Abstract
This study was undertaken to investigate the impact of culture pH (4.5–6.5) and temperature (32–37 °C) on the stress resilience of Lactobacillus reuteri DSM 17938 during freeze-drying and post freeze-drying exposure to low pH (pH 2) and bile salts. Response-surface methodology analysis revealed that freeze-drying survival rates were linearly related to pH with the highest survival rate of 80% when cells were cultured at pH 6.5 and the lowest was 40% when cells were cultured at pH 4.5. The analysis further revealed that within the chosen temperature range the culture temperature did not significantly affect the freeze-drying survival rate. However, fermentation at pH 4.5 led to better survival rates when rehydrated cells were exposed to low pH shock or bile salts. Thus, the effect of pH on freeze-drying survival was in contrast to effects on low pH and bile salts stress tolerance. The rationale behind this irreconcilability is based on the responses being dissimilar and are not tuned to each other. Culturing strain DSM 17938 at pH values higher than 5.5 could be a useful option to improve the survivability and increase viable cell numbers in the final freeze-dried product. However, the dissimilar responses for the process- and application parameters tested here suggest that an optimal compromise has to be found in order to obtain the most functional probiotic product possible.
Keywords: Probiotics, Fermentation technologies, Lactobacillus reuteri DSM 17938, Freeze-drying, Bile salt, Survivability
Introduction
As stated by Rosenstiel and Stange (2010), probiotics are living microorganisms that—if taken in appropriate dosage—may result in a health benefit for the host. The probiotic market has increased in recent years. In 2015, it has exceeded 35 billion USD and is expected to reach revenues of 74 billion USD in 2024 (Grand View Research Inc. 2016). To help realize this expectation, it is essential to better understand how production processes influence product quality. Thus, evaluation of the impact of different production variables on the yield and survivability of the cells in the freeze-dried product is essential to the development of stable probiotics.
Cell stress tolerance is an important issue in the development of stable probiotic products based on freeze-dried formulations of Lactic Acid Bacteria (LAB). Particularly in the case of probiotic bacteria to be ingested, tolerance to low pH in the stomach and bile salts in the intestine is crucial to achieve efficient bioactivity (Yadav and Shukla 2017). Among the different culture variables, temperature, pH, and dissolved oxygen concentration, have previously been shown to be particularly important for the stabilization of freeze-dried bacterial cells of the genera Lactobacillus (Schoug et al. 2008; Liu et al. 2014; Béal and Fonseca 2015) and Bifidobacterium (Mozzetti et al. 2013).
This have also been shown in preconditioning or physiological condition of bacterial biomass subjected to freeze-drying, where mild abiotic stressors applied during or immediately after the fermentation can improve desiccation tolerance and the stability of dried formulations (Palmfeldt and Hahn-Hägerdal 2000; Liu et al. 2014).
To date, the most studied bacterial genera with probiotic properties are Lactobacillus and Bifidobacterium (Sánchez et al. 2017). Lactobacillus reuteri DSM 17938 is the active ingredient of several probiotic products (e.g. BioGaia Protectis) (Rosander et al. 2008; Mu et al. 2018) and this strain is stabilized by freeze-drying to achieve storage stability of the final product.
Although the fermentation process of the strain DSM 17938 has been well characterized (Burgé et al. 2015; Strömberg et al. 2017; van Niel et al. 2017; Mauro and Garcia 2019), the impact of different fermentation variables on the survival of this strain to freeze-drying and several of the subsequent gastro-intestinal tract (GIT) stress conditions (i.e. low pH and bile salts) has not been deeply explored. In this study, we have evaluated the impact of both culture pH and temperature on the survival rate of strain DSM 17938 under these stress conditions. The results shown here will be of relevance for future development of stable desiccated formulations of L. reuteri DSM 17938 with high levels of probiotic activity.
Materials and methods
Microorganisms and culture conditions
Lactobacillus reuteri DSM 17938 (Rosander et al. 2008), kindly provided by BioGaia AB, was used for all experiments. A previously established and controlled cryopreserved cell bank was used (Garcia et al. 2017) as inoculum throughout this study. In total 12 fermentation batches at 1-L were performed. Each experiment was started from cells retrieved from this cell bank, 10 mL vials containing 10 mL MRS medium (Merck) were inoculated with 100 μL of the cell bank. Each vial was capped and incubated statically for 6 h at 37 °C and the resulting culture was used as the inoculum for the next stage. 1 mL from the 10-mL preculture was inoculated to 500 mL flask containing 500 mL MRS and this culture was incubated statically for 16 h at 37 °C. The fermentations at 1-L scale were performed in 1.8 L Jenny bioreactors (Belach Bioteknik AB). One hundred fifty milliliter from the 500-mL preculture were inoculated into 850 mL MRS medium (pH- and temperature-controlled fermentation) (Strömberg et al. 2017).
The starting pH was 6.5 and the pH control was kept at 4.5, 5.5, or 6.5 using NaOH 3.7 M once the culture reached the set value. The culture temperature was set to either 32 °C or 37 °C according to the planned factorial design. Every batch fermentation was performed for a total of 26 h; thus, all cultures were well in the stationary phase at which cells are better adapted to stressful conditions, which ensures that cells survive better to desiccation (Meng et al. 2008), and the growth was determined by OD600 measurement with spectrophotometer Ultrospec 1100 pro.
Freeze-drying procedure
At the end of each fermentation process, 500 mL of the culture was harvested and centrifuged according to Teixeira et al. (1997) but for 30 min instead of 10 min, at 5000×g at 24 °C. The pellet was suspended in 50 mL sucrose 10% (w/v) with a spatula and thoroughly mixed by gentle shaking using a vortex until a homogenous slurry was obtained. One mL of this concentrated cell suspension was dispensed into freeze-drying glass vials (35 vials per batch) and the vials were frozen in an ultrafreezer at − 50 °C for 2 h before being transferred to a Labconco FreeZone® Stoppering Tray Freeze-Dryer. The freeze-drying scheme was set-up as follows: Freezing step: − 40 °C for 3 h; Main drying: − 40 °C for 18 h at 0.2 mbar, − 20 °C for 70 h at 0.2 mbar; secondary drying: 5 °C increments every 2 h up to 20 °C at 0.01 mbar. At the end of the process the vials were capped under vacuum and stored at − 50 °C until further use. For every experimental condition, the freeze-dried cells were rehydrated by triplicate with 1 mL of saline solution (NaCl 9 g/L) for determining the survival rates.
Viable count
For every sample, serial dilutions were done. For each dilution, 10 µL were plated (Jett et al. 1997) (in triplicate) on MRS agar plates and incubated at 37 °C for 18 h. The resulting colonies were counted and the viability (cfu/mL) value was calculated based on the plated dilution.
Bile and low pH stress in survival assays
Porcine bile (B8631; Sigma) and bovine bile (B3883; Fluka) were diluted in MRS (Merck) to a final concentration of 0.5% (w/v) and 1% (w/v) bile salts, respectively. Low pH MRS was prepared by adding 1 M HCl to MRS, lowering the pH to pH 2. Freeze-dried cell samples were rehydrated for 20 min with 1 mL saline solution, vortexed and diluted 100 times in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) to reach a target concentration. One mL samples were spun down for 2 min at 16,100×g. The cells were then resuspended in 1 mL MRS-bile salts or low pH-MRS and vortexed, immediately after which 100 µL samples were taken for flow cytometry analysis. The remaining samples with MRS-bile salt or low pH-MRS were then incubated at 37 °C and new samples for survivability assays were taken every 30 min. The survival of the cells was checked with a flow cytometer (BD Accuri C6 plus flow cytometer with a BD C sampler (BD Biosciences, Franklin Lakes, NJ, USA) using a protocol described below.
Flow cytometry
The flow cytometry protocol is based on the standard staining method of the Swiss Federal Institute of Aquatic Science and Technology, Eawag, Switzerland (Hammes et al. 2008) for analyzing the quality of drinking water. It makes use of the two different dyes SYBR® Green I (Life Technologies) and Propidium Iodide (PI) (Sigma-Aldrich) for staining of the cells. The SYBR Green I penetrates all cells and binds selectively to double-stranded DNA (Zipper et al. 2004) and cells with compromised membranes are stained by PI that binds to DNA and RNA, whereas PI is excluded from cells with intact membranes due to the positive charge of PI (Shapiro 2003). Samples were diluted in PBS with the addition of 1 mM EDTA (Fluka) and 0.01% (v/v) Tween 20 (Sigma), to achieve a cell concentration of 1 × 103–5 × 106 cells/mL. For each sample a mix of 5 μL of 100X SYBR® Green I (diluted in DMSO) and 1 μL PI (1 mg/mL) was used. The final volume of each sample was 506 μL. The samples were vortexed and then incubated in the dark for 15 min at 37 °C. After incubation, the samples were vortexed again and analyzed on the BD Accuri C6 plus flow cytometer with the fast fluid speed (flow rate of 66 μL/min), a sample volume of 50 μL and using a FL1-H and FL3-H threshold of 1000. Tubes with milli-Q-water were run between samples to rinse the system. The blue 488 nm laser was used with the optical filters FL1 (533 ± 30 nm) and FL3 (> 670 nm). The data were collected and analyzed using the BD Accuri C6 Plus software. A log scale density plot of FL1-H vs FL3-H was obtained to visualize the fluorescence of the dyes. Instrument settings and electronic gates were kept the same for all samples to achieve comparable data.
Data analysis
A response surface methodology (RSM) was applied using a script previously written in Python 2.7 by the authors to process the experimental data. The resulting response surface and contours were graphed and fitted to the equation of a plane with intercept 0 assuming only the pure linear effect of these two factors on the survival rate since it was a screening design. The significance of the model coefficients was evaluated with the same script using the package Statsmodels.api (Python 2.7). The data of the post-stress tests were analyzed with ANOVA-single factor in Microsoft Office Excel 2007.
Results
Fermentation behavior at 1 L scale
The initial number of cells is an important variable to be considered when bacterial cells are preserved by desiccation methods (Morgan et al. 2006; García 2011), and most often the aim is to maximize the yield at the end of the fermentation (Alonso 2016). In addition, the physiological state of the bacterial cells is crucial to achieve high survival rates when drying. For instance, the change in unsaturated to saturated fatty acids ratio to keep the membrane fluidity, the active synthesis of stress proteins to protect cell structures, the expression of antioxidative defenses such as superoxide dismutase (SOD), the presence of compatible solutes, and the uptake of Mn2+ are among the mechanisms to prevent the desiccation damage (Potts et al. 2005; Garcia 2011). To evaluate the possible combined effects of pH and temperature on the biomass production of strain DSM 17938, the growth curves (Fig. 1a) and the growth phases were defined as follows: (i) exponential phase (between 2 and 8 h for cells cultivated at 32 °C, and between 2 and 5 h for cells cultivated at 37 °C), (ii) early stationary phase (between 10 and 12 h for cells cultivations at 32 °C, and between 7 and 9 h for cultivations at 37 °C), and (iii) late-stationary phase (after 15–16 h for cultures performed at 32 °C and after 9–10 h for cultivations at 37 °C). The specific growth rate (µMAX) and generation time (tg) were estimated by fitting the growth curves to the logistic model (Peleg and Corradini 2011) (Table 1). For every temperature condition, the target pH was reached and controlled at different stages of the culture: (1) pH 6.5 was controlled since the beginning of the fermentation, (2) pH 5.5, was reached at exponential phase, (3) pH 4.5 was reached at early stationary phase (data not shown).
Fig. 1.
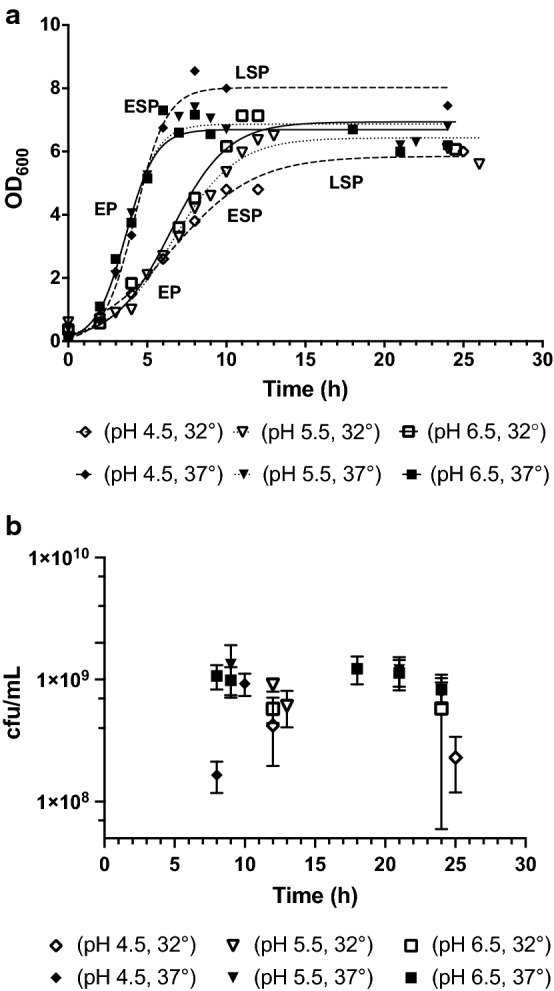
Fermentation of DSM 17938 during the 1-L scale under different conditions of pH and temperature. a Growth curves. b Cell concentration over time. The cells were cultured in MRS under anaerobic conditions and agitation speed 100 rpm. n = 2 replicates. EP exponential phase, ESP early stationary phase, LSP late stationary phase
Table 1.
Growth rate and generation time of Lactobacillus reuteri DSM 17938 cultured under different conditions
| Experimental condition | μmax (h−1) | tg (h) |
|---|---|---|
| pH 4.5, 32 °C | 0.51 ± 0.06 | 1.36 ± 0.16 |
| pH 4.5, 37 °C | 1.09 ± 0.22 | 0.64 ± 0.13 |
| pH 5.5, 32 °C | 0.55 ± 0.06 | 1.26 ± 0.14 |
| pH 5.5, 37 °C | 1.13 ± 0.21 | 0.61 ± 0.11 |
| pH 6.5, 32 °C | 0.59 ± 0.14 | 1.17 ± 0.28 |
| pH 6.5, 37 °C | 1.11 ± 0.23 | 0.62 ± 0.13 |
The fermentation was performed using MRS and different combinations of pH and temperature
The growth rate of the strain was twofold higher at 37 °C compared to 32 °C; moreover, the growth curves were similar at the three pH levels for every temperature in terms of growth rate and generation time (Table 1); although small differences were shown at pH 4.5 for both temperatures regarding OD600: at 32 °C the growth curve showed slightly lower absorbance values than at other pH, and in contrast, at 37 °C the absorbance values were slightly higher.
In addition, the cell viability of the cultures was monitored between 8 and 25 h. Even for the most severe culture conditions tested (pH 4.5 at late-stationary phase) the cell concentration reached between 108 and 109 cfu/mL and in all the variants, the cell viability was above 108 cfu/mL (Fig. 1b).
Effect of temperature and culture pH on freeze-drying survival rates
To determine the impact of the culture variables pH and temperature on the freeze-drying survival of DSM 17938 cells cultured at two temperatures (32 and 37 °C) in combination with three pH levels (4.5, 5.5, and 6.5), a response surface methodology (RSM) was used (Fig. 2). No significant impact of temperature on the survival rates was found for the tested temperatures 32 to 37 °C (control) (P-value = 0.338). However, a highly significant impact of increased culture pH on the survival rate was found (P-value = 0.001) (Table 2). Some fluctuations were observed in the response (Fig. 2), which may be due to the treatment of the samples since they were stored at − 50 °C immediately after every freeze-drying process and rehydrated the next day in the morning at room temperature.
Fig. 2.

Freeze-drying survival rate of DSM 17938 as function of pH and temperature. A RSM was applied and the data were fitted to a linnear model. n = 2 replicates
Table 2.
Statistical analysis corresponding to the factorial design performed
| Dependent variable: survival rate (%) | R-squared: 0.977 |
| Method: least squares | Adj. R-squared: 0.972 |
| Number of observations: 12 | F-statistic: 209.9 |
| Degrees of freedom residuals: 10 | Probability (F-statistic): 6.82 × 10−9 |
| Degrees of freedom: 2 | |
| Coefficient for Temperature: − 0.534 | Probability: 0.338 |
| Coefficient for pH: 14.305 | Probability: 0.001 |
A Python script was written for the analysis. Survival rate was the dependent variable, and pH and temperature the independent factors. The surface was fitted to a plane with intercept 0
Post-stress tests survival rates
To investigate if fermentation conditions had any effect on tolerance to bile salts and low pH, stress survival tests on rehydrated freeze-dried cells were performed and analyzed using flow cytometry. Two different origins of bile salts were chosen. Bovine bile salts are known to be less inhibitory than that of porcine, the latter is similar to human bile salts (Grill et al. 2000). The two bile stress tests showed similar tendency in that cells cultured at higher pH possessed lower survival rates during these stress tests (P-value with porcine and bovine bile salts at 37 °C being 0.018 and 0.011, respectively) (Fig. 3a). The difference between the bile salt stress tests concerned only the inhibition capacity of the two different bile salts, i.e., 0.5% porcine bile killed more cells than 1% bovine bile.
Fig. 3.
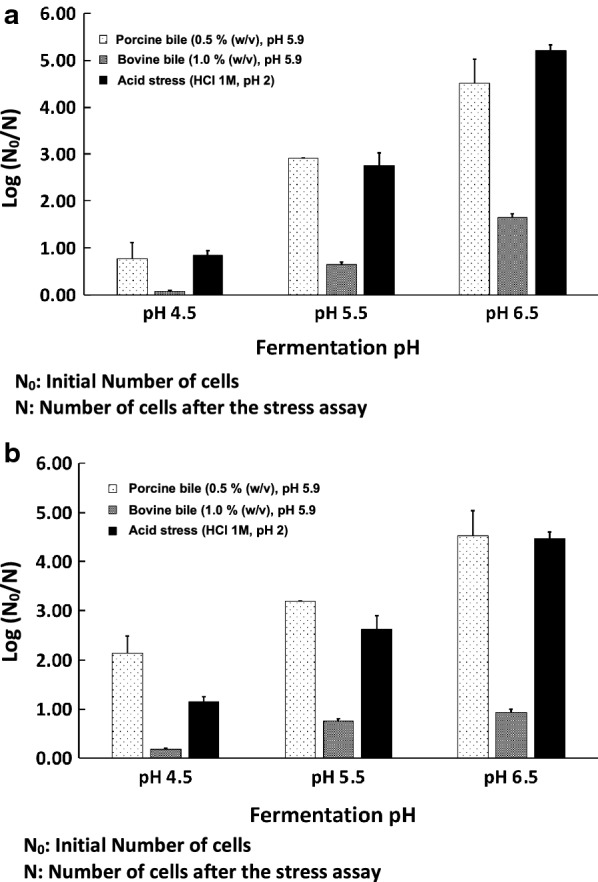
Stress survival tests on rehydrated freeze-dried DSM 17938 as function of pH and temperature. a Cells cultivated at 37 °C. b Cells cultivated at 32 °C
The low pH stress tests led to lower stress survival rates when cells were grown at higher pH (P-value of 0.0005 at 37 °C) which was similar to what was observed with the bile salt test (Fig. 3a).
The same trends for the bile- and low-pH stress tests were observed at 32 °C (Fig. 3b) as for 37 °C, with P-values between 0.003 and 0.041.
Discussion
This study was carried out to determine the effects of temperature and pH as growth parameters on the survivability of L. reuteri DSM 17938 to freeze-drying and to subsequent GIT conditions of rehydrated cells to mimic the process- and application stresses encountered by probiotic bacterial cells in both production and use. Different combinations of growth pH levels and temperatures were tested. The values of the specific growth rate (µMAX) and generation time (tg) of the fermentations at 37 °C and pH 5.5 (Table 1) are in the range previously reported (Burgé et al. 2015; van Niel et al. 2017). In addition, since the growth rate and generation time were similar for every pH condition at the same temperature, it suggests that the growth of the DSM 17938 is not limited by pH when it grows without pH control until the fermentation reaches pH 4.5. Only the OD600 showed differences between the variants at the end of the culture, likely because of the auto-aggregation phenomenon which has been previously reported to be less extensive in another L. reuteri strain (TMW1.106) when cultivated in MRS and exposed to pH 4 (Walter et al. 2008).
The twofold increase in growth rate at 37 °C as compared to 32 °C irrespective of culture pH, reflects that the growth of DSM 17938 is temperature-sensitive in the narrow temperature interval investigated here. In addition, the OD600 at pH 4.5 behaved differently at the two temperatures. The higher absorbance observed at 37 °C might be related to a lower degree of auto-aggregation of the cells than observed at 32 °C. A similar trend has been observed in L. johnsonii CRL 1294 where the auto-aggregation percentage decreased from 76.7% to 67.8% when cultured in MRS at pH 5 at 30 °C and 44 °C, respectively (Juárez Tomás et al. 2005). The distribution of cell aggregates might therefore depend on the combination of pH and temperature. At higher temperature and lower pH, auto-aggregation seems to be less extensive for DSM 17938. The phenomenon of auto-aggregation should be evaluated in further studies since it is of importance for biofilm formation in the colonization of probiotic bacteria (Juárez Tomás et al. 2005; Leccese Terraf et al. 2014).
The viability of strain DSM 17938 remained above 108 cfu/mL despite the exposure of the cells to an acidic environment (pH 4.5) (Fig. 1b). Similar behavior has been observed in the parental strain ATCC 55730 when exposed to more drastic conditions (pH 2.7) for one hour (Wall et al. 2007). It would be interesting in future work to test the survival of DSM 17938 under such drastic conditions and simultaneously perform intracellular pH assays to determine if cells manage to keep their pH homeostasis.
Preconditioning or pre-adaptation of cells during fermentation has notable impact on the survival rates during freeze-drying of probiotic bacteria (Shin et al. 2018; Anandharaj et al. 2017). For instance, the pre-adaptation by both heat and acid stress has shown to improve the freeze-drying survival rates in Enterococcus faecium HL7 (Shin et al. 2018). In our study, a 5 °C variation in culture temperature does not promote additional protection against desiccation by freeze-drying, whereas increased culture pH positively impacts the desiccation tolerance of this probiotic strain in the experimental interval tested (Table 2). Studies with other Lactobacillus species subjected to freeze-drying have shown that an increase of 8 °C in early stationary phase did not influence the freeze-drying survival rate. However, a decrease of 8 °C drastically reduced the freeze-drying survival of this strain as compared to when it was grown at 34 °C and pH 5.5 (Schoug et al. 2008). Regarding the impact of pH on freeze-drying survival, studies performed in L. rhamnosus GG revealed that cells harvested at late stationary phase grown under uncontrolled pH survived freeze-drying better than cells grown under controlled pH; however, the opposite effect was observed when cells were harvested in early stationary phase (Ampatzoglou et al. 2010).
In our study, the survival rates of DSM 17938 after freeze-drying are in the range published for other lactobacilli (Martos et al. 2007; Schoug et al. 2008; Liu et al. 2014). According to these results, formulation of the cells in 10% sucrose provides sufficient protection during freeze-drying to allow for a comparative study of growth condition effects on freeze-drying survival. Our findings differ from that reported by Palmfeldt and Hahn-Hägerdal (2000) for the parental strain ATCC 55730. According to these authors, the freeze-drying survival rate of the parental strain, as assessed by colony forming units, increases when the pH of the culture is decreased. However, their formulation differed since they used skim milk as lyoprotectant instead of sucrose which should be kept in mind when comparing the results. In a more recent study with L. reuteri I5007, Liu et al. (2014) have shown results similar to ours, with higher survival rates at pH 6.7 than at pH 4.7 as determined by viable cell counts. These researchers cultivated their strain in MRS and exposed it to various stress conditions, including pH stress (4.7 to 6.7), heat shock at 47 °C, and cold shock at 27 °C, from which they concluded that the highest survival rate was achieved when cells were subjected to pH 6.7 at late stationary phase. Therefore, culturing strain DSM 17938 at higher pH could be a useful option to improve survivability and therefore higher number of viable cells in the final product.
The pH of the fermentation culture influenced post-desiccation stresses (low-pH stress and bile salt stress) survival in an opposite manner as it did for freeze-drying survival per se. A lower culture pH enabled cells to survive exposure to low pH and bile salts better than for cells cultured at higher pH. Preconditioning through exposure to mild pH stress to boost survivability to a more severe pH shock has been observed for many other microorganisms (Skandamis et al. 2008; Cañamás et al. 2009). Proteomic studies with another L. reuteri strain (ATCC 23272) revealed which key proteins were overexpressed to adapt to these conditions, and included proteins related to transport, energy metabolism, biosynthesis of amino acids and nucleotides, and pH homeostasis (Lee et al. 2008a). Interestingly, similar key proteins were overexpressed in strain ATCC 23272 when exposed to purified bile salts (Lee et al. 2008b). This supports our observation that cells grown at lower pH have better survivability to bile salt exposure. In addition, similar finding has been reported for L. reuteri ATCC 55730, the parental strain of DSM 17938 (Whitehead et al., 2008). These authors found that genes implicated in protection to bile also protect against acid stress in L. reuteri, which could explain our results for freeze-dried DSM 17938.
For the fermentation temperature range studied herein (32 to 37 °C), no significant effect on the survivability in post-desiccation stress exposures was observed. Therefore, larger differences in temperature should be tested in future experiments, or alternatively, the biomass subjected to heat shock at sub-lethal temperatures to verify if significant differences are observed in freeze-drying survival rate.
As discussed above and as observed in other studies, preconditioning at low pH improves survivability during a pH and bile salt shock (Yadav and Shukla 2017; Chen et al. 2017). Moreover, the stress responses to an acidic pH have become better understood, but apparently these responses are detrimental for freeze-drying tolerance. Instead, the improvement of the latter emerges during growth at higher pH (around neutral) and might be related to a higher ratio of unsaturated fatty acids to saturated fatty acids in the cell membrane (Wang et al. 2005; Liu et al. 2014), which will be evaluated in further experiments, as well as the analysis of membrane fluidity by flow cytometry (Marielle and Sarrah 2017). Thus, responses do occur in the cells that are divergent and not completely streamlined for the series of stress events the L. reuteri DSM 17938 cells will have to undergo when produced and used. This demands that an appropriate solution for maximizing survivability over the whole life cycle from production to consumption is found: either (i) a compromise weighing in all the different encountered stress characteristics, or (ii) by providing the probiotic bacteria in capsules to avoid direct exposure to bile and acid in the GIT that would also allow for production of more freeze-drying tolerant biomass by growing at higher pH.
A subsequent comparative and functional proteomics and transcriptomics would be the appropriate tool to analyze how the cells in such a process become more tolerant to the freeze-drying stress.
In conclusion, culture pH in the range of 4.5–6.5 had a strong influence on freeze drying and post-desiccation stress survival whilst growth temperature in the range of 32–37 °C had no significant effects. Survivability to freeze drying is better for cells grown at higher pH, whereas survivability to GIT conditions is better for cells grown at lower pH. Culturing strain DSM 17938 at higher pH values could be a useful option to improve survivability and therefore higher viable cell numbers in the final product, however an optimization of the process should be performed to improve the resistance of these cells to the acidic environment in the stomach.
Acknowledgements
The authors acknowledge financial support from BioGaia AB. The authors also thank Dr. Elke Lohmeier-Vogel for critically reading the manuscript.
Authors’ contributions
SH, SR and EWJvanN designed and supervised the experiments. AH designed and executed the experiments, and also wrote the Python Script to process the data. CL designed and executed the experiments. RS executed part of the experiments. All authors read and approved the final manuscript.
Funding
This research was funded by BioGaia AB.
Availability of data and materials
All data and material are available without any restriction.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Armando Hernández, Phone: +46729402365, Email: armando.hernandez.garcia@slu.se.
Christer U. Larsson, Email: christer.larsson@tmb.lth.se
Radoslaw Sawicki, Email: radoslaw.sawicki@qlinea.com.
Ed W. J. van Niel, Email: ed.van_niel@tmb.lth.se
Stefan Roos, Email: stefan.roos@slu.se.
Sebastian Håkansson, Email: sebastian.hakansson@slu.se.
References
- Alonso S. Novel preservation techniques for microbial cultures. In: Ojha KS, Tiwari BK, editors. Novel food fermentation technologies. Basel: Springer International Publishing Switzerland; 2016. pp. 7–33. [Google Scholar]
- Ampatzoglou A, Schurr B, Deepika G, Baipong S, Charalampopoulos D. Influence of fermentation on the acid tolerance and freeze drying survival of Lactobacillus rhamnosus GG. Biochem Eng J. 2010;52:65–70. doi: 10.1016/j.bej.2010.07.005. [DOI] [Google Scholar]
- Anandharaj M, Rani RP, Swain MR. Microbial functional foods and nutraceuticals. In: Gupta VK, Treichel H, Shapaval VO, de Oliveira LA, Tuohy MG, editors. Production of high‐quality probiotics by fermentation. Chichester: Wiley & Sons; 2017. p. 235. [Google Scholar]
- Béal C, Fonseca F. Freezing of probiotic bacteria. In: Foerst P, Santivarangkna C, editors. Advances in probiotic technology. Boca Raton: CRC Press; 2015. p. 179. [Google Scholar]
- Burgé G, Saulou-Bérion C, Moussa M, Allais F, Athes V, Spinnler HE. Relationships between the use of Embden Meyerhof pathway (EMP) or Phosphoketolase pathway (PKP) and lactate production capabilities of diverse Lactobacillus reuteri strains. J Microbiol. 2015;53:702–710. doi: 10.1007/s12275-015-5056-x. [DOI] [PubMed] [Google Scholar]
- Cañamás TP, Viñas I, Abadias M, Usall J, Torres R, Teixidó N. Acid tolerance response induced in the biocontrol agent Pantoea agglomerans CPA-2 and effect on its survival ability in acidic environments. Microbiol Res. 2009;164:438–450. doi: 10.1016/j.micres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Tang HY, Chiang ML. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017;66:20–27. doi: 10.1016/j.fm.2017.03.020. [DOI] [PubMed] [Google Scholar]
- García AH. Anhydrobiosis in bacteria: from physiology to applications. J Biosci. 2011;36:939–950. doi: 10.1007/s12038-011-9107-0. [DOI] [PubMed] [Google Scholar]
- Garcia AH, Herrmann AM, Håkansson S. Isothermal microcalorimetry for rapid viability assessment of freeze-dried Lactobacillus reuteri. Proc Biochem. 2017;55:49–54. doi: 10.1016/j.procbio.2017.01.012. [DOI] [Google Scholar]
- Grand View Research, Inc. (2016) Probiotics market. In: Market estimates and trend analysis. http://www.grandviewresearch.com/industry-analysis/probiotics-market. Accessed 20 Nov 2018.
- Grill JP, Cayuela C, Antoine JM, Schneider F. Isolation and characterization of a Lactobacillus amylovorus mutant depleted in conjugated bile salt hydrolase activity: relation between activity and bile salt resistance. J Appl Microbiol. 2000;89:553–563. doi: 10.1046/j.1365-2672.2000.01147.x. [DOI] [PubMed] [Google Scholar]
- Hammes F, Berney M, Wang Y, Vital M, Koster O, Egli T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008;42:269–277. doi: 10.1016/j.watres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- Juárez Tomás MS, Wiese B, Nader-Macías ME. Effects of culture conditions on the growth and auto-aggregation ability of vaginal Lactobacillus johnsonii CRL 1294. J Appl Microbiol. 2005;99:1383–1391. doi: 10.1111/j.1365-2672.2005.02726.x. [DOI] [PubMed] [Google Scholar]
- Leccese Terraf MC, Mendoza LM, Juárez Tomás MS, Silva C, Nader-Macías MEF. Phenotypic surface properties (aggregation, adhesion and biofilm formation) and presence of related genes in beneficial vaginal lactobacilli. J Appl Microbiol. 2014;117:1761–1772. doi: 10.1111/jam.12642. [DOI] [PubMed] [Google Scholar]
- Lee K, Lee HG, Pi K, Choi YJ. The effect of low pH on protein expression by the probiotic bacterium Lactobacillus reuteri. Proteomics. 2008;8:1624–1630. doi: 10.1002/pmic.200700663. [DOI] [PubMed] [Google Scholar]
- Lee K, Lee H-G, Choi Y-J. Proteomic analysis of the effect of bile salts on the intestinal and probiotic bacterium Lactobacillus reuteri. J Biotechnol. 2008;137:14–19. doi: 10.1016/j.jbiotec.2008.07.1788. [DOI] [PubMed] [Google Scholar]
- Liu XT, Hou CL, Zhang J, Zeng XF, Qiao SY. Fermentation conditions influence the fatty acid composition of the membranes of Lactobacillus reuteri I5007 and its survival following freeze-drying. Lett Appl Microbiol. 2014;59:398–403. doi: 10.1111/lam.12292. [DOI] [PubMed] [Google Scholar]
- Marielle B, Sarrah G. Assessment of bacterial membrane fluidity by flow cytometry. J Microbiol Meth. 2017;143:50–57. doi: 10.1016/j.mimet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Martos GI, Minahk CJ, de Valdez GF, Morero R. Effects of protective agents on membrane fluidity of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Lett Appl Microbiol. 2007;45:282–288. doi: 10.1111/j.1472-765X.2007.02188.x. [DOI] [PubMed] [Google Scholar]
- Mauro CS, Garcia S. Coconut milk beverage fermented by Lactobacillus reuteri: optimization process and stability during refrigerated storage. J Food Sci and Technol. 2019;56:854–864. doi: 10.1007/s13197-018-3545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XC, Stanton C, Fitzgerald GF, Daly C, Ross RP. Anhydrobiotics: the challenges of drying probiotic cultures. Food Chem. 2008;106:1406–1416. doi: 10.1016/j.foodchem.2007.04.076. [DOI] [Google Scholar]
- Morgan CA, Herman N, White PA, Vesey G. Preservation of micro-organisms by drying; a review. J Microbiol Methods. 2006;66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Mozzetti V, Grattepanche F, Berger B, Rezzonico E, Arigoni F, Lacroix C. Fast screening of Bifidobacterium longum sublethal stress conditions in a novel two-stage continuous culture strategy. Benef Microbes. 2013;4:167–178. doi: 10.3920/BM2012.0026. [DOI] [PubMed] [Google Scholar]
- Mu Q, Tavella VJ, Luo XM. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. 2018;9:757. doi: 10.3389/fmicb.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmfeldt J, Hahn-Hägerdal B. Influence of culture pH on survival of Lactobacillus reuteri subjected to freeze-drying. Int J Food Microbiol. 2000;55:235–238. doi: 10.1016/S0168-1605(00)00176-8. [DOI] [PubMed] [Google Scholar]
- Peleg M, Corradini MG. Microbial growth curves: what the models tell us and what they cannot. Crit Rev Food Sci Nutr. 2011;51:917–945. doi: 10.1080/10408398.2011.570463. [DOI] [PubMed] [Google Scholar]
- Potts M, Slaughter SM, Hunneke FU, Garst JF, Helm RF. Desiccation tolerance of prokaryotes: application of principles to human cells. Integr Comp Biol. 2005;45:800–809. doi: 10.1093/icb/45.5.800. [DOI] [PubMed] [Google Scholar]
- Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol. 2008;74:6032–6040. doi: 10.1128/AEM.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel P, Stange EF. Probiotics and intestinal diseases. Ann Nutr Metab. 2010;57:27–28. doi: 10.1159/000309094. [DOI] [PubMed] [Google Scholar]
- Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- Schoug A, Fischer J, Heipieper HJ, Schnürer J, Håkansson S. Impact of fermentation pH and temperature on freeze-drying survival and membrane lipid composition of Lactobacillus coryniformis Si3. J Ind Microbiol Biotechnol. 2008;35:175–181. doi: 10.1007/s10295-007-0281-x. [DOI] [PubMed] [Google Scholar]
- Shapiro HM. Practical flow cytometry. 4. Hoboken: Wiley; 2003. p. 300. [Google Scholar]
- Shin Y, Kang CH, Kim W, So JS. Heat adaptation improved cell viability of probiotic Enterococcus faecium HL7 upon various environmental stresses. Probiotics Antimicrob Proteins. 2018 doi: 10.1007/s12602-018-9400-4. [DOI] [PubMed] [Google Scholar]
- Skandamis PN, Yoon Y, Stopforth JD, Kendall PA, Sofos JN. Heat and acid tolerance of Listeria monocytogenes after exposure to single and multiple sublethal stresses. Food Microbiol. 2008;25:294–303. doi: 10.1016/j.fm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Strömberg S, Connolly E, Roos S (2017) Method of activating lactic acid bacteria. U.S. Patent 2017/0304376 A1. Infant Bacterial Therapeutics AB
- Teixeira P, Castro H, Mohácsi-Farkas C, Kirby R. Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J Appl Microbiol. 1997;83:219–226. doi: 10.1046/j.1365-2672.1997.00221.x. [DOI] [PubMed] [Google Scholar]
- Van Niel EWJ, Bergdahl B, Hahn-Hägerdal B. Close to the edge: growth restrained by the NAD (P) H/ATP formation flux ratio. Front Microbiol. 2017;8:1149. doi: 10.3389/fmicb.2017.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall T, Båth K, Britton RA, Jonsson H, Versalovic J, Roos S. The early response to acid shock in Lactobacillus reuteri involves the ClpL chaperone and a putative cell wall-altering esterase. Appl Environ Microbiol. 2007;73:3924–3935. doi: 10.1128/AEM.01502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Schwab C, Loach DM, Gänzle MG, Tannock GW. Glucosyltransferase A (GtfA) and inulosucrase (Inu) of Lactobacillus reuteri TMW1. 106 contribute to cell aggregation, in vitro biofilm formation, and colonization of the mouse gastrointestinal tract. Microbiology. 2008;154:72–80. doi: 10.1099/mic.0.2007/010637-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Corrieu G, Béal C. Fermentation pH and Temperature Influence the Cryotolerance of Lactobacillus acidophilus RD758. J Dairy Sci. 2005;88:21–29. doi: 10.3168/jds.S0022-0302(05)72658-8. [DOI] [PubMed] [Google Scholar]
- Whitehead K, Versalovic J, Roos S, Britton RA. Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri ATCC 55730. Appl Environ Microbiol. 2008;74:1812–1819. doi: 10.1128/AEM.02259-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Shukla P. An overview of advanced technologies for selection of probiotics and their expediency: a review. Crit Rev Food Sci Nutr. 2017;57:3233–3242. doi: 10.1080/10408398.2015.1108957. [DOI] [PubMed] [Google Scholar]
- Zipper H, Brunner H, Bernhagen J, Vitzthum F. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004;32:e103. doi: 10.1093/nar/gnh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available without any restriction.


