Abstract
Background
Response prediction of certain biologic agents for the treatment of rheumatoid arthritis (RA) remains an unmet need in real-world clinical practice. The contribution of patient-reported components to the 28-joint Disease Activity Score (DAS28) was termed DAS28-P and investigated as a predictor of response to biologic agents, mostly tumor necrosis factor inhibitors. We aimed to evaluate DAS28-P as a predictor of the European League Against Rheumatism (EULAR) response to abatacept in patients with RA.
Methods
The study population was a prospective, observational, multicenter cohort of Korean patients with RA, who were followed up for a nationwide post-marketing surveillance study of abatacept. Correlation of DAS28-P with DAS28, change of DAS28, and EULAR response groups were evaluated. Logistic regression analysis was used to predict good-to-moderate EULAR response to abatacept in the study population.
Results
A total of 341 patients were involved in the analysis stratified on the EULAR response criteria. Presence of comorbidities, previous exposure to biologic agents, baseline DAS28, three of its components (tender joint counts, global health visual analog scale, erythrocyte sedimentation rate), and baseline DAS28-P were significantly associated with EULAR response to abatacept at 6 months. Stratified upon EULAR response, a group with good-to-moderate response had a higher baseline value and lower interval change for DAS28-P. Logistic regression analysis showed that a DAS28-P cut-off of > 0.44 was more positively associated with good-to-moderate EULAR response with abatacept treatment than naivety to biologic agents.
Conclusions
The DAS28-P could be predictive of response to abatacept. A higher baseline DAS28-P is associated with a favorable therapeutic response to abatacept.
Trial registration
Trial name, Korean Post-marketing Surveillance for Orencia®. Trial registration number, NCT01583244. Registered on April 20, 2012.
Electronic supplementary material
The online version of this article (10.1007/s40271-018-0347-z) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| A high subjective proportion of 28-joint Disease Activity Score (DAS28-P) is associated with a good-to-moderate EULAR response at 6 months after initiating abatacept in a real-world setting. |
| Decrease in DAS28-P is observed in the RA patients who had good-to-moderate EULAR response to abatacept. |
| RA patients with relatively more subjective complaints should not be ignored or excluded from the initiation of abatacept. |
Introduction
Therapeutic strategies for the treatment of rheumatoid arthritis (RA) were diversified by the advent of various biologic agents, but predicting their response in individual patients remains a challenge in real-world clinical practice [1]. To reduce trial and error when selecting a biologic agent, previous studies have attempted to identify clinical or biochemical markers to forecast good or poor response [2, 3]. One of the well-known examples of an association for good response to a particular biologic agent and its predictor is between rituximab and seropositivity status, either rheumatoid factor or anti-cyclic citrullinated peptide antibody (ACPA) [4]. Seropositivity was also shown to have a similar association with abatacept, but not with tocilizumab [5, 6]. However, most of the other proposed predictors have not demonstrated strong enough evidence to support clinical decision-making, or are too complex to be used universally. Discovering easily accessible, cost-effective predictive markers for treatment response would, thus, be beneficial for both patients and healthcare systems.
Recently, patient-reported outcomes (PROs) have been globally applied as endpoints in clinical trials to measure disease activity in patients with RA [7]. PROs are reliable in describing a patient’s perspective and symptoms, but are not as efficient as biomarkers such as C-reactive protein or erythrocyte sedimentation rate (ESR) in reflecting objective inflammation [8]. Considering that biologic agents aim to block specific inflammatory targets, their effect on PROs would be regarded as a relatively indirect outcome of biologic agents. However, recent studies have reported the role of PROs as predictors of objective outcomes, such as radiographic progression or therapeutic response to biologic agents [9, 10].
The patient-reported components of the 28-joint Disease Activity Score (DAS28) comprise tender joint count (TJC) and the global health visual analog scale (VAS-GH) [11], the combination of which is termed DAS28-P [12]. As these patient-reported components can be affected by non-inflammatory conditions, such as combined osteoarthritis or fibromyalgia [12–14], higher DAS28-P was suspected as a factor predicting poor response to biologic agents [15]. However, a study by Jurgens et al. conversely showed, though without statistical significance, an unexpected decrease in DAS28-P in patients with good (DAS28 at endpoint ≤ 3.2 and improvement of DAS28 from baseline ≤ 1.2) or moderate (DAS28 at endpoint ≤ 5.1 and improvement of DAS28 from baseline > 0.6 and ≤ 1.2; DAS28 at endpoint > 3.2 and improvement of DAS28 from baseline ≤ 1.2) European League Against Rheumatism (EULAR) response compared with the non-response group, where treatment was mostly with tumor necrosis factor (TNF) inhibitors [15, 16].
Abatacept (cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin, CTLA4-Ig) is a selective co-stimulation modulator with an inhibitory effect on T lymphocytes via binding to human CD80 and CD86 cells on antigen-presenting cells [16]. Biologic factors, such as ACPA positivity or number of specific circulating T cells, were reported as potential predictors of good therapeutic response to abatacept [5, 17], while previous clinical trials found that abatacept was associated with significant improvements in both conventional measures of disease activity and PROs [18]. In this study, we aimed to evaluate whether baseline DAS28-P is a predictor of EULAR response to abatacept after 6 months’ treatment in patients with RA.
Materials and Methods
Patients
The study population was a prospective, observational, multicenter cohort of Korean patients with RA, who were followed up for a nationwide post-marketing surveillance study of abatacept from March 2010 to March 2016. All of the study subjects met the 1987 American College of Rheumatology criteria of RA [19] and provided written informed consent. This study was reviewed and approved by the institutional and ethical review boards of the 40 participating hospitals, including Seoul National University Hospital (IRB No. 1206-051-414).
Clinical Assessment
Baseline characteristics included age, sex, seropositivity, disease duration, current comorbidities, previous history of disease-modifying antirheumatic drug (DMARD) use, and biologic agents. Injection route of abatacept was subcutaneous or intravenous. Collected components of DAS28 (TJC, VAS-GH, swollen joint count [SJC], and ESR) at 0 and 6 months were included for analysis, and DAS28 and DAS28-P, a contribution of subjective components of DAS28, were calculated by means of the following formulas [11, 12]. Achievement of the criteria for low disease activity (DAS28 < 3.2) and for remission (DAS28 < 2.6) was also evaluated for each patient [11].
Statistical Methods
Univariate analysis including the Chi square test or Kruskal–Wallis test was conducted for each baseline characteristic variable versus three EULAR response criteria groups (good, moderate, and none). Correlation of baseline DAS28-P versus DAS28 or change of DAS28 for the initial 6-month treatment period was evaluated by scatter plot and Pearson’s coefficient (PC). After stratifying the study subjects into the three EULAR response groups, the mean value of baseline DAS28-P, or change in DAS28-P, was compared by Student’s t test. The last-observation-carried-forward (LOCF) method was used for imputation of missing data.
To evaluate baseline DAS28-P as a predictor of EULAR response to abatacept, a receiver operating characteristic (ROC) curve with area under the curve (AUC) was measured. A practical cut-off value to predict favorable response was derived from the ROC curve plus the maximum value for the sum of specificity and sensitivity. Including the cut-off value of DAS28-P, as well as variables from the univariate analysis with significance (p < 0.05), logistic regression analysis was conducted to deduce the predictive power of each variable associated with a good-to-moderate (favorable) EULAR response to abatacept in the study population. Statistical analysis was performed using SPSS version 18.0 (IBM Corp., USA). Odds ratios (ORs) and 95% confidence intervals were calculated, and statistical significance was inferred when p < 0.05.
Results
Baseline Characteristics
Overall, 529 patients were included for efficacy analysis in the post-marketing survey, and 341 adult patients with RA on newly initiated abatacept were included for analysis after applying a 6-month LOCF method (Fig. 1). The baseline characteristics of the study population were analyzed after stratification by EULAR response at 6 months (Table 1). Mean age (55.5 years) and disease duration (5.46 years) at enrollment were not significantly different among good, moderate, and non-response groups. Proportion of seropositivity (95.3%), previous use of DMARDs (85.6%), and subcutaneous administration of abatacept (11.1%) also showed no difference among response groups. However, patients with fewer comorbidities, as listed in Table 1 (p = 0.035), and those who were naive to biologic agents (p = 0.018) had better response to abatacept. Baseline DAS28 was highest in the moderate response group and lowest in the non-response group (p = 0.003). The baseline DAS28-P, SJC, TJC, VAS-GH, and ESR were the variables that deviated substantially from normality when evaluated by the Kolmogorov–Smirnov test (p < 0.05).
Fig. 1.
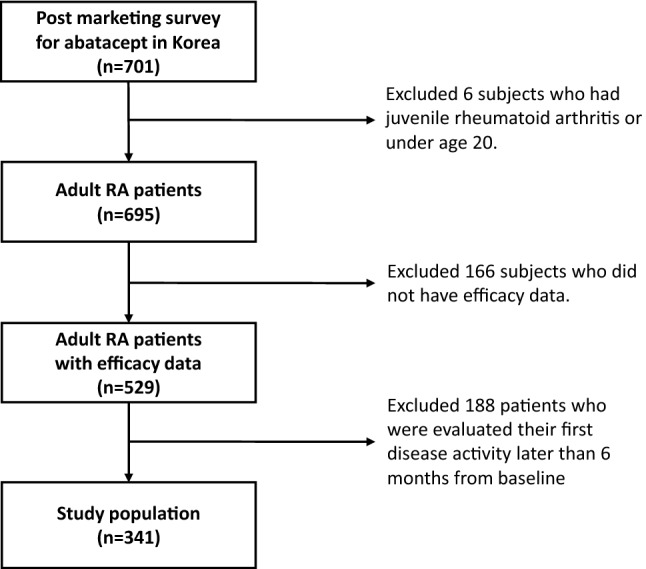
Flow chart for selection of patients included in the final analysis. RA rheumatoid arthritis
Table 1.
Baseline characteristics of the study population
| Total (n = 341) | EULAR response | ||||
|---|---|---|---|---|---|
| Good (n = 72) | Moderate (n = 178) | None (n = 91) | P value | ||
| Age, years | 55.5 ± 12.6 | 53.3 ± 12.0 | 56.0 ± 12.0 | 56.4 ± 13.6 | 0.255 (K) |
| Sex, female (%) | 283 (83.0) | 60 (83.3) | 145 (81.5) | 78(85.7) | 0.677 (C) |
| Body weight, kg | 56.8 ± 9.0 | 57.1 ± 10.4 | 57.1 ± 9.0 | 56.0 ± 7.5 | 0.861 (K) |
| Seropositivity (%) | |||||
| Double negativity | 16 (4.7) | 6 (8.3) | 3 (1.7) | 7 (7.7) | 0.145 (C) |
| Single ACPA positivity | 28 (8.2) | 4 (5.6) | 16 (9.0) | 8 (8.8) | |
| Single RF positivity | 79 (23.2) | 16 (22.2) | 39 (21.9) | 24 (26.4) | |
| Double positivity | 218 (63.9) | 46 (63.9) | 120 (67.4) | 52 (57.1) | |
| Disease duration, years | 5.46 ± 4.64 | 5.54 ± 4.67 | 5.22 ± 4.30 | 5.87 ± 5.30 | 0.820 (K) |
| Comorbidities (current) (%) | 251 (73.6) | 45 (62.5) | 133 (74.7) | 73 (80.2) | 0.035 (C) |
| Hypertension | 93 (27.3) | 19 (26.4) | 50 (28.1) | 24 (26.4) | |
| Diabetes | 42 (12.3) | 5 (6.9) | 20 (11.2) | 17 (18.7) | |
| Renal disease | 11 (3.2) | 3 (4.2) | 5 (2.8) | 3 (3.3) | |
| Osteoporosis | 77 (22.6) | 17 (23.6) | 32 (18.0) | 28 (30.8) | |
| Interstitial lung disease | 23 (6.7) | 2 (2.8) | 18 (10.1) | 3 (3.3) | |
| Liver disease | 15 (4.4) | 3 (4.2) | 9 (5.1) | 3 (3.3) | |
| Sjogren’s or sicca | 18 (5.3) | 4 (5.6) | 8 (4.5) | 6 (6.6) | |
| Fibromyalgia | 12 (3.5) | 2 (2.8) | 6 (3.4) | 4 (4.4) | |
| Previous DMARDs (%) | 292 (85.6) | 63 (87.5) | 148 (83.2) | 81 (89.0) | 0.379 (C) |
| Methotrexate | 57 (16.7) | 18 (25.0) | 25 (14.0) | 14 (15.4) | |
| Leflunomide | 124 (36.4) | 17 (23.6) | 67 (37.6) | 40 (44.0) | |
| Hydroxychloroquine | 152 (44.6) | 37 (51.4) | 76 (42.7) | 39 (42.9) | |
| Sulfasalazine | 105 (30.8) | 26 (36.1) | 48 (27.0) | 31 (34.1) | |
| TNF inhibitors | 135 (39.6) | 23 (31.9) | 65 (36.5) | 47 (51.6) | |
| Tocilizumab | 9 (2.6) | 0 (0.0) | 3 (1.7) | 6 (6.6) | |
| Rituximab | 8 (2.4) | 1 (1.4) | 4 (2.3) | 3 (3.3) | |
| Route of abatacept injection, SQ (%) | 38 (11.1) | 9 (12.5) | 16 (9.0) | 13 (14.3) | 0.391 (C) |
| Biologics exposure (%) | |||||
| Naive | 203 (59.5) | 48 (66.7) | 112 (62.9) | 43 (47.3) | |
| Experienced | 138(40.5) | 24 (33.3) | 66 (37.1) | 48 (52.7) | 0.018 (C) |
| DAS28 | 5.95 ± 1.21 | 5.52 ± 0.89 | 6.23 ± 1.04 | 5.75 ± 1.57 | <0.001 (K) |
| TJC, median (IQR) | 8 (5–14) | 6.5 (4–10) | 9 (5–16) | 6 (3–14) | 0.006 (K) |
| VAS-GH, median (IQR) | 70 (55–80) | 70 (50–80) | 77.5 (60–80) | 65 (50–80) | 0.005 (K) |
| SJC, median (IQR) | 6 (3–10) | 6 (3–9.5) | 6 (4–10) | 5 (2–11) | 0.207 (K) |
| ESR, median (IQR) (mm/h) | 50 (32–73) | 38.5 (26–53) | 55.5 (37–79) | 52 (32–78) | <0.001 (K) |
| DAS28-P, median (IQR) | 0.43 (0.38–0.47) | 0.43 (0.40–0.47) | 0.44 (0.39–0.48) | 0.42 (0.36–0.46) | 0.039 (K) |
The data were expressed as mean ± standard deviation, median (IQR), or number (percentage)
ACPA anti-cyclic citrullinated peptide antibody, (C) Chi square test, DAS28 28-joint Disease Activity Score, DAS28-P patient-reported component of DAS28, DMARD disease-modifying antirheumatic drug, ESR erythrocyte sedimentation rate, EULAR European League Against Rheumatism, IQR interquartile range, (K) Kruskal–Wallis test, RF rheumatoid factor, SJC swollen joint count, SQ subcutaneous, TNF tumor necrosis factor, TJC tender joint count, VAS-GH global health visual analog scale
Correlation Between DAS28-P and Therapeutic Response
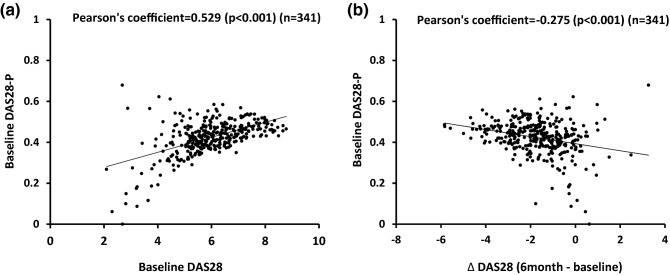
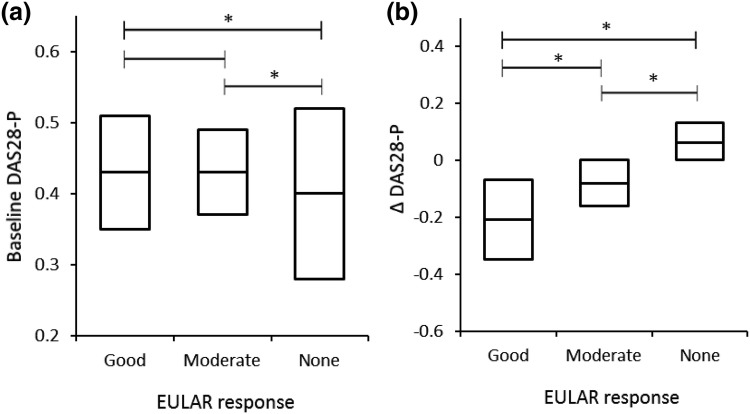
Baseline DAS28-P had modest positive correlation with baseline DAS28 (PC = 0.529, p < 0.001) (Fig. 2a) and a weak negative correlation with a change in DAS28 (PC = − 0.275, p < 0.001) (Fig. 2b). The patients with good or moderate EULAR response after 6 months had slightly higher baseline DAS28-P (median 0.43, interquartile range [IQR] 0.40–0.47; median 0.44, IQR 0.39–0.48, respectively) compared with the non-response group (median 0.42, IQR 0.36–0.46; p < 0.05 vs both groups) (Fig. 3a). Differences in change of DAS28-P at 6 months were more prominent among good (median − 0.22, IQR − 0.30 to − 0.11) and moderate (median −0.08, IQR − 0.13 to − 0.04) responders compared with the non-response group (median −0.01, IQR − 0.03 to 0.02) (Fig. 3b). Therefore, good or moderate response to abatacept after 6 months was associated with higher baseline DAS28-P and a decrease in DAS28-P when compared to the non-response group.
Fig. 2.
Correlations between a baseline DAS28-P and DAS28, and b baseline DAS28-P vs change in DAS28 (Δ DAS28), during the initial 6 months of abatacept treatment. DAS28 28-joint Disease Activity Score, DAS28-P patient-reported component of DAS28
Fig. 3.
Comparison of medians (with interquartile range) of DAS28-P among the groups with good, moderate or no EULAR response after the initial 6 months of treatment. a Baseline DAS28-P; b change in DAS28-P (Δ DAS28-P). DAS28 28-joint Disease Activity Score, DAS28-P patient-reported component of DAS28, EULAR European League Against Rheumatism
Cut-Off Value of DAS28-P to Predict Therapeutic Response
An ROC curve was drawn to estimate the performance of DAS28-P as a predictor of good-to-moderate response to abatacept at 6 months, but the AUC was relatively low (0.590, p = 0.014) (Fig. 4). The best cut-off value of DAS28-P as a predictor of good-to-moderate response was 0.44, which represents the maximum value for the sum of specificity and sensitivity. Specificity (71.4%) of the cut-off value (0.44) was modest, but sensitivity (48.8%) was poor.
Fig. 4.
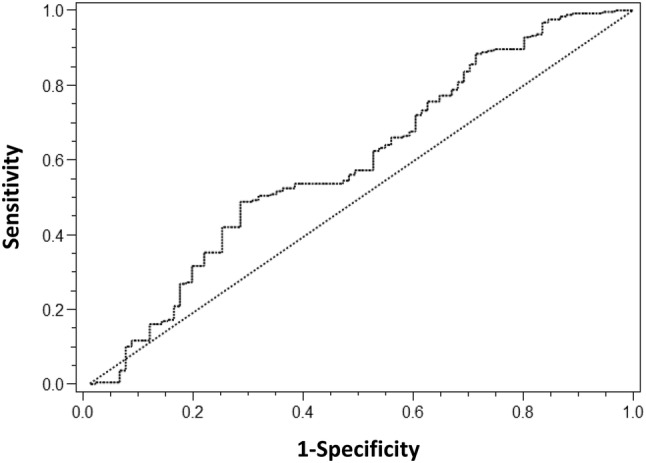
Receiver operating characteristic curve of DAS28-P to predict good-to-moderate EULAR response to abatacept (area under the curve = 0.590, p = 0.014). DAS28 28-joint Disease Activity Score, DAS28-P patient-reported component of DAS28, EULAR European League Against Rheumatism
Multivariate Analysis for Predictive Factors of Favorable EULAR Response
Including the elicited cut-off value of baseline DAS28-P, binary logistic regression analysis was conducted with the variables that had shown significance in the univariate analysis excluding the components of DAS28 (SJC, TJC, VAS-GH, ESR) and DAS28 itself due to evident collinearity with DAS28-P. The presence of fibromyalgia was additionally included as it was reported to have an association with DAS28-P recently (Table 2) [20]. Therefore, naivety to biologic agents, comorbidity except fibromyalgia (absence vs presence), fibromyalgia alone (absence vs presence), and the cut-off (> 0.44) value of DAS28-P were finally included variables for binary logistic regression. The Hosmer–Lemeshow test to measure the goodness of fit of this logistic regression model showed an adequate fit of the model to the data (χ2 = 8.092, p = 0.231).
Table 2.
Binary logistic regression analysis to predict factors indicating good-to-moderate EULAR response to abatacept during the initial 6 months of treatment
| Variables | β | SE | OR (95% CI) | P value |
|---|---|---|---|---|
| Intercept | − 0.218 | 0.663 | 0.742 | |
| Naive to biologic agents | 0.670 | 0.253 | 1.954 (1.190–3.211) | 0.008 |
| Comorbidity except fibromyalgia | 0.528 | 0.308 | 1.696 (0.927–3.102) | 0.087 |
| Fibromyalgia | 0.412 | 0.646 | 1.509 (0.425–5.353) | 0.524 |
| DAS28-P cut-off (> 0.44) | 0.855 | 0.268 | 2.350 (1.390–3.974) | 0.001 |
CI confidence interval, DAS28 28-joint Disease Activity Score, DAS28-P patient-reported component of DAS28, EULAR European League Against Rheumatism, OR odds ratio, SE standard error
As a result, baseline DAS28-P cut-off (0.44) was the strongest predictive factor for favorable (good-to-moderate) EULAR response to abatacept among the related variables (OR 2.350, p = 0.001). Being naive to biologic agents (OR 1.954, p = 0.008) was also associated positively with EULAR response. Comorbidities other than fibromyalgia and fibromyalgia alone were not significantly associated with favorable EULAR response.
Discussion
In this study, DAS28-P was evaluated as a predictor of EULAR response after 6 months of treatment with abatacept in patients with RA as part of post-marketing surveillance in Korea. Patients who had high DAS28-P value at baseline showed good-to-moderate (favorable) EULAR response after 6 months’ use of abatacept. At first glance, higher DAS28-P could be recognized as more complaints of pain and fatigue, which are characteristics of fibromyalgia. That would be a reason why the previous study by Jurgens et al. initially set a hypothesis that high DAS28-P is associated with worse response to biologic agent [15]. To interpret the findings of the current study, the actual meaning of the DAS28-P needs to be discussed. DAS28-P is not just an absolute sum of subjective complaints, but rather a measure of the contribution of subjective endpoints in the calculation of the DAS-28 (i.e., those complaints that can be affected by various kinds of discomfort other than joint pain). For example, higher burden of systemic or extra-articular inflammation of RA would be reflected as a higher DAS28-P because those manifestations could mainly affect VAS-GH. As abatacept restores immune tolerance by targeting T cells rather than neutralizing a single cytokine, it may potentially influence the disease more fundamentally than TNF inhibitors such as adalimumab, and contribute to improvement of VAS-GH. This would be concordant with the results of a phase IIIb trial of abatacept that showed better response of subjective pain or global assessment in the abatacept group compared to the adalimumab group [21].
Compared to previous studies that have reviewed DAS28-P as a predictive factor for therapeutic response of a biologic agent [3, 15], distinctive features of this study were (1) real-world population (in Korea) that initiated biologic therapy, (2) statistical difference of DAS28-P values among the three EULAR response groups, and (3) the study of abatacept, which was not included in the previous studies. Since the data are from an observational post-marketing surveillance study, the characteristics of the patients and quality of data acquisition might be heterogeneous. However, the national health insurance system in Korea strictly regulates the use of biologic agents with specific indications, and requires precise evaluation of therapeutic response of each patient to determine whether to continue co-payment. Approximately 90% of the patients who were using biologic agents met the insurance requirement. Hence, patients included in this study share some characteristics, such as previous failure with two or more DMARDs for at least 6 months, or a high rate of seropositivity, which were required factors of specific reimbursement for Korean RA patients.
This study has a few limitations. First, the predictive power of DAS28-P in abatacept users cannot be generalized in all RA patients. Because all of the RA patients who enrolled in this study were reimbursed a significant portion of the cost for abatacept from the Korean National Health Insurance system, they had to meet strict criteria (DAS28 > 5.1, failure with more than two kinds of DMARDs for 6 months). There was no comparator as this was a surveillance study for abatacept, and therefore, DMARD or other biologic agent users were not available for analysis. It is difficult to differentiate whether these observations on DAS28-P were unique to abatacept or a shared feature of improvement regardless of the therapeutic agent used. Applying DAS28-P as a predictive factor for response in a previously performed, randomized, placebo-controlled trial may reveal whether the marker was specific to a biologic agent. Second, though seropositivity was the most acknowledged predictive factor for therapeutic response of biologic agents, it was not demonstrated in this study. This phenomenon might be associated with the high portion of seropositivity (95.3%) in the patient pool, which would have arisen from the aforementioned strict health insurance policy in Korea. As evidence for this association, the cohort of a previous report described the correlation of seropositivity with response to abatacept, and it may have been possible to compare two groups by serostatus because of the lower seropositivity rates (65–75%) [5]. Third, the long-term efficacy data after the initial 6 months are not available. In this study, 73.3% of patients achieved good-to-moderate EULAR response at 6 months. Real-world efficacy data from the Danish registry showed that the rate was similar (70%) at the same time point, but increasing trends in the rate of good-to-moderate EULAR response or DAS28 remission were still maintained up to 48 weeks [22]. Predicting long-term response by baseline DAS28-P or by DAS28-P after baseline would be interesting topics for future research. Fourth, the cut-off value (0.44) of the DAS28-P derived from the ROC curve was still important in multivariate analysis, but was insufficiently sensitive (48.8%) and was not robust enough for clinical practice. It would be a real message of this study that there is no need to be reluctant to start abatacept because of the high subjective portion of patients with high disease activity.
We suggested DAS28-P as an easily accessible composite disease activity index reflecting subjective complaints, useful for predicting treatment response to abatacept. PROs, such as the Routine Assessment of Patient Index Data 3 (RAPID3), are well validated measurements of disease activity in RA patients, especially for clinical trials [23, 24]. Although PROs are more sophisticated measures of pain, function, and global health status than clinical measures, it is not easy to obtain such questionnaire-based PROs frequently in clinical practice. On the other hand, because DAS28-P is a component of DAS28 and a commonly used and well validated measure of disease activity in RA patients, it can be easily calculated by a clinician for his/her patient without incurring additional costs for biomarker laboratory testing, such as for ACPA, C-reactive protein, or ESR. It would be beneficial to evaluate DAS28-P data in a longitudinal, long-term follow-up study with other biologic agents or DMARDs, such as in insurance claim data. Validating concordance or interaction between other established PROs and DAS28-P can add more credibility for the use of DAS28-P as a unique disease activity index in RA patients.
Conclusions
In conclusion, DAS28-P, the subjective portion of DAS28, could predict response to abatacept. High baseline DAS28-P and a decrease at 6 months after treatment initiation were associated with a favorable (good-to-moderate) EULAR response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to all healthcare professionals and patients for participating in the post-marketing survey of abatacept in Korea.
Authors’ contributions
JSL participated in the study design, obtained data, conducted statistical analysis, and drafted and wrote the manuscript. HA participated in statistical analysis and revised the manuscript. SCS, SCB, and YWS participated in the study design, obtained data, and analyzed the data. EYL participated in the study design, obtained data and revised the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Funding
No funding was received for this study, and only the open access fee for this publication is funded by Bristol-Myers Squibb.
Conflict of interest
Harris Ahmad is an employee and shareholder of Bristol-Myers Squibb. Currently, Lee JS, Shin SC, Bae SC, Song YW, and Lee EY have no conflicts of interest.
Ethics approval and consent to participate
This study was conducted according to the Helsinki declaration and approved by the institutional review boards of the 40 participating hospitals as listed in the Supplementary Table 1 (see the electronic supplementary material). Written informed consent was obtained from all of the enrolled patients.
Data availability statement
All data generated or analyzed during this study are owned by Bristol-Myers Squibb and are not available to the public.
References
- 1.van den Broek M, Visser K, Allaart CF, Huizinga TW. Personalized medicine: predicting responses to therapy in patients with RA. Curr Opin Pharmacol. 2013;13(3):463–469. doi: 10.1016/j.coph.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Hyrich KL, Watson KD, Silman AJ, Symmons DP, British Society for Rheumatology Biologics R Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology. 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 3.McWilliams DF, Walsh DA. Factors predicting pain and early discontinuation of tumour necrosis factor-alpha-inhibitors in people with rheumatoid arthritis: results from the British society for rheumatology biologics register. BMC Musculoskelet Disord. 2016;17:337. doi: 10.1186/s12891-016-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacs JD, Cohen SB, Emery P, Tak PP, Wang J, Lei G, et al. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis. 2013;72(3):329–336. doi: 10.1136/annrheumdis-2011-201117. [DOI] [PubMed] [Google Scholar]
- 5.Gottenberg JE, Ravaud P, Cantagrel A, Combe B, Flipo RM, Schaeverbeke T, et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘Orencia and Rheumatoid Arthritis’ registry. Ann Rheum Dis. 2012;71(11):1815–1819. doi: 10.1136/annrheumdis-2011-201109. [DOI] [PubMed] [Google Scholar]
- 6.Maneiro RJ, Salgado E, Carmona L, Gomez-Reino JJ. Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: systematic review and meta-analysis. Semin Arthritis Rheum. 2013;43(1):9–17. doi: 10.1016/j.semarthrit.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Dehoratius RJ, Brent LH, Curtis JR, Ellis LA, Tang KL. Satisfaction with subcutaneous golimumab and its auto-injector among rheumatoid arthritis patients with inadequate response to adalimumab or etanercept. The Patient. 2018;11(3):361–369. doi: 10.1007/s40271-018-0297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gossec L, Dougados M, Dixon W. Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis. RMD open. 2015;1(1):e000019. doi: 10.1136/rmdopen-2014-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis JR, Churchill M, Kivitz A, Samad A, Gauer L, Gervitz L, et al. A randomized trial comparing disease activity measures for the assessment and prediction of response in rheumatoid arthritis patients initiating certolizumab pegol. Arthritis Rheumatol. 2015;67(12):3104–3112. doi: 10.1002/art.39322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khawaja MN, Bergman MJ, Yourish J, Pei J, Reiss W, Keystone E. Routine Assessment of Patient Index Data 3 and the American College of Rheumatology/European League Against Rheumatism provisional remission definitions as predictors of radiographic outcome in a rheumatoid arthritis clinical trial with tocilizumab. Arthritis Res Ther. 2017;69(5):609–615. doi: 10.1002/acr.23008. [DOI] [PubMed] [Google Scholar]
- 11.van Riel PL. The development of the Disease Activity Score (DAS) and the disease activity score using 28 joint counts (DAS28) Clin Exp Rheumatol. 2014;32(5 Suppl 85):S-65–S-74. [PubMed] [Google Scholar]
- 12.McWilliams DF, Zhang W, Mansell JS, Kiely PD, Young A, Walsh DA. Predictors of change in bodily pain in early rheumatoid arthritis: an inception cohort study. Arthritis Res Ther. 2012;64(10):1505–1513. doi: 10.1002/acr.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joharatnam N, McWilliams DF, Wilson D, Wheeler M, Pande I, Walsh DA. A cross-sectional study of pain sensitivity, disease-activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther. 2015;17:11. doi: 10.1186/s13075-015-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McWilliams DF, Marshall M, Jayakumar K, Doherty S, Doherty M, Zhang W, et al. Erosive and osteoarthritic structural progression in early rheumatoid arthritis. Rheumatology. 2016;55(8):1477–1488. doi: 10.1093/rheumatology/kew197. [DOI] [PubMed] [Google Scholar]
- 15.Jurgens MS, Overman CL, Jacobs JW, Geenen R, Cuppen BV, Marijnissen AC, et al. Contribution of the subjective components of the disease activity score to the response to biologic treatment in rheumatoid arthritis. Arthritis Care Res. 2015;67(7):923–928. doi: 10.1002/acr.22532. [DOI] [PubMed] [Google Scholar]
- 16.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 17.Scarsi M, Ziglioli T, Airo P. Baseline numbers of circulating CD28-negative T cells may predict clinical response to abatacept in patients with rheumatoid arthritis. J Rheumatol. 2011;38(10):2105–2111. doi: 10.3899/jrheum.110386. [DOI] [PubMed] [Google Scholar]
- 18.Massarotti EM. Clinical and patient-reported outcomes in clinical trials of abatacept in the treatment of rheumatoid arthritis. Clin Ther. 2008;30(3):429–442. doi: 10.1016/j.clinthera.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Salaffi F, Di Carlo M, Carotti M, Sarzi-Puttini P. The subjective components of the Disease Activity Score 28-joints (DAS28) in rheumatoid arthritis patients and coexisting fibromyalgia. Rheumatol Int. 2018;38(10):1911–1918. doi: 10.1007/s00296-018-4096-z. [DOI] [PubMed] [Google Scholar]
- 21.Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leffers HC, Ostergaard M, Glintborg B, Krogh NS, Foged H, Tarp U, et al. Efficacy of abatacept and tocilizumab in patients with rheumatoid arthritis treated in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis. 2011;70(7):1216–1222. doi: 10.1136/ard.2010.140129. [DOI] [PubMed] [Google Scholar]
- 23.Ruta DA, Hurst NP, Kind P, Hunter M, Stubbings A. Measuring health status in British patients with rheumatoid arthritis: reliability, validity and responsiveness of the short form 36-item health survey (SF-36) Br J Rheumatol. 1998;37(4):425–436. doi: 10.1093/rheumatology/37.4.425. [DOI] [PubMed] [Google Scholar]
- 24.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol. 2008;35(11):2136–2147. doi: 10.3899/jrheum.080182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are owned by Bristol-Myers Squibb and are not available to the public.