Abstract
Retinal injuries and diseases are major causes of human disability involving vision impairment by the progressive and permanent loss of retinal neurons. During development, assembly of this tissue entails a successive and overlapping, signal-regulated engagement of complex events that include proliferation of progenitors, neurogenesis, cell death, neurochemical differentiation and synaptogenesis. During retinal damage, several of these events are re-activated with both protective and detrimental consequences. Purines and pyrimidines, along with their metabolites are emerging as important molecules regulating both retinal development and the tissue’s responses to damage. The present review provides an overview of the purinergic signaling in the developing and injured retina. Recent findings on the presence of vesicular and channel-mediated ATP release by retinal and retinal pigment epithelial cells, adenosine synthesis and release, expression of receptors and intracellular signaling pathways activated by purinergic signaling in retinal cells are reported. The pathways by which purinergic receptors modulate retinal cell proliferation, migration and death of retinal cells during development and injury are summarized. The contribution of nucleotides to the self-repair of the injured zebrafish retina is also discussed.
Keywords: ATP, Nucleotides, Adenosine, P2 receptors, P1 receptors, Retina diseases
1. Introduction
Neurodegeneration after retinal injury or diseases like retinitis pigmentosa, age-related macular degeneration (AMD), glaucoma and diabetic retinopathy is a major cause of human disability involving vision impairment by the progressive and irreversible loss of retinal neurons. Drug-based therapies, using agonists and antagonists of signaling molecules and enzyme inhibitors are promising strategies for the prevention of damage and/or minimization of disease progression. For these strategies, identification of molecules and their receptors that are activated by tissue damage, as well as the mechanisms of synthesis and release of these substances are of great value. Unfortunately, when neuronal loss is complete, drug-based strategies per se are ineffective in fully restoring visual function.
New strategies for cell-based therapies to replace lost neurons are currently being developed. In the retina, a particular interesting strategy is the induction of endogenous stem-like cells to replace lost neurons. After the seminal work of Fischer and Reh (2001) showing that Müller cells are capable of re-entering cell cycle in response to damage, countless attempts to obtain retinal neurons derived from retinal glia cells were performed. However, although retinal cell generation and differentiation during development is highly conserved across species, the regenerative capacity for an efficient repair of the adult retina is very limited in mammals.
A growing and exciting strategy for retinal repair is coming from the observation that endogenous glia can be reprogrammed to produce neurons. However, many issues need to be addressed for this strategy to be successful. Studies on retinal development will help answer key questions such as which particular environmental conditions and signaling molecules lead to growth of neurons or generation of glia-derived neurons that can assume an appropriate functional connectivity in the mature tissue.
During development, the formation of the retina involves a successive and overlapping engagement of complex events that includes proliferation of progenitors, neurogenesis, cell death, neurochemical differentiation and synaptogenesis. Migration to the correct layer at the right time during the conversion of the developing retinal neuroepithelium into the mature retina is also a critical event for the successful establishment of synaptic connections by the retinal cells. These events are controlled by signaling molecules and ATP and its metabolites, along with pyrimidine analogs, are emerging as important molecules regulating retinal development. They are also involved in the regenerative response of glial cells during retinal injury in non-mammalian vertebrates, notably in zebrafish (Battista et al., 2009), where endogenous glial cells exit their quiescent state to undergo proliferation and neuronal differentiation. Like heparin-binding EGF-like growth factor (HB-EGF), nucleotides and their metabolites can induce the expression of pluripotency and proneural transcription factors such as lin-28, achaete-scute homolog 1a (ascl1a) and sex determining region Y-box 2 (Sox2) in the injured retina (Wan et al., 2012; Todd et al., 2015; Medrano et al., 2017) or cultured spinal cord astrocytes (Xia and Zhu, 2015). In the present review, we report evidence showing that nucleotides are released and metabolized in the retina, that all retinal cells express one or more nucleotide P2Y and P2X receptor, as well as adenosine P1 receptor subtypes, and that purines are implicated in the mechanisms that regulate the proliferation, survival, death, migration and differentiation of developing retinal cells. Evidences showing the involvement of purinergic signaling in retinal responses to damage and disease are equally reported.
2. Purines in the retina
2.1. ATP release in the retina
The release of ATP determines the overall input and impact of purinergic signaling in the retina. The spatial aspects of signaling are coordinated by the activation of release from sites on particular cell types and from the distribution of these sites along the membrane in each cell. The temporal aspects of retinal ATP release are influenced by the triggers leading to this release as well as the release mechanisms, in conjunction with the availability of ectoATPases to dephosphorylate the ATP to ADP, AMP and adenosine. The ability to stimulate specific purinergic receptors is determined by their proximity to ATP release sites and the release mechanism, as this mechanism will influence the amount of ATP released and thus the concentration of ATP reaching the receptor to control the probability of activation. As such, the pattern of ATP release from retinal cells has a considerable impact on the functional consequences of purinergic signaling in the tissue (Sanderson et al., 2014).
While nearly all cells in the body show some form of physiologically- regulated ATP release, the use of distinct release mechanisms enables a refined control and reflects the widespread presence of purinergic signaling. In addition to the release that accompanies cell lysis, ATP can be released from cells via both vesicular and channel-associated mechanisms (Praetorius and Leipziger, 2009; Corriden and Insel, 2010). ATP release has been associated with a range of ion channels and pores including connexin hemichannels, pannexin hemichannels, calcium homeostasis modulator (CALHM), cystic fibrosis transmembrane conductance regulator (CFTR), P2X7 receptor volume- anion and other channels (Cisneros-Mejorado et al, 2015; Dahl, 2015; Ma et al., 2018). The presence of vesicular ATP release has been aided by the discovery of the vesicular nucleoside uptake transporter (VNUT); the presence of VNUT can identify releasable stores of ATP (Oya et al., 2013). Of relevance to the retina is identification of VNUT on lysosomes and other vesicles in addition to identifying ATP stores in traditional synaptic vesicles (Moriyama et al., 2017; Beckel et al., 2018). While channel-mediated and vesicular-mediated ATP represent distinct mechanisms, markers for both types of release may also reflect a vesicular insertion of ATP-release channels into the membrane, masking as a hybrid method (Reigada and Mitchell, 2005).
2.1.1. ATP release from retinal cells
ATP release has been reported from all main types of retinal neuron including photoreceptors, bipolar, horizontal, amacrine and retinal ganglion cells. One of the earliest reports of vesicular ATP release by retinal neurons came from cultures enriched in amacrine-like neurons (Santos et al., 1999). This ATP release was stimulated by depolarization or AMPA-receptor stimulation and inhibited by adenosine acting at A1ARs (A1 adenosine receptors). The expression of VNUT in retinal neurons supports the presence of vesicular ATP release. For example, VNUT colocalizes with vesicular y- aminobutyric acid (GABA) transporter in bipolar and amacrine cells, while in photoreceptors VNUT is associated with synaptophysin and vesicular glutamate transporters (Moriyama and Hiasa, 2016). The presence of VNUT on dopamine-positive inner plexiform cell processes is consistent with a co-release of ATP through Ca2+-dependent vesicular exocytosis that was implicated in modulating the visual response (Ho et al., 2015).
While neurons are traditionally associated with vesicular neurotransmitter release, several reports demonstrate retinal neurons are also capable of channel-mediated ATP release. For example, retinal ganglion cells demonstrated a mechanosensitive ATP release through pannexin hemichannels (Xia et al., 2012); staining identified pannexin channels on both the soma and neurites of the ganglion cells (Dvoriantchikova et al., 2006). ATP release through pannexin channels autostimulated P2X7 receptors for ATP on the retinal ganglion cells to trigger release of IL-3, IL-6 and other cytokines (Lim et al., 2016; Lu et al., 2017). In horizontal cells, ATP release through pannexin hemichannels was recently implicated in synaptic modulation due to the liberation of phosphate groups and protons following the extracellular dephosphorylation of ATP; this acidifies the synaptic cleft which in turn increases glutamate release from cones (Vroman et al., 2014). Interestingly, synaptic signaling was modified by the byproducts of ATP dephosphorylation and not the purines themselves.
2.1.2. ATP release from retinal pigment epithelium (RPE) cells
RPE cells lie between the photoreceptors and the choroid rich in blood vessels and they support the retina in multiple ways. Purinergic signaling contributes to several of these supporting functions, with ATP release from the RPE providing an initiating step in the signaling response. This contribution starts during retinal development, as the release of ATP through hemichannels on RPE cells was associated with the proliferation of retinal progenitor cells (Pearson et al., 2005); the generation of Ca2+ waves across the RPE was implicated in the process (Dale, 2008). Glutamate acting at NMDA receptors leads to channel-mediated ATP release from RPE cells (Reigada et al., 2006). Glutamate is released from RPE cells (McGahan, at al., 2005), suggesting the involvement of an autocrine signaling system. As glutamate is the primary transmitter released from photoreceptors in response to changing light levels, glutamate-dependent ATP release may contribute to the light dependent responses of RPE cells, consistent with the suggestion that ATP is itself the “light peak substance” (Peterson et al., 1997), although it is unclear whether glutamate could diffuse from the photoreceptor synapse to the apical membrane of RPE cells in sufficient concentrations to activate the receptor. ATP release from RPE cells is also triggered by changes in tonicity, ischemia, growth factors and purines themselves (Mitchell, 2001). The ability of UTP and ATP to trigger release suggests an autocrine activation to amplify the impact of ATP release. RPE cells displayed a polarized ATP release across the apical membrane in response to ischemia and glutamate (Mitchell et al., 2008), although detection of ATP release across the basolateral membrane may be thwarted in experiments using permeable supports. Systemic delivery of the P2Y12 receptor antagonist ticagrelor lowers lysosomal pH and lipofuscin levels in RPE cells from the ABCA4−/− mouse model of recessive Stargardt’s disease, suggesting baseline levels of ATP release, and subsequent conversion to endogenous agonist ADP, normally activate the receptor (Lu et al., 2018).
ATP release contributes to inflammatory signaling by RPE cells; as the inflammatory responses of RPE cells contribute to diseases such as age-related macular degeneration, ATP release from RPE cells may play a key role in the pathogenesis. ATP release accompanied the formation of the membrane attack complex and Ca2+ rise in RPE cells, connecting ATP release with complement- mediated cell death (Yang et al., 2014). Increased levels of sodium chloride led to pannexin-mediated ATP release from RPE cells that was implicated in priming the NLRP3 inflammasome (Prager et al., 2016). Vesicular release of ATP from RPE cells was recently described, with exocytosis of lysosomal ATP activated following stimulation of the Toll-Like Receptor 3 by poly(I:C) (Beckel et al., 2018). Of interest, and perhaps concern, was the ability of scrambled “non-targeting” siRNA to trigger vesicular ATP release from RPE cells via this pathway.
2.1.3. ATP release from retinal glia
ATP released from retinal glial cells participates in a complex series of responses implicated in both homeostatic mechanisms and pathophysiology. ATP release from Müller cells has been most thoroughly documented; these glial cells extend from inner to outer limiting retinal membranes and this transretinal span positions them to integrate multiple retinal functions (Reichenback and Bringmann, 2016). ATP release from Müller cells is stimulated by both osmotic/mechanical and neurochemical triggers and the effects are coordinated. Glutamate triggers ATP release from Müller cells following stimulation of either NMDA or AMPA/kainite receptors (Loiola and Ventura, 2011) or group II mGluRs (Uckermann et al., 2006). . At least some of this ATP release is channel mediated, as glia-specific knockout of soluble N- ethylmaleimide-sensitive factor attachment protein receptor (SNARE)- dependent exocytosis displayed no change in ATP release from Müller cells (Wagner et al., 2017). The release of ATP from Müller cells following osmotic membrane stretching is central to maintaining water dynamics in the retina; blocking this release leads to swelling and retinal edema (Reichenbach and Bringmann, 2016). Osmotic release of ATP is decreased by ischemia-hypoxia or inflammation, leading to Muller cells swelling, although the glutamate- dependent release of purines can provide a secondary backup and prevent osmotic swelling under pathological conditions (Wurm et al., 2011). In contrast, stimulation of metabotropic glutamate receptors on Müller cells led to ATP release and conditioned medium from these cells killed retinal ganglion cells (RGCs) via the P2X7 receptor, suggesting reactive Müller cells could kill RGCs by releasing ATP (Xue et al., 2016a). The balance between beneficial and detrimental actions of ATP released from Müller cells to the retina may depend on the duration, magnitude and location of release, as found elsewhere in the eye (Sanderson et al., 2014).
Paracrine interactions between Müller cells and other retinal cells are well documented. For example, ATP from Müller cells acts on A1 receptors after conversion to adenosine and hyperpolarizes RGCs (Newman, 2003; 2004). The corollary, whereby ATP release from inner retinal neurons acts upon purinergic receptor on Müller cells, was also demonstrated (Newman, 2005). Retinal glial cells were suggested as the source of tonic ATP release that lead to the vasoconstriction of retinal vessels, suggesting the vascular tone in retinal arterioles is controlled by released ATP (Kur and Newman, 2014; Newman, 2015).
In addition to the neuromodulatory effects of ATP released from Müller cells, autocrine actions on Müller cells themselves are plentiful, including the induction of Ca2+ waves, gliosis and degeneration (Reichenbach and Bringmann, 2016). Multiple reports implicate ATP release in the Ca2+ waves radiating across Müller cells. Spontaneous calcium waves were eliminated by the soluble ectoATPase apyrase, implicating a baseline release of ATP is necessary for the waves (Kurth-Nelson et al., 2009). Interestingly, the development of these spontaneous waves was associated with a larger ATP release in adult rats than 20 day old rats. Müller cells also release ATP in response to mechanical stimulation; this release initiates both an immediate rise and a second rise in waves and may transmit gliosis signals throughout the retina (Agte et al., 2017).
ATP release has also been documented from astrocytes; the optic nerve head is a focus of the mechanical strains induced by elevation of intraocular pressure (IOP) in glaucoma, and optic nerve head astrocytes display mechanosensitive release of ATP, suggesting this release may provide a response to elevated IOP (Beckel et al., 2014). The release was associated with pannexin hemichannels, although the mechanosensor may be upstream and linked to the pannexin channels through a Rho-kinase-sensitive pathway as found in retinal cells (Krizaj et al., 2014). This mechanosensitive ATP release from optic nerve head astrocytes through pannexin hemichannels led to autostimulation of the P2X7 receptor and priming of inflammatory signals through the NFkB pathway, suggesting ATP release from these astrocytes linked mechanical strain to neuroinflammation (Albalawi et al., 2017). Optic nerve head astrocytes were recently shown to store ATP inside the lumen of lysosomes, and release this ATP in response to stimulation of the TLR3 receptor and lysosomal alkalinization (Beckel et al., 2018). VNUT colocalized with secretory lysosome marker lysosome-associated membrane glycoprotein 3 (CD63), and released lysosomal ATP was sufficient to raise cellular Ca2+ in the astrocytes, suggesting lysosomal ATP release can connect autophagy and the innate immune system.
2.1.4. ATP release in retinal diseases
ATP release is implicated as in numerous retinal diseases, with both protective and detrimental roles attributed to the release. For example, ATP release from retina cells and subsequent stimulation of P2X7 receptors was implicated in photoreceptor death in subretinal hemorrhages found in wet AMD, with elevated levels of extracellular ATP in the vitreous of hemorrhaging AMD patients (Notomi et al., 2013). An increase in ATP release from retinal cells that accompanied nutrient starvation also led to a P2X7 receptor-mediated photoreceptor loss (Notomi et al, 2011). Mixed retinal cells cultured in high glucose showed greater exocytotic release of ATP upon depolarization stimulation than those in mannitol (Costa et al., 2009), implying a possible role for release in diabetes. Osmotic-sensitive ATP release was reduced in in PKD2 1/703 rats, while glutamate-mediated ATP release was found to be unaffected, suggesting a role for the osmotic ATP release in the photoreceptor degeneration found with cilium polycystin-2 defects (Vogler et al, 2016). Retinal detachment triggers a release of ATP that is linked to the cell proliferation and tissue remodeling phases of the recovery (Garweg et al., 2013). Increased ocular ATP release is associated with elevated IOP in humans with glaucoma (Li et al., 2011; Zhang et al., 2007). Levels of ATP and expression of VNUT were increased with age in the DBA/2J mouse model of glaucoma along with a rise in vitreal ATP (Perez de Lara et al., 2015). In as much as VNUT is a marker for vesicular ATP release, changes in VNUT expression in association with disease may reflect defective vesicular ATP release. In mouse, primate and rat models of chronic ocular hypertension levels of ATP were elevated in the vitreous humor, while expression of ectonucleoside triphosphate diphosphohydrolase 1 (NTPDase 1) was increased in the retina; as NTPDase1 expression closely parallels the extracellular levels of ATP bathing astrocytes and RPE cells (Lu et al, 2007; 2015), these observations are consistent with IOP-associated ATP release (Lu et al., 2015). Pannexin hemichannels were also implicated in the release of ATP from the bovine retina in response to mechanical strain (Mitchell et al., 2008; Reigada et al., 2008). Together, these reports implicate altered ATP release in numerous retinal diseases, although the balance between beneficial and detrimental roles remains to be clarified given the potentially differential actions of ATP and adenosine on retinal health (Zhang et al., 2006b).
2.2. Adenosine synthesis and release in the retina
Adenosine is referred as a neuromodulator and not as a classical neurotransmitter. This classification is due to a large amount of evidence indicating that adenosine is not stored or released by synaptic vesicles from presynaptic terminals. However, it is important to note that some reports suggest that adenosine can be released by mechanisms similar to the release of neurotransmitters (Wall and Dale, 2007; Klyuch et al., 2011).
Intracellular levels of adenosine are regulated by its uptake through transporters or by the synthesis of this purine from AMP, which is catalyzed by the enzyme 5’-nucleotidase. The extracellular concentration of adenosine is directly correlated to the activity of its transporters and to the degradation of released adenine nucleotides mediated by ecto-nucleotidases that converts released ATP to adenosine in the synaptic cleft (Dunwiddie et al., 1997). The main enzymes responsible for the metabolism of adenosine are adenosine kinase, which generates AMP, and adenosine deaminase, which metabolizes adenosine into inosine (Latini and Pedata, 2001).
Nucleoside transporters are divided into equilibrative and concentrative transporters. These membrane proteins are responsible for the transport of adenosine, nucleosides, nucleobases, and nucleoside analogues (Kong et al., 2004).
Equilibrative Nucleoside Transporters (ENTs) perform their functions according to the levels of nucleosides in the intra and extracellular media. In contrast, concentrative transporters promote the influx of nucleosides against their concentration gradient with the help of the sodium gradient across cell membranes (Podgorska et al., 2005). ENTs are divided further into transporters that are sensitive or insensitive to the inhibitor nitrobenzylthioinosine (NBTI). The sensitive ones are inhibited at nM concentrations of this drug, while the insensitive ones are not affected at concentrations up to 1μM (Podgorska et al., 2005). Currently, there are 4 subtypes of transporters named ENT1, ENT2, ENT3 and ENT4, and only ENT1 and ENT2 are sensitive to NBTI, the latter being much less susceptible to inhibition (Podgorska et al., 2005). These proteins that have 11 transmembrane domains can be found in a glycosylated form and their N-terminal tail faces the cytoplasm, whereas their C-terminal tail faces the extracellular space. They have a large extracellular loop between the transmembrane domains 1 and 2 and a large intracellular loop interconnecting transmembrane domains 6 and 7 (Sundaram et al., 1998; for review, see Baldwin et al., 2004).
The ENT1 is the adenosine carrier widely expressed in most cells and considered as the main regulator of adenosine levels in tissues (Bone et al., 2007). ENT1 from several species were cloned so far, including the rat, mouse, dog and human protein (Hammond et al., 2004). Previous data showed that inhibition of ENT1 may potentiate the neuroprotective and cardioprotective effects induced by adenosine in situations of injury (Bone and Hammond, 2007).
Concentrative transporters are divided into 3 subtypes (CNT1, CNT2 and CNT3). They have 13 transmembrane domains with a cytoplasmic N-terminal tail and the C-terminal tail with glycosylation sites located iat the extracellular side of the membrane (Kong et al., 2004).
The presence of adenosine, its receptors and transporters was reported in retinas of different species (Schaeffer and Anderson, 1981; Braas et al., 1987; Blazynski, 1991). The presence of an adenosine uptake system in goldfish (Studholme and Yazulla, 1997), rabbit (Perez et al., 1986), rat (Schaeffer & Anderson, 1981) and chicken retinas (Perez and Bruun, 1987; Paes de Carvalho et al., 1990), among others, has been already demonstrated.
In the developing chick retina, binding assays with [3H]-NBTI, a high affinity ligand for ENT1, demonstrated the presence of these transporters in tissues obtained from 8-day-old embryos to post-hatching animals (Paes de Carvalho et al., 1992). In the later stages of development, these transporters were located mainly at the plexiform layers where A1 adenosine receptors are also detected (Paes de Carvalho, 1990). This colocalization was also demonstrated in other structures of the CNS (Jennings et al., 2001).
Perez and colleagues (1986) showed that most of the [3H]-adenosine taken up by rabbit retinal cells is converted into adenine nucleotides and depolarizing stimuli are able to increase the release of radioactivity, most of it found as hypoxanthine, xanthine and inosine. This release is inhibited by dipyridamole, an inhibitor of equilibrative nucleosides transporters, indicating that, at least in part, this release occurs in the form of nucleosides.
In purified cultures of neurons and photoreceptors from the chick retina, a specific high affinity uptake system for [3H]-adenosine was demonstrated and under a depolarizing stimulus, an increase in purines release with most of the extracellular radioactivity as inosine was obtained (Paes de Carvalho et al., 1990). Incubation of these cultures with the ENT1 inhibitor NBTI (10 nM), prevented the uptake of adenosine by more than 80%, indicating that ENT1 is the major transporter of adenosine expressed by these cells. In these cultures, adenosine uptake was independent of sodium ions and blocked by a previous incubation with adenosine deaminase, an enzyme that converts adenosine to inosine, indicating that this transporter does not carry inosine into retinal neurons in culture. Similarly, in mixed cultures of chick retina, containing both neurons and glia cells, more than 90% of [3H]-adenosine taken up by the cells was converted to adenine nucleotides and after activation of ionotropic glutamate receptors, around 80% of extracellular radioactivity was found as inosine and hypoxanthine. This purine release stimulated by glutamate was also blocked by NBTI (Paes de Carvalho et al., 2005).
The presence of the ENT1 transporter through binding assays using [3H]- NBTI was also demonstrated in the mixed cultures of chick retina (Paes de Carvalho, 2002). Their long-term incubation with EHNA, an inhibitor of adenosine deaminase, caused a significant reduction in ENT1 levels, suggesting that activation of adenosine receptors may modulate the expression of this transporter (Paes de Carvalho, 2002),
Adenosine release in the retina is also controlled by a circadian clock. Under the light, rabbit retina releases adenosine, but in the darkness and in the nighttime, there is an increase of extracellular adenosine levels, which comes mainly from the degradation of extracellular ATP to adenosine through ectonucleotidases (Ribelayga & Mangel, 2005).
3. Expression of purinergic receptors in the retina
3.1. P1 Receptors
Adenosine exerts its effects through G protein-coupled receptors named P1 receptors. There are four subtypes described and named A1, A2a, A2b and A3 adenosine receptors (Schulte and Fredholm, 2003; Fredholm et al., 2005). In brief, these receptors are able to modulate adenylate cyclase activity. A1 and A3 receptors decrease cAMP levels via inhibition of this enzyme, while A2a and A2b receptors enhance cAMP levels via an increase in adenylate cyclase activity. However, we should point out that there are other possible kinds of coupling of adenosine receptors with different G proteins (Cunha et al. 1999; Casadó et al. 2010).
Several reports showed the presence of A1 receptors in retinas from different species (Fig. -1). A seminal study, using binding assays with specific agonists, described the expression of A1 receptors in the rat, guinea pig, monkey, and human retina (Braas et al., 1987). During development, the presence of A1 receptors was mainly described in the chick embryo retina (Paes de Carvalho, 1990; Paes de Carvalho et al., 1992; Brito et al., 2012). During development, staining for A1 receptors begins to be observed in retinas from 10- day-old embryos (E10), remaining in this tissue until the post-hatching period (Paes de Carvalho, 1990). Paes de Carvalho and colleagues also showed the expression of these receptors in the intact retina through autoradiography assays. Expression of A1 receptors increases along chick retina development, and at all ages, staining is mainly concentrated at the inner and outer plexiform layers (Paes-de-Carvalho et al., 1992). As plexiform layers are rich in synapses of retinal neurons, this pattern of expression indicates that these receptors might be important modulators of neuronal activity in the retina. Other studies also showed that the expression of A1 receptors in the developing chick retina could be modulated by its long-term treatment of with A1 or A2a receptor agonists and antagonists (Brito et al., 2012; Brito et al., 2016), an effect that could also be observed in retinal cultures (Pereira et al., 2010). Moreover, in good agreement with the well established evidence of regulation of neurotransmitters release by adenosine in the central nervous system (Cunha, 2008), during the development of chick retina, blockade of A1 receptors with caffeine induces an increase in the GABA release induced by D-aspartate (Ferreira et al., 2014), an effect that reinforces the role of these receptors as modulators of synaptic transmission.
Fig-1.
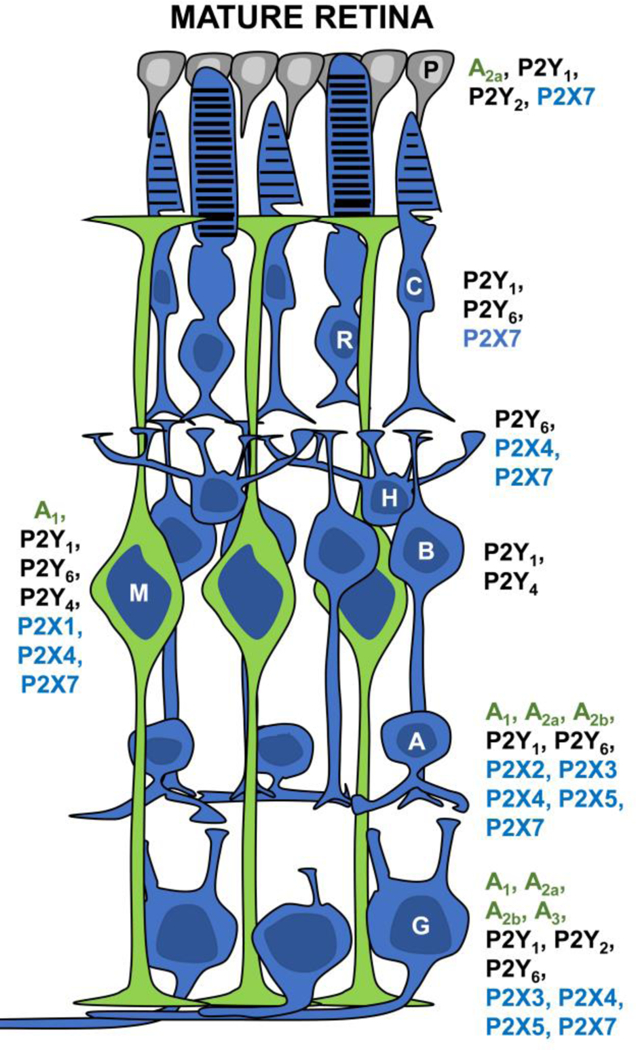
Schematic representation illustrating the expression of purinergic receptors in the adult retina and RPE. Cellular expression of P1 (green), P2Y (black) and P2X (blue) purinergic receptor subtypes is shown close to the indicated cell body of the retina cell type. P, Pigmented epithelium cell; R, rod cell; C, cone cell; H, horizontal cell; B, bipolar cell; M, Müller cell; A, amacrine cell; G, ganglion cell. Receptor subtype expression in the synaptic layers and cell processes is described in text (Section 3).
The expression of A2a adenosine receptors in the retina was described in a wide range of species, such as dog, salamander, mouse, rat and others (for review, see dos Santos-Rodrigues et al., 2015). In rat retina, expression of mRNA of this receptor was mainly observed in the inner nuclear layer and ganglion cell layer (Kvanta et al., 1997). An immunohistochemistry approach showed that the expression of these receptors is higher in the inner plexiform and ganglion cell layers of the postnatal (P2) rat retina (Huang et al., 2014). The presence of mRNA for these receptors was also detected in rat retinal microglia cultures (Liou et al., 2008). In the developing (P9) ferret retina, a similar pattern of immunostaining was observed (Stellwagen et al., 1999). Expression of A2a receptors was also detected in the retina of guinea pigs (Cui et al., 2010). In the chick retina, the first direct evidence for the expression of A2a receptors was obtained in mixed retinal cultures (Pereira et al., 2010) that express these receptors in glial cells and neurons. The presence of A2a receptors in the retina of 16-day-old chick embryos was also observed in the inner plexiform and ganglion cell layers (Brito et al., 2012; 2016).
Few data report the expression of A2b receptors in the retina. An early report describes the presence of mRNA for these receptors in the ciliary processes of rat eye, but not in the retina itself (Kvanta et al., 1997). However, recently, immunoreactivity against these receptors was observed in the inner portion of the rat retina, especially in the retinal ganglion cell layer (Nakashima et al., 2018). The presence of A2b receptors in the retina of guinea pig (Cui et al., 2010) and ferret retina was also reported (Stellwagen et al., 1999).
The first clear evidence for the presence of A3 receptors in the rat retina came out in 2006, where mRNA for this receptor was observed in ganglion cells. These receptors turned out to be functional, since their activation was able to decrease the calcium increase induced by stimulation of P2X7 receptors (Zhang et al., 2006a). Moreover, the increase of calcium levels triggered by activation of NMDA receptors in cultures of rat retinal ganglion cells was also prevented by stimulation of the A3 adenosine receptors (Zhang et al., 2010). Other studies from the same group also demonstrated that A3 receptors agonists block the death of rat retinal ganglion cells triggered by the activation of P2X7 receptors both in vitro (Zhang et al., 2006b) and in vivo (Hu et al., 2010). A similar study also showed that adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration (Galvao et al., 2015). Besides preventing cell death, activation of A3 receptors induces neurite outgrowth and axon regeneration of rat retinal ganglion cells (Nakashima et al., 2018) and may also regulate rat electroretinogram (Jonsson and Eysteinsson, 2017). Collectively, thes evidences strongly indicate the expression of this P1 receptor subtype in the retina.
3.2. P2Y receptors
Expression of several P2 receptors that are G protein-coupled receptors (P2Y) or ligand gated ion channels (P2X) was detected in the retina (Fig. -1). So far, the P2Y1 is the main P2Y receptor characterized in this tissue. In the rodent retina, immunoreactivity against this receptor was detected over ganglion, cholinergic amacrine cells, Müller glia and cells of the pigment epithelium (Fries et al., 2004; 2005; Ward and Fletcher, 2009; Wurm et al., 2009; Dilip et al., 2013). During development, labeling for the P2Y1 receptor was clearly observed over glial cell bodies and processes as well as in cholinergic amacrine cells in the rodent retina (Wurm et al., 2009; Dilip et al., 2013). Moreover, indirect evidence using specific antagonists suggest the expression of this receptor in retinal progenitors in the chick embryo and rat postnatal retina (Jacques et al., 2017; Almeida-Pereira et al., 2017) and in light-damaged retinas, increased expression of P2Y1 receptor subtype was detected in nestin-positive glial cells (Ward and Fletcher, 2009). In experimentally detached rabbit retinas, upregulation of ADP-dependent calcium responses was also observed in reactive retinal glial cells, but the ADP-sensitive receptor subtype was not determined (Uckermann et al., 2003). In chemically injured zebrafish retina, P2Y1 activation increases glia cell proliferation (Battista et al., 2009; Medrano et al., 2017).
High immunoreactivity against the P2Y2 receptor subtype was observed only over ganglion cells bodies in the rat retina (Fries et al., 2004; Wurm et al.,2009), but relevant expression of this receptor was detected in human retinal glial cells (Fries et al., 2005) and in other ocular tissues as, for example, the pigment epithelium (Sullivan et al., 1997), where the specific agonist INS37207 induces calcium responses (Maminishkis et al., 2002) and corneal epithelial cells, where P2Y2 activity is involved in corneal wound healing (Boucher et al.,2010). Recent reviews on the involvement of this receptor subtype in the pathophysiology of ocular tissues are available (Sanderson et al., 2014; Guzman-Aranguez and Pintor, 2012). In the developing retina, expression of P2Y2 receptors was detected in extracts from purified retinal glial cultures and calcium responses in glial cells induced by a specific P2Y2 agonist were observed in scratched retinal cell cultures (Martins-Silva et al., 2015).
Labeling for P2Y4 receptor was noticed over dendrites and axon terminals of bipolar cells in the rat retina (Ward et al., 2008) and in Müller cell endfeet (Wurm et al., 2009). Immunolabeling and mRNA transcripts for P2X4 receptors were also detected in Müller cells of the human retina (Fries et al., 2005). In the developing rat retina, immunoreactivity for P2Y4 receptor in glial cells is clearly observed after the third postnatal week (Wurm et al., 2009). While UTP-induced proliferation of neuronal progenitors was characterized in the chick developing retina (Pearson et al., 2005), the involved receptor subtype remained to be defined.
Expression of P2Y6 receptor subtype was also characterized by immunohistochemistry in the rat retina, over photoreceptor terminals, horizontal cells, several types of amacrine and ganglion cells, but not over glia or horizontal cells (Zhang et al., 2012). However, immunolabeling and mRNA transcripts were observed in glial Müller cells of the human retina (Fries et al.,2005). Evidence for the expression of this receptor in the developing retina is generally missing, but in purified cultures of postnatal rat ganglion cells, UDP- mediated activation of P2Y6 receptors promotes neurite outgrowth and in an in vitro model of glaucoma, these cells express decreased levels of P2Y6 receptors (Taguchi et al., 2016).
Direct evidence for the expression of P2Y11, P2Y12 and P2Y13 receptors in the mammalian adult retina was not obtained so far. However, while mRNA expression for P2Y12 receptors was observed in the neonatal rat retina (Almeida-Pereira et al., 2018), treatment of chick embryo retinal cell cultures with a P2Y13 antagonist prevents the proliferation of glial progenitors (Jacques et al., 2017). Functional and molecular identification of the P2Y12 receptor in RPE cells was recently reported (Lu et al, 2018).
3.3. P2X receptors
While no mRNA for the P2X1 receptor was detected in the rat retina (Brändle et al., 1998), immunoreactivity for this receptor was observed only in the inner plexiform layer of the mouse retina (Kaneda et al., 2004). Recently, high labeling for this receptor subtype was observed over glial Müller cells of the turtle retina (Vitanova and Kupenova, 2014).
In contrast, high labeling for P2X2 receptor was detected in cholinergic amacrine cell bodies and processes in the mouse retina (Kaneda et al., 2004) and Muller cells in the turtle retina (Vitanova and Kupenova, 2014). Recently, choline transport through P2X2 channel in OFF cholinergic amacrine cells was observed in the mouse retina, raising the possibility that these receptors can work as a new choline transport system in the retina (Ishii et al., 2017).
While high immunoreactivity for P2X3 receptor subtype was detected in GABAergic amacrine cells, over the ganglion cell layer and plexiform layers in the rodent retina (Shigematsu et al., 2007; Puthussery and Fletcher, 2007), immunoreactivity against P2X4 receptors was found over horizontal cell bodies and processes, amacrine and ganglion cell processes in the mammalian retina (Kaneda et al., 2004; Ho et al., 2014). P2X4 immunoreactivity was also observed over Müller cell processes in the mouse retina (Ho et al., 2014). Only weak immunoreactivity for the P2X6 receptor subtype was observed over the nerve fiber layer of the mouse retina (Shigematsu et al., 2007).
High immunoreactivity for P2X7 receptor was observed over the plexiform layers in the rat retina. Horizontal cells and photoreceptors were positive for the P2X7 receptor in the outer portion of the retina as well as amacrine cell processes in the inner plexiform layer (Puthussery and Fletcher, 2004). In the monkey retina, immunoreactivity for P2X7 receptor was also observed in ganglion cells (Ishii et al., 2003). In these species, no labeling for P2X7 receptor was detected in Müller glia. However, expression of this receptor was detected in isolated glial cells from the human retina using not only immunocytochemistry but also single cell PCR and whole-cell patch clamp recordings (Pannicke et al., 2000). Indirect evidence for the expression of the P2X7 receptor in Müller glia in the avian retina was obtained in cell cultures containing neurons and glia (Anccasi et al., 2013) and purified glial cultures (Faria et al., 2017). During development of the rat retina, P2X7 immunoreactivity was observed over ganglion, amacrine and horizontal cells and over the plexiform layers in animals from postnatal day 2 and thereafter. Microglial cells located at the ganglion cell layer also express P2X7 receptors, as these cells take up fluorescent dyes after stimulation with P2X7 receptor agonists (Innocenti et al., 2004). Expression of this receptor was also observed in the human pigment epithelium (Yang et al., 2011).
4. Purines and retinal cell proliferation
In retinal histogenesis, progenitors undergo a series of unidirectional changes in their competence to generate the different cell types of the mature tissue (Livesey and Cepko, 2001). Six types of neurons and one type of glia are generated in an early ordered manner that is successive, overlapping and conserved across different species (Martins and Pearson, 2008). To generate retinal cells, progenitors initially undergo repeated cycles of division that are regulated by inductive and inhibitory molecules that control their progression through the phases of the mitotic cycle, a process that is concomitant with the movement of nuclei through the interkinetic nuclear migration (INM) process (Fig.2A). Cell divisions occur at the outer, apical margin of the neuroblastic layer (NL) while DNA synthesis occurs at the inner, basal margin and nuclei migrate during cell cycle phase transitions (Fujita, 1962; Sidman, 1961).
Fig.2.
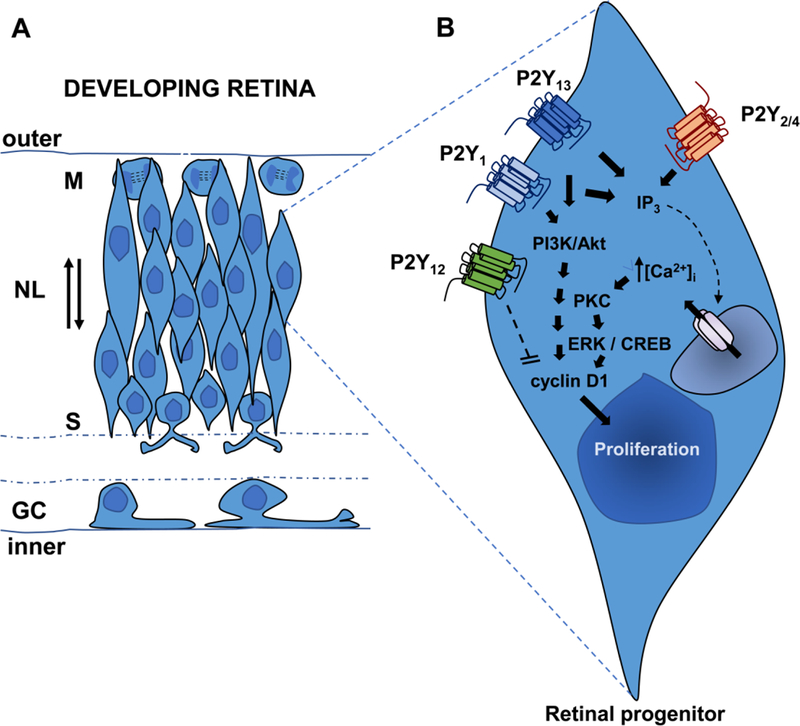
Schematic drawing illustrating progenitors undergoing interkinetic nuclear movement in the neuroblastic layer (NL) of the developing retina (A). Major nucleotide receptor subtypes and intracellular signaling pathways involved in cell proliferation in the developing retina (B). GC, Ganglion Cell Layer; S, S- phase; M, M-phase. See text for detailed discussion on the intracellular signaling pathways activated by nucleotides during retinal cell proliferation (Section 4).
Stimulation of progenitor’s proliferation is a major effect of nucleotides in the developing retina (Fig. 2B). For instance, nucleotide-mediated induction of cell proliferation is observed in cultured Müller cells of the guinea pig (Milenkovic et al., 2003; Moll et al., 2002), progenitors of the chick embryo (Pearson et al., 2002; 2005; Sanches et al., 2002) and mouse retina (Sholl- Franco et al., 2010), as well as in the rat developing retina (Almeida-Pereira et al., 2017).
Nucleotide induced increase in retinal progenitor proliferation is primarily associated with Ca2+ mobilization from intracellular stores and its capacitive entry (Sugioka et al., 1996; 1999a; 1999b). Nucleotide-induced calcium release from intracellular stores occurs as early as the embryonic day 3 in the chick retina (Sugioka et al., 1996), decreasing as progenitors exit cell cycle and begin to differentiate (Yamashita 2013). In the rabbit retina, nucleotide-mediated calcium responses in developing glial cells also decrease during their differentiation in vivo (Uckermann et al., 2002). A similar developmental profile is observed in nucleotide-dependent formation of inositol phosphates in the avian tissue (Nunes et al., 2007). Interestingly, both ATP-induced increase in [3H]-thymidine incorporation and ATP-dependent formation of inositol phosphates are decreased by conditioned medium obtained from cultured postmitotic retinal cells, indicating that nucleotide signaling to proliferation is desensitized by diffusible factors released from post-mitotic cells (França et al., 2007).
Nucleotide receptors involved in the proliferation of retinal progenitors were characterized in the chick embryo retina. In this tissue, activation of P2Y2/4 receptors by exogenous ATP or UTP induces proliferation of early developing progenitors which will generate photoreceptors, amacrine, ganglion and horizontal cells (Pearson et al., 2002; 2005; Sugioka et al., 1999b). In a later stage, activation of ADP-sensitive, UTP-insensitive receptors induces the proliferation of late developing glial/bipolar progenitors (França et al., 2007; Sanches et al., 2002). Interestingly, while the UTP-dependent proliferation of early progenitors is associated with the acceleration of their mitosis (Pearson et al., 2002), ADP-mediated effect is associated with the entry of progenitors in S phase without any change in cell cycle dynamics through G2 and M phases (Sholl-Franco et al., 2010). These findings indicate that different mechanisms are activated by UTP and ADP to induce the proliferation of retinal progenitors with distinct competences to generate retinal cells of the adult tissue.
As nucleotides, cholinergic muscarinic receptors induce phospholipase C activation and calcium mobilization very early in the embryonic retina (Sugioka et al., 1996, Pearson et al., 2002, Calvet and Ventura, 2005), a response that also decreases after retina synaptogenesis. However, in contrast to nucleotides, muscarinic receptor agonists decrease cell proliferation in the developing retina (Pearson et al., 2002; Dos Santos et al., 2003), although it stimulates the proliferation of corneal keratocytes in culture (Sloniecka et al., 2015). The explanation for this apparent contradiction seems to be the activation of different signaling effectors in addition to, or as a consequence of Ca2+ mobilization. The extracellular signal-regulated kinases (ERK) pathway is a membrane-to-nucleus signaling module involved in cell proliferation (Peyssonnaux and Eychène, 2001). Accordingly, in the developing retina, ADP- mediated increase in cell proliferation is inhibited by mitogen-activated protein kinase kinase (MEK) inhibitors (Sanches et al., 2002; Nunes et al., 2007). However, in retinas from 7-day-old chick embryos, while both ADP and UTP induce a transient activation of the ERK pathway, only the response to ADP can be observed over the neuroblastic layer where BrdU-labeled cells are located (Nunes et al., 2007), indicating that a concomitant activation of additional signaling pathways other than ERK is required for nucleotide-mediated induction of retinal progenitor proliferation.
The phosphatidylinositol-4,5-bisphosphate 3-kinase / protein kinase B (PI3K/Akt) pathway is another signaling module implicated in the nucleotide- induced proliferation of retinal progenitors (Ornelas and Ventura, 2010) and Müller cells from the adult retina (Milenkovic et al., 2003, Moon et al., 2009). In developing retina cells in culture, both ADP and ATP can induce the phosphorylation of Akt, an effect that is prevented by PI3K inhibitors and that provokes the increase of cyclin D1, a component of the kinase complex that regulates the progression of the G1 phase of the cell cycle of progenitors (Ornelas and Ventura, 2010). Phosphorylated Akt is also present during mitosis of retinal progenitors and is required for the expression of CDK1, an enzyme that controls cell transition from G2 phase to mitosis (Ornelas et al., 2013).
Cyclic nucleotide responsive element binding protein (CREB) is a transcription factor that integrates diverse extracellular signals to promote gene transcription. It is regulated by phosphorylation induced by multiple intracellular signaling kinases, including cyclicAMP-dependent protein kinase (PKA), Ca2+/calmodulin-dependent kinases, ERKs and PI3K/Akt pathway (Shaywitz and Greenberg, 1999; Lonze and Ginty, 2002). While several signaling molecules induce the phosphorylation of this transcriptional factor in retinal glial cells and progenitors to induce their proliferation and survival (Lamas et al., 2007; Fischer et al., 2009; Ramirez and Lamas, 2009; Socodato et al., 2009; 2011), ADP-dependent phosphorylation of CREB, through an ERK dependent mechanism was also demonstrated recently in developing retinal progenitors in culture, where it is required for ADP-dependent proliferation (Jacques et al., 2017).
Identifying the receptor subtype(s) involved in the proliferation of glial progenitors is a major goal in the study of the purinergic signaling in the developing retina as nucleotides are implicated in reactive gliosis, a glial response that occurs after experimental or pathological injuries of the retina. Since the work of Massé et al., (2007) showing that knocking down P2Y1 receptor expression decreases eye formation in tadpoles of the frog Xenopus laevis, the P2Y1 is considered the principal receptor subtype to regulate eye development. Accordingly, more than that 80% of glial progenitors of the newborn mouse retina do express the P2Y1 receptor subtype (Sholl-Franco et al (2010) and injection of the P2Y1 receptor antagonist MRS2179 in the eyes of newborn rats decreases the number of BrdU positive progenitors (Almeida- Pereira et al., 2017). However, in contrast to the above mentioned studies, Gampe et al., (2015) have shown that eye formation and functional topography of the adult retina is not affected in P2Y1 knockout mice, indicating that other receptor subtypes or mechanisms operate in the absence of the P2Y1 receptor in the developing retina. Accordingly, Jacques et al. (2017) showed that both P2Y1 and P2Y13 receptor antagonists prevent ADP-induced proliferation of retinal glial progenitors in culture and that the stimulation of only the P2Y1 receptor with a specific agonist does not induce their proliferation, indicating that activation of both receptors participates in the proliferative response of chick retinal glial progenitors in culture. However, the expression of this receptor subtype was not detected in the intact newborn rat retina (Almeida-Pereira et al., 2018), raising the possibility that the participation of the P2Y13 receptor occurs only in the avian retina or in glial progenitors in culture.
The participation of the P2Y12 receptor in the proliferation of the retinal progenitors was recently detected. In the intact newborn rat retina, blockade of the P2Y12 receptor subtype induces the increase in cyclin D1 as well as the decrease in p57 protein, two factors that stimulate and inhibit cell progression in the G1 phase of the cell cycle, respectively. Since P2Y12 inhibition does not interfere with S phase of the cell cycle and induces the death of cyclin D1 positive cells, these findings suggest that activation of P2Y12 receptors is required for the successful exit of late developing retinal progenitors from the cell cycle (Almeida-Pereira et al., 2018).
Nucleotide-induced cell proliferation is not only observed in the developing retina but also in cultured glial cells from the mature tissue or in glial cells from tissues under pathological conditions that results in Müller cell gliosis (for review, see Reichenbach and Bringmann, 2016). In these situations, multiple P2 receptors seem to be capable of inducing cell proliferation. For example, in adult guinea pig retinal glial cells in culture, both ATP and UTP are able to induce cell proliferation through a mechanism that involves the activation of PKC, release of growth factors, ERK and PI3K activation (Moll et al., 2002; Milenkovic et al., 2003). In cultured human Müller cells, the selective agonist for P2X7 receptors BzATP is also capable of increasing DNA synthesis in the cultures (Bringmann et al., 2001). Moreover, upregulation of nucleotide- mediated calcium responses is observed in Muller cells during retinal detachment or proliferative vitreoretinopathy (for review, see Reichenbach and Bringmann, 2016).
Collectively, the studies mentioned above suggest that multiple nucleotide receptor subtypes can be involved in the proliferative response of glial cells and progenitors. Since mechanisms of synergism between different nucleotide P2Y receptor subtypes (Grimm et al., 2009) as well as their crosstalk were observed in other types of cells (Suplat et al., 2007), the development of specific agonists and antagonists can contribute to identifying the receptor subtypes that are responsible for inducing the proliferative response of developing glial progenitors as well as progenitors derived from adult dedifferentiated glia from retinas under injury.
5. Purines and cell migration in the retina
Classically, development of the neural tissue occurs through a stereotyped sequence of genesis, migration and differentiation of cell progenitors. During development of the retina, the ganglion cell layer is the first layer to appear, followed by the inner nuclear layer and then by the outer nuclear layer. Albeit this temporal organization, cells destined for each of these layers are not strictly generated in the same sequence and several progenitors for different layers are born concurrently. Since progenitors divide adjacent to the outer limiting membrane (OLM), newborn retinal cells need to migrate properly to their definitive positions in order to differentiate and form their appropriate synaptic connections (for review, Godinho and Link, 2006).
Like cell proliferation, nucleotides were implicated in cell migration and chemotaxis of a broad range of cell types, including immune cells, cardiac fibroblasts, epithelial or tumor cells, keratinocytes and microglia (Corriden and Insel, 2012). In the developing CNS, nucleotides induce the migration of astrocyte progenitors (Striedinger et al., 2007) and neural stem cells (Scemes et al, 2003) that increase cell spreading and formation of stress fibers (Grimm et al., 2010). Accordingly, in cell cultures of the chick embryo retina that were mechanically scratched, activation of UTP-sensitive P2Y2/4 receptors induces the growth of glial cells towards the area of the scratch through a mechanism involving PI3K, proto-oncogene tyrosine-protein kinase (SRC) and focal adhesion kinase (FAK) signaling pathways (Martins-Silva et al., 2015). Moreover, in scratched cultures that were treated with apyrase, glial actin cytoskeleton and microtubules are less organized and vinculin-labeled adhesion sites are hardly found in glial cells at the border of the scratch. Both adhesion and migration are decreased by P2 receptor antagonists when purified glial cultures are used (Martins-Silva et al., 2015).
The observations mentioned above support the idea that nucleotides through activation of UTP-sensitive receptors regulate glia migration in the retina. Although no proliferation or migration of glial cells is observed in the adult intact retina, Müller cells are capable of re-entering cell cycle in response to damage or disease and generate new neurons depending on the species (Fischer and Reh, 2001; Bringmann and Wiedemann, 2012). While the injured teleost fish retina regenerates and Müller glia is the source of regenerated neurons, the injured mammalian retina has very low capacity to regenerate and de-differentiated glia contributes to the formation of glial scars. Following retinal detachment in rabbits, for example, several nuclei of Müller cells migrate to the outer retina, undergo mitosis and some cells grow beyond the OLM and begin to form glial scars in the subretinal space (Lewis et al., 2010), an event that contributes to the degeneration of photoreceptors. Since ATP is released upon mechanical stimulation of retinal glia (Newman 2001; 2003), it is possible that ATP, through the activation of multiple P2 receptors contributes to the formation of glial scars not only by regulating the proliferation but also the migration of Müller glia (for review, see Reichenbach and Bringmann, 2016).
6. Purines and cell death induction in the retina
Cell death is a regulated phenomenon in the developing nervous system that, together with cell proliferation, determines the size of cell populations in the adult tissue. Besides the programmed cell death that is regulated by target- derived trophic factors that protect and determine the survival of projecting neurons, cell death also affects neural precursor cells and young postmitotic neuroblasts during development. This “early” neural cell death was clearly detected in the developing retina of several species, including the avian and rodent retina (for review, see Valenciano et al., 2009).
Activation of cytotoxic mechanisms by nucleotides in the developing retina was demonstrated in newborn rats by Resta et al. (2005). Direct application of ATP to isolated retinas induces the death of cholinergic amacrine cells that express P2X7 receptors. Noteworthy, intraocular injection of P2X7 antagonists increases the population of these cells without modulating other retinal cell populations, indicating that activation of P2X7 receptors controls the density of cholinergic amacrine cells during retinal development.
The neurotoxic effect of activating P2X7 receptors was also investigated in developing avian retinal cells in culture (Anccasi et al., 2013). In this model, although neurons probably do express P2X7 receptors as they take up dyes when stimulated with ATP, developmentally regulated P2X7 receptor-induced death of neuroblasts is dependent on the presence of glial cells and can be blocked by NMDA and non-NMDA receptor antagonists, suggesting that neuronal death mediated by P2X7 in the developing avian retina may be indirect, via activation of glutamate ionotropic receptors.
The cytotoxic mechanisms activated by nucleotides were also demonstrated in more differentiated retinal neurons, both in cell culture and intact retina preparations. ATP-induced death of rat retinal ganglion cells in culture mediated by activation of P2X7 receptor was clearly demonstrated by Zhang et al. (2005). While these cells express P2X7 receptors, their acute stimulation with the P2X7 receptor agonist BzATP provokes large increases in intracellular calcium but not in the permeability of fluorescent dyes, whereas their sustained stimulation provokes their death. ATP-induced retinal ganglion cell death is blocked by P2X7 receptor antagonists and is also observed in the retina in vivo (Hu et al., 2010).
Activation of P2X7 receptors was implicated in the death of retinal neurons promoted by different kinds of injury. Incubation of retinal cell cultures under hypoxia conditions induces a significant increase in the death of retinal neurons, a damage that can be prevented by P2X7 receptor antagonists such as Brillian Blue G (BBG) and oxidized ATP (Sugiyama et al., 2010). Application of high pressure transients to isolated rat retinas induces significant damage to retinal ganglion cells that display soma blebbing and loss of membrane integrity (Resta et al., 2007). Ganglion cell damage is prevented by apyrase and P2X7 receptor antagonists and can be induced in vivo by short increases in the intraocular pressure that leads to increased levels of extracellular ATP in the eye fluids (Resta et al., 2007). In explant cultures of human retina, oxygen/glucose deprivation that mimics ischemia induces the death of ganglion cells, a response that is prevented by the incubation of the explants with the P2X7 antagonist BBG (Niyadurupola et al., 2013). In rats submitted to optic nerve crush (ONC) injury that causes retinal ganglion cell death, these cells are significantly preserved when P2X7 receptor antagonists are applied during 7 days after the injury. Interestingly, an increase in P2X7 immunoreactivity over ganglion cells that is suppressed by P2X7 antagonists is observed in the retina from animals with the ONC injury, suggesting that retinal injury induces the upregulation of this receptor expressed in ganglion cells (Kakurai et al., 2013).
The involvement of P2X7 receptor activation in the death of retinal ganglion cells was also demonstrated in retina models of gliosis (Reichenbach and Bringmann, 2015). For example, in the rat retina, intravitreal injection of (S)- 3,5-dihydroxyphenylglycine (DHPG), an agonist of the group I metabotropic glutamate receptors, induces Müller cell gliosis with increased levels of ATP released from the activated Müller cells. The injection of DHPG also induces an increase in retinal ganglion cell death that is at least partially blocked by the application of the P2X7 receptor antagonist BBG, indicating that reactivation of retinal glial cells can induce the death of ganglion cells through the release of excessive ATP and activation of P2X7 receptors (Xue et al., 2016a). Interestingly, in this model, glia activation induces the upregulation of P2X7 receptor in ganglion cells through a mechanism dependent on ATP released from the activated glia, indicating that gliosis may potentiate the deleterious effect of ATP by upregulating P2X7 receptor expression in ganglion cells (Xue et al., 2016b). Upregulation of P2X7 receptor expression in these cells is also observed in rat retinas from eyes submitted to elevated intraocular pressure (IOP) (Sugiyama et al., 2013) and at the early stages of development of the retina of rds mice, a murine model of retinitinis pigmentosa disease (Franke et al., 2005).
Cytotoxic effect of P2X7 receptor activation was also demonstrated in retina photoreceptors. Intravitreal injection of ATP causes consistent apoptosis of photoreceptors in the rat retina, an effect that is significantly reduced by P2X7 receptor antagonists (Puthussery and Fletcher, 2009; Notomi et al.,2011). A P2X7 antagonist also slows photoreceptor degeneration in the retina of rd1 mouse model of retinitis pigmentosa (Puthussery and Fletcher, 2009). In retinas from humans with age-related macular degeneration (AMD), photoreceptor cell apoptosis is also mediated by P2X7 receptor activation (Notomi et al., 2013).
7. Purines and cell survival in the retina
Adenosine is considered a neuroprotective agent in the retina under different stressful situations, such as ischemia, glaucoma, optic nerve lesion and diabetic retinopathy, but this nucleoside may also act as inducer of neuronal degeneration.
7.1. Glaucoma
Glaucoma is an eye disease associated mainly with an increase of intraocular pressure that damages the optic nerve and leads to vision loss through retinal ganglion cell (RGC) death. Recently, Madeira et al. (2015) reported that the antagonism of A2a receptors is able to block the death of ganglion cells induced by an elevated hydrostatic pressure in organotypic retinal cultures, an ex vivo model of glaucoma. Moreover, activation of A2a receptors expressed in microglia cells from the rat retina is responsible for the release of pro-inflammatory cytokines that causes RGC death. Similar results are observed when primary cultures of retinal microglia are used (Madeira et al., 2016a). Yet, in an in vivo model of glaucoma, long-term treatment of these animals with caffeine ad libitum, which exerts some effects via antagonism of A2a receptors, is able to control retinal neuroinflammation, microglia reactivity and loss of retinal ganglion cells (Madeira et al., 2016b).
7.2. Ischemia
Under ischemic conditions, a significant death of retinal ganglion cells is observed. High intraocular pressure-induced transient ischemia is known to induce microglia activation, an elevation in the release of the pro-inflammatory cytokines, such as IL-1β and TNF and RGC loss. Moreover, when eyes are injected with an antagonist of A2a receptors before the transient ischemia, an increase of ganglion cells survival is obtained, indicating that A2a receptors signaling contributes to neurotoxicity of RGCs under ischemia events (Madeira et al., 2016a).
Retinal ischemic preconditioning is a process where an induced short term ischemia protects the retina against a subsequent strong event of ischemia. It is well established that under ischemia conditions, there are higher levels of extracellular adenosine in cultured retinal cells compared to non-ischemic conditions (Rego et al., 1997). The increase in adenosine induced by ischemic preconditioning is important for retinal protection against ischemia and this effect is dependent on the activation of A1 and A2a receptors that protects retinal functionality as evaluated by electroretinogram recordings (Li et al., 1999). The effects mediated by adenosine receptors are dependent of protein kinase C (PKC) and activation of KATP channels as well as on protein synthesis (Li et al., 2000). Recently, it was shown that blockade of A2a receptors down- regulates the levels of the IL-1β pro-inflammatory cytokine, decreases activation of microglia and increases cell survival against transient retinal ischemia (Boia et al., 2017). Besides A1 and A2a receptors, evidences also came out showing the involvement of A3 receptors in an in vivo model of ischemia-reperfusion injury. Activation of these receptors is able to prevent retinal cell death induced by ischemia (Galvao et al., 2015).
7.3. Diabetic Retinopathy
Diabetic retinopathy is another type of ocular disease that damages blood vessels and neurons of the retina and is the leading cause of blindness in the world. In contrast to the results obtained in ischemia, activation of A2a receptors is protective against the death of retinal neurons induced by experimental diabetes. Signaling triggered by A2a receptors expressed in microglia cells blocks tumor necrosis factor (TNF)-release and also prevents the change in microglia morphology to an activated state (Ibrahim et al., 2011). The beneficial role of adenosinergic system in diabetic retinopathy was also indicated by another study showing that inhibition of adenosine kinase decreases retinal inflammatory markers, retinal microglial activation, oxidative stress and retinal cell death in diabetic mice (Elsherbiny et al., 2013). The inhibition of adenosine kinase increases adenosine extracellular levels, which activates A2a receptors in retinal microglia cells to block the release of TNF-α.
Recently, another work investigating the edema of retinal macular region that occurs in the diabetic retinopathy showed that the non-selective adenosine receptors antagonist caffeine prevents the blood retinal barrier breakdown (Maugeri et al., 2017). Although this study has not checked if this effect was really mediated by A2a receptors, it seems clear that A2a receptors can have dual effects in terms of cell survival.
7.4. Glutamate Excitotoxicity
Glutamate excitotoxicity is involved in a plethora of different pathological conditions such as Alzheimer’s disease, multiple sclerosis, hypoxia and ischemia, among others. In general, this phenomenon is featured by the over-activation of the NMDA subtype of glutamate receptor (NMDAR) with a massive intracellular accumulation of calcium which, in turn, activates calcium- dependent cell death. Some works described that adenosine can protect retinal neurons against glutamate-induced cell death. Chronic activation of A2a adenosine receptors is able to prevent glutamate-induced cell death in purified cultures of chick retinal neurons, an effect that is dependent on cAMP signaling (Ferreira et al., 2001). A similar protective effect of A2a receptors activation and cAMP signaling is observed in a model of cell death of chick retinal neurons and photoreceptors triggered by re-feeding the cultures with fresh medium (Paes de Carvalho et al., 2003). However, A2a receptors stimulation may have dual effects on the cell survival of neuronal progenitors from the chick retina. Socodato et al., (2011) demonstrated that activation of A2a receptors in cultures of retinas from 6-day-old embryos (E6) triggers cell death and the different effects of A2a receptor stimulation observed at the two different developmental stages (E6 vs E8) can be due to coupling of the receptor to different intracellular signaling pathways. Yet, another study showed a protective effect of adenosine against NMDA-induced neurotoxicity in cultured rat retinal neurons, mediated by the activation of A1 receptors (Oku et al., 2004). Moreover, another report, using both dissociated and organotypic rat retinal cell cultures, demonstrated that A3 receptors stimulation can be beneficial against glutamate induced cell death mediated by ionotropic receptors (Galvão et al., 2015).
7.5. Optic Nerve Injury
Optic Nerve Injury (ONI) is a kind of lesion where optic nerve axons may be damaged either directly or indirectly and the visual loss may be partial or complete. This lesion causes the death of a significant number of RGCs due to interruption of trophic support, oxidative stress, high levels of extracellular glutamate and retinal microglia reactivity (Ahmad et al., 2013).
Some studies also pointed to adenosine receptors as regulators of the damage induced by ONI. In an in vivo model of ONI, inosine increases cell survival of axotomized RGCs (Hou et al. 2004). Although a direct demonstration was not provided, the protective effect of inosine might be mediated by its interaction with A3 receptors since inosine can bind these receptors (Jin et al. 1997). Accordingly, another study demonstrated that the A3 selective agonist 2- Cl-IB-MECA decreases RGC loss after the optic nerve transection (Galvao et al. 2015). Activation of A3 receptors is also protective against the death of RGCs induced by P2X7 receptor stimulation in an in vitro model (Zhang et al. 2006b).
Besides A3 receptors, activation of other adenosine receptors was also implicated in the ONI responses of the retina. Activation of A2a receptors can regulate several features of this lesion such as the increase of markers of oxidative stress, microglial reactivity, retinal inflammation and RGCs cell death (Ahmad et al., 2013). In an in vitro model of optic nerve injury, activation of A1 receptors (Perígolo-Vicente et al., 2013) and A2a receptors (Perígolo-Vicente et al., 2014) seems to be required for the survival of axotomized rat RGCs in culture induced by IL-6.
8. Purines and the regeneration of the retina
8.1. The zebrafish (Danio rerio) retina as a model for growth and regeneration
Throughout evolution, teleost fish maintained the capacity of completely repairing organs and structures such as heart, brain, spinal cord and the retina after injury or disease, despite the adaptive specializations of such organs. Among teleost fish, zebrafish has been extensively used as an experimental model system to study organ and tissue growth and regeneration (Gemberling et al., 2013). Of particular interest to this review, the retina of this species is endowed with multipotent progenitor cells (stem-like cells) that slowly proliferate throughout the animal’s life originating derived progenitors that, through a differentiation process, give rise to retina cells. These cells synaptically integrate at the border of the mature retina to form fully functional tissue. These stem-like and derived progenitors form an undifferentiated portion of cuneiform tissue at the periphery of the mature retina denominated ciliary marginal zone (CMZ) (Müller et al., 1952; Johns and Easter, 1977; Fernald et al., 1990; Harris and Perron, 2002). In contrast, rod photoreceptors genesis occurs in the mature retina from another kind of progenitors represented by a population of Müller glia that asymmetrically divides giving rise to monopotent progenitors (Johns et al., 1981). These cells migrate to the outer nuclear layer and differentiate into rod photoreceptors that are incorporated to the synaptic circuit of the outer retina avoiding the loss of sensitivity to light when the retinal tissue expands, accompanying the growth of the eyeball (Fernald, 1990; Lenkowski and Raymond, 2014).
Under injury, the zebrafish retina can fully regenerate through activation of a quiescent Müller glia population that after genetic reprogramming re-enters cell cycle and divides asymmetrically originating multipotent progenitor cells that rapidly proliferate and generate all other cells of the mature retina (Fig. 3). Progenitors form clusters in the mature inner nuclear layer (INL) and migrate to other retinal layers, originating all required interneurons, photoreceptor and ganglion cells to repair the injured tissue (Maier and Wolburg, 1979; Faillace et al., 2002; Fausett and Goldman, 2006; Bernardos et al., 2007). The differentiated cells establish synaptic contacts and a fully functional retina is reconstructed. Optic nerve and connections with brain structures dealing with visual processing are also re-established. Therefore, this vertebrate model system offers unique characteristics to study signaling pathways and genes involved in the control of multipotent progenitor activation and the neurogenic, gliogenic and angiogenic processes that lead to retina repair and visual function recovery (Mensinger and Powers, 2007).
Fig.3.
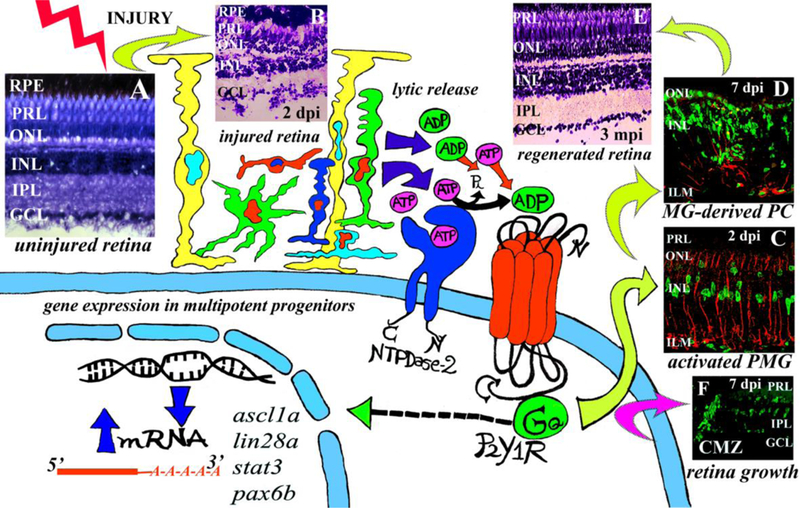
Extracellular adenine nucleotides, NTPDases and P2Y receptors are necessary signals to induce progenitor cell proliferation and retina repair in the injured zebrafish retina. (A) Histological image of an uninjured retina. Injury was induced with one injection within the vitreous of a cytotoxic agent (6 μM ouabain). Injury provokes retina cell death and lytic release of nucleotides. NTPDase2 hydrolyses ATP to produce extracellular ADP. P2Y1 receptor activation chiefly by ADP provokes pluripotency gene expression in progenitor Müller glia (PMG) and Müller glia-derived progenitor cells (MG-derived PC). (B) Histological image of a retina 2 days after injury (dpi) with ouabain. (C) Confocal image of a retina at 2 dpi. PMG mitotically activates shortly after injury. (D) Confocal image of a retina 7 dpi with ouabain. MG-derived progenitors greatly amplify between 5 to 7 dpi. (E) Retina repair is achieved around 3 months after injury (mpi). (F) Confocal image of a retina 7 dpi with ouabain. Progenitor cells in the ciliary marginal zone (CMZ) are activated showing enhanced retina growth after injury. In (C) and (D) GFAP-positive MG is depicted in red to show glial activation. In (C), (D) and (F) BrdU-positive proliferative nuclei are depicted in green. RPE: retinal pigment epithelium; PRL: photoreceptor segment layer; ONL: outer nuclear layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer; ILM: inner limiting membrane.
8.2. Purines and retina growth and regeneration in the zebrafish
Following injury or during a disease, higher than physiological levels of nucleotides and nucleosides are released to the extracellular milieu by damaged or dying cells, which provoke an important impact on the homeostatic and metabolic balance, causing different cells to survive or die (Newman, 2015).
An important family of enzymes that regulate nucleotide and nucleoside availability in the extracellular milieu is the group of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). These enzymes convert ATP (or other nucleotides) to ADP (UDP, CDP, or GDP) or AMP (UMP, CMP, or GMP) (Zimmermann and Braun, 1999). Other plasma membrane enzymes, the ecto- 5’-nucleotidases, catalyse the production of adenosine from AMP in the extracellular environment (Colgan et al., 2006).
In the zebrafish retina, the expression of several components of the purinergic system such as NTPDases 1, 2 and 3 are differentially expressed in retinal layers and cell types, both in the intact and injured tissue. Several P2X and P2Y receptors (P2XR and P2yR) which play important roles in regulating cell growth and tissue regeneration are also differentially expressed (Ricatti et al., 2009; 2011; Battista et al., 2009). In order to characterize the expression of these components, we have used in vivo injections of compounds into the vitreous cavity of the zebrafish eyeball to generate damage and to pharmacologically manipulate different components of the purinergic system.
NTPDase mRNA expression is upregulated in light versus dark conditions, whereas levels of P2Y1 receptor mRNA do not show significant day- night variations (Ricatti et al., 2011). Moreover, the expression of specific P2 receptors and plasma membrane-bound NTPDases 1, 2 and 3 is upregulated at different intervals after in vivo injury of the zebrafish retina with ouabain (Battista et al., 2009, Medrano et al., 2017). Other kinds of cytotoxic injuries also induce changes in the expression of different purinergic receptors (data not published). For instance, P2Y1 receptor transcriptional expression is induced early after injury and shows maximal levels at the time of peak proliferation of progenitor cells. Extracellular nucleotides, mainly ADP, regulate P2Y1 receptor transcriptional and protein expression. The transcriptional expression of ecto- nucleotidases is also increased by injury and these changes are important for cell cycle regulation and cell survival at different intervals after injury.
Injection of apyrase in the vitreous cavity of zebrafish eyes which causes nucleotide depletion in the extracellular environment does not affect significantly the mature retina structure or the morphology of retinal cells. However, depletion of nucleotides with apyrase treatment significantly inhibits the number of progenitor cells entering S-phase in the CMZ, indicating that nucleotide availability and activation of purinergic receptors are necessary for normal retina growth originating from CMZ progenitor cells. Moreover, no deleterious effects on the mature tissue are observed with the apyrase treatment, indicating that depletion of nucleotide basal levels, at least in the short term, does not cause cytotoxic effects in the uninjured retina (Ricatti et al., 2011).
In intact adult retinas, treatment with the hydrolysis-resistant ADP analogue ADPβS significantly enhances the proliferative activity in the CMZ. ADPβS injected within the eye at night induces an increase to mid-light levels in the S-phase activity of progenitor cells in the CMZ. Moreover, blockade of P2Y1 receptor with MRS2179 abolishes mid-light increase of CMZ progenitor cell S- phase activity. The increase in the number of progenitor cells that enter S- phase in a 2 h interval is also prevented by disrupting extracellular nucleotide hydrolysis mediated by NTPDase activity. Therefore, purinergic signaling is essential for inducing S-phase activity of progenitors in the CMZ throughout the light-dark cycle that is necessary to sustain normal growth in the intact retina (Ricatti et al., 2011).
Blockade of P2Y1 receptor significantly diminishes progenitor proliferation induced by damaging the retina with low or intermediate concentrations of ouabain (Fimbel et al, 2007; Battista et al, 2009; Medrano et al, 2017). The inhibition is observed at two intervals post-injury: when progenitor Müller cells are mitotically activated and when Müller glia-derived progenitors are amplified. Furthermore, the number of GFAP-BrdU-positive cells, likely representing multipotent Müller glia, observed at an early interval after injury with ouabain, is significantly reduced by the treatment with the P2Y1 receptor antagonist in vivo.
In the injured CNS, lytic release by damaged and dying cells is a major source of extracellular nucleotides (Pafundo et al., 2008). The increase in extracellular ADP levels, likely caused by lytic release after injury, positively regulates P2Y1 receptor expression in retinal cells. The induction of the expression of multipotency and neurogenic genes is crucial for progenitor cell genetic reprogramming in the zebrafish retina following injury (Ramachandran et al., 2010). In this regard, genes such as lin28a and ascl1a are upregulated by ADPβS in intact retinas and ouabain treatment induces the expression of these genes. Ouabain effect on the transcriptional induction of these multipotency genes is significantly inhibited by blocking P2Y1 receptor activation (Fig. 3). ADPβS is also able to induce by itself progenitor and precursor cell proliferation in the INL and ONL, respectively, and in the intact mature retina this effect is also abolished by antagonizing P2Y1 receptor. Furthermore, while NTPDase expression and activity are necessary to reach enough levels of proliferation of the Müller-derived progenitor cells to achieve retina repair after damage, their activity is not needed at early intervals for Müller glia reprogramming and asymmetric division (Medrano et al., 2017). Moreover, since apoptotic cell death that occurs at the peak of progenitor proliferation is not modified by NTPDase activity inhibitors or P2Y1 receptor antagonists, the reduction in the number of mitotic cells cannot be ascribed to a neuroprotective or deleterious effect of these compounds, but is likely due to an inhibition of the proliferative activity of Müller glia-derived progenitor cells (Medrano et al., 2017). On the other hand, either a neuroprotective or a deleterious effect caused by different members of the purinergic system may provoke a decrease in progenitor proliferation by inducing progenitor death or avoiding excessive deleterious effects in the mature tissue which in turn provokes a lower degree of progenitor mitotic induction.
It is likely that both injury-induced progenitors and differentiated cells survive further in the presence of extracellular nucleotides and nucleosides that could have neuroprotective roles such as those already demonstrated for adenosine (Gessi et al., 2011). Noteworthy, under injury conditions the complete hydrolysis of extracellular nucleotides by apyrase results in an excessively damaged tissue unable to self-repair (Battista et al., 2009; Medrano et al., 2017). The apyrase treatment extremely aggravates the injury induced by ouabain or other cytotoxic compounds and likely prevents recovery by abolishing the proliferative response and inducing the death of progenitors and differentiated cells that survived damage (Battista et al., 2009, Medrano et al., 2017). For instance, extracellular nucleotides are crucial to regulate retina cell volume increase, an event that could be provoked by osmotic imbalance caused by the ouabain treatment. Moreover, nucleotides and their interaction with different purinergic receptors are required to limit microglia inflammatory activation. The gliotic response of Müller cells and astrocytes is also controlled by purinergic signals in the mammalian retina (Puthussery and Fletcher, 2009; Notomi et al., 2011). In the zebrafish retina, the lack of nucleotides in the extracellular environment, such as the one caused by apyrase intraocular treatment, might allow an excessive gliotic response of Müller glia that could prevent multipotent Müller glia reprogramming and retina regeneration (Than- Trong and Bally-Cuif, 2015; Reichenbach and Bringmann, 2016). So, purinergic signaling is indispensable to limit neurodegenerative processes and allow controlled Müller glia mitotic division triggered by a cytotoxic damage independently of the toxic compound used to provoke the injury.
8.3. Injury-induced growth factors, cytokines and purines cooperate to regulate retina regeneration.
At early intervals after different kinds of injury, many types of retina cells release injury-induced factors that regulate Müller glia reprograming and the proliferation of Müller glia-derived multipotent progenitors for retina repair. These factors include, among other molecules, ciliary neurotrophic factor (CNTF), epidermal growth factor (EGF),heparin-binding EGF-like growth factor (HB-EGF), insulin, insulin-like growth factor I (IGF-I) and fibroblast growth factor (FGF) (Faillace et al., 2002; Wan et al., 2014). Moreover, progenitors can be reprogrammed and activated by cytokines, leptins, IL-11 and tumor necrosis factor α (TNFα) (Nelson et al., 2013). Also, Transforming Growth Factor β (TGFβ) inhibits the mitotic activation of Müller glia progenitors (Lenkowski and Raymond, 2014) and GABA neurotransmission is required to induce progenitor cell proliferation and regeneration in the zebrafish retina (Rao et al., 2017).
A variety of injuries can be induced by regulating the concentrations of cytotoxic drugs or performing different types of lesions. So, it is possible to cause a global damage that kills almost all retina cells, a specific damage to a particular retinal layer, an injury to a single patch of cells or the ablation of a single cell type (Raymond et al., 1988; Fimbel et al., 2007; Mensinger and Powers, 2007; Zhao et al., 2009). Through intracellular signaling pathways, injury-induced factors can act in concert to regulate Müller glia genetic reprograming and cell cycle progression. The factors that are activated and their interactions might depend on the nature of the injury and the type of cells that are killed or affected. They may also contribute, like probably is the case of some purinergic signals, to avoiding excessive death of mature cells or to keeping progenitor and precursor cells alive, helping them to reach the right number necessary to replace lost cells in a cell proliferation stimulating but harsh environment.
At the injury zone, molecules such as ATP, ADP and adenosine are released as well as growth and pro-inflammatory factors. Moreover, signals released by damaged or dying cells may directly act on Müller glia, microglia, neural, endothelial or RPE cells at the neighbouring regions next to the injured tissue. In a paracrine way, these signals may induce their own release, including nucleotides and particularly ATP, as well as the release of other factors, enhancing receptor sensitivity and expression and spreading injury- induced signals to other parts of the tissue (Weick et al., 2005). At the injured regions, extracellular nucleotides activate P2Y1 receptors which transactivate tyrosine kinase growth factor receptors. Activation of P2Y1 receptors induces the release of platelet-derived growth factor (PDGF) and HB-EGF from Müller glia (Milenkovic et al., 2003). HB-EGF activates Müller glia reprogramming whereas PDGF is involved in the development of proliferative vitreoretinopathy (Andrews et al., 1999; Wan et al., 2012). In turn, HB-EGF stimulates ATP release from Müller glia (Weuste et al., 2006). Activation of P2Y receptors, including P2Y1, estimulates the phosphoinositide 3 kinase/Akt (PI3-K/Akt) and the Extracellular signal-regulated protein kinase (MAPK/ERK) pathways mediating astrogliosis following injury in the CNS (Franke et al., 2009). Activation of PI3-K/Akt pathway by growth factor receptors promotes cell survival, growth, proliferation, migration, differentiation and angiogenesis and inhibits apoptosis (Cantley, 2002). This pathway controls cell cycle by stimulating progenitor cells to pass G1 phase and G1 to S-phase transition (Wang et al., 2009). Furthermore, P2Y1 receptor activity regulates trophic- inflammatory factor expression in astrocytes (Franke et al., 2009). Trophic and pro-inflammatory factors released by Müller glia, microglia and astrocytes control multipotent progenitor mitotic activation in the injured zebrafish retina (Wan et al., 2014). Thus, the combined action of chiefly ADP acting on P2Y1 receptors with other injury-induced signaling pathways regulates multipotent progenitor cell reprogramming and mitotic activation.
9. Concluding remarks
From the above reported findings, it is clear that purinergic signaling regulates several events in the developing retina as well as in the adult damaged tissue. While adenosine is involved mainly in cell survival, nucleotides through activation of P2Y receptors, induce the proliferation and migration of retinal cells. In an opposite direction, activation of P2X receptors, mainly the P2X7 receptor subtype is capable of inducing cell death both in the developing as well as in the damaged retina. It is also clear, that as in the developing tissue, the release of purines and the expression of purinergic receptors are modified when the retina is damaged or in a pathological condition. Moreover, activation of nucleotide receptors seems to be involved in the response of self-repair in the lesioned zebrafish retina by inducing pluripotency genes in glial cells. Thus, to determine the factors and mechanisms that control the release of purines and the expression of the different purinergic receptor subtypes in the healthy and damaged retina could contribute for better drug-based strategies for the prevention and/or treatment of retinal diseases. To verify if purinergic signaling induces pluripotency genes in the mammalian retina under injury also could contribute and impact the strategies for retina regeneration in mammals.
Highlights.
ATP release from retinal neurons, glia and Pigment Epithelium in health and disease;
Adenosine synthesis, release and cell survival in the developing and damaged retina;
P2Y receptors, cell proliferation and migration in the developing and injured retina;
P2X receptors and cell death in the retina during development and disease;
P2Y receptors, pluripotency genes and self-repair in the injured zebrafish retina;
Acknowledgements
We would like to thank Matias P. Medrano technical assistance and Ramón O. Bernabeu for helpful discussions on section 8.
Funding: This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ-grant E26/202.563/2016), Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - grant 303018/2016–0) and Pró-reitoria de Pesquisa, Pós- graduação e Inovação (Proppi-UFF) to A.L.M.V.; the National Institutes of Health, USA (Grants EY013434 and EY015537) to C.H.M.; Universidad de Buenos Aires (grant UBACYT N° 0061 2016–2018) to M.P.F..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ana Lucia Marques Ventura, Email: almvuff@gmail.com.
Alexandre dos Santos-Rodrigues, Email: rodrigues.alex9@gmail.com.
Claire H. Mitchell, Email: chm@upenn.edu.
Maria Paula Faillace, Email: pfaillace@qb.ffyb.uba.ar.
References
- Agte S, Pannicke T, Ulbricht E, Reichenbach A, Bringmann A, 2017. Two different mechanosensitive calcium responses in Muller glial cells of the guinea pig retina: Differential dependence on purinergic receptor signaling. Glia 65(1), 62–74. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Fatteh N, El-Sherbiny NM, Naime M, Ibrahim AS, El- Sherbini AM, El-Shafey SA, Khan S, Fulzele S, Gonzales J, Liou GI, 2013. Potential role of A2A adenosine receptor in traumatic optic neuropathy. J. Neuroimmunol. 264, 54–64. [DOI] [PubMed] [Google Scholar]
- Albalawi F, Lu W, Beckel JM Lim JC, McCaughey SA, Mitchell CH, 2017. The P2X7 Receptor Primes IL-1beta and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain. Front. Cell Neurosci. 11, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Pereira L, Magalhães CF, Repossi MG, Thorstenberg MLP, Sholl-Franco A, Coutinho-Silva R, Ventura ALM, Fragel-Madeira F, 2017. Adenine nucleotides control proliferation in vivo of rat retinal progenitors by P2Y1 receptor. Molec. Neurobiol. 54(7), 5142–5155. [DOI] [PubMed] [Google Scholar]
- Almeida-Pereira L, Repossi MG, Magalhães CF, Azevedo RF, Corrêa- Velloso JC, Ulrich H, Ventura ALM, Fragel-Madeira L, 2018. P2Y12 but not P2Y13 Purinergic Receptor Controls Postnatal Rat Retinogenesis In Vivo. Molec. Neurobiol. DOI 10.1007/s12035-018-1012-1. [DOI] [PubMed] [Google Scholar]
- Anccasi RM, Ornelas IM, Cossenza M, Persechini PM, Ventura ALM, 2013. ATP induces the death of developing avian retinal neurons in culture via activation of P2X7 and glutamate receptors. Purinergic Signal. 9(1), 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews A, Balciunaite E, Leong FL, Tallquist M, Soriano P, Refojo M, Kazlauskas A, 1999. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci, 40(11), 2683–2689. [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD, 2004. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 447, 735–43. [DOI] [PubMed] [Google Scholar]
- Battista AG, Ricatti MJ, Pafundo DE, Gautier MA, Faillace MP, 2009. Extracellular ADP regulates lesion-induced in vivo cell proliferation and death in the zebrafish retina. J. Neurochem. 111(2), 600–613. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Argallm AJ, Lim JC, Xia J, Lu W, Coffey EE, Macarak EJ, Shahidullah M, Delamere NA, Zode GS, Sheffield VC, Shestopalov VI, Laties AM, Mitchell CH, 2014. Mechanosensitive release of adenosine 5’-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 62(9), 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel JM, Gómez NM, Lu W, Campagno KE, Nabet B, Albalawi F,Lim JC, Boesze-Battaglia K, Mitchell CH, 2018. Stimulation of TLR3 triggers release of lysosomal ATP in astrocytes and epithelial cells that requires TRPML1 channels. Sci. Rep. 8(1), 5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA, 2007. Late- stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J. Neurosci. 27(26), 7028–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazynski C, 1991. The accumulation of [3H]phenylisopropyl adenosine ([3H]PIA) and [3H]adenosine into rabbit retinal neurons is inhibited by nitrobenzylthioinosine (NBI). Neurosci. Lett. 121, 1–4. [DOI] [PubMed] [Google Scholar]
- Boia R, Elvas F, Madeira MH, Aires ID, Rodrigues-Neves AC, Tralhão P, Szabó EC, Baqi Y, Müller CE, Tomé AR, Cunha RA, Ambrésio AF, Santiago AR, 2017. Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis. 8, e3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone DB, Hammond JR, 2007. Nucleoside and nucleobase transporters of primary human cardiac microvascular endothelial cells: characterization of a novel nucleobase transporter. Am. J. Physiol. Heart Circ Physiol. 293, H3325–32. [DOI] [PubMed] [Google Scholar]
- Bone DB, Robillard KR, Stolk M, Hammond JR, 2007. Differential regulation of mouse equilibrative nucleoside transporter 1 (mENTI) splice variants by protein kinase CK2. Mol. Membr. Biol. 24, 294–303. [DOI] [PubMed] [Google Scholar]
- Boucher I, Rich C, Lee A, Marcincin M, Trinkaus-Randall V, 2010. The P2Y2 receptor mediates the epithelial injury response and cell migration. Am. J. Physiol. Cell Physiol. 299(2), C411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, Zarbin MA, Snyder SH, 1987. Endogenous adenosine and adenosine receptors localized to ganglion cells of the retina. Proc. Natl. Acad. Sci. U S A. 84, 3906–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändle U, Guenther E, Irrle C, Wheeler-Schilling TH, 1998. Gene expression of the P2X receptors in the rat retina. Molec. Brain Res. 59(2), 269–272. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P, 2012. Müller glial cells in retinal disease. Ophthalmologica 227, 1–19. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Moll V, Milenkovic I, Faude F, Enzmann V, Wolf S, Reichenbach A, 2001. Upregulation of P2X7 Receptor Currents in Müller Glial Cells during Proliferative Vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 42(3), 860–867. [PubMed] [Google Scholar]
- Brito R, Pereira MR, Paes-de-Carvalho R, Calaza KC, 2012. Expression of A1 adenosine receptors in the developing avian retina: In vivo modulation by A(2A) receptors and endogenous adenosine. J. Neurochem. 123, 239–249. [DOI] [PubMed] [Google Scholar]
- Brito R, Pereira-Figueiredo D, Socodato R, Paes-de-Carvalho R, Calaza KC, 2016. Caffeine exposure alters adenosine system and neurochemical markers during retinal development. J. Neurochem. 138, 557–570. [DOI] [PubMed] [Google Scholar]
- Calvet GA, Ventura ALM, 1995. Accumulation of 3H-phosphoinositides mediated by muscarinic receptors in the developing chick retina: Inhibition of carbachol-induced response by glutamate receptors. J. Neurochem. 64(3), 1064–1070. [DOI] [PubMed] [Google Scholar]
- Cantley LC, 2002. PI3K Pathway, Science’s STKE (Connections Map), http://www.stke.org/cgi/cm/CMP_6557.
- Casadó V, Barrondo S, Spasic M, Callado LF, Mallol J, Canela E, Lluís C, Meana J, Cortés A, Sallés J, Franco R, 2010. Gi protein coupling to adenosine A1-A2A receptor heteromers in human brain caudate nucleus. J. Neurochem. 114, 972–980. [DOI] [PubMed] [Google Scholar]
- Cisneros-Mejorado A, Perez-Samartin A, Gottlieb M, Matute C, 2015. ATP signaling in brain: release, excitotoxicity and potential therapeutic targets. Cell Mol. Neurobiol. 35(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Eltzschig HK, Eckle T, Thompson LF, 2006. Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal. 2:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R, Insel PA, 2012. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 8, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R, Insel PA, 2010. Basal release of ATP: an autocrine- paracrine mechanism for cell regulation. Sci. Signal. 3(104), re1 DOI: 10.1126/scisignal.3104re1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G, Pereira T, Neto AM, Cristovao AJ, Ambrosio AF, Santos PF, 2009. High glucose changes extracellular adenosine triphosphate levels in rat retinal cultures. J. Neurosci. Res. 87(6), 1375–1380. [DOI] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J, Wu K, Yu M, Ge J, 2010. Adenosine receptor protein changes in guinea pigs with form deprivation myopia. Acta Ophthalmologica 88, 759–765. [DOI] [PubMed] [Google Scholar]
- Cunha RA, 2008. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem. Int. 52, 65–72. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Constantino MD, Ribeiro JA, 1999. G protein coupling of CGS 21680 binding sites in the rat hippocampus and cortex is different from that of adenosine A1 and striatal A2A receptors. Naunyn Schmiedebergs Arch. Pharmacol. 359, 295–302. [DOI] [PubMed] [Google Scholar]
- Dahl G, 2015. ATP release through pannexon channels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370(1672). DOI: 10.1098/rstb.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, 2008. Dynamic ATP signalling and neural development. J. Physiol. 586(10), 2429–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilip R, Ishii T, Imada H, Wada-Kiyama Y, Kiyama R, Miyachi E, Kaneda M, 2013. Distribution and development of P2Y1-purinoceptors in the mouse retina. J. Mol. Histol. 44(6), 639–644. [DOI] [PubMed] [Google Scholar]
- Dos Santos AA, Medina SV, Sholl-Franco A, de Araujo EG, 2003. PMA decreases the proliferation of retinal cells in vitro: the involvement of acetylcholine and BDNF. Neurochem. Int. 42(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Dos Santos-Rodrigues A, Pereira MR, Brito R, de Oliveira NA, Paes- de-Carvalho R, 2015. Adenosine transporters and receptors: key elements for retinal function and neuroprotection. Vitam. Horm. 98, 487–523. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR, 1997. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 17, 7673–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI, 2006. Expression of pannexin family of proteins in the retina. FEBS Lett. 580(9), 2178–2182. [DOI] [PubMed] [Google Scholar]
- Elsherbiny NM, Ahmad S, Naime M, Elsherbini AM, Fulzele S, Al- Gayyar MM, Eissa LA, El-Shishtawy MM, Liou GI, 2013. ABT-702, an adenosine kinase inhibitor, attenuates inflammation in diabetic retinopathy. Life Sci. 93, 78–88. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Julian D, Korenbrot JI, 2002. Mitotic activation of proliferative cells in the inner nuclear layer of the mature fish retina: regulatory signals and molecular markers. J. Comp. Neurol, 451(2), 127–141. [DOI] [PubMed] [Google Scholar]
- Faria RX, Freitas HR, Reis RAM, 2017. P2X7 receptor large pore signaling in avian Müller glial cells. J. Bioenerg. Biomembr. 49(3), 215–229. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D, 2006. A role for α1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J. Neurosci. 26(23), 6303–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD, 1990. Teleost vision: seeing while growing. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 256(S5), 167–180. [DOI] [PubMed] [Google Scholar]
- Ferreira DD, Stutz B, de Mello FG, Reis RA, Kubrusly RC, 2014. Caffeine potentiates the release of GABA mediated by NMDA receptor activatio n: Involvement of A1 adenosine receptors. Neurosci. 281, 208–215. [DOI] [PubMed] [Google Scholar]
- Ferreira JM, Paes-de-Carvalho R, 2001. Long-term activation of adenosine A(2a) receptors blocks glutamate excitotoxicity in cultures of avian retinal neurons. Brain Res. 900, 169–76. [DOI] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR, 2007. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 27(7), 1712–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA, 2001. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 4, 247–252. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER, Sherwood P, 2009. Mitogen- activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia 57(14), 1538–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França GR, Freitas RC, Ventura ALM, 2007. ATP-induced proliferation of developing retinal cells: regulation by factors released from postmitotic cells in culture. Int. J. Dev. Neurosci. 25(5), 283–291. [DOI] [PubMed] [Google Scholar]
- Franke H, Klimke K, Brinckmann U, Grosche J, Francke M, Sperlagh B, Reichenbach A, Liebert UG, Illes P, 2005. P2X(7) receptor-mRNA and - protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem. Int. 47(4), 235–242. [DOI] [PubMed] [Google Scholar]
- Franke H, Sauer C, Rudolph C, Krügel U, Hengstler JG, Illes P, 2009. P2 receptor-mediated stimulation of the PI3-K/Akt-pathway in vivo. Glia 57(10), 1031–1045. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM, 2005. Adenosine and brain function. Int. Rev. Neurobiol. 63, 191–270. [DOI] [PubMed] [Google Scholar]
- Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T, 2005. Identification of P2Y receptor subtypes in human muller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest. Ophthalmol. Vis. Sci. 46(8), 3000–3007. [DOI] [PubMed] [Google Scholar]
- Fries JE, Wheeler-Schilling TH, Guenther E, Kohler K, 2004. Expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptor subtypes in the rat retina. Invest. Ophthalmol. Vis. Sci. 45, 3410–3417. [DOI] [PubMed] [Google Scholar]
- Fujita S, 1962. Kinetics of cellular proliferation. Exp. Cell Res. 28, 52–60. [DOI] [PubMed] [Google Scholar]
- Galvao J, Elvas F, Martins T, Cordeiro MF, Ambrosio AF, Santiago AR, 2015. Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp. Eye Res. 140, 65–74. [DOI] [PubMed] [Google Scholar]
- Gampe K, Haverkamp S, Robson CS, Gachet C, Hüser L, Acker-Palmer A, Zimmermann H, 2015. NTPDase2 and the P2Y1 receptor are not required for mammalian eye formation, Purinergic Signal. 11, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garweg JG, Tappeiner C, Halberstadt M, 2013. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv. Ophthalmol 58(4), 321–329. [DOI] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD, 2013. The zebrafish as a model for complex tissue regeneration. Trends Genet. 29(11), 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Varani K, Borea PA (2011) Adenosine receptors in health and disease. Adv. Pharmacol. 61, 41–75. [DOI] [PubMed] [Google Scholar]
- Godinho L, Link B, 2006. Cell Migration In Retinal Development, Sernagor E, Eglen S, Harris B, Wong R, eds. (Cambridge, United Kingdom: Cambridge University Press; ), pp. 59–74. [Google Scholar]
- Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H, 2009. Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J. Cell Sci. 122, 2524–2533. [DOI] [PubMed] [Google Scholar]
- Grimm I, Ullsperger SN, Zimmermann H, 2010. Nucleotides and epidermal Growth Factor Induce Parallel Cytoskeletal Rearrangements and Migration in Cultured Adult Murine Neural Stem Cells. Acta Physiol. 199, 181–189. [DOI] [PubMed] [Google Scholar]
- Guzman-Aranguez A, Pintor J, 2012. Focus on molecules: purinergic P2Y(2) receptor. Exp. Eye Res. 105, 83–84. [DOI] [PubMed] [Google Scholar]
- Hammond JR, Stolk M, Archer RG, McConnell K, 2004. Pharmacological analysis and molecular cloning of the canine equilibrative nucleoside transporter 1. Eur. J. Pharmacol. 491, 9–19. [DOI] [PubMed] [Google Scholar]
- Harris WA, Perron M, 2002. Molecular recapitulation: the growth of the vertebrate retina. International J. Dev. Biol. 42(3), 299–304. [PubMed] [Google Scholar]
- Ho T, Aplin FP, Jobling AI, Phipps JA, de Iongh RU, Greferath U, Vessey KA, Fletcher EL, 2016. Localization and Possible Function of P2X Receptors in Normal and Diseased Retinae. J. Ocul. Pharmacol. Ther. 32(8), 509–517. [DOI] [PubMed] [Google Scholar]
- Ho T, Jobling AI, Greferath U, Chuang T, Ramesh A, Fletcher EL, Vessey KA, 2015. Vesicular expression and release of ATP from dopaminergic neurons of the mouse retina and midbrain. Front. Cell Neurosci. 9, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T, Vessey KA, Fletcher EL, 2014. Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neurosci. 277, 55–71. [DOI] [PubMed] [Google Scholar]
- Hou B, You SW, Wu MM, Kuang F, Liu HL, Jiao XY, Ju G, 2004. Neuroprotective Effect of Inosine on Axotomized Retinal Ganglion Cells in Adult Rats. Invest. Ophthalmol. Vis. Sci. 45, 662–667. [DOI] [PubMed] [Google Scholar]
- Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, Mitchell CH, 2010. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp. Eye. Res. 91, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Hsiao YT, Kao SY, Chen CF, Chen YC, Chiang CW, et al. , 2014. Adenosine A(2A) receptor up-regulates retinal wave frequency via starburst amacrine cells in the developing rat retina. PLoS One 9, e95090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, El-Shishtawy MM, Zhang W, Caldwell RB, Liou GI, 2011. A(2A) adenosine receptor (A(2A)AR) as a therapeutic target in diabetic retinopathy. Am. J. Pathol. 178, 2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Pfeiffer S, Zrenner E, Kohler K, Guenther E, 2004. ATP- induced non-neuronal cell permeabilization in the rat inner retina. J. Neurosci. 24(39), 8577–8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T, 2003. Neuron- specific distribution of P2X7 purinergic receptors in the monkey retina. J. Comp. Neurol. 459(3), 267–277. [DOI] [PubMed] [Google Scholar]
- Ishii T, Homma K, Mano A, Akagi T, Shigematsu Y, Shimoda Y, Inoue H, Kakinuma Y, Kaneda M, 2017. Novel channel-mediated choline transport in cholinergic neurons of the mouse retina. J. Neurophysiol.118(4), 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques FJ, Martins Silva T, Silva FE, Ornelas IM, Ventura ALM, 2017. Nucleotide P2Y13-stimulated phosphorylation of CREB is required for ADP-induced proliferation of late developing retinal glial progenitors in culture. Cell. Signal. 35, 95–106. [DOI] [PubMed] [Google Scholar]
- Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE, 2001. Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacol. 40, 722–731. [DOI] [PubMed] [Google Scholar]
- Jin X, Shepherd RK, Duling BR, Linden J, 1997. Inosine Binds to A 3 Adenosine Receptors and Stimulates Mast Cell Degranulation. J. Clin. Invest. 100, 2849–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PR, Easter SS, 1977. Growth of the adult goldfish eye. II. Increase in retinal cell number. J. Comp. Neurol. 176(3), 331–341. [DOI] [PubMed] [Google Scholar]
- Johns PR, Fernald RD, 1981. Genesis of rods in teleost fish retina. Nature, 293(5828), 141–142. [DOI] [PubMed] [Google Scholar]
- Jonsson G, Eysteinsson T, 2017. Retinal A2A and A3 adenosine receptors modulate the components of the rat electroretinogram. Vis. Neurosci. 34, E001. [DOI] [PubMed] [Google Scholar]
- Kakurai K, Sugiyama T, Kurimoto T, Oku H, Ikeda T, 2013. Involvement of P2X(7) receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neurosci. Lett. 534, 237–241. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Ishii K, Morishima Y, Akagi T, Yamazaki Y, Nakanishi S, Hashikawa T, 2004. OFF-cholinergic-pathway-selective localization of P2X2 purinoceptors in the mouse retina. J. Comp. Neurol. 476(1), 103–111. [DOI] [PubMed] [Google Scholar]
- Klyuch BP, Richardson MJ, Dale N, Wall MJ, 2011. The dynamics of single spike- evoked adenosine release in the cerebellum. J. Physiol. 589, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Engel K, Wang J, 2004. Mammalian nucleoside transporters. Curr. Drug Metabol. 5, 63–84. [DOI] [PubMed] [Google Scholar]
- Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI, 2014. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr. Eye Res. 39(2), 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kur J, Newman EA, 2014. Purinergic control of vascular tone in the retina. J. Physiol. 592(3), 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Nelson ZL, Mishra A, Newman EA, 2009. Spontaneous glial calcium waves in the retina develop over early adulthood. J. Neurosci. 29(36), 11339–11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB, 1997. Localization of adenosine receptor messenger RNAs in the rat eye. Exp. Eye. Res. 65, 595–602. [DOI] [PubMed] [Google Scholar]
- Lamas M, Lee-Rivera I, Ramírez M, López-Colomé AM, 2007. D- serine regulates CREB phosphorylation induced by NMDA receptor activation in Müller glia from the retina. Neurosci Lett. 427(1), 55–60. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F, 2001. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 79, 463–484. [DOI] [PubMed] [Google Scholar]
- Lenkowski JR, Raymond PA, 2014. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 40, 94–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Chapin EA, Luna G, Linberg KA, Fisher SK, 2010. The fate of Müller’s glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol. Vision 16, 1361–1372 [PMC free article] [PubMed] [Google Scholar]
- Li B, Roth S, 1999. Retinal ischemic preconditioning in the rat: requirement for adenosine and repetitive induction. Invest. Ophthalmol. Vis. Sci. 40, 1200–1216. [PubMed] [Google Scholar]
- Li B, Yang C, Rosenbaum DM, Roth S, 2000. Signal transduction mechanisms involved in ischemic preconditioning in the rat retina in vivo. Exp. Eye Res. 70, 755–765. [DOI] [PubMed] [Google Scholar]
- Li A, Zhang X, Zheng D, Ge J, Laties AM, Mitchell CH, 2011. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp. Eye Res. 93(4), 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Lu W, Beckel JM, Mitchell CH, 2016. Neuronal Release of Cytokine IL-3 Triggered by Mechanosensitive Autostimulation of the P2X7 Receptor Is Neuroprotective. Front. Cell. Neurosci. 10, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S, et al. , 2008. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest. Ophthalmol. Vis. Sci. 49, 5526–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL, 2001. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2(2), 109–118. [DOI] [PubMed] [Google Scholar]
- Loiola EC, Ventura AL, 2011. Release of ATP from avian Muller glia cells in culture. Neurochem. Int. 58(3), 414–422. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD, 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. [DOI] [PubMed] [Google Scholar]
- Lu W, Reigada D, Sevigny J, and Mitchell CH 2007. Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells. J. Pharmacol. Exp. Ther. 323, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Hu H, Sévigny J, Gabelt BT, Kaufman PL, Johnson EC, Morrison JC, Zode GS, Sheffield VC, Zhang X, Laties AM, Mitchell CH, 2015. Rat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eye. Invest. Ophthalmol.Vis. Sci. 56(5), 3075–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Albalawi F, Beckel JM, Lim JC, Laties AM, Mitchell CH, 2017. The P2X7 receptor links mechanical strain to cytokine IL-6 up-regulation and release in neurons and astrocytes. J. Neurochem. 141(3), 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Gómez NM, Lim JC, Guha S, Campagno KE, McCaughey SA, Laties AM, Carlsson LG, Mitchell CH, 2018. The P2Y12 receptor antagonist ticagrelor reduces lysosomal pH and autofluorescence in retinal pigmented epithelial cells from the ABCA4−/− mouse model of retinal degeneration. Front. Pharmacol. 9, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, Payne R, Demuro A, Parker I, Mitchell CH, Henao-Mejia J, Tanis JE, Matsumoto I, Tordoff MG, Foskett JK, 2018. CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98(3), 547–561.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MH, Boia R, Elvas F, Martins T, Cunha RA, Ambròsio AF, Santiago AR, 2016a. Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Transl. Res. 169, 112–128. [DOI] [PubMed] [Google Scholar]
- Madeira MH, Elvas F, Boia R, Gongalves FQ, Cunha RA, Ambròsio AF, Santiago AR, 2015. Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure. J. Neuroinflammation 12, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MH, Ortin-Martinez A, Nadal-Nicolas F, Ambrósio AF, Vidal-Sanz M, Agudo-Barriuso M, Santiago AR, 2016b. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci Rep. 6, 27532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Wolburg H, 1979. Regeneration of the goldfish retina after exposure to different doses of ouabain. Cell Tissue Res. 202(1), 99–118. [DOI] [PubMed] [Google Scholar]
- Maminishkis A, Jalickee S, Blaug SA, Rymer J, Yerxa BR, Peterson WM, Miller SS, 2002. The P2Y(2) receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest. Ophthalmol. Vis. Sci. 43(11), 3555–3566. [PubMed] [Google Scholar]
- Martins RAP, Pearson RA, 2008. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 1192, 32–60. [DOI] [PubMed] [Google Scholar]
- Martins-Silva T, França GR, Ornelas IM, Loiola EC, Ulrich H, Ventura ALM, 2015. Involvement of Nucleotides in Glial Growth Following Scratch Injury in Avian Retinal Cell Cultures. Purinergic Signal, 11(2), 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé K, Bhamra S, Eason R, Dale N, Jones EA, 2007. Purine- mediated signalling triggers eye development, Nature 449, 1058–1062. [DOI] [PubMed] [Google Scholar]
- Maugeri G, D’Amico AG, Rasà DM, La Cognata V, Saccone S, Federico C, Cavallaro S, D’Agata V, 2017. Caffeine prevents blood retinal barrier damage in a model, in vitro, of diabetic macular edema. J. Cell Biochem. 118, 2371–2379. [DOI] [PubMed] [Google Scholar]
- McGahan MC, Harned J, Mukunnemkeril M, Goralska M, Fleisher L, Ferrell JB 2005. Iron alters glutamate secretion by regulating mcytosolic aconitase activity. Am. J. Physiol. Cell. Physiol. 288, C1117–1124. [DOI] [PubMed] [Google Scholar]
- Medrano MP, Bejarano CA, Battista AG; Venera GD, Bernabeu RO, Faillace MP, 2017. Injury-induced purinergic signalling molecules upregulate pluripotency gene expression and mitotic activity of progenitor cells in the zebrafish retina. Purinergic Signal. 13(4), 443–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensinger AF, Powers MK, 2007. Visual function in regenerating teleost retina following surgical lesioning. Vis. Neurosci. 24(3), 299–307. [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A, 2003. P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest. Ophthalmol. Vis. Sci. 44, 1211–1220. [DOI] [PubMed] [Google Scholar]
- Mitchell CH, 2001. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J. Physiol. 534(Pt 1), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Lu W, Hu H, Zhang X, Reigada D, Zhang M, 2008. The P2X(7) receptor in retinal ganglion cells: A neuronal model of pressure- induced damage and protection by a shifting purinergic balance. Purinergic Signal. 4(4), 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Lu W, Hu H, Zhang X, Reigada D, Zhang M, 2009. The P2X(7) receptor in retinal ganglion cells: A neuronal model of pressure- induced damage and protection by a shifting purinergic balance. Purinergic Signal. 5(2), 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Reigada D, 2008. Purinergic signalling in the subretinal space: a role in the communication between the retina and the RPE. Purinergic Signal. 4(2), 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll V, Weick M, Milenkovic I, Kodal H, Reichenbach A, Bringmann A, 2002. P2Y receptor-mediated stimulation of Müller glial DNA synthesis. Invest. Ophthalmol. Vis. Sci. 43, 766–773. [PubMed] [Google Scholar]
- Moon SW, Chung EJ, Jung S, Lee JH, 2009. PDGF stimulation of Müller cell proliferation: Contributions of c-JNK and the PI3K/AKT pathway. Biochem. Biophys. Res. Comm. 388, 167–171. [DOI] [PubMed] [Google Scholar]
- Moriyama S, Hiasa M, 2016. Expression of Vesicular Nucleotide Transporter in the Mouse Retina. Biol. Pharm. Bull. 39(4), 564–569. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Hiasa M, Sakamoto S, Omote H, Nomura M, 2017. Vesicular nucleotide transporter (VNUT): appearance of an actress on the stage of purinergic signaling. Purinergic Signal. 13(3), 387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima KI, Iwao K, Inoue T, Haga A, Tsutsumi T, Mochita MI, Fujimoto T, Tanihara H, 2018. Stimulation of the adenosine A3 receptor, not the A1 or A2 receptors, promote neurite outgrowth of retinal ganglion cells. Exp. Eye. Res. 170, 160–168. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Ackerman KM, O’ Hayer P, Bailey TJ, Gorsuch RA, Hyde DR, 2013. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Müller glia proliferation during zebrafish retinal regeneration. J. Neurosci. 33(15), 6524–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2015. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Phil. Trans. R. Soc. B, 370(1672), 20140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2001. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J. Neurosci. 21, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2003. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 23, 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2004. Glial modulation of synaptic transmission in the retina. Glia 47(3), 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2005. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J. Neurosci. 25(23), 5502–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyadurupola N, Sidaway P, Ma N, Rhodes JD, Broadway DC, Sanderson J, 2013. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Invest. Ophthalmol. Vis. Sci. 54(3), 2163–2170. [DOI] [PubMed] [Google Scholar]
- Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, Kroemer G, Ishibashi T, 2011. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am. J. Pathol. 179(6), 2798–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S, Asato R, Takeda A, Ikeda Y, Enaida H, Sakamoto T, Ishibashi T, 2013. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One 8(1), e53338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes PH, Calaza KC, Albuquerque LM, Fragel-Madeira L, Sholl- Franco A, Ventura ALM, 2007. Signal transduction pathways associated with ATP-induced proliferation of cell progenitors in the intact embryonic retina. Intl. J. Dev. Neurosci. 25(8), 499–508. [DOI] [PubMed] [Google Scholar]
- Oku H, Goto W, Kobayashi T, Okuno T, Hirao M, Sugiyama T, Yoneda S, Hara H, Ikeda T, 2004. Adenosine protects cultured retinal neurons against NMDA-induced cell death through A1 receptors. Curr. Eye Res. 29, 449–455. [DOI] [PubMed] [Google Scholar]
- Ornelas IM, Silva TM, Fragel-Madeira L, Ventura ALM, 2013. Inhibition of PI3K/Akt Pathway Impairs G2/M Transition of Cell Cycle in Late Developing Progenitors of the Avian Embryo Retina. Plos One 8(1), e53517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas IM, Ventura ALM, 2010. Involvement of the PI3K/AKT pathway in ATP-induced proliferation of developing retinal cells in culture. Intl. J. Dev. Neurosci. 28, 503–511. [DOI] [PubMed] [Google Scholar]
- Oya M, Kitaguchi T, Yanagihara Y, Numano R, Kakeyama M, Ikematsu K, Tsuboi T, 2013. Vesicular nucleotide transporter is involved in ATP storage of secretory lysosomes in astrocytes. Biochem. Biophys. Res. Commun. 438(1), 145–151. [DOI] [PubMed] [Google Scholar]
- Paes de Carvalho R, 1990. Development of A1 adenosine receptors in the chick embryo retina. J. Neurosci. Res. 25, 236–242. [DOI] [PubMed] [Google Scholar]
- Paes de Carvalho R, Braas KM, Adler R, Snyder SH, 1992. Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Dev. Brain. Res. 70, 87–95. [DOI] [PubMed] [Google Scholar]
- Paes de Carvalho R, Braas KM, Snyder SH, Adler R, 1990. Analysis of adenosine immunoreactivity, uptake, and release in purified cultures of developing chick embryo retinal neurons and photoreceptors. J. Neurochem. 55, 1603–11. [DOI] [PubMed] [Google Scholar]
- Paes de Carvalho R, de Mello FG, 1982. Adenosine-elicited accumulation of adenosine 3’,5’-cyclic monophosphate in the chick embryo retina. J. Neurochem. 38, 493–500. [DOI] [PubMed] [Google Scholar]
- Paes de Carvalho R, de Mello FG, 1985. Expression of A1 adenosine receptors modulating dopamine-dependent cyclic AMP accumulation in the chick embryo retina. J. Neurochem. 44, 845–851. [DOI] [PubMed] [Google Scholar]
- Paes-De-Carvalho R, 2002. Adenosine as a signaling molecule in the retina: biochemical and developmental aspects. An. Acad. Bras. Cienc. 74, 437–451. [DOI] [PubMed] [Google Scholar]
- Paes-de-Carvalho R, Dias BV, Martins RA, Pereira MR, Portugal CC, Lanfredi C, 2005. Activation of glutamate receptors promotes a calcium-dependent and transporter-mediated release of purines in cultured avian retinal cells: possible involvement of calcium/calmodulin-dependent protein kinase II. Neurochem. Int. 46, 441–451. [DOI] [PubMed] [Google Scholar]
- Paes-de-Carvalho R, Maia GA, Ferreira JM, 2003. Adenosine Regulates the Survival of Avian Retinal Neurons and Photoreceptors in Culture. Neurochem. Res. 28, 1583–1590. [DOI] [PubMed] [Google Scholar]
- Pafundo DE, Chara O, Faillace MP, Krumschnabel G, Schwarzbaum PJ, 2008. Kinetics of ATP release and cell volume regulation of hyposmotically challenged goldfish hepatocytes. American Journal of Physiology-Regulatory, Integr. Comp. Physiol. 294(1), R220–R233. [DOI] [PubMed] [Google Scholar]
- Pannicke T, Fischer W, Biedermann B, Schädlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A, 2000. P2X7 receptors in Müller glial cells from the human retina. J. Neurosci. 20(16), 5965–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Catsicas M, Becker D, Mobbs P, 2002. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J. Neurosci. 22, 7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P, 2005. ATP released via gap junction hemichanels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46, 731–744 [DOI] [PubMed] [Google Scholar]
- Pereira MR, Hang VR, Vardiero E, de Mello FG, Paes-de-Carvalho R, 2010. Modulation of A1 adenosine receptor expression by cell aggregation and long-term activation of A2a receptors in cultures of avian retinal cells: Involvement of the cyclic AMP/PKA pathway. J. Neurochem. 113, 661–673. [DOI] [PubMed] [Google Scholar]
- Perez de Lara MJ, Guzman-Aranguez A, de la Villa P, Diaz- Hernandez JI, Miras-Portugal MT, Pintor J, 2015. Increased levels of extracellular ATP in glaucomatous retinas: Possible role of the vesicular nucleotide transporter during the development of the pathology. Mol. Vis. 21, 1060–1070. [PMC free article] [PubMed] [Google Scholar]
- Perez de Lara MJ, Pintor J, 2015. Presence and release of ATP from the retina in an Alzheimer’s disease model. J. Alzheimer’s Dis. 43(1), 177–181. [DOI] [PubMed] [Google Scholar]
- Perez MT, Ehinger BE, Lindstrom K, Fredholm BB, 1986. Release of endogenous and radioactive purines from the rabbit retina. Brain Res. 398, 106–112. [DOI] [PubMed] [Google Scholar]
- Perez MT, Bruun A, 1987. Colocalization of (3H)-adenosine accumulation and GABA immunoreactivity in the chicken and rabbit retinas. Histochemistry 87, 413–417. [DOI] [PubMed] [Google Scholar]
- Perígolo-Vicente R, Ritt K, Gonçalves-de-Albuquerque CF, Castro- Faria-Neto HC, Paes-de-Carvalho R, Giestal-de-Araujo E, 2014. IL-6, A1 and A2aR: A crosstalk that modulates BDNF and induces neuroprotection. Biochem. Biophys. Res. Commun. 449, 477–482. [DOI] [PubMed] [Google Scholar]
- Perígolo-Vicente R, Ritt K, Pereira MR, Torres PM, Paes-de- Carvalho R, Giestal-de-Araujo E, 2013. IL-6 treatment increases the survival of retinal ganglion cells in vitro: The role of adenosine A1 receptor. Biochem. Biophys. Res. Commun. 430, 512–518. [DOI] [PubMed] [Google Scholar]
- Peterson WM, Meggyesy C, Yu K, Miller SS, 1997. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J. Neurosci. 17(7), 2324–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Eychene A, 2001. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 93 (1–2), 53–62. [DOI] [PubMed] [Google Scholar]
- Podgorska M, Kocbuch K, Pawelczyk T, 2005. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochimica Polonica. 52, 749–758. [PubMed] [Google Scholar]
- Portillo JC, Lopez Corcino Y, Dubyak GR, Kern TS, Matsuyama S, Subauste CS, 2016. Ligation of CD40 in Human Muller Cells Induces P2X7 Receptor-Dependent Death of Retinal Endothelial Cells. Invest. Ophthalmol. Vis. Sci. 57(14), 6278–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J, 2009. ATP release from non-excitable cells. Purinergic Signal. 5(4), 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager P, Hollborn M, Steffen A, Wiedemann P, Kohen L, Bringmann A, 2016. P2Y1 Receptor Signaling Contributes to High Salt- Induced Priming of the NLRP3 Inflammasome in Retinal Pigment Epithelial Cells. PLoS One 11(10), e0165653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Fletcher EL, 2004. Synaptic localization of P2X7 receptors in the rat retina. J. Comp. Neurol. 472(1), 13–23. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Fletcher EL, 2007. Neuronal expression of P2X3 purinoceptors in the rat retina. Neurosci. 146(1), 403–414. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Fletcher EL, 2009. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J. Comp. Neurol. 513(4), 430–440. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D, 2010. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28- dependent, let-7 microRNA signalling pathway. Nature Cell Biol. 12(11), 1101 –1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M, Lamas M, 2009. NMDA receptor mediates proliferation and CREB phosphorylation in postnatal Müller glia-derived retinal progenitors. Mol. Vis.15, 713–721. [PMC free article] [PubMed] [Google Scholar]
- Rao MB, Didiano D, Patton JG, 2017. Neurotransmitter-Regulated Regeneration in the Zebrafish Retina. Stem Cell Rep. 8(4), 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Reifler MJ, Rivlin PK, 1988. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. Dev. Neurobiol. 19(5), 431–463. [DOI] [PubMed] [Google Scholar]
- Rego AC, Santos MS, Oliveira CR, 1997. Adenosine triphosphate degradation products after oxidative stress and metabolic dysfunction in cultured retinal cells. J. Neurochem. 69, 1228–1235. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A 2016. Role of Purines in Muller Glia. J. Ocul. Pharmacol. Ther. 32(8), 518–533. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A, 2016. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology 104, 194–211. [DOI] [PubMed] [Google Scholar]
- Reigada D, Lu W, Mitchell CH, 2006. Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J. Physiol. 575(Pt 3), 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang M, Mitchell CH, 2008. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neurosci. 157(2), 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Mitchell CH, 2005. Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am. J. Physiol. Cell Physiol. 288(1), C132–140. [DOI] [PubMed] [Google Scholar]
- Resta V, Novelli E, Di Virgilio F, Galli-Resta L, 2005. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 132, 2873–2882. [DOI] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L, 2007. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur. J. Neurosci. 25(9), 2741–2754. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2005. A Circadian Clock and Light/Dark Adaptation Differentially Regulate Adenosine in the Mammalian Retina. J Neurosci. 25, 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricatti MJ, Alfie LD, Lavoie EG, Sévigny J, Schwarzbaum PJ, Faillace MP, 2009. Immunocytochemical localization of NTPDases1 and 2 in the neural retina of mouse and zebrafish. Synapse 63(4), 291–307. [DOI] [PubMed] [Google Scholar]
- Ricatti MJ, Battista AG, Zorrilla Zubilete M, Faillace MP, 2011. Purinergic signals regulate daily S-phase cell activity in the ciliary marginal zone of the zebrafish retina. J. Biol. Rhythms 26(2), 107–117. [DOI] [PubMed] [Google Scholar]
- Sanches G, Alencar LS, Ventura ALM, 2002. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Intl. J. Dev. Neurosci. 20, 21–27. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH, 2014. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp. Eye Res. 127, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos PF, Caramelo OL, Carvalho AP, Duarte CB, 1999. Characterization of ATP release from cultures enriched in cholinergic amacrine- like neurons. J. Neurobiol. 41(3), 340–348. [PubMed] [Google Scholar]
- Scemes E, Duval N, Meda P, 2003. Reduced Expression of P2Y1 Receptors in Connexin43-Null Mice Alters Calcium Signaling and Migration of Neural Progenitor Cells. J. Neurosci. 23(36), 11444–11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer JM, Anderson SM, 1981. Nucleoside uptake by rat retina cells. Life Sci. 29, 939–946. [DOI] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB, 2003. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 15, 813–827. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME, 1999. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68, 821–861. [DOI] [PubMed] [Google Scholar]
- Shigematsu Y, Shimoda Y, Kaneda M, 2007. Distribution of immunoreactivity for P2X3, P2X5, and P2X6-purinoceptors in mouse retina. J. Mol. Histol. 38(4), 369–371. [DOI] [PubMed] [Google Scholar]
- Sholl-Franco A, Fragel-Madeira L, Macama ACCM, Linden R, Ventura ALM, 2010. ATP controls cell cycle and induces proliferation in the mouse developing retina. Intl. J. Dev. Neurosci. 28, 63–73. [DOI] [PubMed] [Google Scholar]
- Sidman RL, 1961. Histogenesis of mouse retina studied with thymidine- H3 In: Smelser GK. (Ed.), The Structure of the Eye. Academic Press, London, pp. 487–506. [Google Scholar]
- Stoniecka M, Backman LJ, Danielson P, 2015. Acetylcholine enhances keratocyte proliferation through muscarinic receptor activation. Int. Immunopharmacol. 29(1), 57–62. [DOI] [PubMed] [Google Scholar]
- Socodato R, Britto R, Calaza KC, Paes-de-Carvalho R, 2011. Developmental regulation of neuronal survival by adenosine in the in vitro and in vivo avian retina depends on a shift of signaling pathways leading to CREB phosphorylation or dephosphorylation. J. Neurochem. 116, 227–239. [DOI] [PubMed] [Google Scholar]
- Socodato RE, Magalhães CR, Paes-de-Carvalho R, 2009. Glutamate and nitric oxide modulate ERK and CREB phosphorylation in the avian retina: evidence for direct signaling from neurons to Müller glial cells. J. Neurochem.108(2), 417–429. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB, 1999. Dynamics of retinal waves are controlled by cyclic AMP. Neuron 24, 673–685. [DOI] [PubMed] [Google Scholar]
- Striedinger K, Meda P, Scemes E, 2007. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia 55(6), 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme KM, Yazulla S, 1997. 3H-adenosine uptake selectively labels rod horizontal cells in goldfish retina. Vis. Neurosci. 14, 207–212. [DOI] [PubMed] [Google Scholar]
- Sugioka M, Fukuda Y, Yamashita M, 1996. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J. Physiol. 493, 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka M, Zhou WL, Hoffmann HD, Yamashita M, 1999a. Ca2+ mobilization and capacitive Ca2+ entry regulate DNA synthesis in cultured chick retinal neuroepithelial cells. Intl. J. Dev. Neurosci. 17(3), 163–172. [DOI] [PubMed] [Google Scholar]
- Sugioka M, Zhou WL, Hofmann HD, Yamashita M 1999b. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Intl. J. Dev. Neurosci. 17(2), 135–144. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Lee SY, Horie T, Oku H, Takai S, Tanioka H, Kuriki Y, Kojima S, Ikeda T, 2013. P2X7 receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats. Mol. Vis. 19, 2080–2091. [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T, 2010. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Inv. Ophthalmol. Vis. Sci, 51, 3236–3243. [DOI] [PubMed] [Google Scholar]
- Sullivan DM, Erb L, Anglade E, Weisman GA, Turner JT, Csaky KG, 1997. Identification and characterization of P2Y2 nucleotide receptors in human retinal pigment epithelial cells. J. Neurosci. Res. 49, 43–52. [DOI] [PubMed] [Google Scholar]
- Sundaram M, Yao SY, Ng AM, Griffiths M, Cass CE, Baldwin SA, Young JD, 1998. Chimeric constructs between human and rat equilibrative nucleoside transporters (hENT1 and rENT1) reveal hENT1 structural domains interacting with coronary vasoactive drugs. J. Biol. Chem. 273, 21519–21525. [DOI] [PubMed] [Google Scholar]
- Suplat D, Krzeminski P, Pomorski P, Baranska J, 2007. P2Y1 and P2Y12 receptor cross-talk from nonstarved and long-term serum-deprived glioma C6 cells. Purinergic Signal. 3, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi M, Shinozaki Y, Kashiwagi K, Shigetomi E, Robaye B, Koizumi S, 2016. Müller cell-mediated neurite outgrowth of the retinal ganglion cells via P2Y6 receptor signals. J. Neurochem. 136, 741–751. [DOI] [PubMed] [Google Scholar]
- Than-Trong E, Bally-Cuif L, 2015. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 63(8), 1406–1428. [DOI] [PubMed] [Google Scholar]
- Todd L, Volkov LI, Zelinka C, Squires N, Fischer AJ, 2015. Heparin-binding EGF-like growth factor (HB-EGF) stimulates the proliferation of Müller glia-derived progenitor cells in avian and murine retinas. Mol. Cell. Neurosci. 69, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckermann O, Grosche J, Reichenbach A, Bringmann A, 2002. ATP- evoked Calcium Responses of Radial Glial (Mu ¨ ller) Cells in the Postnatal Rabbit Retina. J. Neurosci. Res. 70, 209–218. [DOI] [PubMed] [Google Scholar]
- Uckermann O, Uhlmann S, Weick M, Pannicke T, Francke M, Reichenbach A, Wiedemann P, Bringmann A, 2003. Upregulation of purinergic P2Y receptor-mediated calcium responses in glial cells during experimental detachment of the rabbit retina. Neurosci. Lett. 338, 131–134 [DOI] [PubMed] [Google Scholar]
- Uckermann O, Wolf A, Kutzera F, Kalisch F, Beck-Sickinger AG, Wiedemann P, Reichenbach A, and Bringmann A 2006. Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J. Neurosci. Res. 83, 538–550 [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Boya P, De La Rosa EJ, 2009. Early neural cell death: numbers and cues from the developing neuroretina. Intl. J. Dev. Biol. 53 (8–10), 1515 –1528. [DOI] [PubMed] [Google Scholar]
- Vitanova LA, Kupenova PN, 2014. Ionotropic purinergic receptors P2X in frog and turtle retina: glial and neuronal localization. Acta Histochem. 116(5), 694–701. [DOI] [PubMed] [Google Scholar]
- Vogler S, Pannicke T Hollborn M, Kolibabka M, Wiedemann P, Reichenbach A, Hammes HP, Bringmann A, 2016. Impaired Purinergic Regulation of the Glial (Muller) Cell Volume in the Retina of Transgenic Rats Expressing Defective Polycystin-2. Neurochem. Res. 41(7), 1784–1796. [DOI] [PubMed] [Google Scholar]
- Vroman R, Klaassen LJ, Howlett MH, Cenedese V, Klooster J, Sjoerdsma T, Kamermans M, 2014. Extracellular ATP hydrolysis inhibits synaptic transmission by increasing ph buffering in the synaptic cleft. PLoS Biol. 12(5), e1001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L, Pannicke T, Rupprecht V, Frommherz I, Volz C, Illes P, Hirrlinger J, Jagle H, Egger V, Haydon PG, Pfrieger FW, Grosche A, 2017. Suppression of SNARE-dependent exocytosis in retinal glial cells and its effect on ischemia-induced neurodegeneration. Glia 65(7), 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Vogler S, Grosche A, Pannicke T, Ueffing M, Wiedemann P, Reichenbach A, Hauck SM, Bringmann A, 2013. Osteopontin inhibits osmotic swelling of retinal glial (Muller) cells by inducing release of VEGF. Neurosci. 246, 59–72. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Dale N, 2007. Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release. J. Physiol. 581, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D, 2012. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev. Cell, 22(2), 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhao XF, Vojtek A, Goldman D, 2014. Retinal injury, growth factors, and cytokines converge on β-catenin and pStat3 signaling to stimulate retina regeneration. Cell Reports, 9(1), 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fridman A, Blackledge W, Connelly S, Wilson IA, Pilz RB, Boss GR, 2009. The phosphatidylinositol 3-kinase/akt cassette regulates purine nucleotide synthesis. J. Biol. Chem. 284(6), 3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MM, Fletcher EL, 2009. Subsets of retinal neurons and glia express P2Y1 receptors. Neurosci. 160, 555–566. [DOI] [PubMed] [Google Scholar]
- Ward MM, Puthussery T, Fletcher EL, 2008. Localization and possible function of P2Y(4) receptors in the rodent retina. Neurosci. 155(4), 1262–1274. [DOI] [PubMed] [Google Scholar]
- Weick M, Wiedemann P, Reichenbach A, Bringmann A, 2005. Resensitization of P2Y Receptors by Growth Factor-Mediated Activation of the Phosphatidylinositol-3 Kinase in Retinal Glial Cells. Invest. Ophthalmol. Vis. Sci. 46(4), 1525–1532. [DOI] [PubMed] [Google Scholar]
- Weuste M, Wurm A, landiev I, Wiedemann P, Reichenbach A, Bringmann A, 2006. HB-EGF: increase in the ischemic rat retina and inhibition of osmotic glial cell swelling. Biochem. Biophys. Res. Commun. 347(1), 310–318. [DOI] [PubMed] [Google Scholar]
- Wurm A, Erdmann I, Bringmann A, Reichenbach A, Pannicke T, 2009. Expression and function of P2Y receptors on Müller cells of the postnatal rat retina. Glia 57, 1680–1690. [DOI] [PubMed] [Google Scholar]
- Wurm A, Pannicke T, landiev I, Francke M, Hollborn M, Wiedemann P, Reichenbach A, Osborne NN and Bringmann A, 2011. Purinergic signaling involved in Muller cell function in the mammalian retina. Prog. Retin. Eye Res. 30(5), 324–342. [DOI] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH, 2012. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J. Physiol. 590(10), 2285–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Zhu Y, 2015. The Regulation of Sox2 and Sox9 stimulated by ATP in Spinal Cord Astrocytes. J. Mol. Neurosci. 55, 131–140. [DOI] [PubMed] [Google Scholar]
- Xue B, Xie Y, Xue Y, Hu N, Zhang G, Guan H, Ji M, 2016a. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Müller cells. Exp. Eye Res. 153, 42–50. [DOI] [PubMed] [Google Scholar]
- Xue Y, Xie Y, Xue B, Hu N, Zhang G, Guan H, Ji M, 2016b. Activated Müller Cells Involved in ATP-Induced Upregulation of P2X7 Receptor Expression and Retinal Ganglion Cell Death. Biomed. Res. Int. 2016, 9020715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, 2013. From neuroepithelial cells to neurons: Changes in the physiological properties of neuroepithelial cells. Arch. Biochem. Biophys. 534, 64–70. [DOI] [PubMed] [Google Scholar]
- Yang D, Elner SG, Clark AJ, Hughes BA, Petty HR, Elner VM, 2011. Activation of P2X Receptors Induces Apoptosis in Human Retinal Pigment Epithelium. Invest. Ophthalmol. Vis. Sci. 52(3), 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Baciu P, Kerrigan BC, Etheridge M, Sung E, Toimil BA, Berchuck JE, Jaffe GJ, 2014. Retinal pigment epithelial cell death by the alternative complement cascade: role of membrane regulatory proteins, calcium, PKC, and oxidative stress. Invest. Ophthalmol. Vis. Sci. 55(5): 3012–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH, 2005. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 46, 2183–2191. [DOI] [PubMed] [Google Scholar]
- Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, et al. , 2006a. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol. Vis. 16, 937–948. [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH, 2006b. Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation. J. Neurochem. 98, 566 −575. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH, 2007. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp. Eye Res. 85(5), 637–643. [DOI] [PubMed] [Google Scholar]
- Zhang M, Hu H, Zhang X, Lu W, Lim J, Eysteinsson T, et al. , 2010. The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem. Int. 56, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PP, Yang XL, Zhong YM, 2012. Cellular localization of P2Y6 receptor in rat retina. Neurosci. 220, 62–69. [DOI] [PubMed] [Google Scholar]
- Zhao XF, Ellingsen S, Fjose A, 2009. Labelling and targeted ablation of specific bipolar cell types in the zebrafish retina. BMC Neurosci. 10(1), 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Braun N, 1999. Ecto-nucleotidases--molecular structures, catalytic properties, and functional roles in the nervous system. Prog. Brain Res. 120, 371–385. [PubMed] [Google Scholar]


