical trials (CheckMate-141 and KEYNOTE-040) demonstrated similar survival results and frequencies of adverse events. Pembrolizumab and nivolumab are currently used as second line therapy of R/M HNSCC.
4. Market review
To date, there are no predictive biomarkers to guide treatment selection in HNSCC. Precision medicine aims to utilize the genetic information of the patient’s tumor to precisely tailor their treatment approach. Targeted agents, including immunotherapeutic agents, are likely to facilitate the development of precision medicine approaches for cancer patients. According to a new market intelligence report by BIS Research, titled “Global Precision Medicine Market- Analysis and Forecast, 2017–2026“, the global precision medicine market tallied $43.59 billion in 2016 and is estimated to reach $141.70 billion by 2026, with a compound annual growth rate (CAGR) of 11.23% (https://bisresearch.com/industry-report/global-precision-medicine-market-2026.html). According to the Personalized Medicine Coalition, the market value of targeted agents dependent on companion diagnostics was $25 billion in 2015. Also, the number of precision medicine drugs used, as guided by specific biomarkers has increased from 5 in 2008 to 132 in 2016. The Tufts Centre for Drug Development survey showed that 42% of drugs in the developmental pipeline plan will be evaluated with biomarkers taken into consideration. While several biopharmaceutical companies have doubled their investments in precision medicine over the last five years, it is predicted that they may further increase their investments by an additional 33% over the next five years.
The global market for immunotherapy drugs is estimated to grow from $108 billion in 2016 to more than $200 billion by 2021 (https://www.marketsandmarkets.com/Market-Reports/cancer-immunotherapy-market-197577894.html). In HNSCC, immunotherapeutic agents are likely to expand from use in second-line settings of R/M disease to application as first-line agents, with or without conventional agents, for primary tumors.
5. Current research goals
Clinical trials evaluating new anti-cancer drugs are focused on evaluating safety, efficacy, and biomarkers of response, also known as predictive biomarkers. A key goal of molecular targeting agents is to increase treatment efficacy while reducing toxicities. To date, clinical trials of molecular targeting agents and checkpoint inhibitors in R/M HNSCC have generated only modest improvements in response, and in only a limited subset of patients. The elucidation of biomarkers of response and mechanisms of drug resistance will be critically important for broadening the clinical utility and the pool of responsive patients for all molecular targeting agents.
A number of mechanisms have been shown to contribute to resistance to anti-EGFR agents, including aberrant activation of the EGFR signaling pathway, cross-talk and signaling by other receptor tyrosine kinases, epithelial-to-mesenchymal transition (EMT), and altered cellular activity of hypoxia-inducible factors [70]. Inhibiting key components of the EGFR signaling pathway or parallel signaling pathways provides an opportunity for overcoming the resistance of HNSCC to drugs targeting EGFR. Moreover, components of resistance pathways may serve as predictive markers of nonresponse.
With respect to checkpoint inhibition, PD-L1, PD-L2, and IFN-γ-related gene signatures represent candidate predictive biomarkers. Clinical trials to date, including Checkmate-141, KEYNOTE-012, −040, performed subgroup analyses based on the expression pattern of PD-L1. In these studies, the efficacy of immune checkpoint inhibitors (nivolumab, pembrolizumab) was found to be greater in patients with PD-L1 (+) tumors. However, the precise cutoff value for PD-L1 expression remains incompletely understood [63]. In the nivolumab study (CheckMate-141), the presence of HPV was correlated with improved response independent of PD-L1 expression. In addition, overall survival following nivolumab treatment was found to be higher in cetuximab-naïve versus cetuximab-treated patients (8.2 versus7.1 months) [69]. Further, an abundance of tumor-associated immune cells with PD-L1 expression was associated with longer overall survival and greater likelihood of response to nivolumab [71]. The levels and phenotype of infiltrating immune cells and the mutational burden of tumor also may be important factors predicting response to checkpoint inhibitors [72].
6. Scientific rationale
6.1. PI3K/Akt/mTOR
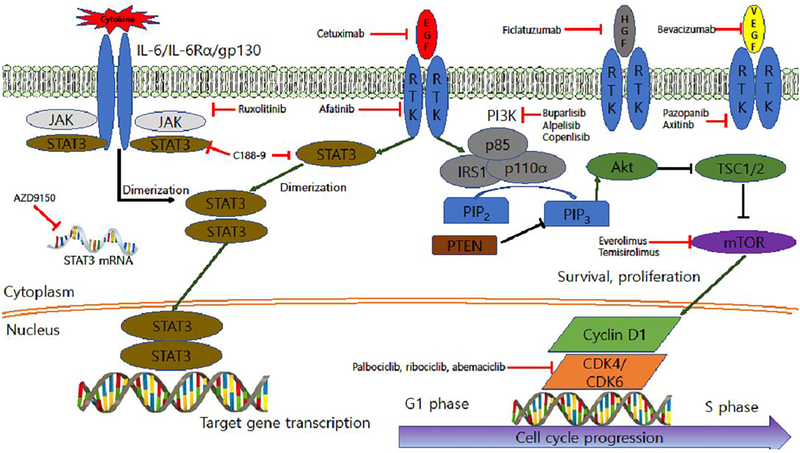
The PI3K/Akt/mTOR signaling pathway is activated by integrin mediated signals, G-protein-coupled receptors or receptor tyrosine kinases (Figure 1). Signaling via the PI3K/Akt/mTOR pathway plays key roles cellular proliferation, viability, metabolism, and motility. Following activation, PI3K phosphorylates plasma membrane phosphatidylinositol 4,5-biphosphate (PIP2) to generate phosphatidylinositol 3,4,5-triphosphate (PIP3), leading to recruitment and phosphorylation/activation of the serine/threonine kinase Akt [73]. Activated Akt fosters the phosphorylation/activation of mammalian target of rapamycin (mTOR) [74]. The tumor suppressor PTEN acts as a negative regulator of Akt signaling by catalyzing dephosphorylation of PIP3. Loss of PTEN expression occurs frequently in HNSCC, as well as other solid tumor malignancies [75]. Overall, dysregulation of components of the PI3K/Akt/mTOR signaling pathway is detected in HNSCC more frequently (30–50%) than in any other tumor type. The most commonly altered gene in this pathway is PIK3CA (encodes the p110α catalytic subunit of PI3K), which is amplified, or mutated to generated constitutively active PI3K, in 30.5% of HNSCC tumors [76,77]. One potential resistance mechanism to anti-EGFR treatment is downstream activation of the PI3K/Akt/mTOR pathway [78]. Thus, the agents targeting this pathway may overcome EGFR inhibitor resistance.
Figure 1. Receptor tyrosine kinase (RTK) and cytokine-related signaling pathways.
RTK and related cytokine signaling pathways are shown in conjunction with agents that target selected nodes in these pathways.
PI3K isoforms are divided into three functional classes according to domain structures and lipid. Class IA and IB are associated with cell survival and inflammation, respectively. Class II is involved in angiogenesis and signal transduction while class III is involved in protein synthesis and autophagy [73]. In HPV(−) tumors, mutations in PIK3CA are relatively rare (7–11%), whereas there are dominant hot spot genomic mutations in about 30% of in HPV (+) disease [79]. Currently available small molecule inhibitors of PI3K block production of PIP3, leading to suppression of Akt, inhibition of cell growth, and induction of apoptosis [80] The PI3K inhibitors BKM120 (Buparlisib), PX-866, BYL719 (Alpelisib), and copanlisib are being actively investigated in HNSCC clinical trials. Buparlisib is an orally bioavailable pan-class I PI3K inhibitor with known adverse effects that include gastrointestinal side effects, hyperglycemia, skin reactions, and stomatitis. A phase II clinical trial in platinum-resistant R/M HNSCC demonstrated improved outcomes with buparlisib plus paclitaxel versus paclitaxel alone [80]. Median PFS was 4.6 months vs. 3.5 months and median OS was 10.4 months vs. 6.5 months. The ORR was 39.2% with the combination vs. 13.9% with paclitaxel alone. Buparlisib efficacy was maintained across patient subgroups and was higher in patients with poor prognostic factors such as HPV (−) disease, progression on prior therapy and non-oropharyngeal cancer. PX-866 is a class I pan-isoform PI3K inhibitor. A clinical trial comparing treatment of advanced, refractory HNSCC with PX-866, plus cetuximab versus cetuximab alone generated negative results. Notably, patients with PIK3CA mutations failed to respond to PX-866 [81]. Further, a survival benefit was not demonstrated for inclusion of PX-866 in regimens of docetaxel or cetuximab [82]. Alpelisib is a specific α isoform inhibitor which has advantage of low toxicity compared with other pan-class PI3K inhibitors. A phase Ib/II trial combining inhibition of alpelisib and cetuximab is ongoing (NCT01602315). Copanlisib is a pan-class I PI3K inhibitor [83]. A phase Ib/II clinical trial is recruiting R/M HNSCC patients to determine efficacy of the combination of copanlisib with cetuximab in patients harboring a PI3KCA mutation/amplification with or without a PTEN loss (NCT02822482).
Rapamycin, also known as sirolimus, is a first generation mTOR inhibitor. The rapamycin analogs everolimus and temsirolimus are also pharmacologic inhibitors of mTOR signaling (Figure 1). All rapamycin analogs (rapalogs) are created to improve bioavailability by replacing the hydrogen at C-40-O position with different moieties. The moieties of everolimus and temisirolimus were a hydroxylethyl group and a hydroxyl group, respectively [84]. Anti-cancer activity of rapamycin was not exploited until late 1990s, when rapalogs were developed. Rapalogs demonstrated limited effect in treating a few cancer types, including renal cell carcinoma and mantle cell lymphoma, not in HNSCC. Temisirolimus was approved by the FDA for the treatment of bladder cancer [85]. mTOR inhibition cannot be combined with full dose cytotoxic chemotherapy in R/M HNSCC, due to myelosuppression, in which has been reported in phase I trials in multiple solid tumors [86,87]. Serious adverse effects such as infection and mucositis, led to closure of a phase I trial of combining cisplatin, cetuximab, and everolimus [88]. The role of mTOR inhibition in induction chemotherapy for treatment naïve locally advanced HNSCC has been reported (NCT01133678, NCT00935961). Rapamycin treatment prior to definite treatment with surgery or CCRT as primary therapy for the patients with stage II-IVA HNSCC showed significant response in 94% of enrolled patients including one patient who demonstrated a complete response (NCT01195922) [89]. The combination of temsirolimus with erlotinib is poorly tolerated suggesting that in simultaneous targeting of mTOR and EGFR may be infeasible [54]. A phase II study of temsirolimus in HNSCC patients refractory to platinum and cetuximab has demonstrated a 40% PFS at 12 weeks of treatment, (TEMHEAD, NCT01172769) [90]. A phase I/II trials of temisirolimus in combination with low dose weekly paclitaxel and carboplatin in R/M HNSCC was performed (NCT01016769). The overall radiologic response rate was 43% with 1 complete remission with 12.9 months of OS [91]. Adverse effects were lymphopenia, leukopenia, and neutropenia. A phase II study of temsirolimus and erlotinib in patients with platinum-refractory R/M HNSCC reported a median PFS and OS 1.9 and 4.0 months, respectively [92]. However, this trial was closed due to toxicity or death, which occurred in half of enrolled patients within 6 weeks. Everolimus is used as an immunosuppressant to prevent rejection from organ transplant and for the treatment of kidney cancer. Another phase II study of everolimus, a mTORC inhibitor, plus erlotinib in patients with platinum-refractory metastatic HNSCC demonstrated an ORR of 2.8% and PFS of 49% at 12 weeks [93]. Disease was stabilized in 77% and 31% of patients at 4 and 12 weeks, respectively. High neutrophil gelatinase, lipocalin, and vascular endothelial growth factor (VEGF) plasma levels were negative predictors of response (NCT00942734). Overall, the use of mTOR inhibitors in HNSCC has been limited by treatment-related toxicities and testing in unselected patient populations. Application of “basket” trial approaches where these agents are given to patients whose tumors harbor alterations that activate the mTOR pathway, may identify those individuals who are likely to benefit from these targeted agents.
6.2. IL-6/JAK/STAT3 signaling pathway
The IL-6/JAK/STAT3 signaling pathway is aberrantly hyperactivated in patients with chronic inflammatory conditions and in patients with hematologic or solid tumor malignancies, including HNSCC. IL-6 activates cellular signaling via two distinct mechanisms: classic signaling and trans-signaling [17]. In classic signaling IL-6 binds to a cell surface receptor that lacks tyrosine kinase activity (IL-6R). Signaling is initiated when a complex form containing two molecules each of IL-6, IL-6R, and gp130. Activation of the tyrosine kinase activity of gp130 leads to recruitment to the complex and activation of JAK enzymes and STAT3 transcription factor (Figure 1). In trans-signaling, IL-6 binds to a secreted form of IL-6Rα which subsequently binds and activates signaling by gp130. Activation of the IL-6/JAK/STAT3 pathway induces the expression of STAT3 target genes, which drive the proliferation and survival of cancer cells. In addition to activating STAT3, IL-6 stimulation of gp130 also leads to activation of the PI3K/Akt/mTOR and RAS/RAF/MEK/ERK pathways, which also promotes cellular proliferation and survival.
Recent evidence indicates that STAT3 is hyperactivated in tumor infiltrating immune cells. Functional studies have determined that STAT3 activity suppresses neutrophils, natural killer cells, effector T cells, and dendritic cells, while inducing myeloid-derived suppressor cells and regulatory T-cells. Hence, STAT3 activation in immune cells has an overall effect of suppressing anti-tumor immunity. This suggests that therapeutic targeting of the IL-6/JAK/STAT3 pathway may have a two-fold benefit: directly inhibiting the growth of tumor cells and enhancing anti-tumor immunity.
Several inhibitors of IL-6, IL-6R, and JAKs have received FDA approval, with multiple other novel inhibitors of the IL-6/JAK/STAT3 pathway under preclinical and/or clinical development. Siltuximab, sirukumab, olokizumab, clazakizumab, MEDI5117 target IL-6, while tocilizumab and sarilumab are IL-6Rα targeting agents. However, none of these agents are currently being evaluated in clinical trials for HNSCC [17]. Ruxolitinib, a JAK1/2 inhibitor, is undergoing evaluation for pharmacodynamic effects in a window-of-opportunity trial in patients with operable HNSCC with planned definitive surgery (NCT03153982). Early phase clinical trials in HNSCC patients have evaluated STAT3 inhibition using a decoy oligonucleotide (NCT00696176), a STAT3 antisense oligonucleotide AZD9150 (NCT02499328), and the small molecule inhibitor C188–9 (NCT03195699). The combination of AZD9150 and durvalumab was found to be well tolerated and induced a higher response rate than durvalumab monotherapy in patients with advanced solid malignancies including HNSCC [94]. In view of the critical role that IL-6/JAK/STAT3 signaling plays in the TME, investigation of combined treatment with IL-6/JAK/STAT3 inhibitors and immune checkpoint inhibitors is warranted.
6.3. Vascular endothelial growth factor
Hypoxia induces the expression of VEGF, which stimulates angiogenesis in necrotic and hypoxic regions of tumor tissue. Overexpression of VEGF modulates the microvessel density in the vicinity of cells, cell migration and the formation of distant metastases. VEGF is overexpressed in a majority of HNSCC tumors, is associated with reduced sensitivity to radiationand evokes tumor cell growth, migration, and metastasis [95]. VEGF signaling can be targeted with a number of agents including bevacizumab, sorafenib, sunitinib, and vandetanib, pazopanib, and axitinib. Bevacizumab, a recombinant humanized monoclonal antibody that binds VEGF-A, was the first anti-angiogenesis agent to receive FDA approval in malignant glioma [96]. Phase I and II clinical trials in R/M HNSCC showed that combination of bevacizumab and erlotinib resulted in an improved complete response rate (15%) and median survival rate (7.1 months) [55]. However, addition of bevacizumab to cetuximab treatment failed to show any survival benefit [97]. The addition of bevacizumab to high-dose cisplatin with intensity modulated RT (IMRT) in patients with advanced HNSCC without distant metastasis was found to result in 2-year PFS of 75.9% and 2-year OS of 88% [98]. A phase III trial in R/M HNSCC comparing bevacizumab plus platinum-based therapy with or without 5-FU versus platinum plus 5-FU revealed improved ORR (36% vs. 25%) and PFS (6.1 months vs. 4.4 months), but no significant difference in OS (NCT00588770) [99]. Sorafenib is an inhibitor of VEGFR and platelet-derived growth factor receptor (PDGFR). Vandetanib is an inhibitor targeting EGFR and VEGFR-2, while sunitinib is an inhibitor targeting VEGFR, PDGFR, and c-kit kinase. There are no ongoing trials of sorafenib, vandetanib, or sunitinib in HNSCC. A phase I trial of pazopanib in combination with cetuximab is active (NCT01716416). A single-arm phase II study of axitinib is recruiting patients for unresectable R/M HNSCC (NCT02762513). Overall, despite evidence that angiogenesis represents a plausible therapeutic target in HNSCC preclinical models, extensive evaluation of these agents in the clinic has been disappointing.
6.4. Cyclin D-CDK4/6-INK4/Rb pathway
Dysregulation of cell division is a hallmark of cancer and represents another target of cancer therapy. The cyclin D/cyclin dependent kinase (CDK) 4/CDK6/retinoblastoma (Rb) pathway is essential for cell cycle regulation. CDKs control the transition from G1 to S phase following activation by interaction with cyclins [100]. Overexpression of cyclin D, amplification of CDK4/6, or loss of the cyclin D/CDK4/6 negative regulator p16INK4A are commonly seen in cancer cells and lead to increased CDK4/6 activity, hyperphosphorylation/inactivation of Rb, and aberrant cell cycle progression (Figure 1) [101]. Thus, CDKs have been regarded as an important therapeutic target. Amplification of CCDN1 (encoding cyclin D1) and inactivating mutations of CDKN2A (encoding p16INK4A) were reported in 31% and 22%, respectively, of the 279 HNSCC tumors analyzed by TCGA [16]. CDK4/6 inhibitors undergoing clinical evaluation include palbociclib, ribociclib, and abemaciclib. Palbociclib and ribociclib target both CDK4 and CDK6, while abemaciclib is more specific for CDK4 [102]. Palbociclib treatment resulted in 27% stable disease in patients with advanced solid tumor [103]. Adverse effects of palbociclib include reversible neutropenia, nausea, fatigue, diarrhea, stomatitis, and asthenia [104]. Major adverse effects of ribociclib are neutropenia, leukopenia, lymphopenia [105]. Abemaciclib is orally bioavailable and is rarely associated with neutropenia, while diarrhea, nausea, and vomiting are frequent [106]. Dysregulation of the CDK4/6/Rb pathway is a characteristic feature of HPV (+) HNSCC. The viral oncoprotein E7 promotes ubiquitination and proteasomal degradation of Rb and subsequent upregulation of p16, which acts like CDK4/6 inhibitor [107]. Thus, CDK4/6 inhibitors are unlikely to be effective in HPV-related SCCs. A phase I trial to evaluate the safety of palbociclib with cetuximab in patients with R/M HNSCC demonstrated that DCR was 89% in cetuximab- or platinum-resistant patients. Myelosuppression was the most common adverse effect [108]. Two patients of 5 patients with p16INK4A (−) tumors showed PR, while 4 patients with p16INK4A (+) tumors showed either SD or PD. This result leads to enroll the patients with p16INK4A (−) HNSCC in next clinical trial. Patients with platinum-resistant, cetuximab-naïve, HPV (−) R/M HNSCC were treated with palbociclib plus cetuximab in a phase II trial (NCT02101034). Tumor response rate was 35% with 6.4 months of PFS and 12.1 months of OS [109]. Recently initiated clinical trials are recruiting patients to evaluate the efficacy of combined treatment of palbocicilib with platinum (NCT03194373), cetuximab with RT (NCT03024489), or gedatolisib (NCT03065062). A phase I trial to evaluate potential biomarkers recruiting patients with resectable oral cavity, HPV (−) oropharyngeal cancer, or larynrx cancer (NCT03179956). A single arm, phase II trial to evaluate the treatment efficacy of abemaciclib is recruiting R/M HNSCC patients refractory to platinum-based treatment (NCT03356587).
6.5. PD-1/PD-L1 pathway
Intense focus in now being placed on the development of immunotherapeutic approaches to cancer. Specific attention is directed at disrupting interactions between checkpoint receptors (PD-1, CTLA-4) on anti-tumor immune cells and ligands for these receptors (PD-L1/L2 and CD80, respectively) that are expressed on cancer cells. Programmed death-1 (PD-1) is an immunoglobulin superfamily member related to CD28 and CTLA-4. PD-1 is induced on the surface of T-cells, B-cells, and monocytes following the activation of these cells [110]. PD-1 has two ligands: PD-1 ligand 1 (PD-L1; B7-H1) and PD-1 ligand 2 (PD-L2; B7-dendritic cells). PD-L1 and PD-L2 negatively regulate cellular and humoral immune responses by engaging PD-1 receptor. PD-L1 is expressed on resting T-cells, B-cells, dendritic cells, macrophages, and parenchymal cells, including vascular endothelial cells and pancreatic islet cells. The expression of PD-L2 is primarily restricted to induced dendritic cells and macrophages [111]. PD-L1 and PD-L2 are also commonly expressed on cancer cells in the TME where they promote tumor growth by inducing apoptosis of tumor-reactive T-cells that express PD-1.
Nivolumab and pembrolizumab target PD-1 and have demonstrated success in the treatment of R/M HNSCC, as well as other solid tumor malignancies leading to FDA approval in 2016. Blockade of PD-L1 also resulted in comparable immunological and clinical outcomes in patients with melanoma and other solid tumors [112]. These results lead to expand trials of anti-PD-L1 agents into HNSCC. Agents targeting PD-L1 have also been developed, including atezolizumab, durvalumab, and avelumab. These IgG monoclonal antibodies that bind to PD-L1 and inhibit the interaction between PD-L1 and PD1, prompting cytotoxic effects of T cell or NK cells [113]. Recently, the European Society for Medical Oncology reported phase Ia trial results for atezolizumab in R/M HNSCC (NCT01375842) [114]. Median PFS and OS were 2.6 months and 6 months, respectively with an ORR of 22%. Clinical responses were independent of PD-L1 expression or HPV status. In the single arm, phase II HAWK trial with durvalumab, patients with high PD-L1 expression levels who had progressed or recurred during/after 1 platinum-based treatment were treated with durvalumab [115]. HPV (+) and HPV (−) patients showed ORRs of 26.5% and 7.9%, respectively. The overall median PFS was 2.3 months. The incidence of serious adverse events (≥ 3) was 9.8%. The phase II CONDOR trial evaluated treatment efficacy and adverse effects of durvalumab monotherapy, as well as combination with tremelimumab (anti-CTLA-4 agent), for the patients with PD-L1 (−) R/M HNSCC (NCT02319044) [116]. Durvalumab monotherapy yielded a 9.2% of ORR in this heavily pretreated population of R/M HNSCC with low or negative PD-L1 expression. Median OS was 6.0 months and 7.6 months in the monotherapy and combination therapy groups, respectively. Clinical trial results for avelumab in HNSCC have not been reported. Most of the ongoing clinical studies evaluating anti-PD-L1 targeting agents in HNSCC involve combination of conventional chemotherapy or with other immunotherapeutic targeting agents in HNSCC (NCT02952586, NCT03260023, NCT02999087, and NCT01772004) (Table 2).
Table 2.
Emerging immunotherapeutic agents currently active, under investigation, or recruiting in head and neck squamous cell carcinoma
| Compound | Company | Target | Mechanism of action | Stage of development | Setting |
|---|---|---|---|---|---|
| Inhibition of immune suppression | |||||
| Nivolumab | Ono, Bristol-Myers Squibb | PD-1 | Activation of cytotoxic T-cells by inhibiting binding of PD-1 to PD-L1 | I/II/III | Monotherapy or combined with other immunotherapeutic agents |
| Pembrolizumab | Merck | I/II/III | Monotherapy or combined with other immunotherapeutic agents | ||
| Atezolizumab | Roche | PD-L1 | Activation of cytotoxic T-cells by inhibiting binding of PD-L1 to PD | II/III | Monotherapy or combined with RT |
| Durvalumab | AstraZeneca | I/II/III | Monotherapy or combined with docetaxel/cisplatin/5-FU | ||
| Avelumab | Pfizer, Merck, Eli Lilly | I/II/III | Combined with CCRT or cetuximab/RT | ||
| Ipilimumab | Bristol-Myers Squibb | CTLA-4 | Activation of cytotoxic T-cells by inhibiting binding of CTLA-4 to CD80/86 | I/II/III | Combined with conventional chemotherapy, RT, or anti-PD-1 agents |
| Tremelimumab | AstraZeneca | I/II/III | Monotherapy or combined with durvalumab or RT | ||
| Relatlimab | Bristol-Myers Squibb | LAG-3 | Activation of cytotoxic T-cells by inhibiting LAG-3 on tumor infiltrating lymphocytes | I/II | Combined with nivolumab |
| Lirilumab | Bristol-Myers Squibb | KIR | Activation of NK cells by inhibiting binding of KIR to KIR ligand | II | Combined with nivolumab or ipilimumab and epacadostat |
| Epacadostat | Incyte | Indoleamine 2,3-idoxygenase (IDO) | Activation of T-cell, APC, and NK cell by inhibiting IDO1 and decreasing kynurenine | II | Combined with pembrolizumab |
| Pexidartinib (PLX3397) | Plexxikon, Daiichi Sankyo, Merck | CSF1R | Inhibition of TAM by inhibiting binding of CSF-1 to CSF1R | I | Combined with pembrolizumab |
| Cabiralizumab (FPA008) | Five Prime | I | Combined with nivolumab | ||
| Stimulation of immunity | |||||
| MEDI6469 | AstraZeneca | OX40 | Activation of effector T cell by stimulating OX40 | I | Neoadjuvant setting |
| INCAGN01949 | Incyte | I/II | Monotherapy or combined with nivolumab or ipilimumab | ||
| MEDI6383 | AstraZeneca | I | Monotherapy or combined with durvalumab or RT | ||
| PF04518600 | Pfizer | I/II | Monotherapy or combined with utomilumab or avelumab | ||
| INCAGN01876 | Incyte | GITR | Activation of effector T cell and inhibition of Treg by stimulating GITR, | I/II | Combined with epacadostat or anti-PD-1 or anti-CTLA-4 agents |
| TRX518 | Leap Therapeutics | I | Monotherapy or combined with gemcitabine, anti-PD-1 agents | ||
| MEDI1873 | AstraZeneca | I | Monotherapy | ||
| Varlilumab | Celldex Therapeutics | CD27 | Activation of cytotoxic T-cells and NK cells by stimulating CD27 | I/II | Combined with nivolumab |
| CDX-1140 | Celldex Therapeutics | CD40 | Activation of APC and cytotoxic T-cells by stimulating CD40 | I | Monotherapy |
| Utomilumab | Pfizer | 4–1BB (CD137) | Activation of APC, NK cells and cytotoxic T-cells by stimulating CD137 | Ib/II | Combined with avelumab or pembrolizumab or PF04518600 |
| Motolimod | Celgene | TLR-8 | Activation of dendritic cells, monocytes, and NK cells by stimulating TLRs | Ib | Combined with cetuximab with or without nivolumab |
| SD-101 | Dynavax | TLR-9 | I/II | Intratumoral injection with systemic pembrolizumab | |
| ISA101 | ISA | HPV-16 | Vaccination of HPV (+) cancer and stimulation of antigen presentation to T-cell | II | Vaccination with utomilumab or nivolumab in HPV (+) cancer |
| Talimogene Laherparepvec (T-VEC) | Amgen | HSV-1 | Lysis by infecting tumor cells and expression of immune stimulating GM-CSF | I | Combined with pembrolizumab |
RT; radiation therapy
APC; antigen presenting cells
Tregs; regulatory T-cells
TAM; tumor-associated macrophage
MDSC: myeloid-derived stem cell
GITR: glucocorticoid-induced TNF receptor
TLR: toll-like recptor
LAG-3; lymphocyte-activation gene-3
KIR: killer-cell immunoglobulin-like receptors
6.6. The CTLA-4 pathway
Ipilimumab is a fully humanized IgG1 mAb that inhibits CTLA-4. Co-treatment with ipilimumab and nivolumab has been reported to improve treatment efficacy in advanced melanoma [117]. Based on these impressive outcomes in melanoma, the combination of ipilumumab and nivolumab is currently being investigated in other advanced solid tumors, including HNSCC ((NCT02919683, NCT03126110, NCT03241173) [118]. Tremelimumab, another CTLA-4 blocking antibody, is being studied in several phase I and II trials in combination with durvalumab for treatment naïve (NCT02262741, NCT03450967) or platinum refractory HNSCC (NCT02319044, NCT02369874). The treatment efficacy of durvalumab (anti-PD-L1), alone and in combination with tremelimumab or the EXTREME regimen (carboplatin or cisplatin plus 5-FU and cetuximab) is being evaluated in phase III trials for R/M HNSCC (NCT02551159; KESTREL, NCT02369874: EAGLE).
7. Other classes of immunotherapeutic agents
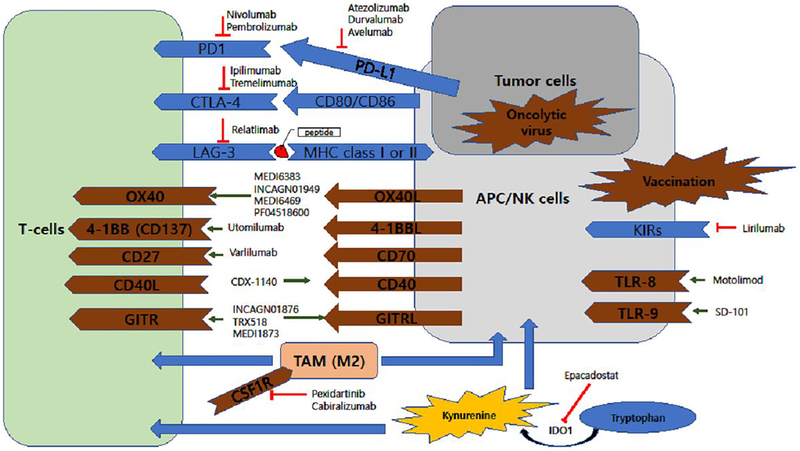
In addition to blockade of immune checkpoints, agonistic antibodies against costimulatory molecules such as the TNF receptor family members OX40, 4–1BB, GITR, CD27, and CD40 are currently being developed as anti-cancer agents (Table 2). Specific recognition of major histocompatibility (MHC) molecules expressed at the surface of antigen presenting cells (APC) by the T-cell receptor (TCR) is the first step for T-cell activation, the process of adaptive immunity. Involvement of costimulatory molecules is required for full T-cell activation in the process of adaptive immunity against tumor cells (Figure 2). OX40 (CD134) can be expressed by activated immune cells, such as tumor-infiltrating lymphocytes, mostly on memory T cells (CD4+ and CD8+). OX40 has also been found on polymorphonuclear cells and dendritic cells, which have biologic (proinflammatory) effect in hosts [119]. An OX40 agonist is associated with increased production of T cells and inflammatory cytokines to kill tumor cells. The agonistic anti-OX40-antibody MEDI6383 is being evaluated as monotherapy or in combination with durvalumab in advanced solid tumors, including HNSCC (NCT02221960) [120]. Phase I trials of 9B12, a murine-derived agonistic antibody against OX40, demonstrated only moderate toxicities in patients, and a maximum tolerated dose was not reached (NCT01644968). This study drove the development of the humanized/mouse chimeric antibody, MEDI6469 [121]. An ongoing phase I trial is evaluating the safety of MEDI6469 in patients with advanced HNSCC (NCT02274155). INCAGN01949, another OX40 agonist, is being evaluated in combination with nivolumab, ipilimumab, or both (NCT03241173, phase I/II). A phase I trial evaluating treatment with the OX40 agonist PF-04518600, alone and in combination with an agonistic antibody against 4–1BB, is currently recruiting HNSCC patients (NCT02554812).
Figure 2. HNSCC tumor microenvironment.
The HNSCC tumor microenvironment is shown in conjunction with agents that target different components of the microenvironment.
Glucocorticoid-induced tumor necrosis factor receptor (GITR) is a costimulatory receptor expressed on the surface of APCs and CD4+ T-cells. Targeting GITR with an agonistic antibody leads to inhibition of Treg-mediated immunosuppression via downregulation of FoxP3 and reduction of IL-10 secretion from Tregs. In addition, GITR agonistic antibody also induces the CD8+ T cell expansion [122]. Two clinical trials investigating a combination of agonistic anti-GITR antibody (INCAGN01876) with anti-PD-1 and/or anti-CTLA4 agents are actively recruiting patients with advanced solid tumors including HNSCC (NCT03277352, NCT03126110). Phase I studies of other anti-GITR agents (TRX518 and MEDI1873) are recruiting patients with advanced solid tumors (NCT02628574, NCT01239134).
The 4–1BB (CD137) protein is expressed on CD8+ and CD4+ T cells and natural killer (NK) cells. Utomilumab is agonistic antibody that targets 4–1BB and induces T cell-mediated anti-tumor activity [123]. An ongoing phase Ib/II trial of utomilumab in combination with avelumab (anti-PD-L1) is accruing patients with advanced HNSCC (NCT02554812).
Varlilumab, an agonistic anti-CD27 antibody, is being studied in combination with nivolumab in a phase I/II trial for patients with advanced refractory solid tumors, including R/M HNSCC (NCT02335918). Preclinical studies have shown promising immune stimulation with agonistic CD40 mAb, as well as with recombinant CD40L, which enhance the ability of APC to cross-prime naïve T-cells to tumor antigen [124]. Dacetuzumab and lucatumumab, agonistic CD40 mAbs, are not yet being studied in HNSCC.
Lymphocyte-activation gene-3 (LAG-3) and the killer-cell immunoglobulin-like receptors (KIRs) are additional checkpoint proteins that can be a targeted for immunotherapy. LAG-3 and the majority of KIRs are receptor proteins that suppress lymphocyte- and NK cell-mediated cytotoxicity, respectively. Phase I/II trials of relatlimab (anti-LAG-3) plus nivolumab (NCT01968109) are currently recruiting immunotherapy-naïve patients with advanced solid tumors, including HNSCC. A phase I trial of lirilumab (anti-KIRs) in combination with nivolumab or ipilimumab and epacadostat, an indoleamine 2,3-dioxygenase (IDO) inhibitor, is recruiting patients with advanced or metastatic solid tumors, including HNSCC (NCT03341936, NCT03347123). Preliminary results indicate an ORR of 24% in the nivolumab plus lirilumab group, with higher response rates higher in patients with PL-L1 (+) tumors [125].
Indoleamine-pyrrole 2,3-dioxygenase (IDO) depletes tryptophan in the TME and leads to kynurenine production. The activity of IDO acts to stimulate immunosuppressive Tregs and MDSCs, which results in enhanced immune tolerance in the TME. Oral epacadostat, a IDO inhibitor, in combination with pembrolizumab, is being evaluated in patients with HNSCC (NCT02178722; ECHO-202/KEYNOTE-037) [126]. Preliminary results from this phase I/II trial demonstrated an ORR of 34% and a DCR of 62%, with tolerable adverse effects. Based on these preliminary findings, a phase III trial is planned.
Toll-like receptors (TLRs) are important mediators of both innate and adaptive immunity. Motolimod (VTX-2337) is a small molecule TLR-8 agonist that activates myeloid dendritic cells, monocytes, and natural killer cells. A phase Ib study of motolimod plus cetuximab in R/M HNSCC showed response and disease control rates of 17% and 54%, respectively, with an acceptable toxicity profile [127]. A randomized phase II study comparing EXTREME regimen (carboplatin or cisplatin plus 5-FU and cetuximab) with or without motolimod has been launched (NCT01836029) [120]. SD-101 is a TLR-9 agonist that leads to interferon-α production activating NK cells and promoting migration of cytotoxic CD8+ T cells into the tumor. The safety and efficacy of intratumoral SD-101 in combination with pemebrolizumab in R/M HNSCC is being evaluated (NCT02521870).
In view of the complications associated with locoregional growth of HNSCC tumors (bleeding, superimposed infection, airway obstruction, dysphagia), locally-delivered immunomodulatory therapies may have considerable value. Potential regional therapies include vaccines, chimeric antigen receptor (CAR) T cells, and the use of genetically-modified viruses. ISA101, a synthetic long peptide derived from HPV-16, was combined with nivolumab in a phase II study of incurable HPV-16 (+) cancer patients [128]. Patients with oropharyngeal cancer who received combination treatment of ISA101 with nivolumab showed an ORR of 36%, which is higher than was achieved with nivolumab monotherapy in the CHECKMATE-141 trial. Talimogene Laherparepvec (T-VEC, IMLYGIC™) is a genetically modified virus derived from HSV-1, and is the first oncolytic viral therapy approved by the FDA (for local treatment of unresctable cutaneous, subcutaneous and nodal lesions of recurrent melanoma) [123]. The combination of T-VEC with pembrolizumab is currently being evaluated in R/M HNSCC (MASTERKEY232/KEYNOTE-137, NCT02626000).
8. Potential development issues
8.1. Predictive markers and resistance mechanism for anti-EGFR therapy
The modest survival benefit of EGFR-targeted therapy in HNSCC is likely due to de novo or acquired resistance [129]. Activation of ErbB2/HER2 in multiple HNSCC cell lines has been associated with resistance to cetuximab and overexpression of ErbB2/HER2 was detected in 39% of treatment-naïve HNSCC primary lesions [130]. Expression of the mutant type III variant of EGFR (EGFRvIII) has also been implicated as a potential cetuximab resistance mechanism. However, analysis of TCGA data indicates that this mutation is found in only 1 of 279 HNSCC patients and exome sequencing did not identify EGFRvIII mutations at the DNA level in 74 patient samples [15,16]. Overexpression of EMT-related proteins, such as cortactin repressor delta crystallin enhancer binding factor (ZEB1) was found in erlotinib-resistant HNSCC cell lines. EMT-mediated resistance can also affect resistance to anti-EGFR treatment [131, 132]. Compensatory activation of VEGF, MET, and NOTCH signaling pathways, or aberrant activation downstream signaling (PI3K/Akt/mTOR, JAK/STAT3, aurora kinase), has been shown to cause resistance to EGFR inhibitors [133, 134]. Treatment with a PI3K inhibitor in a cetuximab-resistant HNSCC cell line harboring a mutation in PIK3CA restored growth inhibition [135]. The role of EGFR copy number and EGFR expression levels were evaluated in the EXTREME and CRYSTAL trials and neither were found to predict response to cetuximab therapy [136]. Biomarker analyses were performed using samples of tissue from patients treated with cisplatin and cetuximab for R/M HNSCC on ECOG-E5397 and the results are pending (NCT01466244). One potential resistance mechanism to anti-EGFR therapy in HNSCC is activation of c-Met, bound by HGF. A phase Ib/II trial to evaluate the efficacy of inhibiting HGF/c-MET signaling pathway by ficlatuzumab (AV-299) is ongoing for the patients with R/M HNSCC (NCT02277197, NCT03422536). To date, many resistance mechanisms have been identified in preclinical models but without clear validation of these mechanisms in HNSCC patients who demonstrate cetuximab resistance, predictive biomarkers remain poorly understood.
The PI3K signaling pathway is commonly activated as a result of PIK3CA mutation or amplification or loss of PTEN. Ongoing basket trials are studying the impact of PI3K inhibition in HNSCC patients whose tumors harbor these alterations with encouraging results [137]. Taselisib is a selective inhibitor of class I PI3K with enhanced activity against PIK3CA-mutated cancer. Phase I study enrolled patients with PIK3CA-muatated solid tumors refractory to at least one prior treatment. Taselisib monotherapy demonstrated that RR was 19% in HNSCC (NCT01296555).
8.2. Predictive markers and resistance mechanism of immunotherapy
Many different cell types and functional states contribute to the overall immunosuppressive or immunopermissive status of the tumor microenvironment. The immunosuppressive state within tumors can be influenced by tumor cells, stromal cells, or other infiltrating immune cells [138]. Although multiple technologies, including flow cytometry and expression profiling, can be used to provide an understanding of the immunologic status of tumors, the inherent heterogeneity of most tumors complicates precise determinations [139]. In addition to expressing ligands (eg. PD-L1) for T cell checkpoint receptors, tumor cells also secret immunosuppressive cytokines and chemokines (eg. TGF-β, IL-6, and IL-10) that foster the recruitment of immunosuppressive immune cells such as myeloid-derived suppressor cells (MDSCs) [140]. MDSCs (CD11b+CD14+CD33+) are recruited into the HNSCC tumor microenvironment by elevated expression of GM-CSF, MCP-1, CXCL1, IL-8, and CSF1. MDSCs suppress the activity of T cells by production of arginase 1 (Arg-1), nitric oxide synthase (iNOS), and reactive oxygen species (ROS) [141]. L-arginine is a common substrate for Arg-1 and iNOS. Upregulation of these enzymes depletes L-arginine from the tumor microenvironment, which leads to T-cell suppression [142]. iNOS also converts L-arginine into nitric oxide, which is subsequently converted into peroxynitrite, suppressing T-cell function. Vitamin D3 analogs, IDO inhibitors (eg. epacadostat), STAT3 inhibitors (decoy, OPB-51602, OPB-31131, AZD9150), targeted antibodies, and phosphodiesterase 5 (PDE5) inhibitors (taldalafil, sildenafil) are candidates to inhibit the immunosuppressive features of MDSCs. Vitamin D3 analogs stimulate maturation of immature MDSCs into antigen-presenting dendritic cells [143]. Tumor-associated macrophages (TAMs) are categorized as tumor limiting M1 cells and tumor enhancing M2 cells. M2 TAMs secrete cytokines (IL-1β, IL-6, IL-10, and TGF-β) that promote HNSCC tumor growth and immunosuppression in the TME [144]. Similar to MDSCs, M2 TAMs deplete L-arginine levels via expression of Arg-1 and iNOS, resulting in suppression of T cell function. TAMs also express PD-L1 and tend to be converted toward the immunosuppressive M2 subtype under hypoxic conditions [145]. The recruitment of TAMs is stimulated by CSF1/CSF1R interactions, which can serve as a target for anti-TAM therapy. Anti-CSF1R antibodies and small molecule inhibitors of CSF1R has been shown to deplete TAMs from the TME and reprogram M2 cells to M1 cells [146]. A phase I/II trial combining an anti-CSF1R antibody (PLX3397) with pembrolizumab (NCT02452424) and phase I trial combining a different anti-CSF1R antibody (cabiralizumab; FPA008) with nivolumab (NCT02526017) are currently ongoing in patients with advanced solid tumors.
Tregs are a subset of CD4+ T-cells that express the transcription factor FoxP3 and commonly express CD25. Tregs foster the immunosuppressive tumor microenvironment in HNSCC. Tregs also commonly express CTLA-4, GITR, OX40, and CCR4 [147]. In addition, Tregs promote inhibition of CD8+ effector T cells and NK cells via IL-10 and TGF-β [148]. Blockade (CD25, CCR4, CTLA-4) or stimulation (OX40, GITR) are potential strategies to target Tregs. Agents targeting CTLA-4, GITR, and OX40 may exert their effects, in part, by inhibiting the activity of Tregs [149]. Ex vivo depletion of CCR4+ T cells from PBMCs of melanoma patients resulted in induction of effector T-cells [150]. Mogamulizumab, an anti-CCR4 mAb, is being evaluated as a monotherapy in advanced solid tumors, including HNSCC (NCT02281409), or in combination with other immune activating therapies, such as durvalumab or tremelimumab (NCT02301130) and nivolumab ((NCT02705105).
8.3. Combinational therapeutic strategies
In this era of immune-oncology, a number of combination trials using immune checkpoint inhibitors and other immunotherapeutic agents such as agonistic antibodies targeting costimulatory molecules are being investigated in advanced or R/M HNSCC (Table 2). In addition, conventional chemotherapy, radiation, and other molecular targeting agents are candidates for combination with immunotherapy in the primary treatment setting. Radiation therapy, in addition to inducing abscopal effects such as recruitment of CD8+ T-cells into the TME, secretion of cytokines, and enhanced antigen presentation, also stimulates upregulation of PD-L1 [151]. Moreover, DNA damage cause by RT is a potent proinflammatory trigger, and micronuclear DNA is recognized as an immunostimulant. These effects of radiation therapy have provided the bases for a number of clinical trials evaluating radiation or chemoradiation in combination with anti-PD-1 for locally advanced HNSCC (NCT02609503, NCT02586207, NCT02289209, and NCT02318771).
8.4. Curative immunotherapy
In addition to R/M HNSCC, trials to evaluate the role of immunotherapy in surgically resectable HNSCC are ongoing. The impact of pembrolizumab on the HNSCC was evaluated (NCT02296684). Pembolizumab treatment reduced tumor volume in 48% of patients. Preoperative tumor biopsies demonstrated PD-L1 (+) (>1% of tumor cells) expression in 58% and was correlated with response in the neoadjuvant setting, which is aimed to shrink a tumor before the main curative treatment [152]. A similar study of nivolumab was performed. The safety and feasibility of nivolumab prior to surgery in patients with resectable HNSCC was evaluated in CheckMate-358 (NCT02488759). Grade 3–4 treatment-related adverse effects identified in 16.7% did not delay surgery. Tumor reduction before surgery was observed in 48% and 13% showed tumor reduction ≥ 40% [153].
9. Conclusion
Despite expression of EGFR on the surface of most HNSCC cells, EGFR targeting agents have not proven highly effective in the treatment of this cancer. Cetuximab is the only FDA-approved EGFR inhibitor and today, its use is largely relegated to combining with radiation therapy for patients who cannot tolerate platinum chemotherapy. The addition of cetuximab to curative CCRT regimens has not prolonged survival. Further, clear definitions of cetuximab resistance (primary or acquired) are still lacking and in predictive biomarkers to guide treatment selection remain incompletely understood. EGFR TKIs have been extensively studied in unselected HNSCC populations with limited benefit reported. Anti-angiogenic agents, particularly bevacizumab, have been studied extensively in HNSCC with negative phase III results. The major developments in HNSCC over the past decade have been the increased recognition of HPV-positive HNSCC as a distinct, and increasingly common entity, the characterization of the molecular landscape of this malignancy through the TCGA and other efforts, and the FDA-approval of immune checkpoint inhibitors in 2016. Thus, we are poised to integrate our extensive understanding of the biologic underpinnings of HNSCC to select therapies and achieve the promise of personalized cancer medicine. Expert opinion
10. Expert opinion
Increased recognition of HPV as a major and independent cause of HNSCC has largely led to clinical trials that test modifications of standard CCRT regimens. Given the generally more favorable prognosis of HPV-positive HNSCC, the majority of studies are investigating the efficacy of reduced radiation and chemotherapy dosing. However, the morbidity of CCRT and the increased recognition of the distinct biology of HPV-positive HNSCC, underscore the need to develop HPV-selective therapies.
The explosion of information regarding the genetic and epigenetic landscape of HNSCC lays the groundwork for the development of precision medicine approaches in HNSCC. Ongoing “basket” trials are testing the response to targeted agents in HNSCC patients whose tumors harbor specific mutations with encouraging preliminary results. In fact, the limitation of testing molecular targeting agents in unselected HNSCC populations is increasingly recognized. Ideally, application of any targeted drug will require that the patient’s tumor harbor specific features that have been shown to predict response in appropriate preclinical models.
The immune system has been recognized as an important factor in mediating cancer development and response to therapy for decades. The FDA-approval of pembrolizumab and nivolumab for the treatment of R/M HNSCC in 2016 heralded the current era of immune-oncology. While these agents are highly effective in a subgroup of HNSCC patients, they are not HPV-selective and predictive biomarkers are lacking. Ideally, an increased understanding of the tumor microenvironment in the context of HNSCC therapy will accelerate our ability to apply treatments that target both the tumor cell and the immune system to achieve cures.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (R01 DE024728 and P50 CA097190 to D.E. Johnson., R01 DE023685 and P50 CA097190 to J.R. Grandis), the American Cancer Society (CRP-13-308-06-COUN to J.R. Grandis.), and National Research Foundation of Korea (NRF) (MSIP; 2016R1C1B1014827 to Y. S. Lee).
Abbreviations:
- FDA
food and drug administration
- EGFR
epidermal growth factor receptor
- HNSCC
head and neck squamous cell carcinoma
- R/M
recurrent and/or metastatic
- PI3K
phosphoinositide-3kinase
- JAK
Janus kinase
- STAT3
signal transduce and activator of transcription 3
- HPV
human papillomavirus
- ICGC
international cancer genome consortium
- TCGA
the Cancer Genome Atlas
- WES
whole exome sequencing
- CTLA4
cytotoxic T lymphocyte-associated antigen 4
- TME
tumor microenvironment
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death-ligand 1
- CCRT
concurrent chemoradiation therapy
- DFS
disease free survival
- PFS
progression free survival
- OS
overall survival
- TKI
tyrosine kinase inhibitor
- ECOG
eastern cooperative oncology group
- RR
response rate
- ORR
overall response rate
- DCR
disease control rate
- PR
partial remission
- CR
complete remission
- EMA
European medicine agency
- MAPK
mitogen-activated protein kinase
- SOC
standard of care
- 5-FU
5-fluoruracil
- mTOR
mammalian target of rapamycin
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- RT
radiation therapy
- CDK
cyclin dependent kinase
- MHC
major histocompatibility
- GITR
Glucocorticoid-induced tumor necrosis factor receptor
- APC
antigen presenting cell
- Treg
regulatory T cell
- NK cell
natural killer cell
- LAG-3
lymphocyte-activation gene-3
- KIRs
killer-cell immunoglobulin-like receptors
- IDO
indoleamine 2,3-dioxygenase
- MDSC
myeloid-derived suppressor cell
- TLR
Toll-like receptor
- CAR T cell
chimeric antigen receptor T cell
- TNF
tumor necrosis factor
- EMT
epithelial to mesenchymal transition
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Bibliography
- 1.Stewart B, Wild CP. World cancer report 2014. Health 2017. [Google Scholar]
- 2.Schlecht NF, Franco EL, Pintos J et al. Effect of smoking cessation and tobacco type on the risk of cancers of the upper aero-digestive tract in Brazil. Epidemiology 1999:412–8. [DOI] [PubMed] [Google Scholar]
- 3.Benson E, Li R, Eisele D et al. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 2014; 50:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, D’Souza G, Gillison ML et al. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral Oncol 2016; 60:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong AM, Dobbins TA, Lee CS et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer 2010; 103:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rischin D, Young RJ, Fisher R et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010; 28:4142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gospodarowicz MK, Brierley JD, Wittekind C. TNM classification of malignant tumours. John Wiley & Sons, 2017. [Google Scholar]
- 9.Ndiaye C, Mena M, Alemany L et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014; 15:1319–31. [DOI] [PubMed] [Google Scholar]
- 10.Chen AM, Felix C, Wang P-C et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol 2017; 18:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colevas AD. Systemic Therapy for Metastatic or Recurrent Squamous Cell Carcinoma of the Head and Neck. J Natl Compr Canc Netw 2015; 13:e37–48. [DOI] [PubMed] [Google Scholar]
- 12.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs 2010; 15:355–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium ICG. International network of cancer genome projects. Nature 2010; 464:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal N, Frederick MJ, Pickering CR et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011:1206923.** This reference discusses the mutational characteristics of HNSCCs.
- 15.Stransky N, Egloff AM, Tward AD et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011; 333:1157–60.** This reference discusses the mutational characteristics of HNSCCs.
- 16.Network CGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517:576–82.** This reference introduces the genomic characteristics of a large cohort of HNSCCs and highlights a future for precision medicine.
- 17.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018; 15:234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blasco MA, Svider PF, Raza SN et al. Systemic therapy for head and neck squamous cell carcinoma: Historical perspectives and recent breakthroughs. Laryngoscope 2017; 127:2565–9. [DOI] [PubMed] [Google Scholar]
- 19.Zumsteg ZS, Luu M, Yoshida EJ et al. Combined high-intensity local treatment and systemic therapy in metastatic head and neck squamous cell carcinoma: An analysis of the National Cancer Data Base. Cancer 2017; 123:4583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabó B, Nelhűbel GA, Kárpáti A et al. Clinical significance of genetic alterations and expression of epidermal growth factor receptor (EGFR) in head and neck squamous cell carcinomas. Oral Oncol 2011; 47:487–96. [DOI] [PubMed] [Google Scholar]
- 21.Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–78.* This reference highlights the clinical study which lead to US FDA approval of cetuximab in advanced HNSCC.
- 22.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015; 33:1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015; 373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbst RS, Baas P, Kim D-W et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387:1540–50. [DOI] [PubMed] [Google Scholar]
- 26.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–30. [DOI] [PubMed] [Google Scholar]
- 27.Forastiere AA, Goepfert H, Maor M et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349:2091–8. [DOI] [PubMed] [Google Scholar]
- 28.Popovtzer A, Burnstein H, Stemmer S et al. Phase II organ-preservation trial: Concurrent cisplatin and radiotherapy for advanced laryngeal cancer after response to docetaxel, cisplatin, and 5-fluorouracil-based induction chemotherapy. Head Neck 2017; 39:227–33. [DOI] [PubMed] [Google Scholar]
- 29.Posner MR, Hershock DM, Blajman CR et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007; 357:1705–15. [DOI] [PubMed] [Google Scholar]
- 30.Vermorken JB, Remenar E, Van Herpen C et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007; 357:1695–704. [DOI] [PubMed] [Google Scholar]
- 31.Haddad R, O’Neill A, Rabinowits G et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 2013; 14:257–64. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Jiang N, Shi Y et al. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Scientific reports 2015; 5:10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernier J, Cooper JS, Pajak TF et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005; 27:843–50. [DOI] [PubMed] [Google Scholar]
- 34.Network NCC. NCCN guidelines: Head and neck cancer, version 1.2018. Fort Washington, PA, National Comprehensive Cancer Network; 2018. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Zhang J, Shi C et al. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC cancer 2016; 16:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008; 83:489–501. [DOI] [PubMed] [Google Scholar]
- 37.Saloura V, Cohen EE, Licitra L et al. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2014; 73:1227–39. [DOI] [PubMed] [Google Scholar]
- 38.Bossi P, Alfieri S. Investigational drugs for head and neck cancer. Expert Opin Investig Drugs 2016; 25:797–810. [DOI] [PubMed] [Google Scholar]
- 39.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11:9. [DOI] [PubMed] [Google Scholar]
- 40.Chung CH, Parker JS, Ely K et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-κB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res 2006; 66:8210–8. [DOI] [PubMed] [Google Scholar]
- 41.Schneider-Merck T, van Bueren JJL, Berger S et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010; 184:512–20. [DOI] [PubMed] [Google Scholar]
- 42.Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11:21–8. [DOI] [PubMed] [Google Scholar]
- 43.Burtness B, Goldwasser MA, Flood W et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 2005; 23:8646–54.* This reference highlights the proof-of-priciple trial for cetuximab as first-line treatment for R/M HNSCC
- 44.Vermorken JB, Trigo J, Hitt R et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 2007; 25:2171–7. [DOI] [PubMed] [Google Scholar]
- 45.Vermorken JB, Mesia R, Rivera F et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359:1116–27. [DOI] [PubMed] [Google Scholar]
- 46.Vermorken JB, Stöhlmacher-Williams J, Davidenko I et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol 2013; 14:697–710. [DOI] [PubMed] [Google Scholar]
- 47.Wirth LJ, Dakhil SR, Kornek G et al. PARTNER: A randomized phase II study of docetaxel/cisplatin (doc/cis) chemotherapy with or without panitumumab (pmab) as first-line treatment (tx) for recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): American Society of Clinical Oncology, 2013. [Google Scholar]
- 48.Sacco AG, Worden FP. Molecularly targeted therapy for the treatment of head and neck cancer: a review of the ErbB family inhibitors. OncoTargets Ther 2016; 9:1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen EE, Licitra LF, Fayette J et al. Biomarker analysis in recurrent and/or metastatic head and neck squamous cell carcinoma (R/M HNSCC) patients (pts) treated with second-line afatinib versus methotrexate (MTX): LUX-Head & Neck 1 (LUX-H&N1): American Society of Clinical Oncology, 2015. [Google Scholar]
- 50.Seiwert TY, Fayette J, Cupissol D et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol 2014; 25:1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung CH, Rudek MA, Kang H et al. A phase I study afatinib/carboplatin/paclitaxel induction chemotherapy followed by standard chemoradiation in HPV-negative or high-risk HPV-positive locally advanced stage III/IVa/IVb head and neck squamous cell carcinoma. Oral Oncol 2016; 53:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdul Razak AR, Soulieres D, Laurie SA et al. A phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neck. Ann Oncol 2013; 24:761–9. [DOI] [PubMed] [Google Scholar]
- 53.Kim HS, Kwon HJ, Jung I et al. Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clinical Cancer Res 2014:clincanres. 1756.2014. [DOI] [PubMed] [Google Scholar]
- 54.Bauman JE, Arias-Pulido H, Lee S-J et al. Phase II study of temsirolimus and erlotinib in patients (pts) with recurrent/metastatic (R/M), platinum-refractory head and neck squamous cell carcinoma (HNSCC): American Society of Clinical Oncology, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen EE, Davis DW, Karrison TG et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol 2009; 10:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Allen EM, Lui VW, Egloff AM et al. Genomic correlate of exceptional erlotinib response in head and neck squamous cell carcinoma. JAMA oncology 2015; 1:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart J, Cohen E, Licitra L et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27:1864–71. [DOI] [PubMed] [Google Scholar]
- 58.de Souza JA, Davis DW, Zhang Y et al. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clinical Cancer Research 2012; 18:2336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Limaye S, Riley S, Zhao S et al. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol 2013; 49:835–41. [DOI] [PubMed] [Google Scholar]
- 60.Duray A, Demoulin S, Hubert P et al. Immune suppression in head and neck cancers: a review. Clin Dev Immunol 2011; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fessas P, Lee H, Ikemizu S et al. A molecular and preclinical comparison of the PD-1–targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab Seminars in oncology: Elsevier, 2017:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen E, Harrington K, Le Tourneau C et al. LBA45_PRPembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): Phase 3 KEYNOTE-040 trial. Ann Oncol 2017; 28. [Google Scholar]
- 63.Ferris RL, Blumenschein G Jr., Fayette J et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016; 375:1856–67.** This reference reported the efficacy of nivolumab in R/M HNSCC leading to FDA approval.
- 64.Bauml J, Seiwert TY, Pfister DG et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol 2017; 35:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seiwert TY, Burtness B, Mehra R et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17:956–65.** This reference reports the efficacy of pembrolizumab in R/M HNSCC, which led to its FDA approval for HNSCC.
- 66.Chow LQ, Haddad R, Gupta S et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016; 34:3838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klochikhin A, Greil R, Cohen E et al. 11TiP Phase 3 trial of pembrolizumab as a first-line treatment in subjects with recurrent/metastatic head and neck squamous cell carcinoma: KEYNOTE-048. Ann Oncol 2015; 26:viii5–viii. [Google Scholar]
- 68.Gillison ML, Blumenschein G, Fayette J et al. Nivolumab (nivo) vs investigator’s choice (IC) for recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CheckMate-141. AACR Annual Meeting; New Orleans, LA, 2016;CT099a. [Google Scholar]
- 69.Harrington KJ, Ferris RL, Blumenschein G Jr et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017; 18:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boeckx C, Baay M, Wouters A et al. Anti-epidermal growth factor receptor therapy in head and neck squamous cell carcinoma: focus on potential molecular mechanisms of drug resistance. Oncologist 2013; 18:850–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferris RL, Blumenschein G, Harrington K et al. Abstract CT021: Tumor-associated immune cell PD-L1 expression and peripheral immune profiling: Analyses from CheckMate 141: AACR, 2017. [Google Scholar]
- 72.Topalian SL, Taube JM, Anders RA et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Felice F, Urbano TG. New drug development in head and neck squamous cell carcinoma: The PI3-K inhibitors. Oral Oncol 2017; 67:119–23. [DOI] [PubMed] [Google Scholar]
- 74.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Squarize CH, Castilho RM, Abrahao AC et al. PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia 2013; 15:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lui VW, Hedberg ML, Li H et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 2013; 3:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seiwert TY, Zuo Z, Keck MK et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res 2015; 21:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wheeler DL, Huang S, Kruser TJ et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene 2008; 27:3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chung CH, Guthrie V, Masica D et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol 2015; 26:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soulieres D, Faivre SJ, Mesia R et al. BERIL-1: A phase II, placebo-controlled study of buparlisib (BKM120) plus paclitaxel in patients with platinum-pretreated recurrent/metastatic head and neck squamous cell carcinoma (HNSCC): American Society of Clinical Oncology, 2016. [Google Scholar]
- 81.Jimeno A, Shirai K, Choi M et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann Oncol 2014; 26:556–61. [DOI] [PubMed] [Google Scholar]
- 82.Jimeno A, Bauman JE, Weissman C et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Oral Oncol 2015; 51:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markham A Copanlisib: First Global Approval. Drugs 2017; 77:2057–62. [DOI] [PubMed] [Google Scholar]
- 84.Zheng Y, Jiang Y. mTOR Inhibitors at a Glance. Mol Cell Pharmacol 2015; 7:15. [PMC free article] [PubMed] [Google Scholar]
- 85.Hudes G, Garducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 86.Kollmannsberger C, Hirte H, Siu L et al. Temsirolimus in combination with carboplatin and paclitaxel in patients with advanced solid tumors: a NCIC-CTG, phase I, open-label dose-escalation study (IND 179). Ann Oncol 2011; 23:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramalingam SS, Harvey RD, Saba N et al. Phase 1 and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer 2010; 116:3903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung CH, Wang H, Tsottles N et al. A phase I study of everolimus in combination with cetuximab and cisplatin as first-line therapy in recurrent and metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): American Society of Clinical Oncology, 2012. [Google Scholar]
- 89.Shirai K, Day TA, Szabo E et al. A pilot, single arm, prospective trial using neoadjuvant rapamycin prior to definitive therapy in head and neck squamous cell carcinoma: American Society of Clinical Oncology, 2015. [Google Scholar]
- 90.Grünwald V, Keilholz U, Boehm A et al. TEMHEAD: a single-arm multicentre phase II study of temsirolimus in platin-and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO). Annal Oncol 2014; 26:561–7. [DOI] [PubMed] [Google Scholar]
- 91.Fury MG, Xiao H, Baxi SS et al. A phase II trial of temsirolimus plus low-dose weekly carboplatin and paclitaxel for recurrent/metastatic HNSCC: American Society of Clinical Oncology, 2014. [Google Scholar]
- 92.Bauman JE, Arias-Pulido H, Lee S-J et al. A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum-refractory head and neck squamous cell carcinoma. Oral Oncol 2013; 49:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Massarelli E, Lin H, Ginsberg L et al. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2015; 26:1476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen E, Hong D, Wise Draper T et al. 1135OPhase 1b/2 Study (SCORES) assessing safety, tolerability, and preliminary anti-tumor activity of durvalumab plus AZD9150 or AZD5069 in patients with advanced solid malignancies and squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol 2017; 28. [Google Scholar]
- 95.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res 2005; 11:1434–40. [DOI] [PubMed] [Google Scholar]
- 96.McGee MC, Hamner JB, Williams RF et al. Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys 2010; 76:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Argiris A, Kotsakis A, Hoang T et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 2012; 24:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fury MG, Lee NY, Sherman E et al. A phase 2 study of bevacizumab with cisplatin plus intensity‐modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer 2012; 118:5008–14. [DOI] [PubMed] [Google Scholar]
- 99.Argiris A, Ghebremichael M, Gilbert J et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. J Clin Oncol 2013; 31:1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 2006; 24:1770–83. [DOI] [PubMed] [Google Scholar]
- 101.Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016; 45:129–38. [DOI] [PubMed] [Google Scholar]
- 102.Asghar U, Witkiewicz AK, Turner NC et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 2015; 14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flaherty KT, LoRusso PM, DeMichele AM et al. Phase 1, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 2011:clincanres. 0509.2011. [DOI] [PubMed] [Google Scholar]
- 104.Patnaik A, Rosen LS, Tolaney SM et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non–small cell lung cancer, and other solid tumors. Cancer Discov 2016. [DOI] [PubMed] [Google Scholar]
- 105.Infante JR, Shapiro G, Witteveen P et al. A phase I study of the single-agent CDK4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas: American Society of Clinical Oncology, 2014. [Google Scholar]
- 106.Shapiro G, Rosen LS, Tolcher AW et al. A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer: American Society of Clinical Oncology, 2013. [Google Scholar]
- 107.Choschzick M, Hess S, Tennstedt P et al. Role of cyclin D1 amplification and expression in vulvar carcinomas. Hum Pathol 2012; 43:1386–93. [DOI] [PubMed] [Google Scholar]
- 108.Michel L, Ley J, Wildes TM et al. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol 2016; 58:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adkins D, Oppelt PJ, Ley JC et al. Multicenter phase II trial of palbociclib, a selective cyclin dependent kinase (CDK) 4/6 inhibitor, and cetuximab in platinum-resistant HPV unrelated (−) recurrent/metastatic head and neck squamous cell carcinoma (RM HNSCC): American Society of Clinical Oncology, 2018. [Google Scholar]
- 110.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23:515–48. [DOI] [PubMed] [Google Scholar]
- 111.Latchman Y, Wood CR, Chernova T et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2:261. [DOI] [PubMed] [Google Scholar]
- 112.Cavalieri S, Rivoltini L, Bergamini C et al. Immuno-oncology in head and neck squamous cell cancers: News from clinical trials, emerging predictive factors and unmet needs. Cancer Treat Rev 2018. [DOI] [PubMed] [Google Scholar]
- 113.Chen DS, Irving BA, Hodi FS. Molecular pathways: Next generation immunotherapy: Inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012:clincanres. 1362.2012. [DOI] [PubMed] [Google Scholar]
- 114.Bahleda R, Braiteh F, Balmanoukian A et al. 1044OLong-Term Safety and Clinical Outcomes of Atezolizumab in Head and Neck Cancer: Phase Ia Trial Results. Ann Oncol 2017; 28. [DOI] [PubMed] [Google Scholar]
- 115.Zandberg D, Algazi A, Jimeno A et al. 1042ODurvalumab for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Preliminary results from a single-arm, phase 2 study. Ann Oncol 2017; 28. [Google Scholar]
- 116.Alfieri S, Cavalieri S, Licitra L. Immunotherapy for recurrent/metastatic head and neck cancer. Curr Opin Otolaryngol Head Neck Surg 2018; 26:152–6. [DOI] [PubMed] [Google Scholar]
- 117.Larkin J, Chiarion-Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer–review of current data. Oral Oncol 2015; 51:12–5. [DOI] [PubMed] [Google Scholar]
- 119.Aspeslagh S, Postel-Vinay S, Rusakiewicz S et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016; 52:50–66. [DOI] [PubMed] [Google Scholar]
- 120.Dogan V, Rieckmann T, Munscher A et al. Current studies of immunotherapy in head and neck cancer. Clin Otolaryngol 2018; 43:13–21. [DOI] [PubMed] [Google Scholar]
- 121.Curti BD, Kovacsovics-Bankowski M, Morris N et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013; 73:7189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schaer DA, Budhu S, Liu C et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T-cell lineage stability. Cancer Immunol Res 2013; 1:320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fisher TS, Kamperschroer C, Oliphant T et al. Targeting of 4–1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother 2012; 61:1721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy: AACR, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14:561. [DOI] [PubMed] [Google Scholar]
- 126.Hamid O, Bauer TM, Spira AI et al. Epacadostat plus pembrolizumab in patients with SCCHN: Preliminary phase I/II results from ECHO-202/KEYNOTE-037: American Society of Clinical Oncology, 2017. [Google Scholar]
- 127.Chow LQ, Morishima C, Eaton KD et al. Phase Ib trial of the toll-like receptor 8 agonist, motolimod (VTX-2337), combined with cetuximab in patients with recurrent or metastatic SCCHN. Clin Cancer Res 2017; 23:2442–50. [DOI] [PubMed] [Google Scholar]
- 128.Glisson B, Massarelli E, William W et al. 1136ONivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol 2017; 28. [Google Scholar]
- 129.Rabinowits G, Haddad RI. Overcoming resistance to EGFR inhibitor in head and neck cancer: a review of the literature. Oral Oncol 2012; 48:1085–9. [DOI] [PubMed] [Google Scholar]
- 130.Yonesaka K, Zejnullahu K, Okamoto I et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011; 3:99ra86–99ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res 2009; 15:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Timpson P, Wilson AS, Lehrbach GM et al. Aberrant expression of cortactin in head and neck squamous cell carcinoma cells is associated with enhanced cell proliferation and resistance to the epidermal growth factor receptor inhibitor gefitinib. Cancer Res 2007; 67:9304–14. [DOI] [PubMed] [Google Scholar]
- 133.Fernandez-Mateos J, Seijas-Tamayo R, Mesia R et al. Epidermal growth factor receptor (EGFR) pathway polymorphisms as predictive markers of cetuximab toxicity in locally advanced head and neck squamous cell carcinoma (HNSCC) in a Spanish population. Oral Oncol 2016; 63:38–43. [DOI] [PubMed] [Google Scholar]
- 134.Zhang X, Fan J, Li Y et al. Polymorphisms in epidermal growth factor receptor (EGFR) and AKT1 as possible predictors of clinical outcome in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Tumour Biol 2016; 37:1061–9. [DOI] [PubMed] [Google Scholar]
- 135.Rebucci M, Peixoto P, Dewitte A et al. Mechanisms underlying resistance to cetuximab in the HNSCC cell line: role of AKT inhibition in bypassing this resistance. Int J Oncol 2011; 38:189–200. [PubMed] [Google Scholar]
- 136.Licitra L, Storkel S, Kerr KM et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer 2013; 49:1161–8. [DOI] [PubMed] [Google Scholar]
- 137.Jhaveri K, Juric D, Saura C et al. Abstract CT046: A phase I basket study of the PI3K inhibitor taselisib (GDC-0032) in PIK3CA-mutated locally advanced or metastatic solid tumors: AACR, 2018. [Google Scholar]
- 138.Zou W Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263. [DOI] [PubMed] [Google Scholar]
- 139.Keck MK, Zuo Z, Khattri A et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res 2015; 21:870–81. [DOI] [PubMed] [Google Scholar]
- 140.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davis RJ, Van Waes C, Allen CT. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol 2016; 58:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodriguez PC, Hernandez CP, Quiceno D et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 2005; 202:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garrity T, Pandit R, Wright MA et al. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer 1997; 73:663–9. [DOI] [PubMed] [Google Scholar]
- 144.Lee YS, Park JY, Cho KJ et al. Composition of inflammatory cells regulating the response to concurrent chemoradiation therapy for HPV (+) tonsil cancer. Oral Oncol 2015; 51:1113–9. [DOI] [PubMed] [Google Scholar]
- 145.Chanmee T, Ontong P, Konno K et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014; 6:1670–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mok S, Koya RC, Tsui C et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res 2014; 74:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu C, Workman CJ, Vignali DA. Targeting regulatory T cells in tumors. The FEBS journal 2016; 283:2731–48. [DOI] [PubMed] [Google Scholar]
- 148.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res 2017; 27:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Selby MJ, Engelhardt JJ, Quigley M et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013; 1:32–42. [DOI] [PubMed] [Google Scholar]
- 150.Sugiyama D, Nishikawa H, Maeda Y et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+ CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 2013; 110:17945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park B, Yee C, Lee K-M. The effect of radiation on the immune response to cancers. International J Mol Sci 2014; 15:927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Uppaluri R, Zolkind P, Lin T et al. Neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV negative head and neck squamous cell carcinoma (HNSCC): American Society of Clinical Oncology, 2017. [Google Scholar]
- 153.Ferris R, Gonçalves A, Baxi S et al. LBA46An open-label, multicohort, phase 1/2 study in patients with virus-associated cancers (CheckMate 358): Safety and efficacy of neoadjuvant nivolumab in squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol 2017; 28. [Google Scholar]