Abstract
Background:
Although several studies linked adolescent cannabis use to long-term cognitive dysfunction, there are negative reports too. The fact that not all users develop cognitive impairment suggests a genetic vulnerability to adverse effects of cannabis, which are attributed to action of delta-9-tetrahydrocannabinol (Δ9-THC), a cannabis constituent and partial agonist of brain cannabinoid receptor 1 (CNR1). As both neurons and glial cells express CNR1, genetic vulnerability could influence Δ9-THC-induced signaling in a cell type-specific manner.
Methods:
Here we use an animal model of inducible expression of dominant-negative Disrupted-In-Schizophrenia-1 (DN-DISC1) selectively in astrocytes to evaluate the molecular mechanisms whereby an astrocyte genetic vulnerability could interact with adolescent Δ9-THC exposure to impair recognition memory in adulthood.
Results:
Selective expression of DN-DISC1 in astrocytes and adolescent treatment with Δ9-THC synergistically affected recognition memory in adult mice. Similar deficits in recognition memory were observed following knockdown of endogenous Disc1 in hippocampal astrocytes in mice treated with Δ9-THC during adolescence. At the molecular level, DN-DISC1 and Δ9-THC synergistically activated the NF-kB-COX-2 pathway in astrocytes and decreased immunoreactivity of parvalbumin-positive pre-synaptic inhibitory boutons around pyramidal neurons of the hippocampal CA3 area. The cognitive abnormalities were prevented in DN-DISC1 mice exposed to Δ9-THC by simultaneous adolescent treatment with the COX-2 inhibitor, NS389.
Conclusions:
Our data demonstrate that individual vulnerability to cannabis can be exclusively mediated by astrocytes. Results of this work suggest that genetic predisposition within astrocytes can exaggerate Δ9-THC-produced cognitive impairments via convergent inflammatory signaling, suggesting possible targets for preventing adverse effects of cannabis within susceptible individuals.
Keywords: cannabis, astrocytes, adolescence, cognitive dysfunction, hippocampus, gene-environment interaction
One sentence summary:
Adolescent GxE in astrocytes impairs adulthood memory
Introduction
Cannabis is the most commonly used illicit drug of abuse in the United States (1). Although several studies have reported no long-term cognitive impairments after cannabis use, chronic cannabis exposure during adolescence has been associated with persistent deficits in some cognitive domains, including attention, memory, and processing speed (2–4). Cannabis sativa plant includes more than 400 different chemical constituents, of which about 70 are cannabinoids (5). Cannabis-induced adverse effects are mediated by delta-9-tetrahydrocannabinol (Δ9-THC), the principal psychoactive constituent of cannabis and a partial agonist of brain cannabinoid receptors (CNR1) (6). However, the mechanisms underlying Δ9-THC-induced long-lasting behavioral and cognitive abnormalities remain unknown.
Although neurons highly express CNR1, the role of glial CNR1 is being increasingly appreciated (7, 8). Two recent studies have shown that detrimental effects of Δ9-THC on learning and memory in mice are mediated by astrocyte CNR1 (9, 10), activation of NF-κB signaling and up-regulation of cyclooxygenase-2 (COX-2) that might lead to excessive glutamate release by astrocytes (11).
Notably, not all cannabis users demonstrate cognitive impairment suggesting a genetic vulnerability to adverse effects of cannabis (12–14). Similarly, preclinical studies have reported that mice carrying mutations in candidate genes for psychiatric disorders exhibit greater responses to adverse effects of Δ9-THC on memory (15–19). However, the underlying molecular mechanisms of how genetic mutations could moderate cognitive effects of cannabis remain unknown. Further, although astrocytes appear to play a major role in mediating effects of Δ9-THC on memory (9, 10), the molecular underpinning of how genetic risk factors could interact with Δ9-THC in a cell type specific manner to impair cognitive abilities have never been studied. In order to address these questions, we used an animal model of selective astrocyte expression of a rare, highly penetrant mutation, a dominant-negative form of Disrupted in Schizophrenia 1 (DISC1) (18, 20–28).
DISC1 is a gene disrupted by the balanced (1;11) (q42.1; q14.3) translocation, segregating in a Scottish family with several major psychiatric disorders (29, 30). Although the DISC1 locus has not been reported in recent genome-wide association studies (GWAS)(31), rare mutations of large effects contribute to behavioral and cognitive abnormalities (32) and have important roles in mechanistic studies (33, 34). It is in this context that we use a C-terminus truncated form of full-length DISC1 as a dominant-negative molecular tool (DN-DISC1). In this study, we sought to determine the molecular basis of gene-environment interaction (GxE) in astrocytes and elucidate how GxE interplay could shape up individual vulnerability to adverse cognitive effects of cannabis on cognitive abilities.
Materials and Methods
Animals
In order to evaluate the cell-specific role of astrocytes in gene environment interaction (GxE), mice expressing dominant negative form of DISC1 (DN-DISC1) in astrocytes were exposed to chronic Δ9-THC treatment (8 mg/kg, sc, daily) for three weeks from postnatal day (P) P30 and on. 21 days later, mice were assessed in a series of behavioral tests. All procedures were approved by the JHU ACUC.
Behavioral tests
The following tests were used: open field, spontaneous alternation, spatial recognition in Y maze, novel object recognition test, novel place recognition test and fear conditioning as previously described (24, 35–37).
AAV injections
AAV-Gfa-mir30-Disc1-EGFP or AAV-Gfa mir30-control-EGFP were injected in the CA2-CA3 areas of the hippocampus at P15–17.
Isolation of RNA and RNAseq analyses
Total RNA was purified from mouse hippocampus upon completion of Δ9-THC. RNAseq analysis was done as described in supplemental 1.
Biochemistry
Expression of Phospho-p65, Phospho-IκBα and COX-2 was assessed with standard Western blotting in primary astrocytes or hippocampal tissue samples. DISC1-IκBα binding was analyzed with co-immunoprecipitation as previously described (38).
Immunohistochemistry
GAD67-positive (GAD67+) presynaptic boutons within parvalbumin-positive (PV+) branches were evaluated on the surface of pyramidal neurons of the CA1 and CA3 areas of hippocampus.
Pharmacological treatment with the COX-2 inhibitor
Effects of COX-2 inhibition on cognitive deficits were assessed with the COX-2 inhibitor, NS398, (10mg/kg, daily sc).
Measurement of glutamate in hippocampal tissue and in culture medium
Glutamate concentration in the hippocampus or primary astrocyte culture were assayed using Kusakabe’s method (39) or glutamate assay, respectively.
For detailed information, please see Supplemental 1.
Results
Astrocyte DN-DISC1 and adolescent Δ9-THC impair memory in adult mice
Based on our prior studies (26, 28), we hypothesized that expression of DN-DISC1 in astrocytes (aDN-DISC1) would synergistically interact with adolescent Δ9-THC exposure to affect learning and memory in adult mice. In order to test this hypothesis, we treated control or aDN-DISC1 male and female mice with single daily injections of Δ9-THC (8mg/kg; SC) starting at postnatal day 30 (P30) for three weeks (14) to span mouse adolescence (P30–51) that corresponds to human adolescence from 12 to 19 years of age (40–43). Upon completion of treatment, the mice were left undisturbed for another three weeks before behavioral testing was commenced (Fig.1A).
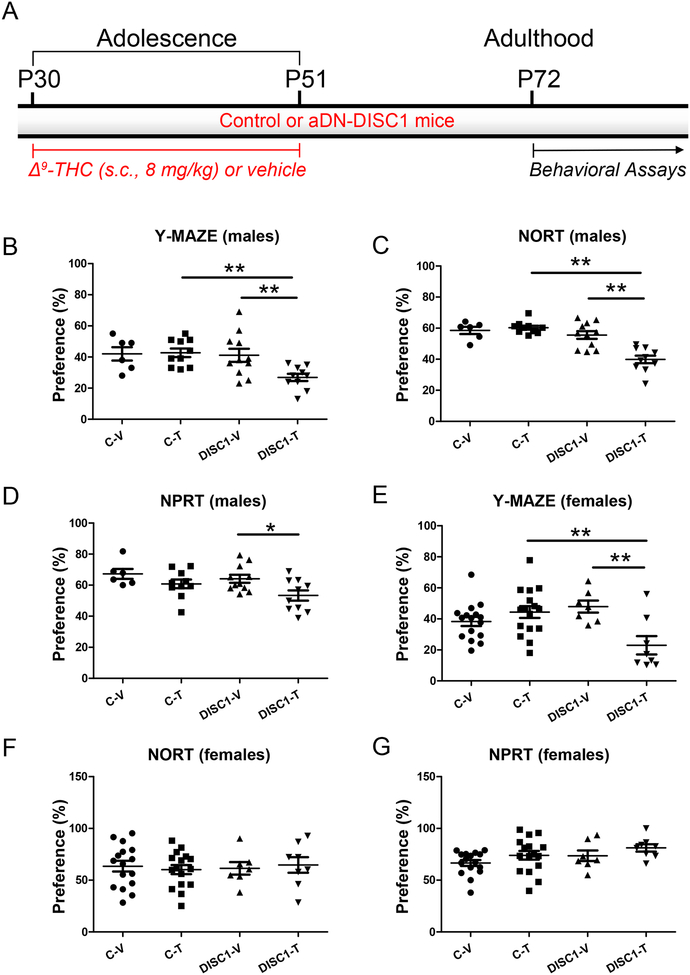
Figure 1. Cognitive impairments in astrocyte DN-DISC1 mice.
A - Schematic diagram of the treatment protocol
In all graphs the Y-axes depict the preference (%); the X-axes depict the experimental groups: C-V – control mice treated with the vehicle (6 males and 16 females); C-T – control mice treated with Δ9-THC (10 males and 16 females); DISC1-V – aDN-DISC1 mice treated with vehicle (11 males and 7 females); DISC1-T - aDN-DISC1 mice treated with Δ9-THC (10 males and 8 females)
Male mice
B- Spatial recognition memory in the Y maze. Compared to other groups, aDN-DISC1 male mice treated with Δ9-THC exhibited the significantly decreased preference for the previously blocked arm. Two-way ANOVA of the preference data revealed a significant effect of DN-DISC1, F(1,33)=5.49, p=0.025 and the significant aDN-DISC1 by Δ9-THC interaction, F(1,33)=4.46, p = 0.045; Fisher LSD post-hoc test showed that aDN-DISC1-Δ9-THC mice were significantly different from both C-T and DISC1-V mice, ** p<0.01.
C- Novel object recognition test (NORT). Compared to other groups, aDN-DISC1 male mice treated with Δ9-THC exhibited the significantly decreased preference for the novel object. Two-way ANOVA of the preference data revealed a significant effect of DN-DISC1, F(1,33)=26.12, p<0.001; a significant effect of Δ9-THC, F(1,33)=9.26, p=0.005 and the significant aDN-DISC1 by Δ9-THC interaction, F(1,33)=14.48, p<0.001; Fisher LSD post-hoc test showed that DN-DISC1- Δ9-THC mice were different from both C-T and DISC1-V mice, ** p<0.01.
D- Novel place recognition test (NPRT). Compared to other groups, aDN-DISC1 male mice treated with Δ9-THC exhibited the significantly decreased preference for the novel place of one of two identical objects. Two-way ANOVA of the preference data revealed a significant effect of Δ9-THC, F(1,33)=7.89, p=0.008. Planned post-hoc tests showed that significantly decreased preference in aDN-DISC1 mice treated with Δ9-THC compared to vehicle-treated aDN-DISC1 mice (DISC1-V) but there was no difference in the preference between Δ9-THC –treated- aDN-DISC1 (DISC1-T) and control (C-T) mice (p=0.074). * p<0.05.
Female mice
E- Spatial recognition memory in the Y maze. Compared to other groups, aDN-DISC1 female mice treated with Δ9-THC exhibited the significantly decreased preference for the previously blocked arm. Two-way ANOVA of the preference data revealed a significant effect of Δ9-THC, F(1,43)=4.74, p=0.035 and the significant aDN-DISC1 by Δ9-THC interaction, F(1,43)=12.88, p<0.001; Fisher LSD post-hoc test showed that aDN-DISC1-Δ9-THC (DISC1-T) mice were different from vehicle-treated - aDN-DISC1-vehicle (DISC1-V) mice and Δ9-THC -treated control (C-T) mice, ** p<0.01.
F- NORT. No group differences were found.
G- NPRT. No group differences were found.
For male mice, compared to other groups, aDN-DISC1 mice treated with Δ9-THC exhibited synergistically impaired performance in the spatial recognition test in the Y maze (the significant aDN-DISC1 by Δ9-THC interaction, F(1,33)=4.46, p = 0.045) and the novel object recognition test (NORT) (the significant aDN-DISC1 by Δ9-THC interaction, F(1,33)=14.48, p<0.001), as well as significantly worse performance in the novel place recognition test (NPRT) compared to aDN-DISC1 mice treated with vehicle, p<0.05 (Fig.1B–D). For female mice, the aDN-DISC1-Δ9THC combination produced synergistic impairment in the spatial recognition test in the Y-maze only (the significant aDN-DISC1 by Δ9-THC interaction, F(1,43)=12.88, p<0.001) (Fig.1E–G). Memory deficits were not associated with group differences in general exploratory activity during the training phase for the above tests, distance traveled in the Y maze, or novelty-induced activity. No effects of Δ9-THC were found in the forced swim test, or context- or cue-dependent delay fear conditioning either (Fig.S1–S3 in Supplement 1). Thus, our results suggest that adolescent treatment with Δ9-THC and expression of DN-DISC1 in astrocytes synergistically affect recognition memory in adult mice. As the most robust behavioral effects were found in aDN-DISC1 male mice, we focused on male mice in all subsequent tests.
In order to examine whether the above synergistic effects were dependent on adolescent Δ9-THC exposure (39), adult control and aDN-DISC1 mice were treated using the same protocol. We found no significant effects of adult Δ9-THC exposure on recognition memory in aDN-DISC1 mice (Fig.S4 in Supplement 1).
Although expression of aDN-DISC1 in the brain reaches the maximum by late adolescence, there is expression of aDN-DSC1 during late gestation and early postnatal period (28) that coincides with astrocyte proliferation and maturation (44, 45). In order to evaluate a possible contribution of early expression of aDN-DISC1 to the cognitive phenotypes, we turned off expression of aDN-DISC1 using doxycycline-containing food beginning at P21 and on (Fig.S5A in Supplement 1). We observed no significant cognitive effects in any group (Fig.S5BD in Supplement 1, suggesting that developmental expression of aDN-DISC1 unlikely contributed to the cognitive abnormalities.
We next wondered whether a different psychoactive compound could also interact with aDN-DISC1 to impair recognition memory (46, 47). In order to evaluate this possibility, control and aDN-DISC1 adolescent male mice were treated with amphetamine (1 mg/kg, ip). No significant changes in the same memory tests were found in either group (Fig.S6 in Supplement 1).
We also evaluated whether expression of DN-DISC1 in neurons could also lead to the cognitive deficits after adolescent Δ9-THC treatment. We generated mice with neuronal expression of DN-DISC1 (nDN-DISC1) by crossing TRE-DN-DISC1 mice with CAMKII-tTA mice to express DN-DISC1 in forebrain neurons (23). Control and nDN-DISC1 male mice were treated with the same treatment prFig.S7 in Supplement 1), suggesting a cell type specific GxE to impair recognition memory.
The hippocampus is sufficient for mediating the major effects of interaction
The hippocampus plays the critical role in spatial recognition memory (48–50). The cognitive effects of exogenous cannabinoids have been linked to adverse effects on hippocampal neuronal circuits (51–54). In addition, we previously reported strong expression of astrocyte DN-DISC1 in the hippocampus compared to the frontal cortex (28). Thus, we evaluated the contribution of hippocampal aDN-DISC1 to memory deficits observed in Δ9-THC-treated mice. In order to address this question and alter expression of DISC1 in astrocytes with a different genetic tool, we engineered an adeno-associated viral (AAV) vector to knockdown (KD) endogenous Disc1 selectively in astrocytes, Gfa-GFP-mir30-Disc1, or control (scrambled) vector, Gfa-GFP-mir30-Ctrl (Fig.S8 in Supplement 1). Gfa-GFP-mir30-Disc1 AAV decreased expression of Disc1 in vivo (Fig.S8A) and transduced astrocytes only (Fig.S8B) (see Supplement 1).
Since prior studies have suggested that Δ9-THC exposure may affect CA1-CA3 circuits (9, 55, 56), control and mir30-Disc1 AAV were injected in the CA2-CA3 areas of the hippocampus at P16 to let the effects of KD take place by P30 when Δ9-THC treatment was commenced. Three weeks later behavioral testing was initiated (Fig.2A). Consistent with our earlier findings (Fig.1), compared to the Gfa-GFP-mir30-Ctrl AAV, Gfa-GFP-mir30-Disc1 AAV synergistically impaired spatial memory in the Y-maze test (the significant Disc1-KD by Δ9-THC interaction, F(1,22)=13.90, p=0.001), NORT (the significant the Disc1-KD by Δ9-THC interaction, F(1,22)=4.35, p=0.05) but not NPRT in mice treated with Δ9-THC during adolescence (Fig.2B–D). These effects were unlikely related to non-specific changes in locomotion or exploratory activity (Fig.S9 in Supplement 1). Upon completion of behavioral tests (P90), we confirmed that AAV transduction was present in astrocytes of the CA2-CA3 areas (Fig.S10 in Supplement 1). Thus, expression of aDN-DISC1 or Disc1 KD in hippocampal astrocytes synergistically exacerbated the adverse cognitive effects of adolescent Δ9-THC in adult mice.
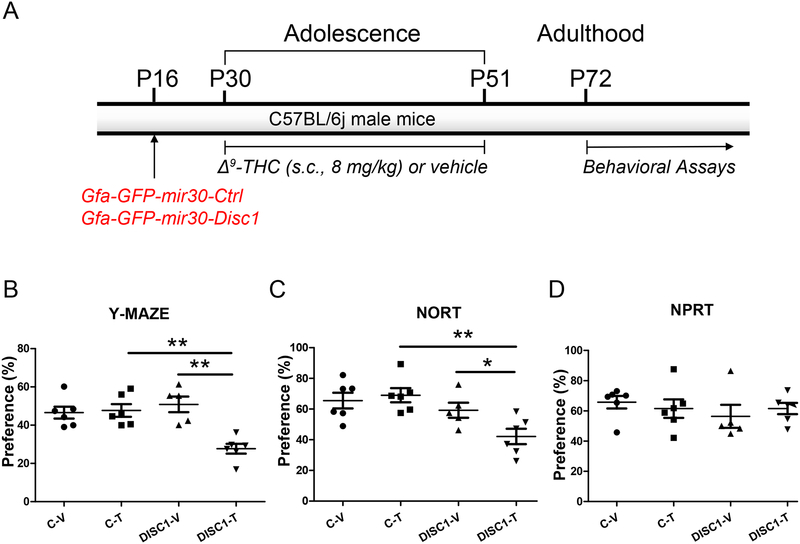
Figure 2. Disc1 KD in hippocampal astrocytes.
A- Schematic diagram of the AAV vector injections and treatment protocol;
On all data panels: the Y-axes depict the preference (%); the X-axes depict the experimental groups: C-V – mice injected with Gfa-GFP-mir30-Ctrl AAV and treated with the vehicle (N=6); C-T - mice injected with Gfa-GFP-mir30-Ctrl AAV and treated with Δ9-THC (N=6); DISC1-V – mice injected with Gfa-GFP-mir30-Disc1 AAV and treated with vehicle (N=5); DISC1-T - mice injected with Gfa-GFP-mir30-Disc1 AAV and treated with Δ9-THC (N=6).
B- Spatial recognition memory in the Y maze. Compared to other groups, Disc1 AAV mice treated with Δ9-THC exhibited the significantly decreased preference for the previously blocked arm. Two-way ANOVA of the preference data revealed a significant effect of Disc1-KD, F(1,22)=5.77, p=0.027; a significant effect of Δ9-THC, F(1,22)=11.43, p=0.003, and the significant Disc1-KD by Δ9-THC interaction, F(1,22)=13.90, p=0.001. Fisher LSD post-hoc test showed that Disc1-KD- Δ9-THC mice were different from Δ9-THC-treated control mice (p<0.001) and Disc1-KD vehicle-treated mice (p<0.001); ** p<0.001.
C- Novel object recognition test (NORT). Compared to other groups, Disc1 AAV mice treated with Δ9-THC exhibited the significantly decreased preference for the novel object. Two-way ANOVA of the preference data revealed a significant effect of Disc1-KD, F(1,22)=11.20, p=0.003; and the borderline significance for the Δ9-THC by Disc1-KD, F(1,22)=4.35, p=0.051; Fisher LSD post-hoc test showed that Disc1-KD-THC mice were different from Δ9-THC-treated control mice (p<0.001) and Disc1-KD vehicle-treated mice (p=0.027); **p<0.01, * p<0.05.
D - Novel place recognition test (NPRT). No significant effects of Disc1-KD were found in NPRT.
Δ9-THC activates pro-inflammatory signaling in DN-DISC1 astrocytes
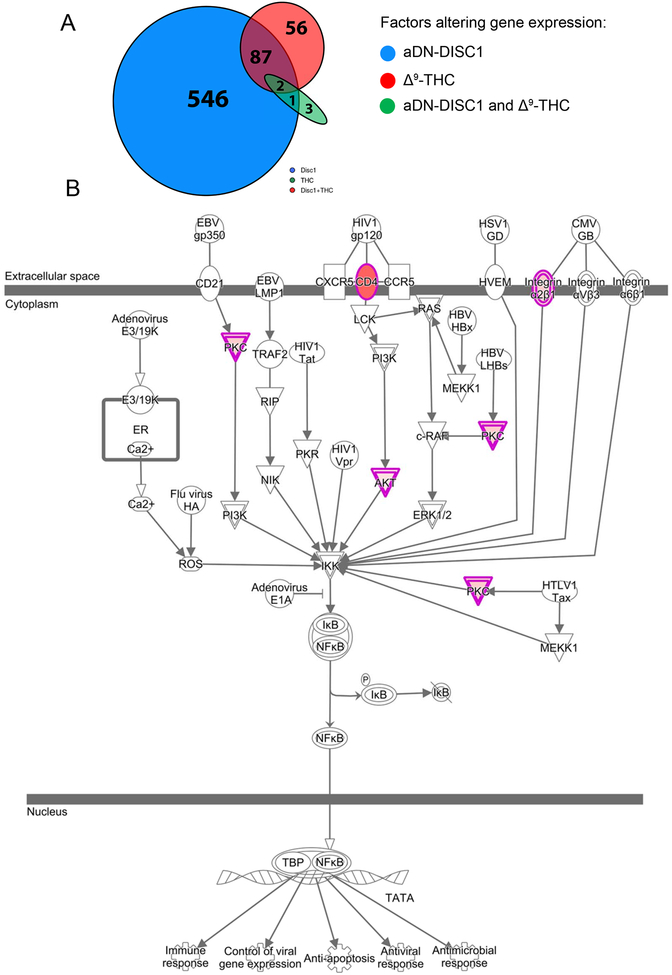
In order to gain an unbiased insight in the mechanisms of GxE, we performed RNA-seq analyses of hippocampal tissue samples derived from control and aDN-DISC1 mice treated with vehicle or Δ9-THC as above. Our analyses focused on the 56 genes differentially expressed in both the aDN-DISC1 and Δ9-THC conditions, but not in either condition alone (Fig.3A). This analysis revealed a significant up-regulation of genes involved in the inflammatory pathways, including NF-kB signaling (Fig.3B). Full lists of differentially expressed genes and pathways are presented in Tables S2 and S3 (in Supplement 2), respectively (the NCBI accession number is GSE116813). Together with prior studies demonstrating that both Δ9-THC and DISC1 influence NF-kB signaling (10), our results suggest that this inflammatory pathway may be a convergent target of DN-DISC1 and Δ9-THC in astrocytes.
Figure 3. RNA-seq identifies synergistic (GxE) genes, which are enriched in NF-kB signaling pathway.
A – In aDN-Disc1 mice exposed to Δ9-THC, there were 145 differentially expressed genes (FDR < 0.20; see circle at upper right). Over a third of the 145 genes (56, 38.6%; see red shaded portion of circle) were synergistic (GxE) genes since they were not found to be differentially expressed by aDN-Disc1 or THC alone. Two of the genes (Ddit4, Sgk1) were also differentially expressed by aDN-Disc1 or THC alone, and 87 genes were differentially expressed by aDN-Disc1 alone. The oval represents six genes differentially expressed after THC treatment: three were differentially expressed in wild type mice, and three in the aDNn-Disc1 mice of which two were in aDNA-Disc1 mice treated with THC.
B – GxE genes are enriched for membership in the NF-kB activation by viruses pathway (z-score = 2.00; p = 1.74E-03; BH-adjusted p-value [FDR] = 0.0253, 4 genes; all up-regulated: Akt2, Cd4, Itga5, and Prkch).
DISC1 regulates activation of NF-kB-COX-2 signaling in astrocytes
We focused on evaluation of expression of Ptgs2 (a.k.a. Cox-2) that encodes for a constitutively expressed and inducible enzyme, cyclooxygenase-2 (COX-2), that converts arachidonic acid to prostaglandins (57, 58). We chose to assess altered expression of Ptgs2 in our GxE model because Δ9-THC induces a robust increase in activity and expression of COX-2 in astrocytes and mediates synaptic and cognitive effects of Δ9-THC (10, 59). Since tissue astrocytes and cultured cells exhibit significant transcriptome differences (60), we assessed Ptgs2 expression in astrocytes acutely isolated from the hippocampus followed by Δ9-THC treatment. We found a synergistic up-regulation of Ptgs2 in astrocytes of aDN-DISC1 mice treated with Δ9-THC compared to other groups (the significant DN-DISC1 × Δ9-THC treatment interaction, F(1,8)=87.43, p=0.001) (Fig.4A).
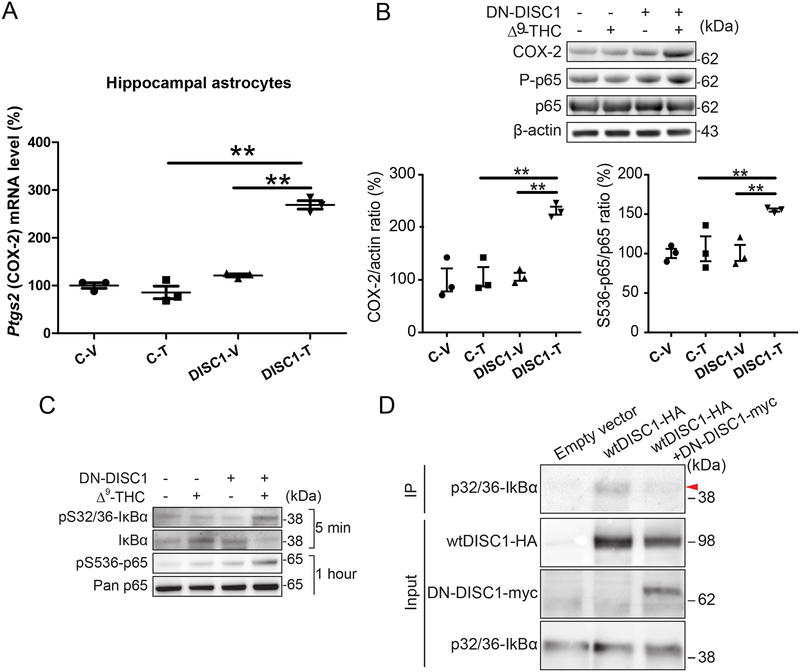
Figure 4. Synergistic effects of DN-DISC1 and Δ9-THC on the NF-kB- COX-2 signaling in astrocytes.
A - DISC1 and Δ9-THC synergistically increased Ptgs2 (gene encoding for COX2 protein) expression in hippocamplal tissue astrocytes. The graph plots the individual data points and superimposes the mean and error bars. Each point represents an independent sample from a single animal assayed in triplicates. Two-way ANOVA revealed the significant effects of DN-DISC1 (F(1,8)=139.52, p<0.001), Δ9-THC (F(1,8)=59.15, p<0.001) and significant DN-DISC1 × Δ9-THC treatment interaction, F(1,8)=87.43, p=0.001. Fisher LSD post-hoc analysis showed that Ptgs2 expression in the DN-DISC1-Δ9-THC group was significantly greater compared to DN-DISC1-vehicle (DISC1-T vs. DISC1-V; p<0.001) or control- Δ9-THC group (DISC1-T vs. C-T; p<0.001); ** - denotes p<0.01.
B – Representative Western blot images and densitometric analysis showing that DISC1 and Δ9-THC treatment synergistically increased expression of COX-2 protein and phosphorylation of p65 (S536-p65/p65) in hippocampal astrocytes. Two-way ANOVA revealed the significant effects on COX-2 protein level (main effects: DISC1 (F(1,8)=18.76, p=0.003, Δ9-THC F(1,8)=18.79, p=0.002 and the significant DN-DISC1 × Δ9-THC interaction F(1,8) =15.52, p=0.004) and on phosphorylation of NF-κB p65 (main effects: DISC1 (F(1,8)=9.29, p=0.016, Δ9-THC F(1,8)=6.32, p=0.036 and the significant DN-DISC1 × Δ9-THC interaction: F(1,8)=6.03, p=0.040. Fisher LSD post-hoc analysis showed that protein level of COX-2 (protein encoded by Ptgs2 gene) in DN-DISC1-Δ9-THC group was significantly greater compared to control-Δ9-THC (DISC1-T vs. C-T; p<0.001) and DN-DISC1-Vehicle group (DISC1-T vs. DISC1-V; p<0.001). Fisher LSD post-hoc analysis showed protein level of Phospho-NF-κB p65 in DN-DISC1-Δ9-THC group was significantly greater compared to control-Δ9-THC (DISC1-T vs. C-T; p=0.005) or DN-DISC1-Vehicle group (DISC1-T vs. DISC1-V; p=0.008). No other significant differences were detected in expression of COX-2 or Phospho-NF-κB p65. Data are presented as the mean ± SEM; n=3 independent samples in each group; ** - denotes p<0.01.
C – Δ9-THC treatment of primary DN-DISC1 astrocytes up-regulated phosphorylation of IκBα and decreased IκBα expression (5 minutes interval) and increased phosphorylation of NF-κB p65 (one hour interval).
D- Protein interaction of DISC1 with phospho-IκBα (p32/36-IkBα) was assessed by coimmunoprecipitation (IP) in HEK293 cells. HA-tagged wild-type DISC1 interacts with endogenous phospho-IκBα. Overexpression of myc-tagged DN-DISC1 reduced wild-type DISC1- phospho-IkBα interaction. Input for each protein is presneted.
We then assessed the protein levels of COX-2 and phospho-NF-κB p65 in astrocytes isolated from the hippocampus one day after in vivo Δ9-THC or vehicle treatment. Consistent with mRNA data, we found a significant and synergistic increase in COX-2 level (the significant DN-DISC1 × Δ9-THC interaction, F(1,8) =15.52, p=0.004) and phosphorylation of NF-κB p65 compared to other groups (the significant DN-DISC1 × Δ9-THC interaction, F(1,8)=6.03, p=0.040) (Fig.4B).
To further evaluate activation of NF-κB p65 signaling in astrocyte, we measured the phosphorylation levels of NF-κB p65 and IκBα, an upstream signaling protein of NF-κB p65, in primary DN-DISC1 or control astrocytes treated with Δ9-THC (5 M for 5 min or 1 hour) or vehicle. Consistent with in vivo data, DN-DISC1 astrocytes treated with Δ9-THC had synergistically increased phosphorylation of NF-κB p65 (Δ9-THC treatment for an hour) and enhanced phosphorylation of IκBα (Δ9-THC treatment for 5 min), compared with other conditions (Fig.4C).
Given that phosphorylation of IκBα leads to its dissociation from p65 and degradation, an event that requires prior to nuclear translocation of the liberated p65 (61, 62), DISC1 may interact with phospho-IκBα for stabilization of cytoplasmic IκBα:p65 complex. Indeed, coimmunoprecipitation experiments confirmed protein interaction between wild-type DISC1 and phospho-IκBα. Importantly, this interaction was disrupted by over-expression of DN-DISC1 (Fig. 4D). Our results suggest that binding of DISC1 to phospho-IκBα may stabilize IκBα activity, supporting inhibitory action of IκBα on NF-kB. Expression of DN-DISC1 in astrocytes leads to decreased levels of endogenous DISC1 (26) that may facilitate degradation of phosphorylated-IκBα and activation of NF-kB as a result of its release from binding to IκBα.
As up-regulation of COX-2 could increase glutamate secretion by astrocytes (11), we examined the effects of Δ9-THC on levels of glutamate in the hippocampus and culture medium collected from primary astrocytes. Compared to other groups, there was a significant increase in glutamate levels in the hippocampus of aDN-DISC1 mice treated with Δ9-THC mice (p<0.05) (Fig.S11A in Supplement 1). In addition, we found a synergistically increased secretion of glutamate by primary DN-DISC1 astrocytes following stimulation of Δ9-THC (the significant aDN-DISC1 by Δ9-THC interaction, F(1,18)=6.74, p=0.02) (Fig.S11B in Supplement 1). Collectively, these results suggest that DN-DISC1 and Δ9-THC interact to activate the pro-inflammatory NF-kB-COX-2 signaling and increase secretion of glutamate by astrocytes.
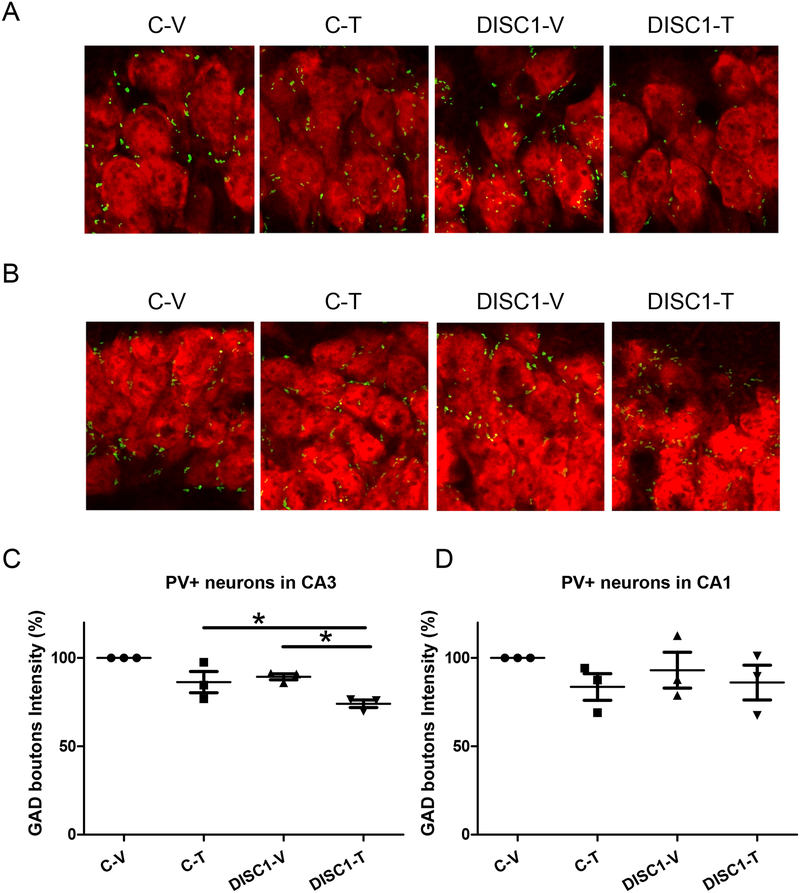
Decreased GAD+PV+ immunoreactivity in the CA3 area of the hippocampus
Chronic exposure to Δ9-THC during adolescence was associated with decreased GAD67 expression in parvalbumin-positive (PV+) interneurons (63–67). Given enhanced vulnerability of GABA+PV+ neurons to adverse effects of chronic excitotoxicity that may be a result of increased secretion of glutamate by DN-DISC1 astrocytes (68–71), we assessed the integrity of pre-synaptic GAD+PV+ boutons in control and aDN-DISC1 mice. Since our AAV results strongly suggested that the CA areas of the hippocampus could be critically involved in producing deficient recognition memory, we focused on examination of pre-synaptic GAD+PV+ boutons in these areas (Fig.S12 in Supplement 1). Compared to aDN-DISC1 mice treated with vehicle or control mice treated with Δ9-THC, we found significantly decreased intensity of GAD+PV+ boutons on the surface of pyramidal neurons of the CA3 but not CA1 area in aDN-DISC1 mice treated with Δ9-THC (p<0.05) (Fig.5). No significant changes of GAD+PV+ boutons size or density were found (Fig.S13 in Supplement 1). Reduced immunoreactivity of GAD+PV+ boutons was not associated with a general decrease in GAD+ immunoreactivity (Fig.S14 in Supplement 1), suggesting PV+ GABA neurons could be selectively and synergistically affected in aDN-DISC1 mice treated with Δ9-THC during adolescence.
Figure 5. Astrocyte DN-DISC1 and Δ9-THC treatment synergistically decrease GAD+PV+ immunoreactivity in the CA3 area of the hippocampus.
On all data panels: the Y-axes depict the percentage of GAD boutons intensity in CA3 or CA1 PV neurons in relation to the level for the control-vehicle group; the X-axes depict the experimental groups (C-V – control mice treated with the vehicle; C-T – control mice treated with Δ9-THC; DISC1-V – aDN-DISC1 mice treated with vehicle; DISC1-T - aDN-DISC1 mice treated with Δ9-THC).
A- Representative images of GAD boutons intensity in pyramidal neurons of the CA3 area of the hippocampus; note decreased intensity in the DISC1+T group
B- Representative images of GAD boutons intensity in pyramidal neurons of the CA1 area of the hippocampus; note comparable intensity in all groups
C- Quantitative analyses of the intensity of GAD+PV+ presynaptic boutons around pyramidal neurons of the CA3 area of the hippocampus; n=3 sections per mouse, 3 mice per group; each data point represents one mouse. Two-way ANOVA of the intensity data revealed a significant effect of the group, F(1,8)=12.06, p=0.008 and significant effect of the Δ9-THC, F(1,8)=19.16, p=0.002; Fisher LSD post-hoc test showed that aDN-DISC1-Δ9-THC mice were significantly different from both C-T and DISC1-V mice, * p<0.05.
D- Quantitative analyses of the intensity of GAD+PV+ presynaptic boutons around pyramidal neurons of the CA1 area of the hippocampus. Two-way ANOVA of the intensity data revealed no significant effects of the DN-DISC1, F(1,8)=0.075, p=0.791, no significant effects of Δ9-THC, F(1,8)=2.129, p=0.183 and no DN-DISC1 by Δ9-THC treatment interaction, F(1,8)=0.347, p=0.572; N=3 sections per mouse, 3 mice per group; each data point represents one mouse.
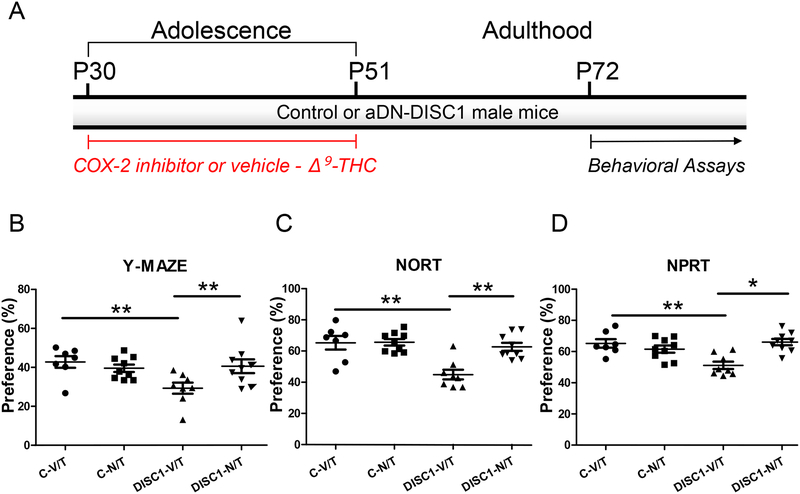
Blockade of NF-kB-COX-2 activation prevents cognitive impairment
Since our results had suggested synergistic elevation of the pro-inflammatory NF-kBCOX-2 signaling in aDN-DISC1 treated with Δ9-THC, we hypothesized that inhibition of COX-2 activity concurrently with Δ9-THC injections during adolescence may prevent the development of cognitive impairment in adult mice. In order to test this prediction, we assessed the effects of COX-2 inhibition (NS398, 10mg/kg, daily S.C. injections 30 min before Δ9-THC injections) on different types of recognition memory in control and aDN-DISC1 treated with Δ9-THC at adolescence (Fig.6). We found that NS398 prevented the development of memory deficits in aDN-DISC1 mice treated with Δ9-THC (the significant aDN-DISC1 by NS398 interaction for Y maze, F(1,29)=5.03, p=0.033; for NORT, F(1,29)=8.99, p=0.006; and for NPRT, F(1,29)=19.81, p<0.001). No effects of NS398 were found on locomotor activity in mice in the Y maze (Fig.S15 in Supplement 1).
Figure 6. Rescuing the memory deficits with the COX-2 inhibitor.
A- Schematic diagram of the treatment protocol;
On all data panels: the Y-axes depict the preference (%); the X-axes depict the experimental groups: C-V/T– control mice treated with vehicle and Δ9-THC (N=7); C-N/T – control mice treated with the selective COX-2 inhibitor (NS398) and Δ9-THC (N=9); DISC1-V/T – aDN-DISC1 mice treated with vehicle and Δ9-THC (N=8); DISC1-N/T -aDN-DISC1 mice treated with the NS398 and Δ9-THC (N=9). NS398 (10 mg/kg SC injections) was administrated daily 30 minutes prior Δ9-THC injections (10 mg/kg SC injections).
B- Spatial recognition memory in the Y maze. Significantly decreased preference for the previously blocked arm in aDN-DISC1 mice treated with Δ9-THC was significantly restored by NS398 co-treatment. Two-way ANOVA of the preference data revealed a significant effect of aDN-DISC1, F(1,29)=4.68, p=0.039; no effect of NS398, F(1,29)=9.26, p=0.205 and the significant aDN-DISC1 by NS398 interaction, F(1,29)=5.03, p=0.033; Fisher LSD post-hoc test showed that aDN-DISC1 significantly reduced preference for the previously blocked arm in Δ9-THC-treated mice (DISC1-V/T vs. C-V/T, p =0.004) and NS398 co-treatment significantly increased this preference (DISC1-V/T vs. DISC1-N/T, p =0.007); ** - denotes p<0.01; N=7–9 per group.
C- Novel object recognition test (NORT). Significantly decreased preference for the novel object in aDN-DISC1 mice treated with Δ9-THC was significantly restored by NS398 co-treatment. Two-way ANOVA of the preference data revealed a significant effect of aDN-DISC1, F(1,29)=11.64, p=0.002; NS398, F(1,29)=8.61, p=0.006 and the significant aDN-DISC1 by NS398 interaction, F(1,29)=8.99, p=0.006; Fisher LSD post-hoc test showed that aDN-DISC1 significantly reduced preference for the novel object in Δ9-THC-treated mice (DISC1-V/T vs. C-V/T, p <0.001) and NS398 co-treatment significantly increased this preference (DISC1-V/T vs. DISC1-N/T, p<0.001); ** - denotes p<0.01; N=7–9 per group.
D- Novel place recognition test (NPRT). Significantly decreased preference for the novel place of one of two identical objects in aDN-DISC1 mice treated with Δ9-THC was significantly restored by NS398 co-treatment. Two-way ANOVA of the preference data revealed no effect of aDN-DISC1, F(1,29)=2.05, p=0.163; no effect of NS398, F(1,29)=2.89, p=0.100 and the significant aDN-DISC1 by NS398 interaction, F(1,29)=19.81, p<0.001; Fisher LSD post-hoc test showed that aDN-DISC1 significantly reduced preference for the novel object in Δ9-THC-treated mice (DISC1-V/T vs. C-V/T, p <0.001) and NS398 co-treatment significantly increased this preference (DISC1-V/T vs. DISC1-N/T, p=0.043); * - denotes p<0.05, ** - denotes p<0.01; N=7–9 per group.
We also evaluated whether the COX-2 inhibition would prevent elevated secretion of glutamate by primary DN-DISC1 astrocytes following stimulation with Δ9-THC. We found that NS398 reversed increased secretion of glutamate by primary DN-DISC1 astrocytes treated Δ9-THC (Fig.S16 in Supplement 1). These results suggest convergence of effects of DN-DISC1 and Δ9-THC on NF-kB-COX-2 signaling in astrocytes, leading to increased production of glutamate by astrocytes.
Discussion
We report that inducible expression of dominant-negative Disrupted-In-Schizophrenia-1 (DN-DISC1) in astrocytes but not neurons or knockdown of endogenous Disc1 in hippocampal astrocytes interact with adolescent Δ9-THC exposure to impair recognition memory in adult mice. The present findings suggest that DN-DISC1 and Δ9-THC synergistically activate the NF-kB-COX-2 pathway in astrocytes, leading to increased secretion of glutamate and decreased immunoreactivity of parvalbumin-positive pre-synaptic boutons around pyramidal neurons of the CA3 area of the hippocampus. Deficient recognition memory could be prevented with the COX-2 inhibitor. Our data demonstrate that astrocyte genetic risk factors can exacerbate cognitive effects of adolescent cannabis use and indicate a putative target for preventive treatment.
Adolescent not adult exposure to Δ9-THC was required for the development of deficient recognition memory in adult mice with expression of DN-DISC1 in astrocytes. These results are consistent with other pre-clinical reports on effects of adolescent exposure to cannabinoids and resulting cognitive impairments (3, 72–74). Lack of effects of Δ9-THC in aDN-DISC1 mice on fear conditioning is in line with the unaltered performance in the Morris water maze (75–77) or aversive memory tasks in mice following adolescent treatment with cannabinoids (2, 72, 78–80). This selectivity in cognitive effects of cannabinoids could be related to differential distribution of CNR1 in the neural circuits underlying various cognitive tasks.
The current work is congruent with human studies that demonstrate that cannabis use during adolescence could have lasting effects on cognition (2, 72, 80–82) that is likely related to continuing maturation of the brain in general (83–86) and cannabinoid receptors in particular (87–89). Our results are consistent with human studies that adolescent cannabis use tend to affect working memory (WM) in adulthood (90, 91), particularly spatial processing that is dependent on the integrity of the hippocampus (92, 93). While human studies suggest an association (94–96), animal models enable us to establish a causal relationship and neurobiological mechanisms. In this context, our study significantly extend the existing literature on effects of cannabinoids on spatial of WM as evaluated in rodents with spatial recognition tests (97).
Our data clearly demonstrate that expression of the same risk factor in different brain cells types produces differential neurobehavioral outcomes in mice treated with Δ9-THC. Astrocyte but not neuronal expression of DN-DISC1 interacts with adolescent Δ9-THC to lead to recognition memory impairment in adult mice. In contrast, neuronal expression of DN-DISC1 and Δ9-THC treatment seem to have greater effects on fear conditioning, consistent with our prior studies with constitutive DN-DISC1 model (18)
The effects on recognition memory in astrocyte DN-DISC1 mice are unlikely dependent on early developmental effects of DN-DISC1 as turning off expression of DN-DISC1 after P21 completely eliminates the cognitive effects observed in our model. This appears in line with our prior reports on differential effects of DN-DISC1 on various behaviors depending on time this risk factor was expressed in neurons or astrocytes (98, 99). In addition, our data with DOX manipulation suggests that expression of DN-DISC1 during adolescent exposure to Δ9-THC is critical for the cognitive effects observed in our model. However, one cannot completely rule out the potential effects of DOX itself on neuroinflammatory processes in astrocytes that may have contributed to the preventive effects of DOX treatment.
GFAP-tTA;DN-DISC1 model has some limitations that make identification of underlying neural circuits mechanisms challenging. The GFAP promoter is active in the hippocampus and subcortical regions (100), and in addition to astrocytes, it is active in progenitor cells of the dentate gyrus of the hippocampus and the olfactory bulbs (101–103). Our findings with the viral knockdown of Disc1 in the CA areas were designed to address these limitations and suggest that the bulk of cognitive effects observed in DN-DISC1 mice treated with Δ9-THC are related to altered expression of Disc1 in hippocampal astrocytes. Additionally, the similar outcomes of Disc1 KD and DN-DISC1 suggest that the observed behavioral outcomes are likely due to altered expression of endogenous Disc1 rather than “off-target” or so-called gain-of-function effects of DN-DISC1 (30).
Although previous research and our current work clearly indicate that adolescent cannabis exposure can produce long-lasting behavioral and cognitive problems, there has been no direct comparison made between cognitive effects of cannabis and other psychoactive drugs. Indeed, there are numerous reports on long-term effects of psychostimulants used during adolescence (104–107). We found that chronic treatment with amphetamine of DN-DISC1 mice did not replicate the phenotypes produced by Δ9-THC, suggesting some selectivity in behavioral outcomes of adolescence exposure to cannabinoids vs. psychostimulants. Future studies will need to perform a more comprehensive dose-dependent comparative analysis.
The majority of pre-clinical research on cannabis has focused on GABA or glutamatergic neurons (108–113). However, there is a growing appreciation that glial cells also contribute to the detrimental behavioral effects associated with cannabis (2–4) as glial cells also express CNR1 and other factors of the endocannabinoid system (114). A recent study has shown that deletion of Cnr1 in mouse astrocytes prevents acute effects of Δ9-THC on spatial working memory and long-term depression (LTD) at hippocampal CA3-CA1 synapses. Critically, abolition of the same receptor on GABA or glutamate neurons does not lead to the same rescue phenomenon, suggesting that deficits in working memory triggered by acute administration of Δ9-THC could be due to the activation of CNR1 signaling in astrocytes (9). In a further support of the major role of astrocytes in the mechanisms of cognitive impairment following Δ9-THC exposure, another study has demonstrated that chronic Δ9-THC triggers a sustained activation of COX-2 and increased production of prostaglandin E2 (PGE2) in the brain. The activation of this signaling mechanism is initiated via CNR1-coupled G protein βγ subunits (10). However, astrocytes also express CNR2 (115, 116). Thus, it is conceivable that at least some of the cognitive effects of THC may have been mediated by CNR2. Future research will address this critical question.
There are significant variations in response to cannabis among users, suggesting genetic disposition (78, 117–119). Consistent with human findings, pre-clinical studies with mouse models carrying mutations in Neuregulin 1 (NRG1), COMT or DISC1 have shown that the effects of Δ9-THC on adolescent or adult mutant mice can dramatically differ from those on control littermates (15, 120). However, the neurobiological and molecular underpinnings of how genetic variants could moderate effects of Δ9-THC remain poorly understood (16, 121, 122). Moreover, there are very few if any studies of the molecular mechanism of cell type-specific GxE that mediate adverse effects of environmental risk factors, including cannabis. We have studied the neurobiological mechanisms of gene-environment interaction relevant to major psychiatric conditions using a rare mutation of a neurodevelopmental risk factor, DN-DISC1(123) as an experimental genetic tool to identify the molecular mechanisms whereby DN-DISC1 in astrocytes influences the signaling pathways activated by Δ9-THC. Based on the results of an unbiased RNA-seq analysis and prior studies (9), we identified the neuroinflammatory signaling in astrocytes that appears to be a convergent target for DN-DISC1 and inflammatory factors up-regulated by Δ9-THC. Specifically, we found that DN-DISC1 and Δ9-THC synergistically activate NF-kB-COX-2 signaling that might lead to increased secretion of glutamate by astrocytes. In order to test this molecular hypothesis, we inhibited activation of COX-2 with the selective inhibitor and were able to prevent the development of cognitive deficits in aDN-DISC1 mice treated with Δ9-THC. We believe this pharmacological approach could be applied to other GxE rodent models with the goal to use COX-2 inhibitors to counteract and/or ameliorate psychosis-like behavioral alterations associated with neuroinflammatory conditions produced by several environmental factors, including chronic THC exposure during adolescence. This would be congruent with several studies that demonstrated that ad-on treatment with COX-2 inhibitors had some beneficial anti-psychotic and cognitive effects (124–129). However, given cell and regional heterogeneity of the hippocampus, future studies will need to validate the above molecular events using isolated tissue astrocytes from different areas of the hippocampus.
Consistent with prior rodent studies (108–113), we also found synergetic adverse effects of Δ9-THC on the integrity of GABA neurons. Our findings indicate that the intensity of PV+ pre-synaptic boutons around pyramidal neurons of the CA3 area are predominantly affected, suggesting that inhibitory influence of PV+ cells in the hippocampus could be compromised in aDN-DISC1 mice treated with Δ9-THC, potentially leading to altered excitatory-inhibitory balance underlying aspects of cognitive dysfunction. In addition, in line with the recent publication, decreased PV+ could also lead to abnormal long-term depression (LTD) at hippocampal CA3-CA1 synapses (10). Future studies will address these possibilities in detail.
In conclusion, our work for the first time demonstrates that a genetic predisposition and adolescent Δ9-THC exposure could synergistically produce a sustained activation of NF-kB-COX-2 signaling in astrocytes. This leads to elevated secretion of glutamate, reduced immunoreactivity of parvalbumin-positive pre-synaptic boutons around pyramidal neurons of the CA3 area of the hippocampus and deficient memory. The observed cognitive deficits can be prevented with the COX-2 inhibitor, suggesting future targets for therapeutic interventions.
Supplementary Material
Acknowledgements:
The authors would like to thank Dr. Akira Sawa and Dr. Christopher A. Ross (Johns Hopkins University) for their thoughtful suggestions and comments. The authors acknowledge Ms. Olga Mychko’s expert technical help with immunostaining. The authors also acknowledge a superb service provided by the JHU Next Generation Sequencing Core. The authors would like to thank Dr. Sun-Hong Kim (Johns Hopkins University) for his expert assistance with generating AAV vectors. We are also grateful to the National Institute on Drug Abuse (NIDA) Drug Supply Program for providing THC.
Funding: This work was supported by DA-041208 (AK, MVP), MH-094268 the Conte Center grant (AK, MVP), MH-083728 (MVP), and The Brain and Behavior Research Foundation (AK, MVP, AS). The authors declare no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. (2016): Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA psychiatry. 73:292–297. [DOI] [PubMed] [Google Scholar]
- 2.Chadwick B, Miller ML, Hurd YL (2013): Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Frontiers in psychiatry. 4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szutorisz H, Hurd YL (2018): High times for cannabis: Epigenetic imprint and its legacy on brain and behavior. Neurosci Biobehav Rev. 85:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N (2016): Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biological psychiatry. 79:557–567. [DOI] [PubMed] [Google Scholar]
- 5.Atakan Z (2012): Cannabis, a complex plant: different compounds and different effects on individuals. Therapeutic advances in psychopharmacology. 2:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990): Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 346:561–564. [DOI] [PubMed] [Google Scholar]
- 7.Navarrete M, Araque A (2010): Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 68:113–126. [DOI] [PubMed] [Google Scholar]
- 8.Stella N (2010): Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 58:1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. (2012): Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 148:1039–1050. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Zhang J, Fan N, Teng ZQ, Wu Y, Yang H, et al. (2013): Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 155:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, et al. (1998): Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 391:281–285. [DOI] [PubMed] [Google Scholar]
- 12.Hasin DS (2018): US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco C, Hasin DS, Wall MM, Florez-Salamanca L, Hoertel N, Wang S, et al. (2016): Cannabis Use and Risk of Psychiatric Disorders: Prospective Evidence From a US National Longitudinal Study. JAMA psychiatry. 73:388–395. [DOI] [PubMed] [Google Scholar]
- 14.Levine A, Clemenza K, Rynn M, Lieberman J (2017): Evidence for the Risks and Consequences of Adolescent Cannabis Exposure. Journal of the American Academy of Child and Adolescent Psychiatry. 56:214–225. [DOI] [PubMed] [Google Scholar]
- 15.O’Tuathaigh CM, Hryniewiecka M, Behan A, Tighe O, Coughlan C, Desbonnet L, et al. (2010): Chronic adolescent exposure to Delta-9-tetrahydrocannabinol in COMT mutant mice: impact on psychosis-related and other phenotypes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 35:2262–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T (2013): Transmembrane domain Nrg1 mutant mice show altered susceptibility to the neurobehavioural actions of repeated THC exposure in adolescence. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 16:163–175. [DOI] [PubMed] [Google Scholar]
- 17.Tantra M, Krocher T, Papiol S, Winkler D, Rockle I, Jatho J, et al. (2014): St8sia2 deficiency plus juvenile cannabis exposure in mice synergistically affect higher cognition in adulthood. Behavioural brain research. 275:166–175. [DOI] [PubMed] [Google Scholar]
- 18.Ballinger MD, Saito A, Abazyan B, Taniguchi Y, Huang CH, Ito K, et al. (2015): Adolescent cannabis exposure interacts with mutant DISC1 to produce impaired adult emotional memory. Neurobiol Dis. 82:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal-Gavish H, Gazit N, Barhum Y, Ben-Zur T, Taler M, Hornfeld SH, et al. (2017): BDNF overexpression prevents cognitive deficit elicited by adolescent cannabis exposure and host susceptibility interaction. Human molecular genetics. 26:2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. (2005): A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nature cell biology. 7:1167–1178. [DOI] [PubMed] [Google Scholar]
- 21.Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, Kubo A, et al. (2008): Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatry. 65:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. (2010): Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 65:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. (2008): Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Molecular psychiatry. 13:173–186, 115. [DOI] [PubMed] [Google Scholar]
- 24.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, et al. (2011): Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Molecular psychiatry. 16:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. (2010): Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biological psychiatry. 68:1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, et al. (2013): Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Molecular psychiatry. 18:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogorelov VM, Nomura J, Kim J, Kannan G, Ayhan Y, Yang C, et al. (2012): Mutant DISC1 affects methamphetamine-induced sensitization and conditioned place preference: a comorbidity model. Neuropharmacology. 62:1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrillion CE, Abazyan B, Yang Z, Crawford J, Shevelkin AV, Jouroukhin Y, et al. (2017): DISC1 in Astrocytes Influences Adult Neurogenesis and Hippocampus-Dependent Behaviors in Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42:2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. (2000): Disruption of two novel genes by a translocation co-segregating with schizophrenia. Human molecular genetics. 9:1415–1423. [DOI] [PubMed] [Google Scholar]
- 30.Brandon NJ, Sawa A (2011): Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nature reviews Neuroscience. 12:707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schizophrenia Working Group of the Psychiatric Genomics C (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O’Donovan MC, et al. (2015): Evaluating historical candidate genes for schizophrenia. Molecular psychiatry. 20:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan PF, Daly MJ, O’Donovan M (2012): Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature reviews Genetics. 13:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geschwind DH, Flint J (2015): Genetics and genomics of psychiatric disease. Science. 349:1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. (2008): Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 13:173–186, 115. [DOI] [PubMed] [Google Scholar]
- 36.Abazyan B, Dziedzic J, Hua K, Abazyan S, Yang C, Mori S, et al. (2014): Chronic exposure of mutant DISC1 mice to lead produces sex-dependent abnormalities consistent with schizophrenia and related mental disorders: a gene-environment interaction study. Schizophr Bull. 40:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. (2010): Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 68:1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito A, Taniguchi Y, Kim SH, Selvakumar B, Perez G, Ballinger MD, et al. (2017): Developmental Alcohol Exposure Impairs Activity-Dependent S-Nitrosylation of NDEL1 for Neuronal Maturation. Cerebral cortex. 27:3918–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koga M, Serritella AV, Messmer MM, Hayashi-Takagi A, Hester LD, Snyder SH, et al. (2011): Glutathione is a physiologic reservoir of neuronal glutamate. Biochem Biophys Res Commun. 409:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spear L (2000): Modeling adolescent development and alcohol use in animals. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 24:115–123. [PMC free article] [PubMed] [Google Scholar]
- 41.Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003): Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 27:19–31. [DOI] [PubMed] [Google Scholar]
- 42.Casey BJ, Pattwell SS, Glatt CE, Lee FS (2013): Treating the developing brain: implications from human imaging and mouse genetics. Annual review of medicine. 64:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutta S, Sengupta P (2016): Men and mice: Relating their ages. Life sciences. 152:244–248. [DOI] [PubMed] [Google Scholar]
- 44.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY (2012): Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 484:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanski R, van Strien ME, van Tijn P, Hol EM (2014): A star is born: new insights into the mechanism of astrogenesis. Cellular and molecular life sciences : CMLS. 71:433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundqvist T (2010): Imaging cognitive deficits in drug abuse. Current topics in behavioral neurosciences. 3:247–275. [DOI] [PubMed] [Google Scholar]
- 47.Barkus E, Murray RM (2010): Substance use in adolescence and psychosis: clarifying the relationship. Annual review of clinical psychology. 6:365–389. [DOI] [PubMed] [Google Scholar]
- 48.Barbizet J (1963): Defect of memorizing of hippocampal-mammillary origin: a review. Journal of neurology, neurosurgery, and psychiatry. 26:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meissner WW (1967): Hippocampus and learning. International journal of neuropsychiatry. 3:298–310. [PubMed] [Google Scholar]
- 50.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S (2004): NMDA receptors, place cells and hippocampal spatial memory. Nature reviews Neuroscience. 5:361–372. [DOI] [PubMed] [Google Scholar]
- 51.Miller LL, Branconnier RJ (1983): Cannabis: effects on memory and the cholinergic limbic system. Psychological bulletin. 93:441–456. [PubMed] [Google Scholar]
- 52.Schulz S (2011): MDMA & cannabis: a mini-review of cognitive, behavioral, and neurobiological effects of co-consumption. Current drug abuse reviews. 4:81–86. [DOI] [PubMed] [Google Scholar]
- 53.Kutlu MG, Gould TJ (2016): Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learning & memory. 23:515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hudson R, Rushlow W, Laviolette SR (2018): Phytocannabinoids modulate emotional memory processing through interactions with the ventral hippocampus and mesolimbic dopamine system: implications for neuropsychiatric pathology. Psychopharmacology (Berl). 235:447–458. [DOI] [PubMed] [Google Scholar]
- 55.Chye Y, Suo C, Yucel M, den Ouden L, Solowij N, Lorenzetti V (2017): Cannabis-related hippocampal volumetric abnormalities specific to subregions in dependent users. Psychopharmacology (Berl). 234:2149–2157. [DOI] [PubMed] [Google Scholar]
- 56.Ghaderi M, Rezayof A, Vousooghi N, Zarrindast MR (2016): Dorsal hippocampal NMDA receptors mediate the interactive effects of arachidonylcyclopropylamide and MDMA/ecstasy on memory retrieval in rats. Progress in neuro-psychopharmacology & biological psychiatry. 66:41–47. [DOI] [PubMed] [Google Scholar]
- 57.Kirkby NS, Chan MV, Zaiss AK, Garcia-Vaz E, Jiao J, Berglund LM, et al. (2016): Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-kappaB and NFAT transcriptional pathways. Proceedings of the National Academy of Sciences of the United States of America. 113:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricciotti E, FitzGerald GA (2011): Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and vascular biology. 31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamberletti E, Gabaglio M, Grilli M, Prini P, Catanese A, Pittaluga A, et al. (2016): Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacological research. 111:459–470. [DOI] [PubMed] [Google Scholar]
- 60.LoVerso PR, Cui F (2016): Cell type-specific transcriptome profiling in mammalian brains. Frontiers in bioscience. 21:973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perkins ND (2006): Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 25:6717–6730. [DOI] [PubMed] [Google Scholar]
- 62.Christian F, Smith EL, Carmody RJ (2016): The Regulation of NF-kappaB Subunits by Phosphorylation. Cells. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Behan AT, Hryniewiecka M, O’Tuathaigh CM, Kinsella A, Cannon M, Karayiorgou M, et al. (2012): Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: impact on indices of dopaminergic, endocannabinoid and GABAergic pathways. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 37:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eggan SM, Hashimoto T, Lewis DA (2008): Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 65:772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomes FV, Issy AC, Ferreira FR, Viveros MP, Del Bel EA, Guimaraes FS (2014): Cannabidiol attenuates sensorimotor gating disruption and molecular changes induced by chronic antagonism of NMDA receptors in mice. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friend L, Weed J, Sandoval P, Nufer T, Ostlund I, Edwards JG (2017): CB1-Dependent Long-Term Depression in Ventral Tegmental Area GABA Neurons: A Novel Target for Marijuana. The Journal of neuroscience : the official journal of the Society for Neuroscience. 37:10943–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY (2014): CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Molecular psychiatry. 19:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang L, Zhao S, Lu W, Guan S, Zhu Y, Wang JH (2015): Acidosis-Induced Dysfunction of Cortical GABAergic Neurons through Astrocyte-Related Excitotoxicity. PloS one. 10:e0140324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao YL, Xiang Q, Shi QY, Li SY, Tan L, Wang JT, et al. (2011): GABAergic excitotoxicity injury of the immature hippocampal pyramidal neurons’ exposure to isoflurane. Anesthesia and analgesia. 113:1152–1160. [DOI] [PubMed] [Google Scholar]
- 70.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. (2012): GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desfeux A, El Ghazi F, Jegou S, Legros H, Marret S, Laudenbach V, et al. (2010): Dual effect of glutamate on GABAergic interneuron survival during cerebral cortex development in mice neonates. Cerebral cortex. 20:1092–1108. [DOI] [PubMed] [Google Scholar]
- 72.Rubino T, Parolaro D (2016): The Impact of Exposure to Cannabinoids in Adolescence: Insights From Animal Models. Biological psychiatry. 79:578–585. [DOI] [PubMed] [Google Scholar]
- 73.Rubino T, Parolaro D (2008): Long lasting consequences of cannabis exposure in adolescence. Molecular and cellular endocrinology. 286:S108–113. [DOI] [PubMed] [Google Scholar]
- 74.Davies SN, Pertwee RG, Riedel G (2002): Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 42:993–1007. [DOI] [PubMed] [Google Scholar]
- 75.Varvel SA, Hamm RJ, Martin BR, Lichtman AH (2001): Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl). 157:142–150. [DOI] [PubMed] [Google Scholar]
- 76.Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS (2006): Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacology, biochemistry, and behavior. 83:448–455. [DOI] [PubMed] [Google Scholar]
- 77.Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH (2012): Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS chemical neuroscience. 3:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silveira MM, Arnold JC, Laviolette SR, Hillard CJ, Celorrio M, Aymerich MS, et al. (2017): Seeing through the smoke: Human and animal studies of cannabis use and endocannabinoid signalling in corticolimbic networks. Neurosci Biobehav Rev. 76:380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, et al. (2012): Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PloS one. 7:e39243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris CV, DiNieri JA, Szutorisz H, Hurd YL (2011): Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. The European journal of neuroscience. 34:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barthelemy OJ, Richardson MA, Cabral HJ, Frank DA (2016): Prenatal, perinatal, and adolescent exposure to marijuana: Relationships with aggressive behavior. Neurotoxicology and teratology. 58:60–77. [DOI] [PubMed] [Google Scholar]
- 82.Alegria AA, Hasin DS, Nunes EV, Liu SM, Davies C, Grant BF, et al. (2010): Comorbidity of generalized anxiety disorder and substance use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 71:1187–1195; quiz 1252–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. (1999): Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl). 142:295–301. [DOI] [PubMed] [Google Scholar]
- 84.Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL (2004): Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biological psychiatry. 56:86–94. [DOI] [PubMed] [Google Scholar]
- 85.Pope HG Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D (2003): Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and alcohol dependence. 69:303–310. [DOI] [PubMed] [Google Scholar]
- 86.Schneider M, Koch M (2003): Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 28:1760–1769. [DOI] [PubMed] [Google Scholar]
- 87.Belue RC, Howlett AC, Westlake TM, Hutchings DE (1995): The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and teratology. 17:25–30. [DOI] [PubMed] [Google Scholar]
- 88.McLaughlin CR, Martin BR, Compton DR, Abood ME (1994): Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug and alcohol dependence. 36:27–31. [DOI] [PubMed] [Google Scholar]
- 89.Seeman P (1999): Images in neuroscience. Brain development, X: pruning during development. The American journal of psychiatry. 156:168. [DOI] [PubMed] [Google Scholar]
- 90.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA (2004): Maturation of cognitive processes from late childhood to adulthood. Child development. 75:1357–1372. [DOI] [PubMed] [Google Scholar]
- 91.Luna B, Padmanabhan A, O’Hearn K (2010): What has fMRI told us about the development of cognitive control through adolescence? Brain and cognition. 72:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hassabis D, Kumaran D, Vann SD, Maguire EA (2007): Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences of the United States of America. 104:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kesner RP (2007): Behavioral functions of the CA3 subregion of the hippocampus. Learning & memory. 14:771–781. [DOI] [PubMed] [Google Scholar]
- 94.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S (2013): Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in psychiatry. 4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Padula CB, Schweinsburg AD, Tapert SF (2007): Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 21:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE (2014): Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Human brain mapping. 35:2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Realini N, Rubino T, Parolaro D (2009): Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological research. 60:132–138. [DOI] [PubMed] [Google Scholar]
- 98.Xia M, Broek JA, Jouroukhin Y, Schoenfelder J, Abazyan S, Jaaro-Peled H, et al. (2016): Cell Type-Specific Effects of Mutant DISC1: A Proteomics Study. Molecular neuropsychiatry. 2:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia M, Zhu S, Shevelkin A, Ross CA, Pletnikov M (2016): DISC1, astrocytes and neuronal maturation: a possible mechanistic link with implications for mental disorders. J Neurochem. 138:518–524. [DOI] [PubMed] [Google Scholar]
- 100.Lee Y, Messing A, Su M, Brenner M (2008): GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 56:481–493. [DOI] [PubMed] [Google Scholar]
- 101.Weber T, Baier V, Pauly R, Sahay A, Baur M, Herrmann E, et al. (2011): Inducible gene expression in GFAP+ progenitor cells of the SGZ and the dorsal wall of the SVZ-A novel tool to manipulate and trace adult neurogenesis. Glia. 59:615–626. [DOI] [PubMed] [Google Scholar]
- 102.Casper KB, McCarthy KD (2006): GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Molecular and cellular neurosciences. 31:676–684. [DOI] [PubMed] [Google Scholar]
- 103.Namba T, Mochizuki H, Suzuki R, Onodera M, Yamaguchi M, Namiki H, et al. (2011): Time-lapse imaging reveals symmetric neurogenic cell division of GFAP-expressing progenitors for expansion of postnatal dentate granule neurons. PloS one. 6:e25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berman SM, Kuczenski R, McCracken JT, London ED (2009): Potential adverse effects of amphetamine treatment on brain and behavior: a review. Molecular psychiatry. 14:123–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park TM, Haning WF 3rd (2016): Stimulant Use Disorders. Child and adolescent psychiatric clinics of North America. 25:461–471. [DOI] [PubMed] [Google Scholar]
- 106.Marshall BD, Werb D (2010): Health outcomes associated with methamphetamine use among young people: a systematic review. Addiction. 105:991–1002. [DOI] [PubMed] [Google Scholar]
- 107.Levine DA (2007): “Pharming”: the abuse of prescription and over-the-counter drugs in teens. Current opinion in pediatrics. 19:270–274. [DOI] [PubMed] [Google Scholar]
- 108.Cortes-Briones JA, Cahill JD, Skosnik PD, Mathalon DH, Williams A, Sewell RA, et al. (2015): The psychosis-like effects of Delta(9)-tetrahydrocannabinol are associated with increased cortical noise in healthy humans. Biological psychiatry. 78:805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Radhakrishnan R, Skosnik PD, Cortes-Briones J, Sewell RA, Carbuto M, Schnakenberg A, et al. (2015): GABA Deficits Enhance the Psychotomimetic Effects of Delta9-THC. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 40:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cortes-Briones J, Skosnik PD, Mathalon D, Cahill J, Pittman B, Williams A, et al. (2015): Delta9-THC Disrupts Gamma (gamma)-Band Neural Oscillations in Humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 40:2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skosnik PD, Krishnan GP, D’Souza DC, Hetrick WP, O’Donnell BF (2014): Disrupted gamma-band neural oscillations during coherent motion perception in heavy cannabis users. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 39:3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zamberletti E, Beggiato S, Steardo L Jr., Prini P, Antonelli T, Ferraro L, et al. (2014): Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis. 63:35–47. [DOI] [PubMed] [Google Scholar]
- 113.Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S (2016): Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci Biobehav Rev. 64:359–381. [DOI] [PubMed] [Google Scholar]
- 114.Stella N (2004): Cannabinoid signaling in glial cells. Glia. 48:267–277. [DOI] [PubMed] [Google Scholar]
- 115.Fernandez-Trapero M, Espejo-Porras F, Rodriguez-Cueto C, Coates JR, Perez-Diaz C, de Lago E, et al. (2017): Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Disease models & mechanisms. 10:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S (2017): Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Frontiers in neuroscience. 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benyamina A, Bonhomme-Faivre L, Picard V, Sabbagh A, Richard D, Blecha L, et al. (2009): Association between ABCB1 C3435T polymorphism and increased risk of cannabis dependence. Progress in neuro-psychopharmacology & biological psychiatry. 33:1270–1274. [DOI] [PubMed] [Google Scholar]
- 118.Spiro AS, Wong A, Boucher AA, Arnold JC (2012): Enhanced brain disposition and effects of Delta9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PloS one. 7:e35937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saito A, Ballinger MD, Pletnikov MV, Wong DF, Kamiya A Endocannabinoid system: potential novel targets for treatment of schizophrenia. Neurobiol Dis. 53:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Tuathaigh CM, Clarke G, Walsh J, Desbonnet L, Petit E, O’Leary C, et al. (2012): Genetic vs. pharmacological inactivation of COMT influences cannabinoid-induced expression of schizophrenia-related phenotypes. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 15:1331–1342. [DOI] [PubMed] [Google Scholar]
- 121.Calvignac-Spencer S, Merkel K, Kutzner N, Kuhl H, Boesch C, Kappeler PM, et al. (2013): Carrion fly-derived DNA as a tool for comprehensive and cost-effective assessment of mammalian biodiversity. Molecular ecology. 22:915–924. [DOI] [PubMed] [Google Scholar]
- 122.Du H, Kwon IK, Kim J (2013): Neuregulin-1 impairs the long-term depression of hippocampal inhibitory synapses by facilitating the degradation of endocannabinoid 2-AG. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33:15022–15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ayhan Y, McFarland R, Pletnikov MV (2016): Animal models of gene-environment interaction in schizophrenia: A dimensional perspective. Progress in neurobiology. 136:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng W, Cai DB, Yang XH, Ungvari GS, Ng CH, Muller N, et al. (2017): Adjunctive celecoxib for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. Journal of psychiatric research. 92:139–146. [DOI] [PubMed] [Google Scholar]
- 125.Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. (2002): Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. The American journal of psychiatry. 159:1029–1034. [DOI] [PubMed] [Google Scholar]
- 126.Marini S, De Berardis D, Vellante F, Santacroce R, Orsolini L, Valchera A, et al. (2016): Celecoxib Adjunctive Treatment to Antipsychotics in Schizophrenia: A Review of Randomized Clinical Add-On Trials. Mediators of inflammation. 2016:3476240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nitta M, Kishimoto T, Muller N, Weiser M, Davidson M, Kane JM, et al. (2013): Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophrenia bulletin. 39:1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B (2007): Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophrenia research. 90:179–185. [DOI] [PubMed] [Google Scholar]
- 129.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D (2005): Celecoxib augmentation of continuously ill patients with schizophrenia. Biological psychiatry. 57:1594–1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.