Abstract
Introduction:
Sexual dysfunction affects many people, with 33–60% of women reporting sexual dysfunction and 8–52% of men with erectile dysfunction (ED) or premature ejaculation (PE). In an effort to determine the constellation of factors responsible for sexual dysfunction, the effect of thyroid hormone derangements has been of recent interest.
Aim:
To investigate the associations between thyroid hormones and sexual dysfunction in women and men.
Methods:
Review of the literature examining effects of hypo- and hyperthyroidism on sexual function.
Main Outcome Measures:
Summary of effects of thyroid dysfunction on domains of sexual functioning.
Results:
Most studies demonstrate that hypothyroid and hyperthyroid men have increased rates of sexual dysfunction, including erectile dysfunction (ED) in hypothyroid men. However, studies vary on the strength of correlation between hormonal derangement and level of sexual dysfunction. In both hyper- and hypothyroid men, treatment of thyroid disorder at least partially reverses sexual dysfunction. In contrast, the current literature provides no consensus on the effect of hypothyroidism, hyperthyroidism, or Hashimoto’s thyroiditis on female sexual function. In studies that observed increased rates of sexual dysfunction in women with thyroid disorders, correction of the thyroid derangement resulted in resolution of some sexual dysfunction. Studies are also conflicted on whether there is a relationship between degree of sexual dysfunction and degree of hormone derangement in women. However, prior work has demonstrated a relationship between thyroid autoantibodies and sexual dysfunction in women.
Conclusion:
Thyroid dysfunction is an important factor in the pathogenesis of sexual dysfunction in men and possibly women. Evidence suggests a reversibility of sexual dysfunction with correction of thyroid dysfunction, though the exact pathophysiology of thyroid-mediated sexual dysfunction remains unknown. However, current evidence supports thyroid derangements rather than autoantibodies as the causative factor in men, while autoantibodies appear to play a more prominent role in women.
Keywords: Thyroid Hormones, Erectile Dysfunction, Sexual Dysfunction, Physiological, Premature Ejaculation, Hypothyroid, Hyperthyroid
Introduction:
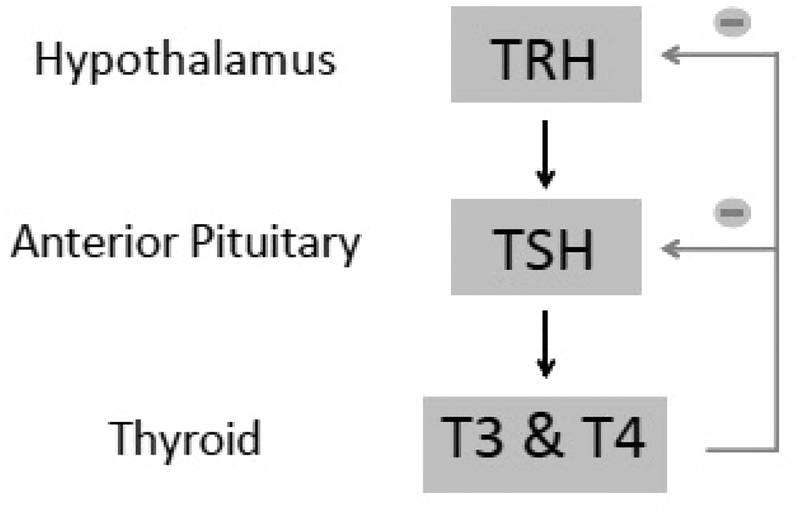
The thyroid is a hormonally active gland that is part of the hypothalamic-pituitary-thyroid axis. The axis includes thyroid releasing hormone (TRH) which is secreted by the hypothalamus. TRH stimulates the release of thyroid stimulating hormone (TSH) from the anterior pituitary gland. TSH, in turn, stimulates the thyroid to secrete thyroxine (T4) and triiodothyronine (T3), which are present in a free, active form and a bound, inactive form (Figure 1).1 Thyroid derangements most commonly arise from primary thyroid dysfunction, rarely being caused by secondary etiologies such as a TSH secreting pituitary adenoma, pituitary failure or hypothalamic failure (Table 1).1 Thus, hypothyroidism is generally associated with decreased T3 and T4 and increased TSH. In contrast, hyperthyroidism is associated with increased T3 and T4, and decreased TSH levels. Graves’ disease and Hashimoto’s thyroiditis (HT) result in autoimmune hyper-and hypothyroidism, respectively. Hyperfunctioning “toxic” multinodular goiter, and hyperfunctioning adenoma are common non-autoimmune causes of hyperthyroidism Non-autoimmune hypothyroidism often results secondary to surgery, radiation or radioiodine administration for the treatment of hyperthyroidism. Hyperthyroidism results in a hypermetabolic state typified by sympathetic overactivity resulting in tachycardia, tremor, anxiety, diarrhea and weight loss. Conversely, hypothyroidism is a slowing of physical and mental activity with resultant fatigue, decreased cardiac output, constipation, and weight gain (Table 2).1,2 While the effects of thyroid function on metabolism and anthropomorphic parameters are well known, the effects of these hormones and thyroid disease on sexual functioning has been less thoroughly elucidated. In part, this is due to the long-held belief that genitalia are non-responsive to thyroid hormone. However, recent studies have identified the presence of thyroid hormone receptors in both the male and female genitalia including the testis, corpora cavernosa, ovary, and vagina.3
Figure 1.
Thyroid axis. Secretion of thyroid releasing hormone (TRH) from hypothalamus causes downstream secretion of thyroid stimulating hormone (TSH) from anterior pituitary then T3 and T4 from thyroid. T3 and T4 cause negative feedback at both the level of the hypothalamus and anterior pituitary.
Table 1.
Causes of hypo- and hyperthyroidism by level of dysfunction
| Hypothyroidism | Hyperthyroidism |
|---|---|
Primary - level of thyroid
|
Primary - Level of thyroid
|
Secondary - level of pituitary
|
Secondary - level of pituitary
|
Tertiary - Level of hypothalamus
|
Table 2.
Common symptoms of hypo- and hyperthyroidism
| Hypothyroidism | Hyperthyroidism |
|---|---|
|
|
Sexual dysfunction is composed of a constellation of often overlapping disorders and symptoms4. The International Index of Erectile Function (IIEF) was developed in 1997, and assesses five domains of sexual function—erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. The survey has been validated across cultures and is regarded as the current gold standard for assessing male sexual function, despite its limitations.5, 6
Several sexual dysfunctions have been described in men including premature ejaculation (PE), erectile dysfunction (ED), ejaculatory dysfunction, and hypoactive sexual desire (HSD). Erectile dysfunction is defined as “the consistent or recurrent inability to attain or maintain penile erection adequate for sexual intercourse”.7 The Massachusetts Male Aging Study observed that 52% of men ages 40–70 had ED, with rates increasing with age.8 While the majority of ED results from vascular compromise, an increased understanding of hormonal regulation of erectile function is making apparent the subtle, but important, influences of the endocrine system on erectile function.9 Premature ejaculation (PE) is defined as a shortened ejaculatory latency time from vaginal penetration: less than 1 minute for lifelong PE and less than 3 minutes for acquired PE.10 The prevalence of PE varies widely, ranging from 8–30%.11, 12, 13 The pathophysiology of PE is not completely elucidated, but prevailing theories suggest both psychological and biological mechanisms are at play.14 Specifically, “disruptions in central serotonergic neurotransmission, potent cortical representation of the pudendal nerve, prostatitis, hypersensitivity of the penis, recreational drugs, and hormonal disorders” have been implicated.15 On the other end of the spectrum is delayed ejaculation (DE), which is more poorly defined and studied. While no specific duration of ejaculatory latency has been agreed upon, a prevailing definition is a complete lack of or delay in ejaculation longer than normal in the setting of appropriate stimulation.16 In order to quantify derangement of erectile function, intravaginal ejaculation latency time (IELT) is often used.17 Finally, HSD is defined as “persistently or recurrently deficient (or absent) sexual fantasies and desire for sexual activity.”18 Similar to other disorders of sexual function, HSD has been associated with several conditions and medications that alter neurotransmitter and hormone levels.19
Female sexual dysfunction (FSD) is present in 33–60% of women.20, 21, 22, 23. In spite of a prevalence that rivals and perhaps surpasses that in men, the conversation of sexual dysfunction in women has been far quieter, which Rosen et al. hypothesize is at least in part due to a lack of diagnostic framework until 2000.18,24 Mechanisms of many female sexual dysfunctions remain incompletely understood, although menopause and low income have been identified as risk factors.25 In women, sexual dysfunction can currently best be evaluated using the Female Sexual Function Index (FSFI), which has been translated into several languages and validated across cultures.26 It contains 6 subcategories: desire, arousal, lubrication, orgasm, satisfaction, and pain.18
Sexual dysfunction affects a large portion of the male and female population and has an incompletely defined etiology. In this review we explore the impact of thyroid dysfunction on female and male sexual function. We discuss the impact of correcting thyroid derangements and the pathophysiologic basis of thyroid hormone on the genitalia, and suggest future directions for research into the impact of thyroid disease on sexual functioning.
Methods
To conduct this review, a search of PubMed was performed for all English literature published on or before January 2018 using the following search terms and their combinations: male sexual function, female sexual function, sexual dysfunction, erectile dysfunction, premature ejaculation, persistent genital arousal, desire, climax, impotence, hyposexual, hypersexual, thyroid, thyroid disease, thyroiditis, hypothyroid, hyperthyroid, Graves, and HT. Articles were included if they assessed the impact of thyroid disease on sexual function. Abstracts were excluded from this review.
Males
Hypothyroidism in Men
Carani et al. (2005) found that men with hypothyroidism are more likely to have HSD (64.3 vs 17.6%), DE (64.3 vs 2.9%) and ED (64.3 vs 14.7%) and less likely to have PE (7.1 vs 50%) when compared to hyperthyroid men. Two to four months after normalization of thyroid hormone levels, the fraction of originally hypothyroid men with ED significantly decreased (64.3 to 21.4%, p<0.05), as did the fraction of men with HSD (64.3% to 35.7%, p<0.05), and DE (64% to 28.6%, p<0.05). The authors further found that IELT significantly decreased from 21.8±10.9 to 7.4±7.2 minutes (p<0.01) after normalization of thyroid levels. All IIEF domains demonstrated improvement but only the intercourse satisfaction domain significant improved (p<0.05). It is important to note that this study was limited by a very small sample and included only 14 hypothyroid men.17
In a slightly larger study, Krassas et al. (2008) observed a significantly increased frequency of ED in hypothyroid men than in controls (p<0.0001). Additionally, they reproduced Carani et al.’s findings and found a significant decrease in prevalence of ED after thyroid treatment (p<0.0001). Unlike Carani et al., they did not use the entire IIEF, but rather focused on the 5 question Sexual Health Inventory for Males (SHIM), derived from the IIEF. After treatment, SHIM scores increased from 14.5 to 23.0 (p<0.0001), indicating improvement in erectile function. Additionally, in hypothyroid men, a positive correlation between SHIM scores and free T3 levels (r=0.413, p=0.005), and a negative correlation with TSH levels (r=−0.669, p<0.001) were observed. However, no differences in SHIM scores in hypothyroid patients with or without thyroid antibodies were observed.27 This supports the conclusion that the hypothyroid state, and not the antibodies that result in this state, was responsible for sexual dysfunction in this population.
Krysiak et al. observed an even more severe impact of hypothyroidism on sexual function in men. They compared thyroid function in 12 men with overt hypothyroidism and 12 with subclinical hypothyroidism to 12 euthyroid controls. Overt hypothyroidism was defined within this study as plasma TSH greater than 20 mU/L with a free T3 and T4 below the lower limit of normal. Men with overt hypothyroidism had lower scores in all IIEF domains compared with controls (all p<0.001). 83% of men with overt hypothyroidism met criteria for ED—42% mild, 17% mild to moderate and 25% moderate—while only 8% of euthyroid men met criteria for ED, which was mild in all cases. The overall rate of ED was significantly different between the two groups (p<0.001). After 6 months of optimal treatment with L-thyroxine titrated until TSH levels 0.45–4.5 mU/L, all IIEF domain scores increased significantly from baseline (p<0.01 for all), although erectile function domain scores remained significantly lower than controls (p<0.05). The incidence of ED in the study population decreased to 25%, which was significantly lower than baseline (p<0.0001) and not significantly different than the rate in controls (p>0.05). In this cohort, erectile function, intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction were positively correlated with free T3 and T4 levels both before and after treatment (r=0.23–0.42, p<0.05). The same parameters were negatively correlated with TSH in both treated and untreated patients (r=−0.49− −0.34, p<0.01). The findings in men with subclinical hypothyroidism, defined as TSH 4.5–20 mU/L and normal free T4 levels, were slightly different. Compared to controls, men with subclinical hypothyroidism only had lower erectile function IIEF domain scores (p<0.05). The rate of ED in this group was 42%, which is significantly lower than that in the overt hypothyroidism group (p<0.001) and significantly higher than that in the control group (p<0.05). Similar to the overt hypothyroidism group, men in the subclinical hypothyroidism group experienced normalization of all IIEF scores except erectile function (p<0.05 vs. control) after 6 months of optimal treatment (p>0.05 all others). Further, ED rates in the subclinical hypothyroidism group decreased significantly after treatment to 16% (p<0.05 vs baseline; p>0.05 vs control). In men with subclinical hypothyroidism, IIEF scores for erectile function negatively correlated with TSH (r=−0.46–4.3, p<0.01) and positively correlated with free T3 and T4 (r=0.26–0.35, p<0.05) both before and after treatment. All other IIEF domain scores were only correlated with TSH prior to treatment (r=−0.26 - −0.23, p<0.05), but not T4 or T3 levels either before or after treatment.28
Similarly, Nikoobakht et al. observed significant differences in IIEF score between hypothyroid and control men (p=0.005). Unlike Krysiak et al., however, they did not find a relationship between IIEF score and TSH levels in hypothyroid men (r=0.088, p=0.681).29
In contrast, a large cross-sectional study of 5,471 patients by Corona et al. found slightly different results. The authors studied two different populations: 2,269 men from the general population 40–79 years old who participated in the European Male Aging (EMAS) study and 3,202 patients from a sexual medicine clinic. The authors found that 1.1% of men in the general population were hypothyroid, in contrast to 2.5% of the sexual clinic population. Within the EMAS population, no difference in patients reporting no erections between euthyroid and hypothyroid men (p>0.05) was observed. In the sexual medicine clinic population, however, a significant inverse relationship between TSH levels and ability to have erections sufficient for intercourse, even when controlling for age, smoking status, testosterone levels and chronic disease (r=−0.044, p=0.035) was observed. In the same population, no significant differences were observed in HSD rates in patients with thyroid dysfunction and those without, but a significant difference in the prevalence of severe ED in men with overt hypothyroidism when compared with euthyroid men was observed (p=0.003). However, when controlling for confounding factors such as smoking, testosterone levels, prolactin levels and chronic disease score, the association was not confirmed (p=0.023).30 (Table 3)
Table 3.
Thyroid derangements and sexual function in men
| Study | N | Findings |
|---|---|---|
| Carani et al.15 | 48 (34 hyperthyroid, 14 hypothyroid) | When compared to hyperthyroid men, hypothyroid men were more likely to have HSD (64.3 vs 17.6%), DE (64.3 vs 2.9%) and ED (64.3 vs 14.7%). Hyperthyroid men were more likely to have PE (7.1 vs 50%) 2–4 months after normalization of hormone levels, hypothyroid men had a decrease in ED (p<0.05), HSD (p<0.05), and DE (p<0.05). IELT increased from 21.8 ±10.9 to 7.4±7.2 (p<0.01). All IIEF domain scores increased but only intercourse satisfaction attained significance (p<0.05). 2–4 months after normalization of hormone levels, PE rate in hyperthyroid men fell to nearly the rate reported in the general population (15% vs. 14%). DE (2.9% to 0%), HSD (17.6% to 5.8%) and ED (14.7% to 2.9%) also decreased. IELT increased from 2.4±2.1 to 4.0±2.0 minutes (p<0.01) and IIEF scores for intercourse satisfaction and erectile function increased (p<0.05). |
| Krassas et al.25 | 142 (27 hyperthyroid, 44 hypothyroid, 71 control) | Increased levels of ED as measured by SHIM scores in both hypothyroid (p<0.0001) and hyperthyroid (p<0.0001) men compared to controls. After 1 year of treatment, no difference in SHIM scores between thyroid patients and controls was observed (p>0.05). Hypothyroid men had decreased rates of ED after thyroid treatment (p<0.0001). SHIM scores increased from 14.5 to 23.0 (p<0.0001). There was a positive correlation between SHIM scores and free T4 levels (r=0.413, p=0.005) and negative correlation with TSH levels (r=−0.669, P<0.001) in hypothyroid men. No differences between patients with or without thyroid antibodies were observed. Hyperthyroid men had decreased ED rates after thyroid treatment (p<0.0001). SHIM scores increased from 17.0 to 24.0 (p<0.001). There was no correlation between free T4 levels or TSH and SHIM scores in hyperthyroid patients. There was no difference in SHIM score between Graves’, nodular goiter and multinodular toxic goiter patients. |
| Krysiak et al.26 | 36 (12 overt hypothyroid, 12 subclinical hypothyroid, 12 control) | ED incidence was 83% in overt hypothyroidism and 42% in subclinical hypothyroidism (p<0.001 for both vs. controls; p<0.05 overt vs. subclinical) Men with overt hypothyroidism had decreased IIEF scores in all domains compared to controls (all p<0.001). Those with subclinical hypothyroidism only had decreased erectile function domain scores compared to controls (p<0.05). After 6 months of optimal treatment, both groups had all IIEF scores normalize to control values except erectile function domain (p<0.05 in both groups vs controls). However, post-treatment rate of ED was not statistically different than the rate in controls in either group (p>0.05 both). In men with overt hypothyroidism, erectile function, intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction domain scores were positively correlated with free T3 and T4 both before and after treatment (r=0.23–0.42, p<0.05). The same parameters were negatively correlated with TSH in both treated and non-treated patients (r=−0.49− −0.34, p<0.01). In men with subclinical hypothyroidism erectile function domain score was negatively correlated to TSH (r=−0.46–4.3, p<0.01) and positively correlated with free T3 and T4 (r=0.26–0.35, p<0.05) both before and after hormone treatment. All other domains were only correlated to TSH prior to treatment (r=− 0.26 − −0.23, p<0.05), but not T4 or T3 either before or after treatment. |
| Veronelli et al.7 | 122 (13 hyperthyroid, 109 controls) | ED incidence was higher in hyperthyroid men (76.9%) than controls (30.2%) (p=0.0001) There was no relationship between severity of ED and severity of thyroid derangement, duration of illness, or presence of thyroid antibodies (p>0.05 |
| Nikoobakht et al.27 | 40 (24 hypothyroid, 66 controls) | Significant difference in IIEF score between hypothyroid men and control patients (p=0.005). No correlation between IIEF score and TSH level in hypothyroid men (r=0.088, p=0.681) |
| Corona et al.28 | 5271 (2269 general population (EMAS), 3202 sexual medicine clinic) | Rate of hypothyroidism was 1.1% in the general population and 2.5% in the sexual clinic population. Rate of hyperthyroidism was similar in the two populations (3.5 vs 3.4%). In both populations, overt hyperthyroidism was associated with higher rates of severe ED even when controlling for smoking status, BMI, and testosterone level (HR=14 general, 16 sexual medicine, p<0.05 for both). Overt hypothyroidism was not associated with severe ED when controlling for confounders (p=0.023). In both populations, a negative correlation between TSH and ED severity was observed (r=−0.060, p=0.009 EMAS; r=−0.044, p=0.035 sexual medicine). In the EMAS group, a positive correlation between free T4, never having an erection (r=0.050, p=0.033), but no difference between patients who were euthyroid and hypothyroid in regards to never having an erection (p>0.05). Treatment of hyperthyroidism in the sexual medicine group significantly reduced rates of severe ED (p<0.05). In the sexual medicine clinic population, no significant difference in HSD between men with thyroid dysfunction and those without was observed. An inverse relationship between TSH and ability to have erection sufficient for intercourse even when controlling for age, smoking status, testosterone levels and chronic disease was observed (r=−0.044, p=0.035). |
| Keller et al31 | 25240 (6310 ED patients, 18930 controls) | Patients with ED were 1.64× more likely to have hyperthyroidism than controls (OR 1.64, (1.37–1.96), p<0.001). In age-based subgroups, men ages 4049 (OR 1.61 (1.14–2.26), p<0.01), 50–59 (1.24–2.43), p<0.01) and 60–69 (OR 1.84, p<0.001) had an increased rate of hyperthyroidism compared to controls. Men ages 18–29, 30–39, and 70–80 did not have a significantly increased rate of hyperthyroidism. |
| Corona et al.33 | 755 sexual dysfunction clinic patients | Patients with IELT <1 minute had significantly higher prevalence of hyperthyroidism than those without (p<0.05) |
| Ozturk et al.34 | 201 (107 PE, 94 control) | Levels of free T4, but not free T3 were significantly higher in the PE group compared to controls (p<0.05, p>0.05 respectively). TSH levels were not different between the two groups (p>0.05). |
| Corona et al.35 | 2652 sexual dysfunction clinic patients | Patients with hyperthyroidism had significantly higher rates of PE that the general sexual dysfunction clinic population (HR=2.98, p<0.05). TSH was found to affect ejaculation independent of prolactin and testosterone (r=0.047, p=0.019), with an increase in TSH associated with an increase in IELT (p>0.05) |
| Canat et al.36 | 102 (63 PE, 39 controls) | No differences in free T3 or free T4 and PE were observed (p>0.05 both). However, TSH levels were significantly lower in men with PE (p=0.017) |
| Waldinger et al.37 | 620 men with lifelong PE | Rates of hypo- and hyperthyroidism in men with lifelong PE were not significantly different than expected based on general population prevalence (p>0.05). |
| Cihan et al.38 | 49 men with untreated hyperthyroidism | 72% of men with untreated hyperthyroidism had PE. A direct correlation between TSH and IELT score was observed (r=0.37, p=0.04). Patients were then treated with medical or surgical management and retested 8–16 weeks after reaching euthyroid state. Post-treatment, IELT increased from 75.8 to 123 seconds (p=0.004) and rate of PE decreased to 25%. The IIEF erectile function, orgasmic function, sexual desire and overall satisfaction domain scores increased significantly (p=0.04, p=0.04, p=0.03, p=0.03 respectively). The increase in intercourse satisfaction domain score did not reach significance (p=0.09). |
| Maseroli et al32 | 4049 (3847 ED clinic patients, 202 general population) | No significant difference in the rate of overt hypo or hyperthyroidism between men with ED and the general population (p>0.05). In fact, subclinical hyperthyroidism had higher prevalence in the general population group (p<0.05). |
Sexual Dysfunction Outcomes After Normalization of Hormone Levels in Hypothyroid Men
Viewed together, the treatment of hypothyroidism in men shows some emerging trends in its effect on sexual function. In all studies, the rate of ED decreased after treatment.17, 27, 28 In studies using IIEF scores, total IIEF scores increased significantly.17, 28 However, there was difference in which IIEF domain scores improved with treatment. Carini et al. found a significant increase in only the intercourse satisfaction domain, with all other domains increasing but not significantly.17 In contrast, Kyrsiak et al. found in both men with overt and subclinical hypothyroidism that all domains except erectile function normalized to control levels after treatment.28 Thus, it is clear that the treatment of hypothyroidism improves male sexual function, especially ED. However, more research is needed to determine exactly how normalization of thyroid hormone levels affects other aspects of sexual functioning.
Animal Models of Hypothyroidism
Further evidence of hypothyroidism resulting in impaired erectile function has been observed in basic laboratory studies. Amadi et al. studied corpus cavernosum tissue in response to prostaglandin E1 (PGE1) and sildenafil as a proxy for erection in 40 rabbits: 10 that underwent thyroidectomy alone, 10 that underwent thyroidectomy and were treated with thyroxine, 10 that were given thyroxine alone and 10 control rabbits. Penile tissue from thyroidectomized rabbits did not respond to PGE1 and had a decreased response to sildenafil. Thyroidectomized rabbits treated with thyroxine, in contrast, had a normal response to PGE1 and decreased response to sildenafil. Rabbits who were treated with thyroxine but did not undergo thyroidectomy did not differ from control rabbits. Thus, the authors hypothesized that thyroid hormone is necessary for normal PGE1 activity in modulating erectile function.31
To more specifically assess how thyroid function affects sexual function, Yildirim et al. investigated the smooth muscle relaxation response in rabbit penile tissue. In normal physiology, penile smooth muscle relaxes, in part due to nitric oxide (NO) release from penile parasympathetic nerves and endothelial cells, and allows blood to flow into the trabecular spaces of the corpora cavernosa, resulting in penile tumescence. The authors compared 20 rabbits, 10 thyroidectomized, hypothyroid rabbits and 10 with normal thyroid function. Rabbits were sacrificed after 6 weeks and numerous substrates were used to induce smooth muscle relaxation. In hypothyroid rabbits, smooth muscle relaxation was diminished when compared to controls, and when adenosine, which increases NO release from endothelial cells, was added to penile tissue, no difference in smooth muscle relaxation was observed. Because of these results, the authors theorize that hypothyroidism impairs erectile function by down-regulating NO release, resulting in impaired smooth muscle relaxation.32 (Table 4)
Table 4.
Animal models of the effect of thyroid hormones of sexual functioning
| Study | N | Findings |
|---|---|---|
| Amadi et al29 | 40 (10 status post thyroidectomy alone, 10 status post thyroidectomy and repleated with thyroxine, 10 thryoxine treate alone, 10 contol) | Penile tissue from thyroidectomized rabbits had no response to PGE1 and decreased response to sildenafil. Thyroidectomized rabbits treated with thyroxine had normal response to PGE1 and decreased response to sildenafil. Rabbits who were treated with thyroxine but did not undergo thryoidectomy did not differ from control rabbits. |
| Yildirim et al.30 | 20 (10 status post thyroidectomy, 10 contol) | Relaxation of penile smooth muscle from hypothyroid rabbits was diminished when compared to controls (p<0.05). When adenosine, which increases NO release from endothelial cells, was added to the rabbit penile tissue, no difference in smooth muscle relaxation was observed (p>0.05). |
| Cihan et al.39 | 28 (7 thyroxine injected, 7 thyroxine injected + washout period, 7 saline injected, 7 with no injections) | Ejaculation model was based on seminal vesicle pressure and bulbospongiosus muscle contractility in response to para-chloramphetamine. Latency period between para-chloroamphetamine injection and ejaculation was significantly shorter in hyperthyroid group compared to other groups (202 vs 480, 465, 444 seconds, p<0.001 for all). The number of SV contractions, which studied the emission phase, was higher in hyperthyroid group than other 3 groups (p<0.05). In hyperthyroid rats, the number of SV contractions was inversely correlated with TSH (p=0.012, r=−0.94). However, all other emission phase parameters were the same in all 4 groups: baseline SV pressure, increase pressure from baseline after PCA, max amplitude of SV pressure in contractions (all p>0.05). The EMG activity area under the curve modeled expulsion and was greater in hyperthyroid group than other 3 groups (p<0.001). It also correlated with TSH values (p=0.016, r=−.094). Time separating first SV pressure increase (emission phase) and first organized BS EMG activity (expulsion phase) was not different between any of the groups (p>0.05 |
| Shifren et al.40 | 31,581 | A positive correlation between all domains of sexual dysfunction and a history of thyroid disorder was observed: desire (OR=1.18, p<0.05), orgasm (OR=1.22, p<0.05), and arousal (OR=1.19, p<0.05) |
Hyperthyroidism in Men
Several studies have investigated the relationship between male sexual dysfunction and hyperthyroidism. Researchers have sought to examine this relationship both by looking for sexual dysfunction in hyperthyroid men as well as looking for hyperthyroidism in men with sexual dysfunction.
In the same prospective study by Carani et al. discussed above, normalization of thyroid hormone levels in hyperthyroid patients for 2–4 months decreased the prevalence of PE from 50% to 15%, an incidence approximating that in the general population (14%). Further, the incidence of DE fell from 2.9% to 0%, HSD from 17.6% to 5.8% and ED 14.7% to 2.9%. IELT significantly increased from 2.4 ±2.1 to 4.0±2.0 minutes (p<0.01) and IIEF scores for intercourse satisfaction and erectile function increased significantly (p<0.05).17
A cross-sectional study of 13 hyperthyroid men and 109 controls found significantly higher rates of ED in hyperthyroid men using the IIEF: 76.9% compared to 30.2% (p=0.0001). Interestingly, no relationships between the severity of ED and that of thyroid derangement, duration of illness, or presence of thyroid antibodies were observed.9 Krassas et al. compared men with thyroid dysfunction to healthy controls using the SHIM and found that 70% of patients with hyperthyroidism vs. 34% of controls had ED (p<0.0001). Unlike what was observed in hypothyroid men, the level of hormonal derangement did not affect sexual functioning, as SHIM scores did not correlate with either free T4 or TSH levels. The authors found no difference in SHIM scores between different thyroid diseases, including Graves’ disease, multinodular toxic goiter and nodular toxic goiter. Patients were retested after one year of treatment, with an increase in SHIM scores from 14.5 to 23.0 (p<0.0001). At this point, men with thyroid dysfunction, which co-mingled both hypo- and hyperthyroid patients, did not have a statistically significant incidence of ED when compared to baseline control patients (p>0.05).27
A Taiwanese study by Keller et al. of 6,310 patients newly diagnosed with ED and 18,930 matched controls found that men with ED were 1.64 times more likely to have a prior diagnosis of hyperthyroidism when controlling for income, location, hypertension, diabetes mellitus, coronary artery disease, hyperlipidemia, obesity, and alcohol abuse. Of note, in subgroups of patients 18–29 (OR 2.05, 0.87–4.85, p>0.05), 30–39 (OR 1.03, 0.58–1.85, p>0.05) and 70–80 years old (OR 1.46 (0.86–2.49), p>0.05) no significant association between ED and hyperthyroidism was observed, but in men 40–49 (OR 1.61 (1.14–2.26), p<0.01), 50–59 (OR 1.74 (1.24–2.43), p<0.01) and 60–69 years old (OR 1.84, (1.20–2.84) p<0.001) a significant association was observed.33
In Corona et al.’s study of both EMAS and sexual medicine clinic populations discussed above, similar rates of overt primary hyperthyroidism in the men with sexual dysfunction and those in the general population were observed (0.2% vs 0.3%). In both populations, overt hyperthyroidism was strongly associated with ED (HR=14 EMAS, HR=16 sexual clinic, p<0.05 both). In the EMAS group, a positive correlation between free T4 levels and patients reporting never having an erection was observed (r=0.050, p=0.033). In both groups, TSH levels were inversely related to ED rates (r=−0.060, p=0.009 EMAS; r=−0.044, p=0.035). In the sexual health clinic population, treatment of hyperthyroidism decreased the incidence of severe ED from 28.6% to 0% (p<0.05).30
While the majority of studies support a relationship between both hypo- and hyperthyroidism and sexual dysfunction in men, Maseroli et al. published contrasting results. The authors compared 202 men from the Florentine spin-off of the EMAS comprising 3,847 patients from an outpatient ED clinic, finding no significant differences in the rate of overt hypo- or hyperthyroidism between the two groups (p>0.05). In fact, subclinical hyperthyroidism was more prevalent in the EMAS general population group (p<0.05).34 (Table 3)
Taken together, these studies demonstrate the lack of consensus in the association of hyperthyroidism and ED. Most studies point to a relationship, but dissenting studies should not be ignored. Additionally, studies disagree on whether there is a relationship between the severity of ED and the severity of hormone derangement. Other sexual dysfunctions such as DE and HSD are less researched and require further investigation to reach a meaningful consensus on their relationship with hyperthyroidism.
Premature Ejaculation and Hyperthyroidism
An area of special interest in the relationship between sexual function and hyperthyroidism has been premature ejaculation (PE). In a different study by Corona et al. of 755 men presenting to a sexual dysfunction clinic, those with rapid ejaculation—defined as ejaculation within 1 minute of vaginal intromission—had a significantly higher prevalence of hyperthyroidism (p<0.05).35 Similarly, Ozturk et al. observed in a case-control study of 107 men with PE and 94 healthy controls that levels of free T4, but not free T3, were significantly increased in the PE group when compared with controls (p<0.05, p>0.05 respectively). TSH levels were not different between the two groups (p>0.05). Of note, prolactin was also increased in the same population (p<0.05), though the authors did not control for this or other endocrinopathies.36
In a retrospective analysis of 2,652 patients at a sexual medicine clinic, the prevalence of PE in patients with overt hyperthyroidism was 42.4%, which was significantly higher than that in the general cohort of men at the sexual medicine clinic (HR=2.98, p<0.05). TSH level affected ejaculation time independent of age, testosterone or prolactin level (r=0.047, p=0.019) with an increase in TSH associated with an increase in IELT (p>0.05).37 In another case-control study of 63 men with PE and 39 controls by Canat et al., no differences in free T3 or free T4 (p>0.05) were observed, but TSH levels were significantly lower in men with PE (p=0.017).38 Waldinger et al. performed an observational study of 620 men with lifelong PE, which was defined as IELT less than 1 minute since first sexual intercourse experience with all partners >90% of the time. The authors observed that rates of hypo- and hyperthyroidism in men with lifelong PE were not significantly different than those in the general population (p>0.05). It is important to note that this study may have failed to find a relationship because it only included men with lifelong PE, excluding men who may have developed PE later in life as a result of thyroid dysfunction.39
Finally, Cihan et al. examined the relationship between hyperthyroidism and PE from the opposite perspective, starting with men with hyperthyroidism rather than sexual dysfunction. In a single center, prospective observational study of 49 men with untreated hyperthyroidism, 72% had PE, and a direct correlation between TSH levels and IELT was observed (r=0.37, p=0.04). Patients were managed medically or surgically and retested 8–16 weeks after achieving a euthyroid state. Post-treatment, a significant increase in IELT from 75.8 to 123 seconds (p=0.004), and a decrease in PE incidence to 25%, were observed. Furthermore, an increase in all IIEF domain scores was observed, with significant increases in erectile function, orgasmic function, sexual desire and overall satisfaction domain scores (p=0.04, p=0.04, p=0.03, p=0.03, respectively), while intercourse satisfaction scores increased, but not significantly (p=0.09). This study was limited by a high attrition rate; only 24 of 49 patients attended follow-up.40
Taken together, these studies support a relationship between PE and thyroid disease, though thyroid dysfunction does not appear to be the only contributing factor. This explains why studies that investigated only men with PE found variable results, as only a fraction of subjects likely had PE resulting from thyroid derangement. (Table 3)
Sexual Dysfunction Outcomes After Normalization of Hormone Levels in Hyperthyroid Men
There are several trends in how normalization of hormone levels affect hyperthyroid men. In all studies investigating ED, there was a decrease in ED rate after normalization of thyroid hormone levels17, 27, 30, 40. Two studies found a decrease in PE after treatment, with rates similar to the general population in the Carani study. Similarly, both studies saw increase in IELT and several IIEF domain scores. However, these studies differed in which domains of the IIEF were affected by treatment.17, 40 In Carani et al. intercourse satisfaction and erectile function significantly increased,17 whereas Cihan et al. observed increases in all domain scores, with no significant increase in intercourse satisfaction domain score.40 Carani et al. further saw a decrease in DE and HSD rates in their popualtion, but these metrics were not investigated by other studies.17
Animal Models and Hyperthyroidism
To further investigate the relationship between hyperthyroidism and PE, Cihan et al. used a rat model with 4 groups of 7 rats each. Hyperthyroidism was induced in one group using 14 days of L-thyroxine injections. A recovery group was given the same treatment followed by a 28-day washout. A control group was injected with saline for 7 days while the sham group got no injections. An ejaculation model was based on seminal vesicle pressure and bulbospongiosus muscle contractility in response to para-chloramphetamine. The latency period between para-chloroamphetamine injection and ejaculation was significantly shorter in the hyperthyroid rat group compared to the other groups (202 vs. 480, 465, 444 seconds, respectively, p<0.001 for all). These data demonstrate not only that hyperthyroidism can result in PE, but also that the effect is reversible. The emission phase was investigated by examining seminal vesicle (SV) contractions, with the number of SV contractions being significantly higher in the hyperthyroid group than the other 3 groups (p<0.05). In hyperthyroid rats, the number of SV contractions was inversely correlated with TSH levels (p=0.012, r=−0.94). However, baseline SV pressure, increase in pressure from baseline after PCA, and the maximal amplitude of SV pressure in contractions were the same in all 4 groups (p>0.05). The seminal expulsion phase was modeled using EMG activity, with the area under the curve being greater in the hyperthyroid than the other 3 groups (p<0.001), and again correlated with TSH levels (p=0.016, r=−0.094).41 (Table 4)
Women
While work examining the effects of thyroid hormone levels on male sexual dysfunction is more advanced, only recently has the link between thyroid derangement and female sexual function been examined. In 2008 Shifren et al. performed a cross-sectional study of 31,581 adult women, examining the relationship between sexual function and thyroid disease. The authors used the Changes in Sexual Functioning Questionnaire short form, which contains subset of questions for sexual desire, arousal and orgasm. Thyroid disease was determined using patient reported medical history. The authors observed an increased risk of sexual dysfunction in women with a history of thyroid disorder for all questionnaire domains: desire (OR 1.18 (1.03–1.34), p<0.05), orgasm (OR 1.22 (1.02–1.45), p<0.05), and arousal (OR 1.19 (1.01–1.40), p<0.05).42 In a similar cross-sectional study of 1,119 Chinese women, Han et al. used the Chinese version of the FSFI and found that elevated free T3 was an independent risk factor for FSD (p<0.001) with TSH and free T4 also significantly affecting FSFI score (p=0.484 and p=0.17, respectively). However, women with overt hyper- or hypothyroidism were excluded from this study.43 Pasquali et al. compared 104 women with thyroid disease to 53 control patients. They found the prevalence of FSD in participants with thyroid disease was 46.1% compared to 20.7% in controls (p>0.05). Interestingly, 53% of post-menopausal women with thyroid disease compared to 55% without thyroid disease had FSD. In the pre-menopausal group, 42% of women with thyroid disease had FSD compared to 20% of those without thyroid disease. While the authors did not note the significance of these figures, they appear to suggest that thyroid disease increase the rate of FSD in pre-menopausal but not post-menopausal women.44
Hypothyroidism in Women
Several studies have shown that hypothyroidism has pervasive effects across domains of female sexual function. Veronelli et al. performed a case-control study with 24 hypothyroid women and 26 healthy controls. Using the FSFI questionnaire, the authors found significantly decreased scores in every FSFI domain (all p<0.05) and overall scores (p<0.01) in hypothyroid women when compared to controls. They also found that the presence of anti-thyroid antibodies was inversely correlated with FSFI score (p<0.05). Of note, when hypothyroid women were compared to euthryoid women who were obese or diabetic, the hypothyroid women had higher rates of sexual dysfunction.45
Oppo et al. performed a similar case-control study, including 17 hypothyroid women and 30 controls. Similar to the Veronelli study’s results, the authors found all FSFI domain scores were significantly reduced in hypothyroid women when compared with controls (p<0.0001 for desire, arousal/lubrication, orgasm and pain; p<0.0005 for satisfaction). Additionally, patients were contacted after 3 months of treatment, at which point they had reached a euthyroid state and desire, satisfaction and pain domains had normalized to control levels (p>0.05). However, arousal/lubrication and orgasm domain scores remained decreased when compared with control patients (p<0.01 for both), although the orgasm domain score did increase significantly from pre-treatment level (p<0.05). In contrast with Veronelli et al., the authors found an inverse correlation between FSFI domain scores and TSH levels (p=0.001–0.03) and a positive correlation between T4 levels and FSFI domain scores (p=0.0001–0.01).46
In the same Pasquali et al. study described above, they stratified by thyroid state and compared 22 hypothyroid women to 53 controls. They found that only desire, arousal and lubrication were significantly decreased in the hypothyroidism cohort (p<0.05, p<0.05, p<0.01 respectively). While they found increased rates of FSD in the hypothyroid group compared to controls (41% vs 20.7%), these were not significant (p>0.05).44 While the data remain limited, studies have overall shown that women with overt untreated hypothyroidism have higher levels of sexual dysfunction. Unlike in men, a connection between thyroid autoantibodies and sexual dysfunction was observed in at least one study. Studies differ on whether sexual effects are more pronounced as hormonal derangements worsen.
Subclinical Hypothyroidism in Women
While overt hypothyroidism appears to have a significant effect on female sexual function, the effect of subclinical hypothyroidism is less clear. Hong et al. studied 138 middle-aged women with subclinical hypothyroidism compared to 948 aged matched, healthy controls and found no difference in total FSFI or any domain scores (p>0.05 all). Similarly, the frequency of FSD was 68.4% in controls and 67.4% in subclinical hypothyroidism patients, which was not significantly different even after controlling for confounding variables (p>0.05).25 Conversely, in a smaller study by Krysiak et al. stratifying subclinical hypothyroidism by autoimmune and non-autoimmune mechanisms, significant differences in sexual function compared to healthy controls were observed. The rate of FSD was 41% in the non-autoimmune subclinical hypothyroid group and 59% in the autoimmune subclinical hypothyroid group, both significantly higher than the control group rate of 17% (p<0.01, p<0.001, respectively). For women with nonautoimmune subclinical hypothyroidism, significant differences in total FSFI score (p<0.01) and desire (p<0.01), arousal (p<0.05), lubrication (p<0.01) and dyspareunia (p<0.01) scores were observed when compared to healthy women. For women with autoimmune disease, total FSFI (p<0.01) and all domain scores were significantly lower than those in euthyroid women (p<0.05 for all). For women with both autoimmune and non-autoimmune subclinical hypothyroidism, an inverse relationship between TSH level and total FSFI score (p<0.05), along with the sexual desire (p<0.001 and p<0.01 respectively), sexual arousal (p<0.05), lubrication (p<0.05 and p<0.01 respectively), and dyspareunia (p<0.05 and p<0.01 respectively) domains was observed. No relationship was observed in either group between sexual satisfaction or orgasm domains and TSH levels (p>0.05).47 Thus, the above studies indicate that the data regarding the impact of subclinical hypothyroidism on female sexual function are conflicting and incomplete.
Hashimoto’s Thyroiditis in Women
While others have demonstrated that overt and subclinical hypothyroidism can impact sexual function, Oppo et al., Krysiak et al. and Pasquali et al. investigated if the presence of Hashimoto’s Thyroiditis (HT), even in euthyroid women, resulted in sexual dysfunction. In another subset of the Oppo et al. study discussed above, researchers compared 17 women with HT to 30 controls. They found that only the FSFI desire domain score was lower when compared with controls (p<0.0005; all others p>0.05). No associations between any FSFI domains and TSH or T4 levels were observed (p>0.05).46 In the study by Krysiak et al. discussed above, the authors also compared 16 euthyroid women with HT with 18 controls, finding that the rate of FSD as measured by FSFI score was significantly lower in patients with subclinical autoimmune hypothyroidism (37% vs 59%, p<0.05) but higher than that in controls (17% p<0.01). This implies that both the effect of the autoantibodies and the hormonal derangement result in thyroid dysfunction. The authors further supported the effect of autoantibodies on sexual function by demonstrating an inverse relationship in thyroid peroxidase (TPO) antibody titers and total FSFI scores along with the sexual desire, lubrication, and sexual satisfaction domains in both euthyroid HT and autoimmune subclinical hypothyroidism (p<0.05 for all).47 Pasquali et al. compared 45 euthyroid women with HT to 53 healthy controls. Of note, 15 of the HT women were treated with thyroxine. The authors found no significant differences in rate of FSD, FSFI score or any single domain for FSFI between the HT group and the controls.44 The above studies show that current data on the impact of euthyroid HT on sexual function in women are conflicting. However, as with hypothyroidism in women, the presence of autoantibodies is again implicated in the pathogenesis of sexual dysfunction in this patient group.
Hyperthyroidism in Women
In a study by Atis et al. of 40 Turkish women with newly diagnosed primary hyperthyroidism and 40 aged matched controls, sexual dysfunction was evaluated using the Turkish version of the FSFI, with 60% of hyperthyroid patients having FSD in comparison with 32.5% of controls (p=0.014). In addition, women with hyperthyroidism had significantly worse total FSFI scores as well as across all individual domains (p<0.04 for all). Overall, an inverse correlation between FSFI score and free T3, free T4 and a direct correlation between FSFI score and TSH level was observed (p=0.0001 for all).23 In yet another subset of patients from the Oppo et al. study, 22 pre-treatment hyperthyroid women—20 with graves disease and 2 with toxic multinodular goiter—were compared to the same 30 controls. Using the FSFI, the authors observed that all sexual function domains had significantly reduced scores in hyperthyroid women: desire (p<0.005), arousal/lubrication (p<0.001), orgasm (p<0.001), satisfaction (p<0.05) and pain (p<0.005). The domains of desire, arousal/lubrication, and orgasm were directly correlated with TSH (p=0.02, p=0.0s03, p=0.03, respectively). While only desire was inversely correlated to T4 (p=0.003). After 3 months of treatment with methimazole, FSFI domains for sexual desire, arousal/lubrication, satisfaction, and pain normalized to control levels (p<0.05 for all). No significant increase in orgasm scores was observed when compared to pretreatment scores (p>0.05) and scores continued to be higher than in control patients (p<0.05).46 Pasquali et al. compared 53 hyperthyroid to 53 control women. There were no significant differences in rate of FSD, total FSFI score or any FSFI domain, except for desire, which was significantly lower than in controls (p<0.05). When looking specifically at 19 women with hyperthyroidism secondary to nodular goiter compared to the same 53 control women, the prevalence of FSD was 68.4%, which was significantly higher than that in the control group (20.7%, p<0.005). Additionally, there were decreases in arousal (p<0.01), desire (p<0.01), lubrication (p<0.01), orgasm (p<0.05), and satisfaction (p<0.05). Finally, the authors observed a significant inverse correlation between TSH level and FSFI score (r =−0.7, P= 0.01) in the nodular goiter group.44
Thus, there is conflicting information on whether hyperthyroidism affects female sexual function, with two studies finding a relationship and one failing to find a relationship (Table 5).
Table 5.
Thyroid derangements and sexual functioning in women
| Study | N | Findings |
|---|---|---|
| Han et al.41 | 1,119 Chinese women without overt hyper or hypothyroidism | Elevated free T3 was an independent risk factor for FSD (p<0.001). TSH and free T4 did significantly affect FSFI score (p=0.484 and p=0.17 respectively). |
| Veronelli et al.43 | 50 (24 hypothyroid 26 controls) | Hypothyroid women had significantly decreased scores in all FSFH domains (all p<0.05) and overall FSFI score (p<0.01), when compared to controls. The presence of antithyroid antibodies was inversely correlated with FSFI score (p<0.05). |
| Oppo et al.44 | 86 (17 hypothyroid, 22 hyperthyroid, and 17 euthyroid Hashimoto’s and 30 controls) | All FSFI domain scores were significantly reduced in hypothyroid and hyperthryoid women compared to controls (p=0.0001–0.05). In euthyroid HT only the desire domain of the FSFI was decreased compared to controls (p<0.0005). In hypothyroid women, an inverse correlation between FSFI domain scores and TSH levels was observed (p=0.001–0.03). In hyperthyroid women, a direct correlation between TSH and only the desire, arousal/lubrication and orgasm domains was observed (p=0.003–0.03). Hypothyroid women demonstrated a positive correlation between T4 and all FSFI domain scores (p=0.0001–0.01), while hyperthyroid women had a negative correlation between T4 and only the desire domain (p=0.003). No associations between any FSFI domain score and TSH or T4 in euthyroid HT women were observed(p>0.05) After 3 months of treatment to achieve euthyroid state, desire, satisfaction and pain domain scores normalized to control levels in previously hypothyroid women (p<0.05). In hyperthyroid women sexual desire, arousal/lubrication, satisfaction, and pain normalized (p<0.05) |
| Pasquali et al.42 | 157 (53 control, 18 hyperthyroid, 22 hypothyroid, 45 Hashimoto’s thyroiditis, 19 nodular goiter) | Overall rate of FSD in women with thyroid disease was 46.1% versus 20.7% in controls (p>0.05). FSFI score was significantly higher overall 20.1 ±7.1 vs 25.6±4.7 (P<0.001), but no single group had difference in FSFI score from control that reached significance. The hyperthyroid group had decreased desire (p<0.05). The hypothyroid group had decreased desire (<0.05), arousal (p<0.05) and lubrication (p<0.01). The Hashimoto’s thyroiditis group had no differences in FSFI domains than control. The nodular goiter group had decreased arousal (p<0.01), desire (P<0.01), lubrication (p<0.01), orgasm (p<0.05) and satisfaction (p<0.05). In the nodular group there was an inverse relationship between TSH and FSFI score (r=−0.7, P=0.01) |
| Krysiak et al.45 | 68 (16 euthyroid Hashimoto’s Thryoiditis, 17 nonautoimmune subclinical hypothyroidism, 17 autoimmune hypothyroidism, 18 control) | The rate of FSD was 41% in the non-autoimmune subclinical hypothyroid group and 59% in the autoimmune subclinical hypothyroid group, compared to 17% in the control group (p<0.01, p<0.001, respectively). In women with autoimmune disease all FSFI domains were lower than euthryoid women compared to only desire, arousal, lubrication and dyspareunia in nonautoimmune disease women (p<0.05 all). In boht groups there was an inverse relationship between TSH level and total FSFI score (p<0.05). In euthryoid HT women, the rate of FSD, 17%, was significantly higher than controls but lower than subclinical autoimmune hypothryoidism (p<0.01 and p<0.05 respectively). There was an inverse relationship between thyroid peroxidase antibody titiers and total FSFI scores (p<0.05). |
Sexual Dysfunction Outcomes After Normalization of Thyroid Hormone Levels in Women
The primary study examining sexual dysfunction in women after thyroid hormone normalization is by Oppo et al. The authors found that after 3 months of treatment, desire, satisfaction and pain domain scores of the FSFI increased to control group levels in both hypo and hyperthyroid women. In both groups, the orgasm domain score failed to increase to control level. However, the hypothyroid group had a significant increase from baseline while the hyperthyroid group did not. The hyperthyroid, but not the hypothyroid group, had arousal/lubrication domain scores increase to similar values as the control group.46 Less directly, Pasqueli et al. observed that euthyroid women with HT, 33% of whom were on thyroid treatment, had no difference in FSD or FSFI scores when compared to controls.44 Together, these studies point to the reversibility of some, but likely not all, aspects of sexual dysfunction in women with thyroid derangement. Notably, the lack of orgasm remained an issue in both hypo- and hyperthyroid groups, suggesting lack of reversibility of this symptom of FSD. Additional study of this relationship is needed to reach definitive conclusions.
Conclusions
Most studies to date have demonstrated a relationship between thyroid and sexual dysfunction in both men and women. This relationship is further supported by several basic research studies in animals highlighting specific molecular mechanisms that define this relationship. In addition, several studies showed at least partial resolution of sexual dysfunction symptoms after treatment of thyroid disease, which is compatible with research confirming the presence of thyroid hormone receptors in the male and female genitalia.3 When treating patients with sexual dysfunction, physicians should consider endocrine causes such as hyper and hypothyroidism.48
However, more research is needed to fully understand the relationship between thyroid and sexual dysfunction. Agreement regarding which parameters of sexual function are affected by thyroid disease remains elusive. Additionally, many of the studies are limited by small sample size, which may result in an underappreciation of the effects of thyroid dysfunction on sexual function. In fact, studies such as that by Veronelli et al., where women with thyroid disease were observed to have higher rates of sexual dysfunction than either obese or diabetic women suggest that the extent to which thyroid disease impacts sexual function has likely been underestimated.45
In addition to conducting larger studies, there are several avenues that future research could investigate. First, future work is needed to examine specific thyroid conditions to better understand if it is the hormonal derangement, the presence of autoantibodies, or other factors that disrupt sexual functioning. While several studies have shown that normalization of thyroid function improves sexual functioning, normal scores were not achieved in several FSFI and IIEF domains in several studies. Longer follow-up is needed to determine if levels will normalize over time or if there is another factor at play that prevents normalization of sexual functioning upon treatment. Additionally, in euthyroid women with HT, 2 of 3 studies observed sexual dysfunction, and the presence of TPO antibodies was associated with lower FSFI scores.44, 46, 47 Conversely in a study of hypothyroid men, there was no difference in sexual function between those with and without autoantibodies.27 These studies support the conclusion that not only hormone levels, but auto-antibodies as well, might negatively impact sexual function in women but not men.
Another avenue of future research involves improving our understanding of the role of menopause in the relationship between female sexual function and thyroid function. Pasquali et al. hinted at a relationship between thyroid disease, FSD and menopause when they observed higher rates of FSD in premenopausal women with thyroid disease, but conversely in the postmenopausal women, it was the control group that had a slightly higher rate of sexual dysfunction.44 A more detailed examination into the interplay between the hormonal changes of menopause and those of thyroid disease is needed to fully understand the relationship between these disorders.
Acknowledgments
Role of the Funding Source
This work is supported in part by a Mentored Career Development Award (K08DK115835–01) from the National Institute of Diabetes and Digestive and Kidney Diseases to A.W.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper DS, Ladenson PW. The Thyroid Gland In: Gardner DG, Shoback D. eds.Greenspan’s Basic & Clinical Endocrinology, 10e, McGraw-Hill; New York, NY, 2018. [Google Scholar]
- 2.Maitra A The endocrine system In: Kumar V, Abbas AK, Aster JC, ed. Pathologic basis of disease, 9th edition, Elsevier Saunders: 2015, pp 1073–1139. [Google Scholar]
- 3.Carosa E Lenzi A Jannini EA Thyroid hormone receptors and ligands, tissue distribution and sexual behavior. Mol Cell Endorinol, 2018;467:49–59. [DOI] [PubMed] [Google Scholar]
- 4.McCabe MP Sharlip ID, Atalla E, Balon R, Fisher AD, Laumann E, Lee SW, Lewis R, Segraves RT. Definitions of sexual dysfunctions in women and men: A consensus statemen from the fourth Internal Consultation on Sexual Medicine 2015. J Sex Med, 2016;13(2):135–43. [DOI] [PubMed] [Google Scholar]
- 5.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urol 1997, 49(6):822–30. [DOI] [PubMed] [Google Scholar]
- 6.Rosen RC, Cappelleri JC. Gendrano N 3rd: the international index of erectile function (IIEF): a state-of-the-science review. Int J Impot Res 2002;14:226–44. [DOI] [PubMed] [Google Scholar]
- 7.Lizza EF, Rosen RC 1999. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res 11:141–143. [DOI] [PubMed] [Google Scholar]
- 8.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB 2000. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol 163:460–463. [PubMed] [Google Scholar]
- 9.Veronelli A, Masu A, Ranieri R, Rognoni C, Laneri M, Pontiroli AE. Prevalence of erectile dysfunction in thyroid disorders: comparison with control subjects and with obese and diabetic patients. Int J Impot Res 2006;18:111–4. [DOI] [PubMed] [Google Scholar]
- 10.Serefoglu EC, McMahon CG, Waldinger MD, Althof SE, Shindel A, Adaikan G, et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. Sex Med. 2014;2:41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarty EJ, Dinsmore WW: Premature ejaculation: treatment update. Int J STD AIDS 2010; 21: 77–81. [DOI] [PubMed] [Google Scholar]
- 12.Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J: The premature ejaculation relevanceand attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol 2007; 51: 816. [DOI] [PubMed] [Google Scholar]
- 13.Jannini EA, Lenzi A. Epidemiology of premature ejaculation. Curr Opin Urol. 2005;15:399–403. [DOI] [PubMed] [Google Scholar]
- 14.Rowland DL and Motofei IG: The aetiology of premature ejaculation and the mind—body problem: implications for practice Int J Clin Pract 2006; 61: 77. [DOI] [PubMed] [Google Scholar]
- 15.Canat L, Erbin A, Canat M, Dinek M, Çaşkurlu T. Assessment of hormonal activity in patients with premature ejaculation. Int Braz J Urol 2017: 43:311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldinger MD Schweitzer DH. Retarded ejaculation in men: an overview of psychological and neurobiological insights. World J Urol, 2005;23(2):76–81. [DOI] [PubMed] [Google Scholar]
- 17.Carani C, Isidori AM, Granata A, Carosa E, Maggi M, Lenzi A, Jannini EA. Multicenter study on the prevalence of sexual symptoms in male hypo and hyperthyroid patients. J Clin. Endocrinol Metab 2005;90(12)6472–9. [DOI] [PubMed] [Google Scholar]
- 18.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R Jr. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther, 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein I, Kim NN, Clayton AH, DeRogatis LR, Giraldi A, Parish SJ, Pfaus J, Simon JA, Kingsberg SA, Meston C, Stahl SM, Wallen K, Worsley R. Hypoactive sexual desire disorder: International society for the study of women’s sexual health (ISSWSH) expert consensus panel review. Mayo Clin Proc, 2017;92(1):114–128. [DOI] [PubMed] [Google Scholar]
- 20.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA 1999;281:537–44. [DOI] [PubMed] [Google Scholar]
- 21.Aslan E, Beji NK, Gungor I, Kadioglu A, Dikencik BK. Prevalence and risk factors for low sexual function in women: A study of 1,009 women in an outpatient clinic of a university hospital in Instabul. J Sex Med 2008;5:2044–52. [DOI] [PubMed] [Google Scholar]
- 22.Bargiota A, Dimitropoulos K, Tzortzis V, Koukoulis GN. Sexual dysfunction in diabetic women. Hormones (Athens). 2011, 3:196–206. [DOI] [PubMed] [Google Scholar]
- 23.Atis G, Dalkilinc A, Altuntas Y, Atis A, Gurbuz C, Ofluoglu Y, Cil E, Caskurlu T. Hyperthyroidism: a risk factor for female sexual dysfunction. J Sex Med 2011; 8: 2327–33. [DOI] [PubMed] [Google Scholar]
- 24.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999, 281: 537–44. [DOI] [PubMed] [Google Scholar]
- 25.Hong H, Lee HJ, Kim SM, Jeon MJ, Shin DW, Choi HC, Lee H, Yun JM, Cho B, Lee SM. Subclinical hypothyroidism is not a risk factor for female sexual dysfunction in Korean middle-aged women. Thyroid 2015;25(7):784–8. [DOI] [PubMed] [Google Scholar]
- 26.Rehman KU, Asif Mahmood M, Sheikh SS, Sultan T, Khan MA. The Female Sexual Function Index (FSFI): Translation, Validation, and Cross-Cultural Adaptation of an Urdu Version “FSFI–U.” Sexual Medicine. 2015;3(4):244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krassas GE, Tziomalos K, Papadopoulou F, Pontikides N, Perros P. Erectile Dysfunction in Patients with Hyper- and Hypothyroidism: How Common and Should We Treat? J Clin Endocrinol Metab 2008; 93(5): 1815–9. [DOI] [PubMed] [Google Scholar]
- 28.Krysiak R, Szkróbka W, Okopień. The effect of L-thyroxine treatment on sexual function and depressive symptoms in men with autoimmune hypothyroidism. Pharmacol Rep 2017; 69:432–7. [DOI] [PubMed] [Google Scholar]
- 29.Nikoobakht MR, Aloosh M, Nikoobakht N, Mehrsay A, Biniaz F, Karjalian MA. The role of hypothyroidism in male infertility and erectile dysfunction. Urol J 2012;9(1):405–9. [PubMed] [Google Scholar]
- 30.Corona G, Wu FCW, Forti G, Lee DM, O’Connor DB, O’Neill TW, Pendleton N, Bartfai G, Boonen S, Casaneuva FF, Finn JD, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean MEJ, Punab M, Vanderschueren D, Jannini EA, Mannucci E, Maggi M, EMAS Study Group. Thyroid hormones and male sexual function. Int J Androl 2012; 35: 668–79. [DOI] [PubMed] [Google Scholar]
- 31.Amadi K, Sabo MA, Sagay AS. Thryoid hormone: the modulator of erectile function in the rabbit. Niger J Physiol Sci 2006; 21:83–9. [DOI] [PubMed] [Google Scholar]
- 32.Yildirim MKm Bagcivan I, Sarac B, Kilicarslan, Yildirim S, Kaya T. Effect of hypothyroidism on the purinergic response of corpus caversonal smooth muscle in rabbits. Int Urol Nephrol 2008;40:691–9. [DOI] [PubMed] [Google Scholar]
- 33.Keller J, Chen YK, Lin HC. Hyperthyroidism and erectile dysfunction: a population-based case-control study. Int J Impot Res 2012; 24:242–6. [DOI] [PubMed] [Google Scholar]
- 34.Maseroli E, Corona G, Rastrelli G, Lotti F, Cipriani S, Forti G, Mannucci E, Maggi M. Prevalence of endocrine and metabolic disorders in subjects with erectile dysfunction: a comparative study. J Sex Med 2015;12:956–65. [DOI] [PubMed] [Google Scholar]
- 35.Corona G, Petrone L, Mannucci E, Jannini EA, Mansani R, Magini A, Giommi R, Forti G, Maggi M. Pyscho-biological correlates of rapid ejaculation in patients attending andrologic unit for sexual dysfunctions. Eur Urol 2004; 46: 615–622. [DOI] [PubMed] [Google Scholar]
- 36.Oztürk MI, Koca O, Tüken M, Keleş MO, İlktaç A, Karaman MI. Hormonal evaluation in premature ejaculation. Urol Int 2012; 88:454–8. [DOI] [PubMed] [Google Scholar]
- 37.Corona G, Jannini EA, Lotti F, Boddi V, De Vita G, Forti G, Lenzi A, Mannucci E, Maggi M. Premature and delayed ejaculation: two ends of a single continuum influenced by hormonal milieu. Int J Andro 2010; 34: 41–8. [DOI] [PubMed] [Google Scholar]
- 38.Canat L, Erbin A, Canat M, Dinek M, Çaşkurlu T. Assessment of hormonal activity in patients with premature ejaculation. Int Braz J Urol 2017: 43:311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldinger MD, Zwinderman AH, Olivier B, Schweitzer DH. Thyroid-stimulating hormone assessments in a Dutch cohort of 620 men with lifelong premature ejaculation without erectile dysfunction. J Sex Med 2005; 2:865–70. [DOI] [PubMed] [Google Scholar]
- 40.Cihan A, Demir O, Demir T, Aslan G, Comlekci A, Esen A. The relationship between premature ejaculation and hyperthyroidism. J Urol 2009; 181:1273–80. [DOI] [PubMed] [Google Scholar]
- 41.Cihan A, Murat N, Demir O, Aslan G, Demir T, Gidener S, Esen AA. An experimental approach to the interrelationship between hyperthyroidism and ejaculation latency time in male rats. J Urol 2009; 181:907–12. [DOI] [PubMed] [Google Scholar]
- 42.Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women. Obstet Gynecol 2008; 112(5):970–8. [DOI] [PubMed] [Google Scholar]
- 43.Han L, Yang H, Zhao Q, Han Q, Zeng L, Tan H, Zhu J. Elevated free triiodothyronine may lead to female sexual dysfunction in Chinese urban women: A hospital-based survey. Sci Rep 2017; 7: 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasquali D,MI Maiorino MI, Renzullo A, Bellastella G, Accardo G, Esposito D, Barbato F, Esposito K. Female sexual dysfunction in women with thyroid disorders J Endocrinol Invest 2013;36(9):729–33. [DOI] [PubMed] [Google Scholar]
- 45.Veronelli A, Mauri C, Zecchini B, Peca MG, Turri O, Valitutti MT, dall’Asta C, Pontiroli AE. Sexual dysfunction is frequent in premenopausal women with diabetes, obesity, and hypothyroidism and correlates with markers of increased cardiovascular risk. A preliminary report. J Sex Med 2009;6:1561–8. [DOI] [PubMed] [Google Scholar]
- 46.Oppo A, Franceschi E, Atzeni F, Taberlet A, Mariotti S. Effects of hyperthyroidism, hypothyroidism, and thyroid autoimmunity on female sexual function. J Endocrinol Invest 2011;34:449–53. [DOI] [PubMed] [Google Scholar]
- 47.Krysiak R, Drosdzol-Copt A, Skrzypulec-Plintat S, Okopien B. Sexual function and depressive symptoms in young women with thyroid autoimmunity and subclinical hypothyroidism. Clin Endocrinol; 2016, 84: 925–31. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen HMT, Gabrielson AT, Hellstrom WJG. Erectile Dysfunction in Young Men—A Review of the Prevalence and Risk Factors. Sex Med Rev 2017;5:508–520. [DOI] [PubMed] [Google Scholar]